Abstract
Interferon alpha-producing plasmacytoid dendritic cells (pDC) are crucial contributors to pro-inflammatory or tolerogenic immune responses and are important in autoimmune diseases such as psoriasis. pDC accumulate in the lesional skin of psoriasis patients, but are rarely found in the affected skin of patients with atopic dermatitis (AD). While homeostatic chemokine CXCL12 and inducible pro-inflammatory CXCR3 chemokine ligands may regulate pDC influx to psoriatic skin, the mechanism responsible for selective pDC recruitment in psoriasis vs. AD remains unknown. Circulating pDC from normal donors express a limited number of chemoattractant receptors, including CXCR3 and CMKLR1 (chemokine-like receptor 1). In this work, we demonstrate that circulating pDC from normal donors as well as psoriasis and AD patients express similar levels of CXCR3 and responded similarly in functional migration assays to CXCL10. We next found that blood pDC from normal, AD, and psoriasis patients express functional CMKLR1. In contrast to normal skin, however, lesional skin from psoriasis patients contains the active form of the CMKLR1 ligand chemerin. Furthermore, in affected skin from psoriatic patients the level of active chemerin was generally higher then in AD skin. Taken together, these results indicate that local generation of active chemerin may contribute to pDC recruitment to psoriatic skin.
Keywords: immunology, chemoattractant, dendritic cell, psoriasis, atopic dermatitis
Introduction
Myeloid and plasmacytoid dendritic cells (mDC and pDC, respectively) are the two principal subpopulations of dendritic cells. pDC are morphologically similar to plasma cells and are distinguished functionally from mDC by their ability to produce massive amounts of type I interferons in response to viral and microbial stimuli [1]. Both mDC and pDC can be differentially recruited to promote variable types of immunologic reactions [2]. Blood pDC express several chemokine receptors, including CCR2, CCR5, CCR7, CXCR3 and CXCR4 [3]. However, a consistent migratory response has been demonstrated only for CXCR4 ligand-CXCL12, and CXCR3 ligands, such as CXCL10, when given in combination with CXCL12 [4]. The CXCR3 ligands, such as inflammatory, IFN-induced chemokine, CXCL10, may synergize with constitutively expressed CXCL12 to direct pDC to inflamed skin [4]. In addition, recent data indicate that immature pDC are attracted by chemerin, the only known protein ligand for serpentine, chemokine-like receptor 1 (CMKLR1), also known as ChemR23 [5].
pDC and type I interferons have been implicated in the pathogenesis of lupus erythematosus (SLE) and psoriasis [6, 7]. Although pDC are absent in normal skin, lesional skin from patients suffering from SLE are characterized by substantial numbers of pDC [8, 9]. In contrast, few infiltrating pDC are present in skin lesions of atopic dermatitis (AD) patients [10-13]. pDC are CLA+ (cutaneous lymphocyte antigen), and may use this skin homing adhesion molecule to infiltrate the dermis [14]. In addition, pDC-responsive chemokines such as CXCL12, CXCL9, CXCL10, and CXCL11 are found in lesional skin [4]. However, given that CXCR3 ligands are present in both psoriatic and AD skin, it is unlikely that CXCR3 defines a singular selective chemoattractant receptor responsible for pDC recruitment to psoriatic skin.
High levels of mRNA encoding chemerin, also known as tazarotene-induced gene 2 have been reported in psoriatic skin [15]. More recently, chemerin has also been detected on dermal endothelial vessels of SLE skin lesions [9]. Chemerin circulates as an inactive precursor in blood but can be converted into a potent chemoattractant upon proteolytic cleavage of its C-terminus by several host and bacterial proteinases [16-19]. Proteolytically processed chemerin selectively supports pDC migration [5]. These data suggest that chemerin may contribute to pDC and recruitment to psoriatic skin.
In this work we demonstrate that CMKLR1 is expressed by circulating pDC in normal individuals and patients suffering from skin diseases, such as psoriasis and AD. Moreover, chemotactically active chemerin is present in lesional skin of psoriasis patients and to a lesser extent in AD patients, suggesting that this chemoattractant may contribute to pDC influx to psoriatic skin.
Materials and methods
Chemoattractants
Recombinant human CXCL12 (SDF-1α) and CXCL10 (IP-10) were purchased from R&D Systems. StaphopainB (SspB) -activated -chemerin was prepared as described previously [16].
Patients
All human studies were performed in accordance with guidelines established by Jagiellonian University Institutional Bioethics Committee under approved protocols. Declaration of Helsinki protocols were followed. A total of 46 psoriasis patients (age 34.4±10 years; F:M, 23:23), 28 AD patients (age 25.9±8.4 years; F:M, 12:16) and 42 healthy individuals (age 26.6±7.4 years; F:M, 20:22) were enrolled into these studies. The severity of the psoriasis skin lesions was assessed according to the Psoriasis Area Severity Index score (PASI) (minimum point 0, maximum point 72) and ranged from 9.2 to 45.1 (mean±SD, 20.7±9.9). AD patients were diagnosed according to the criteria defined by Hanifin and Rajka [20]. Disease severity in AD was measured according to the Severity Scoring of Atopic Dermatitis (SCORAD) (minimum point 0, maximum point 100) and ranged from 12 to 80 (mean±SD, 43.7±21.4). Patients on UV therapy, systemic or local corticosteroid treatment, or with viral infections were excluded from the studies. Healthy control subjects had no clinical signs of dermatologic or allergic diseases.
Flow cytometry analysis
Peripheral blood mononuclear cells (PBMC) were harvested from human blood following LSM1077 (PAA Laboratories) gradient separation. Where indicated pDC were prepared from PBMC using negative selection with biotinylated mAb directed against CD3, CD14, CD16, CD19, CD20, CD56 and anti-biotin MACS Microbeads (Milteney), according to the manufacturer's recommendations. Cells were blocked with 50-80% of autologous plasma and then stained for flow cytometry using mAbs to CD123, BDCA-2 (Miltenyi Biotech), CXCR3 (BD Pharmingen), ChemR23 (R&D Systems) and mouse IgG (Jackson ImmunoResearch). Stained cells were analyzed on a FACSCalibur or LSRII (Becton Dickinson).
Skin biopsy samples
Approximately 6 mm punch biopsies were taken from lesional skin from psoriasis & AD patients. Unchanged areas of skin surrounding moles were obtained from healthy individuals undergoing cosmetic surgery and served as a negative control. Skin samples were immediately homogenized in a buffer containing 0.5M NaCl, 0.05M Tris pH 7.4 and protease inhibitors (Complete, Roche), followed by incubation o/n at 4°C. Extracts were centrifuged at 10,000g for 15 min and then normalized based on protein concentration as determined by BCA assay (Sigma). Equal amounts of protein were subjected to chemotaxis analysis or Western Blot analysis.
Chemotaxis to skin samples
0.35 mg of protein skin extracts was tested in an in vitro chemotaxis assay using 5 μm pore Transwell inserts (Costar) and murine pre-B lymphoma cell line L1.2 or L.1.2 cells stably transfected with human recombinant CMKLR1 (CMKLR1/L1.2). Chemotaxis assay was performed using chemotaxis media (RPMI + 10% FCS, supplemented with L-glutamine, gentamycin, sodium pyruvate, and non-essential amino acids). 100 μl of cells (2.5×105 cells/well) were added to the top well and tested samples were added to the bottom well in a 600 μl volume. Migration was assayed for 2 h at 37°C. The inserts were then removed and cells that had migrated through the filter to the lower chamber were collected and counted by flow cytometry. The results are presented as % input migration.
Western Blot analysis
0.5 mg of skin protein extracts and 200 microliters of plasma were subjected to Heparin-sepharose chromatography to enrich for chemerin. Samples were resolved by SDS-10% PAGE. Chemerin was visualized by ECL following electrotransfer to PVDF membranes and incubation with biotin-labeled goat anti-human chemerin Ab (R&D Systems) and peroxidase-conjugated streptavidin (Jackson Immunoresearch).
pDC chemotaxis assay
PBMC and pDC were harvested from blood as described above. Chemotaxis assay was performed as described above using 0.4×-0.5×106 of PBMC or 0. 2×106 of pDC. The number of gated pDC defined as CD123+ BDCA-2+ counted in 90 s was used as the migration output.
Statistical analysis
Two-tailed Student's t test and Mann Whitney rank sum test were performed.
Results
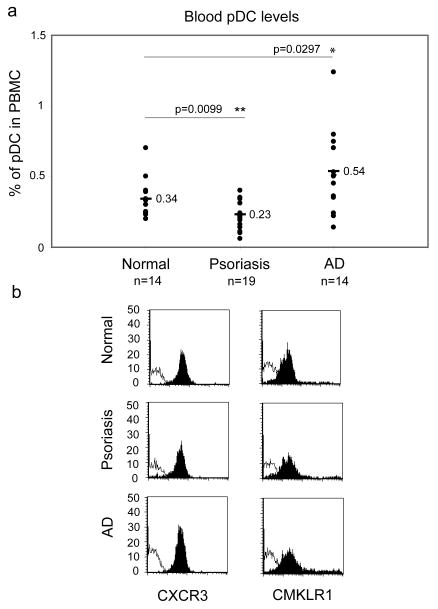
In order to better understand the function of pDC in skin diseases, we characterized certain phenotypic and functional properties of pDC isolated from peripheral blood of psoriasis and AD patients. In agreement with previous reports, the percentage of circulating pDC was found to be higher in AD patients compared to healthy individuals, whereas we noted a decreased frequency of pDC in psoriasis patients [8, 11] (Fig. 1a). The differences in the frequency of circulating pDC may reflect disease-associated variations in pDC homing behaviour. We next examined circulating pDC for expression of key chemoattractant receptors CXCR3 and CMKLR1. pDC from normal donors as well as psoriasis and AD patients expressed comparable levels of CXCR3 and CMKLR1, indicating that the surface levels of these receptors are unlikely to account for differences in pDC migration (Fig. 1b).
Fig. 1. Circulating pDC from normal, psoriasis and AD patients express similar surface levels of CXCR3 and CMKLR1.
PBMC were isolated from the blood of indicated donors and stained for pDC using anti-CD123 and anti-BDCA2 mAbs. The cells were then analyzed by flow cytometry. (a) Data are shown as percentage of pDC among PBMC. Lines indicate the mean value for each data set. Statistically significant differences between normal and psoriasis or AD pDC levels are indicated by asterisks (*, p<0.05; **, p<0.01; Student's t test). (b) Gated pDC were plotted for CXCR3 (left column, black histograms) or CMKLR1 (right column, black histograms). Thin open histograms represent profiles of negative control mAbs. Fluorescence intensity from one representative experiment out of five independent experiments is shown.
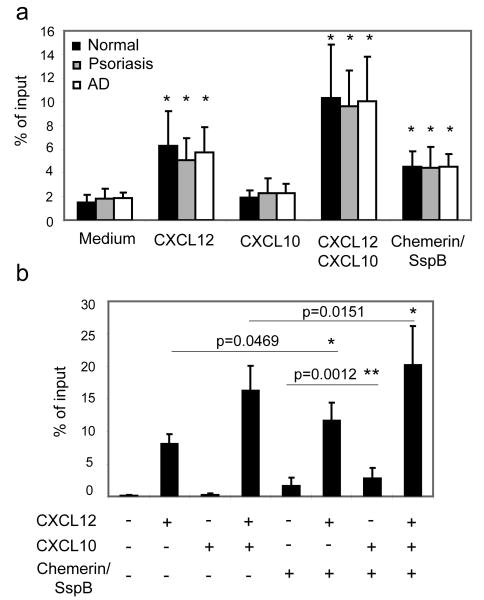
To assess functional migratory responses, we performed in vitro transwell chemotaxis assays on freshly isolated PBMC from normal, psoriasis, and AD patients, which were stained for pDC after migration. pDCs from the three patient groups migrated to CXCL12 and protease-activated chemerin (chemerin/SspB), and the combination of CXCL10 and CXCL12 elicited the most robust response (Fig. 2a). CXCL10 alone did not induce a chemotactic response in any of the pDC. There were no differences in migratory responses among the groups to these attractants (Fig. 2a). Thus circulating pDC in normal donors as well as in psoriasis and AD patients all display functional CXCR3, CXCR4 and CMKLR1.
Fig. 2. pDC derived from the blood of normal donors as well as psoriasis and AD patients express functional CXCR3 and CMKLR1.
PBMC (a) or enriched pDC (b) were tested in transwell chemotaxis, and the migrated cells were stained for CD123 and BDCA2. Migration was assessed to the following chemoattractants, as indicated: CXCL12 (10nM), CXCL10 (115nM) and chemerin/SspB (50pM). Migration to chemotaxis medium served as a negative control (Medium). A live cell gate based on forward and side light scatter was set, and then pDCs were identified based on CD123 and BDCA2 staining. (a) pDC migration is displayed as the mean of eight independent experiments ±S. D. Statistically significant differences in the migration to the negative control (chemotaxis medium) vs. various chemoattractants in pairwise comparisons within patient groups was determined by Student's t test (p<0.05) and indicated by “*”. (b) Migration of pDC (aprox. 90% pure) isolated from eight normal donors is displayed as the mean ± S. D. Statistically significant differences in the migration to CXCL12 vs. CXCL12+chemerin; CXCL12+CXCL10 vs. CXCL12+CXCL10+chemerin; or chemerin vs. chemerin+CXCL10 are indicated by asterisks (*, p<0.05; **, p<0.01; Student's t test).
In order to examine if there is any synergy between chemerin and CXCL12 and/or CXCL10, we used magnetic bead-selected blood pDC from normal donors. Although the pDC from total PBMC migration experiments and the enriched pDC responded in a similar fashion to the chemoattractants, the amplitude of the measured migratory response was more pronounced for purified pDC. As shown in Fig. 2b, chemerin given in combination with CXCL12, CXCL10 or CXCL12+CXCL10, in each case increased the pDC migratory response. These data suggest that all three chemoattractants may play additive effects in recruiting pDC.
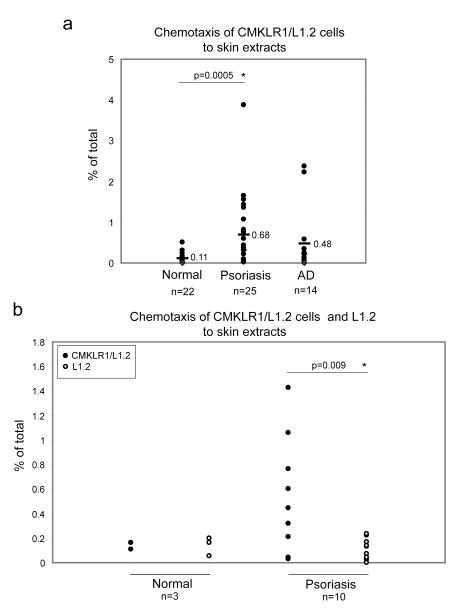
While CXCL12 and CXCL10 are present in lesional skin from psoriasis and AD patients [4, 21, 22], it is unknown whether or not chemerin is present in psoriatic and AD skin. Our attempts to show the presence of chemerin in skin tissue by immunocytochemistry have failed due to lack of anti-chemerin Ab suitable for this application (data not shown). However, Western Blot analysis revealed that chemerin is present in protein extracts obtained from lesional skin of some psoriasis and AD patients (Fig. 3). Plasma samples from psoriasis and AD patients all contained similar levels of chemerin, and six out of eight skin samples form psoriasis patients, and one out of three skin samples from AD patients were immunoreactive with anti-chemerin Abs (Fig. 3 and data not shown). We also observed chemerin in one skin extract out of three normal individual tested (Fig. 3). Moreover, similarly to plasma chemerin, the skin-extracted chemerin was primarily detected in full-length form in all skin samples, suggesting that this chemoattractant might not be chemotactively active. However, since chemerin exerts chemoattractant activity in as low as pM concentration [16], Western Blot analysis might not be sensitive enough to detect active form of this chemoattractant. In order to determine whether chemerin in lesional skin is able to support chemotaxis of CMKLR1 positive cells, we tested skin extracts for chemerin-mediated migration using CMKLR1-transfected L1.2 cells (CMKLR1/L1.2) [5]. CMKLR1/L1.2 cells showed little migration to proteins extracted from the skin of normal individuals (0.11±0.11), but migrated significantly to protein extracts from lesional skin of psoriasis patients (0.68±0.88). In the case of skin extracts from AD patients, some migration was also observed when compared with healthy individuals (0.48±0.85), although this response was not statistically significant (Fig. 4a). As a positive control, 50-200 pM chemerin/SspB was used, giving a response ranging from 10 to 30 % of total CMKLR1/L1.2 cells (data not shown) [16].
Fig. 3. Chemerin protein is present in diseased skin.
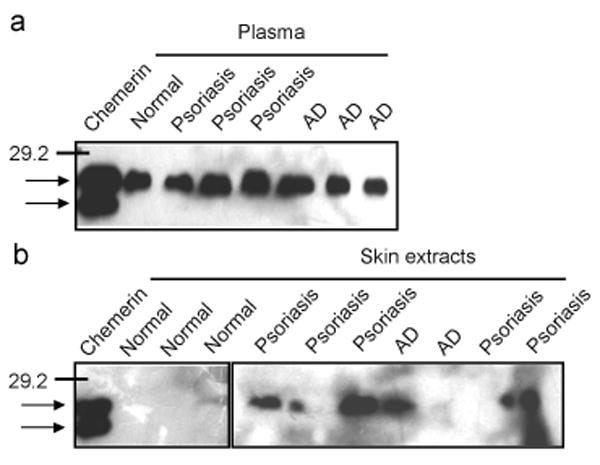
Heparin sepharose enriched plasma fractions (a) and skin-derived protein extracts (b) from the indicated donors were resolved by SDS-PAGE and immunoblotted for chemerin. The position of a molecular weight marker (29.2 kDa) is indicated. Arrows indicate full-length and truncated forms of recombinant chemerin (20 ng), used as a control.
Fig. 4. Chemotactically active chemerin is present in lesional skin of psoriasis patients.
Skin-derived extracts containing 0.35 mg of protein were tested in a chemotaxis assay with CMKLR1/L1.2 cells (a) and either CMKLR1/L1.2 or L1.2 parental cells (b). Lines indicate the mean value for each data set. The difference between chemotactic responses to protein extracts derived from normal individuals vs. psoriasis patients in panel (a) was statistically significant as determined by Mann-Whitney test (p<0.05). The difference between chemotactic responses of CMKLR1/L1.2 vs. L1.2 cells to skin extracts derived from psoriasis patients (panel b) was statistically significant as determined by Student's t test (p<0.01).
To confirm that the observed response was chemerin-dependent, we compared chemotaxis of CMKLR1/L1.2 cells and CMKLR1-negative parental L1.2 cells to the same skin extracts derived from psoriasis patients. Only CMKLR1+ L1.2 cells were able to respond to psoriatic skin extracts, indicating that chemerin present in extracts was responsible for cell migration (Fig. 4b). The response of parental L1.2 cells to 50-200 pM chemerin/SspB did not exceed 0.1% (data not shown).
Discussion
pDC are a relatively newly defined subset of DC equipped with the unique functional capability to respond to viral or microbial nucleic acids by rapid and robust production of type I interferons (INFα/β). Interestingly, dysregulated production of INFα/β is strongly associated with autoimmune diseases such as SLE and psoriasis [6, 7], implicating activated pDC in these pathologic conditions. Therefore, pDC recruitment to the skin may be important to the development of autoimmune skin diseases.
The cellular content of the inflammatory infiltrate in skin diseases is largely defined by chemotactic molecules [23]. CXCR3 is highly expressed on pDC [3, 4], suggesting that this receptor might play a dominant role in chemokine-mediated pDC trafficking. Consistent with high CXCR3 expression on pDC, CXCR3 ligands have been proposed to play a role in directing pDC to inflamed skin, including psoriasis [4]. In addition, circulating pDC selectively express CMKLR1 and respond to active chemerin [5]. In the present work, we demonstrate that blood pDC from normal individuals and patients with psoriasis and AD express similar levels of CXCR3 and CMKLR1. These findings suggest that both receptors might support pDC mobilization to diseased skin, provided the pDC-recruiting chemoattractant is present. CXCR3-specific ligands are known to be expressed in psoriatic and AD lesions [4, 22]. In the present study we show that active chemerin protein is also present in psoriatic skin. Therefore chemerin might provide an alternative or additional signal to regulate pDC homing to lesional skin in psoriasis. The lack of detection of proteolytically processed (active) form of chemerin by Western blot analysis most likely results from lower sensitivity of this assay compared to chemotaxis assay and/or poor extraction of the chemoattractant from the skin biopsies.
In contrast to a previous report [9], our findings indicate that pDC migration to CXCL10 and a combination of CXCL10 and CXCL12 is increased by chemerin. Thus chemerin may cooperate with CXCL12 and CXCL10 in directing pDC to skin in psoriasis. The discrepancies between previous and present studies are unknown at present. Different methods of pDC purification (positive, BDCA-4-based selection, in the case of the previous report, versus negative pDC sorting as in our case) may have unintended functional consequences. For instance, BDCA-4 binding may lead to some level of pDC activation, in which case the pDC would be expected not to respond to chemerin or the combination of chemerin and other chemoattractants, since activated pDC downregulate CMKLR1 [5]. Alternatively, differences in the methods used for detecting migratory responses (transmigration through endothelial cell-coated filters vs. migration through bare filters) might contribute to the observed differences.
In contrast to psoriatic skin, AD skin appears to be largely chemerin-negative. The involvement of pDC in AD seems to be more complex and controversial; in some reports, pDC are present in AD skin lesions, and in other reports they are absent [10, 11, 13]. In contrast to psoriasis and SLE, which are characterized as TH1 cell-mediated inflammatory diseases, AD shares characteristics of both TH1and TH2 immune responses [24, 25]. Patients with AD exhibit a biphasic TH cell pattern, in which TH2–like profile predominates during the acute phase, but switches to a more TH1-like involvement during the chronic or maintenance phase [24]. Therefore, the apparent discrepancies on mobilization of pDC to atopic skin might result from the dual character of this disorder, with pDC infiltrating into AD lesions during a specific stage of the disease. It will be interesting to see if chemerin is associated with chronic AD lesions and/or skin healing.
Chemerin message is expressed at high levels in the non-lesional skin adjacent to lesional skin from psoriasis patients [15]. In addition, plasma is a significant source of this chemoattractant [26], likely contributing to local availability of chemerin during skin inflammation. Also, proteinases required for chemerin activation are likely present in psoriatic skin, as the associated inflammatory infiltrate is rich in neutrophils, which release the chemerin-activating serine proteases elastase and cathepsin G [17, 19, 25]. Thus our results suggest a model in which local generation of active chemerin contributes to the recruitment of pDC to psoriatic skin.
Acknowledgments
We thank Drs. M. Dutka and J. Pułka for help with statistical analysis.
This work was supported in part by grants from the Polish Ministry of Scientific Research 2P04A07629 and SPUB3088 (to JC), by Fogarty International Research Collaborative Award R03TW007174-01 (to ECB and JC) and by grant from EU 6th FP project SP6MTKD-CT-2006-042586 (to JC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 2.Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, Narumi S, Morikawa S, Ezaki T, Lu B, Gerard C, Ishikawa S, Matsushima K. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol. 2004;16:915–928. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 3.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol. 2001;167:1862–1866. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- 4.Vanbervliet B, Bendriss-Vermare N, Massacrier C, Homey B, de Bouteiller O, Briere F, Trinchieri G, Caux C. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med. 2003;198:823–830. doi: 10.1084/jem.20020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zabel BA, Silverio AM, Butcher EC. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J Immunol. 2005;174:244–251. doi: 10.4049/jimmunol.174.1.244. [DOI] [PubMed] [Google Scholar]
- 6.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, Zal T, Mellman I, Schroder JM, Liu YJ, Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 7.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol. 2006;18:676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermi W, Riboldi E, Wittamer V, Gentili F, Luini W, Marrelli S, Vecchi A, Franssen JD, Communi D, Massardi L, Sironi M, Mantovani A, Parmentier M, Facchetti F, Sozzani S. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med. 2005;201:509–515. doi: 10.1084/jem.20041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Whynot J, Novitskaya I, Cardinale I, Haider A, Khatcherian A, Carucci JA, Bergman R, Krueger JG. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J Allergy Clin Immunol. 2007;119:1210–1217. doi: 10.1016/j.jaci.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Hashizume H, Horibe T, Yagi H, Seo N, Takigawa M. Compartmental imbalance and aberrant immune function of blood CD123+ (plasmacytoid) and CD11c+ (myeloid) dendritic cells in atopic dermatitis. J Immunol. 2005;174:2396–2403. doi: 10.4049/jimmunol.174.4.2396. [DOI] [PubMed] [Google Scholar]
- 12.Novak N, Allam JP, Hagemann T, Jenneck C, Laffer S, Valenta R, Kochan J, Bieber T. Characterization of FcepsilonRI-bearing CD123 blood dendritic cell antigen-2 plasmacytoid dendritic cells in atopic dermatitis. J Allergy Clin Immunol. 2004;114:364–370. doi: 10.1016/j.jaci.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Wollenberg A, Wagner M, Gunther S, Towarowski A, Tuma E, Moderer M, Rothenfusser S, Wetzel S, Endres S, Hartmann G. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol. 2002;119:1096–1102. doi: 10.1046/j.1523-1747.2002.19515.x. [DOI] [PubMed] [Google Scholar]
- 14.Facchetti F, Vermi W, Mason D, Colonna M. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 2003;443:703–717. doi: 10.1007/s00428-003-0918-8. [DOI] [PubMed] [Google Scholar]
- 15.Nagpal S, Patel S, Jacobe H, DiSepio D, Ghosn C, Malhotra M, Teng M, Duvic M, Chandraratna RA. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J Invest Dermatol. 1997;109:91–95. doi: 10.1111/1523-1747.ep12276660. [DOI] [PubMed] [Google Scholar]
- 16.Kulig P, Zabel BA, Dubin G, Allen SJ, Ohyama T, Potempa J, Handel TM, Butcher EC, Cichy J. Staphylococcus aureus-derived staphopain B, a potent cysteine protease activator of plasma chemerin. J Immunol. 2007;178:3713–3720. doi: 10.4049/jimmunol.178.6.3713. [DOI] [PubMed] [Google Scholar]
- 17.Wittamer V, Bondue B, Guillabert A, Vassart G, Parmentier M, Communi D. Neutrophil-mediated maturation of chemerin: a link between innate and adaptive immunity. J Immunol. 2005;175:487–493. doi: 10.4049/jimmunol.175.1.487. [DOI] [PubMed] [Google Scholar]
- 18.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, Butcher EC. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem. 2005;280:34661–34666. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]
- 20.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 21.Gombert M, Dieu-Nosjean MC, Winterberg F, Bunemann E, Kubitza RC, Da Cunha L, Haahtela A, Lehtimaki S, Muller A, Rieker J, Meller S, Pivarcsi A, Koreck A, Fridman WH, Zentgraf HW, Pavenstadt H, Amara A, Caux C, Kemeny L, Alenius H, Lauerma A, Ruzicka T, Zlotnik A, Homey B. CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J Immunol. 2005;174:5082–5091. doi: 10.4049/jimmunol.174.8.5082. [DOI] [PubMed] [Google Scholar]
- 22.Klunker S, Trautmann A, Akdis M, Verhagen J, Schmid-Grendelmeier P, Blaser K, Akdis CA. A second step of chemotaxis after transendothelial migration: keratinocytes undergoing apoptosis release IFN-gamma-inducible protein 10, monokine induced by IFN-gamma, and IFN-gamma-inducible alpha-chemoattractant for T cell chemotaxis toward epidermis in atopic dermatitis. J Immunol. 2003;171:1078–1084. doi: 10.4049/jimmunol.171.2.1078. [DOI] [PubMed] [Google Scholar]
- 23.Alaibac M, Berti E, Chizzolini C, Fineschi S, Marzano AV, Pigozzi B, Riboldi E, Sozzani S, Kuhn A. Role of cellular immunity in the pathogenesis of autoimmune skin diseases. Clin Exp Rheumatol. 2006;24:S14–19. [PubMed] [Google Scholar]
- 24.Abramovits W. Atopic dermatitis. J Am Acad Dermatol. 2005;53:S86–93. doi: 10.1016/j.jaad.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 26.Zabel BA, Zuniga L, Ohyama T, Allen SJ, Cichy J, Handel TM, Butcher EC. Chemoattractants, extracellular proteases, and the integrated host defense response. Exp Hematol. 2006;34:1021–1032. doi: 10.1016/j.exphem.2006.05.003. [DOI] [PubMed] [Google Scholar]