Abstract
Study Objectives
Depressive symptoms following adenotonsillectomy (AT) relative to controls were examined in children with obstructive sleep apnea syndrome (OSAS).
Methods
The Childhood Adenotonsillectomy Trial (CHAT) multisite study examined the impact of AT in 453 children aged 5 to 9.9 years with polysomnographic evidence of OSAS without prolonged desaturation, randomized to early adenotonsillectomy (eAT) or watchful waiting with supportive care (WWSC). One hundred seventy-six children (eAT n = 83; WWSC n = 93) with complete evaluations for depressive symptomatology between baseline and after a 7-month intervention period were included in this secondary analysis.
Results
Exact binomial test assessed proportion of depressive symptomatology relative to norms, while effects of AT and OSAS resolution were assessed through linear quantile mixed-models. Treatment group assignment did not significantly impact depression symptoms, although self-reported depression symptoms improved over time (p < 0.001). Resolution of OSAS symptoms demonstrated a small interaction effect in an unexpected direction, with more improvement in parent ratings of anxious/depressed symptoms for children without resolution (p = 0.030). Black children reported more severe depressive symptoms (p = 0.026) and parents of overweight/obese children reported more withdrawn/depressed symptoms (p = 0.004). Desaturation nadir during sleep was associated with self-report depressed (r = −0.17, p = 0.028), parent-reported anxious/depressed (r = −0.15, p = 0.049), and withdrawn/depressed (r = −0.24, p = 0.002) symptoms.
Conclusions
Increased risk for depressed and withdrawn/depressed symptoms was detected among children with OSAS, and different demographic variables contributed to risk in self-reported and parent-reported depression symptoms. Arterial oxygen desaturation nadir during sleep was strongly associated with depressed symptoms. However, despite improvements in child-reported depressed symptoms over time, changes were unrelated to either treatment group or OSAS resolution status.
Trials Registration
Childhood Adenotonsillectomy Study for Children with OSAS (CHAT), https://clinicaltrials.gov/show/NCT00560859, NCT00560859.
Keywords: obstructive sleep apnea, pediatrics – sleep apnea, depression, mental health, neuropsychology, sleep and psychiatric conditions
Statement of Significance
Few randomized obstructive sleep apnea syndrome (OSAS) treatment studies have examined depressive symptoms following adenotonsillectomy (AT) relative to controls; even fewer studies obtained reports of depression directly from children. This sample, a subsample of the multisite Childhood Adenotonsillectomy Trial (CHAT) study which assessed 453 children aged 5 to 9.9 years with polysomnographic evidence of OSAS without prolonged desaturation, represents the largest known sample of children randomized to AT versus watchful waiting with supportive care (WWSC) in which self-report of depression was obtained. One hundred seventy-six children (eAT n = 83; WWSC n = 93) were eligible for this subsample analysis by age. Although the treatment groups did not show differential change in symptoms across follow-up, degree of oxygen desaturation prior to treatment assignment was related to increased depressed symptoms across all raters. Results support higher rates of severe depressive symptoms in children with OSAS referred for AT compared to normative data, and highlight the differences between parent and child report and the importance of considering demographic factors.
Introduction
Pediatric obstructive sleep apnea syndrome (OSAS) is estimated to occur in 2%–3% of children [1]. This disorder is associated with impaired cognition, academic functioning, and general quality of life [2]. Although attention-deficit/hyperactivity disorder symptomatology is the most commonly studied behavior in children with OSAS, increases in the prevalence of symptoms of depression have also been reported [3–8]. However, inconsistencies about the relationship between depression and OSAS have been found in both adults and children [4, 9, 10]. A recent meta-analysis of 11 studies assessing depressive symptoms in children with OSAS versus controls before and after adenotonsillectomy (AT) found a medium effect size between baseline depressive symptoms and OSAS (Hedges’ g = 0.43; p = 0.0005) [4]. However, none of these studies involved a large cohort with a randomized, controlled design.
AT is the primary treatment for childhood OSAS [2]. Although previous data suggested that OSAS improves after AT in the majority of children, prior to the Childhood Adenotonsillectomy Trial (CHAT; a randomized controlled trial examining the impact of AT compared to watchful waiting with supportive care [WWSC]), rigorous evaluation of outcomes had not been conducted in a large-scale randomized study [11, 12]. Additionally, few studies have examined depressive symptoms as an outcomes measure following AT relative to controls. Even fewer studies obtained a report of depression symptoms directly from the child; indeed, Yilmaz et al. [4] identified only three studies in which child self-report was included.
The primary objective of this study was to examine depressive symptomatology between baseline and study completion of children in the CHAT study who provided self-report at both time points. CHAT data provided a large sample size in a randomized trial. The secondary objective was to determine effects of demographic (race, body mass index, maternal education) factors on prevalence of depressive symptomatology reported by both children and parents, an important area that is lacking in the literature [13]. Due to deliberate disproportionate recruitment, the CHAT study had good representation of racial minorities and overweight/obese children. Inclusion of child self-report, as well as considering the impact of demographic characteristics, is increasingly important as recent research demonstrates that not only do parent and child self-report of depressive symptomatology differ in important ways, but these differences can be moderated by ethnicity and other demographic variables [14–16].
Methods
Participants
The CHAT trial details, including methods, have been previously published, including details regarding recruitment representativeness, blinding, quality control, and safety monitoring [11]. In brief, a total of 453 children ranging in age from 5 to 9.9 years with polysomnographic evidence of OSAS without prolonged desaturation were randomized to early adenotonsillectomy (eAT) or WWSC at one of six research sites. Polysomnographic inclusion criteria included OSAS defined as an obstructive apnea index (OAI) ≥ 1/hour or obstructive apnea-hypopnea index (AHI) ≥ 2/hour of sleep. Children with an OAI > 20/hour or an AHI > 30/hour, or arterial oxyhemoglobin saturation < 90% for ≥2% of total sleep time were not eligible due to severity of findings. As one of our outcome measures of interest, the Child Depression Inventory (CDI) [17], is validated for children ≥7 years of age, the current study was limited to the CHAT participants who were 7 years or older at the time of recruitment.
Procedures
The study was approved by local sites’ institutional review boards, and parents provided consent and children assent to participate. Children underwent polysomnography, completed tests of cognitive functioning, and parent-, teacher- and self-report of behavioral and emotional functioning. Any reported elevations in depressive symptomatology or endorsement of critical items related to self-harm were addressed with participants and families. After their first study visit, children were assigned to either eAT or WWSC groups, with the eAT group undergoing surgery within 4 weeks of the first study visit. Follow-up assessments of behavioral, emotional and cognitive functioning were repeated approximately 7 months after baseline visits (mean time between baseline and follow-up visits = 7.1 months). Behavioral and cognitive findings from the study, other than the self-report depressive outcomes, have been published elsewhere [12, 18, 19].
Measures of depressive symptomatology
Children’s depression inventory
The children’s depression inventory (CDI) is a reliable and well-validated self-report measure of depression symptoms in children [17]. This scale is comprised of 27 items and is validated for use in a diverse sample of children ranging in age from 7 to 17 years. Each item has three statements that range from no symptoms (0-point value) to definite symptoms (2-point value). Children select the response options that best characterized their feelings over the previous 2 weeks. The scale includes a total score (with score values that range from 0 to 54) and five “factors” that evaluate childhood depression symptoms including: Negative Mood, Interpersonal Problems, Ineffectiveness, Anhedonia, and Negative Self-Esteem. Scores are summed and transformed to standardized T-scores (mean = 50, SD = 10) for age and sex. Scores in the range between 61 and 65 correspond to “above average” symptoms (≥85th percentile), scores from 66 to 70 correspond to “much above average” symptoms (≥95th percentile), and scores ≥70 correspond to “very much above average” symptoms. For the purposes of these analyses, in addition to “above average” and “much above average” or greater depressive symptoms endorsement, children endorsing scores of 66 or above are referred to as having elevated symptoms of depression. Of note, two of the 27 items from the CDI directly relate to sleep, both of which load onto the Anhedonia subscale: item 16: “I have trouble sleeping every night / I have trouble sleeping many nights / I sleep pretty well” and item 17: “I am tired once in a while / I am tired many days / I am tired all the time.” Parents were notified if children endorsed “above average” or “much above average” overall symptoms of depression, or if they endorsed critical items related to self-harm. Data in this study were compared to the CDI normative sample of 1463 ethnically diverse public school students from the United States.
Child behavior checklist
The child behavior checklist (CBCL) is a widely-used, caregiver-completed questionnaire designed to assess multiple aspects of behavioral, emotional, and adaptive functioning in children [20]. The scale contains two subscales related to depressive symptomatology: Anxious/Depressed and Withdrawn/Depressed. Standardized T-scores (mean = 50, SD = 10) are generated based on age- and sex-specific norms. Higher scores indicate increased severity, with scores ≥63 (90th percentile) generally considered clinically elevated [20]. In a prior paper, seven items from the CBCL were identified as being clearly related to sleep, although not necessarily OSAS [18]. None of these items, however, are included in either the Anxious/Depressed or Withdrawn/Depressed subscales, loading instead onto subscales related to somatic complaint, thought problems, or other problems, and therefore sleep items were not felt to represent a potential confound for the current analyses. Data in this study are compared to the CBCL normative data of 1753 ethnically diverse individuals collected through multistage national probability sampling.
Statistical analysis
Rank and percentile-based methods were used to accommodate the skewness in the majority of the study variables. Baseline demographic characteristics were examined by treatment arm using Wilcoxon rank sum tests. An exact binomial test was conducted to assess the proportion of depressive symptomatology (dichotomized as normal vs. above average or higher/normal vs. much above average or higher for total CDI, and normal versus elevated for CBCL Anxious/Depressed and Withdrawn/Depressed) in this sample relative to expected population/test norms. Depression symptoms were examined by demographic characteristics (Black vs. other race, sex, maternal education above high school vs. maternal education high school or below, overweight/obese vs. normal weight or below), using Fisher’s exact test for count data. Similar trends were noted for overweight and obese children, and thus these two categories were combined. It should be noted that as the measures are normed by sex, differences in sex would only be observed in this sample if they were evident at rates disproportionate to same-sex norms. Correlations in T-score scale between self-report of depressive symptoms (CDI) and parent-report of depressive symptoms (CBCL) were evaluated using Spearman correlation coefficients. Relationships between baseline sleep variables and reported depression were also examined with Spearman’s rank correlation tests.
The effects of AT on depressive symptoms were assessed through the linear quantile mixed-models to estimate the conditional median of depression variables by group (eAT and WWSC), time (baseline and follow-up), and interaction. Depression variables were analyzed in their original T-score scale in this analysis. Models were adjusted for race, sex, maternal education and overweight status. In addition to the total CDI and both CBCL total scores (Anxious/Depressed and Withdrawn/Depressed), this analysis was repeated for all subscales of the CDI to determine the impact of the Anhedonia scale.
Finally, the effects of OSAS resolution (defined in the study as a reduction in both the AHI score to fewer than two events per hour and the OAI score to fewer than one event per hour) on depressive symptoms were assessed through the linear quantile mixed-models to estimate the conditional median of depression variables by group (eAT and WWSC), time (baseline and follow-up), and interaction. Depression variables were analyzed in their original T-score scale in this analysis. Models were adjusted for race, sex, maternal education and overweight status (yes/no). This analysis was repeated for all subscales of the CDI to determine the impact of the Anhedonia scale (which includes sleep-related items).
Analyses were performed in R version 3.4.0. A p-value < 0.05 was considered statistically significant. Analyses were based on available data and did not impute the missing data.
Results
Characteristics of the subsample
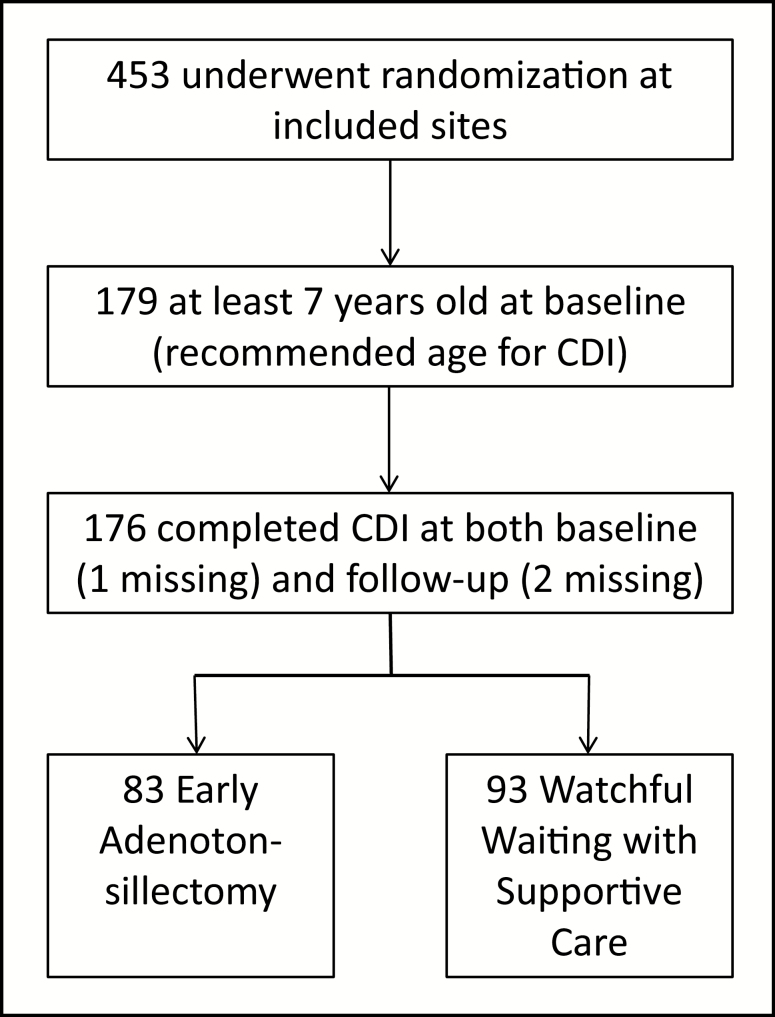
Of the 453 children randomized in the larger CHAT sample, 179 children who were at least 7 years old at baseline (the minimum age recommended by test authors for the self-report CDI) completed the CHAT study. Of these, the CDI was not available for one child at baseline and an additional two children at follow-up. Thus, 176 children (eAT n = 83; WWSC n = 93) were included in the final analyses (Figure 1). There were no significant differences at baseline between eAT and WWSC for any demographic or polysomnographic variables (Table 1). Missing data was minimal (four values among CBCL scales and two values for maternal education).
Figure 1.
Participants of larger CHAT study included in this secondary analysis.
Table 1.
Baseline demographic variables by treatment arm
| Overall (N = 176) | eAT (N = 83) | WWSC (N = 93) | |
|---|---|---|---|
| Age (years) at baseline | 8.30 (0.96) | 8.37 (0.91) | 8.24 (1.00) |
| Sex (male) | 71 (40.34%) | 28 (33.73%) | 43 (46.24%) |
| BMI z-score | 1.30 (1.14) | 1.45 (1.12) | 1.17 (1.16) |
| Overweight/obese | 115 (65.34%) | 59 (71.08%) | 56 (60.22%) |
| Maternal education | |||
| High school or less | 62 (35.23%) | 31 (37.35%) | 31 (33.33%) |
| Associates’ degree or some college | 71 (40.34%) | 29 (34.94%) | 42 (45.16%) |
| Bachelor’s degree or more | 41 (23.3%) | 21 (25.30%) | 20 (21.51%) |
| Missing | 2 (1.14%) | 2 (2.41%) | 0 (0.00%) |
| Child’s race | |||
| Black | 106 (60.23%) | 49 (59.04%) | 57 (61.29%) |
| White | 59 (33.52%) | 29 (34.94%) | 30 (32.26%) |
| Other | 11 (6.25%) | 5 (6.02%) | 6 (6.45%) |
| Site | |||
| Philadelphia | 56 (31.82%) | 25 (30.12%) | 31 (33.33%) |
| Cincinnati | 17 (9.66%) | 8 (9.64%) | 9 (9.68%) |
| Cleveland | 53 (30.11%) | 27 (32.53%) | 26 (27.96%) |
| St. Louis | 33 (18.75%) | 15 (18.07%) | 18 (19.35%) |
| New York | 2 (1.14%) | 1 (1.20%) | 1 (1.08%) |
| Boston | 15 (8.52%) | 7 (8.43%) | 8 (8.60%) |
| Annual household income | |||
| <$20K | 57 (32.39%) | 20 (24.10%) | 37 (39.78%) |
| $20–$40K | 35 (19.89%) | 18 (21.69%) | 17 (18.28%) |
| >$40K | 51 (28.98%) | 22 (26.51%) | 29 (31.18%) |
| Missing | 33 (18.75%) | 23 (27.71%) | 10 (10.75%) |
BMI z-score = body mass Index z-score. Data are shown as mean (SD) or N (%). There were no statistical differences between groups.
Symptoms of depression at baseline
At baseline, 26 (14.8%) of children reported an “above average” level of depressive symptomatology (CDI ≥ 61, 85th percentile of normative group), whereas 19 (10.8%) reported a “much above average” or greater level of depressive symptoms (CDI ≥ 66, 95th percentile of normative group). By parent report, 25 children (14.3%) were elevated for Anxious/Depressed symptoms on the CBCL, and 28 children (16.0%) were elevated for Withdrawn/Depressed symptoms. Based on the normative percentiles, these percentages are consistent with the expected prevalence of both child-reported “above average” depressive symptomatology and parent-reported elevations in Anxious/Depressed symptoms, but indicate significantly higher levels of “much above average” depressive symptoms among self-reports (p = 0.002) and parent-reported elevations in Withdrawn/Depressed symptoms (p = 0.012) in this population of children with OSAS.
For self-reported symptoms of depression on the CDI, Black children reported higher levels of “much above average” depressive symptoms in comparison to White/other children (p = 0.026; Table 2). There were no significant differences in the rate of elevated symptoms endorsed by participant sex, maternal education, or obesity level. We did not find demographic associations among elevated parent-reported depression symptoms on the Anxious/Depressed subscale of the CBCL. In contrast, parent-reported depression symptoms from the Withdrawn/Depressed subscale of the CBCL were significantly elevated in overweight/obese children relative to their normal or underweight peers (p = 0.004) (Table 2).
Table 2.
Relationship of demographic characteristics to self-reported depressive symptoms at baseline
| Demographic characteristic | N (%) of sample overall | N (%) of category with CDI ≥ 61 | N (%) of category with CDI ≥ 66 | N (%) of category with CBCL Anxious/ Depressed ≥ 63 | N (%) of category with CBCL Withdrawn/ Depressed ≥ 63 | |
|---|---|---|---|---|---|---|
| Total sample | 176 (100%) | 26 (14.77%) | 19 (10.80%) | 25 (14.29%) | 28 (16.00%) | |
| Weight | Overweight/Obese | 115 (65.34%) | 19 (16.52%) | 14 (12.17%) | 19/114 (16.67%) | 25/114 a (21.93%) |
| Normal / Underweight | 61 (34.66%) | 7 (11.48%) | 5 (8.20%) | 6/61 (9.84%) | 3/61 (4.92%) | |
| Race | Black | 106 (60.23%) | 20 (18.87%) | 16 b (15.09%) | 13/106 (12.26%) | 13/106 (12.26%) |
| White/Other | 70 (39.77%) | 6 (8.57%) | 3 (4.29%) | 12/69 (17.39%) | 15/69 (21.74%) | |
| Sex | Female | 105 (59.66%) | 19 (18.10%) | 13 (12.38%) | 16/105 (15.24%) | 17/105 (16.19%) |
| Male | 71 (40.34%) | 7 (9.86%) | 6 (8.45%) | 9/70 (12.86%) | 11/70 (15.71%) | |
| Maternal education* | High school or less | 62/174 (35.63%) | 8 (12.90%) | 5 (8.06%) | 5/62 (8.06%) | 12/62 (19.35%) |
| More than high school | 112/174 (64.37%) | 17 (15.18%) | 13 (11.61%) | 20/111 (18.02%) | 16/111 (14.41%) |
a p = 0.004.
b p = 0.026.
*Maternal education missing for n = 2, CBCL missing for n = 1; denominators shown when full sample not represented. Significant data are shown in bold.
Correlation between parent and child-reported depressive symptomatology at baseline
At baseline, the total CDI had a weak but significant correlation with parent reports of Anxious/Depressed symptoms (r = 0.21, p = 0.006) but no significant correlation with Withdrawn/Depressed symptoms (r = 0.09, p = 0.258).
Relationship between depressive symptoms and sleep variables at baseline
There were no significant correlations between depressive symptoms and AHI or degree of hypercapnia (percentage of total sleep time with end-tidal CO2 > 50 mmHg), but there was an inverse correlation between SpO2 nadir and higher reported depression for all reports, that is, the child CDI total score (r = −0.17, p = 0.028) and both parent-report scales—CBCL Anxious/Depressed (r = −0.15, p = 0.049) and Withdrawn/Depressed (r = −0.24, p = 0.002). That is, children with more severe desaturation had more depressive symptoms across all ratings (Table 3).
Table 3.
Correlations between depressive symptom report and sleep variables at baseline
| CDI total score (self) | CBCL Anxious/Depressed (parent) | CBCL Withdrawn/Depressed (parent) | |
|---|---|---|---|
| rho (p value) | rho (p value) | rho (p value) | |
| AHI | 0.13 (0.080) | 0.01 (0.910) | 0.08 (0.281) |
| SpO2 nadir | −0.17 (0.028) | −0.15 (0.049) | −0.24 (0.002) |
| % Sleep time with CO2 > 50 mmHg | −0.01 (0.866) | −0.06 (0.497) | 0.06 (0.506) |
| Sleep efficiency | 0.13 (0.09) | −0.08 (0.29) | −0.11 (0.17) |
| Arousal index | −0.19 (0.80) | −0.03 (0.73) | −0.03 (0.71) |
| % Stage 1 Sleep | −0.05 (0.50) | −0.07 (0.35) | 0.08 (0.28) |
| % Stage 2 Sleep | 0.008(0.92) | −0.05 (0.50) | −0.13 (0.08) |
| % Stage 3 sleep | .02 (0.79) | 0.15 (0.05) | 0.11 (0.15) |
| % REM sleep | −0.03 (0.69) | −0.18 (0.02) | −0.02 (0.77) |
Significant correlations shown in bold. SpO2 = arterial oxygen saturation, CO2 = end-tidal carbon dioxide.
Additional analyses of secondary sleep variables revealed no relationship at baseline between depressive symptoms endorsed by children or parents and sleep efficiency (percentage of time in bed that was spent sleeping), arousal index (total arousals per hour of sleep), or percentage of sleep time spent in stage 1, stage 2, or slow wave (stage 3) sleep. Parents reported decreased Anxious/Depressed symptoms associated with a greater percentage of time in REM sleep, but no relationship was noted for Withdrawn/Depressed or child self-report depressive symptoms and this sleep variable.
Effects of OSAS treatment on depression
Analysis for the effects of OSAS treatment on depression symptoms showed no significant group differences, and no significant interaction between group and visit, indicating that both eAT and WWSC groups demonstrated similar changes in depression symptoms from baseline to follow-up. Notably, although there were no significant differences in child-reported depression scores between groups at baseline or follow-up study periods, both eAT and WWSC groups showed an improvement in self-reported (CDI) but not parent-reported (CBCL) depression over time (p < 0.001) (Table 4). The associations between the changes in depression outcomes and group at 25th and 75th percentiles were consistent with those for the median, as shown in Table 4. We also performed sensitivity analyses to explore whether the results were moderated by ethnicity or other demographic variables. No significant interactions between group, visit and interaction factors were identified (data not shown).
Table 4.
Analysis of depressive symptoms by group, time and interaction: eAT versus WWSC
| Measure | eAT | WWSC | P value | ||||
|---|---|---|---|---|---|---|---|
| Baseline median (IQR) | Change | Baseline median (IQR) | Change | Group | Time | Group × time | |
| CDI total score | 47 (43, 57) | −3 (−8, 0) | 49 (42, 56) | −3 (−7, 1) | 0.753 | <0.001 | 0.403 |
| CBCL Anxious/Depressed | 50 (50, 54) | 0 (−1, 0) | 51 (50, 60) | 0 (−3, 1) | 0.226 | 0.149 | 0.870 |
| CBCL Withdrawn/Depressed | 52 (50, 60) | 0 (−4, 0) | 52 (50, 58) | 0 (−4, 0) | 0.387 | 0.105 | 0.436 |
IQR = interquartile range. Significant data are shown in bold. CDI improved significantly over time in both groups.
Effects of OSAS resolution on depression
Although self-reported depressive symptoms (CDI) improved over time, no significant effect was evident for resolution of OSAS (yes/no) or the interaction between resolution of OSAS and time. In contrast, parent-reported CBCL Anxious/Depressed symptoms demonstrated a significant time effect, with decreased depressive symptomatology reported over time. A group × time interaction for resolution was noted as well, but in an unexpected direction, with the group not demonstrating resolution showing a small decrease in depressive symptomatology over time while the group with resolved OSAS remained unchanged. (Table 5).
Table 5.
Analysis of depressive symptoms by group, time and interaction: OSAS resolution versus no resolution
| Measure | Resolution (N = 98) | No resolution (N = 78) | P value | ||||
|---|---|---|---|---|---|---|---|
| Baseline median (IQR) | Change | Baseline median (IQR) | Change | Group | Time | Group × time | |
| CDI total score | 47 (43, 54) | −4 (−8, 0) | 49 (42.25, 57) | −3 (−6.75, 1.75) | 0.111 | <0.001 | 0.474 |
| CBCL Anxious / Depressed | 51 (50, 54) | 0 (−2, 0) | 51 (50, 60) | 0 (−2, 0) | 0.041 | 0.030 | 0.030 |
| CBCL Withdrawn /Depressed | 52 (50, 56) | 0 (−2, 0) | 54 (50, 61.5) | 0 (−4, 0.5) | 0.094 | 0.121 | 0.204 |
IQR = interquartile range. Significant data are shown in bold.
Impact of sleep-specific items from the CDI
As described previously, two items from the CDI relate specifically to sleep and tiredness, both of which load on to the Anhedonia subscale of this measure. To determine the impact of these sleep-related items, analyses were repeated for all subscales of the CDI for both eAT versus WWSC and resolution of OSAS (yes/no). In the eAT versus WWSC analysis, although the Anhedonia subscale demonstrated a significant improvement over time (p < 0.001), the Negative Mood subscale demonstrated a significant improvement over time as well (p = 0.009). No other CDI subscales revealed effects for treatment group, time, or the interaction of treatment group by time (Table 6). In analyses of the effect of resolution of OSAS (yes/no), a similar improvement over time was observed for both the Anhedonia (p < 0.001) and Negative Mood (p = 0.014) subscales (Table 7). In this analysis, a group effect was also noted for the Negative Self-Esteem subscale (p = 0.027), with the group with resolved OSAS having lower baseline scores on this subscale, but no statistically significant group differences in changes from baseline.
Table 6.
Analysis of self-report depressive symptom subscales by group, time and interaction: eAT versus WWSC
| CDI subscale | eAT | WWSC | P value | ||||
|---|---|---|---|---|---|---|---|
| Baseline median (IQR) | Change | Baseline median (IQR) | Change | Group | Time | Group × time | |
| Negative Mood | 50 (44, 59) | −5 (−10, 3) | 49 (44, 59) | 0 (−10, 5) | 0.411 | 0.009 | 0.256 |
| Interpersonal Problems | 45 (45, 56) | 0 (−11, 0) | 47 (45, 57) | 0 (−8, 0) | 0.642 | 0.206 | 0.425 |
| Ineffectiveness | 44 (41, 52) | 0 (−6, 1) | 41 (41, 52) | 0 (−6, 0) | 1.000 | 0.487 | 0.969 |
| Anhedonia | 54 (46, 61) | −7 (−12, 0) | 52 (46, 64) | −4 (−11, 0) | 0.782 | <0.001 | 0.341 |
| Negative Self-Esteem | 40 (40, 46) | 0 (−5, 0) | 46 (40, 51) | 0 (−6, 0) | 0.190 | 0.627 | 0.340 |
IQR = interquartile range. Significant data are shown in bold.
Table 7.
Analysis of self-report depressive symptom subscales by group, time and interaction: OSAS resolution versus no resolution
| CDI subscale | Resolution (N = 98) | No resolution (N = 78) | P value | ||||
|---|---|---|---|---|---|---|---|
| Baseline median (IQR) | Change | Baseline Median (IQR) | Change | Group | Time | Group × time | |
| Negative Mood | 50 (44, 59) | −5 (−10, 3) | 49 (44, 59) | 0 (−10, 5) | 0.968 | 0.014 | 0.643 |
| Interpersonal Problems | 45 (45, 56) | 0 (−11, 0) | 49 (45, 57) | 0 (−8, 0) | 0.183 | 0.048 | 0.513 |
| Ineffectiveness | 41 (41, 51) | 0 (−6, 0) | 44 (41, 52) | 0 (−5, 0) | 0.366 | 0.509 | 0.924 |
| Anhedonia | 52 (46, 61) | −4 (−11, 0) | 53 (46, 65) | −4 (−12, 0) | 0.488 | <0.001 | 0.930 |
| Negative Self-Esteem | 40 (40, 46) | 0 (−6, 0) | 46 (40, 46) | 0 (−6, 0) | 0.027 | 0.960 | 0.450 |
IQR = interquartile range. Significant data are shown in bold.
Discussion
This large pediatric study of AT versus WWSC for pediatric OSAS demonstrated a significant improvement in self-reported depression symptoms over time, but symptoms of depression did not improve differentially with treatment of OSAS versus watchful waiting by either parent- or self-report. Additionally, resolution of OSAS, regardless of eAT versus WWSC group, was associated only with parent-reported Anxious/Depressed symptoms and in an unexpected direction, with greater improvement in Anxious/Depressed symptoms in the group without OSAS resolution. Potential reasons for the lack of consistent improvement in depression symptoms for either eAT or resolution of OSAS may be that the increased risk of depression potentially precipitated by OSAS is not reversible (or is not reversible over the time course of this study) through resolution of the OSAS alone, or that the depression is not secondary to the OSAS but is rather a comorbidity. Further study is needed. Main effects for improvement in depression over time across the study population as a whole may reflect identification of depressive symptoms by the study team and subsequent intervention for individuals determined to be at risk at baseline, as elevations in depressed symptoms and/or endorsement of critical items was addressed with participants and families at all timepoints.
Results revealed a high prevalence of self-reported depression symptoms in school-aged children with OSAS, with a disproportionate number of children reporting elevated depressive symptoms in the most severe category (i.e. depressive symptoms falling “much above average”). Rates of self-reported “much above average” depressed symptoms in this OSAS population as a whole were double the expected rate based on CDI normative samples. This more severe category of depressed symptomatology was associated also with race, with Black children reporting “much above average” rates of depressive symptomatology at three times the rate of White/other children. Maternal education, sex, and overweight/obese status did not demonstrate an impact on self-reported depression. Additionally, parent-reported levels of Withdrawn/Depressed symptoms were significantly elevated relative to population/test norms, and overweight/obese status was a risk factor for parent-reported Withdrawn/Depressed symptoms, highlighting the potential impact of weight on social isolation and withdrawal in overweight/obese children with OSAS.
Consistent with prior studies [14–16], child self-report of depression symptoms was only modestly correlated with parent reports even for similar constructs, highlighting the importance of including child self-report in future studies.
The relationship between nocturnal desaturation and depression at baseline point to hypoxemia as a potential mechanism for increased depression in this population. Continued research into the relationship between polysomnographic findings and depressive symptomatology in a pediatric population is warranted, and may have implications for asthma, cystic fibrosis and other pediatric respiratory disorders that are also characterized by oxygen desaturation.
The inclusion of questions related to sleep and tiredness on depression measures (in this case, on the CDI Anhedonia subscale but not CBCL depression scales) creates a potential confound for the use of these measures within an OSAS population. Additional analyses of CDI subscales revealed improvement over time in both Anhedonia and Negative Mood subscales. Thus, parallel changes between the Anhedonia subscale (which includes sleep-related items) and the Negative Mood subscale (which does not include sleep-related items) suggest that these study results were not significantly skewed by a change in sleep complaints only. Nonetheless, these analyses highlight the need for consideration of item-level analysis when using broad depression measures in a sleep-disordered population. This consideration of sleep-related items was noted in a prior analysis of CHAT CBCL data, in which greater improvements in some CBCL scales (Internalizing Problems, Somatic Complaints, Thought Problems) in the eAT versus WWSC group were found to be no longer significant when sleep-related items from those scales were excluded, with only Total Problems showing greater improvement in the eAT group after sleep items were eliminated [18].
Strengths of this study include a relatively large cohort, the inclusion of both self-report and caregiver-report measures of depression, and the randomized study design. Limitations include the fact that this study was restricted to school-aged children, and children with severe desaturation were not included. Thus, study findings may not be applicable to different age ranges or children with more severe OSAS. Additionally, results may not be applicable to the larger population of children with OSAS, who may differ from those who participate in randomized clinical trials. Further, depressive symptomatology was evaluated by questionnaires rather than by comprehensive clinical evaluations. Despite the relatively large sample size in relation to other studies of childhood depressive symptoms and OSAS [4], the sample size was predicated by the size of the CHAT study, and an even larger study may be needed to definitively determine the impact of OSAS on childhood depression. This subanalysis was also not adequately powered to examine the potential moderating effects of demographic factors on treatment or resolution effects of OSAS and depressive symptoms. Additionally, a non-OSAS group was not included in the study design, and therefore comparisons to published norms in a community sample were necessary to determine whether depressive symptoms were elevated for the study population as a whole. Demographic differences between the questionnaire normative samples and the current sample could impact the rates of participants exceeding published score cutoffs, but would not be expected to affect findings within our sample related to treatment, time, treatment × time interactions, or correlation with sleep variables.
In summary, this study has shown increased risk for depressed and withdrawn/depressed symptoms in this sample of children with OSAS, and revealed different demographic variables contributing to risk in self-reported versus parent-reported depression symptoms. Additionally, arterial oxygen desaturation nadir during sleep was strongly associated with both self-report and parent-reported depressed symptoms. Although child-reported depressed symptoms improved over time, this improvement was unrelated to either treatment group or OSAS resolution status. Parent-reported Anxious/Depressed symptoms also showed improvement in time but not for treatment group, and a time by resolution interaction was in an unexpected direction, with improved depressive symptomatology in the group without resolution of OSAS. Future studies of depression and pediatric OSAS would benefit from inclusion of child self-report, as well as similar recruitment of minority and overweight/obese participants, in order to further examine the impact of these variables. Additionally, longer-term studies, as well as studies in younger populations, would be beneficial in determining whether the relationship between low saturation and depressive symptoms can be mitigated by earlier treatment or reversed after longer follow-up periods.
Funding
The study was funded by National Institutes of Health grants HL083075, HL083129, and UL 1 TR000003. The study was also supported by a Scientific Coordinating Center / Sleep Reading Center at Brigham and Women’s Hospital (Boston, MA).
Conflict of interest statement. E.H., C.L.M., J.Y.K., J.S., B.G., D.W.B., R.B.M., E.S.K., D.G., S.R., L.E., R.A., R.M., H.G.T., J.R., and N.H.T. have no financial or nonfinancial disclosures. M.X. has received salary support through a research agreement from Eisai Inc. to the Children’s Hospital of Philadelphia for a project unrelated to the current manuscript. C.L.R. is on the board of directors of the American Academy of Sleep Medicine, is involved with investigational drugs/devices with JazzPharmaceutical and Flamel, and has been a consultant for Jazz Pharmaceutical and Advance-Medical; not related to the current article. R.D.C. receives research support from the NIH and Michigan Medicine. He serves on the boards of directors for the International Pediatric Sleep Association, the nonprofit Sweet Dreamzzz, Inc, and the American Academy of Sleep Medicine. He is President of the Associated Professional Sleep Societies. He serves as an editor and author for UpToDate.
Acknowledgments
We thank the children and their families for participating in the study. We thank all the members of the CHAT research team for their assistance. The CHAT study was a multi-site study with enrollment at the Children’s Hospital of Philadelphia (Philadelphia, PA), C (Cleveland, OH), Cardinal Glennon Children’s Medical Center (St. Louis, MO), Cincinnati Children’s Hospital Medical Center (Cincinnati, OH), Boston Children’s Hospital (Boston, MA), and Children’s Hospital at Montefiore and Montefiore Medical Center (New York, NY). Data was coordinated through a Data Coordination Center (University of Pennsylvania; Philadelphia, PA) with a Data and Safety Monitoring Board and quality assurance through surgical and neuropsychology cores that operated at the University of Michigan (Ann Arbor, MI).
References
- 1. Redline S, et al. . Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1527–1532. [DOI] [PubMed] [Google Scholar]
- 2. Marcus CL, et al. ; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. [DOI] [PubMed] [Google Scholar]
- 3. Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29(9):1115–1134. [DOI] [PubMed] [Google Scholar]
- 4. Yilmaz E, et al. . The relationship between depressive symptoms and obstructive sleep apnea in pediatric populations: a meta-analysis. J Clin Sleep Med. 2013;9(11):1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang CH, et al. . Pediatric sleep apnea and depressive disorders risk: a population-based 15-year retrospective cohort study. PLoS One. 2017;12(7):e0181430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stojek MMK, et al. . Fitness, sleep-disordered breathing, symptoms of depression, and cognition in inactive overweight children: mediation models. Public Health Rep. 2017;132(2_suppl):65S–73S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carotenuto M, et al. . Depressive symptoms and childhood sleep apnea syndrome. Neuropsychiatr Dis Treat. 2012;8: 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crabtree VM, et al. . Health-related quality of life and depressive symptoms in children with suspected sleep-disordered breathing. Sleep. 2004;27(6):1131–1138. [DOI] [PubMed] [Google Scholar]
- 9. Harris M, et al. . Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13(6):437–444. [DOI] [PubMed] [Google Scholar]
- 10. Dillon JE, et al. . DSM-IV diagnoses and obstructive sleep apnea in children before and 1 year after adenotonsillectomy. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Redline S, et al. . The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34(11):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcus CL, et al. ; Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Twenge JM, et al. . Age, gender, race, socioeconomic status, and birth cohort differences on the children’s depression inventory: a meta-analysis. J Abnorm Psychol. 2002;111(4): 578–588. [DOI] [PubMed] [Google Scholar]
- 14. De Los RA. Introduction to the special section: more than measurement error: discovering meaning behind informant discrepancies in clinical assessments of children and adolescents. J Clin Child Adolesc Psychol. 2011;40(1):1–9. [DOI] [PubMed] [Google Scholar]
- 15. Moretti MM, et al. . Childhood and adolescent depression: child-report versus parent-report information. J Am Acad Child Psychiatry. 1985;24(3):298–302. [DOI] [PubMed] [Google Scholar]
- 16. Kim J, et al. . Parent-Child discrepancies in reporting of child depression in ethnic groups. J Nurse Pract. 2016;12(6):374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kovacs M. Children’s Depression Inventory (CDI) Technical Manual Update. North Tonawanda, NY: Multi-Health Systems (MHS); 2003. [Google Scholar]
- 18. Thomas NH, et al. . Effects of adenotonsillectomy on parent-reported behavior in children with obstructive sleep apnea. Sleep. 2017;40(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor HG, et al. . Cognitive effects of adenotonsillectomy for obstructive sleep apnea. Pediatrics. 2016;138(2):e20154458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Achenbach TM, et al. . Manual for the ASEBA School-age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families; 2001. [Google Scholar]