Abstract
Stroke instigates a dynamic process of repair and remodelling of remaining neural circuits, and this process is shaped by behavioural experiences. The onset of motor disability simultaneously creates a powerful incentive to develop new, compensatory ways of performing daily activities. Compensatory movement strategies that are developed in response to motor impairments can be a dominant force in shaping post-stroke neural remodelling responses and can have mixed effects on functional outcome. The possibility of selectively harnessing the effects of compensatory behaviour on neural reorganization is still an insufficiently explored route for optimizing functional outcome after stroke.
Behavioural compensation refers to alternative behavioural strategies that circumvent impairments to enable the performance of tasks and the attainment of goals. The development of compensatory strategies after CNS damage is observed across diverse impairment modalities. For example, patients who have undergone callosotomy (and who are referred to as ‘split-brain’ individuals) develop peripheral self-cuing strategies to compensate for impaired interhemispheric communication1. People with partial visual field loss use compensatory head and eye movements to help to fill in the field2. Those with agrammatic aphasia compensate for impaired syntax comprehension by relying on semantics to understand speech3.
The compensatory strategies for stroke-induced motor disability are a prevalent category owing to the overwhelming prevalence of stroke. As of 2013, there were more than 25 million stroke survivors worldwide4, and this population is predicted to reach 70 million by 2030 (REF. 5). Up to 85% of stroke survivors have hemiparesis that affects the upper extremity (hand and arm) of one side6. Humans use both hands together most of the time7, and the loss of the function of either hand requires major adjustments to our interactions with the physical world. A common response to this loss after stroke is to learn compensatory ways of relying on the better-functioning, non-paretic hand8,9. There are also compensatory changes in the coordination of movements of both hands and of the paretic forearm with the trunk, as explained below.
Compensation is often mistaken for recovery (BOX 1). One goal of this Review is to highlight the importance of distinguishing between the two in research on the neural mechanisms of recovery. Motor recovery is defined as the return of more-normal movement, or reductions in movement impairment. Some forms of compensation are obvious, but others are subtle enough to go undetected in the absence of sensitive behavioural measures, making them easily confused with recovery10. If not for the clever assays developed by Gazzaniga and colleagues1, the use of self-cuing strategies in patients who have undergone callosotomy would be easy to miss and could be interpreted as support for the idea that these patients recovered interhemispheric communication without a key anatomical pathway for it. The failure to recognize compensatory strategies has a strong potential to confuse our understanding of the neural mechanisms of functional improvement after brain injury and to hamper the usefulness of basic neuroscience research for guiding treatment.
Box 1 | Recovery versus compensation.
Recovery refers to the restoration of a function back to a more-normal, pre-injured state, whereas compensation refers to the substitution for, or circumvention of, impaired functions. In this Review, the term recovery describes the return of more-normal movements, and the term compensation describes the use of alternative movement strategies. The terms refer only to their behavioural-level meaning in this Review. Note, however, that in the stroke literature it is common to use the term recovery to refer to performance improvements in tasks that do not distinguish between compensation and recovery. For example, rodents are often said to recover in skilled motor tasks as measured, for example, by success rates in retrieving food rewards, despite the well-established potential for compensation to underlie these improvements16,69. Behavioural compensation and recovery, as strictly defined above, often overlap in their contributions to performance improvements in such tasks.
The neural mechanisms of behavioural recovery and compensation also overlap. Stroke survivors who have sufficient remaining corticomotor function follow a ‘proportional recovery rule’; that is, their initial motor impairments improve over the first 3 months to a maximum that can be predicted on the basis of the initial impairment level181. The mechanisms underlying this spontaneous behavioural recovery are not well understood, but such recovery is frequently attributed to spontaneous repair and restoration at the CNS level. For example, it may involve the resolution of acute dysfunction in neural activity and cellular metabolism in regions connected to the injury site182,183, the return of more-normal blood flow in peri-infarct tissue184 and the reorganization of the connectivity patterns of surviving neurons106.
Behavioural compensation depends, in part, on learning-related neural plasticity mechanisms126. However, learning-related neural plasticity can depend on the sufficient resolution of neural dysfunction and on the restoration of blood flow, both of which are presumed to also be involved in behavioural recovery13. In addition, the resolution of neural dysfunction can depend on neural plasticity. For example, the formation of new synapses (a learning-related plasticity mechanism) contributes to the restoration of more-normal electrophysiological responses in denervated neurons185.
Further complicating the matter is that some behavioural recovery is practice dependent73,95,96, implicating learning-related neural plasticity as an underlying mechanism. Motor skill training even long after stroke can promote some recovery of more-normal movement17,83. Practice can drive sufficient reorganization of neural connectivity in surviving brain regions to permit a degree of vicariation (take-over) of the original function of these regions97,186. However, the same types of motor training can promote both recovery and compensation16,73,95,96. It is likely that the distinction between the neural mechanisms of recovery and compensation is related more to the specific collection of circuits that is changed than to the general nature of the change. As a result, it could prove challenging for the neural substrates of the subtlest forms of behavioural compensation, as described in the text, to be distinguished from those of recovery. However, many of the frequently observed strategies for compensating for upper-limb hemiparesis are far from subtle (see the first four examples in TABLE 1).
These overlaps in the neural mechanisms of behavioural recovery and compensation create strong potential for their mechanisms to influence, as well as to be mistaken for, one another, thus necessitating a clear distinction between compensation and recovery at the behavioural level in research on their mechanisms.
Another reason to attend to compensation is that it is both a mechanism for improved function and a contributor to persisting impairment after CNS injury8,10,11. There is currently much interest in the potential to optimize functional outcome after CNS damage by capitalizing on early endogenous mechanisms of neural repair and remodelling after stroke, which are sensitive to behavioural manipulation and could facilitate the efficacy of motor rehabilitation12–15. Behavioural compensation is often a major contributor to the functional improvements that result from motor rehabilitative training16,17. Compensatory behaviours are also self-taught. As they are developed early and are well practised7, these selftaught behaviours may normally be a dominant force in shaping post-stroke brain reorganization; however, they may do so in suboptimal, or even maladaptive, ways.
From the perspective of a researcher of rodent models of chronic stroke, this Review first attempts to jointly summarize findings from human and animal studies on the nature of motor compensation after strokes that impair upper-limb function. I describe findings from animal models on the influence of motor compensation on neural reorganization after stroke and its maladaptive consequences, and briefly consider the potential impact of motor compensation on the role of the contralesional hemisphere in functional outcome.
Compensatory movement strategies
Stroke damage and its effects on movement.
The high prevalence of motor impairments after stroke may be due to the propensity for strokes to damage motor regions of cortex, the subcortical projection pathways of these regions or both18,19, thus disrupting the cortical control of movement (FIG. 1). Post-stroke motor impairments are characterized by weakness, diminished muscle activation, abnormal muscle co-activation and other abnormalities that diminish movement capacity and disrupt the spatiotemporal coordination of movements20–23. There is often abnormal coupling of shoulder and elbow movements (muscle synergies) — for example, the elbow may bend as the arm is lifted20,21,24. Reduced wrist stability, diminished finger and grasp control, and impaired grasp release all impair hand dexterity25–27. Movements are slower and more variable27,28, reach trajectories are less direct29, and more-extraneous movements occur30 in stroke survivors than in healthy controls. Although the movements of both the left and the right hands are typically impaired after unilateral stroke, the non-paretic side is less severely so31–34. Spasticity due to hyperexcitability of the stretch reflex can develop after stroke35, and its severity is correlated with the severity of motor impairments36. Motor impairments co-occur to varying degrees with impaired somatosensation37,38, which is associated with damage to ascending somatosensory pathways39.
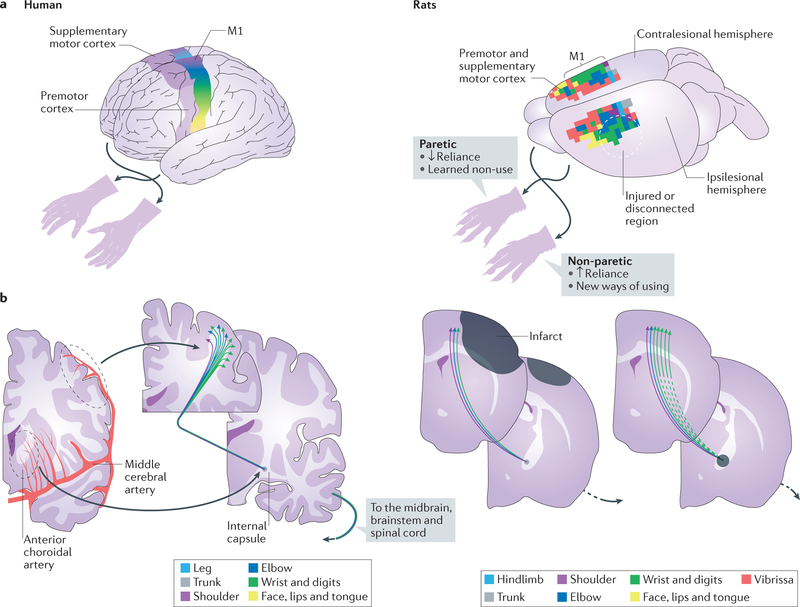
Figure 1 |. The motor cortex and its descending projection pathways are often affected by strokes that result in upper-extremity impairments.
a | Simplified illustrations of motor cortical regions of a human (left), and of motor cortical regions of a naive rat, derived using intracortical microstimulation (right), are shown. The colours show the cortical territories that are responsible for the movement of different body parts. The motor cortical control of the upper limbs is mostly crossed, such that the left hemisphere controls movement of the right side and vice versa. b | Occlusions or ruptures in the cerebral vasculature (red) that supplies motor cortical regions (the distal middle cerebral artery) and its projection pathways (for example, the anterior choroidal and striate arteries) position strokes (the dark grey regions on the rat coronal sections on the right) that either kill the cortical neurons that control upper-limb movement or disconnect their projections to the spinal cord and other subcortical structures, such as the red nucleus and reticular formation. The degree of disruption of the descending motor cortical pathway due to either cortical or subcortical strokes predicts the severity of motor impairment18–20. The colours of the corticospinal neurons correspond to the body parts that they control as in the motor cortical regions in part a. The rat coronal sections show the locations of ischaemic infarcts that have been used to model post-stroke upper-extremity impairments; these infarcts are located in the forelimb region of the primary motor cortex69,73 (M1; which is supplied by the distal middle cerebral artery) and in the posterior limb of internal capsule77,190 (which is supplied by the anterior choroidal artery). The dashed lines indicate the disconnection of descending cortical projections.
Movement adaptations after stroke.
There are normally many different ways (or degrees of freedom) in which individual joint movements can be combined to perform a task, and this diversity allows for adaptation to changing environmental conditions and movement constraints. Motor impairments after stroke can demand adaptation for a greatly diminished repertoire of movement combinations20,40,41. Some of the compensatory movements that have been observed in stroke survivors or in animal models of stroke are described in TABLE 1. To perform reaching or pointing tasks with the paretic upper limb in laboratory settings, stroke survivors compensate for limited arm extension and stability using movements of the trunk and scapula42–45 (FIG. 2). The aim and orientation of the hand tend to be controlled more proximally by trunk and scapular movements, with elbow and hand positions fixed46,47, a strategy that may reduce the complexity of movement control and increase stability42,48,49. To grasp an object, stroke survivors use an excess of forward movements when reaching towards it50 and use the resistance of the object to wind their fingers around it25, presumably to compensate for impairments in opening the fingers to grasp. Those with milder impairments reach for differently sized objects using greater-than-normal adjustment at proximal, in place of distal, finger joints and make these adjustments later in the reach than normal, possibly reflecting a greater reliance on visual feedback to compensate for impaired feedforward control51. Those with milder impairments open the hand excessively to grasp objects and hold them with excessive grip force31,52,53, which may help to ensure a successful grasp and hold in the presence of diminished hand control53.
Table 1.
Examples of compensatory movement strategies for upper-limb hemiparesis
| Normal strategy | Compensatory strategy | Type of study | Refs |
|---|---|---|---|
| Use of both hands together | Dominant reliance on the non-paretic hand | Clinical | 7–9, 54–57 |
| Rodent | 70–72 | ||
| Hand extended via elbow extension | Hand extended via trunk displacement | Clinical | 25,42,43 |
| Hand oriented via wrist movements | Hand oriented via trunk rotation | Clinical | 46,47 |
| Rodent | 63,69 | ||
| Palm to mouth via wrist supination | Palm to mouth via trunk and head rotation | Rodent | 16,63,69 |
| Scaling of finger opening to object size | Excess finger opening to grasp | Clinical | 31,52,53 |
| Grip force varied with object properties | Excessive grip force | Clinical | 31,53 |
| Grasp via flexion at distal, and extension at proximal, finger joints | Grasp via flexion at proximal finger joints | Clinical | 51 |
| Precision grip | Grasp between fingertip and palm or proximal thumb | Monkey | 75,76,96 |
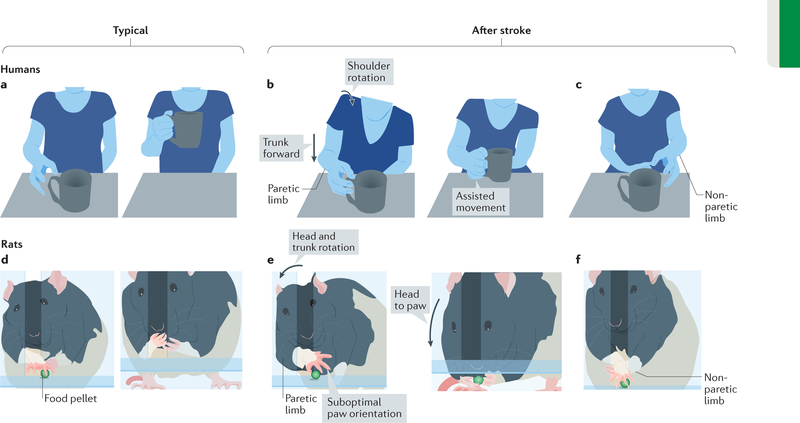
Figure 2 |. Illustrations of compensatory movement strategies for upper-limb hemiparesis.
a | The illustration shows typical reach and grasp movements in healthy humans. b | When reaching with the paretic upper limb after stroke, forward trunk and shoulder displacement and rotation compensate for the diminished control of more-distal movements46,47. If not instructed otherwise, the non-paretic side often assists movements of the paretic side. c | More commonly, the non-paretic side is used for unimanual tasks54,55. d | The illustration shows typical movements used by healthy rats to reach for and consume a palatable food pellet (green sphere) in an apparatus that forces the use of one forelimb (that is, an inner chamber wall placed close to the reaching window on the side of the trained limb blocks the body position needed to reach the pellet with the other forelimb. An alternative method is to place bracelets on the wrist of the other forelimb that prevent its extension through the reaching window82 (not shown)). e | After unilateral motor system injury, rats perform reaching tasks with the paretic forelimb using compensatory movements of the trunk, head and non-paretic forelimb13,16,63. This includes the use of trunk rotation to control paretic paw position (left) and greater head movement (right) to bring the mouth to the paretic paw (rather than the paw to the mouth). The paw orientation is suboptimal for grasping the pellet. f | The non-paretic forelimb is often used to assist in paretic limb movements.
In the laboratory, stroke survivors who are not told which hand to use will typically perform unimanual tasks with the non-paretic hand54,55. Outside of the laboratory, increased reliance on the non-paretic hand is a dominant strategy9. Based on data recorded with movement monitors worn on the wrists, healthy controls perform most daily activities using both hands7,56. After strokes affecting the dominant body side, bilateral hand use is diminished, and sole use of the formerly non-dominant hand is increased in these patients relative to healthy controls7,56. After strokes affecting the non-dominant side, use of only the dominant hand predominates7,56,57. The non-paretic side is also used to help to move the paretic leg during walking, via amplified swinging movements with the non-paretic arm and leg48.
Compensatory movement strategies are likely to be mediated by CNS regions that are less severely affected by the injury. Relying on the better-functioning hand puts the relatively intact circuitry of the contralesional hemisphere mostly in charge of task completion. In healthy individuals, there is more bilateral control of trunk, relative to limb, movements58,59, and trunk movements are also used to reach for objects outside of arm’s reach60; thus, trunk movements may offer a ready solution for impaired arm extension after stroke.
Movement adaptations in animal models.
There are remarkable homologies in the movement patterns used by rodents and humans to perform reach-to-grasp tasks61,62. There is also resemblance in the impairments and compensatory movement patterns that result from unilateral motor system damage63–66 (FIG. 2d–f). Slower, more-variable and more-extraneous movements are observed in the paretic forelimb of rodents after motor system damage than in intact animals65,67,68. To reach for food rewards with the paretic forelimb, rodents use compensatory rotational movements of the trunk to extend and control paw position, and use compensatory trunk and head movements to bring the mouth to the paw16,63,69. If not constrained, the non-paretic limb is often used to assist paretic limb movements — for example, to support the paretic forelimb during its extension (FIG. 2f) or to help to open its digits so that food can be released into the mouth13,16. In addition, rats rely more on the non-paretic forelimb for activities such as food handling70,71 and postural support during exploration72.
Impairments in fine manual dexterity have been characterized in monkeys after focal motor system damage73,74 — for example, in tasks in which food rewards are most efficiently retrieved using a precision grip. After ischaemic lesions of the digit region of the primary motor cortex (M1)75,76 or of the posterior limb of internal capsule77, macaques compensate for impairments in precision grip by scooping food rewards with one or more digits towards the palm or proximal thumb. However, these movement strategies can change with task practice, as described below.
Learning to compensate
Compensation is likely to begin whenever a stroke survivor first attempts to perform an activity with the paretic body side in the normal way that they did before the stroke, at which point it becomes evident that the normal way no longer works. That is, compensation can be expected to begin with the resumption of movement very early after stroke, but the process of becoming adept in the new ways of moving involves motor skill learning, which is practice dependent78. A right-hander can manage to eat and dress using only the left hand, but the speed and efficiency in doing so is likely to improve over time with practice. Even long after stroke, new compensatory movement patterns can be developed in response to motor training10.
Learned non-use.
The counterpart of the compensatory strategy of relying mostly on the non-paretic hand is disuse of the paretic upper limb. This disuse has been proposed to result from ‘learned non-use’, whereby repeated experience with the weakness and ineptitude of stroke-impaired functions encourages their disuse8. This idea was originally based on observations in macaque monkeys with peripheral sensory deafferentation of one arm, which the monkeys continued to disuse even after its capacity to move returned79. Findings in rats suggest that paretic limb disuse develops with repeated experiences in attempting to use it. Most rats have a strong preference for performing unimanual skilled reaching tasks with one limb. If this limb becomes paretic owing to large motor cortical or mixed cortical–subcortical lesions, rats continue to attempt to retrieve food rewards with it at first80. However, after repeated failures, they start attempting to retrieve rewards with the other limb. With continued practice, most rats with substantial injuries completely switch ‘handedness’ for reaching80,81. Even rats that are not allowed to switch limbs (FIG. 2) transiently reduce their attempts to retrieve food rewards with the paretic limb after initial attempts are unsuccessful82.
In people who have survived stroke, the reliance on the trunk and scapula to control the position of the paretic hand can also encourage the disuse of more-distal movements of the paretic upper limb. Stroke survivors with moderate impairments who practise a reaching task with the paretic upper limb while the trunk is restrained regain more-normal elbow movement during arm extension, whereas those who do so without trunk restraint increase their reliance on compensatory trunk movements instead83.
Training-induced compensation.
The compensatory movement patterns that develop after stroke can be self-taught or encouraged by interventions. Stroke rehabilitation is focused on improving functional abilities, and this improvement can be achieved through either the development of new compensatory strategies or the recovery of more-normal function. The relative contribution of the two varies with impairment level, with compensation making a larger contribution in those with more severe impairment42,84, and with different rehabilitation approaches — for example, depending on the extent of guided movement and the restriction of compensatory strategies83,85,86. However, most motor rehabilitation approaches are focused on successful task completion, which is permissive of compensation. In fact, some interventions encourage reliance on the non-paretic hand to perform basic daily tasks, such as dressing87,88.
Rehabilitation efficacy can depend on compensation even when use of the non-paretic arm is restricted. Constraint-induced movement therapy (CIMT) involves constraint of the non-paretic arm during most waking hours while the paretic side undergoes intense task-oriented practice. CIMT has mostly been studied in stroke survivors who have some residual capacity to move the wrist and fingers. In one such study89, CIMT improved performance on the Action Research Arm Test (which does not discriminate between recovery and compensation) but led to no notable improvements in measures of movement quality and impairment recovery. Several studies have found that CIMT improves the speed, efficiency and smoothness of paretic arm movements90–92, but one of these studies92 found that it does so without diminishing reliance on compensatory trunk movements; in fact, reliance on such movements was increased after CIMT. Together, these findings suggest that CIMT may promote the refinement of compensatory movement strategies that improve the functional capacity of the paretic arm.
Several studies in rodent models of stroke have found that a few weeks of daily training of the paretic forelimb in skilled reaching tasks can improve performance (as measured by the successful retrieval of food rewards), even when abnormalities in the paretic limb movements that are used to perform the task persist, suggesting that the improvements result from compensatory strategies68,69,93,94. In rats with substantial motor cortical infarcts, the performance improvements were greatly diminished if the non-paretic forelimb was kept in a sling during daily training, indicating that assistive movements of this limb probably contributed to the improvements16. After smaller motor cortical infarcts that result in less-severe impairments, extensive training of the paretic limb in skilled reaching tasks promoted the normalization of some movements, whereas abnormalities in others — including wrist movements — persisted16,95.
Findings in macaques with small M1 lesions indicate that the relative contributions of compensation and recovery to motor performance improvements can depend on the amount of task practice96. M1 lesions impaired the performance of a precision grip task, but with daily post-lesion training on the task, the monkeys initially improved their performance using an alternative grip pattern that involved holding the food between the index fingertip and the proximal thumb. Over the course of weeks of daily training, the opposition moved more distally on the thumb surface, and eventually the original precision grip pattern (fingertip to thumb tip) returned. By contrast, there was no return of the original precision grip pattern in monkeys that received no training over the same time span. Thus, practising compensatory strategies that sufficiently approximate the original movement pattern might encourage the recovery of the original movement pattern.
Compensation-driven neural plasticity
Compensation involves learning and can be expected to depend on learning-associated neural plasticity mechnisms, but it begins while the brain is also undergoing widespread repair, regrowth and remodelling of the connectivity patterns of surviving neurons after stroke. Learning-related neural changes can interact with these regenerative responses to stroke13.
Regenerative responses to stroke.
The degeneration of neurons and their processes during and after a stroke instigates widespread regenerative counter-reactions, the end result of which is a reorganization of the synaptic connectivity patterns of surviving neurons. Axonal degeneration is a trigger for remaining axons near denervated regions to sprout new collaterals and to form synapses with newly denervated neurons97–99. This is accompanied by dendritic restructuring100–102 and highly coordinated changes in glia and vasculature13,103,104, as reviewed elsewhere105,106. This regenerative process proceeds in many different brain regions to variable degrees and follows various time courses, depending on the anatomical connectivity of the injured region107. Tissue near the core region of damage, and all areas that previously had substantial direct afferent or efferent projections to or from the damaged area, can be expected to undergo regenerative responses, such that even focal stroke results in widespread and bihemispheric changes in synaptic connectivity patterns. These regenerative processes occur over long time spans (months or longer), but they are particularly dynamic early (days to weeks) after stroke105,108. The resulting alterations in synaptic connectivity patterns presumably contribute to clinical observations of altered intrahemispheric and interhemispheric inhibitory and excitatory activity109–111, and changes in functional activation, as detected by, for example, functional MRI112,113.
Effects of experience on regenerative responses.
Neural, as well as glial and vascular, remodelling responses to stroke are very sensitive to behavioural manipulations13. For example, in rodent studies, axonal sprouting patterns99,114,115, synaptogenesis116, dendritic growth responses117 and astrocytic reactions118 are affected by post-injury manipulation of forelimb use. This sensitivity to behavioural experience may be explained by the neural activity dependence of post-stroke regenerative responses. More-active neural pathways are more likely than less-active ones to sprout new axons that contribute to re-innervation, as supported by findings that electrical stimulation promotes, and neural activity blockade reduces, the sprouting of specific pathways119–121. Dendritic, synaptic, astrocytic122,123 and vascular124 remodelling responses all depend on neural activity. Thus, it seems reasonable to predict that any behavioural experiences that sufficiently alter activity in circuits undergoing remodelling will influence the resulting pattern of neural connectivity.
Effects of compensation with the non-paretic limb.
Compensation with the non-paretic upper limb has a strong potential to influence neural remodelling responses after stroke, given that it is practised often7 and begins to develop very early after stroke9, when regenerative responses are likely to be particularly dynamic. Findings in rodent models of post-stroke upper-extremity impairments support this possibilty (FIG. 3). Unilateral lesions in the forelimb region of M1 in rats result in compensatory reliance on the non-paretic forelimb, as well as a robust amount of dendritic growth and synapse proliferation in the contralesional homotopic cortex72,125. Neuronal growth responses in the contralesional M1 are initiated by the degeneration of transcallosal projections from the opposite M1, making the contralesional M1 a point of convergence for regenerative responses and for the influence of behavioural compensation with the non-paretic forelimb. A series of studies established that neuronal growth responses in the contralateral M1 do in fact result from this convergence, as reviewed elsewhere126. For example, forcing the use of one forelimb, to mimic compensatory reliance on one limb alone, or transecting callosal fibres, to reproduce the degenerative signals alone, each results in only subtle dendritic changes in the motor cortex101,127. By contrast, forced use of one forelimb after callosal transection results in a dendritic growth response in the M1 contralateral to this forelimb that resembles the response found contralateral to unilateral M1 lesions.
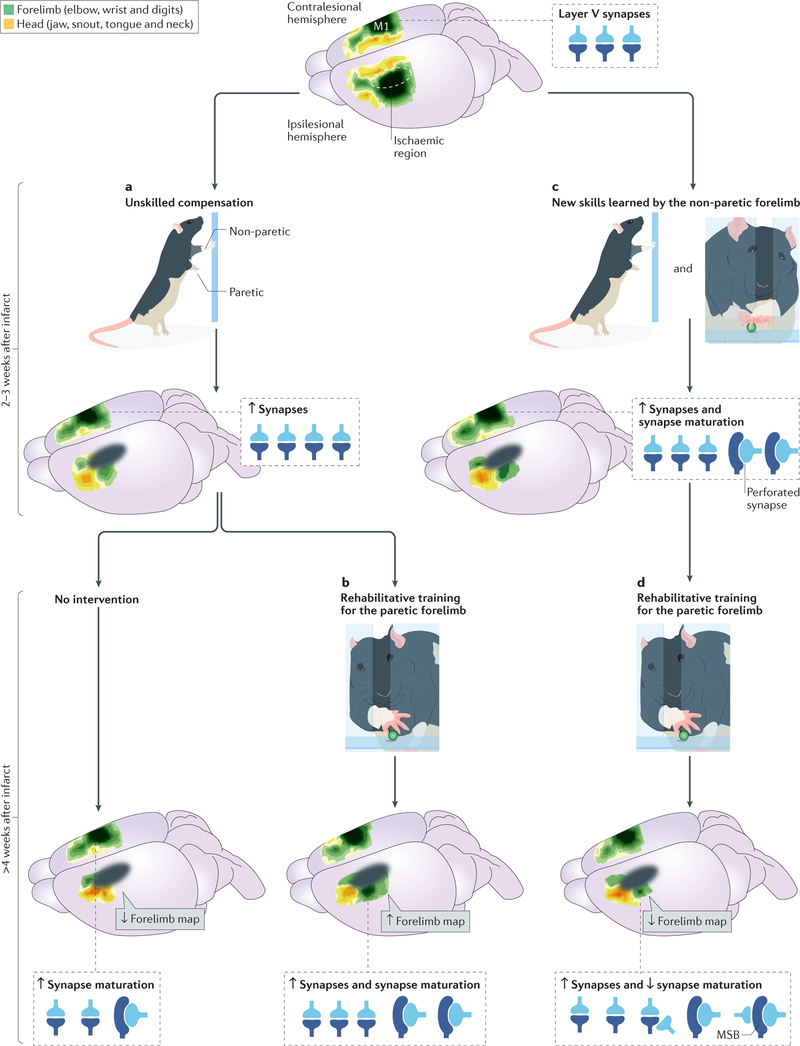
Figure 3 |. Different trajectories of cortical reorganization depending on forelimb behavioural experiences after motor cortical infarcts in rodent models.
Rodents in a standard laboratory cage have limited opportunity to practice fine motor skills with the forelimbs, affording a strong degree of experimental control over these experiences. a | In the absence of interventions, increased reliance on the non-paretic forelimb for movements in the home cage (known as unskilled compensation) promotes synapse addition and selective synapse maturation in the contralesional primary motor cortex (M1)126. These changes in the contralesional cortex have no known functional relevance for the paretic limb. b | The changes in the contralesional cortex are amplified if animals are trained with the non-paretic forelimb on a skilled reaching task that is novel for this limb129. The same training worsens impairment severity in the paretic forelimb145. c | Rehabilitative training of the paretic forelimb in a skilled reaching task promotes the maintenance and re-emergence of the remaining forelimb motor maps in the peri-infarct M1 and improves paretic limb reaching performance131,132. The performance improvements can reflect the recovery of more-normal movements, the refinement of compensatory movements or both16. d | The performance improvements and forelimb motor map maintenance that result from rehabilitative training are diminished as a result of prior skill learning with the non-paretic forelimb116. This is associated with the addition of multisynaptic boutons (MSBs) in the peri-infarct M1, which may reflect competitive influences of the two limbs on synapse addition and maturation in the peri-infarct cortex. The motor cortical map illustrations on the top and bottom-right brains are adapted with permission from REF. 116, Society for Neuroscience.
Regenerative reactions also influence behavioural change. As explained above, the neuronal growth response to increased reliance on one forelimb is amplified in the corresponding M1 shortly after the transection of its transcallosal afferents101,127. The neuronal growth response in the contralesional M1 to skill training of the non-paretic limb is similarly amplified, and this, in turn, is reflected in an increase in the learning capacity for this limb. Despite the presence of subtle motor impairments in the non-paretic forelimb, animals with M1 injuries show an accelerated and augmented acquisition of skilled reaching tasks with this limb compared with sham-operated animals128,129. Thus, regenerative reactions can facilitate the development of compensatory strategies involving the use of the non-paretic limb.
Effects of paretic limb compensation?
Unlike the non-paretic limb, movements of the paretic limb are not normally well practised during the early periods of neural remodelling. However, there has been much interest in initiating rehabilitative interventions earlier after stroke, to capitalize on the pro-growth environment of the early remodelling period13–15,108. Training the paretic forelimb in skilled motor tasks can promote new dendrite and synapse formation in peri-infarct motor cortex in rats117 and can induce the reorganization of surviving movement representations of the paretic forelimb in residual motor cortex of rodents and monkeys116,130–133 (FIG. 3). In the chronic period (>3 months) after stroke in humans, CIMT increases the size of motor cortical output maps of the paretic hand in the injured hemisphere, as assessed using transcranial magnetic stimulation (TMS) mapping134,135. These neural substrates of rehabilitative training-induced improvements may be facilitated in the early pro-neuronal growth environment12,99. Consistent with this, animal and clinical findings indicate that earlier post-stroke onsets of motor rehabilitative training tend to be more effective in improving behavioural function on the paretic side136–138 and in promoting reorganization of peri-infarct motor cortex139 than do later ones. However, whether earlier interventions promote greater recovery, or simply more-effective compensation, has not yet been determined. Given that compensation is often a major contributor to motor training-induced performance improvements after stroke, it is reasonable to suspect that the time sensitivity of its efficacy could reflect time sensitivity in learning effective ways of compensating, rather than, or in addition to, recovery.
At present, there is not a precise understanding of the distinction between the neural mechanisms of practice-dependent improvements in the performance of the paretic upper limb that are due to the recovery of more-normal movement versus those that are due to the development of compensatory strategies. In squirrel monkeys with focal M1 infarcts, improvements in the performance of the paretic hand in a rehabilitative training task were due to the establishment of new movement patterns in some monkeys and to the recovery of the original movement patterns in others73. There was not a discernible difference in the reorganization of the remaining M1 movement representations of the paretic forelimb between the two groups. However, the compensatory strategies included those that could be considered to be normal for the task, despite not being the monkeys’ originally preferred strategies. It is conceivable that the neural correlates of subtler forms of compensation are nearly indistinguishable from those of recovery. The strategy described above in which macaques grasp food rewards between the fingertip and the mid-thumb rather than the thumb tip96 could be considered a subtler form of compensation than are strategies such as using trunk movements to extend and rotate the paw (FIG. 2). The use of trunk movements to control hand position presumably involves neural changes that are quite distinct from those of the recovery of more-normal elbow and wrist movements. Nevertheless, the distinction of their mechanisms from those of training-dependent recovery has not yet received prominent research attention in animal models. One obstacle is that it is currently very challenging to precisely quantify movement patterns in manual skill tasks in rodents, which are the most popular model species for chronic stroke (BOX 2). Approaches that are in development for automated measurement of such movement patterns140,141 hold promise for remedying this situation.
Box 2 | Distinguishing motor recovery from compensation in rodent models.
Most of the popular measures of motor function in rodent models of stroke do not distinguish between recovery and compensation. This includes grid-walking, ladder-walking and beam-walking tests, skilled reaching tests and rotarod tests, as they are normally performed. The gold standard for distinguishing motor recovery from compensation is to perform kinematic (geometry of movement) analyses65,68,140, but this is currently challenging to do routinely (because the skin markers that are commonly used in kinematic systems to automatically track joint positions do not work well on rodent forelimbs). Automated measures of rodent movement that are in development140,141 may help to overcome this obstacle. In the meantime, there are several other ways to probe for the presence of impairments and compensation. Alaverdashvili and Whishaw93 have established qualitative measures of the movements used in reaching tasks, and these measures can be used to infer whether performance improvements reflect recovery from impairment or compensation93. Some behavioural tests probe for impairments by measuring the use of compensatory strategies. The ledged tapered beam test reveals limb impairment when animals resort to stepping down to a wider ledge (compensation) as they traverse a progressively tapering beam187. The Schallert cylinder test, in which the animal is placed into a transparent cylinder where they rear and support themselves with their forepaws, reveals impairment in one forelimb by measuring the compensatory reliance on the other forelimb for upright exploratory movements188. Similarly, the vermicelli-handling test reveals a preferential reliance on the movements of one forepaw to move a long pasta piece into the mouth71. The contribution of the non-paretic forelimb in performance improvements can also be probed by retesting the animals after this limb has been constrained16 or peripherally anaesthetized128,189. For example, if peripheral anaesthetization of the non-paretic forelimb reinstates impairments of the paretic limb in a grid-walking task, whereas the same manipulation in intact animals has little effect, the pre-anaesthetization performance improvements are inferred to be dependent on the non-paretic limb and thus compensatory.
Maladaptive compensation
It is tempting to count any means of resuming more-normal life activities after stroke as a victory, but relying on compensatory strategies can come with costs. The strategies of relying mostly on the non-paretic hand and of relying on proximal body movements instead of distal arm movements can encourage the disuse of any residual capacity for a greater range of paretic limb movements that might be achieved with practice to enable better overall functionality47,67,142. Such forms of compensation could be the best option for those with the most-severe impairments; for those with milder impairments, however, they are unlikely to be. Nevertheless, even stroke survivors who have a good functional capacity in the paretic limb, as measured in the laboratory, persist in disusing the paretic limb in everyday life143. Given that compensatory strategies that counter the remaining capacity for better overall functionality can be considered to be maladaptive, why do such strategies persist? As described next, one culprit underlying the persistence of maladaptive compensation could be the neural plasticity that is driven by it.
Neural substrates of maladaptive compensation.
Recent findings from rodent studies suggest that some of the neural changes that are driven by compensatory reliance on the non-paretic forelimb counter improvements in the function of the paretic side. This was revealed in studies that modelled the effects of learning to compensate with the non-paretic forelimb by training this limb daily in skilled reaching tasks, beginning between 4 days to 3 weeks after subtotal M1 infarcts11,116 (FIG. 3). Rats that received a brief (10–15-day) period of daily training of the non-paretic forelimb showed exacerbated impairments, and disuse of, the paretic forelimb144,145, and diminished performance improvements in subsequent rehabilitative training of the paretic forelimb145–147 compared with rats that received non-training control procedures during the non-paretic limb training period. The worsening of paretic limb performance lasts for at least 2 months after non-paretic forelimb training ceases148. By contrast, bilateral-reach training (that is, training of each limb in alternating trials of the same sessions) does not exacerbate impairments or diminish improvements in the performance of the paretic side146.
In the absence of prior training of the non-paretic forelimb, motor skill training of the paretic forelimb improves its performance and increases its movement representation area in the peri-infarct motor cortex116,130–133. Both effects are greatly reduced in animals that receive a period of non-paretic forelimb training before the onset of rehabilitative training of the paretic limb, even though the number of attempts to use the paretic limb in the rehabilitation task is not diminished116. Thus, a well-established correlate of improvement in the performance of the paretic side — the reorganization of movement representations — is disrupted by learning new ways of using the non-paretic forelimb, at least after strokes involving M1. It remains to be determined whether these effects generalize to other injury loci.
The effects of learning new skills with the non-paretic forelimb on the reorganization of motor maps in the peri-infarct cortex may reflect experience-dependent competition in the reorganization of synaptic connectivity in this region116. Motor skill training normally promotes the addition and maturation of synapses in the M1 contralateral to the trained limb149–151. The maturation of synapses is reflected by the proliferation of a mature and efficacious subtype of excitatory synapses known as perforated synapses152. Training the non-paretic forelimb also promotes synapse addition in the peri-infarct M1 but not the addition of perforated synapses. Instead, it increases the formation of synapses by multisynaptic boutons (MSBs)116. The presence of MSBs may reflect ongoing competition for survival between synapses153 and, in this case, between synapses that were created in response to the experiences of the non-paretic limb versus those created in response to experiences of the paretic limb. If so, and given that callosal transections in rats attenuate the deleterious effects of training the non-paretic forelimb154, it seems possible that projections from the contralesional motor cortex are a source of at least some of the competing synapses. The broader implication is that the experiences of either forelimb may ‘compete’ with one another in shaping neural reorganization patterns after stroke.
Potential clinical implications.
The findings discussed above have the disturbing implication that the neural mechanisms of improved function in the paretic side are typically subverted by the usual response to hemiparesis of learning to rely on the non-paretic upper limb. The findings also raise the possibility that analogous mechanisms of synaptic competition contribute to the persistence of suboptimal compensatory movement strategies that involve using the paretic body side. For example, an over-reliance on trunk movements to extend the arm and control hand position might shape synaptic connectivity in the motor cortex in a manner that interferes with new connections that could support a greater range of movement of the elbow and wrist. If so, given the sensitivity of neural remodelling responses to behavioural experience, it could be particularly deleterious for such strategies to be well practised in the early post-stroke period, when the neural changes can be amplified by the early pro-neuronal-growth environment.
Roles of the contralesional hemisphere
There has been much recent debate about the role of the contralesional hemisphere in functional outcome after stroke. Various clinical studies suggest that the contralesional hemisphere may contribute to function of the paretic side, or may be disruptive of this function, depending on the severity of damage to the corticospinal system, the time since stroke and other factors, as reviewed elsewhere113,155–159. For example, in support of disruptive influences, the contralesional M1 can exert an excessive inhibitory influence over the ipsilesional hemisphere during paretic hand movements, as detected using paired-pulse protocols with TMS155,160,161. Manipulations such as low-frequency TMS that reduce excitability or disrupt activity in the contralesional cortex improve the performance of the paretic hand in motor tasks in some patients155,162–164, but there is considerable variability in this effect165–167. In addition, disrupting the contralesional M1 or premotor cortex during the performance of more-complex motor tasks with the paretic hand disrupts the timing of movement with the paretic hand more so than it does in healthy controls168, suggesting that the contralesional hemisphere positively contributes to paretic hand function. Similarly contradictory roles of the contralesional hemisphere can be found in the animal literature112,169–171.
Contralesional contributions to re-innervation.
The contralesional motor cortex contributes to the reinnervation of regions denervated by stroke. Many studies in animal models of stroke have found that axonal projections of neurons in the contralesional motor cortex can sprout crossed collaterals to form synapses in denervated regions of the spinal cord, red nucleus, striatum, peri-infarct cortex and other regions, as recently reviewed elsewhere106. This promotes the formation of new connections in regions that could give the contralesional hemisphere greater control over movements of the paretic side157. Several studies in rodent models have used gain-of-function and loss-of-function manipulations that support causal relationships between sprouting from the contralesional motor cortex and improvements in motor function, including the recovery of more-normal paretic forelimb movement172 and reduced compensatory reliance on the non-paretic forelimb172–174. For example, after large motor cortical infarcts, treatment with an antibody that targets the axon-growth inhibitor Nogo-A (also known as reticulon 4A) increases sprouting from the spared corticospinal tract into the denervated spinal cord, and promotes improved performance and the recovery of more-normal movements of the paretic forelimb in a skilled reaching task172. Transections of the spared corticospinal tract reinstate forepaw movement abnormalities172. Surviving cortical neurons of the injured hemisphere can also contribute to the re-innervation of the same targets97,98,115. In macaques, the sprouting of ipsilesional and contralesional projections into denervated regions of the spinal cord has been found to vary depending on cortical lesion size and territory175. The relative importance of the two sources of re-innervation has not been thoroughly dissected. However, some findings suggest that, given a sufficient remaining capacity for ipsilesional projections to contribute to re-innervation, the contralesional cortex may be the less relevant source of these projections for the function of the paretic limb97,98.
Potential influence of compensation.
There is no debate about the role of the contralesional cortex in controlling compensatory movements, including those of the non-paretic hand and trunk. However, the possibility that its involvement in doing so could be a source of variability in how it contributes to paretic limb function has not received very much attention. Bilateral patterns of cortical functional activation113 and interhemispheric inhibition176 during paretic hand movement vary with the severity of motor impairments. That behavioural experiences can influence these patterns is supported by findings that rehabilitative training of the paretic upper limb can increase the functional activation of motor regions of the ipsilesional cortex177,178 and reduce interhemispheric inhibition from the contralesional motor cortex179. People with more-severe motor impairments rely more heavily on compensatory strategies42,84. This leaves open the possibility that the experience of relying on compensatory strategies contributes to impairment-related variance in cortical activation and inhibition patterns.
As reviewed above, the use of compensatory movement strategies can influence neuroanatomical reorganization of the contralesional cortex: compensatory reliance on the non-paretic limb promotes the remodelling of dendrites and synapses in the contralesional motor cortex of rats126. In humans after stroke, the use of compensatory shoulder abduction during simulated eating movements correlates with functional activation of contralesional cortex during these movements180. This involvement of the contralesional motor cortex in compensatory movements, and the neuroanatomical changes that result from using such movements, might alter the potential of the contralesional motor cortex to make adaptive contributions to movements of the paretic side. This possibility awaits research attention.
Perspectives and challenges
The extent of reorganization of remaining motor cortex and of its afferent and efferent projections has been strongly linked to improved functional capacity in the paretic upper limb, but these improvements can reflect recovery, compensation or both. Interventions such as motor rehabilitative training that drive motor cortical reorganization promote varying degrees of recovery of more-normal movement and more-effective compensatory movement in the paretic limb. There is not currently a clear distinction between the neural mechanisms of the recovery that can be promoted by motor rehabilitative training and those of the compensatory strategies that can be encouraged by the same training.
Compensation is often viewed as a suboptimal counterpart to recovery, but compensatory strategies for moving the paretic upper limb can contribute to improvements in its functional capacity. Nevertheless, there are more-optimal and less-optimal ways of compensating. There is clear evidence that reliance on the trunk to move the paretic arm can counter the recovery of more-normal arm movement in individuals with moderate impairments83. Such compensation may be the best option after strokes that leave no remaining capacity for the recovery of more-normal movements; however, the ‘cutoff point’ for the absence of such capacity is unclear at present.
Dominant reliance on the non-paretic upper limb is a particularly troublesome compensatory strategy. It encourages the disuse of the paretic side and thus limits the return of more-functional use, through recovery or compensation. Rodent studies have found that learning new ways of using the non-paretic forelimb promotes synaptic changes that interfere, and probably compete, with the ipsilesional motor cortical changes that could otherwise contribute to functional improvements on the paretic side. This may help to explain the persistence of paretic limb disuse, even in individuals with relatively modest impairments. Encouraging damaged neural circuits to change in a manner that subserves better function on the paretic side is challenging enough. It cannot help if the same circuits have already been driven to change in a manner that impedes further change.
The important role of the contralesional hemisphere in mediating compensatory strategies is not currently a central topic in the ongoing debates about the role of the contralesional hemisphere in paretic limb function. Given the findings reviewed above, it seems reasonable to suspect that exactly how the contralesional hemisphere contributes to, or interferes with, paretic hand and arm function varies greatly depending on how involved it has already become in compensating for the loss of this function. The inter-relationship between impairment severity and reliance on compensation poses a challenge to the investigation of this possibility in clinical populations, but it is quite feasible to study through the manipulation of behavioural experiences in animal models101,114,115,145.
Finally, compensation has not received the amount of consideration in animal models of CNS injury that would seem to be warranted by its reliable clinical manifestations. This effort would benefit from further refinement of measures of compensation in rodent models (BOX 2). The findings of potent and lasting influences of compensatory behavioural experiences on post-stroke brain reorganization and behavioural outcome further motivate research into compensation. They suggest that new treatments for injury-induced impairments may lie within the neural mechanisms and influences of compensatory behaviour, awaiting discovery.
Callosotomy
The surgical procedure of severing the corpus callosum at the midline.
Non-paretic
Refers to the side of the body that is ipsilateral to the stroke.
Paretic
Showing weakness, ineptitude and partial loss of voluntary movement. Usually most severe on the side of the body that is contralateral to the stroke.
Rehabilitation
A treatment designed to improve functional capacities. It includes physical therapy for motor impairments.
Precision grip
Grasp between the tips of the thumb and a finger.
Motor skill learning
The practice-dependent refinement of novel movement sequences.
Action Research Arm Test
A test that measures the ability to perform various actions with the paretic upper limb, such as grasping differently sized objects and pouring water from a glass.
Homotopic
Describes an anatomical region corresponding to that of the other hemisphere or body side.
Movement representations
The territories in the motor cortex that control discrete movements, such as of the wrist or of a digit. They are usually defined on the basis of movements evoked by electrical stimulation and are also known as a ‘motor map’.
Perforated synapses
Synapses with discontinuous postsynaptic densities that are on average larger and more potent in evoking postsynaptic depolarizations than are other synapses.
Multisynaptic boutons (MSBs).
Single boutons that form synapses with more than one postsynaptic dendritic surface.
Paired-pulse protocols
Tests for the facilitation or depression of a stimulation-evoked neural response due to a preceding stimulation.
Crossed collaterals
Branches of an axon that cross the midline to terminate in the hemisphere opposite to that of the originating axon.
Acknowledgements
The author is supported by NS056839 and NS078791. The author thanks the Bergeron Family Foundation (Vermont, USA) for enabling experiences at the Georgetown University (Washington DC, USA) and the US National Rehabilitation Hospital Center for Brain Plasticity and Recovery (Washington DC, USA) that informed the content of this Review, and S. K. Subramanian for helpful suggestions on figure 2a.
Footnotes
Competing interests statement
The author declares no competing interests.
References
- 1.Gazzaniga MS Forty-five years of split-brain research and still going strong. Nat. Rev. Neurosci 6, 653–659 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Bahnemann M et al. Compensatory eye and head movements of patients with homonymous hemianopia in the naturalistic setting of a driving simulation. J. Neurol 262, 316–325(2015). [DOI] [PubMed] [Google Scholar]
- 3.Hagoort P, Wassenaar M & Brown C Real-time semantic compensation in patients with agrammatic comprehension: electrophysiological evidence for multiple-route plasticity. Proc. Natl Acad. Sci. USA 100, 4340–4345 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin VL et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology 45, 161–176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feigin VL et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 383, 245–254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayo NE et al. Disablement following stroke. Disabil. Rehabil 21, 258–268 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Bailey RR, Klaesner JW & Lang CE Quantifying real-world upper-limb activity in nondisabled adults and adults with chronic stroke. Neurorehabil. Neural Repair 29, 969–978 (2015).This study provides a clear demonstration, based on data from accelerometers worn on the wrists, of how dramatically stroke alters the use of the paretic and non-paretic hands.
- 8.Taub E, Uswatte G & Mark VW The functional significance of cortical reorganization and the parallel development of CI therapy. Front. Hum. Neurosci 8, 396 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakayama H, Jørgensen HS, Raaschou HO & Olsen TS Compensation in recovery of upper extremity function after stroke: the Copenhagen Stroke Study. Arch. Phys. Med. Rehabil 75, 852–857 (1994). [DOI] [PubMed] [Google Scholar]
- 10.Levin MF, Kleim JA & Wolf SL What do motor ‘recovery’ and ‘compensation’ mean in patients following stroke? Neurorehabil. Neural Repair 23, 313–319 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Jones TA et al. Motor system plasticity in stroke models: intrinsically use-dependent, unreliably useful. Stroke 44, S104–S106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeiler SR & Krakauer JW The interaction between training and plasticity in the poststroke brain. Curr. Opin. Neurol 26, 609–616 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allred RP, Kim SY & Jones TA Use it and/or lose it — experience effects on brain remodeling across time after stroke. Front. Hum. Neurosci 8, 379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahl A-S & Schwab ME Finding an optimal rehabilitation paradigm after stroke: enhancing fiber growth and training of the brain at the right moment. Front. Hum. Neurosci 8, 381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dromerick AW et al. Critical periods after stroke study: translating animal stroke recovery experiments into a clinical trial. Front. Hum. Neurosci 9, 231 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whishaw IQ Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology 39, 788–805 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Krakauer JW Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol 19, 84–90 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Puig J et al. Decreased corticospinal tract fractional anisotropy predicts long-term motor outcome after stroke. Stroke 44, 2016–2018 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Sterr A, Dean PJA, Szameitat AJ, Conforto AB & Shen S Corticospinal tract integrity and lesion volume play different roles in chronic hemiparesis and its improvement through motor practice. Neurorehabil. Neural Repair 28, 335–343(2014). [DOI] [PubMed] [Google Scholar]
- 20.Latash ML Progress in Motor Control: Structure– Function Relations in Voluntary Movements (Human Kinetics, 2002).
- 21.Twitchell TE The restoration of motor function following hemiplegia in man. Brain J. Neurol 74, 443–480 (1951). [DOI] [PubMed] [Google Scholar]
- 22.McCrea PH, Eng JJ & Hodgson AJ Saturated muscle activation contributes to compensatory reaching strategies after stroke. J. Neurophysiol 94, 2999–3008 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner JM, Dromerick AW, Sahrmann SA & Lang CE Upper extremity muscle activation during recovery of reaching in subjects with post-stroke hemiparesis. Clin. Neurophysiol 118, 164–176 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roh J, Rymer WZ, Perreault EJ, Yoo SB & Beer RF Alterations in upper limb muscle synergy structure in chronic stroke survivors. J. Neurophysiol 109, 768–781 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roby-Brami A, Fuchs S, Mokhtari M & Bussel B Reaching and grasping strategies in hemiparetic patients. Motor Control 1, 72–91 (1997). [Google Scholar]
- 26.Lang CE et al. Deficits in grasp versus reach during acute hemiparesis. Exp. Brain Res 166, 126–136 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Nowak DA The impact of stroke on the performance of grasping: usefulness of kinetic and kinematic motion analysis. Neurosci. Biobehav. Rev 32, 1439–1450 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Alt Murphy M, Willén C & Sunnerhagen KS Responsiveness of upper extremity kinematic measures and clinical improvement during the first three months after stroke. Neurorehabil. Neural Repair 27, 844–853 (2013). [DOI] [PubMed] [Google Scholar]
- 29.van Dokkum L et al. The contribution of kinematics in the assessment of upper limb motor recovery early after stroke. Neurorehabil. Neural Repair 28, 4–12 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Foroud A & Whishaw IQ Reaching-to-eat in humans post-stroke: fluctuating components within a constant pattern. Behav. Neurosci 124, 851–867 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Nowak DA et al. Dexterity is impaired at both hands following unilateral subcortical middle cerebral artery stroke. Eur. J. Neurosci 25, 3173–3184 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Kaeser M et al. Effects of unilateral motor cortex lesion on ipsilesional hand’s reach and grasp performance in monkeys: relationship with recovery in the contralesional hand. J. Neurophysiol 103, 1630–1645 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Bowden JL, Taylor JL & McNulty PA Voluntary activation is reduced in both the more- and less- affected upper limbs after unilateral stroke. Front. Neurol 5, 239 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart JC, Gordon J & Winstein CJ Control of reach extent with the paretic and nonparetic arms after unilateral sensorimotor stroke II: planning and adjustments to control movement distance. Exp. Brain Res 232, 3431–3443 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Nielsen JB, Crone C & Hultborn H The spinal pathophysiology of spasticity — from a basic science point of view. Acta Physiol. (Oxf.) 189, 171–180 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Levin MF, Selles RW, Verheul MH & Meijer OG Deficits in the coordination of agonist and antagonist muscles in stroke patients: implications for normal motor control. Brain Res 853, 352–369 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Torre K et al. Somatosensory-related limitations for bimanual coordination after stroke. Neurorehabil. Neural Repair 27, 507–515 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Borich MR, Brodie SM, Gray WA, Ionta S & Boyd LA Understanding the role of the primary somatosensory cortex: opportunities for rehabilitation. Neuropsychologia 79, 246–255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer S et al. Voxel-based lesion-symptom mapping of stroke lesions underlying somatosensory deficits. Neuroimage Clin 10, 257–266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung VCK et al. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl Acad. Sci. USA 109, 14652–14656 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SW, Triandafilou K, Lock BA & Kamper DG Impairment in task-specific modulation of muscle coordination correlates with the severity of hand impairment following stroke. PLoS ONE 8, e68745 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cirstea MC & Levin MF Compensatory strategies for reaching in stroke. Brain 123, 940–953 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Levin MF, Michaelsen SM, Cirstea CM & Roby-Brami A Use of the trunk for reaching targets placed within and beyond the reach in adult hemiparesis. Exp. Brain Res 143, 171–180 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Buma F, Kwakkel G & Ramsey N Understanding upper limb recovery after stroke. Restor. Neurol. Neurosci 31, 707–722 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Shaikh T, Goussev V, Feldman AG & Levin MF Arm–trunk coordination for beyond-the-reach movements in adults with stroke. Neurorehabil. Neural Repair 28, 355–366 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Michaelsen SM, Jacobs S, Roby-Brami A & Levin MF Compensation for distal impairments of grasping in adults with hemiparesis. Exp. Brain Res 157, 162–173 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Levin MF, Liebermann DG, Parmet Y & Berman S Compensatory versus noncompensatory shoulder movements used for reaching in stroke. Neurorehabil. Neural Repair 30, 635–646 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Kwakkel G, Kollen B & Lindeman E Understanding the pattern of functional recovery after stroke: facts and theories. Restor. Neurol. Neurosci 22, 281–299 (2004). [PubMed] [Google Scholar]
- 49.Latash ML Motor synergies and the equilibrium- point hypothesis. Motor Control 14, 294–322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang CE, Wagner JM, Edwards DF, Sahrmann SA & Dromerick AW Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabil. Neural Repair 20, 444–454 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Raghavan P, Santello M, Gordon AM & Krakauer JW Compensatory motor control after stroke: an alternative joint strategy for object- dependent shaping of hand posture. J. Neurophysiol 103, 3034–3043 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tretriluxana J, Gordon J, Fisher BE & Winstein CJ Hemisphere specific impairments in reach-to-grasp control after stroke: effects of object size. Neurorehabil. Neural Repair 23, 679–691 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Schaefer SY, DeJong SL, Cherry KM & Lang CE Grip type and task goal modify reach-to-grasp performance in post-stroke hemiparesis. Motor Control 16, 245–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sterr A, Freivogel S & Schmalohr D Neurobehavioral aspects of recovery: assessment of the learned nonuse phenomenon in hemiparetic adolescents. Arch. Phys. Med. Rehabil 83, 1726–1731 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Han CE et al. Quantifying arm nonuse in individuals poststroke. Neurorehabil. Neural Repair 27, 439–447 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rinehart JK, Singleton RD, Adair JC, Sadek JR & Haaland KY Arm use after left or right hemiparesis is influenced by hand preference. Stroke 40, 545–550 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Haaland KY et al. Relationship between arm usage and instrumental activities of daily living after unilateral stroke. Arch. Phys. Med. Rehabil 93, 1957–1962 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Dickstein R, Heffes Y, Laufer Y & Ben-Haim Z Activation of selected trunk muscles during symmetric functional activities in poststroke hemiparetic and hemiplegic patients. J. Neurol. Neurosurg. Psychiatry 66, 218–221 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carr LJ, Harrison LM & Stephens JA Evidence for bilateral innervation of certain homologous motoneurone pools in man. J. Physiol 475, 217–227 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossi E, Mitnitski A & Feldman AG Sequential control signals determine arm and trunk contributions to hand transport during reaching in humans. J. Physiol 538, 659–671 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whishaw IQ, Pellis SM & Gorny BP Skilled reaching in rats and humans: evidence for parallel development or homology. Behav. Brain Res 47, 59–70 (1992). [DOI] [PubMed] [Google Scholar]
- 62.Sacrey L-AR, Alaverdashvili M & Whishaw IQ Similar hand shaping in reaching-for-food (skilled reaching) in rats and humans provides evidence of homology in release, collection, and manipulation movements. Behav. Brain Res 204, 153–161 (2009).This study establishes that there are major homologies between humans and rats in the upper-limb movements used in reach-to-grasp tasks.
- 63.Whishaw IQ, Pellis SM, Gorny BP & Pellis VC The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, videorecording, and movement notation analysis. Behav. Brain Res 42, 77–91 (1991). [DOI] [PubMed] [Google Scholar]
- 64.Whishaw IQ, Gorny B & Sarna J Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav. Brain Res 93, 167–183 (1998). [DOI] [PubMed] [Google Scholar]
- 65.Braun RG, Andrews EM & Kartje GL Kinematic analysis of motor recovery with human adult bone marrow-derived somatic cell therapy in a rat model of stroke. Neurorehabil. Neural Repair 26, 898–906 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Klein A, Sacrey L-AR, Whishaw IQ & Dunnett SB The use of rodent skilled reaching as a translational model for investigating brain damage and disease. Neurosci. Biobehav. Rev 36, 1030–1042 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Alaverdashvili M, Foroud A, Lim DH & Whishaw IQ “Learned baduse” limits recovery of skilled reaching for food after forelimb motor cortex stroke in rats: a new analysis of the effect of gestures on success. Behav. Brain Res 188, 281–290 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Lai S et al. Quantitative kinematic characterization of reaching impairments in mice after a stroke. Neurorehabil. Neural Repair 29, 382–392 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Metz GA, Antonow-Schlorke I & Witte OW Motor improvements after focal cortical ischemia in adult rats are mediated by compensatory mechanisms. Behav. Brain Res 162, 71–82 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Whishaw IQ & Coles BL Varieties of paw and digit movement during spontaneous food handling in rats: postures, bimanual coordination, preferences, and the effect of forelimb cortex lesions. Behav. Brain Res 77, 135–148 (1996). [DOI] [PubMed] [Google Scholar]
- 71.Allred RP et al. The vermicelli handling test: a simple quantitative measure of dexterous forepaw function in rats. J. Neurosci. Methods 170, 229–244 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones TA & Schallert T Use-dependent growth of pyramidal neurons after neocortical damage. J. Neurosci 14, 2140–2152 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friel KM & Nudo RJ Recovery of motor function after focal cortical injury in primates: compensatory movement patterns used during rehabilitative training. Somatosens. Mot. Res 15, 173–189 (1998). [DOI] [PubMed] [Google Scholar]
- 74.Plautz EJ, Milliken GW & Nudo RJ Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol. Learn. Mem 74, 27–55 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Murata Y et al. Effects of motor training on the recovery of manual dexterity after primary motor cortex lesion in macaque monkeys. J. Neurophysiol 99, 773–786 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Moore TL et al. Recovery from ischemia in the middle-aged brain: a nonhuman primate model. Neurobiol. Aging 33, 619.e9–619.e24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murata Y & Higo N Development and characterization of a macaque model of focal internal capsular infarcts. PLoS ONE 11, e0154752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stanley J & Krakauer JW Motor skill depends on knowledge of facts. Front. Hum. Neurosci 7, 503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knapp HD, Taub E & Berman AJ Movements in monkeys with deafferented forelimbs. Exp. Neurol 7, 305–315 (1963). [DOI] [PubMed] [Google Scholar]
- 80.Peterson GM Mechanisms of Handedness in the Rat (Johns Hopkins Press, 1934). [Google Scholar]
- 81.Döbrössy MD & Dunnett SB The effects of lateralized training on spontaneous forelimb preference, lesion deficits, and graft-mediated functional recovery after unilateral striatal lesions in rats. Exp. Neurol 199, 373–383 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Erickson CA, Gharbawie OA & Whishaw IQ Attempt-dependent decrease in skilled reaching characterizes the acute postsurgical period following a forelimb motor cortex lesion: an experimental demonstration of learned nonuse in the rat. Behav. Brain Res 179, 208–218 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Michaelsen SM, Dannenbaum R & Levin MF Task- specific training with trunk restraint on arm recovery in stroke: randomized control trial. Stroke 37, 186–192 (2006).This study finds that training stroke survivors who have moderate impairments on a reaching task while the trunk is restrained increases the range of elbow movement, whereas training without trunk restraint decreases it and instead promotes a greater reliance on trunk movement for arm extension.
- 84.Subramanian SK, Yamanaka J, Chilingaryan G & Levin MF Validity of movement pattern kinematics as measures of arm motor impairment poststroke. Stroke 41, 2303–2308 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Cirstea CM, Ptito A & Levin MF Feedback and cognition in arm motor skill reacquisition after stroke. Stroke 37, 1237–1242 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Lum PS et al. Gains in upper extremity function after stroke via recovery or compensation: potential differential effects on amount of real-world limb use. Top. Stroke Rehabil 16, 237–253 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Poole JL, Sadek J & Haaland KY Ipsilateral deficits in 1-handed shoe tying after left or right hemisphere stroke. Arch. Phys. Med. Rehabil 90, 1800–1805 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Walker CM, Walker MF & Sunderland A Dressing after a stroke: a survey of current occupational therapy practice. Br. J. Occup. Ther 66, 263–268 (2003). [Google Scholar]
- 89.Kitago T et al. Improvement after constraint-induced movement therapy recovery of normal motor control or task-specific compensation? Neurorehabil. Neural Repair 27, 99–109 (2013).Findings from this study indicate that compensation underlies the improvements in paretic upper-limb function that result from CIMT.
- 90.Wu C, Chen C, Tang SF, Lin K & Huang Y Kinematic and clinical analyses of upper-extremity movements after constraint-induced movement therapy in patients with stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil . 88, 964–970 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Caimmi M et al. Using kinematic analysis to evaluate constraint-induced movement therapy in chronic stroke patients. Neurorehabil. Neural Repair 22, 31–39 (2008). [DOI] [PubMed] [Google Scholar]
- 92.Massie C, Malcolm MP, Greene D & Thaut M The effects of constraint-induced therapy on kinematic outcomes and compensatory movement patterns: an exploratory study. Arch. Phys. Med. Rehabil 90, 571–579 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Alaverdashvili M & Whishaw IQ A behavioral method for identifying recovery and compensation: hand use in a preclinical stroke model using the single pellet reaching task. Neurosci. Biobehav. Rev 37, 950–967 (2013). [DOI] [PubMed] [Google Scholar]
- 94.O’Bryant AJ et al. Enduring poststroke motor functional improvements by a well-timed combination of motor rehabilitative training and cortical stimulation in rats. Neurorehabil. Neural Repair 30, 143–154 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moon S-K, Alaverdashvili M, Cross AR & Whishaw IQ Both compensation and recovery of skilled reaching following small photothrombotic stroke to motor cortex in the rat. Exp. Neurol 218, 145–153 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Murata Y et al. Temporal plasticity involved in recovery from manual dexterity deficit after motor cortex lesion in macaque monkeys. J. Neurosci 35, 84–95 (2015).Findings from this study indicate that, over the course of training in a fine motor skills task, the use of compensatory movement strategies can be a stage that precedes the recovery of more-normal movements.
- 97.Starkey ML et al. Back seat driving: hindlimb corticospinal neurons assume forelimb control following ischaemic stroke. Brain 135, 3265–3281 (2012).This study finds that training-induced motor performance improvements after infarcts of the forelimb region of the motor cortex in rats depend on the reorganization of the corticospinal projections of surviving neurons of the ipsilesional motor cortex (also see reference 172).
- 98.Liu Z, Zhang RL, Li Y, Cui Y & Chopp M Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke 40, 2546–2551 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carmichael ST, Kathirvelu B, Schweppe CA & Nie EH Molecular, cellular and functional events in axonal sprouting after stroke. Exp. Neurol 287, 384–394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng HW et al. Differential spine loss and regrowth of striatal neurons following multiple forms of deafferentation: a Golgi study. Exp. Neurol 147, 287–298 (1997). [DOI] [PubMed] [Google Scholar]
- 101.Adkins DL, Bury SD & Jones TA Laminar- dependent dendritic spine alterations in the motor cortex of adult rats following callosal transection and forced forelimb use. Neurobiol. Learn. Mem 78, 35–52 (2002). [DOI] [PubMed] [Google Scholar]
- 102.Vuksic M et al. Unilateral entorhinal denervation leads to long-lasting dendritic alterations of mouse hippocampal granule cells. Exp. Neurol 230, 176–185 (2011). [DOI] [PubMed] [Google Scholar]
- 103.Muramatsu R et al. Angiogenesis induced by CNS inflammation promotes neuronal remodeling through vessel-derived prostacyclin. Nat. Med 18, 1658–1664 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Hermann DM, Buga A-M & Popa-Wagner A Neurovascular remodeling in the aged ischemic brain. J. Neural Transm. (Vienna) 122 (Suppl. 1), S25–S33 (2015). [DOI] [PubMed] [Google Scholar]
- 105.Murphy TH & Corbett D Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev. Neurosci 10, 861–872 (2009). [DOI] [PubMed] [Google Scholar]
- 106.Jones TA & Adkins DL Motor system reorganization after stroke: stimulating and training toward perfection. Physiology (Bethesda) 30, 358–370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hinman JD The back and forth of axonal injury and repair after stroke. Curr. Opin. Neurol 27, 615–623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krakauer JW, Carmichael ST, Corbett D & Wittenberg GF Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil. Neural Repair 26, 923–931 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manganotti P, Acler M, Zanette GP, Smania N & Fiaschi A Motor cortical disinhibition during early and late recovery after stroke. Neurorehabil. Neural Repair 22, 396–403 (2008). [DOI] [PubMed] [Google Scholar]
- 110.Hummel FC et al. Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology 72, 1766–1772 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carmichael ST Brain excitability in stroke: the yin and yang of stroke progression. Arch. Neurol 69, 161–167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dijkhuizen RM et al. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J. Neurosci 23, 510–517 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grefkes C & Ward NS Cortical reorganization after stroke: how much and how functional? Neuroscientist 20, 56–70 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Overman JJ et al. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc. Natl Acad. Sci. USA 109, E2230–E2239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ishida A et al. Causal link between the cortico-rubral pathway and functional recovery through forced impaired limb use in rats with stroke. J. Neurosci 36, 455–467 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim SY et al. Experience with the ‘good’ limb induces aberrant synaptic plasticity in the perilesion cortex after stroke. J. Neurosci 35, 8604–8610 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang L, Conner JM, Nagahara AH & Tuszynski MH Rehabilitation drives enhancement of neuronal structure in functionally relevant neuronal subsets. Proc. Natl Acad. Sci. USA 113, 2750–2755 (2016).This article provides an excellent example of the major neuronal structural changes in ipsilesional cortex that can result from motor skill training focused on the paretic forelimb, as examined in rats.
- 118.Bury SD, Eichhorn AC, Kotzer CM & Jones TA Reactive astrocytic responses to denervation in the motor cortex of adult rats are sensitive to manipulations of behavioral experience. Neuropharmacology 39, 743–755 (2000). [DOI] [PubMed] [Google Scholar]
- 119.Brus-Ramer M, Carmel JB, Chakrabarty S & Martin JH Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J. Neurosci 27, 13793–13801 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Friel KM & Martin JH Bilateral activity- dependent interactions in the developing corticospinal system. J. Neurosci 27, 11083–11090 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang Y-Q, Zaaimi B & Martin JH Competition with primary sensory afferents drives remodeling of corticospinal axons in mature spinal motor circuits. J. Neurosci 36, 193–203 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Theodosis DT, Poulain DA & Oliet SHR Activity-dependent structural and functional plasticity of astrocyte–neuron interactions. Physiol. Rev 88, 983–1008 (2008). [DOI] [PubMed] [Google Scholar]
- 123.Perez-Alvarez A, Navarrete M, Covelo A, Martin ED & Araque A Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J. Neurosci 34, 12738–12744 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lacoste B et al. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron 83, 1117–1130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jones TA Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J. Comp. Neurol 414, 57–66 (1999). [PubMed] [Google Scholar]
- 126.Jones TA & Jefferson SC Reflections of experience-expectant development in repair of the adult damaged brain. Dev. Psychobiol 53, 466–475 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bury SD et al. Denervation facilitates neuronal growth in the motor cortex of rats in the presence of behavioral demand. Neurosci. Lett 287, 85–88 (2000). [DOI] [PubMed] [Google Scholar]
- 128.Bury SD & Jones TA Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the ‘unaffected’ forelimb and training-induced dendritic structural plasticity in the motor cortex. J. Neurosci 22, 8597–8606 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hsu JE & Jones TA Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp. Neurol 201, 479–494 (2006). [DOI] [PubMed] [Google Scholar]
- 130.Nudo RJ, Wise BM, SiFuentes F & Milliken GW Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272, 1791–1794 (1996). [DOI] [PubMed] [Google Scholar]
- 131.Conner JM, Chiba AA & Tuszynski MH The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron 46, 173–179 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Nishibe M, Urban ETR, Barbay S & Nudo RJ Rehabilitative training promotes rapid motor recovery but delayed motor map reorganization in a rat cortical ischemic infarct model. Neurorehabil. Neural Repair 29, 472–482 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tennant KA et al. Age-dependent reorganization of peri-infarct ‘premotor’ cortex with task-specific rehabilitative training in mice. Neurorehabil. Neural Repair 29, 193–202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liepert J et al. Treatment-induced cortical reorganization after stroke in humans. Stroke 31, 1210–1216 (2000). [DOI] [PubMed] [Google Scholar]
- 135.Sawaki L et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil. Neural Repair 22, 505–513 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Biernaskie J, Chernenko G & Corbett D Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J. Neurosci 24, 1245–1254 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lang KC, Thompson PA & Wolf SL The EXCITE Trial: reacquiring upper-extremity task performance with early versus late delivery of constraint therapy. Neurorehabil. Neural Repair 27, 654–663 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Zeiler SR et al. Paradoxical motor recovery from a first stroke after induction of a second stroke: reopening a postischemic sensitive period. Neurorehabil. Neural Repair 30, 794–800 (2016).This study in a mouse model provides particularly compelling support for the idea that there is a period after stroke during which there is increased sensitivity to the effects of rehabilitative training on paretic forelimb performance.
- 139.Barbay S et al. Behavioral and neurophysiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Exp. Brain Res 169, 106–116 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Palmér T, Tamtè M, Halje P, Enqvist O & Petersson P A system for automated tracking of motor components in neurophysiological research. J. Neurosci. Methods 205, 334–344 (2012). [DOI] [PubMed] [Google Scholar]
- 141.Lambercy O et al. Sub-processes of motor learning revealed by a robotic manipulandum for rodents. Behav. Brain Res 278, 569–576 (2015). [DOI] [PubMed] [Google Scholar]
- 142.Michaelsen SM, Luta A, Roby-Brami A & Levin MF Effect of trunk restraint on the recovery of reaching movements in hemiparetic patients. Stroke 32, 1875–1883 (2001). [DOI] [PubMed] [Google Scholar]
- 143.Dromerick AW et al. Relationships between upper-limb functional limitation and self-reported disability 3 months after stroke. J. Rehabil. Res. Dev 43, 401–408 (2006). [DOI] [PubMed] [Google Scholar]
- 144.Allred RP, Maldonado MA, Hsu JE & Jones TA Training the ‘less-affected’ forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restor. Neurol. Neurosci 23, 297–302 (2005). [PubMed] [Google Scholar]
- 145.Allred RP & Jones TA Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Exp. Neurol 210, 172–181 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kerr AL, Wolke ML, Bell JA & Jones TA Post- stroke protection from maladaptive effects of learning with the non-paretic forelimb by bimanual home cage experience in C57BL/6 mice. Behav. Brain Res 252, 180–187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kerr AL, Cheffer KA, Curtis MC & Rodriguez A Long-term deficits of the paretic limb follow post-stroke compensatory limb use in C57BL/6 mice. Behav. Brain Res 303, 103–108 (2016). [DOI] [PubMed] [Google Scholar]
- 148.MacLellan CL, Langdon KD, Botsford A, Butt S & Corbett D A model of persistent learned nonuse following focal ischemia in rats. Neurorehabil. Neural Repair 27, 900–907 (2013). [DOI] [PubMed] [Google Scholar]
- 149.Xu T et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jones TA, Chu CJ, Grande LA & Gregory AD Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J. Neurosci 19, 10153–10163 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kleim JA et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol. Learn. Mem 77, 63–77 (2002). [DOI] [PubMed] [Google Scholar]
- 152.Ganeshina O, Berry RW, Petralia RS, Nicholson DA & Geinisman Y Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience 125, 615–623 (2004). [DOI] [PubMed] [Google Scholar]
- 153.Lee KJ et al. Motor skill training induces coordinated strengthening and weakening between neighboring synapses. J. Neurosci 33, 9794–9799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Allred RP, Cappellini CH & Jones TA The ‘good’ limb makes the ‘bad’ limb worse: experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav. Neurosci 124, 124–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hummel FC & Cohen LG Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 5, 708–712 (2006). [DOI] [PubMed] [Google Scholar]
- 156.Bütefisch CM, Kleiser R & Seitz RJ Post-lesional cerebral reorganisation: evidence from functional neuroimaging and transcranial magnetic stimulation. J. Physiol. Paris 99, 437–454 (2006). [DOI] [PubMed] [Google Scholar]
- 157.Jankowska E & Edgley SA How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist 12, 67–79 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bradnam LV, Stinear CM & Byblow WD Ipsilateral motor pathways after stroke: implications for non-invasive brain stimulation. Front. Hum. Neurosci 7, 184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dancause N, Touvykine B & Mansoori BK Inhibition of the contralesional hemisphere after stroke: reviewing a few of the building blocks with a focus on animal models. Prog. Brain Res 218, 361–387 (2015). [DOI] [PubMed] [Google Scholar]
- 160.Murase N, Duque J, Mazzocchio R & Cohen LG Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol 55, 400–409 (2004). [DOI] [PubMed] [Google Scholar]
- 161.Harris-Love ML, Chan E, Dromerick AW & Cohen LG Neural substrates of motor recovery in severely impaired stroke patients with hand paralysis. Neurorehabil. Neural Repair 30, 328–338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fregni F et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 16, 1551–1555 (2005). [DOI] [PubMed] [Google Scholar]
- 163.Nowak DA et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch. Neurol 65, 741–747 (2008). [DOI] [PubMed] [Google Scholar]
- 164.Grefkes C et al. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage 50, 233–242 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bradnam LV, Stinear CM, Barber PA & Byblow WD Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb. Cortex 22, 2662–2671 (2012).The findings of this study strongly indicate that the influence of the contralesional cortex on paretic limb function in chronic stroke varies with impairment severity and corticospinal tract integrity.
- 166.Talelli P et al. Theta burst stimulation in the rehabilitation of the upper limb:a semirandomized, placebo-controlled trial in chronic stroke patients. Neurorehabil. Neural Repair 26, 976–987 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mohapatra S et al. Role of contralesional hemisphere in paretic arm reaching in patients with severe arm paresis due to stroke: a preliminary report. Neurosci. Lett 617, 52–58 (2016). [DOI] [PubMed] [Google Scholar]
- 168.Lotze M et al. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J. Neurosci 26, 6096–6102 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Biernaskie J, Szymanska A, Windle V & Corbett D Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur. J. Neurosci 21, 989–999 (2005). [DOI] [PubMed] [Google Scholar]
- 170.Shanina EV, Schallert T, Witte OW & Redecker C Behavioral recovery from unilateral photothrombotic infarcts of the forelimb sensorimotor cortex in rats: role of the contralateral cortex. Neuroscience 139, 1495–1506 (2006). [DOI] [PubMed] [Google Scholar]
- 171.O’Bryant A, Bernier B & Jones TA Abnormalities in skilled reaching movements are improved by peripheral anesthetization of the less-affected forelimb after sensorimotor cortical infarcts in rats. Behav. Brain Res 177, 298–307 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lindau NT et al. Rewiring of the corticospinal tract in the adult rat after unilateral stroke and anti-Nogo-A therapy. Brain 137, 739–756 (2014).This study provides compelling evidence that axonal sprouting from contralesional corticospinal neurons can contribute to motor training-dependent improvements in paretic forelimb performance and recovery, as examined in rats with large motor cortical infarcts.
- 173.Ueno M, Hayano Y, Nakagawa H & Yamashita T Intraspinal rewiring of the corticospinal tract requires target-derived brain-derived neurotrophic factor and compensates lost function after brain injury. Brain 135, 1253–1267 (2012). [DOI] [PubMed] [Google Scholar]
- 174.Tanaka T, Fujita Y, Ueno M, Shultz LD & Yamashita T Suppression of SHP-1 promotes corticospinal tract sprouting and functional recovery after brain injury. Cell Death Dis 4, e567 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morecraft RJ et al. Frontal and frontoparietal injury differentially affect the ipsilateral corticospinal projection from the nonlesioned hemisphere in monkey (Macaca mulatta). J. Comp. Neurol 524, 380–407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Harris-Love ML, Chen E Dromerick AW & Cohen LG Neural substrates of motor recovery in severely impaired stroke patients with hand paralysis. Neurorehabil. Neural Repair 30, 328–338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hodics T, Cohen LG & Cramer SC Functional imaging of intervention effects in stroke motor rehabilitation. Arch. Phys. Med. Rehabil 87, S36–S42 (2006). [DOI] [PubMed] [Google Scholar]
- 178.Hubbard IJ et al. A randomized controlled trial of the effect of early upper-limb training on stroke recovery and brain activation. Neurorehabil. Neural Repair 29, 703–713 (2015). [DOI] [PubMed] [Google Scholar]
- 179.Harris-Love ML, Morton SM, Perez MA & Cohen LG Mechanisms of short-term training-induced reaching improvement in severely hemiparetic stroke patients: a TMS study. Neurorehabil. Neural Repair 25, 398–411 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee MY et al. Cortical activation pattern of compensatory movement in stroke patients. NeuroRehabilitation 25, 255–260 (2009). [DOI] [PubMed] [Google Scholar]
- 181.Byblow WD, Stinear CM, Barber PA, Petoe MA & Ackerley SJ Proportional recovery after stroke depends on corticomotor integrity. Ann. Neurol 78, 848–859 (2015). [DOI] [PubMed] [Google Scholar]
- 182.Feeney DM & Baron JC Diaschisis. Stroke 17, 817–830 (1986). [DOI] [PubMed] [Google Scholar]
- 183.Cho J et al. Remodeling of neuronal circuits after reach training in chronic capsular stroke. Neurorehabil. Neural Repair 30, 941–950 (2016). [DOI] [PubMed] [Google Scholar]
- 184.Ergul A, Alhusban A & Fagan SC Angiogenesis: a harmonized target for recovery after stroke. Stroke 43, 2270–2274 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Reeves TM, Prins ML, Zhu J, Povlishock JT & Phillips LL Matrix metalloproteinase inhibition alters functional and structural correlates of deafferentation-induced sprouting in the dentate gyrus. J. Neurosci 23, 10182–10189 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nudo RJ Recovery after brain injury: mechanisms and principles. Front. Hum. Neurosci 7, 887 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schallert T, Woodlee MT & Fleming SM in Pharmacology of Cerebral Ischemia 201–216 (Stuttgart, 2002). [Google Scholar]
- 188.Schallert T, Fleming SM, Leasure JL, Tillerson JL & Bland ST CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39, 777–787 (2000). [DOI] [PubMed] [Google Scholar]
- 189.Schallert T, Kozlowski DA, Humm JL & Cocke RR Use-dependent structural events in recovery of function. Adv. Neurol 73, 229–238 (1997). [PubMed] [Google Scholar]
- 190.Blasi F, Whalen MJ & Ayata C Lasting pure-motor deficits after focal posterior internal capsule white-matter infarcts in rats. J. Cereb. Blood Flow Metab 35, 977–984 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]