Abstract
Study Objective
Sleep deprivation significantly reduces the ability to maintain a consistent alertness level and impairs vigilant attention. Previous studies have shown that longer inter-stimulus interval (ISI) are associated with faster reaction times (RTs) on the Psychomotor Vigilance Test (PVT). However, whether and how sleep deprivation interacts with this ISI effect remains unclear.
Methods
N = 70 healthy adults (age range 20–50 years, 41 males) participated in a 5-day and 4-night in-laboratory controlled sleep deprivation study, including N = 54 in the experimental group with one night of total sleep deprivation and N = 16 in the control group without sleep loss. All participants completed a neurobehavioral test battery every 2 hours while awake, including a 10-minute standard PVT (PVT-S, N = 1626) and a 3-minute brief PVT (PVT-B, N = 1622). The linear approach to threshold with ergodic rate (LATER) model was used to fit the RT data.
Results
RT decreased significantly with longer ISI on the PVT-S and PVT-B. Increased ISI effect was found for both PVT-S and PVT-B during sleep deprivation compared to baseline or recovery sleep in the experimental group, whereas no differences in the ISI effect were found in the control group. The LATER model fitting indicated that changes in perceptual sensitivity rather than threshold adjustment may underlie the ISI effect.
Conclusions
Both standard and brief PVT showed a similar ISI effect on vigilant attention performance. Sleep deprivation increased the ISI effect on both PVT-S and PVT-B, which may be due to impaired temporal resolution and time estimation after sleep loss.
Keywords: sleep deprivation, Psychomotor Vigilance Test, inter-stimulus interval, reaction time, LATER model
Statement of Significance
Psychomotor Vigilance Test (PVT) is a highly sensitive task for assessing vigilant attention deficits in sleep deprivation studies. Previous studies have shown that longer inter-stimulus intervals (ISIs) are associated with faster reaction times on the PVT. However, how sleep deprivation interferes with the ISI effect remains unclear. Using a large PVT dataset from a well-controlled in-laboratory sleep deprivation study, we showed a similar ISI effect on both standard and brief PVT performance, which was increased during sleep deprivation. To our knowledge, this is the first demonstration that sleep loss modulates the ISI effect on PVT performance. These findings have implications for future research regarding the impact of variable ISI durations on vigilant attention performance, especially after sleep loss.
Introduction
Sleep deprivation is highly prevalent throughout the world, which may be due to work demands, lifestyles, family obligations, and medical conditions [1]. Sleep deprivation increases sleep propensity, induces wake state instability, slows psychomotor responses, impairs cognitive processing, and causes considerable social, financial and health-related costs [2–4]. Among a range of neurobehavioral functions affected by sleep loss, sustained or vigilant attention is the most severely impaired cognitive domain [5–7].
The Psychomotor Vigilance Test (PVT) is a highly sensitive test for assessing vigilant attention deficits in sleep deprivation studies [7, 8]. In this test, participants are instructed to maintain their focus of attention on a small rectangular box on the screen and monitor it for the appearance of a red millisecond counter, which appears after a random inter-stimulus interval (ISI). Participants are instructed to respond with a button press as quickly as possible to each stimulus, while avoiding premature responses (i.e. false starts) when the counter is not on the screen. Reaction times (RTs) are recorded in milliseconds for each stimulus to evaluate participants’ ability to sustain attention and respond in a timely manner during the test. Unlike other cognitive tests, performance on the PVT has been shown to be highly reliable, uncontaminated by aptitude, and has no practice or learning effects [9]. These features make the PVT a widely used test to track the interaction of sleep homeostatic and circadian processes on wakefulness over a broad range of conditions, and numerous studies have consistently demonstrated the detrimental effects of sleep loss and circadian misalignment on PVT performance, including slower RTs, increased numbers of lapses (i.e. errors of omission), more premature responses (i.e. errors of commission) and enhanced time-on-task effects (e.g. see Lim, Dinges [7] for a review).
Two different versions of the PVT have been developed to measure vigilant attention deficits in studies of sleep deprivation [5–7]. A variable ISI is employed in both the standard PVT (PVT-S) and the brief PVT (PVT-B). The PVT-S has a duration of 10 minutes with a random ISI range of 2 to 10 seconds (the range is 3 to 11 seconds if the 1 second feedback after participants’ motor response is included in the ISI), while the PVT-B lasts 3 minutes with a random ISI range of 1 to 4 seconds (the range is 2 to 5 seconds if the 1 second feedback after participants’ motor response is included in the ISI). Despite the ISI differences, PVT-S and PVT-B tests were comparable for assessing the effects of both acute total sleep deprivation (TSD) and chronic partial sleep restriction [5, 6]. A few studies have shown that longer ISIs were associated with shorter RTs on the PVT-S, and less likely to produce lapses (RT > 500 ms) [10, 11]. However, the underlying mechanism of this ISI effect on RT is unclear. Based on previous studies on temporal probabilities and expectations [12–14], we hypothesized that participants may implicitly use the temporal pattern of ISI to anticipate the upcoming stimulus, which subsequently facilitates the next response to a PVT stimulus. That is, when ISI varies during the PVT, the participants’ temporal expectation (i.e. the expectation of an event about to occur given that it has not occurred yet) may change accordingly, leading to an altered response speed for different ISI durations. During temporal expectation, participants might use subjective time differences rather than objective durations of ISI to guide their responses. Consequently, PVT-S and PVT-B would display similar longer ISI facilitating effects. In order to test this hypothesis, we used the linear approach to threshold with ergodic rate (LATER) model [15–18] to examine the role of temporal expectation in the ISI effect (i.e. RT decreases with increasing ISI). Specifically, we divided the variable ISI in the PVT-S/PVT-B into 7 equal time intervals (on the log scale) and fitted the RT data in PVT-S and PVT-B tasks to determine whether a change in sensory information processing speed or in the decision threshold would underlie the longer ISI facilitating effect.
In addition, previous studies have demonstrated that sleep loss impairs temporal perception, such as reducing temporal resolution and disturbing the ability to estimate short-time intervals [19–23]. It is likely that sleep deprivation may affect the temporal expectation of the ISI during the PVT and influence its effect on vigilant attention. Using a large set of PVT data from a well-controlled in-laboratory sleep deprivation study, we also test the hypothesis that TSD interferes with the ISI effect on vigilant attention performance.
Methods
Participants
A total of 70 healthy adults (age range 20–50 years, 41 males) participated in a 5-day and 4-night in-laboratory controlled sleep deprivation study [24]. Fifty-four healthy adults (33.4 ± 8.6 years old, 22 females, right-handed) were recruited for the TSD group, and 16 healthy adults (35.7 ± 8.9 years old, seven females, right-handed) were recruited for the control group. The present study focused on the PVT behavioral data, which included a total of 1626 PVT-S test data and 1622 PVT-B test data from all 70 participants. All participants were nonsmokers and had no acute or chronic medical and psychological conditions, as established by interviews, clinical history, questionnaires, physical examinations, and blood and urine tests. Participants had habitual bedtime 2200–0000 hours and habitual awake time 0600–0900 hours, 6.5–8 hours sleep duration, and no evidence of habitual napping, as assessed by sleep and circadian rhythm questionnaires [25] and actigraphy. Participants could not have participated in trans-meridian travel or shift work, nor had irregular sleep-week routines in 60 days prior to the in-lab study. Sleep disorders were excluded by a night of laboratory polysomnography and oximetry measurements. Bedtime and wake time of enrolled participants were assessed by actigraphy, sleep-wake diaries, and time-stamped call-ins during the week before and after the in-laboratory phase. No caffeine, alcohol, tobacco, and medications (except oral contraceptives) were permitted in the week before and during the in-laboratory study. This study was approved by the Institutional Review Board of the University of Pennsylvania. All participants provided written informed consent before enrollment, which was in accordance with the Declaration of Helsinki. Participants were compensated for participating in the study.
Study protocol and experimental design
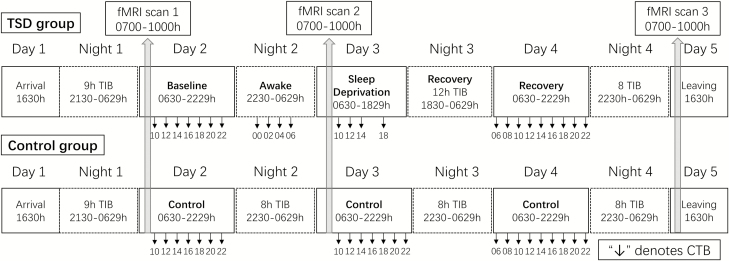
The in-laboratory study lasted 5 consecutive days and 4 nights (see Figure 1 for the study protocol). All participants stayed in a laboratory room of the Clinical Translational Research Center at the Hospital of the University of Pennsylvania, where they were continuously monitored by trained staff during the study. Participants arrived at the laboratory on the late afternoon of day 1 and were provided with a 9-hour time-in-bed (TIB) sleep opportunity, from 2130 to 0630 hours, to ensure that the participants obtained sufficient habitual sleep durations in the unfamiliar laboratory environment [26]. Participants in the TSD group were kept awake until 1830 hours on day 3, followed by a night of 12-hour recovery sleep on day 3 (from 1830 to 0630 hours). On day 4, participants in the TSD group were provided with an 8-hour TIB sleep from 2230 to 0630 hours. The control group was provided with an 8-hour TIB sleep each night on days 2–4 (2230—0630 hours). All participants completed a 30-min neurobehavioral test battery every 2 hours during the protocol when they were awake and not doing fMRI scans during day 2–4. The test battery included the Forward and Backward Digit Span Task (DST), the PVT-S, the Digit Symbol Substitution Task (DSST), the Karolinska Sleepiness Scale (KSS), the Profile of Mood States (POMS) Questionnaire, and the PVT-B.
Figure 1.
Protocol summary. Participants arrived at the laboratory on late afternoon of day 1 and were provided 9 hours TIB sleep for night 1. Day 2 was the baseline day for TSD group (upper panel). TSD group were kept awake throughout night 2. Day 3 was the sleep deprivation day. Then, TSD group were allowed 12 TIB recovery sleep at night 3. Day 4 was the recovery day. For control group (bottom panel), 8 hours TIB sleep was provided every night at nights 2, 3, and 4. All participants completed a cognitive test battery (CTB) that includes PVT-S and PVT-B every 2 hours while they were awake and not doing fMRI scans during days 2–4 (except for 1600 hours of day 3 in TSD group). Each time point of cognitive battery was depicted in the figure (i.e. 10 denotes test time around 1000 hours). Three fMRI scans were scheduled between 0700 and 1000 hours on day 2, 3, and 5 (scan duration for each participant was about an hour).
PVT tests
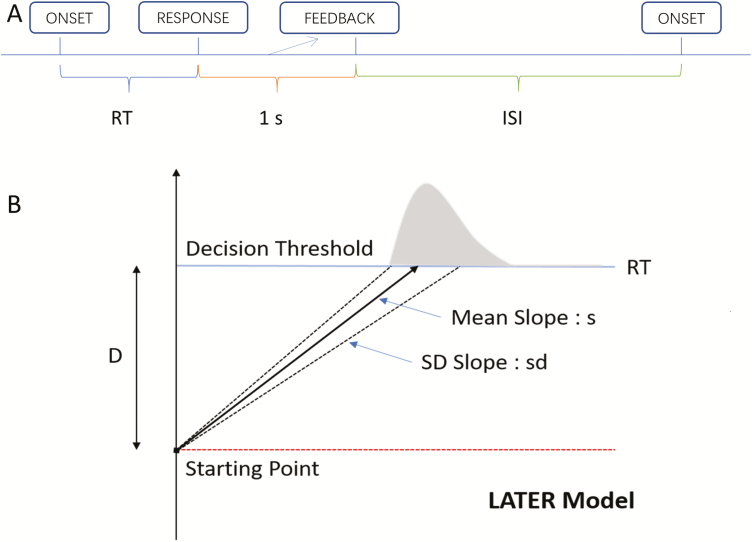
The PVT is a RT test that has been widely employed to assess vigilant attention deficits in numerous studies [5–8, 27]. During the PVT, participants were required to maintain their attention on a red-outlined rectangle located in the center of a dark screen and press the space bar to a cue (millisecond counter) that was presented at random ISI (ranging from 2 to 10 seconds in the PVT-S and from 1 to 4 seconds in the PVT-B, not including the 1 seconds feedback after response) [5, 7, 28]. They were instructed to respond as fast as they could whenever they perceived a millisecond counter inside a red rectangular area without false starts. The millisecond counter stopped after participants’ button press and remained on the screen for one more second to allow participants to see their most recent RTs (Figure 2A). Failure to respond within 30 seconds lead to a timeout, whereas button presses without a stimulus were counted as false starts. When false starts or timeouts occurred, corresponding texts appeared on the screen for 1 second before the trial ended.
Figure 2.
Panel A: An illustration of a single trial on PVT-S (PVT-B). PVT-S (PVT-B) requires a simple response to a cue, i.e. the onset of a millisecond counter. The millisecond counter stops after participant’s response and remains for another 1 second to allow participants to see their reaction time, i.e. 1 second feedback. The next cue presents after a random ISI ranging from 2 to 10 seconds (1 to 4 seconds). Panel B: The LATER model. When the stimulus onset, a decision signal rise linearly from a certain starting point to a decision threshold (D). If the decision signal reaches the decision threshold, a response is initiated. The slope of the decision signal is followed a Gaussian distribution with mean slope s and with SD slope sd. The RT of each trial reflects the time elapsed before decision signal reaches the threshold decision.
Temporal expectations
Since the ISIs of PVT-S and PVT-B were randomly drawn from a discrete uniformly distributed time interval, the probability of stimuli appearing was calculated as 1/8000 (ISI range between 2000 and 10000 ms) and 1/3000 (ISI range between 1000 and 4000 ms) for each millisecond in PVT-S and PVT-B, respectively. The hazard rate was used to define temporal expectations of ISI [13], which reflected the likelihood that the next stimulus was about to appear, given it has not occurred yet. Therefore, temporal expectation monotonically increased as the ISI became longer (i.e. if a stimulus has not occurred after a certain amount of time, the temporal expectation of upcoming stimulus increases). Since participants had no prior knowledge about the absolute ISI distribution (participants were only informed to respond to the visual cue as soon as possible and to avoid false start and timeout), perceived differences on elapsed time might be used to distinguish two stimuli followed by different ISIs.
According to the Weber–Fechner law, the just noticeable difference (JND) should be proportional to the reference time duration. The first reference time duration is the duration between the stimulus offset (after 1 second feedback) and the shortest ISI (i.e. 2 seconds in PVT-S and 1 second in PVT-B). We used JND as the time unit to divide the ISI distributions of PVT-S and of PVT-B into seven ISI sub-groups (i.e. seven equidistant ISI on the log scale), respectively. In theory, there should be no subjective time difference within an ISI sub-group, while the difference between two adjacent ISI sub-groups should be noticeable. The advantages of dividing the ISI this way include: (1) the ISI effects can be directly compared across PVT-S and PVT-B; and (2) the Weber fractions used here, 0.2586 for PVT-S and 0.2214 for PVT-B, were comparable to the Weber fraction of time estimation used in the literatures [13, 29]. Specifically, for the PVT-S, the ranges of the 7 ISI sub-groups were 2000–e7.83 ms, e7.83–e8.06 ms, e8.06–e8.29 ms, e8.29–e8.52 ms, e8.52–e8.75 ms, e8.75–e8.98 ms, and e8.98–10000 ms. For the PVT-B, the ranges of the 7 ISI sub-groups were between 1000–e7.1 ms, e7.1–e7.3 ms, e7.3–e7.5 ms, e7.5–e7.7 ms, e7.7–e7.9 ms, e7.9–e8.1 ms, and e8.1–4000 ms. Similar to previous studies [10, 11], we also divided ISI distributions of PVT-S and of PVT-B into ISI sub-groups on the linear scale, that is, each second was defined as one ISI sub-group, and compared to those of the log scale (Supplementary Figure S1).
The LATER Model of RT
The LATER (linear approach to threshold with ergodic rate) model is a simple while elegant model for explaining the RTss and the underlying decision mechanisms [15–18]. In this model, there are two independent parameters that will cause RT changes, that is, the distance to decision threshold (D) and the sensory information processing speed (mean slope s). According to the LATER model (Figure 2B), the onset of a stimulus (e.g. the millisecond counter) leads to the linear rise of a decision or action signal start from a certain starting point to a decision or action threshold (D). If the decision or action signal reaches the decision or action threshold, a response is initiated. The slope of the decision or action signal follows a Gaussian distribution with mean slope (s) and with SD slope (sd) [30]. The RT of each trial reflects the time elapsed before the decision or action signal reaches the threshold D. Therefore, the reciprocal RT (1/RT) follows a Gaussian distribution with mean s/D and SD sd2/D2. Given that 1/RT is normally distributed, we can compute a z-score to reflect the difference between the observed 1/RT and the mean 1/RT. Next, a reciprobit plot is created by plotting the z-score of reciprocal RT’s cumulative distribution (i.e. RT’s cumulative percent probability on the probit scale) against RT plotted on the reciprocal scale (straight lines in Figure 3, right panel) [15, 18, 31]. The resulting reciprobit line intersects the line z-score = 0 at the median reciprocal RT s/D, whereas the resulting line intersects the line RT = ∞ at s/sd (Figure 3, right panel).
Figure 3.
Two hypotheses about how temporal expectation of stimulus modulates reaction time. Left panel shows the rise of decision signal (mean slope of this linear rise: s, standard deviation of the slope: sd) towards the decision threshold (D). Right panel shows the reciprobit analysis of RT distribution. For decision threshold (DT) modulation hypothesis: increased temporal expectation (e.g. under long ISI) leads to lower decision threshold (Dl < Ds, top left panel), which is captured by the swivel effect on reciprobit line (top right panel). For gain control of processing speed hypothesis (GAIN): increased temporal expectation (e.g. under long ISI) leads to faster processing speed (sl > ss, bottom left panel), which is captured by the shift effect on reciprobit line (bottom right panel). Note: Z-score reflects Z-score of the cumulative distribution of 1/RT.
The mathematical features of the reciprobit plots can be used to distinguish between the two independent modulation mechanisms in LATER model by changes in parameter D or s through graphical representations (Figure 3). These two competing mechanisms are: (1) a reduction of decision threshold D, which would lead to a swivel of the reciprobit line toward shorter RT (Figure 3, top panel); and (2) an increase of the slope s (gain control of information processing speed), which would cause the reciprobit line to shift (Figure 3, bottom panel).
The LATER model fitting was conducted by fitting the model for each of the PVT-S and PVT-B test data. Trials with RT < 130 ms were considered as coincident false start (less than 0.1% of total trials), and were excluded in further analysis. First, we performed a reciprocal transformation [15, 18] on the RT data and normalized each participant’s reciprocal RT data to the population’s average and SD [31]. Next, we pooled all the participants’ reciprocal RT data together and then sorted reciprocal RT data into 7 ISI sub-groups with increasing levels of temporal expectations.
Statistical analysis
First, in order to examine the effects of TSD on PVT performance, a mixed model analysis of variance (ANOVA) was conducted to analyze median RT and percentage of lapses (%lapse, the number of lapses divided by the total trials) of each of PVT-S/PVT-B test with the group condition (two levels, TSD vs. control group) and the test day (three levels: day 2, day 3, and day 4) as two independent variables. For the TSD group, day 2 is the baseline sleep condition, day 3 is the sleep deprivation condition, and day 4 is the recovery sleep condition. In order to avoid potential confounding time-of-day effects, only PVT data collected during daytime in both baseline day and TSD/control day (1000–1800 hours) were used in this analysis.
Second, a Bayesian model selection strategy was used to identify which of the two competing modulation mechanisms could better explain the changes observed in RT distributions across the 7 log-transformed ISI sub-groups. Specifically, we fitted the LATER model using the maximum likelihood estimation under the hypotheses that ISI effect on RT was either from changes in sensory information processing speed (Gain hypothesis, Figure 3, bottom panel) or from changes in the distance to decision threshold (DT hypothesis, Figure 3, top panel). Model comparison was performed in a way that either the slope or the distance to threshold was fixed across the ISI sub-groups, depending on the hypothesis tested. Finally, the log likelihood ratio between the two hypotheses (LDT-LGain) was calculated. The cutoff value of the Bayesian factor (LDT-LGain ≥ 2.3) was used to assess the significance level [32].
Third, in order to test the ISI effect on RT and %lapse, the median RT and percentage of lapses were calculated for each of the 7 ISI sub-groups in each of the PVT-S and PVT-B tests. Median RT of each ISI sub-group was obtained by model-fitting results (s/D = 1/RT). Percentage of lapses were calculated by the number of lapses divided by the total trials. The ISI effect on both median RT and %lapses were tested using linear regression. Only data collected during the daytime (1000–1800 hours) were used in this analysis.
Fourth, moderated multiple regression analysis [33] was conducted to examine the influences of TSD on the ISI effect on PVT-S and PVT-B performance, respectively. Because there was no difference in PVT-S or PVT-B performance between the baseline and recovery sleep days, these two conditions were combined. Only data collected during the daytime (1000–1800 hours) were used in this analysis.
Finally, in order to examine the potential effect of day (1000–1200 hours of days 2 and 4 in TSD group) versus night (2000–2200 hours of days 2 and 4 in TSD group), we performed an additional moderated multiple regression analysis to examine the influences of day versus night on the ISI effect on PVT-S and PVT-B performance, respectively.
Results
We first examined the effects of TSD on PVT-S and PVT-B performance, respectively. Consistent with findings from previous studies, TSD significantly impaired the overall performance on both PVT-S and PVT-B tests. There were significant group × day interactions for the median RT (for PVT-S: F(2, 18) = 34.81, p < 0.001; for PVT-B: F(2,18) = 58.08, p < 0.001) and percentage of lapses (for PVT-S: F(2,18) = 78.68, p < 0.001; for PVT-B: F(2,18) = 78.59, p < 0.001). Tests of simple effects showed that in the TSD group, participants had significantly longer RTs (p < 0.001) and greater number of lapses (p < 0.001) after TSD (day 3) compared to those following baseline sleep (day 2) or recovery sleep (day 4), whereas no differences were found between baseline sleep and recovery sleep. No differences were found for the PVT-S and PVT-B performance in the control group across 3 days (all p > 0.1). Baseline sleep and recovery sleep were combined as the rested wakefulness (RW) condition in further analyses.
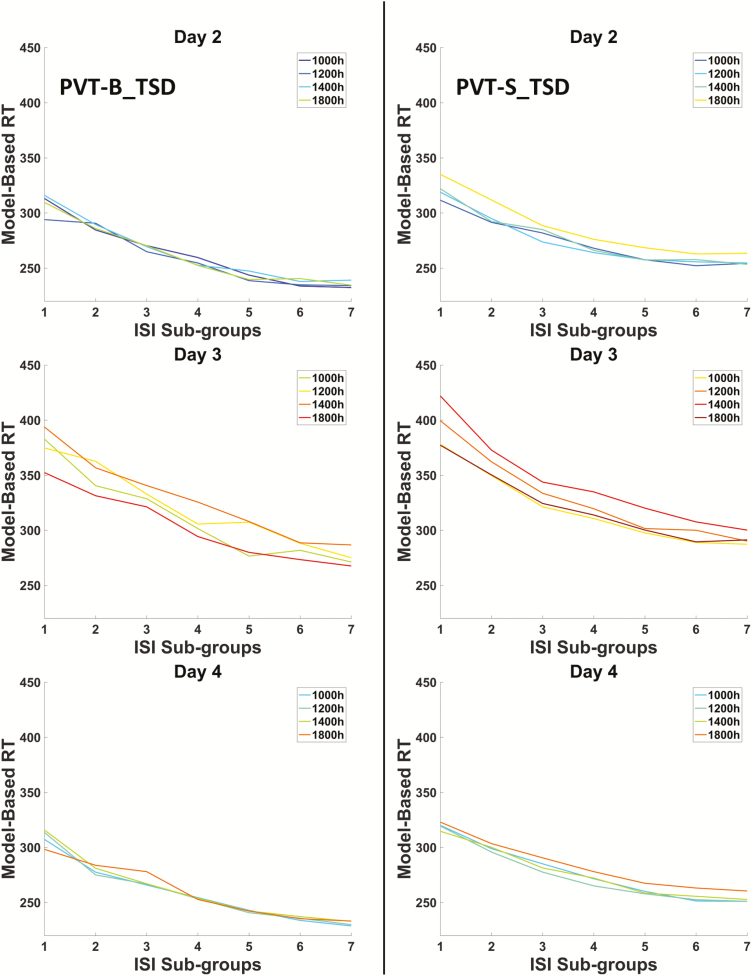
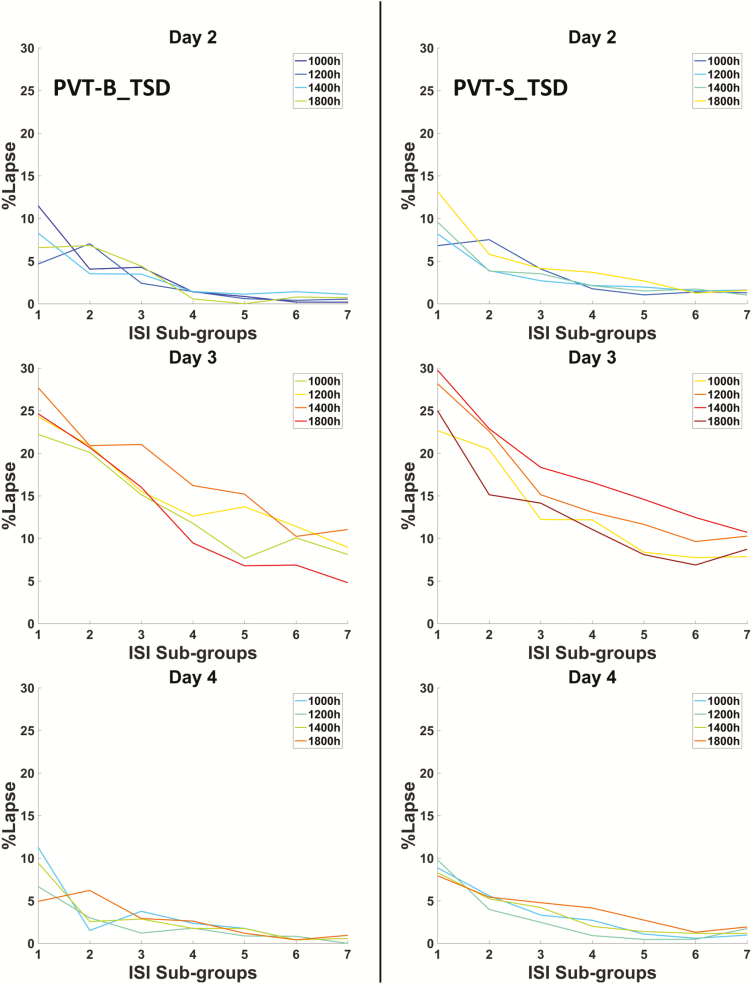
For the TSD group, the ISI effects on PVT-S and PVT-B performance, including the median RT and percentage of lapses, are displayed in Figures 4 and 5, respectively (data from control group do not show). A LATER model was fitted to the distributions of the RT to explain the variable RT associated with the different ISIs in the PVT-S and PVT-B tests, respectively. As shown in Figure 6, the representative examples of reciprobit plot of PVT-S and PVT-B demonstrated a shift toward shorter RT as temporal expectation increases, suggesting Gain hypothesis but not DT hypothesis underlying the ISI effect. The likelihood of the Gain hypothesis was then compared with that of the DT hypothesis. Results showed that the Gain hypothesis was significantly more likely to explain the RT data than the DT hypothesis for all PVT-S and PVT-B tests. For the TSD group, the log-likelihood ratio (LLR) between these two mechanisms were consistently higher than 40 (Figure 7), which is “very strong” (LLR > 32) or “decisive” (LLR > 100) according to Bayesian inference theory [32]. Moreover, compared to the RW condition, TSD significantly decreased LLR (p < 0.05 for PVT-S and p < 0.001 for PVT-B, see Figure 7).
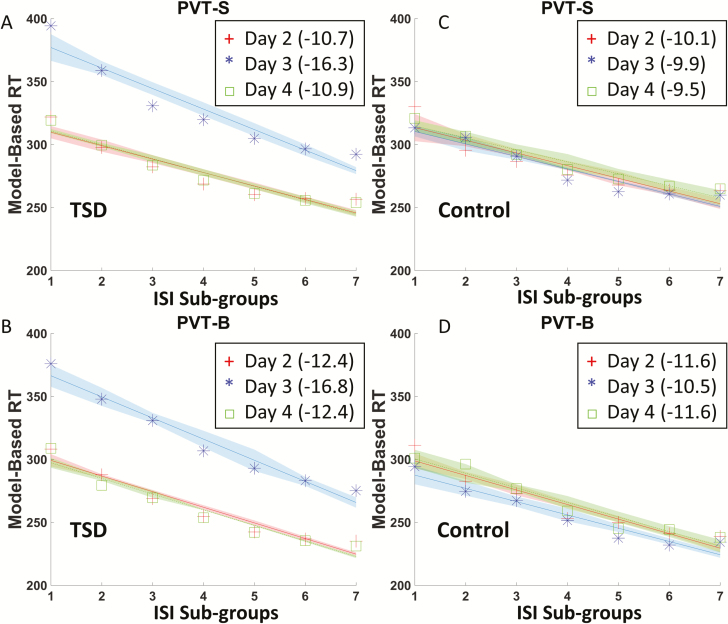
Figure 4.
The relationship between model-based RT and ISI sub-groups in PVT-B (left column) and PVT-S (right column) of the TSD group during day 2 (baseline), day 3 (sleep deprivation), and day 4 (recovery). Different colored line represents data collected from different time-of-day.
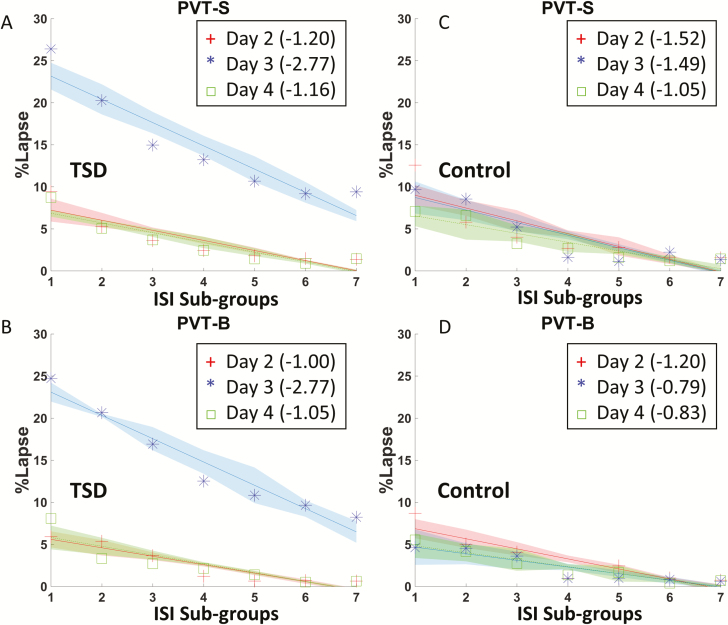
Figure 5.
The relationship between percentage of lapse (%lapse) and ISI sub-groups in PVT-B (left column) and PVT-S (right column) of the TSD group during day 2 (baseline), day 3 (sleep deprivation), and day 4 (recovery). Different colored line represents data collected from different time-of-day.
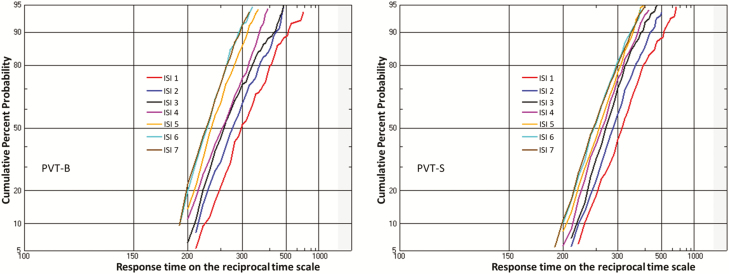
Figure 6.
Representative examples of reciprobit plot for both PVT-B (left panel, data from test at 1000 hours, day 2) and PVT-S (right panel, data from test at 1000 hours, day 2) showing a shift towards shorter RT as temporal expectation increases (i.e. from ISI sub-group 1 to ISI sub-group 7). This is consistent with Gain control of processing speed hypothesis.
Figure 7.
Differences in the log-likelihood ratio (LLR) between the two LATER hypotheses (Gain-DT) when fitting the PVT-S and PVT-B data from the TSD group and control group, respectively. Sleep deprivation significantly reduced LLR for the TSD group (p < 0.05 for PVT-S and p < 0.001 for PVT-B), whereas no LLR differences were found across 3 days for the control group (all p > 0.1). Note: error bar denotes standard error.
As shown in Figure 8, reliable ISI effect on vigilant attention was found in PVT-S and PVT-B in both TSD and control groups. That is, RT decreased linearly with longer ISI, regardless of being sleep deprived or not (all p < 0.001). When calculating the slopes of linear relationships between ISI and RT for the PVT-S and PVT-B, there were significant differences between the slopes for the RW and TSD conditions for both PVT-S (b = −11.1 at RW vs. b = −15.5 at TSD, see Figure 8A) and PVT-B (b = −12.6 at RW vs. b = −17.7 at TSD, see Figure 8B). However, no differences were found for the slopes for the PVT-S and PVT-B tests across the three test days in the control group (Figure 8, C and D; Figure 9, C and D).
Figure 8.
Sleep deprivation changed the relationships between the ISI sub-groups and RT on both PVT-S and PVT-B. Red cross in each picture represents median RT of each ISI sub-group from day 2 (baseline), red line is a linear regression of red cross, and shadow represents standard error of each data point. Likewise, blue and green denote data from day 3 (sleep deprivation) and day 4 (recovery), respectively. Slopes of each line are shown in the bracket. (A and B) Sleep deprivation significantly changed the slopes of regression line for both PVT-S and PVT-B (p < 0.001). (C and D) No differences were found among the slopes in 3 days in the control group for both PVT-S and PVT-B (all p > 0.1).
Figure 9.
Sleep deprivation changed the relationships between the ISI sub-groups and Lapse% on both PVT-S and PVT-B. Red cross in each picture represents Lapse% of each ISI sub-group from day 2 (baseline), red line is a linear regression of red cross, and shadow represents standard error of each data point. Likewise, blue and green denote data from day 3 (sleep deprivation) and day 4 (recovery), respectively. Slopes of each line are shown in the bracket. (A and B) Sleep deprivation significantly changed the slopes of regression line for both PVT-S and PVT-B (p < 0.001). (C and D) No differences were found among the slopes in 3 days in the control group for both PVT-S and PVT-B (all p > 0.1).
The moderated multiple regression was conducted to further examine the potential moderation effects of TSD on the relationships between ISI and RT. Data from day 2 (baseline) and day 4 (recovery) were pooled and combined as the RW condition. In the first regression model, both ISI (p < 0.001) and TSD (p < 0.001) predicted RT in the PVT-S and PVT-B, and the model explained 89% of RT variance for the PVT-S and 87.0% of RT variance for the PVT-B. In the second regression model, the interaction between the TSD and ISI was added, which accounted for a significant additional RT variance in both PVT-S (Fchange(1,80) = 17.40, ΔR2 = 0.02, p < 0.001) and PVT-B (Fchange(1,80) = 13.88, ΔR2 = 0.011, p < 0.001).
The slope calculation and moderated multiple regression analyses were also conducted for the percentage of lapses in the PVT-S and PVT-B tests. Similar ISI effect was found for the percentage of lapses, such that the percentage of lapses decreased linearly with longer ISI, regardless of sleep or TSD (Figure 9). TSD significantly skewed the negative slopes between ISI and lapses from b = −1.26 (b = −1.30 for day 2 and b = −1.22 for day 4) to b = −2.54 in PVT-S (Figure 9A), and from b = −1.16 (b = −1.18 for day 2 and b = −1.14 for day 4) to b = −3.04 in PVT-S (Figure 9B). A significant interaction was also found between the TSD and ISI, which accounted for additional variance of the percentage of lapses in PVT-S (Fchange(1,80) = 36.67, ΔR2 = 0.047, p < 0.001) and PVT-B (Fchange(1,80) = 55.85, ΔR2 = 0.054, p < 0.001).
We also calculated the slopes of linear relationships between ISI and RT for the PVT-S and PVT-B tests collected at different time-of-day (Supplementary Figure S2). There were no significant interactions between data collection time (day vs. night) and ISI effect on RT for both PVT-S (Fchange(1,52) = 0.50, ΔR2 = 0.001, p = 0.484) and PVT-B (Fchange(1,52) = 0.18, ΔR2 = 0.000, p = 0.675).
Discussion
In the present study, we analyzed a large PVT data set from a well-controlled in-laboratory sleep deprivation study and examined how different ISI durations affect PVT performance both following baseline/recovery sleep and during one night of TSD. We observed a consistent and reliable ISI effect on vigilant attention performance in both standard and brief PVT tests, in which RT decreased linearly with increasing ISI, regardless of sleep deprivation. By fitting the RT data with the LATER model, the results suggest that the longer ISI facilitating effect on RT in PVT tests could be better explained by the Gain hypothesis (changes in the sensory information processing rate) than the decision threshold hypothesis (changes in the distance to decision threshold). In addition, when comparing sleep deprivation to rested wakefulness, increased ISI effects on RT and percentage of lapses were observed in both standard and brief PVT tests, whereas no such ISI effect differences were found in the PVT performance across multiple days in the control group. These findings suggest an interaction between sleep loss and the ISI effect on vigilant attention.
The robust ISI effect on PVT performance replicated findings from previous studies showing that both RT and percentage of lapses are subject to exponential decay when ISI increased from 2 to 10 seconds [10, 11]. Surprisingly, comparable ISI effect was also observed in the brief PVT. Given that the ISI ranges in the brief and standard PVT tests are very different (yet similar ISI effects were observed), these findings support our hypothesis that RT is influenced by the relative ISI time frames rather than the absolute ISI time.
In contrast to a previous study, which failed to find an interaction between sleep deprivation and ISI effect [10], we observed significantly enhanced ISI effects after sleep deprivation. This discrepancy may be explained by potential differences in data acquisition and data analysis approaches. For example, the PVT dataset in the present study is bigger and more homogeneous (i.e. a total of 1622 PVT-B tests and 1626 PVT-S tests from a single sleep deprivation experiment lasting 5 days and 4 nights) compared to the dataset used in the previous study [10] (the standard 10-minute PVT data from 3 different experiments lasting 54 hours). The ISI grouping and modeling methods were also different in the two studies. For example, in the previous study [10], the ISI data were divided to 3 sub-groups (short, medium, and long) based on the absolute duration of ISI and a curvilinear relationship was observed between ISI duration and RT. In the current study, the hazard rate [13] and Weber’s law were used to define temporal expectations of ISI and divide the ISI into seven sub-groups based on the log scale, whereupon a more linear relationship between ISI duration and RT was observed.
A potential explanation for the longer ISI facilitating effect on PVT performance is the “psychological relative refractory state” hypothesis, which suggests that participants’ psychological capability of responding may be reduced after a response and gradually go back to normal [34, 35]. This explanation is supported by results from the choice serial RT task with an ISI range between 0 and 600 ms [35]. In these tasks, RT decreased when ISI increased to 480 ms and remained unchanged until 600 ms. However, the ranges of ISI in both standard (2–10 seconds) and brief PVT tests (1–4 seconds) are well beyond the ISI ranges used in the choice serial RT task. Therefore, the “psychological relative refractory state” hypothesis is likely not applicable to the ISI effect observed in the PVT-S and PVT-B.
Another possible explanation for ISI effect is the “checking and preparation time” proposed in previous information processing speed studies [36]. In a consecutive RT task, checking and preparation time refers to the minimum time needed to check the accuracy of the current response and prepare for the next stimulus. Previous studies have suggested that the checking and preparation time for the consecutive RT task is usually around 1100 ms for young adults [36], which is much shorter than the minimum time interval between the response and the next stimulus (including the 1 second feedback and the ISI) in our PVT tests. Therefore, our participants should have more than enough time to check the response and prepare for the next stimulus during both PVT-S and PVT-B, and the checking and preparation time may not explain the ISI effect on PVT performance.
Previous behavioral studies have consistently shown that temporal expectations of upcoming events may speed motor behavior [12, 13, 37]. During PVT, participants were not instructed to adjust their performance based on the ISI; however, the temporal information of ISI may still be implicitly used by participants to speed their responses. In order to explain the longer ISI facilitating effect on PVT performance, we used the LATER model to fit the PVT data with potential changes in two independent parameters, including the distance to decision threshold (the DT hypothesis) and the sensory information processing speed (the Gain hypothesis). Our findings suggested that the Gain hypothesis better explained the PVT-S and PVT-B RT data, which are in line with previous studies [37] and suggest that gain control of sensory information from temporal expectations may underlie the ISI effect. Moreover, sleep deprivation did not change the LATER model fitting results, although the LLR ratio for the Gain hypothesis was slightly decreased after sleep deprivation compared to baseline/recovery sleep.
The enlarged ISI effects during sleep loss are in line with impaired temporal perception and estimation findings in several previous studies [19–23]. For example, a study used a dichotic temporal order judgment (TOJ) paradigm to examine the impact of 24 hours sleep deprivation on auditory temporal resolution and found that sleep deprivation increased dichotic TOJ threshold from about 57 ms to 74 ms and reduced temporal resolution by about 28% [19]. It was suggested that reduced temporal resolution may have a greater negative impact on short-time intervals, and make it more difficult for participants to discriminate between shorter ISIs. Moreover, several other studies [20, 22, 23] further showed that sleep deprivation has an effect on the internal clock and the estimation of time duration, making participants more likely to underestimate subjective elapsed time and overestimate real elapsed time. Such underestimated subjective elapsed time could be more evident in short ISIs, resulting in a larger impact of sleep deprivation on the shorter ISI trials than the longer ISI trials.
There are several weakness and limitations in the current study. First, all of our participants stayed in a controlled laboratory room during the entire experiment. Although this ensured control of the environment, it remains to be determined whether our findings generalize to more complex/distracting real-life situations. Second, the present study only examined the interactions between one night of acute TSD and the ISI effects, it remains to be determined whether chronic partial sleep restriction (e.g. sleep less than 6.5 hours per night for several nights) would increase the ISI effect on vigilant attention performance in a comparable way. Third, we found that perceptual sensitivity but not decision threshold was changed as ISI increased in the PVT-S and PVT-B, both of which are simple RT tasks. Future studies may need to further explore the ISI effect and its underlying mechanisms in more complex tasks or paradigms designed to specifically examine such processes (e.g. experimental paradigms manipulating threshold adjustment or temporal order, or modulating perceptual sensitivity). Last but not least, this behavioral design ought to be complemented by neuroimaging investigations to examine the brain regions and networks involved in mediating the ISI effects and affected by sleep deprivation. Indeed, a recent neuroimaging study suggests that higher temporal expectations may be associated with greater activity in the early visual cortex [12], which is in line with the changes in perceptual sensitivity from LATER modeling in our results.
In summary, we used a large PVT performance dataset and showed a similar and robust ISI effect on vigilant attention for both PVT-S and PVT-B, regardless of the test duration or sleep loss. Our findings suggest that the consistently observed ISI effect on PVT performance may reflect increased temporal expectations for upcoming events. Moreover, sleep deprivation increased the ISI effect on vigilant attention, which may be due to impaired temporal resolution and time estimation after sleep loss. Since randomized ISI are widely used in numerous psychology studies, our findings have implications regarding the non-negligible impact of variable ISI durations on vigilant attention performance, especially after sleep loss. For example, future studies may alter the ISI distribution to attenuate the ISI effect on attention performance by increasing the probabilities and temporal expectations of shorter ISI trials.
Funding
This research was supported in part by National Institutesof Health (NIH) grants R01 HL102119, R01 MH107571, P30 NS045839, CTRC UL1RR024134, and Sun Yat-sen University.
Conflict of interest statement. None declared.
Supplementary Material
References
- 1. Hublin C, et al. Insufficient sleep–a population-based study in adults. Sleep. 2001;24(4):392–400. [DOI] [PubMed] [Google Scholar]
- 2. Goel N, et al. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29(4):320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dinges DF. An overview of sleepiness and accidents. J Sleep Res. 1995;4(S2):4–14. [DOI] [PubMed] [Google Scholar]
- 4. Leger D. The cost of sleep-related accidents: a report for the National Commission on Sleep Disorders Research. Sleep. 1994;17(1):84–93. [DOI] [PubMed] [Google Scholar]
- 5. Basner M, et al. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 2011;69(11–12):949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basner M, et al. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34(5):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lim J, et al. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. [DOI] [PubMed] [Google Scholar]
- 8. Dinges DF, et al. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods. 1985;17(6):652–655. [Google Scholar]
- 9. Basner M, et al. Repeated administration effects on psychomotor vigilance test performance. Sleep. 2017;41(1). [DOI] [PubMed] [Google Scholar]
- 10. Tucker AM, et al. The variable response-stimulus interval effect and sleep deprivation: an unexplored aspect of psychomotor vigilance task performance. Sleep. 2009;32(10):1393–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matthews RW, et al. Using interstimulus interval to maximise sensitivity of the psychomotor vigilance test to fatigue. Accid Anal Prev. 2017;99(Pt B):406–410. [DOI] [PubMed] [Google Scholar]
- 12. Bueti D, et al. Encoding of temporal probabilities in the human brain. J Neurosci. 2010;30(12):4343–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janssen P, et al. A representation of the hazard rate of elapsed time in macaque area LIP. Nat Neurosci. 2005;8(2):234–241. [DOI] [PubMed] [Google Scholar]
- 14. Coull J, et al. Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol. 2008;18(2):137–144. [DOI] [PubMed] [Google Scholar]
- 15. Carpenter RH, et al. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377(6544):59–62. [DOI] [PubMed] [Google Scholar]
- 16. Reddi BA, et al. The influence of urgency on decision time. Nat Neurosci. 2000;3(8):827–830. [DOI] [PubMed] [Google Scholar]
- 17. Noorani I, et al. The LATER model of reaction time and decision. Neurosci Biobehav Rev. 2016;64:229–251. [DOI] [PubMed] [Google Scholar]
- 18. Reddi BA, et al. Accuracy, information, and response time in a saccadic decision task. J Neurophysiol. 2003;90(5):3538–3546. [DOI] [PubMed] [Google Scholar]
- 19. Babkoff H, et al. Effect of the diurnal rhythm and 24 h of sleep deprivation on dichotic temporal order judgment. J Sleep Res. 2005;14(1):7–15. [DOI] [PubMed] [Google Scholar]
- 20. Casini L, et al. How does one night of sleep deprivation affect the internal clock?Neuropsychologia. 2013;51(2):275–283. [DOI] [PubMed] [Google Scholar]
- 21. Miró E, et al. Time estimation during prolonged sleep deprivation and its relation to activation measures. Hum Factors. 2003;45(1):148–159. [DOI] [PubMed] [Google Scholar]
- 22. Kuriyama K, et al. Diurnal fluctuation of time perception under 30-h sustained wakefulness. Neurosci Res. 2005;53(2):123–128. [DOI] [PubMed] [Google Scholar]
- 23. Soshi T, et al. Sleep deprivation influences diurnal variation of human time perception with prefrontal activity change: a functional near-infrared spectroscopy study. PLoS One. 2010;5(1):e8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fang Z, et al. Altered salience network connectivity predicts macronutrient intake after sleep deprivation. Sci Rep. 2015;5:8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith CS, et al. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74(5):728–738. [DOI] [PubMed] [Google Scholar]
- 26. Paterson JL, et al. What happens to mood, performance and sleep in a laboratory study with no sleep deprivation?Sleep Biol Rhythms. 2013;11(3):200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jewett ME, et al. Dose-response relationship between sleep duration and human psychomotor vigilance and subjective alertness. Sleep. 1999;22(2):171–179. [DOI] [PubMed] [Google Scholar]
- 28. Dinges DF, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- 29. Gibbon J, et al. Toward a neurobiology of temporal cognition: advances and challenges. Curr Opin Neurobiol. 1997;7(2):170–184. [DOI] [PubMed] [Google Scholar]
- 30. Hanes DP, et al. Neural control of voluntary movement initiation. Science. 1996;274(5286):427–430. [DOI] [PubMed] [Google Scholar]
- 31. Domenech P, et al. Decision threshold modulation in the human brain. J Neurosci. 2010;30(43):14305–14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jeffreys H. The Theory of Probability. Oxford, UK: OUP Oxford; 1998. [Google Scholar]
- 33. Aguinis H. Regression Analysis for Categorical Moderators. New York City, NY: Guilford Press; 2004. [Google Scholar]
- 34. Rabbitt P. Psychological refractory delay and response-stimulus interval duration in serial, choice-response tasks. Acta Psychol (Amst). 1969;30:195–219. [Google Scholar]
- 35. Wilkinson RT. Response-stimulus interval in choice serial reaction time: interaction with sleep deprivation, choice, and practice. Q J Exp Psychol A. 1990;42(2):401–423. [DOI] [PubMed] [Google Scholar]
- 36. Kirby NH, et al. Speed of information processing and age. Personality and Individual Differences. 1991;12(2):183–188. [Google Scholar]
- 37. Vangkilde S, et al. Great expectations: temporal expectation modulates perceptual processing speed. J Exp Psychol Hum Percept Perform. 2012;38(5):1183–1191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.