Abstract
Mitochondria are dynamic organelles that can form complex networks in the cell. These networks can be rapidly remodeled in response to environmental changes or to support cellular needs. Mitochondrial dynamics are dependent on interactions with the cellular cytoskeleton – both microtubules and actin filaments. Mitochondrial-cytoskeletal interactions have a well-established role in mitochondrial motility. Recent progress indicates that these interactions also regulate the balance of mitochondrial fission/fusion, as well as mitochondria turnover and mitochondrial inheritance during cell division. We review these advances, and how this work has deepened our understanding of mitochondrial dynamics in the cell.
Mitochondria are complex organelles that coordinate numerous cellular functions, including reactive oxygen species signaling, Ca2+-buffering, lipid synthesis, and perhaps most notably, the generation of ATP by oxidative phosphorylation [1]. In contrast to the archetypal bean-shaped structure often seen in biology textbooks, mitochondria are in fact morphologically diverse and can form dynamic, highly complex networks that can rapidly rearrange their architecture in order to support cellular needs. This plasticity allows cells to quickly adapt to local changes in the extracellular milieu. Mitochondria network architecture is determined by multiple factors, including (1) the regulation of dynamin-like proteins on individual mitochondria [2], (2) interactions between mitochondria and neighboring organelles [3], and (3) direct associations between mitochondria and the cytoskeleton (Figure 1). In the current review, we will examine recent developments in our understanding of how the microtubule and actin cytoskeletons regulate mitochondria structure and function.
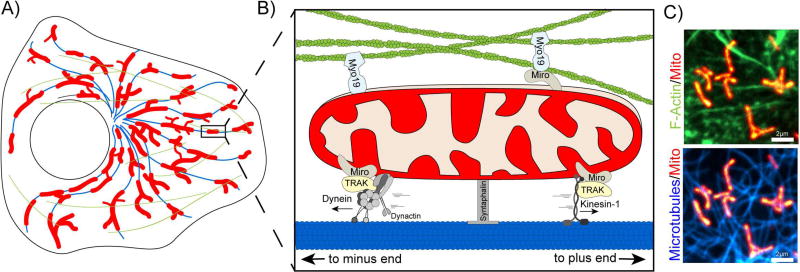
Figure 1.
A. Schematic of mitochondria (red), microtubules (blue), and f-actin (green) distribution in an undifferentiated cell. B. Mitochondria associate with microtubules (blue, bottom) and with actin (green, top) via motor/adaptor complexes. Dynein/dynactin associate with mitochondria via TRAK and Miro to drive retrograde mitochondrial motility. In contrast, Kinesin-1 coordinates anterograde motility towards the cell periphery. Myo19 can associate with the mitochondria outer membrane either directly or through Miro. Syntaphilin anchors mitochondria to microtubules. C. Spinning disk confocal image of a HeLa cell expressing a mitochondria matrix marker (Mito-DsRed2) as w3ell as markers for filamentous actin (top, LifeAct-GFP) and microtubules (bottom, SiR-Tubulin).
Dynamic interactions of mitochondria with microtubules
Mitochondria account for a small fraction (>10%) of the overall cell volume of interphase cells, but are highly dynamic, exploring ~80% of the cell volume within 15 min in some cell types [4]. In mammalian cells, these dynamics are primarily driven by molecular motors actively translocating mitochondria along the microtubule cytoskeleton. In undifferentiated cells, microtubules are radially organized with their minus ends clustered at or near the microtubule-organizing center, and their more dynamic plus ends radiating outward toward the cell periphery. Cytoplasmic dynein and its activator dynactin drive the motility of mitochondria inward, toward the microtubule minus-end, while kinesin-1 is the major plus-end directed motor on mitochondria [5]. The importance of mitochondrial-microtubule interactions has been highlighted by genetic and cellular studies, reviewed below, and also explored in theoretical work highlighting the role of the microtubule cytoskeleton in promoting structural heterogeneity and adaptive responsiveness of the mitochondrial network [6].
A genetic screen for mutants with impaired neuronal function in Drosophila led to the discovery of Milton [7], a kinesin-binding protein that is critical for the regulation of mitochondrial motility. The mammalian homologs of Milton, TRAK1 and TRAK2, similarly mediate the motility of mitochondria through interactions with both kinesin and dynein/dynactin [8]. Milton and TRAKs 1 and 2 are scaffolding proteins that interact with mitochondria via the Ca2+-binding GTPase Miro, which is anchored to the outer membrane of mitochondria via its tail domain [9]. Increased cytosolic Ca2+ inhibits mitochondrial motility in a Miro-dependent manner [10,11].
Both genetic screens and targeted disruptions indicate that mitochondrial dynamics are critically important in differentiated cells with highly polarized morphologies such as neurons. In Drosophila, Miro mutants are lethal due to the impairment of mitochondrial trafficking in neurons [9], and knockout of Miro1 in mouse is sufficient to cause early postnatal death, while neuron-specific deletion of Miro1 results in both neurodevelopmental and neurodegenerative defects [12].
The neuronal phenotypes observed for Miro and Milton mutations indicate that the active translocation of mitochondria along microtubules is critically important for neuronal health. Mitochondrial motility is especially important during neuronal outgrowth, but this motility is dramatically reduced in mature neurons, with <10% of mitochondria motile along the axons of mature mammalian neurons both in vitro and in vivo [13,14].
In axons, the mitochondrially-targeted protein syntaphilin contributes to the anchoring of stationary mitochondria to microtubules [15] leading to enrichment at presynaptic sites [13]. The protein Disrupted-In-Schizophrenia 1 (DISC1) interacts with syntaphilin, TRAK1, and Miro [16,17] to modulate the anchoring activity of syntaphilin, and thus the Ca2+-dependent regulation of mitochondrial motility. Loss of this anchoring leads to defects in short-term facilitation, likely through defects in local mitochondrial-dependent Ca2+-buffering [15].
Turnover of aging or damaged mitochondria is essential in long-lived, post-mitotic cells such as neurons. Multiple studies have linked changes in mitochondrial motility to mitochondrial quality control pathways such as mitophagy. In one pathway, damaged mitochondria are immobilized along the axon through the PINK1- and Parkin-dependent degradation of Miro [18], promoting localized mitophagy [19]. Parkinson’s disease-related mutations in PINK1, Parkin or LRRK2 slow the degradation of Miro, leading to the hypothesis that delayed arrest of damaged mitochondria along the axon also delays their degradation [20]. However, it remains unclear if the localized degradation of stalled mitochondria via axonal mitophagy is the major mechanism for mitochondrial quality control in neurons, as in vivo studies indicate that most PINK1- and Parkin-dependent mitophagy occurs within the soma [21]. A novel mechanism linking syntaphilin to the removal of stressed mitochondria has recently been described, in which the application of the respiratory complex III inhibitor Antimycin A1 to primary neurons induces the budding of syntaphilin-positive carrier vesicles from the ends of damaged mitochondria along axons. These vesicles are then transported in association with late endosomes toward the soma for lysosomal degradation [22]. The increased prevalence of syntaphilin-positive vesicles in neurons from neurodegenerative disease models suggests that the formation of these vesicles may be a response to chronic stress, but the mechanism leading to their generation remains to be determined, as it is independent of both Parkin and Drp1 activity [22].
While most studies to date have focused on the role of Ca2+ in regulating mitochondrial motility along microtubules, two other regulatory pathways have also been explored. Schwarz and colleagues looked at mechanisms connecting local energy state to mitochondrial motility, and found that the direct modification of Milton (TRAK1) by the enzyme O-GlcNAc transferase (OGT) in response to changes in the extracellular glucose concentration modulates mitochondrial motility in neurons [23]. This mechanism allows the cell to relocalize mitochondria in response to changes in the overall energy landscape. Recent work has also shown that ROS production can affect mitochondrial dynamics. Either exogenous or endogenously generated ROS inhibits mitochondrial motility in a pathway that is independent of changes in Ca2+, at least in mammalian cells, but is dependent on the MAP kinase p38a, potentially working through the Miro/TRAK adaptor complex [24]. Of note, the mechanism is not limited to neurons, but also affects mitochondrial motility in cardiomyocytes. This observation is of potential interest, due to the importance of mitochondrial localization in cardiomyocytes [25]. In failing heart cells, changes in the alignment of cellular microtubules lead to altered mitochondrial organization, which is correlated with defects in calcium release in response to mechanical stimuli. Thus, in both neurons and cardiomyocytes, the regulated interactions of mitochondria with microtubules are required to maintain normal calcium dynamics, highlighting the key role of mitochondria in maintaining cellular Ca2+ homeostasis, and further emphasizing the importance of mechanisms that allow the cell to dynamically relocalize mitochondria in response to changes in the external environment.
A major role of mitochondria is to produce ATP, and recent work has provided new insights into the connection between mitochondrial motility along microtubules and energy gradients in cells. Schuler et al. [26] used 3D fluorescence spinning disc confocal and lattice light sheet microscopy to monitor the intracellular response of the biosensor Perceval HR, which provides a ratiometric measure of ATP/ADP levels. In wild type MEFs, a gradient was observed with highest ATP:ADP ratios in the perinuclear region and a gradual decline in the ratio toward the cell periphery. A steeper gradient was observed in Miro −/− MEFs, where mitochondria were more strictly localized to the perinuclear region due to defective mitochondrial transport along microtubules. Deficits in energy-dependent processes such as membrane ruffling, leading edge protrusion, and focal adhesion dynamics at the cell periphery were observed, that led to marked differences in cell migration. These observations are further confirmation of the long-held hypothesis that the active positioning of mitochondria near sites of high energy demand are required for normal cellular functions.
A dramatic change in the interaction of mitochondria with microtubules occurs as dividing cells enter mitosis. Prior to mitosis, many mitochondria are closely associated with the microtubule cytoskeleton, through the motor-dependent interactions described above. However, as cells enter prophase, the association of mitochondria with microtubules is significantly decreased, due to the CDK1- and Aurora A-dependent shedding of kinesin and dynein motors [27]. This shedding has been proposed to allow for the passive segregation of mitochondria between daughter cells [27]. Alternatively, mitochondrial distribution into the two daughter cells at the end of mitosis may be more active. As dividing cells initiate cytokinesis, mitochondria rebind to microtubules in a Miro- and CENP-F dependent manner, and were observed to track with dynamic microtubule plus ends in a dynamic mechanism that favors the equal inheritance of these organelles by the daughter cells [28]. Also, as discussed below, actin dynamics also facilitate equitable organelle partitioning following cell division in mammalian cells.
Actin dynamics in mitochondrial motility and remodeling
Across the tree of life, the actin cytoskeleton plays important and diverse roles in regulating mitochondrial network structure and function. In simple eukaryotes, such as budding yeast, the actin cytoskeleton directs mitochondrial movement [29] and is essential for the faithful inheritance of mitochondria upon cytokinesis [30]. In metazoans, long range mitochondrial movement is coordinated by microtubules, but the actin cytoskeleton plays an important role in regulating mitochondrial distribution, coordinating short range mitochondrial motility [31], anchoring [32], and fission [33–36].
Perhaps the most direct evidence for actin-based mitochondrial motility came with the discovery of the unconventional myosin motor, Myosin XIX (Myo19). A plus-end directed motor with sequence similarity to MyoV, Myo19 was shown to specifically localize to the mitochondrial outer membrane where it drives mitochondrial movement [31,37]. Overexpression of fluorescent tagged Myo19 induced a nearly two-fold increase in mitochondrial velocity, which was reversed upon depolymerization of actin but not microtubules. Since its initial discovery, Myo19 has been implicated in a range of functions, including targeting mitochondria to stress induced filopodia [38,39] and coordinating mitochondrial inheritance upon cytokinesis [40]. Most recently, Myo19 localization to mitochondria was shown to be dependent on Miro proteins, suggesting that Miro may regulate both actin and microtubule based mitochondrial motility [41]. In addition to Myo19, there is evidence that other myosin motors facilitate mitochondria dynamics. Work in Drosophila motor neurons indicates that Myosin V and VI can localize to axonal mitochondria in order to anchor mitochondria to actin filaments and oppose microtubule based motility [32].
INF2/Spire1C mediated mitochondrial fission
In addition to its role in coordinating short-range mitochondrial motility, filamentous actin has also been implicated in the regulation of mitochondrial fission. Within the last few years, at least two distinct mechanisms of actin-mediated mitochondrial fission have been described (Figure 2). In nearly all cases, mitochondria fission occurs via the assembly and hydrolysis-mediated constriction of ring-shaped Drp1 oligomers on the mitochondrial outer membrane [42]. Drp1 rings tighten and constrict mitochondria, facilitating the subsequent recruitment of dynamin-2 which ultimately coordinates membrane scission [43]. However, because mitochondria are often thicker than the diameter of Drp1 rings, they must first undergo a pre-constriction step to decrease their cross-sectional diameter. This pre-constriction is achieved by wrapping and tightening endoplasmic reticulum tubules around mitochondria [44].
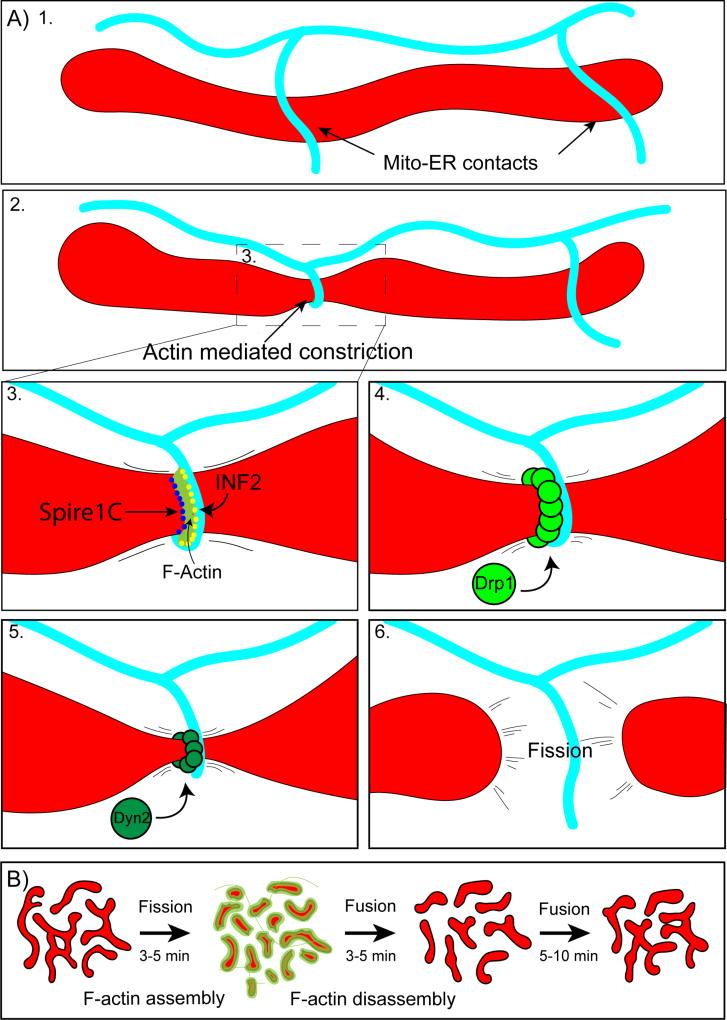
Figure 2.
A) INF2/Spire1C mediated mitochondrial fission. 1. Mitochondria make frequent contacts with endoplasmic reticulum tubules (Mito-ER contacts). 2. A subset of Mito-ER contact sites mark prospective sites of mitochondrial fission. At these sites, ER tubules wrap around mitochondria generating a constriction event. 3. ER constriction around mitochondria is driven by actin polymerization by INF2 on the ER and Spire1C on mitochondria. 4. Once mitochondria undergo constriction, Drp1 forms rings at the constriction site which pinch down the organelle, decreasing the cross sectional diameter. 5. Next, Dyn2 assembles and completes the process of mitochondrial fission (6). B. Arp2/3 mediated mitochondrial fission. Actin transiently assembles on locally hyperfused regions of the mitochondrial network. Actin assembly promotes rapid fission over 3–5 minutes. Mitochondria then fuse back together and the process repeats.
Converging evidence suggests that ER bending around mitochondria is driven by the force of actin polymerization [33,34]. Work from the Higgs lab determined that an ER-anchored isoform of the formin INF2 is absolutely critical for this process. Depletion of INF2 led to an increase in mitochondrial length [33]. Subsequent work showed that INF2 cooperates with a mitochondria-localized Spire protein, Spire1C, in order to generate short actin filaments that span Mitochondria-ER contacts [34]. The force of this actin assembly tightens the ER tubule around mitochondria. Myosin II likely contributes to actin-mediated mitochondrial constriction, as the motor localizes to fission sites and its depletion leads to mitochondrial elongation and loss of mitochondrial Drp1 [45,46].
In addition to driving constriction, F-actin at Mito/ER contacts may also prime Drp1 to form functional oligomers on the mitochondrial outer membrane [47,48]. In vitro GTPase assays show that Drp1 activity is greatly enhanced in the presence of actin filaments [48]. More recent work has shown that INF2 driven actin assembly at mitochondria-ER contacts also triggers calcium uptake into the mitochondria matrix which then synchronizes outer and inner membrane fission [49]. Taken together, these observations suggest that filamentous actin acts as an important regulator of inner and outer mitochondrial membrane fission.
Arp2/3 mediated mitochondrial fission
Stress-induced mitochondrial fission through treatment with the mitochondrial poison CCCP was shown to promote rapid actin assembly around mitochondria [35]. Strikingly, this actin polymerization is not dependent on INF2 and does not specifically occur at mito-ER contact sites. Instead, actin transiently assembles around whole mitochondria in an Arp2/3 dependent manner. These actin assemblies deform mitochondria and facilitate Drp1 recruitment and subsequent fission.
While CCCP treatment triggers rapid and reproducible mitochondrial fission, it nonetheless represents a non-physiological mechanism of mitochondria division. Importantly, there is strong evidence that Arp2/3 actin clouds may also play a role in homeostatic mitochondrial fission, even in the absence of a stressor. In wildtype MEFs, the actin binding proteins Arp3, cortactin, and cofilin were shown to localize to mitochondria by immunofluorescence [35,50]. Depletion of these proteins led to increased mitochondrial length and connectivity due to lower rates of fission [35]. Arp2/3-mediated mitochondrial fission may facilitate mitochondria network fragmentation at the G2/M transition as mitochondrial fission at prophase is linked to increased mitochondrial F-actin [35].
Most recently, the dynamic, Arp2/3-dependent assembly of F-actin on mitochondrial subpopulations has been observed in multiple cell types, including HeLa cells, Cos7 cells, and human epidermal keratinocytes [36]. Actin filaments transiently assemble in a ‘cloud’ around ~25% of cellular mitochondria, and then disassemble and subsequently reassemble around a neighboring subpopulation of mitochondria. Thus the actin cloud travels in a wave-like manner that propagates through the entire mitochondrial network over approximately 15 minutes. Upon actin assembly, mitochondria undergo rapid, Drp1-dependent fragmentation [36]. This fragmentation is reversed following F-actin disassembly when fusion of neighboring mitochondria reinitiates. Constitutive actin cycling has been proposed to function as a homeostatic mechanism by which the actin cytoskeleton regulates the mitochondrial network [36]. Each round of transient fission prevents local hyperfusion of the mitochondrial network and the subsequent fusion following actin disassembly facilitates mixing of mitochondria contents.
Conclusions
Dynamic interactions between mitochondria and the cytoskeleton are critically important to maintain mitochondria network structure and function. There has been a long-standing appreciation for the role of the cytoskeleton in mitochondrial motility, essential for intracellular shuttling to regions of high-energy demand or to locally buffer calcium. More recent work has highlighted the role of actin filaments and microtubules in the regulation of the mitochondrial fission/fusion balance, as well as mitochondrial quality control and turnover, and mitochondrial inheritance during cell division. Over the next few years, further advances in light microscopy techniques that allow for high-speed three-dimensional imaging of cells and tissues will continue to deepen our understanding of how these dynamic networks interact.
Highlights.
Mitochondrial dynamics depend on interactions with the cytoskeleton
Interactions with microtubules mediate motility, turnover, and organelle inheritance
Interactions with actin filaments mediate motility, fission/fusion, and network homeostasis
Acknowledgments
The authors gratefully acknowledge Sydney Cason and Pedro Guedes Dias for thoughtful comments on the manuscript, and funding from NIH (P01 GM087253 to EH and F31 GM123644 to AM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: None.
References
- 1.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Bliek AM, Shen Q, Kawajiri S. Mechanisms of mitochondrial fission and fusion. Mitochondria. 2014:127–142. doi: 10.1101/cshperspect.a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murley A, Nunnari J. The Emerging Network of Mitochondria-Organelle Contacts. Mol Cell. 2016;61:648–653. doi: 10.1016/j.molcel.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E, Lippincott-Schwartz J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature. 2017;546:162–167. doi: 10.1038/nature22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz TL. Mitochondrial trafficking in neurons. Mitochondria. 2014:163–178. [Google Scholar]
- 6.Sukhorukov VM, Meyer-Hermann M. Structural Heterogeneity of Mitochondria Induced by the Microtubule Cytoskeleton. Sci Rep. 2015;5:13924. doi: 10.1038/srep13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- 8.van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, Wulf PS, Keijzer N, Demmers J, Kapitein LC, et al. TRAK/Milton Motor-Adaptor Proteins Steer Mitochondrial Trafficking to Axons and Dendrites. Neuron. 2013;77:485–502. doi: 10.1016/j.neuron.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TT, Oh SS, Weaver D, Lewandowska A, Maxfield D, Schuler MH, Smith NK, Macfarlane J, Saunders G, Palmer CA, et al. Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc Natl Acad Sci U S A. 2014;111:E3631–3640. doi: 10.1073/pnas.1402449111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Lewis TL, Jr, Turi GF, Kwon SK, Losonczy A, Polleux F. Progressive Decrease of Mitochondrial Motility during Maturation of Cortical Axons In Vitro and In Vivo. Curr Biol. 2016;26:2602–2608. doi: 10.1016/j.cub.2016.07.064. The authors characterize a pronounced loss of mitochondrial motility during neuronal maturation, both in primary neurons and in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misgeld T, Schwarz TL. Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron. 2017;96:651–666. doi: 10.1016/j.neuron.2017.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa F, Malavasi EL, Crummie DK, Eykelenboom JE, Soares DC, Mackie S, Porteous DJ, Millar JK. DISC1 complexes with TRAK1 and Miro1 to modulate anterograde axonal mitochondrial trafficking. Hum Mol Genet. 2014;23:906–919. doi: 10.1093/hmg/ddt485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park C, Lee SA, Hong JH, Suh Y, Park SJ, Suh BK, Woo Y, Choi J, Huh JW, Kim YM, et al. Disrupted-in-schizophrenia 1 (DISC1) and Syntaphilin collaborate to modulate axonal mitochondrial anchoring. Mol Brain. 2016;9:69. doi: 10.1186/s13041-016-0250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlevkov E, Kramer T, Schapansky J, LaVoie MJ, Schwarz TL. Miro phosphorylation sites regulate Parkin recruitment and mitochondrial motility. Proc Natl Acad Sci U S A. 2016;113:E6097–E6106. doi: 10.1073/pnas.1612283113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh CH, Shaltouki A, Gonzalez AE, Bettencourt da Cruz A, Burbulla LF, St Lawrence E, Schule B, Krainc D, Palmer TD, Wang X. Functional Impairment in Miro Degradation and Mitophagy Is a Shared Feature in Familial and Sporadic Parkinson's Disease. Cell Stem Cell. 2016;19:709–724. doi: 10.1016/j.stem.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sung H, Tandarich LC, Nguyen K, Hollenbeck PJ. Compartmentalized Regulation of Parkin-Mediated Mitochondrial Quality Control in the Drosophila Nervous System In Vivo. J Neurosci. 2016;36:7375–7391. doi: 10.1523/JNEUROSCI.0633-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **22.Lin MY, Cheng XT, Tammineni P, Xie Y, Zhou B, Cai Q, Sheng ZH. Releasing Syntaphilin Removes Stressed Mitochondria from Axons Independent of Mitophagy under Pathophysiological Conditions. Neuron. 2017;94:595–610. e596. doi: 10.1016/j.neuron.2017.04.004. This work reports a novel mechanism for the removal of dysfunctional mitochondria along axons in response to cellular stress, involving the generation of syntaphilin-positive vesicles that bud from mitochondrial ends and are then trafficked toward the soma in association with late endosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell. 2014;158:54–68. doi: 10.1016/j.cell.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debattisti V, Gerencser AA, Saotome M, Das S, Hajnoczky G. ROS Control Mitochondrial Motility through p38 and the Motor Adaptor Miro/Trak. Cell Rep. 2017;21:1667–1680. doi: 10.1016/j.celrep.2017.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miragoli M, Sanchez-Alonso JL, Bhargava A, Wright PT, Sikkel M, Schobesberger S, Diakonov I, Novak P, Castaldi A, Cattaneo P, et al. Microtubule-Dependent Mitochondria Alignment Regulates Calcium Release in Response to Nanomechanical Stimulus in Heart Myocytes. Cell Rep. 2016;14:140–151. doi: 10.1016/j.celrep.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuler MH, Lewandowska A, Caprio GD, Skillern W, Upadhyayula S, Kirchhausen T, Shaw JM, Cunniff B. Miro1-mediated mitochondrial positioning shapes intracellular energy gradients required for cell migration. Mol Biol Cell. 2017;28:2159–2169. doi: 10.1091/mbc.E16-10-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Chung JY, Steen JA, Schwarz TL. Phosphorylation-Induced Motor Shedding Is Required at Mitosis for Proper Distribution and Passive Inheritance of Mitochondria. Cell Rep. 2016;16:2142–2155. doi: 10.1016/j.celrep.2016.07.055. This work identifies a cell cycle-regulated, phosphorylation-dependent mechanism leading to the shedding of microtubule-based motors kinesin and dynein from mitochondria during mitosis, preventing the association of mitochondria with microtubules within the mitotic spindle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Kanfer G, Courtheoux T, Peterka M, Meier S, Soste M, Melnik A, Reis K, Aspenstrom P, Peter M, Picotti P, et al. Mitotic redistribution of the mitochondrial network by Miro and Cenp-F. Nat Commun. 2015;6:8015. doi: 10.1038/ncomms9015. The authors propose that the association of mitochondria with dynamic microtubule plus-ends promotes the proper segregation of organelles between daughter cells following cell division. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon VR, Swayne TC, Pon LA. Actin-dependent mitochondrial motility in mitotic yeast and cell-free systems: identification of a motor activity on the mitochondrial surface. J Cell Biol. 1995;130:345–354. doi: 10.1083/jcb.130.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boldogh IR, Yang HC, Nowakowski WD, Karmon SL, Hays LG, Yates JR, 3rd, Pon LA. Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proc Natl Acad Sci U S A. 2001;98:3162–3167. doi: 10.1073/pnas.051494698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quintero OA, DiVito MM, Adikes RC, Kortan MB, Case LB, Lier AJ, Panaretos NS, Slater SQ, Rengarajan M, Feliu M, et al. Human Myo19 is a novel myosin that associates with mitochondria. Curr Biol. 2009;19:2008–2013. doi: 10.1016/j.cub.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neurosci. 2010;30:8984–8992. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Manor U, Bartholomew S, Golani G, Christenson E, Kozlov M, Higgs H, Spudich J, Lippincott-Schwartz J. A mitochondria-anchored isoform of the actin-nucleating spire protein regulates mitochondrial division. Elife. 2015;4 doi: 10.7554/eLife.08828. This article identifies a novel splice isoform of Spire1 (Spire1C) which localizes to mitochondria. The authors demonstrate that Spire1C on mitochondria interacts with INF2 on the endoplasmic reticulum in order to promote mitochondrial constriction required for fission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Li S, Xu S, Roelofs BA, Boyman L, Lederer WJ, Sesaki H, Karbowski M. Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. J Cell Biol. 2015;208:109–123. doi: 10.1083/jcb.201404050. This article uses live imaging and immunofluorescence to demonstrate that F-actin can assemble on the surface of mitochondria in HeLa cells and MEFs. The authors find that actin assembly on mitochondria is enhanced upon depletion of the fission protein Drp1. Additionally, they find that induction of fission with the drug CCCP drives actin assembly on mitochondria. They show that mitochondrial actin assembly is dependent on Arp2/3 complex, and depletion of Arp2/3, cortactin, or cofilin leads to mitochondrial elongation. Critically, they show that this mitochondrial elongation is due to decreased fission and not increased fusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Moore AS, Wong YC, Simpson CL, Holzbaur EL. Dynamic actin cycling through mitochondrial subpopulations locally regulates the fission-fusion balance within mitochondrial networks. Nat Commun. 2016;7:12886. doi: 10.1038/ncomms12886. This article uses live-cell microscopy to identify a previously uncharacterized phenomenon whereby actin clouds cycle onto and off of mitochondria subpopulations. They identify actin clouds in wildtype HeLa cells, keratinocytes, and Cos7 cells. Over time, they observe that actin clouds cycle through the mitochondrial network, transiently enhancing mitochondria dynamics. In HeLa cells, they find that actin clouds cycle through the entire mitochondria network every 15 minutes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Z, Ma XN, Zhang HM, Ji HH, Ding H, Zhang J, Luo D, Sun Y, Li XD. Mouse myosin-19 is a plus-end-directed, high-duty ratio molecular motor. J Biol Chem. 2014;289:18535–18548. doi: 10.1074/jbc.M114.569087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shneyer BI, Usaj M, Henn A. Myo19 is an outer mitochondrial membrane motor and effector of starvation-induced filopodia. J Cell Sci. 2016;129:543–556. doi: 10.1242/jcs.175349. [DOI] [PubMed] [Google Scholar]
- 39.Shneyer BI, Usaj M, Wiesel-Motiuk N, Regev R, Henn A. ROS induced distribution of mitochondria to filopodia by Myo19 depends on a class specific tryptophan in the motor domain. Sci Rep. 2017;7:11577. doi: 10.1038/s41598-017-11002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohn JL, Patel JV, Neumann B, Bulkescher J, McHedlishvili N, McMullan RC, Quintero OA, Ellenberg J, Baum B. Myo19 ensures symmetric partitioning of mitochondria and coupling of mitochondrial segregation to cell division. Curr Biol. 2014;24:2598–2605. doi: 10.1016/j.cub.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Domenech G, Covill-Cooke C, Ivankovic D, Halff EF, Sheehan DF, Norkett R, Birsa N, Kittler JT. Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J. 2018 doi: 10.15252/embj.201696380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JE, Westrate LM, Wu H, Page C, Voeltz GK. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016;540:139–143. doi: 10.1038/nature20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korobova F, Gauvin TJ, Higgs HN. A role for myosin II in mammalian mitochondrial fission. Curr Biol. 2014;24:409–414. doi: 10.1016/j.cub.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curchoe CL, Manor U. Actin Cytoskeleton-Mediated Constriction of Membrane Organelles via Endoplasmic Reticulum Scaffolding. ACS Biomater Sci Eng. 2017;3:2727–2732. doi: 10.1021/acsbiomaterials.6b00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DuBoff B, Gotz J, Feany MB. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron. 2012;75:618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji WK, Hatch AL, Merrill RA, Strack S, Higgs HN. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife. 2015;4:e11553. doi: 10.7554/eLife.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Chakrabarti R, Ji WK, Stan RV, de Juan Sanz J, Ryan TA, Higgs HN. INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division. J Cell Biol. 2018;217:251–268. doi: 10.1083/jcb.201709111. This article explores the mechanism by which calcium uptake is linked to mitochondrial fission. The authors find that treatment of U2OS cells with ionomycin drives assembly of actin in the cytoplasm as well as subsequent calcium uptake by mitochondria. Actin depolymerization by Latrunculin A treatment or INF2 knockout blocks ionomycin induced calcium uptake by mitochondria. The authors go on to show that calcium uptake drives inner mitochondrial membrane constriction and that this process requires the mitochondrial calcium uniporter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagliuso A, Tham TN, Stevens JK, Lagache T, Persson R, Salles A, Olivo-Marin JC, Oddos S, Spang A, Cossart P, et al. A role for septin 2 in Drp1-mediated mitochondrial fission. EMBO Rep. 2016;17:858–873. doi: 10.15252/embr.201541612. [DOI] [PMC free article] [PubMed] [Google Scholar]