Abstract
Background
Patient-derived tumor xenografts (PDX) are considered as a more reliable experiment model for screening chemotherapeutic drugs. However, the tumorigenic rate differs depending on mouse strains, which generates the experimental variability.
Materials and methods
In this study, we built PDX models of human non-small-cell lung cancer (NSCLC) in NOD/SCID mice in comparison with BALB/c mice.
Results
The result showed that the tumorigenesis rate of NOD/SCID mice (46.2%, 18/39) was higher than that of BALB/c mice (17.39%, 4/23). Latent times of tumorigenesis of NOD/SCID mice (41±18 days) were shorter than these of BALB/c mice (53±17 days). Times of tumorigenesis of NOD/SCID mice (85±25 days) were shorter than that of BALB/c mice (104±14 days). In addition, squamous carcinoma tissues were more likely to form tumors than adenocarcinoma tissues in NOD/SCID mice (P=0.008) and BALB/c mice (P=0.09). Also tumors could retain patients’ tumor characteristics in NOD/SCID mice and BALB/c mice xenograft models.
Conclusion
It is worth mentioning that the result of the drug experiment in the PDX models was consistent with the effect of clinical chemotherapy. As a result, NOD/SCID mice have advantages in a higher rate of tumorigenesis, shorter latent times of tumorigenesis and times of tumorigenesis over BALB/c mice in PDX models. It can provide a more reliable model of drug screening.
Keywords: NOD/SCID mice, BALB/c mice, PDX models, chemotherapeutic drugs, NSCLC
Introduction
Although target-specific drug therapy has benefited many lung cancer patients, lung cancer is still the leading cause of death among human cancers worldwide.1 Researchers have been exploring new drugs to prolong the life expectancy of patients with non-small-cell lung cancer (NSCLC).2 However, only 5% of new anti-cancer drugs are approved for clinical trials, which indicates that most of the current preclinical methods are limited in predicting successful outcomes.3–5 At present, constructing preclinical models is a feasible method. It requires that these models should faithfully simulate the major features of human cancers.
Patient-derived tumor xenograft (PDX) models are established by inoculating fresh patient tumor tissues subcutaneously or in situ into immune-deficient mice. The model is a prevalent experimental model for predicting tumor characteristics in tumor occurrence or development.5–8 It can stably inherit the tumor biological characteristics and maintain the tumor pathological traits and tumor heterogeneity.9 This experimental model will provide a method for clinically personalized treatment. But some studies showed that the rate of tumorigenesis is around 20%–60% in first generation and beyond 75% in the second to fifth generation PDX model.4 The difference in the tumorigenesis rates can be related to difference among mice strains. In the abovementioned studies, NOD/SCID mice and BALB/c mice were applied to build a PDX model, but the rates of tumorigenesis were significantly different. This study was aimed at selecting a better mouse line between NOD/SCID mice and BALB/c mice to build a PDX model by comparing the rates of tumorigenesis, the latent times of tumorigenesis, the times of tumorigenesis and pathological features. It will provide a better base for screening chemotherapeutic drugs.
Materials and methods
Animals and chemotherapeutic drugs
All animal experiments and maintenance conformed to the guidelines of both the Animal Care and Use Committee and the Chinese Association of Laboratory Animal Care. Female NOD/SCID and BALB/c nude mice (Vital River, Beijing, People’s Reublic of China) aged 6–8 weeks (average weight 23 g) were used in this study. These mice were raised in a pathogen-free environment at a temperature of 21±2°C and a relative humidity of 30%–70% at the animal center in Beijing Chest Hospital. Specialized personnel were responsible for their feeding.
Cisplatin (Sigma-Aldrich, St Louis, MO, USA) was used in the concentration of 2.5 mg/kg. Docetaxel (Selleck, Houston, TX, USA) was used in the concentration of 12 mg/kg. Paclitaxel (Selleck) was used in the concentration of 25 mg/kg. Pemetrexed (Selleck) was used in the concentration of 75 mg/kg. Gemcitabine (Selleck) was used in a concentration of 120 mg/kg. These drugs were administered twice a week. Gefitinib (Selleck) was used at a concentration of 75 mg/kg. The drug was administered five times a week.
Patients and their tissue specimens
This study was approved by the Beijing Chest Hospital Ethics Committee, and all patients provided written informed consent in accordance with the Declaration of Helsinki. The tissue specimens of 44 lung cancer patients at Beijing Chest Hospital between July 2016 and December 2017 were obtained by a surgical operation. Patients were confirmed as NSCLC by pathology. They had not received chemotherapy, radiotherapy or other kinds of therapies relating to NSCLC treatment. If the patients had suffered from another type of tumor within the previous 5 years, or infectious diseases like active tuberculosis, HIV 1&2, HBV and HCV (the elimination of these four diseases was aimed at to protect the manipulators), they were excluded from this study. Patients were classified by the UIUC Eighth Edition Lung Cancer Stage Classification in 2017.
Building patient-derived tumor xenograft models
The experiments were approved by the Animal Research Committee Guidelines of Beijing Chest Hospital, Capital Medical University. Surgical specimens of 39 patients were inoculated in NOD/SCID mice to build PDX models. Surgical specimens of 23 patients were inoculated in BALB/c mice to build PDX models. Also, specimens of 18 patients were inoculated in NOD/SCID mice and BALB/c mice. All tissues were stored in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) within 30 minutes of resection of the tumor tissues. The procedure associated with tumor implantation was carried out in specific pathogen-free conditions at the experimental animal center. First of all, the manipulators removed the non-tumor tissues and necrosis areas, and washed them three times with RPMI-1640 medium supplemented with 20% fetal bovine serum. After that, tissues were divided into smaller pieces at an average volume of 2×2×2 mm, then four pieces were loaded into the trocars. Next, 75% medical alcohol was used to sterilize the midline of the lower abdomen. Tissues were vaccinated under the underarm through the abdomen subcutaneously. All the patients’ information and experimental animals’ information were registered.
The conditions of the mice such as their activity states, psychological condition, fur gloss, changes in animal skin where the tumor was embedded were observed regularly. The time taken from the tumor inoculation to the growth of the tumor to longer than 3 mm in width or length is defined as the latent time of tumorigenesis. Then the length and short diameters of the tumors were measured by vernier caliper twice a week. Tumor volumes were estimated by the formula: V = [rw2*rL]/2. If the volumes of the tumors were greater than or equal to 1 cm3 within 4 months, it indicated that the model was successful. If not, the model had failed.
Generations of xenografts
When the tumor tissues of lung cancer patients were inoculated into the axilla of mice and formed xenografts, we defined these tumors as F1 generation; when the tumors of F1 generation were inoculated into the axilla of mice and formed xenografts, we defined these tumors as F2 generation, and so on. Xenografts were passaged to the fifth generation.
Detection of pathological features of xenografts
Each generation of xenografts were fixed with formalin and then embedded with paraffin. The morphology of the tumor cells was observed by H&E staining. Mouse anti-TTF1 monoclonal antibodies (Maixin, Fuzhou, People’s Republic of China), rabbit anti-Naspin-A monoclonal antibodies (Maixin) and rabbit anti-P40 monoclonal antibodies (Maixin), mouse anti-cytokeratin 5/6 monoclonal antibodies (Maixin) were used to identify adenocarcinoma or squamous cell carcinoma. Mouse anti-ki-67 monoclonal antibodies (Maixin), mouse anti-cytokeratin 7 monoclonal antibodies (Maixin) and mouse anti-TTF1 monoclonal antibodies (Maixin) were used to identify small-cell lung cancer.
Drug sensitivity experiments
When the length and short diameters of the F5 generation xenografts were greater than 3 mm, mice were divided randomly into six groups. The six groups were the control group (normal saline), the cisplatin+docetaxel group, the cisplatin+paclitaxel group, the cisplatin+pemetrexed group, the cisplatin+gemcitabine group and the gefitinib group. Cisplatin, paclitaxel, docetaxel, pemetrexed and gemcitabine were injected into the abdominal cavity at 2.5 µg/g, 25 µg/g, 12 µg/g, 75 µg/g and 120 µg/g body weight two times a week respectively. Gefitinib was given by gavage at 75 µg/g body weight five times a week. All mice were sacrificed after 40 days. Animal care was given and experiments were conducted in accordance with the Animal Research Committee Guidelines of Beijing Chest Hospital, Capital Medical University.
Statistical analysis
All data were analyzed by using the SPSS, 22.0 software (IBM Corporation, Armonk, NY, USA). A chi-squared test (including Fisher’s exact test) was used to evaluate the relationship between the rates of tumorigenesis and clinical pathological parameters. The inhibition rates of colony formation and tumor weights among the different groups were analyzed using one-way analysis of variance. Differences were considered to be significant at P-value less than or equal to 0.05.
Results
Comparison of the tumorigenesis rates between NOD/SCID and BALB/c mice
In NOD/SCID mice, the tumorigenesis rate was 73.33% in squamous carcinoma and 27.27% in adenocarcinoma. The difference was statistically significant (P=0.008). In BALB/c nude mice, the tumorigenesis rate was 30.00% in squamous carcinoma and 0.00% in adenocarcinoma. They were different but not statistically significant (P=0.09). The tumorigenesis rates of other characteristic factors were not different between NOD/SCID mice and BALB/c nude mice as shown in Table 1. The tumorigenesis rate of NOD/SCID mice was 46.15% and that of BALB/c mice was 17.39%. They were different (P=0.029). Although the latent time of tumorigenesis and time of tumorigenesis were not significantly different between NOD/SCID mice and BABL/c mice, the former was shorter than the latter as shown in Table 2.
Table 1.
Rates of tumorigenesis and clinical characteristics in NOD/SCID mice and BALB/c mice
| Characteristic | NOD/SCID mice
|
P-value | BALB/c mice
|
P-value | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
|
| ||||||
| Age (years) | 0.748 | 0.590 | ||||
| <60 | 7 | 10 | 3 | 9 | ||
| ≥60 | 11 | 11 | 1 | 10 | ||
| Gender | 1.000 | 1.000 | ||||
| Male | 12 | 15 | 3 | 13 | ||
| Female | 6 | 6 | 1 | 6 | ||
| Smoking | 0.748 | 0.590 | ||||
| Yes | 11 | 11 | 3 | 9 | ||
| No | 7 | 10 | 1 | 10 | ||
| Pathological types | ||||||
| Squamous carcinoma | 11 | 4 | 0.008 | 3 | 7 | 0.090 |
| Adenocarcinoma | 6 | 16 | 0 | 11 | ||
| Small cell carcinoma | 1 | 1 | 1 | 1 | ||
| TNM staging | 0.433 | 0.408 | ||||
| I | 8 | 12 | 1 | 9 | ||
| II | 2 | 2 | 1 | 4 | ||
| III | 8 | 6 | 2 | 5 | ||
| IV | 0 | 1 | 0 | 1 | ||
| T stage | 0.237 | 0.456 | ||||
| T1 | 5 | 9 | 0 | 6 | ||
| T2 | 8 | 9 | 3 | 8 | ||
| T3 | 4 | 1 | 1 | 3 | ||
| T4 | 1 | 2 | 0 | 2 | ||
| N stage | 0.108 | 0.557 | ||||
| N0 | 9 | 16 | 2 | 14 | ||
| N1 | 2 | 2 | 0 | 4 | ||
| N2 | 7 | 3 | 2 | 1 | ||
Table 2.
Comparison of tumors in NOD/SCID mice and BALB/c nude mice
| Mouse strains | Tumorigenesis rates (%) | P-value | Latent times of tumorigenesis (days) | P-value | Times of tumorigenesis (days) | P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| NOD/SCID mice | 46.15 (18/39) | 0.029 | 41±17 | 0.207 | 85±24 | 0.134 |
| BALB/c mice | 17.39 (4/23) | 53±19 | 104±14 | |||
Notes: Data are presented as mean±SD.
Comparison of PDX models between NOD/SCID mice and BABL/c mice with the same specimens
In order to exclude the effect of difference in patients on the tumorigenesis rate, we compared the tumorigenesis rate of the same specimens in NOD/SCID mice and BABL/c mice. In NOD/SCID mice (see Table 3), although the tumorigenesis rate difference was not statistically significant between squamous carcinoma and adenocarcinoma, the tumorigenesis rate of squamous carcinoma was higher than that of adenocarcinoma. In BALB/c nude mice, the tumorigenesis rate was 50.00% in squamous carcinoma and 0.00% in adenocarcinoma. They were statistically significant (P=0.036). The tumorigenesis rates of other characteristic factors were not different between NOD/SCID mice and BALB/c nude mice.
Table 3.
Comparison of building PDX models in NOD/SCID mice and BABL/c mice with the same specimens
| Characteristic | NOD/SCID mice
|
P-value | BALB/c mice
|
P-value | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
|
| ||||||
| Age (years) | 1.000 | 1.000 | ||||
| <60 | 4 | 4 | 2 | 6 | ||
| ≥60 | 4 | 6 | 2 | 8 | ||
| Gender | 0.321 | 0.569 | ||||
| Male | 4 | 8 | 2 | 10 | ||
| Female | 4 | 2 | 2 | 4 | ||
| Smoking | 0.637 | 0.576 | ||||
| Yes | 3 | 6 | 3 | 6 | ||
| No | 5 | 4 | 1 | 8 | ||
| Pathological types | ||||||
| Squamous carcinoma | 4 | 2 | 0.302 | 3 | 3 | 0.036 |
| Adenocarcinoma | 3 | 7 | 0 | 10 | ||
| Small cell carcinoma | 1 | 1 | 1 | 1 | ||
| TNM staging | 1.000 | 1.000 | ||||
| I+II | 5 | 7 | 3 | 9 | ||
| III+IV | 3 | 3 | 1 | 5 | ||
Abbreviation: PDX, patient-derived tumor xenografts
The tumorigenesis rate of NOD/SCID mice was 38.89% and that of BALB/c mice was 16.67% (see Table 4). They were different (P=0.017). Although the latent time of tumorigenesis and time of tumorigenesis were not significantly different between NOD/SCID mice and BABL/c mice, the former was shorter the latter.
Table 4.
Comparison of tumor formation in NOD/SCID mice and BALB/c nude mice
| Mouse strains | Tumorigenesis rates (%) | P-value | Latent times of tumorigenesis (days) | P-value | Times of tumorigenesis (days) | P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| NOD/SCID mice | 38.89 (7/18) | 0.017 | 45±17 | 0.718 | 94±19 | 0.546 |
| BALB/c mice | 16.67 (3/18) | 50±22 | 102±16 | |||
Notes: Data are presented as mean±SD.
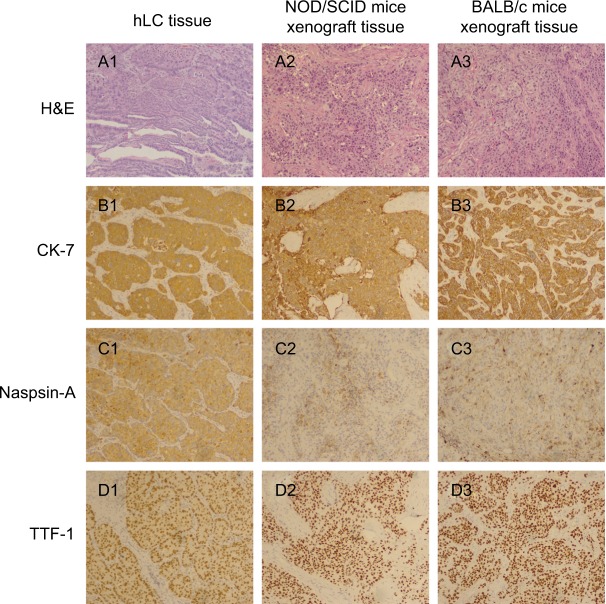
Characteristics and stability of PDX model
To estimate whether xenografted mice could retain histological features of the patients, we compared the gross morphology and histology types by H&E staining and checking the expression of the biomarkers (eg, CK-7, Naspin A, TTF-1 for adenocarcinoma; P40, P63, and CK56 for squamous carcinoma). Figure 1 shows a comparison of building PDX models in NOD/SCID mice and BABL/c mice with the corresponding adenocarcinoma case. H&E staining of the patient tissue showed that there were multiple histopathological patterns, multiple subtypes and differentiation. The mice tissue was usually poorly differentiated and did not have the structure of acinus and nipple. The expressions of CK-7, Naspin A, and TTF-1 were positive and coincident in the patient tissue and mice tissues. Figure 2 shows the comparison of building PDX models in NOD/SCID mice and BABL/c mice with the corresponding squamous cancer patients. H&E staining of the patient tissue showed that there were multiple histopathological patterns, multiple subtypes and differentiation. The mice tissue was usually poorly differentiated and did not have the keratinized structure of squamous cell carcinoma. The expressions of P40, P63 and CK56 were positive and coincident in the patient tissue and mice tissues. In addition, the structure of terminal bronchioles was clearly found in NOD/SCID mice, which confirmed that the PDX mice could inherit the features of the patient’s tumor.
Figure 1.
Comparison of building PDX models in NOD/SCID mice and BABL/c mice with the corresponding adenocarcinoma patient.
Notes: (A1) H&E staining of human lung cancer tissue; (A2) H&E staining of NOD/SCID mice xenograft tissue; (A3) H&E staining of BALB/c mice xenograft tissue; (B1) CK-7 staining of human lung cancer tissue; (B2) CK-7 staining of NOD/SCID mice xenograft tissue; (B3) CK-7 staining of BALB/c mice xenograft tissue; (C1) Naspin A staining of human lung cancer tissue; (C2) Naspin A staining of NOD/SCID mice xenograft tissue; (C3) Naspin A staining of BALB/c mice xenograft tissue; (D1) TTF-1 staining of human lung cancer tissue; (D2) TTF-1 staining of NOD/SCID mice xenograft tissue; (D3) TTF-1 staining of BALB/c mice xenograft tissue. Magnification ×100.
Abbreviations: hLC, human lung cancer; PDX, patient-derived tumor xenografts.
Figure 2.
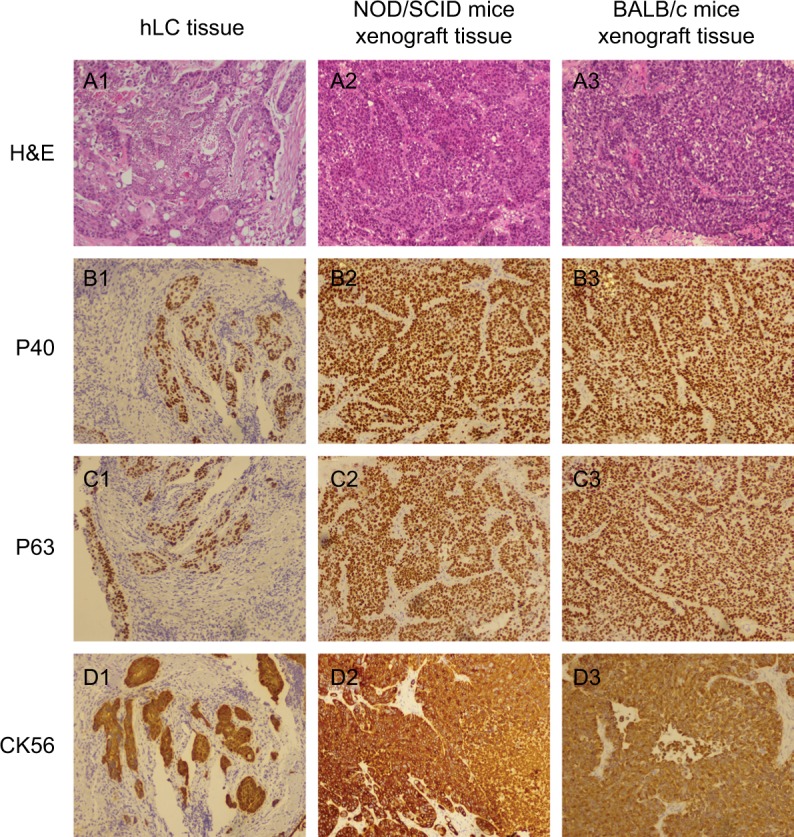
Comparison of building PDX models in NOD/SCID mice and BABL/c mice with the corresponding squamous cancer patient.
Notes: (A1) H&E staining of human lung cancer tissue; (A2) H&E staining of NOD/SCID mice xenograft tissue; (A3) H&E staining of BALB/c mice xenograft tissue; (B1) P40 staining of human lung cancer tissue; (B2) P40 staining of NOD/SCID mice xenograft tissue; (B3) P40 staining of BALB/c mice xenograft tissue; (C1) P63 staining of human lung cancer tissue; (C2) P63 staining of NOD/SCID mice xenograft tissue; (C3) P63 staining of BALB/c mice xenograft tissue; (D1) CK56 staining of human lung cancer tissue; (D2) CK56 staining of NOD/SCID mice xenograft tissue; (D3) CK56 staining of BALB/c mice xenograft tissue. Magnification ×100.
Abbreviations: hLC, human lung cancer; PDX, patient-derived tumor xenografts.
Drug sensitivity experiments
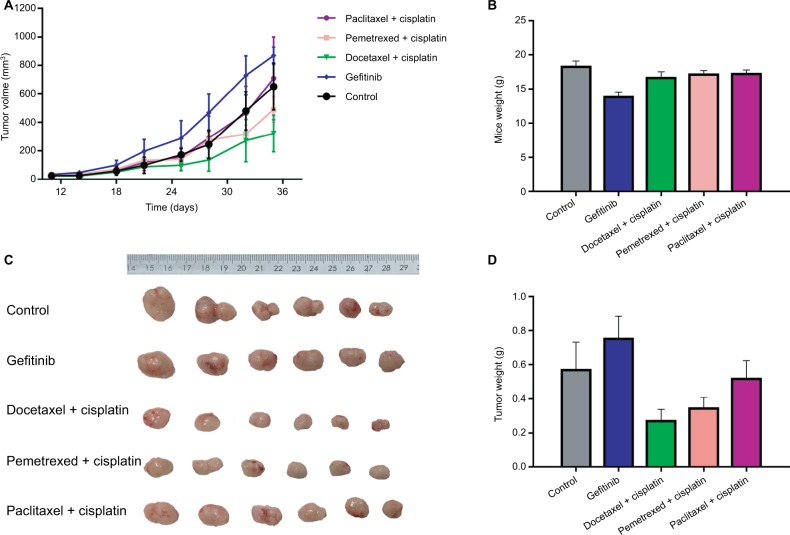
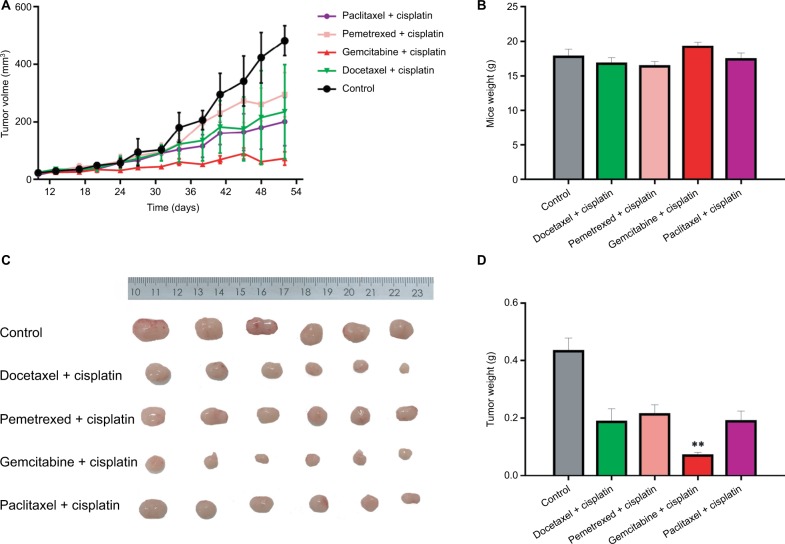
We selected xenografts of two F5 generations (an adenocarcinoma patient and a squamous cancer patient) to carry out the drug experiment (see Figures 3 and 4). Figure 3 shows the drug experiments of the adenocarcinoma patient in PDX model. Whether the growth curve was observed or the average weight of the tumor was observed, the effect of the gefitinib group was the worst. The effect of the cisplatin+ paclitaxel group was worse. The effects of the cisplatin+ docetaxel group and the cisplatin+pemetrexed group were almost the same and were better. The effect of the cisplatin+ gemcitabine group was best. The corresponding patient was followed up. He was treated with the scheme of cisplatin+ paclitaxel for four times. He had a relapse recurrence on the 191st day and died 12 months after the operation. The result was consistent with that of the animal experiment. Figure 4 shows drug experiments of the squamous cancer patient in the PDX model. Whether the growth curve was observed or the average weight of the tumor was observed, the effects of the cisplatin+docetaxel group, the cisplatin+pemetrexed group and the cisplatin+paclitaxel group were almost the same and had a certain effect. The effect of the cisplatin+gemcitabine group was best. The corresponding patient was followed up. So far, the patient is stable and no progression has been noted.
Figure 3.
Drug sensitivity assay of PDX model with the corresponding adenocarcinoma patient.
Notes: (A) Growth curves of transplanted tumor in five groups of mice. (B) Body weight comparison in five groups of mice. (C) Tumor weight comparison in five groups of mice. (D) Columnar diagram of tumor weight in five groups of mice.
Abbreviation: PDX, patient-derived tumor xenografts.
Figure 4.
Drug sensitivity assay of PDX model with the corresponding squamous cancer patient.
Notes: (A) Growth curves of transplanted tumor in five groups of mice. (B) Body weight comparison in five groups of mice. (C) Tumor weight comparison in five groups of mice. (D) Columnar diagram of tumor weight in five groups of mice. **P<0.01.
Abbreviation: PDX, patient-derived tumor xenografts.
Discussion
Since the update of NCCN guideline on NSCLC in 2008, targeted therapy has become a hot spot in the treatment of NSCLC.1 Although individualized targeted therapy has become common in NSCLC, a considerable number of patients are not suitable for targeted therapy. How is individualized treatment possible for them? PDX model is the one that inherits the features of histology, immunohistochemistry and genomic mutation from the patients.6,8,9 So it recapitulates the original characteristics of the patient’s tissues and provides a convincing platform for exploring new drugs and preclinical trials especially in the case of drug efficacy, pharmacokinetics and pharmacodynamics. But it is a notable problem because mouse strains have an influence on the rate of tumorigenesis.
In this study, we selected NOD/SCID mice and BALB/c mice to build a PDX model. Compared with BALB/C mice, the rate of tumorigenesis was obviously high and the latent time of tumorigenesis was short in NOD/SCID mice. The difference was related to mice strains. NOD/SCID mice were derived from hybridization of SCID mice with NOD/Lt mice. Their immune system is destroyed and deficient in three typical kinds of immune cells (T-cell, B-cell, NK-cell), and circulating complements, macrophage cell and antigen processing cell. As for BALB/c mice, they just lack T-cells. If existing B cells and NK cells are active, the immune system can still resist the implantation of the external tumors.10,11 In other words, the immune system of NOD/SCID mice is more defective than it is in BALB/c mice. So xenografts are more successful in NOD/SCID mice. The result was consistent with previous reports.6,7
Except for the influence of mice strains, analysis of the relationship between the tumorigenesis rate and clinical characteristics showed squamous carcinoma form tumors more easily than adenocarcinoma. Other clinical characteristics were not related to the tumorigenesis rate. In order to exclude the effect of different patients on the tumorigenesis rate, we compared the tumorigenesis rate of the same specimens in NOD/SCID mice and BABL/c mice. Although the tumorigenesis rate was not statistically different between squamous carcinoma and adenocarcinoma, the tumorigenesis rate of squamous carcinoma was higher than that of adenocarcinoma in NOD/SCID mice. The tumorigenesis rate was statistically different between squamous carcinoma and adenocarcinoma in BALB/c nude mice. This result was consistent with some studies.8,10–12 But, there have also been contradicting reports.8,13 There is no direct report on the difference in the incidence of squamous cell carcinoma and adenocarcinoma in a PDX model of lung cancer. Some studies show adenocarcinoma cells can be induced to form squamous cell carcinoma cells in the tumor microenvironment provided by specific genotype mice.14–19 In addition, squamous cell carcinoma mostly exists in human skin, central bronchus and esophagus, which are more exposed to external stimuli and may be more adaptable to the environment.20
To estimate whether xenografted mice could retain histological features of the patients, we compared the gross morphology and histology types by H&E staining and checked the expression of the biomarkers. H&E staining of the patient tissue showed that there were multiple histopathological patterns, multiple subtypes and differentiation. But the mice tissue was usually poorly differentiated and did not have the structure of acinus and nipple in adenocarcinoma and the keratinized structure in squamous cell carcinoma. This suggests that poorly differentiated parts are more likely to form tumors in the PDX model. The expressions of related biomarkers were positive and coincident in the patient tissue and mice tissues. In addition, the structure of terminal bronchioles was clearly found in NOD/SCID mice. These confirm that PDX mice could inherit the features of the patient’s tumor. Here, it is worth noting that only poorly differentiated parts are preserved and form tumors and highly differentiated parts are not seen in mice tissues. However, these poorly differentiated parts may more accurately reflect drug efficacy and prognosis of the patient.12,21
So we selected two patients (an adenocarcinoma patient and a squamous cell carcinoma patient) and used their xenografts of the F5 generation to carry out the drug experiment. The result of the squamous cell carcinoma showed the effect of the cisplatin+gemcitabine group was best. The effects of the other groups (cisplatin+docetaxel, cisplatin+paclitaxel and cisplatin+pemetrexed) were almost the same and worse than that of the cisplatin+gemcitabine group. The patient was followed up. He was treated with cisplatin+paclitaxel four times in clinical practice. He had a relapse recurrence on the 191st day and died 12 months after the operation. In other words, the scheme of cisplatin+ paclitaxel was not best treatment for the patient. This was consistent with that of the animal experiment. The animal experiment of the adenocarcinoma patient showed the effect of the cisplatin+docetaxel group was best; the effects of the cisplatin+paclitaxel group and the cisplatin+pemetrexed group were better; but the effect of the gefitinib group was very poor because the EGFR of the patient was wild type. So far, the patient is good after the operation and we cannot compare whether the animal experiment and clinical effects are consistent.
In conclusion, NOD/SCID mice have a higher rate of tumorigenesis, shorter latent times of tumorigenesis and times of tumorigenesis than BALB/c mice. To a certain extent, both NOD/SCID and BALB/c mice could inherit the characteristics of original tumor patients in histology, immunohistochemistry and pathology. It is worth noting that these poorly differentiated parts may more accurately reflect drug efficacy and prognosis of the patient and can provide a more reliable model of drug screening.
Acknowledgments
The work was financed by Beijing Municipal Science and Technology Committee (No. Z151100002115049) and Beijing Natural Science Foundation (7174289).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day CP, Merlino G, van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. 2015;163(1):39–53. doi: 10.1016/j.cell.2015.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fichtner I, Rolff J, Soong R, et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res. 2008;14(20):6456–6468. doi: 10.1158/1078-0432.CCR-08-0138. [DOI] [PubMed] [Google Scholar]
- 7.John T, Kohler D, Pintilie M, et al. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2011;17(1):134–141. doi: 10.1158/1078-0432.CCR-10-2224. [DOI] [PubMed] [Google Scholar]
- 8.Ilie M, Nunes M, Blot L, et al. Setting up a wide panel of patient-derived tumor xenografts of non-small cell lung cancer by improving the preanalytical steps. Cancer Med. 2015;4(2):201–211. doi: 10.1002/cam4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Pham NA, Tong J, et al. Molecular heterogeneity of non-small cell lung carcinoma patient-derived xenografts closely reflect their primary tumors. Int J Cancer. 2017;140(3):662–673. doi: 10.1002/ijc.30472. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XC, Zhang J, Li M, et al. Establishment of patient-derived non-small cell lung cancer xenograft models with genetic aberrations within EGFR, KRAS and FGFR1: useful tools for preclinical studies of targeted therapies. J Transl Med. 2013;11:168. doi: 10.1186/1479-5876-11-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bankert RB, Egilmez NK, Hess SD. Human-SCID mouse chimeric models for the evaluation of anti-cancer therapies. Trends Immunol. 2001;22(7):386–393. doi: 10.1016/s1471-4906(01)01943-3. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki S, Vicini P, Shen Z, et al. Pharmacokinetic/pharmacodynamic modeling of crizotinib for anaplastic lymphoma kinase inhibition and antitumor efficacy in human tumor xenograft mouse models. J Pharmacol Exp Ther. 2012;340(3):549–557. doi: 10.1124/jpet.111.188870. [DOI] [PubMed] [Google Scholar]
- 13.Anderson TM, Hess SD, Egilmez NK, Nwogu CE, Lenox JM, Bankert RB. Comparison of human lung cancer/SCID mouse tumor xenografts and cell culture growth with patient clinical outcomes. J Cancer Res Clin Oncol. 2003;129(10):565–568. doi: 10.1007/s00432-003-0473-3. [DOI] [PubMed] [Google Scholar]
- 14.Gazdar AF, Savage TK, Johnson JE, et al. The comparative pathology of genetically engineered mouse models for neuroendocrine carcinomas of the lung. J Thorac Oncol. 2015;10(4):553–564. doi: 10.1097/JTO.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumi H, Yamasaki A, Ueda Y, et al. Squamous cell carcinoma transformation from EGFR-mutated lung adenocarcinoma: a case report and literature review. Clin Lung Cancer. 2018;19(1):e63–e66. doi: 10.1016/j.cllc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Longo L, Mengoli MC, Bertolini F, Bettelli S, Manfredini S, Rossi G. Synchronous occurrence of squamous-cell carcinoma “transformation” and EGFR exon 20 S768I mutation as a novel mechanism of resistance in EGFR-mutated lung adenocarcinoma. Lung Cancer. 2017;103:24–26. doi: 10.1016/j.lungcan.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Hou S, Zhou S, Qin Z, et al. Evidence, mechanism, and clinical relevance of the transdifferentiation from lung adenocarcinoma to squamous cell carcinoma. Am J Pathol. 2017;187(5):954–962. doi: 10.1016/j.ajpath.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Wang X, Curtiss C, et al. Monoglyceride lipase gene knockout in mice leads to increased incidence of lung adenocarcinoma. Cell Death Dis. 2018;9(2):36. doi: 10.1038/s41419-017-0188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matrka MC, Cimperman KA, Haas SR, et al. Dek overexpression in murine epithelia increases overt esophageal squamous cell carcinoma incidence. PLoS Genet. 2018;14(3):e1007227. doi: 10.1371/journal.pgen.1007227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard SC, Hull SR, Huggins JW, Carraway CA, Carraway KL. Relationship between xenotransplantability and cell surface properties of ascites sublines of a rat mammary adenocarcinoma. J Natl Cancer Inst. 1982;69(1):33–40. [PubMed] [Google Scholar]
- 21.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6(10):813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]