Abstract
Study Objectives
To determine whether high-frequency heart rate variability (HF-HRV) during sleep differs between those with and without posttraumatic stress disorder (PTSD) as a function of sleep type (non-rapid eye movement [NREM] vs. rapid eye movement [REM]), and to explore this relationship across successive sleep cycles. Participants with PTSD were hypothesized to have lower HF-HRV across both REM and NREM sleep.
Methods
Sixty-two post-9/11 military veterans and service members completed self-report measures of sleep quality, insomnia severity, and disruptive nocturnal behaviors. Participants then completed a laboratory-based polysomnographic study night with concurrent HRV assessment.
Results
Participants with PTSD (N = 29) had lower HF-HRV in overall NREM sleep relative to those without PTSD (N = 33) (F(1, 54) = 4.24, p = .04). Groups did not differ on overall HF-HRV during REM sleep. HF-HRV increased over the night for the sample as a whole during both NREM and REM sleep. PTSD status did not moderate the association between HF-HRV and sleep cycles. However, the PTSD group had lower HF-HRV in the first t(155) = 2.67, p = .008, and fourth NREM cycles, t(155) = 2.11, p = .036, relative to participants without PTSD.
Conclusions
Findings suggest blunted parasympathetic modulation during NREM sleep in a young cohort of military veterans and service-members with PTSD. Findings are concerning considering the increased risk of incident cardiovascular events associated with impaired parasympathetic nervous system function. Reduced parasympathetic modulation may be one mechanism underlying the increased prevalence of cardiovascular disease (CVD) among veterans with PTSD.
Keywords: nocturnal heart rate variability, parasympathetic nervous system, posttraumatic stress disorder, veterans, sleep quality
Statement of Significance
Reduced parasympathetic modulation is associated with increased risk of incident cardiovascular events, and those with posttraumatic stress disorder (PTSD) symptoms and diagnoses show dysregulated autonomic nervous system function across both wake and sleep. Only one prior study has examined high-frequency heart rate variability (HF-HRV) as a function of both sleep type and across successive sleep cycles in PTSD, and the current study is the first to do so in a sample of military veterans and service members. Findings contribute to the literature by providing evidence for a potential mechanism underlying the increased cardiovascular morbidity observed in military veterans with PTSD.
Introduction
Owing to technological advances and greater consensus on methodology, assessment of heart rate variability (HRV) has become easier and more accessible for evaluation in recent years [1–3]. Accordingly, HRV has been increasingly used to examine autonomic functioning among those with psychiatric conditions [2]. As a condition defined by interacting physiological and cognitive features, the assessment of HRV in those with posttraumatic stress disorder (PTSD) is becoming an area of prominent focus within this growing literature.
HRV, an index of autonomic nervous system (ANS) activity [1] can be assessed by deconstructing variability of the beat-to-beat heart rate into its underlying spectral components [4], including low-frequency power (LF-HRV), high-frequency power (HF-HRV), and the ratio of these measures (LF/HF-HRV) [5]. Generally speaking, HF-HRV is a marker of parasympathetic nervous system function, or vagal tone, whereas LF-HRV reflects a combination of both sympathetic and parasympathetic activity. The ratio of LF/HF-HRV was previously considered to reflect the balance between sympathetic and parasympathetic nervous systems [6], but this perspective has been challenged [2] due to complex physiological origins which confound the interpretability of the measure [7]. Thus, the ratio of LF/HF HRV has been recently discounted for this purpose [1]. In addition, a recent study documented low reproducibility of both LF and the LF/HF ratio across all sleep stages [8]. As such, it appears that HF-HRV remains as the most reliable index of ANS activity derived from variation in beat-to-beat heart rate.
Some studies have documented ANS dysregulation among those with PTSD and PTSD symptoms, but others have not. Specifically, in some studies those with PTSD were found to have lower HF-HRV components on power spectrum analysis [9, 10], and in other studies no differences were found between those with and without PTSD [11]. In a 2014 meta-analysis of HRV in anxiety disorders [12], research participants with PTSD (N = 192) had significantly lower HF-HRV than controls (N = 525) (effect size = −0.29, p = .049). Similar findings have been documented for respiratory sinus arrhythmia (RSA) among those with PTSD. RSA is HRV in synchrony with respiration [13] and is an additional marker of parasympathetic function that is highly correlated with HF-HRV [14]. Individuals with PTSD tend to have lower RSA than those without PTSD [15].
In addition to the impaired autonomic function discussed above, most individuals with PTSD have disrupted sleep, including poor sleep quality, short sleep duration, fragmented sleep, and nightmares [16]. In healthy adult sleepers, both heart rate and time and frequency domain HRV decrease progressively during non-rapid eye movement (NREM) sleep and increase during rapid eye movement (REM) sleep, and heart rate during REM is higher than during inactive wakefulness [4]. Findings to date further suggest that vagal control of heart rate is predominant in NREM sleep and the sympathetic nervous system is predominant during REM sleep [4].
Since HF-HRV is an approximate index of cardiac vagal control only at rest, assessing HF-HRV during objectively-verified sleep represents ideal conditions for HRV assessment. A small literature has examined parasympathetic nervous system activity during sleep in those with PTSD. Table 1 is a summary of the available research findings on HF-HRV and RSA during sleep in adults with PTSD. We found only one prior study examining HF-HRV during sleep in those with PTSD or PTSD symptoms wherein findings were examined as a function of sleep type (NREM vs. REM) and time of night. Kobayashi and colleagues [17] examined ANS activity during sleep in a sample of healthy young African American adults (age 18–35 years) who had been exposed to a high impact traumatic event. They found that individuals meeting either full diagnostic criteria for PTSD or subthreshold PTSD criteria had greater REM-NREM differences in laboratory-based ANS activity as indexed by LF/HF HRV and HF-HRV compared to resilient individuals (those who never met diagnostic criteria for PTSD despite exposure to trauma). After adjusting for REM sleep percentage, these group differences were no longer present. Within-group findings showed that REM-NREM differences in ANS activity were associated with REM% in the resilient group, but not in the PTSD group. The authors discuss their findings in light of the role of sleep in regulating ANS activity [18]. Specifically, they posited that since REM percentage explained group differences in REM-NREM ANS activity in their sample, REM sleep may play a role in the decrements in ANS regulation during sleep observed in those with PTSD. They also suggested that, since their laboratory-based polysomnography (PSG) findings [17] differed from their prior findings employing ambulatory PSG [19], the assessment environment (home vs. lab) may be an important consideration when examining sleep ANS activity in those with PTSD.
Table 1.
Summary of studies evaluating HF-HRV and RSA during sleep in PTSD
| First author, year | PTSD status | Assessment technologies | Components reported | HRV findings | ||
|---|---|---|---|---|---|---|
| HRV | Sleep | HF | RSA | |||
| Kobayashi, 2016 | Dx/Sxa | PSG | Yes | No | REM–NREM difference greater in PTSD, but not after adjusting for REM%. | |
| Woodward, 2009 | Dx | Ambulatory | Actigraphyb | No | Yes | Lower RSA for both PTSD and PTSD + Panic DO groups relative to both Panic DO only and Controls. |
| Kobayashi, 2014 | Dx | ambulatory | Actigraphyc | Yes | No | HF-HRV lower for PTSD than controls. |
| Bertram, 2014 | Dx | Ambulatory | Actigraphyc | No | Yes | +PTSD did not differ from – PTSD on RSA. |
Dx = diagnosis; Sx = symptoms; + = positive; − = negative.
aParticipants met at least 2 of 3 PTSD symptom clusters.
bMattress actigraphy.
cWrist actigraphy.
The remaining available studies of parasympathetic activity during sleep in PTSD employed ambulatory devices for the assessment of both ANS activity and sleep. Woodward and colleagues [20] compared RSA in 59 community-residing individuals without sleep apnea or restless legs movement disorder across four categories: panic disorder (N = 14); PTSD (N = 14); co-morbid panic disorder and PTSD (N = 13); and controls (N = 8). Both sleep and ANS activity were assessed using accelerometers embedded in a mattress topper. The authors note that within the actigraphy signal, “small movements include the kinetocardiogram (KCG), which, for movement-free periods, closely tracks the ECG.” Participants were assessed in their homes for a median of 12 nights. Those with PTSD or comorbid PTSD plus panic disorder had lower RSA relative to both panic disorder alone and psychiatric controls. In a study of male veterans, those with PTSD (N = 56) were compared to those without PTSD (N = 54) using ambulatory measures of autonomic arousal [21]. Veterans with and without PTSD did not differ on RSA, actigraphy-derived sleep duration, or sleep fragmentation, but heart rate was higher during both wake and sleep in those with PTSD. In another more recent study, young adult trauma-exposed African Americans with (N = 20) and without PTSD (N = 18), HF-HRV was lower in those with PTSD than without PTSD, and total sleep time was significantly associated with HF-HRV (r = 0.75) and LF/HF-HRV (r = −0.72) in those without PTSD only [19].
As heart rate is known to decline over the course of sleep, several studies have also examined trends in parasympathetic activity across nighttime sleep. In healthy adults, findings for trends in HF-HRV have been mixed. Some studies found an increase in HF-HRV across successive NREM cycles and a decrease across successive REM cycles [22, 23], whereas others found no relationship between HF-HRV and sleep timing [24]. Only one study has examined trends in HF-HRV across sleep in those with PTSD. Kobayashi and colleagues (discussed above) [17] found a significant decline in HF-HRV across successive NREM cycles across the night, but the rate of decline did not differ between groups. No changes were found in HF-HRV activity across successive REM sleep cycles.
The research reviewed above is inconsistent in regards to parasympathetic modulation during sleep among those with PTSD, with some studies finding reduced relative parasympathetic modulation of cardiac activity during sleep and others not. The objective of the current study was to contribute to the existing literature by comparing military veterans and service members with and without PTSD on HF-HRV during sleep as a function of sleep stage (REM vs. NREM) and time using laboratory-based assessment. Based on the preponderance of findings for reduced HF-HRV in those with PTSD in prior studies, we hypothesized that study participants with PTSD would have lower HF-HRV relative to controls across both REM and NREM. We also conducted exploratory analyses to examine HF-HRV across successive sleep cycles.
Methods
Participants
Participants included in the current study were assembled from three parent studies [25–27] employing identical recruitment strategies, populations, and laboratory procedures. Military veterans and active duty service members between the ages of 18 and 65 years old, with and without PTSD, were enrolled. Study 1 [28] was a randomized trial designed to investigate prazosin or behavioral intervention compared to placebo on sleep, mental health and health-related quality of life in men and women veterans across 8-week intervention and 4-month follow-up periods. Study 2 [26, 27, 29] was a four-night laboratory-based study of military veterans and service members designed to examine: (1) the neurobiology of PTSD during REM and NREM sleep relative to wakefulness, (2) the neurobiological changes associated with sleep treatment response during REM sleep and NREM sleep relative to wakefulness; and (3) to explore the neurobiological predictors of sleep treatment response. Eligible participants were studied for 4 nights in the sleep laboratory and assessed with positron emission tomography (PET) scans during morning wakefulness, REM sleep, and NREM sleep. Participants with PTSD were then randomized into Study 1 where they received either prazosin or placebo. Study 3 (NIH MH083035) was designed to explore the neurobiological correlates of PTSD during REM sleep by using neuroimaging [18F]-fluoro-2-deoxy-D-glucose (FDG) PET in military veterans or service members with and without PTSD. All participants completed at least one deployment in support of Operation Enduring Freedom or Operation Iraqi Freedom. Participants were recruited via public advertisement and word of mouth. Potential study participants were excluded if they met diagnostic criteria for comorbid psychiatric disorders (e.g. bipolar disorder, psychotic disorders, or current substance use disorder), or they screened positive for drugs or alcohol on a toxicology screen. Similarly, participants using agents know to affect autonomic control (e.g. beta blockers) and those with unstable medical conditions (e.g. diabetes, hospitalization within the past 2 weeks for medical or psychiatric reasons, severe anemia) or chronic medical conditions (e.g. cardiovascular and cardiac conditions) were excluded from the study. Individuals with a history of seizure disorder, open skull brain injury, or concurrent concussive symptoms were excluded. Finally, pregnant or breastfeeding women were excluded.
The current sample was comprised of sixty-two post-9/11 military veterans or active duty service members (M age = 32.29 years, 87.1% male, 88.7% Caucasian, BMI = 27.87, AHI = 2.71, and 47% with PTSD). Potential participants using antidepressants were eligible if they had been using an antidepressant for at least 2 months at the same dose prior to study entry. In this sample, 6.5% met this criterion for stable antidepressant use, 4.8% endorsed current use of sedative-hypnotic medications, and 41.9% of the sample endorsed current tobacco use. Groups (PTSD vs. no-PTSD) did not differ by percent female (p = .85), antidepressant use (p = .25), sedative-hypnotic use (p = .49), or tobacco use (p = .46).
Study procedures
A standardized and common protocol was employed across studies. After providing written informed consent, participants completed an extensive screening intake to evaluate the presence of co-morbid psychiatric and sleep disorders. The Clinician-Administered PTSD Scale was used to assess the presence and severity of PTSD and the Structured Clinical Interview for DSM-IV Axis I disorders (SCID [30]) was used to screen out veterans with comorbid psychiatric disorders. A structured clinical interview for sleep disorders and overnight sleep apnea screening was used to rule out potential participants meeting criteria for obstructive sleep apnea, restless legs syndrome, or periodic limb movement disorder. The protocol involved one night of lab-based overnight apnea screening study followed by one baseline PSG recording night. For the present study, data collected on the baseline PSG recording nights were aggregated across the parent studies. Prior to sleeping in the laboratory, enrolled participants also completed self-report measures including the Pittsburgh Sleep Quality Index (PSQI), the Insomnia Severity Index (ISI), and PSQI Addendum for PTSD (PSQI-A). The PSQI [31] assesses overall sleep quality over the previous 1-month period and the PSQI-A assesses disruptive nocturnal behaviors related to trauma exposure and/or PTSD symptoms, and has been validated in a sample of sample military veterans [32]. The ISI [33] assesses insomnia severity.
Polysomnography and electrocardiography procedures
As part of their study participation, enrolled participants completed questionnaires, followed by a two-night laboratory sleep study including screening for sleep apnea (night 1) and assessment of HRV during NREM and REM sleep (night 2). HRV was assessed on night 2 only, as we have previously reported high temporal stability of HRV across consecutive nights [34]. Grass Telefactor M15 bipolar Neurodata amplifiers and Stellate Harmonie collection software were used for recording and monitoring overnight PSG. The Night 2 PSG montage included bilateral central referential EEG (C3 and C4, referenced to A1 + A2), bilateral electro-oculograms (EOG), submentalis electromyogram (EMG), and electrocardiogram (EKG) using a two-lead electrode placement.
EEG signals were sampled at 256 Hz and filtered during acquisition (high- and low-pass frequency filters of 0.3 and 100 Hz, respectively and 60-Hz notch filter). Raw, digitized data were band-limited to 64 Hz using a low-pass, finite-impulse, response filter and signals were then decimated to 128 Hz for offline visual sleep stage scoring in 20-second epochs using Rechtschaffen and Kales (1968) criteria, as these data were collected and scored prior to publication of the American Academy of Sleep Medicine manual [35]. Visual sleep stage scoring on NREM and REM sleep across individual sleep cycles and the night as a whole were used to generate descriptive sleep characteristics including sleep duration (total sleep time, in minutes), fragmentation (sleep latency defined as minutes from good night time to sleep onset; wakefulness after sleep onset defined as total minutes of wakefulness between sleep onset and offset; number of awakenings, defined as the number of individual bouts of wakefulness after sleep onset of ≥1 minute; and sleep efficiency, defined as total sleep time/time in bed × 100), and sleep staging (minutes of sleep in each stage of NREM sleep and REM sleep/total minutes of sleep × 100), shown as percent in Table 2, and for identifying NREM and REM periods for evaluation of HF-HRV during sleep and across individual sleep cycles.
Table 2.
Participant Characteristics
| No PTSD Dx | PTSD Dx | ||
|---|---|---|---|
| Demographic characteristics | |||
| Mean (SD) | Mean (SD) | Significance ( p) | |
| Age | 33 (7.4) | 31 (8.7) | 0.6 (0.4) |
| BMI | 28 (3.3) | 28 (4.9) | 0.2 (0.7) |
| Insomnia severity and sleep quality | |||
| Mean (SD) | Mean (SD) | ||
| ISI Score | 10.6 (6.9) | 14.6 (3.8) | 7.5** |
| PSQI Score | 6.9 (4.5) | 8.7 (3.4) | 2.7 (0.11) |
| PSQI-A Score | 1.3 (1.6) | 4.6 (2.5) | 40.9** |
| Mental health characteristics | |||
| Mean (SD) | Mean (SD) | ||
| CAPS Score past month | 19.7 (12.9) | 58.8 (13.5) | 137.1*** |
| CES Score | 14.8 (10.8) | 16.9 (11.3) | 0.5 (0.5) |
| # Traumatic events as child | 1.2 (1.5) | 1.6 (1.6) | 0.9 (0.4) |
| # Traumatic events as adult | 5.4 (2.7) | 7.2 (3.4) | 4.7* |
| # Lifetime traumatic events | 6.6 (3.6) | 8.7 (3.7) | 4.6* |
| BDI | 5.0 (5.2) | 10.3 (7.4) | 10.4** |
| BAI | 4.0 (5.1) | 7.5 (6.9) | 4.6* |
| n (%) | n (%) | ||
| Depression | 0 (0) | 7 (24.1) | 8.98** |
| Polysomnographic sleep characteristics | |||
| Apnea-hypopnea Index | 2.92 (3.2) | 2.47 (3.1) | 0.4 (0.5) |
| Sleep onset latency, minutes | 24.6 (27.6) | 19.7 (15.6) | 0.7 (0.4) |
| Total sleep time, minutes | 396.7.0 (55.7) | 377.6 (68.2) | 1.7 (0.2) |
| Wake after sleep onset, minutes | 28.6 (26.3) | 35.3 (37.5) | 0.7 (0.4) |
| Sleep efficiency, percent | 88.2 (8.3) | 87.5 (9.5) | 0.1 (0.7) |
| Number of awakenings | 22.2 (6.4) | 21.7 (5.7) | 0.2 (0.7) |
| NREM stage 1, percent | 4.9 (2.7) | 5.0 (3.6) | 0.0 (0.97) |
| NREM stage 2, percent | 61.9 (5.7) | 62.0 (8.5) | 0.0 (0.86) |
| NREM stage 3 + 4, percent | 6.8 (6.2) | 9.3 (7.8) | 1.8 (0.19) |
| REM, percent | 26.3 (5.1) | 23.6 (5.4) | 3.8 (0.06) |
*=p<.05, **=p<.01. ***=p<.001
ReBAI=Beck Anxiety Inventory; BDI=Beck Depression Inventory; BMI=body mass index; CAPs=Clinician-Administered PTSD Scale; CES=Combat Exposure Scale; Dx=diagnosis; ISI=insomnia severity index; NREM=non-rapid eye movement; PSQI-Pittsburgh Sleep Quality Index; PSQI-A= Pittsburgh Sleep Quality Index PTSD Addendum; PTSD=posttraumatic stress disorder; REM=rapid eye movement; SD=standard deviation.
Power spectral analysis of HRV was estimated from the EKG signal, collected continuously throughout sleep at a sampling rate of 1024 Hz [5]. A commercially-available software (Mindware Heart Rate Variability Scoring Module, Mindware Technologies Ltd., Gahanna, OH) was used to estimate HRV during NREM and REM sleep, as previously described [36] Briefly, EKG signals were inspected and edited for suspected artifacts pursuant to Mindware’s automated artifact detection and visual inspection. Inter-beat intervals were then calculated for each successive pair of R waves and the Fast Fourier Transform (FFT) was used to derive HRV power spectral estimates for each 2-minute epoch of NREM and REM sleep. For the purposes of this report, we focused on HRV power in the high-frequency band (0.15–0.40 Hz) [5]. HRV data were time-aligned with visually-scored sleep to estimate average HF-HRV during each cycle of NREM and REM sleep [36]. For example, Non-REM HRV epochs included six 20-second epochs of homogenous stage 2 or 3 + 4 sleep. If a 2-minute epoch included any 20-second epochs of wake, stage 1, or REM sleep, it was not included in analyses of HF-HRV during NREM sleep. Similarly, REM sleep HF-HRV epochs included only REM epochs; any 2-minute epochs containing any 20-second epochs of wake or NREM sleep was not used to estimate HF-HRV during REM sleep. Respiratory belts were not included in the PSG montage, which precluded the use of respiration as a covariate in HRV analyses. The lack of respiration data is unlikely to have significantly affected our results in light of evidence that uncorrected measures of HRV are accurate during states such as sleep characterized by stable respiratoration [37].
Statistical analyses
Groups were compared on demographic characteristics, polysomnographic characteristics and questionnaire outcomes using t-tests and the chi-square statistic. Multilevel modeling (MLM) was used to examine differences across groups and sleep type (REM vs. NREM). MLM is analogous to repeated measures analysis of variance (ANOVA), but it uses all available data and can accommodate unequal variances. In the present analysis, a three-level model was specified, which accounted for nesting of periods of REM and NREM sleep within cycles, of which there were up to four per person, and nesting of cycles within individuals. Thus, this model could account not only for shared variance among observations within individuals, but also for potential correlations of HF-HRV estimates corresponding to REM and NREM sleep from the same sleep cycle. Consistent with prior research [38], high-frequency power was log-transformed to normalize its distribution. These values were then modeled as a function of PTSD status, sleep type, and the interaction between PTSD status and sleep type. Age, sex, apnea-hypopnea index (AHI), and total BDI scores (with sleep items omitted) were covaried. Given potential confounds of BMI, BAI scores, total sleep time, use of benzodiazepines, and REM percentage on HF-HRV, we conducted sensitivity analyses to determine if findings differed across models covarying for these factors.
We also examined whether changes in HF-HRV across successive sleep cycles differed by PTSD status. To do so, MLM was used to model log HF-HRV as a function of PTSD status, sleep cycle (1, 2, 3, and 4), and the interaction between the PTSD status and sleep cycle. Two separate models were examined, one for NREM sleep and one for REM sleep. As in the prior analysis, age, sex, AHI, and total BDI scores (with sleep items omitted) were covaried.
Results
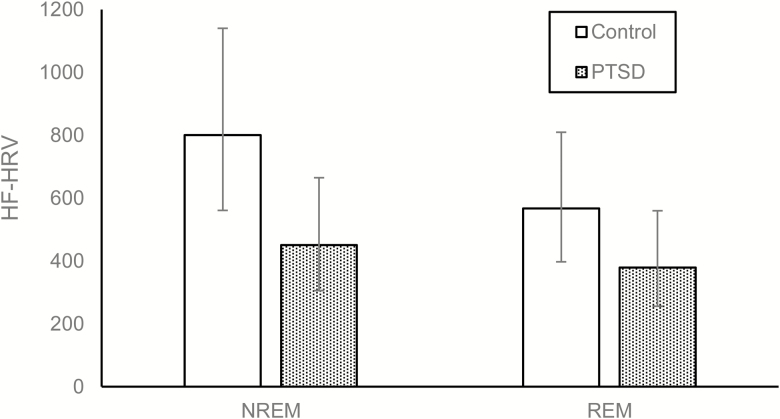
As shown in Table 2, participants with PTSD reported significantly more trauma-related sleep disturbances (p < .01) on the PSQI-A and higher insomnia severity (p < .01) on the ISI. The sample recorded higher HF-HRV during NREM sleep compared to REM sleep. After adjusting for age, sex, AHI, and total BDI score (sleep items omitted), there was no main effect of PTSD on HF-HRV (Table 3). This finding, namely the interaction effect, did not change substantially from the above model (B = −0.17, SE = 0.09, p = .074) when BMI (B = −0.17, SE = 0.09, p = .073), BAI (B = −0.20, SE = 0.10, p = .058), total sleep time (B = −0.17, SE = 0.09, p = .076), use of benzodiazepines (B = −0.17, SE = 0.09, p = .073), or REM percentage (B = −0.17, SE = 0.09, p = .074) were added as additional covariates in separate adjusted analyses. However, participants recorded higher HF-HRV during NREM sleep compared to REM sleep. Moreover, there was a trend suggesting that PTSD status may moderate this association. Examination of the simple slopes indicated that participants with PTSD had lower HF-HRV during NREM sleep than controls, t(58) = −2.03, p = .047 (Figure 1). In contrast, participants did not differ in HF-HRV during REM sleep based on PTSD status, t(58) = −1.42, p = .16.
Table 3.
Multilevel model of HF-HRV (logged values) by PTSD status and sleep type (NREM vs. REM)
| Parameter | Coeff (SE) | p |
|---|---|---|
| Fixed effects | ||
| Within-person predictors | ||
| Intercept | 8.26 (0.55) | <.001 |
| Sleep type (NREM vs. REM) | 0.34 (0.06) | <.001 |
| Between-person predictors | ||
| Age | −0.06 (0.02) | <.001 |
| Gender | 0.28 (0.42) | .51 |
| AHI | 0.02 (0.04) | .68 |
| BDI Score | 0.01 (0.02) | .63 |
| PTSD status | −0.40 (0.28) | .16 |
| Cross-level interaction | ||
| PTSD status × sleep type | −0.17 (0.09) | .074 |
| Random effects | ||
| Interindividual differences | 0.88 (0.18) | <.001 |
| Variability between sleep cycles | 0.04 (0.02) | .020 |
| Variability within sleep cycles | 0.22 (0.02) | <.001 |
Figure 1.
Modeled contrasts of HF-HRV by PTSD status and sleep type (NREM vs. REM). Error bars represent 95% confidence intervals. HF power is represented as milliseconds squared/hertz.
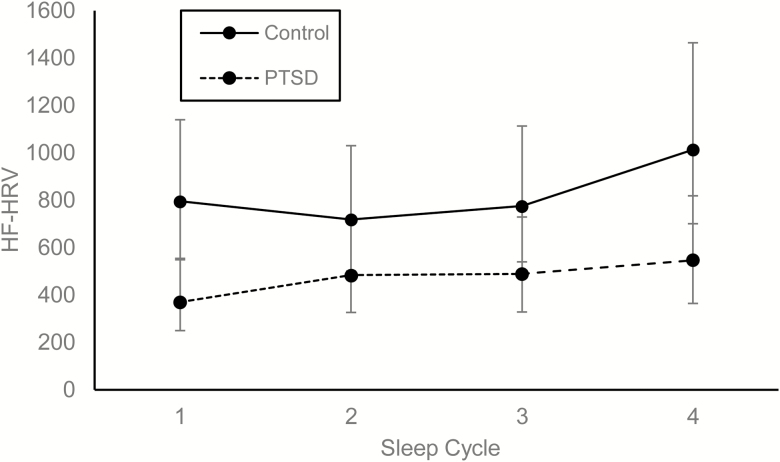
We also examined the relationship between diagnostic status and HF-HRV profiles across successive cycles of NREM and REM sleep. Controlling for the effects of age, F(1, 54) = 15.95, p < .001, sex, F(1, 54) = 0.27, p = .61, AHI, F(1, 54) = 0.40, p = .53, and total BDI scores (minus sleep items), F(1, 54) = 0.27, p = .60, a significant main effect for PTSD status indicated that participants with PTSD had lower overall HF-HRV than controls during NREM sleep, F(1, 54) = 4.24, p = .04. A significant main effect of sleep cycle revealed that HF-HRV increased during NREM over the course of the night, F(3, 155) = 5.39, p = .002. In turn, there was a trend suggesting that PTSD status moderated the main effect of sleep cycle, F(3, 155) = 2.48, p = .063. Examination of the point contrasts revealed that controls had higher HF-HRV during the first, t(155) = 2.67, p = .008, and fourth cycles, t(155) = 2.11, p = .036, of NREM than participants with PTSD (Figure 2). These contrasts were not significant during the second, t(155) = 1.39, p = .17, and third cycles, t(155) = 1.60, p = .11 of NREM.
Figure 2.
Modeled contrasts in HF-HRV by PTSD status across NREM sleep cycles. Error bars represent 95% confidence intervals. HF power is represented as milliseconds squared/hertz.
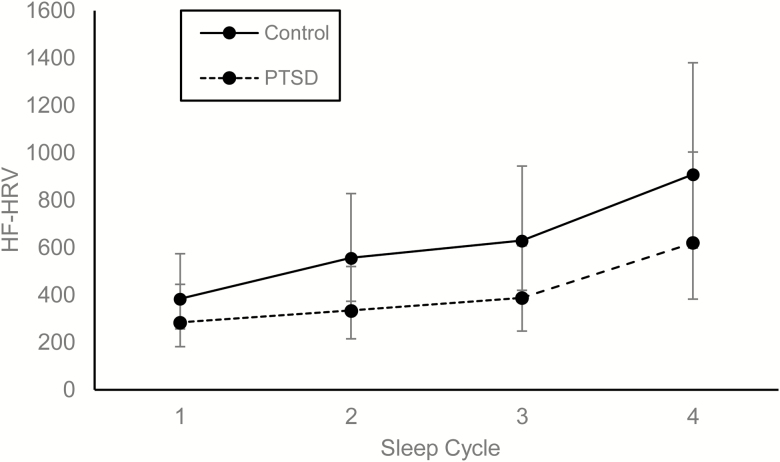
Turning to REM sleep, controlling for the effects of age, F(1, 54) = 11.16, p = .002, sex, F(1, 54) = 0.52, p = .48, AHI, F(1, 54) = 0.00, p = .99, and total BDI scores (minus sleep items), F(1, 54) = 0.19, p = .67, a significant main effect of sleep cycle revealed that HF-HRV increased during REM over the course of the night, F(3, 130) = 18.36, p < .001. Neither the main effect of PTSD status, F(1, 54) = 1.95, p = .17, nor its interaction with sleep cycle, F(3, 130) = 0.52, p = .67, was significant during REM sleep (Figure 3).
Figure 3.
Modeled contrasts in HF-HRV by PTSD status across REM sleep cycles. Error bars represent 95% confidence intervals. HF power is represented as milliseconds squared/hertz.
Discussion
This is the second study to use laboratory-based PSG to compare individuals with and without PTSD diagnosis on high-frequency domain HRV parameters across both NREM and REM sleep, and the first to do so in a population of military veterans and service members. Consistent with findings for normal sleepers, we found higher HF-HRV during NREM than REM sleep in all participants [4]. Although participants with PTSD did not have lower overall HF-HRV across the night or during REM sleep as we hypothesized, they did have lower HF-HRV during NREM sleep than controls. This finding is generally consistent with prior studies suggesting reduced parasympathetic function during sleep in those with PTSD [19, 20]. As discussed above, Kobayashi and colleagues [17] conducted the only other study examining HF-HRV by sleep type in those with PTSD. In contrast with our study, Kobayashi and colleagues found a group-by-sleep-type interaction with the PTSD group having greater REM-NREM differences in HF-HRV than resilient individuals. This effect was no longer significant, when adjusted for the higher percentage of REM sleep in resilient individuals.
In addition to examining overall HF-HRV across the night, we examined HF-HRV by group across successive cycles of NREM and REM across the night. HF-HRV increased across the night for the sample as a whole. In contrast with our findings, Kobayashi and colleagues [17] found decreasing HF-HRV in NREM across the night in the sample, with no differences between groups on the rate of change in NREM HF-HRV across the night.
Several factors could explain differences between our study findings and those of Kobayashi and colleagues [17]. First, their sample was comprised entirely of African American adults, whereas African Americans comprised only 9.7% of our sample. As such, racial differences in sample composition could explain inconsistent findings across studies. Indeed, some studies have found differences on both the time and frequency HRV domains between European Americans and African Americans [39, 40]. Second, our samples differed by other demographic characteristics as well, including the percentage of males (higher percentage of males in our sample), age (our sample was older) and BMI (our sample had higher BMI). Yet, analyses in both studies were adjusted for these demographic variables. Third, our sample was comprised entirely of military veterans and service members as compared to the civilian sample of Kobayashi and colleagues [17]. Since the groups in our sample did not differ in terms of combat exposure, exposure to combat alone does not appear to explain our findings. However, our PTSD group did endorse a higher number of lifetime and adulthood traumatic experiences. As such, it’s not clear if or how military versus civilian status might be relevant to differing findings across studies. Our findings also parallel those of a prior study [41] examining HF-HRV as a function of PTSD severity in a sample of young adults (N = 209; 95 with current PTSD) across actigraphically-assessed wake, rest, and sleep. Those with higher PTSD symptom severity had lower HF-HRV than those with lower PTSD symptom severity, but only during periods of sleep.
Few studies have examined HF-HRV by sleep type and across successive sleep cycles in those with either acute stress or stress-related mental health disorders (i.e. PTSD). The impact of acute stress on HF-HRV during sleep was examined in a study by Hall and colleagues [42]. In this experimental study, 59 healthy men and women were randomized to either control or a condition involving stress induction prior to sleep. Compared to the acute stress condition, overall HF-HRV across the night was increased in controls during both REM and NREM. In addition, parasympathetic activity increased across successive NREM cycles in the control condition, whereas it remained largely unchanged in the acute stress condition. This finding of blunted parasympathetic activity in overall NREM and across successive NREM periods in acutely stressed adults parallels our findings in chronically stressed adults. Unlike the Hall and colleagues study, however, our groups did not differ in parasympathetic modulation during averaged REM bouts. This divergence points to the likelihood that acute and chronic stress have differential impacts on nocturnal HF-HRV, and that this difference may manifest differentially by sleep type. This possibility is consistent with the proposition by other researchers [17, 43] that REM, specifically, may play an important role in regulation of autonomic function during sleep in those with PTSD.
There are a number of clinical implications for our findings. Unlike normal sleepers, parasympathetic nervous system modulation did not increase across successive NREM sleep cycles in military veterans and service members with PTSD. This finding is of great concern because reduced parasympathetic modulation is associated with increased risk of incident cardiovascular events [44], and because cardiovascular events such as acute myocardial infarctions occur mainly in the early morning [43]. Moreover, since this sample was relatively young and largely without co-morbid medical conditions, the decrements in ANS function observed during NREM sleep in this younger sample may be even more pronounced in the typical military Veteran having greater medical morbidity than the average US adult [45]. Prior studies have also found a link between depressed parasympathetic activity and non-dipping nocturnal blood pressure [46], a known risk factor for cardiovascular events in hypertensive patients and a predictor of mortality in both hypertensive patients and population-based samples [47]. In fact, it has been proposed that non-dipping nocturnal blood pressure and impaired autonomic function are “different aspects of the same pathophysiology” [48]. In a sample of women with and without PTSD, we found that “non-dippers” endorsed more traumatic event categories, more PTSD hyperarousal symptoms, greater sleep-related daytime dysfunction, and longer sleep onset latencies than dippers [49].
Laborde and colleagues [1] emphasized the importance of “correct methodological process” in assessing and interpreting HRV. In the current study, we employed a consensus-derived approach to data collection and interpretation. An additional strength of our study is the concurrent assessment of HF-HRV and sleep using laboratory-based PSG rather than ambulatory devices, and that assessment of HF-HRV during sleep eliminates the confounds of wake HRV assessment that are attributable to postural changes and activity level. In contrast with wake HRV, sleep HRV can be compared against sleep staging data and allows concurrent examination of circadian variation across parameters.
Compiling data across several studies offered the opportunity to examine a larger sample in these secondary analyses. However, a limitation of this approach is that we did not evaluate differences in the number of ectopic beats, arrhythmias, wakefulness, and movement artifacts in NREM and REM sleep between the groups. As such, our findings should be interpreted with caution. Our study is also subject to the limitations of cross-sectional study design for predicting causation. Also, since PTSD is often co-morbid with MDD [50] and medical conditions [51], our sample may not be representative of many Veterans with longstanding PTSD.
In military service members, combat exposure and deployment are risk factors for incident hypertension [52], and combat deployers are at increased risk for coronary heart disease [53]. Our findings suggest that impaired parasympathetic function during NREM sleep may represent one pathway by which these increased risks are conferred. Future studies are needed employing concurrent assessment of variation in circadian ANS activity (including both sympathetic and parasympathetic activity) and related biomarkers (e.g. nocturnal blood pressure patterns). Future studies on sleep HRV should also be conducted with Veterans and military service members having comorbid medical and mental health conditions, and should be adequately powered to examine the impact of the presence/absence of comorbid conditions in addition to important demographic variables such as race and sex. Clinical trials are also needed to examine the possibility that health behavior change interventions promote greater HRV in those with PTSD during both wake and sleep. Thayer and colleagues [54] reviewed associations between HRV, autonomic imbalance, and cardiovascular disease (CVD) risk factors. In their review, they make a persuasive argument for a bidirectional relationship between HRV and CVD such that intervening on certain modifiable risk factors can lead to increased HRV. Specifically, interventions such as smoking cessation, exercise, dietary changes and stress reduction are associated with post-intervention increases in HRV.
In summary, our findings extend the literature examining parasympathetic modulation among those with PTSD during sleep. Reduced parasympathetic modulation during sleep may be one mechanism underlying the increased prevalence of CVD among military Veterans with PTSD [55]. The existing literature has relied largely on secondary analyses to examine sleep HRV in those with PTSD as a function of sleep timing and type. Future studies are needed that are specifically designed to examine sleep HRV in those with PTSD and explore the mechanisms by which ANS dysregulation may confer increased CVD risk.
Funding
This work was supported by DoD Awards # PR054093-W81XWH-07-PTSD-IIRA and PT073961-W81XWH-07-PTSD-IIRA (PI: A.G.) and NIH R01 HL104607 (PI: M.H.H.). CU was supported by a VA Office of Research and Development (ORD) Research Career Development Award CDA 09-218 while preparing this manuscript. This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Conflict of interest statement. None declared.
The research described herein was conducted at the University of Pittsburgh.
References
- 1. Laborde S, et al. . Heart rate variability and cardiac vagal tone in psychophysiological research - recommendations for experiment planning, data analysis, and data reporting. Front Psychol. 2017;20(8):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Billman GE, et al. . An introduction to heart rate variability: methodological considerations and clinical applications. Front Physiol. 2015;25(6):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sassi R, et al. . Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace. 2015;17(9):1341–1353. [DOI] [PubMed] [Google Scholar]
- 4. Stein PK, et al. . Heart rate variability, sleep and sleep disorders. Sleep Med Rev. 2012;16(1):47–66. [DOI] [PubMed] [Google Scholar]
- 5. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 6. Reyes del Paso GA, et al. . The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50(5):477–487. [DOI] [PubMed] [Google Scholar]
- 7. Berntson GG, et al. . Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. [DOI] [PubMed] [Google Scholar]
- 8. Herzig D, et al. . Reproducibility of heart rate variability is parameter and sleep stage dependent. Front Physiol. 2017;8:1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen H, et al. . Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res. 2000;96(1):1–13. [DOI] [PubMed] [Google Scholar]
- 10. Cohen H, et al. . Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biol Psychiatry. 1997;41(5):627–629. [DOI] [PubMed] [Google Scholar]
- 11. Keary TA, et al. . Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. Int J Psychophysiol. 2009;73(3):257–264. [DOI] [PubMed] [Google Scholar]
- 12. Chalmers JA, et al. . Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry. 2014;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yasuma F, et al. . Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm?Chest. 2004;125(2):683–690. [DOI] [PubMed] [Google Scholar]
- 14. Moldovan M, et al. . The relationship between respiratory sinus arrhythmia and heart rate during anesthesia in rat. Rom J Physiol. 2004;41(1–2):31–39. [PubMed] [Google Scholar]
- 15. Sack M, et al. . Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: heart rate dynamics and individual differences in arousal regulation. Biol Psychiatry. 2004;55(3):284–290. [DOI] [PubMed] [Google Scholar]
- 16. Troxel WM, et al. . Sleep in the Military: Promoting Healthy Sleep Among U.S. Servicemembers. Santa Monica, CA: RAND Corporation; 2015. [PMC free article] [PubMed] [Google Scholar]
- 17. Kobayashi I, et al. . The impact of posttraumatic stress disorder versus resilience on nocturnal autonomic nervous system activity as functions of sleep stage and time of sleep. Physiol Behav. 2016;164(Pt A):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viola AU, et al. . Sleep processes exert a predominant influence on the 24-h profile of heart rate variability. J Biol Rhythms. 2002;17(6):539–547. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi I, et al. . Nocturnal autonomic balance and sleep in PTSD and resilience. J Trauma Stress. 2014;27(6):712–716. [DOI] [PubMed] [Google Scholar]
- 20. Woodward SH, et al. . Autonomic activation during sleep in posttraumatic stress disorder and panic: a mattress actigraphic study. Biol Psychiatry. 2009;66(1):41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bertram F, et al. . Autonomic arousal during actigraphically estimated waking and sleep in male veterans with PTSD. J Trauma Stress. 2014;27(5):610–617. [DOI] [PubMed] [Google Scholar]
- 22. Bonnet MH, et al. . Cardiovascular Implications of Poor Sleep. Sleep Medicine Clinics. 2007;2:529–538. [Google Scholar]
- 23. Burgess HJ, et al. . Estimating cardiac autonomic activity during sleep: impedance cardiography, spectral analysis, and Poincaré plots. Clin Neurophysiol. 2004;115(1):19–28. [DOI] [PubMed] [Google Scholar]
- 24. Trinder J, et al. . Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10(4):253–264. [DOI] [PubMed] [Google Scholar]
- 25. Cohen DJ, et al. . Quantitative electroencephalography during rapid eye movement (REM) and non-REM sleep in combat-exposed veterans with and without post-traumatic stress disorder. J Sleep Res. 2013;22(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ebdlahad S, et al. . Comparing neural correlates of REM sleep in posttraumatic stress disorder and depression: a neuroimaging study. Psychiatry Res. 2013;214(3):422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stocker RP, et al. . Combat-related blast exposure and traumatic brain injury influence brain glucose metabolism during REM sleep in military veterans. Neuroimage. 2014;99:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Germain A, et al. . Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J Psychosom Res. 2012;72(2):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Germain A, et al. . A window into the invisible wound of war: functional neuroimaging of REM sleep in returning combat veterans with PTSD. Psychiatry Res. 2013;211(2):176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. First MB, et al. . Structured Clinical Interview for the DSM-IV Axis I Disorders. New York, NY: Biometrics Research, New York State Psychiatric Institute;1996. [Google Scholar]
- 31. Buysse DJ, et al. . The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 32. Insana SP, et al. . Validation of the Pittsburgh sleep quality index addendum for posttraumatic stress disorder (PSQI-A) in U.S. male military veterans. J Trauma Stress. 2013;26(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bastien CH, et al. . Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 34. Israel B, et al. . Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: for some measures, one night is enough. Sleep. 2012;35(9):1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iber C, et al. . The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 36. Hall MH, et al. . Racial differences in heart rate variability during sleep in women: the study of women across the nation sleep study. Psychosom Med. 2013;75(8):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Houtveen JH, et al. . Contribution of tonic vagal modulation of heart rate, central respiratory drive, respiratory depth, and respiratory frequency to respiratory sinus arrhythmia during mental stress and physical exercise. Psychophysiology. 2002;39(4):427–436. [DOI] [PubMed] [Google Scholar]
- 38. Pagani M, et al. . Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95(6):1441–1448. [DOI] [PubMed] [Google Scholar]
- 39. Sloan RP, et al. . Cardiac autonomic control and the effects of age, race, and sex: the CARDIA study. Auton Neurosci. 2008;139(1–2):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X, et al. . Ethnic differences and heritability of heart rate variability in African- and European American youth. Am J Cardiol. 2005;96(8):1166–1172. [DOI] [PubMed] [Google Scholar]
- 41. Rissling MB, et al. . Circadian contrasts in heart rate variability associated with posttraumatic stress disorder symptoms in a young adult cohort. J Trauma Stress. 2016;29(5):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hall M, et al. . Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66(1):56–62. [DOI] [PubMed] [Google Scholar]
- 43. Takeda N, et al. . Circadian clock and the onset of cardiovascular events. Hypertens Res. 2016;39(6):383–390. [DOI] [PubMed] [Google Scholar]
- 44. Hillebrand S, et al. . Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. 2013;15(5):742–749. [DOI] [PubMed] [Google Scholar]
- 45. Tansey CM, et al. . Veterans’ physical health. Epidemiol Rev. 2013;35:66–74. [DOI] [PubMed] [Google Scholar]
- 46. Kurpesa M, et al. . Myocardial ischemia and autonomic activity in dippers and non-dippers with coronary artery disease: assessment of normotensive and hypertensive patients. Int J Cardiol. 2002;83(2):133–142. [DOI] [PubMed] [Google Scholar]
- 47. Hansen TW, et al. . Predictive role of the nighttime blood pressure. Hypertension. 2011;57(1):3–10. [DOI] [PubMed] [Google Scholar]
- 48. Okutucu S, et al. . Circadian blood pressure pattern and cardiac autonomic functions: different aspects of same pathophysiology. Anadolu Kardiyol Derg. 2011;11(2): 168–173. [DOI] [PubMed] [Google Scholar]
- 49. Ulmer CS, et al. . Nocturnal blood pressure non-dipping, posttraumatic stress disorder, and sleep quality in women. Behav Med. 2013;39(4):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rytwinski NK, et al. . The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J Trauma Stress. 2013;26(3):299–309. [DOI] [PubMed] [Google Scholar]
- 51. Sledjeski EM, et al. . Does number of lifetime traumas explain the relationship between PTSD and chronic medical conditions? Answers from the National Comorbidity Survey-Replication (NCS-R). J Behav Med. 2008;31(4):341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Granado NS, et al. ; Millennium Cohort Study Team. Newly reported hypertension after military combat deployment in a large population-based study. Hypertension. 2009;54(5):966–973. [DOI] [PubMed] [Google Scholar]
- 53. Crum-Cianflone NF, et al. . Impact of combat deployment and posttraumatic stress disorder on newly reported coronary heart disease among US active duty and reserve forces. Circulation. 2014;129(18):1813–1820. [DOI] [PubMed] [Google Scholar]
- 54. Thayer JF, et al. . The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;28(141):2. [DOI] [PubMed] [Google Scholar]
- 55. Brudey C, et al. . Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am J Physiol Regul Integr Comp Physiol. 2015;309(4):R315–R321. [DOI] [PMC free article] [PubMed] [Google Scholar]