Abstract
Purpose:
In this study, we examine the expression of corneal epithelium-derived thrombospondin-1 (TSP-1) and its immunomodulatory functions in a validated murine model of dry eye disease (DED).
Methods:
DED was induced in female C57BL/6 using a controlled environment chamber (CEC) for 14 days. mRNA and protein expression of TSP-1 by corneal epithelial cells was quantified using real-time PCR and flow cytometry. Corneal epithelial cells from either naïve or DED mice were cultured with bone marrow derived dendritic cells (BMDCs) in the presence of IFNγ for 48 hours, and BMDC expression of MHC-II and CD86 was determined using flow cytometry. Next, either recombinant TSP-1 or anti-TSP-1 antibody was added to the co-culture, and BMDC expression of above activation markers was evaluated. Finally, either DED mice were topically treated with either recombinant TSP-1 or human serum albumin (HSA), and maturation of corneal DCs, expression of inflammatory cytokines, and DED severity were investigated.
Results:
mRNA expression of TSP-1 by the corneal epithelium was upregulated in DED. Corneal epithelial cells derived from mice with DED demonstrated an enhanced capacity in suppressing BMDC expression of MHC-II and CD86 relative to wild type mice, and this effect was abrogated by TSP-1 blockade and potentiated by recombinant TSP-1. Finally, topical application of recombinant TSP-1 significantly suppressed corneal DC maturation and mRNA expression of pro-inflammatory cytokines, and ameliorated disease severity in mice with DED.
Conclusions:
Our study elucidates the function of epithelium-derived TSP-1 in inhibiting DC maturation and shows its translational potential to limit corneal epitheliopathy in DED.
Keywords: corneal epithelial cells, dry eye disease, dendritic cell, epitheliopathy, thrombospondin-1
INTRODUCTION
Dry eye disease (DED) is a multifactorial disease of the ocular surface, characterized by a loss of homeostasis of the tear film, which is perpetuated by ocular surface inflammation and accompanied by symptoms of discomfort and visual disturbance [1]. DED is arguably the most common ophthalmologic condition for which patients seek professional eye care [2]. It is estimated that over 16 million US adults have diagnosed DED, with higher prevalence identified amongst women (8.8% compared to 4.5% of men) and the elderly (18.6% of those aged ≥75 years) [3]. There is evidence to suggest that millions more experience undiagnosed symptoms of DED [3].
Although the pathogenesis of DED has not been fully elucidated, there is increasing evidence indicating that DED is an immune-mediated inflammatory disorder [4]. Our previous studies demonstrate that desiccating stress activates the CD4+ T helper-17 (Th17) effector cells and induces the expression of pro-inflammatory cytokines, such as IL-1, IL-6, TNF-α, IL-17 and IL-23, all of which lead to epithelial damage in DED [5, 6]. The role of inflammation in DED is further corroborated by studies showing that administration of topical anti-inflammatory agents such as corticosteroids [7], cyclosporine A [8] or IL-1 receptor antagonists [9] markedly attenuate the signs and symptoms of DED.
Ocular surface epithelial cells play a critical role in the inflammatory/immune cycle of DED [10]. In homeostatic conditions, corneal epithelial cells produce high levels of immunoregulatory factors, including thrombospondin-1 (TSP-1) [11, 12], programmed death-ligand 1 (PD-L1) [13], pigment epithelium-derived factor (PEDF) [14], IL-10 [15] and Fas ligand (FasL) [16], which can suppress innate and adaptive immune responses. TSP-1 is a 145 kDa matricellular protein that is secreted by a range of normal cell types, including endothelial cells, adipocytes, smooth muscle cells, fibroblasts, monocytes and macrophages [17]. Generally elevated in inflammatory microenvironments, TSP-1 has diverse context-dependent immunoregulatory functions, interacting with CD36, CD47, nitric oxide and transforming growth factor-β (TGF-β) [17, 18]. TSP-1 is recognized as a major activator of latent TGF-β both in vitro and in vivo [19, 20], thereby suppressing dendritic cell (DC) maturation and activation [21]. TSP-1 deficiency has been shown to result in a spontaneous form of chronic DED and aberrant histopathology associated with Sjögren's syndrome[22].
Dysfunction and increased apoptosis of corneal epithelial cells has been demonstrated in DED [23-25]. It has been previously reported that TSP-1 is expressed by corneal epithelial cells [26], keratocytes [27] and corneal endothelial cells [28], and may play a pivotal role in the maintenance of corneal avascularity and integrity. However, the expression of corneal epithelium-derived TSP-1 and its regulatory impact on immune responses in DED remains to be elucidated. In the present study, we investigated DED-induced alterations in corneal epithelium-derived TSP-1. We determined the regulatory function of TSP-1 on the phenotype of bone marrow-derived dendritic cells (BMDCs) and evaluated the effect of local TSP-1 treatment in ameliorating DED. Our data establish a progressive increase in expression of TSP-1 in the course of DED. In addition, we demonstrate that corneal epithelium-derived TSP-1 exerts a suppressive effect on BMDC maturation. Finally, we show that topical TSP-1 significantly suppresses DED-associated inflammation and reduces disease severity.
MATERIALS AND METHODS
Animals
Female 6- to 8-week-old C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were used for this study. The experimental protocol was approved by the Schepens Eye Research Institute Animal Care and Use Committee, and all animals were managed according to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of Dry Eye Disease
Acute DED was induced by housing mice in a controlled environment chamber (CEC) for 14 days, as described previously[29]. The CEC allows continuous regulation of relative humidity below 20%, a constant temperature of 21 to 23°C, and an airflow of 15 L/min. Age- and sex-matched mice placed in the standard vivarium served as controls. To evaluate the effects of desiccating stress on the ocular surface, corneal fluorescein staining (CFS) was performed. A total of 1 μL of 1% fluorescein (Sigma-Aldrich, St. Louis, MO) was applied to the lateral conjunctival sac of the mice with a micropipette, and eyes were examined after 3 minutes for fluorescein staining using slit-lamp biomicroscopy under a cobalt blue light. Punctate staining was evaluated in a masked fashion using the National Eye Institute (Bethesda, MD, USA) grading system, giving a score of 0 to 3 to each of the five areas of the cornea[30].
Generation of Bone Marrow Derived Dendritic Cells (BMDC)
Bone marrow cells were collected from the femurs and tibiae of 6- to 8-week-old female C57BL/6 mice and cell suspension was prepared. Cells were incubated with Red blood cell (RBC) lysis buffer (Sigma-Aldrich, St. Louis, MO) at 37°C for 10 minutes. Cells were then seeded in six-well plates at 5×105 cells/well concentration, and were cultured in RPMI-1640 medium supplemented with 20 ng/mL of granulocyte-macrophage colony-stimulating factor (GM-CSF; Biolegend, San Diego, CA), 10% fetal bovine serum (FBS; Atlanta biologicals, Atlanta, GA), 100U/ml penicillin and 100μg/ml streptomycin. On day 3, the culture medium was changed and on day 5, about Π107 bone marrow derived dendritic cells (BMDCs) were harvested.
BMDC and Corneal Epithelial Cell Co-culture
Corneas were harvested from naïve mice and DED mice at day 14 after desiccating stress. Excised corneas were incubated with 20 mM Ethylenediamine-tetraacetic acid (EDTA; Sigma-Aldrich, St. Louis, MO) at 37°C for 40 minutes. The epithelial layer was peeled off and subsequently digested in 0.25% trypsin-0.02% EDTA. Cell suspension was filtered through a 70-μm cell strainer. In vitro-expanded BMDCs were then co-cultured with or without naïve or DED corneal epithelial cells in a 1:2 ratio (100,000 BMDCs and 200,000 corneal epithelial cells per well) in 96-well plates in the presence of 0.5ng/ml IFNγ (Peprotech, Rocky Hill, NJ) for 48 hours.
Real-Time PCR
Total RNA was extracted from corneal or conjunctival tissue using a commercial reagent (TRIzol; Invitrogen, Carlsbad, CA) and an RNA purification kit (RNeasy Micro Kit; Qiagen, Germantown, MD).First-strand cDNA was synthesized with random hexamers using reverse transcriptase (SuperScript III; Invitrogen), and quantitative real-time polymerase chain reaction (PCR) was performed using predesigned primers (Taqman PCR Mastermix and FAM dye-labeled primers; Applied Biosystems, Foster City, CA) for TSP-1 (Mm00449032_g1), IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), IL-23 ((Mm00518984_m1), IL-17A (Mm00439619_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Mm99999915_g1). The GAPDH gene was used as the endogenous reference for each reaction. The results were analyzed by the comparative threshold cycle (CT) method using a commercial analysis software (LightCycler, version 3; Roche Diagnostics Corp., Indianapolis, IN).
Single Cell Isolation from the Cornea and Draining Lymph Nodes
Excised corneas were digested in 2 mg/mL collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 0.05 mg/mL DNase I (Roche, Basel, Switzerland) for 1 hour at 37°C, as previously described[31]. Cell suspension was filtered through a 70-μm cell strainer (BD Falcon; Becton-Dickinson, Franklin Lakes, NJ). Submandibular and cervical draining lymph nodes were harvested and single cell suspensions were prepared.
Flow Cytometry
Non-specific staining was blocked by incubating cells with an anti-FcR antibody in 0.5% BSA at 37°C for 30 minutes. Cells were subsequently immunostained with the following antibodies: PE/cy5-conjugated anti-CD45, Alexa Fluor 488-conjugated anti-CD11c, APC-conjugated anti-CD86 and PE/cy7-conjugated anti-MHC-II (Biolegend) For TSP-1 staining, cells were incubated with primary TSP-1 antibody (abcam) at 4°C overnight, and stained with the goat anti-mouse IgG conjugated with Dylight 488 (abcam). To quantify IL-17-secreting CD4+ cells, single cell suspension was prepared from cervical LNs harvested from human serum albumin (HSA)-treated and recombinant TSP-1-treated DED mice on day 14. Cells were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Sigma-Aldrich Corp.) in the presence of GolgiStop (BD Biosciences), and subsequently stained with an FITC-conjugated anti-CD4 antibody (Biolegend). After fixation and permeabilization (buffers from eBioscience, San Diego, CA, USA), cells were stained with a PE-conjugated anti-IL-17A antibody (eBioscience). Appropriate isotype-matched control antibodies were used in all experiments. Cells were analyzed using the LSR II flow cytometer (BD Biosciences, San Jose, CA) and data were analyzed using FlowJo vX.0.7 software (FlowJo LLC., Ashland, OR, USA).
TSP-1 Treatment Regimen
Following DED induction, mice were randomly divided into two treatment groups (five mice per group), receiving either 2ng/μl topical recombinant human TSP-1 (R&D Systems, Minneapolis, MN) or Human serum albumin (HSA) (Sigma-Aldrich Corp.) as the control. Each treatment group received 3 μl of its respective medication via ocular surface instillation three times per day over a period of 14 days.
Immunohistochemical Staining
For whole-mount corneal staining, freshly excised corneas were washed in PBS and fixed in acetone at −20°C for 15 minutes. Nonspecific staining was blocked with anti-FcR CD16/CD32 antibody (BD Biosciences). Specimens were immunostained with FITC-conjugated rat anti-mouse MHC-II (BD Biosciences,) or isotype-matched antibody overnight, and mounted with Vector Shield mounting medium (Vector Laboratories; Burlingame, CA). Corneas were analyzed using confocal microscopy at ×40 magnification. MHC-II+ cells were enumerated in four areas in the periphery (0.5 μm area from the limbus) of each cornea and the mean cell density was calculated.
Statistical analysis
Experiments presented in the figures are representative of at least three independent experiments. Data are expressed as mean ± standard error of mean (SEM). The SPSS software package (version 17.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. One-way ANOVA followed by Bonferroni tests were used to compare the difference among more than two groups. Independent Student’s t-test was used to compare the results between HSA and recombinant TSP-1-treated groups. A p value of <0.05 was considered statistically significant.
RESULTS
Expression of corneal epithelium-derived TSP-1 is increased in the course of DED
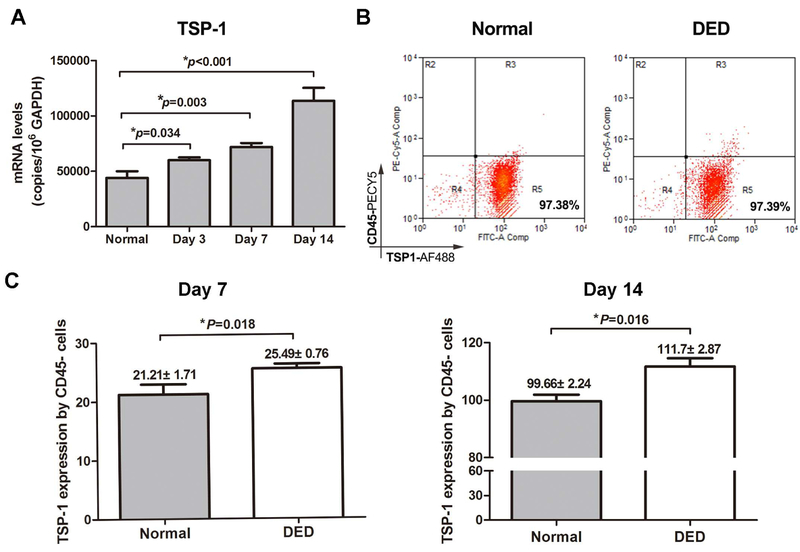
To investigate the expression of corneal epithelium-derived TSP-1 in naïve and DED mice, corneal tissue was harvested from naïve mice or on days 3, 7, 14 and 28 after dry eye induction. mRNA and protein expression levels of TSP-1 were measured using real-time PCR and flow cytometry. As shown in Figure 1A, mRNA expression levels of TSP-1 progressively increased in the cornea of mice with DED compared to healthy mice. Although our flow cytometry results did not show any differences in the frequencies of TSP-1-expressing corneal epithelial cells (CD45− TSP-1+ cells) (Figure 1B), expression levels (mean fluorescent intensity [MFI]) of TSP-1 by corneal epithelial cells were significantly higher in DED mice compared to healthy controls on day 7 (p=0.018) and day 14 (p=0.016) (Figure 1C).
Figure 1. Expression of corneal epithelial TSP-1 is increased in DED mice.
Corneas were harvested on days 3, 7 and 14 after DED induction. The epithelial layer was peeled off and single cell suspension was prepared. Healthy mice served as the control. (A) TSP-1 mRNA expression was quantified using real-time PCR. (B) Representative flow cytometry plots showing the frequencies of TSP-1+CD45− epithelial cells in healthy and DED mice. (C) Protein expression (MFI) of TSP-1 by corneal epithelial cells was assessed in DED mice on days 7 and 14 after desiccating stress using flow cytometry. Data are presented as mean ± SEM of three independent experiments, each consisting of six corneas per group. MFI: mean fluorescence intensity.
Corneal epithelial cells derived from mice with DED demonstrate an amplified ability to suppress BMDC maturation
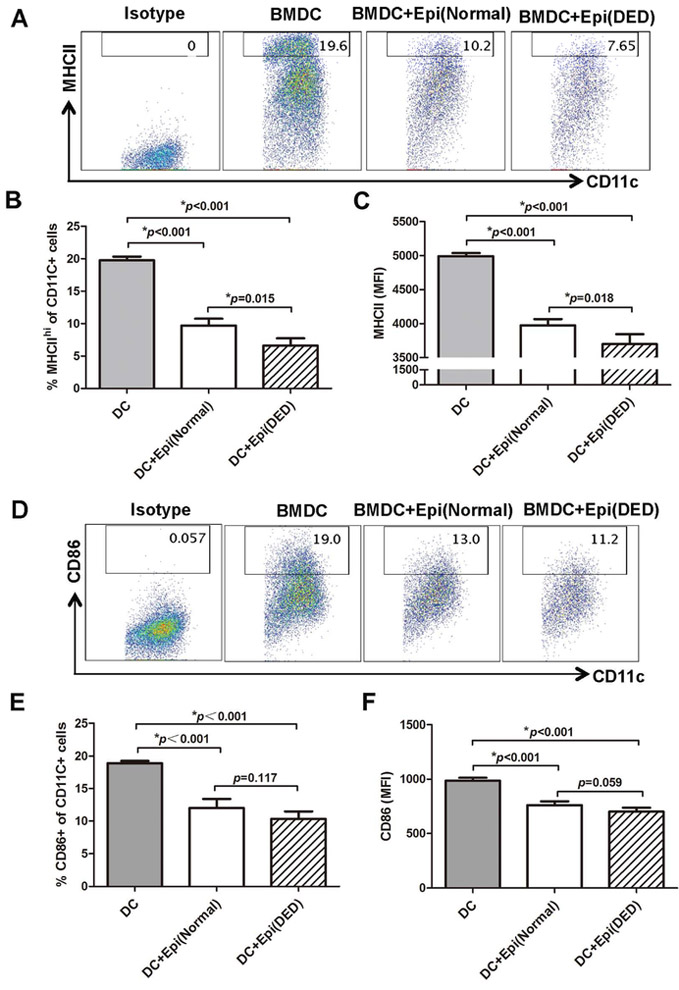
In order to investigate the effect of corneal epithelial cells on the maturation of BMDCs, we co-cultured corneal epithelial cells from either naïve or DED mice with BMDCs, and evaluated the expression of MHC-II and CD86 by BMDCs. Our flow cytometry results revealed that the frequencies of MHC-IIhi BMDCs and BMDC expression of MHC-II were significantly lower in BMDCs co-cultured with either normal (9.703±1.266% and 3974.25±69.419) or DED corneal epithelium (6.605±1.02% and 3697±78.105) compared to BMDCs cultured alone (19.767±0.55% and 4990.667±47.648). Interestingly, this effect was significantly pronounced in BMDCs co-cultured with corneal epithelial cells derived from mice with DED (15.67% more reduction in MHC IIhi BMDC frequencies, p=0.015; and 5.56% more reduction in MHC II MFI, p=0.018) (Figure 2, A-C). A similar trend was noted when the expression of CD86 by BMDCs was examined, although in this case the tendency for the frequencies of CD86+ BMDCs and BMDC expression of CD86 to be decreased in co-culture with DED epithelium relative to naïve epithelium did not reach statistical significance (Figure 2, D-F).
Figure 2. Corneal epithelium from DED mice shows an enhanced suppressive function on bone marrow derived dendritic cell (BMDC) maturation.
Corneal epithelial cells were harvested from healthy or DED mice and were cultured with bone marrow derived dendritic cells (BMDCs) for 48 hours. (A) Representative flow cytometry plots showing the frequencies of MHC-IIhi BMDCs co-cultured with normal or DED corneal epithelial cells. Bar charts showing (B) the frequencies of MHC-IIhi BMDCs and (C) BMDC expression (MFI) of MHC-II in BMDCs cultured alone or in the presence of normal or DED corneal epithelial cells. (D) Representative flow cytometry plots showing the frequencies of CD86+ BMDCs co-cultured with normal or DED corneal epithelial cells. Bar charts showing (E) CD86+ BMDC frequencies and (F) expression levels (MFI) of CD86 by BMDCs cultured alone or in the presence of normal or DED corneal epithelial cells. Data are presented as mean ± SEM of three independent experiments, each consisting of 5 mice per group. Epi: Corneal epithelial cells; MFI: mean fluorescence intensity.
TSP-1 derived from corneal epithelial cells inhibits the maturation of BMDCs.
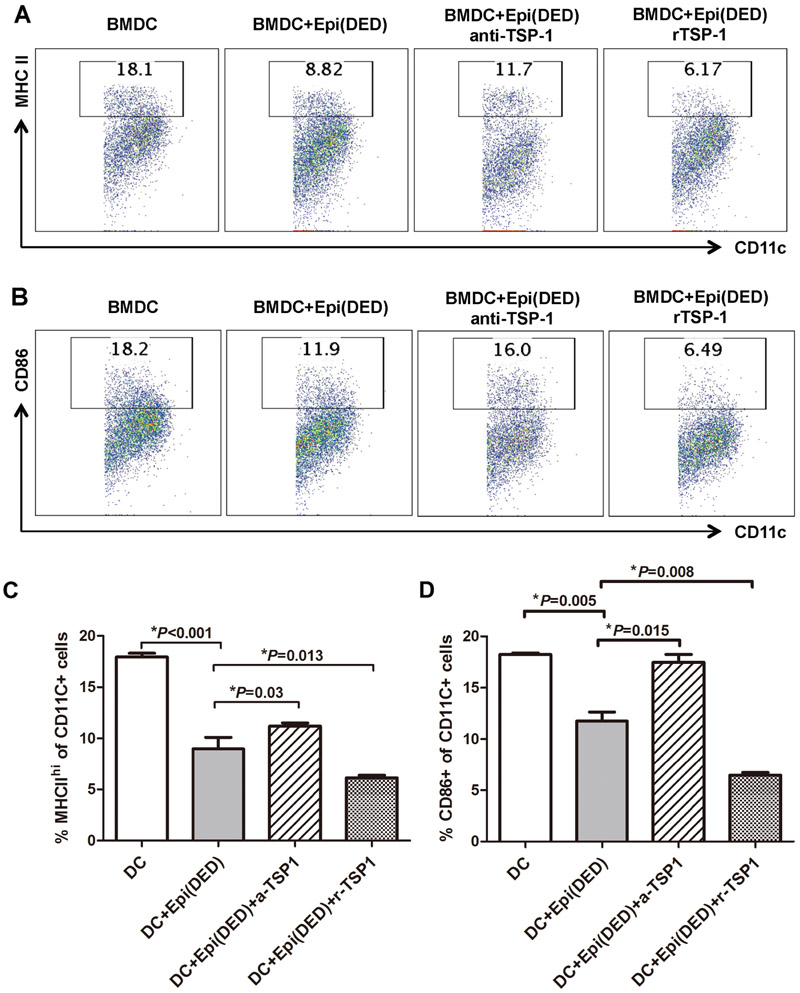
To investigate whether corneal epithelium-derived TSP-1 is responsible for the observed epithelial cell-induced suppression in BMDC maturation, we further added either recombinant TSP-1 or TSP-1 blocking antibodies to our in vitro BMDC and DED corneal epithelium co-culture system. Our flow cytometry results showed that TSP-1 blockade significantly abrogated the suppressive effect of DED corneal epithelium on BMDC maturation (Figure 3, A-D). However, addition of recombinant TSP-1 further potentiated the suppressive function of DED corneal epithelium and led to a more dramatic decrease in the frequencies of MHC-IIhi and CD86+ BMDCs as compared to BMDCs co-cultured with corneal epithelial cells alone (p=0.013 and p=0.008, respectively) (Figure 3, A-D).
Figure 3. TSP-1 derived from corneal epithelial cells inhibits the maturation of BMDCs.
Representative flow cytometry plots showing the frequencies of (A) MHC-IIhi and (B) CD86+ BMDCs co-cultured with DED corneal epithelial cells in the presence of either anti-TSP-1 antibody (anti-TSP-1) or recombinant TSP-1 (rTSP-1). (C & D) Bar charts demonstrating the frequencies of MHC-IIhi and CD86+ BMDCs cultured alone, or with DED corneal epithelial cells in the presence of anti-TSP-1 antibody or recombinant TSP-1. Data are presented as mean ± SEM of three independent experiments, each consisting of 5 mice per group. Epi: Corneal epithelial cells.
Topical TSP-1 inhibits corneal dendritic cell maturation and ocular inflammation, and ameliorates DED
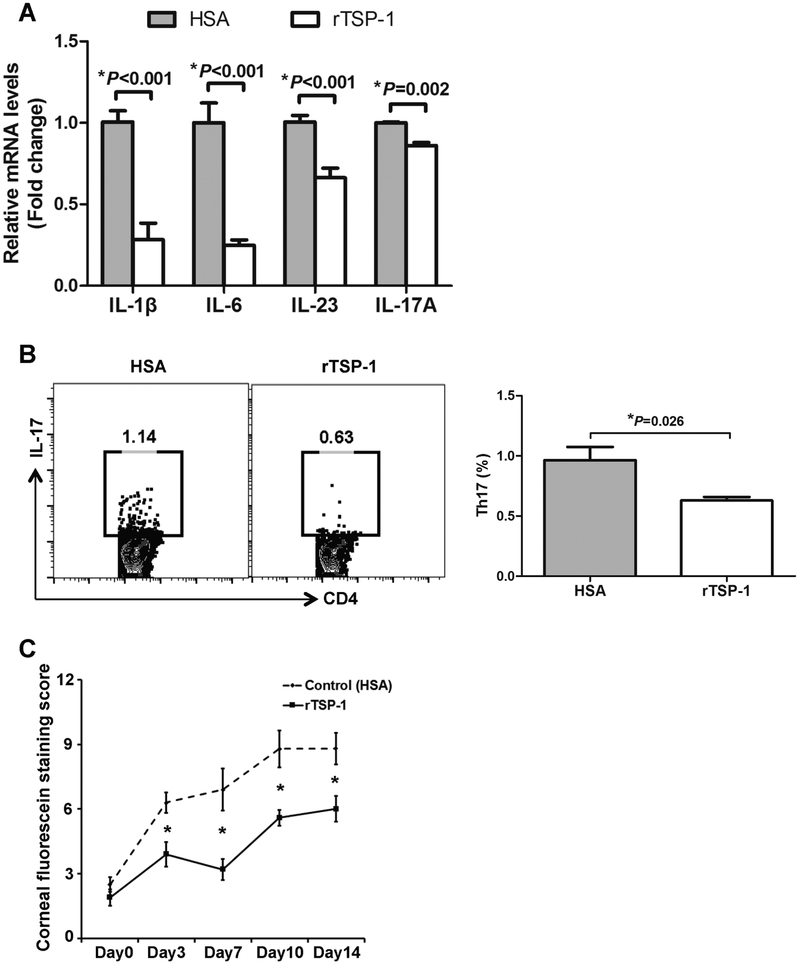
Next, we evaluated the effect of topical recombinant TSP-1 in mice with DED. Topical HSA was used as the control. As shown in Figure 4A, our immunohistochemistry results demonstrated lower number of MHCII+ cells infiltrating the DED corneas in the recombinant TSP-1-treated group compared to the HSA-treated controls. Furthermore, expression of MHC-II and CD86 by corneal CD11c+ cells were significantly suppressed in recombinant TSP-1 treated mice compared to the control group (MHC-II MFI: 117.667±4.619 vs. 172.667±20.133, p=0.034; CD86 MFI: 20.5±1.203 vs. 24.5±0.748, p=0.039) (Figure 4B). In order to investigate the clinical effect of topical recombinant TSP-1 in DED, we evaluated the frequencies of Th17 cells and the expression of DED-associated cytokines in the conjunctivae and draining lymph nodes of mice treated with either recombinant TSP-1 or HSA on day 14 after DED induction. Furthermore, we performed corneal fluorescein staining (CFS) to determine the severity of epitheliopathy. Our results demonstrated that compared to the HSA-treated group, TSP-1-treated mice had significantly lower mRNA levels of IL-1β (p<0.001), IL-6 (p<0.001), IL-23 (p<0.001) and IL-17A (p=0.002) (Figure 5A). Furthermore, topical recombinant TSP-1 treatment significantly suppressed the frequencies of Th17 cells in draining lymph nodes (0.63±0.057 vs. 0.965±0.219, p=0.026), and led to significantly lower CFS scores compared to the control group (p<0.05) (Figure 5B and 5C).
Figure 4. Topical TSP-1 treatment inhibits corneal dendritic cell (DC) maturation in DED.
(A) Left: Representative confocal images showing MHC-II+ cells (green) in the corneas of DED mice treated with topical recombinant TSP-1 (rTSP-1) or HSA (control). Scale bar=100μm. Right: Bar chart showing the density of MHCII+ cells in the cornea of rTSP-1-treated mice compared to HSA-treated controls. (B) Left: Representative flow cytometry histograms showing the expression of MHC-II and CD86 by corneal CD11c+ cells after treatment with topical rTSP-1 or HSA. Right: Bar charts showing expression levels (MFI) of MHC-II and CD86 by corneal CD11c+ cells in rTSP-1 and HSA-treated DED mice. Data are presented as mean ± SEM of three independent experiments, with four corneas in each group.
Figure 5. Topical TSP-1 suppresses ocular inflammation and reduces the severity of DED.
(A) Conjunctivae of DED mice treated with either rTSP-1 or HSA were collected on day 14 after DED induction. The mRNA expression of IL-1β, IL-6, IL-23 and IL-17A cytokines was quantified using real-time PCR (n=3 conjunctivae per group). (B) Flow cytometry plots and bar chart demonstrating the frequencies of CD4+IL-17+ (Th17) cells in the draining lymph nodes of rTSP-1 or HSA-treated DED mice on day 14. (C) Clinical severity of DED after treatment with either rTSP-1 or HSA was evaluated using corneal fluorescein staining (*p < 0.05; n=4 mice/8 eyes per group).
DISCUSSION
DED is a multifactorial disorder characterized by autoimmune inflammation of the ocular surface[1]. Despite significant advances in our understanding of the innate and adaptive immune responses that govern the development and progression of the disease, the pathogenesis of DED still poses a number of unanswered questions. The interface between epithelial cells and immune cells is critical in the pathogenesis of DED, yet the complex interactions between these cells have not been fully elucidated[10]. Herein, we demonstrate that expression of TSP-1 by the corneal epithelium is increased in DED. We show that corneal epithelial cells from mice exposed to desiccating stress have an enhanced capacity to suppress BMDC maturation, and that this effect is abrogated by TSP-1 blockade and potentiated by recombinant TSP-1. Finally, we show that topical administration of recombinant TSP-1 in vivo inhibits corneal DC maturation, reduces expression of pro-inflammatory cytokines and ameliorates disease severity in mice with DED.
Corneal epithelial cells secrete a variety of immunoregulatory factors that help to maintain immune homeostasis at the ocular surface, including TSP-1, PD-L1, PEDF, IL-10 and FasL [11-16]. TSP-1 influences a wide range of cellular interactions in the eye including angiogenesis, cell migration and wound healing, in addition to immunoregulation [32]. TSP-1 mRNA has been identified in corneal epithelium and stroma, and the protein has been immunolocalized in corneal tissue [12, 33]. Using a validated murine model of DED, our study demonstrates a progressive increase in TSP-1 mRNA expression levels over time in mice exposed to desiccating stress [34, 35]. We hypothesized that enhanced production of TSP-1 by the corneal epithelium may be a compensatory mechanism that curbs the vigor of the immune response in DED, thus protecting the integrity of the ocular surface. Interestingly, TSP-1-deficient mice have been shown to exhibit a similar phenotype to Sjögren’s syndrome, developing a spontaneous corneal epitheliopathy that correlates with lacrimal gland inflammation and reduced goblet cell density [22]. Moreover, DCs derived from these mice, but not from wild-type mice, activate T cells to promote IL-17A secretion – a cytokine fundamental to the pathogenesis of DED [5, 22].
The novelty of our work lies in demonstrating the immunoregulatory functional significance of corneal epithelial-derived TSP-1. Mechanistically, the regulation of DC function by ligation of TSP-1 (via CD36 and/or CD47) has been established [36, 37]. DC-derived TSP-1 has been shown to negatively regulate the expression of pro-inflammatory cytokines by DCs in response to inflammatory stimuli [38]. The inhibitory effect of TSP-1 on DC maturation has also been identified in a murine model of corneal transplantation [39]. In this study, Saban et al. showed that nearly all TSP-1-null allografts succumbed to rejection compared with only 50% of wild-type allografts [39]. The investigators demonstrated heightened expression of MHCII, CD80 and CD86 maturation markers on TSP-1-null DCs during inflammation, implicating these cells in the generation of a pro-inflammatory graft microenvironment and higher allograft rejection rates [39]. While these studies demonstrate the autocrine regulation of DC activation by endogenous TSP-1 during inflammation, the function of corneal epithelium as another source of TSP-1 and its role in the pathogenesis of DED has remained mostly unknown. In addition to corroborating the immunomodulatory effects of TSP-1 in DED, our study is the first to our knowledge to demonstrate the effect of epithelial TSP-1 and its altered expression in response to desiccating stress on DC activation.
DCs play a critical role in the sensitization phase of the immune response. Desiccating stress provokes the maturation and mobilization of DCs, with subsequent chemotaxis to the draining lymph nodes [40]. There, activated corneal DCs prime naive T cells to ocular surface antigens, resulting in generation of IL-17 and interferon gamma (IFNγ)-expressing effector cells[40]. Our in vivo results show that topical administration of recombinant TSP-1 significantly inhibits the infiltration of corneal DCs and their expression of MHC II and CD86 maturation markers, and suppresses the expression of pro-inflammatory cytokines at the ocular surface. This is in agreement with a previous report showing that TSP-1-deficient mice exhibit increased expression of Th1 (IFN-γ, TNF-α) and Th17 (IL-6, IL-17A) inflammatory cytokines and related transcription factors (Tbet and RORγt) in their conjunctivae and draining lymph nodes relative to wild type mice [41]. In their study, the investigators note that the presence of inflammatory mediators in TSP-1-deficient conjunctivae correlates with both the spontaneous disruption of the conjunctival epithelium and reduced goblet cell density observed in these mice [41]. A recent study by Contreras Ruiz et al. has shown that 4N1K, a TSP-1 derived peptide, inhibits the secretion of Th17-promoting cytokine IL-23 by macrophages in vitro. Using intraperitoneal injections of TSP-1 peptide, the investigators demonstrate reduced frequencies of Th17 cells accompanied by increased Treg frequencies in TSP-1-treated mice [42]. Consistent with these observations, our in vivo data demonstrate that topical recombinant TSP-1 significantly suppresses the frequencies of Th17 cells in draining lymph nodes of DED mice. Importantly, our work supports the consideration of TSP-1 as a potential therapeutic agent in DED, given the significantly suppressed pro-inflammatory cytokine expression and lower epitheliopathy observed in recombinant TSP-1-treated mice relative to controls. Despite the demonstrated function of epithelial TSP-1 in suppressing DC maturation in DED as shown herein, APCs do maturate in dry eye disease and drive the immunopathogenesis by mediating Th17 priming [43]. In DED, similar to many other immunopathologies, there are countering effects of regulatory factors that suppress inflammation and inflammatory mediators that amplify inflammation. Multiple other factors, including high levels of IL-1, IL-6, IFN-γ, among others, can drive APC maturation [44]. Similarly, while epithelium-derived TSP-1 plays an immunomodulatory function, it is noteworthy that de Paiva’ s group showed DC-derived TSP-1 to be critical in induction of the Th17 response [45]. This is not dissimilar from the principal mediator of TSP-1 function, TGF-β, that has been shown to be potently immunoregulatory [46] or profibrotic [47], depending on the context in which it functions.
In summary, these findings provide new insights on the immunomodulatory function of epithelium-derived TSP-1 in a validated mouse model of DED. We demonstrate increased expression of TSP-1 by corneal epithelial cells in response to desiccating stress, and propose this as a regulatory mechanism for maintaining immune homeostasis at the stressed ocular surface. Our novel data show that corneal epithelial cells from mice exposed to desiccating stress have a heightened ability to suppress BMDC maturation, an effect that is abrogated by TSP-1 blockade, and amplified by recombinant TSP-1. Finally, we have validated the immunoregulatory function of recombinant TSP-1 in vivo, and exhibited its translational potential to limit corneal epitheliopathy in DED.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Susanne Eiglmeier, PhD for her editorial assistance with the manuscript.
FUNDING SUPPORT
This study was supported by the National Eye Institute/National Institutes of Health Grant R01 EY020889, National Eye Institute/National Institutes of Health Core Grant P30EY003790, the National Natural Science Funds of China for Young Scholar (No. 81600707) and the Natural Science Funds of Guangdong province of China (No. 2017A030313458).
Footnotes
FINANCIAL DISCLOSURE
The authors declare no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf 2017;15:438–510. [DOI] [PubMed] [Google Scholar]
- [2].Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Advances in experimental medicine and biology. 2002;506:989–98. [DOI] [PubMed] [Google Scholar]
- [3].Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol 2017. [DOI] [PubMed] [Google Scholar]
- [4].Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Archives of ophthalmology (Chicago, Ill : 1960). 2012;130:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol 2009;2:375–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol 2009;182:1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jung HH, Ji YS, Sung MS, Kim KK, Yoon KC. Long-Term Outcome of Treatment with Topical Corticosteroids for Severe Dry Eye Associated with Sjogren's Syndrome. Chonnam medical journal. 2015;51:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhou XQ, Wei RL. Topical cyclosporine A in the treatment of dry eye: a systematic review and meta-analysis. Cornea 2014;33:760–7. [DOI] [PubMed] [Google Scholar]
- [9].Amparo F, Dastjerdi MH, Okanobo A, Ferrari G, Smaga L, Hamrah P, et al. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA ophthalmology. 2013;131:715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pflugfelder SC, de Paiva CS, Li DQ, Stern ME. Epithelial-immune cell interaction in dry eye. Cornea 2008;27 Suppl 1:S9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med 2011;208:1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, et al. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci 2004;45:1117–24. [DOI] [PubMed] [Google Scholar]
- [13].El Annan J, Goyal S, Zhang Q, Freeman GJ, Sharpe AH, Dana R. Regulation of T-cell chemotaxis by programmed death-ligand 1 (PD-L1) in dry eye-associated corneal inflammation. Invest Ophthalmol Vis Sci 2010;51:3418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kenchegowda S, He J, Bazan HE. Involvement of pigment epithelium-derived factor, docosahexaenoic acid and neuroprotectin D1 in corneal inflammation and nerve integrity after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2013;88:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yan XT, Zhuang M, Oakes JE, Lausch RN. Autocrine action of IL-10 suppresses proinflammatory mediators and inflammation in the HSV-1-infected cornea. J Leukoc Biol. 2001;69:149–57. [PubMed] [Google Scholar]
- [16].Morris JE, Zobell S, Yin XT, Zakeri H, Summers BC, Leib DA, et al. Mice with mutations in Fas and Fas ligand demonstrate increased herpetic stromal keratitis following corneal infection with HSV-1. J Immunol. 2012;188:793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bornstein P Thrombospondins as matricellular modulators of cell function. J Clin Invest 2001;107:929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–70. [DOI] [PubMed] [Google Scholar]
- [20].Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274:13586–93. [DOI] [PubMed] [Google Scholar]
- [21].Strobl H, Knapp W. TGF-beta1 regulation of dendritic cells. Microbes Infect. 1999;1:1283–90. [DOI] [PubMed] [Google Scholar]
- [22].Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjogren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol. 2009;175:1136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fabiani C, Barabino S, Rashid S, Dana MR. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp Eye Res. 2009;89:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003;44:124–9. [DOI] [PubMed] [Google Scholar]
- [25].Luo L, Li DQ, Pflugfelder SC. Hyperosmolarity-induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathways. Cornea. 2007;26:452–60. [DOI] [PubMed] [Google Scholar]
- [26].Sekiyama E, Nakamura T, Cooper LJ, Kawasaki S, Hamuro J, Fullwood NJ, et al. Unique distribution of thrombospondin-1 in human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2006;47:1352–8. [DOI] [PubMed] [Google Scholar]
- [27].Choudhary A, Hiscott P, Hart CA, Kaye SB, Batterbury M, Grierson I. Suppression of thrombospondin 1 and 2 production by herpes simplex virus 1 infection in cultured keratocytes. Molecular vision. 2005;11:163–8. [PubMed] [Google Scholar]
- [28].Scheef EA, Huang Q, Wang S, Sorenson CM, Sheibani N. Isolation and characterization of corneal endothelial cells from wild type and thrombospondin-1 deficient mice. Molecular vision. 2007;13:1483–95. [PubMed] [Google Scholar]
- [29].Chen Y, Chauhan SK, Lee HS, Stevenson W, Schaumburg CS, Sadrai Z, et al. Effect of desiccating environmental stress versus systemic muscarinic AChR blockade on dry eye immunopathogenesis. Invest Ophthalmol Vis Sci. 2013;54:2457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–32. [PubMed] [Google Scholar]
- [31].Shen L, Jin Y, Freeman GJ, Sharpe AH, Dana MR. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J Immunol. 2007;179:3672–9. [DOI] [PubMed] [Google Scholar]
- [32].Masli S, Sheibani N, Cursiefen C, Zieske J. Matricellular protein thrombospondins: influence on ocular angiogenesis, wound healing and immuneregulation. Curr Eye Res. 2014;39:759–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hiscott P, Paraoan L, Choudhary A, Ordonez JL, Al-Khaier A, Armstrong DJ. Thrombospondin 1, thrombospondin 2 and the eye. Prog Retin Eye Res 2006;25:1–18. [DOI] [PubMed] [Google Scholar]
- [34].Chen Y, Chauhan SK, Shao C, Omoto M, Inomata T, Dana R. IFN-gamma-Expressing Th17 Cells Are Required for Development of Severe Ocular Surface Autoimmunity. J Immunol. 2017;199:1163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen Y, Chauhan SK, Tan X, Dana R. Interleukin-7 and -15 maintain pathogenic memory Th17 cells in autoimmunity. J Autoimmun. 2017;77:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol. 2000;164:2193–9. [DOI] [PubMed] [Google Scholar]
- [37].Tabib A, Krispin A, Trahtemberg U, Verbovetski I, Lebendiker M, Danieli T, et al. Thrombospondin-1-N-terminal domain induces a phagocytic state and thrombospondin-1-C-terminal domain induces a tolerizing phenotype in dendritic cells. PLoS One. 2009;4:e6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Doyen V, Rubio M, Braun D, Nakajima T, Abe J, Saito H, et al. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J Exp Med. 2003;198:1277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Saban DR, Bock F, Chauhan SK, Masli S, Dana R. Thrombospondin-1 derived from APCs regulates their capacity for allosensitization. J Immunol. 2010;185:4691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res 2012;31:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Contreras-Ruiz L, Regenfuss B, Mir FA, Kearns J, Masli S. Conjunctival inflammation in thrombospondin-1 deficient mouse model of Sjogren's syndrome. PLoS One. 2013;8:e75937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Contreras Ruiz L, Mir FA, Turpie B, Masli S. Thrombospondin-derived peptide attenuates Sjogren's syndrome-associated ocular surface inflammation in mice. Clin Exp Immunol. 2017;188:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–25. [DOI] [PubMed] [Google Scholar]
- [44].Hamrah P, Liu Y, Zhang Q, Dana MR. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol. 2003;121:1132–40. [DOI] [PubMed] [Google Scholar]
- [45].Gandhi NB, Su Z, Zhang X, Volpe EA, Pelegrino FS, Rahman SA, et al. Dendritic cell-derived thrombospondin-1 is critical for the generation of the ocular surface Th17 response to desiccating stress. J Leukoc Biol. 2013;94:1293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. [DOI] [PubMed] [Google Scholar]
- [47].Zhou Q, Yang L, Wang Y, Qu M, Chen P, Wang Y, et al. TGFbeta mediated transition of corneal fibroblasts from a proinflammatory state to a profibrotic state through modulation of histone acetylation. J Cell Physiol. 2010;224:135–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.