Abstract
Nibrin (NBN) is a member of a DNA repair complex together with MRE11 and RAD50. The complex is associated particularly with the repair of DNA double strand breaks and with the regulation of cell cycle check points. Hypomorphic mutation of components of the complex leads to human disorders characterised by radiosensitivity and increased tumour occurrence, particularly of the lymphatic system. We have examined here the relationship between DNA damage, mutation frequency and mutation spectrum in vitro and in vivo in mouse models carrying NBN mutations and a lacZ reporter plasmid. We find that NBN mutation leads to increased spontaneous DNA damage in fibroblasts in vitro and high basal mutation rates in lymphatic tissue of mice in vivo. The characteristic mutation spectrum is dominated by single base transitions rather than the deletions and complex rearrangements expected after abortive repair of DNA double strand breaks. We conclude that in the absence of wild type nibrin, the repair of spontaneous errors, presumably arising during DNA replication, makes a major contribution to the basal mutation rate. This applies also to cells heterozygous for an NBN null mutation. Mutation frequencies after irradiation in vivo were not increased in mice with nibrin mutations as might have been expected considering the radiosensitivity of NBS patient cells in vitro. Evidently apoptosis is efficient, even in the absence of wild type nibrin.
Keywords: DNA damage, Mismatch repair, Haploinsufficiency
1. Introduction
Nijmegen Breakage Syndrome (NBS) belongs to a group of DNA-repair deficiency syndromes in which the mutated gene is directly involved in the repair of DNA double strand breaks (DSBs), in the case of NBS, the gene nibrin (NBN) [1]. Patients with NBS show symptoms such as immunodeficiency, sensitivity to ionising irradiation (IR) and an extreme risk for cancers, particularly of the lymphoreticular system [2]. The incidence for cancer among patients is approximately 1000 times higher than for the normal population [3]. Heterozygote carriers of mutations in NBN also have an apparently higher cancer risk, although cancer occurs much later and shows no obvious organ specificity [4]. Over 95% of NBS patients carry the major NBN mutation, a 5 bp deletion (c.657 661del5, p.K219fsX19), which leads to a truncated amino terminal fragment and, significantly, alternative translation from a cryptic start codon upstream of the deletion [5]. As a result, a 70 kDa carboxy-terminal protein fragment, p70-nibrin, is produced. This protein fragment maintains partial nibrin function [6,7]. In contrast, null mutations in mice are embryonically lethal due to massive apoptosis at the blastocyst stage [8,9].
Nibrin is a component of a highly conserved trimeric complex, together with MRE11 and RAD50, which is involved through its DNA binding and nuclease activities in the repair of DSBs by both non-homologous end joining (NHEJ) and homologous recombination (HR). In addition, its function in the DNA damage-induced activation of ATM contributes to the regulation of the cell cycle as part of the DNA damage response. Although the consequences of a failure in DSB repair for tumorigenesis are understandable, little is actually known about the correlation between DNA damage, mutation frequency and mutation type in the absence of wild type nibrin.
In order to analyse mutation frequencies and mutation spectra when nibrin is disrupted, we have utilised a conditional null mutant mouse [6] and a humanised NBS mouse which carries the human c.657 661del5 NBN-allele on a null mutant murine Nbn background [7]. Mice homozygous and heterozygous for nibrin mutations were examined since we have previously suggested that NBN is a haploinsufficient tumour suppressor gene [10]. We have bred these mice with a transgenic mutagenesis mouse harbouring 21 tandem copies of the bacterial lacZ gene, stably integrated on chromosome 11 [11]. The mutation frequency and mutation spectrum was determined in DNA extracted from fibroblasts or lymphatic tissues by rescuing the reporter plasmid and transformation of E. coli.
We find that null mutation of Nbn leads to increased DNA damage in vitro, even in the absence of an external genotoxic treatment. Distinct mutation spectra indicate that the molecular origin of spontaneous and radiation induced base mutations is different. In vivo we find the basal mutation frequency measured in lymphatic tissue of the humanised NBS mice is approximately 2.5-fold higher than in control mice. We conclude that spontaneous DNA damage, incurred probably during replication, is a major source of mutations in nibrin deficient cells. Surprisingly, after irradiation, in vivo mutation frequencies were not significantly increased in the humanised NBS mice. This is attributed to efficient apoptosis even in the absence of wild type nibrin and suggests that spontaneous base mutations may have a more significant role in tumorigenesis in NBS patients than previously appreciated.
2. Materials and methods
2.1. Mice breeding and irradiation
For the in vivo experiments, the pUR288 lacZ-transgenic mouse line 30 [12], harbouring 21 copies of the bacterial lacZ gene, was crossbred with the humanised NBS mouse which harbours the human NBN gene with the 5 bp founder mutation on a null-mutant murine Nbn background (Nbn−/−NBNdel5; [7]). The lacZ-transgenic mouse was also crossbred with our previously described cre recombinase inducible null mutant mouse (Nbnins6/lox6; [6]) and fibroblasts isolated for in vitro experiments in the complete absence of nibrin. All mice had a mixed C57BL/6/129Sv background. Geno-typing was conducted by PCR analysis on DNA isolated from tail biopsies of the mice. Whole-body irradiation of mice was performed with the Cs137-irradiation device OB29/4 S/N 010 (Gamma-Service Medical GmbH) after sedation with Isoflurane. Mice were irradiated on 5 consecutive days with 0.5 Gy per day. Two days after the last irradiation animals were sacrificed and organs frozen in liquid nitrogen until extraction of DNA for the lacZ mutation assay.
2.2. Cell culture and cre recombinase treatment
Fibroblasts were derived from ear explants of control wild type mice (lacZ-Nbn+/+), humanised mice (lacZ-Nbn−/−NBNdel5) and heterozygous mice (lacZ-Nbnins6/lox6). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco Life Technologies, Germany) supplemented with 5% glucose and 10% foetal calf serum (FCS). Cell culture conditions were 37 °C and 5% CO2. Environmental oxygen was reduced to 4%. To generate homozygous null mutant fibroblasts in vitro, lacZ-Nbnins6/lox6 cells were incubated in suspension on two consecutive days with 1 μM cre recombinase (Excellgen, Rockville) for 2 h. Loss of nibrin was determined by PCR and western blot analysis as previously described [6]. Fibroblast cultures were irradiated in 75 cm2 flasks on ice with a single dose of 5 Gy and cells subsequently examined in the comet assay or lacZ assay.
2.3. Determination of mutation frequency and mutant classification
For the mutation frequency assay, cells were harvested 24 h after irradiation. The cell pellets were washed with PBS once and the DNA was extracted using standard methods. DNA was extracted from mouse organs flash frozen in liquid nitrogen until use. DNA was isolated by standard phenol/chloroform extraction from pulverised tissues treated previously with proteinase K.
Mutant frequencies were determined as described earlier [12]. Briefly, 10–20 μg of genomic DNA from lacZ-transgenic mice or fibroblasts was digested with HindIII and the lacZ-reporter plasmids recovered on magnetic beads pre-coated with a lacZ/lacI fusion protein. After washing, the plasmids were eluted with isopropylthio-β-galactoside (IPTG) and recircularised with T4-ligase. Plasmid DNA was electroporated into the E. coli C strain (GalE−, ΔLacZ). To determine the mutation frequency, the bacteria were plated on two different agars containing either 5-Brom-4-chlor-3-indoxyl-β-d-galactopyranoside (X-gal) or phenyl-β-d-galactopyranoside (P-gal). As mutant plasmids will only grow on the selective P-gal plate, the mutation frequency is calculated as the number of mutants divided by the number of total recovered plasmids (X-gal plate) multiplied by the appropriate dilution factor.
Mutant plasmids from the P-gal plate were transferred to 150 μl LB Medium in 96-well plates and cultured for at least 8 h before a colony-PCR for the lacZ gene was performed. PCR products were digested with AvaI and HindIII and the fragments were resolved on a 1% agarose gel. The mutation rate was corrected as previously described for HindIII star activity and cloning artefacts based on the restriction enzyme patterns [13]. Positive clones were sequenced directly or after mini preparation of plasmid DNA using the previously described overlapping primers in the lacZ sequence [14]. Identical mutations due to clonal expansion of cells were counted only once.
2.4. Single cell gel electrophoresis assay (comet assay)
The comet assay was performed under alkaline conditions as described [15]. Briefly, frosted-end slides were coated with one layer of 1% normal melting agarose (NMA) in PBS, dried overnight and then covered with a second layer of 0.5% NMA. 5 × 105 harvested fibroblasts were mixed with 0.7% low melting agarose and layered onto the prepared slides. Lysis carried out in the dark at 4 °C for 1 h followed by unwinding of the DNA under alkaline conditions for 20 min. Electrophoresis was for 20 min followed by neutralisation. After drying, slides were stained with 1 μM 4′,6-diamidin-2-phenylindol (DAPI). Each data point is based on the analysis of at least 150 comets per coded sample. The scoring of the comets and the calculation of the tail moment ([percent of DNA in the tail] × [tail length]) was performed with the CASP software [16].
3. Results
3.1. Null and hypomorphic nibrin mutation increases replication based DNA damage and increases the induced mutation frequency in vitro
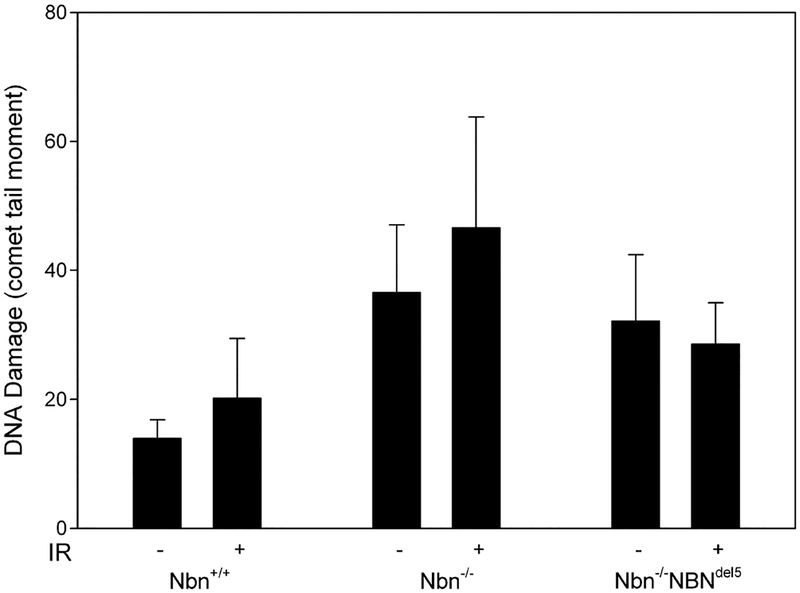
The trimeric Mre11/Rad50/Nibrin complex is involved in the detection and repair of DNA DSBs as indicated by the increased chromosomal breakage typical for NBS patient cells. As shown by the comet assay in Fig. 1, both our conditional null mutant mouse fibroblasts and the humanised mouse fibroblasts display a comparatively high initial comet tail moment in comparison to control cells (p = 0.0424, two-tailed Mann–Whitney U test). The alkaline comet assay used here detects not only single and double-strand breaks but also apurinic and apyrimidinic sites, which are thus alkali labile, and similarly, some damaged bases [17]. Surprisingly, DNA damage after irradiation with 5 Gy is only slightly increased for control cells and the null mutant cells, however, not at all for the humanised mouse cells (Fig. 1). This difference between null mutant cells and cells expressing the hypomorphic nibrin fragment may indicate the residual DNA repair capacity of the mutant fragment. These data suggest that spontaneous DNA damage, probably incurred during DNA replication, is increased in the absence of wild type nibrin and could contribute significantly to an increased mutation frequency.
Fig. 1.
DNA damage in untreated and irradiated Nbn null mutant and control fibro-blasts. The columns give DNA damage as measured with the comet assay (comet tail moment) for murine fibroblasts with the given genotypes. Cells were either untreated (−) or irradiated 10 minutes before lysis with 5 Gy (+). At least 150 nuclei were measured per sample. The chart shows the average results of three experiments, the error bars give the standard deviation.
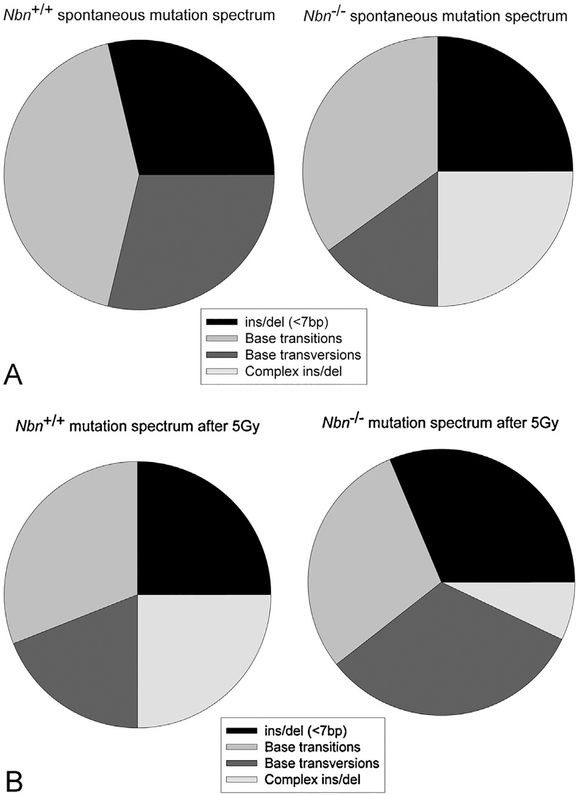
To examine this question in more detail, we measured mutation frequency and mutation spectra in vitro for untreated and irradiated null mutant Nbn mouse cells using the lacZ reporter assay. As detailed in supplementary Table S1a, the basal mutation frequency in null mutant Nbn mouse cells in vitro is 3.2 × 10−5 and rises slightly to 5.2 × 10−5 after 5 Gy irradiation. Control wild type cells had comparable mutation frequencies of 2.8 × 10−5 and 4.6 × 10−5, respectively. Whilst wild type cells had mostly base substitutions and small indels as spontaneous mutations, the null mutant Nbn mouse cells had a large proportion (25%) of complex insertions and deletions involving mouse sequences (Fig. 2A). Such mutations are likely the result of erroneous homologous recombination or non-homologous end joining. A comparably high level of complex mutations in wild type cells was only seen after 5 Gy irradiation (Fig. 2B). Whilst such mutations are also found in null mutant Nbn mouse cells after irradiation, there is a striking increase in the proportion of base transversions in comparison to wild type cells (Fig. 2B).
Fig. 2.
Basal and induced mutation in Nbn null mutant fibroblasts. Conditional null mutant Nbnins6/lox6 fibroblasts carrying the lacZ reporter were treated with cre recombinase to induce homozygosity for Nbn null mutations before measuring mutation frequency of the lacZ reporter as described in Materials and Methods. Mutation spectra were determined by sequencing of rescued lacZ plasmids (A) Basal mutation rate in wild type control mouse cells and Nbn null mutant mouse cells. (B) Mutation rate in wild type control mouse cells and Nbn null mutant mouse cells 24 h after irradiation with 5 Gy. Full data are given in supplementary Tables S1 and S2.
Examining the specificity of base changes indicated that in unir-radiated null mutant Nbn cells the transition G:C>A:T dominates whilst in irradiated cells the transversion C:G>A:T is particularly frequent (supplementary Table S1b). The transversions T:A>G:C and C:G>G:C were observed after irradiation of null mutant Nbn cells but not wild type cells. These characteristic mutation spectra indicate a distinct molecular origin for mutations incurred in the absence of wild type nibrin during normal cell growth or after a mutagenic treatment.
3.2. Hypomorphic mutation of nibrin leads to an increased basal mutation frequency in vivo with an increased proportion of single base transitions rather than transversions
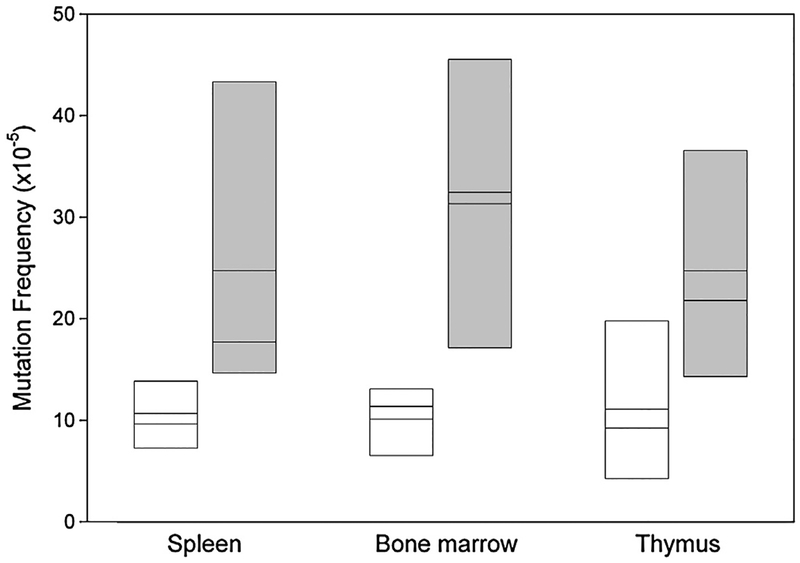
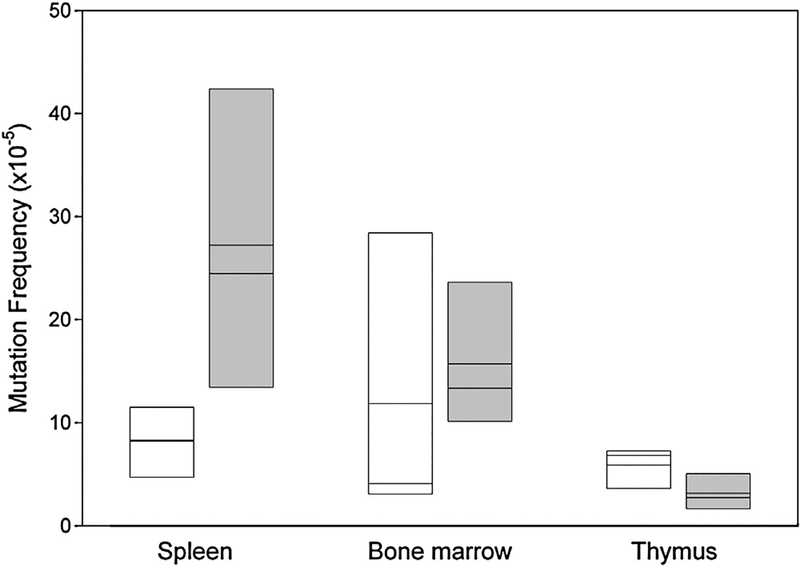
Considering the increased mutation frequency measured in vitro, we turned to an in vivo analysis more relevant for the disease NBS which is characterised by lymphatic malignancies arising early in childhood with no known associated environmental stress [18]. We examined the major lymphatic organs of control mice and humanised mice for mutation frequency and spectrum using the lacZ assay (see supplementary Table S2a). As shown in Fig. 3, the mutation frequency was on average 10.5 × 10−5 in the spleen, bone marrow and thymus of control mice, a value comparable to that measured in other mice using the lacZ assay [19–21]. In comparison, the same organs in the humanised mice had more than double this mutation frequency at 23.9 × 10−5 (p = 0.0018 two-tailed Mann–Whitney U test).
Fig. 3.
Basal in vivo mutation frequency in spleen, thymus and bone marrow for Nbn+/+ and Nbn−/−NBNdel5 mice. Control mice (Nbn+/+, white boxes) and humanised mice (Nbn−/−NBNdel5, grey boxes) carrying the lacZ reporter were examined for mutation frequency as described. The box plot shows the results from 3 to 5 control and 4 to 6 humanised mice. The mean and median are indicated.
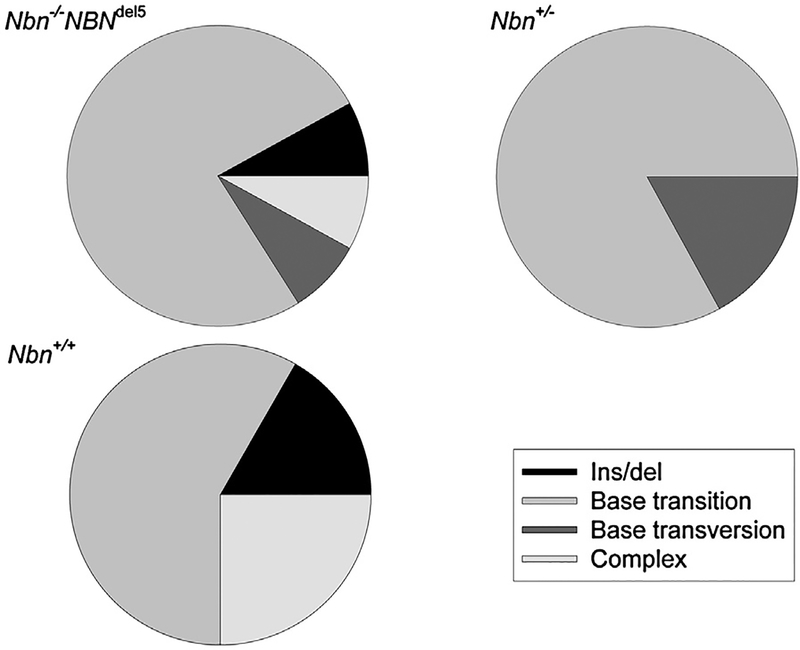
In the absence of wild type nibrin in fibroblasts, irradiation leads to an increased proportion of base transversions (Fig. 2B), suggesting that this particular mutation can be attributed to the DSB repair deficiency caused by nibrin loss. In contrast, as shown in Fig. 4A, the increased basal mutation frequency in the humanised NBS mice in vivo is characterised by base transitions rather than transversions. Again, fewer complex mutations, the consequence of recombination events, are observed in the humanised mice than in control mice.
Fig. 4.
Basal in vivo thymus mutation spectra in control, humanised and heterozygous NBS mice. Mutations were determined by DNA sequencing of plasmids rescued from the thymus of mice carrying the lacZ reporter as in the experiments shown in Fig. 3. (A) Control mice (Nbn+/+) and humanised mice (Nbn−/−NBNdel5). (B) Heterozygous Nbn+/− mice. The legend gives the four types of mutation detected.
3.3. The in vivo radiation induced mutation frequency and mutation spectrum is not altered by hypomorphic mutation of nibrin
Considering the increased mutation frequency observed after irradiation in the null mutant fibroblasts, we examined the mutation frequency in vivo in the humanised mice after repeated low-dose irradiation. As detailed in supplementary Table S3 and shown in Fig. 5, mutation rates dropped in comparison to unirradiated humanised mice (supplementary Table S2 and Fig. 3) from 22.12 × 10−5 to 5.91 × 10−5 in the thymus and from 25.65 × 10−5 to 14.14 × 10−5 in bone marrow. In the spleen, mutation frequencies were comparable with and without irradiation, 23.79 × 10−5 and 23.1 × 10−5, however, significantly higher after irradiation than in the control mice (p = 0.0087, two-tailed Mann–Whitney U test). Although the proportions of base mutations (no change) and complex (size change) mutations varied amongst the three organs examined, they were in each case comparable between humanised mice and control mice (supplementary Table S3).
Fig. 5.
Irradiation induced in vivo mutation frequency in spleen, thymus and bone marrow for Nbn+/+ and Nbn−/−NBNdel5 mice. Control mice (Nbn+/+, white boxes) and humanised mice (Nbn−/−NBNdel5, grey boxes) carrying the lacZ reporter were examined for mutation frequency as described. The box plot shows the results from 3 to 6 control and 3 to 5 humanised mice. The mean and median are indicated.
3.4. The in vivo basal mutation spectrum in the thymus of Nbn heterozygous mice resembles that of mice with a with a hypomorphic mutation in Nbn
Since heterozygous relatives of NBS patients have a significantly increased lifetime cancer risk [4] we were interested to see whether this is reflected in a measurably increased mutation frequency in vivo in mice carrying just one functional Nbn allele. In the lacZ assay, such heterozygous mice had an average mutation frequency of 14.3 × 10−5 in the three lymphatic organs examined, which is not significantly different from that of control mice (10.4 × 10−5). However, the spectrum of mutations was indeed shifted from that of control mice (supplementary Table S2b). As shown in Fig. 4B, complex rearrangements, a mutation which represented 25% of mutations in the thymus of control animals were not found at all amongst the 4 heterozygous mice examined. In contrast, as in the case of Nbn−/−NBNdel5 mice, base transitions dominated the basal mutation spectrum.
4. Discussion
Although the spontaneous chromosomal breakage observed in lymphocytes and fibroblasts from NBS patients indicates increased DNA damage due to repair failure, most biochemical analyses find no gross deficiency in the rejoining of DSBs induced by ionising radiation [22,23]. This can reflect insensitivity of the assays used, but more likely stems from the fact that NBS cells have a hypomorphic mutation in the NBN gene and that a functionally partially active nibrin protein is indeed produced [6,7]. Using a conditional null mutant in murine cells we have previously shown extreme chromosomal breakage, massive cell cycle defects and rapid cell death in the complete absence of nibrin. However, exogenous expression of human nibrin with the hypomorphic mutation prolonged survival of null mutant cells in vitro [6]. Similarly, lymphocytes from the humanised mice expressing hypomorphic nibrin [7] show about half the chromosomal breakage observed in lymphocytes from our null mutant mice.
Although we did detect here a high level of DNA damage, DSBs, SSBs and alkali labile sites, in these null mutant cells, their mutation spectrum did not reflect erroneous repair of this kind of DNA lesion. Small deletions, due mainly to NHEJ, and complex rearrangements, due in general to HR, were not significantly increased in irradiated cells in comparison to unirradiated cells. However, the base mutation spectrum of irradiated null mutant Nbn cells differed clearly from that of untreated null mutant Nbn cells: transversions dominate in the irradiated cells, transitions in the untreated cells. Transversions have been previously reported as common in irradiated cells [24,25]. The transversions C:G>A:T and C:G>G:C, observed in the irradiated nibrin deficient cells are believed to originate from the oxidation product of guanine, 8-oxo-deoxyguanosine and its own oxidation product, imidazolone, respectively [26]. Such oxidation products in DNA are expected by an indirect effect after formation of radicals from water through ionising radiation, indeed, for every DSB generated by ionising radiation there are approximately 100 damaged bases [27].
The common transition, G:C>A:T, in unirradiated cells in vitro may be caused by the tautomer of guanine, enol guanine, which pairs with thymine during replication leading to G>A transitions unless detected by the mismatch repair pathway. In cells lacking the mismatch repair gene Msh6, the proportion of G:C>A:T transitions is strongly increased [21]. The transition T:A>C:G, previously reported as a consequence of UV radiation was not observed in the cells examined here.
The determination of mutation frequency in vivo demonstrated a significantly increased basal mutation rate in the humanised Nbn−/−NBNdel5 mouse compared to control mice in all three lymphatic organs analysed. The two-fold increase in mutation rate is comparable to the 1.2-fold increase found in the liver of NER deficient Xpa−/− mice [28] or the 1.3-fold increase in NHEJ deficient Ku80−/− mouse spleens [19]. In contrast, mismatch repair deficient Mlh1−/− and Pms2−/− mice had 18-fold and 13-fold more spontaneous mutations, respectively [29]. These differences illustrate dramatically the importance of mismatch repair proteins in removing base incorporation errors after replication.
The increased mutation rates reported for Xpa and Ku80 mutants are not expected to be due to misincorporation during replication. In the case of Nbn mutants, however, replication errors may indeed play a role. The mutation spectrum in the thymus of Nbn−/−NBNdel5 mice showed an increased proportion of base mutations and a reduction in complex mutations and small deletions in comparison to control mice. Again the major base mutation was the transition, G:C>A:T. Since there were relatively few base transversions, the mutation so characteristic for mouse cells completely lacking nibrin, we can conclude that the p70-nibrin fragment generated from the hypomorphic NBNdel5 allele is functional in at least this aspect of DNA repair.
The transition G:C>A:T can arise when enol-guanine mispairs during replication suggesting that replication errors are a major source of DNA mutation in Nbn−/−NBNdel5 mouse cells and, presumably, NBS patients too. Why should a hypomorphic mutation in a gene associated usually with the repair of DNA double-strand breaks lead to this type of mutation? The answer may be the involvement of nibrin in mismatch repair. Several lines of evidence have previously suggested that nibrin and the mismatch repair proteins are molecular partners. Firstly, the trimeric complex MRE11/RAD50/NBN is part of a larger complex, BASC, in which the major mismatch repair proteins, MLH1, MSH2 and MSH6 are also located [30]. Secondly, nibrin was found specifically bound to MLH1 in cells treated with temozolomide, an agent which generates the guanine adduct, O6-methylguanine [31]. Thirdly, MNNG treatment of cells leads to replication independent accumulation and association of PCNA and mismatch repair proteins accompanied by phosphorylation of chromatin-bound nibrin [32].
Although the exact role of nibrin in the recognition and repair of mismatches remains to be determined, the reports cited above suggest that mutation of nibrin could result in mismatch repair failure and a concomitant increase in transitions, such as G:C>A:T. Considering the lack of complex rearrangements and small deletions seen in humanised NBS mouse cells in the analyses reported here, the role of nibrin in DSB repair may well be less relevant for mutagenesis than its role in repair of mismatches during DNA replication.
The in vivo mutation frequency in the bone marrow and thymus of control and humanised mice was not significantly increased 7 days after irradiation. This clearly illustrates that elimination of damaged cells by apoptosis is functional in these organs in the presence of hypomorphic nibrin. Clearly nibrin is required for radiation induced apoptosis and the p70-nibrin product of the hypomorphic c.657 661del5 mutation is sufficient to uphold this function. Indeed, even enhanced apoptosis has been previously reported in irradiated NBS patient cells [33] and in a haploinsufficient mouse model [34]. Furthermore, the early embryonic lethality of Nbn null mutants was due to massive apoptosis [8]. Expression of null mutant Nbn specifically in the brain lead to cerebellar defects due to apoptosis of post mitotic neurons, an effect which was rescued on a p53−/− background [35]. Interestingly apoptosis would seem to be somewhat less efficient in the spleen since there is a significantly higher mutation frequency in this organ in the humanised mouse than in controls. We propose that while the radiosensitivity of NBS patients can be attributed to the increased DNA damage response in terms of apoptosis, the increased cancer risk in the absence of environmental mutagenesis is due to the failure to repair spontaneous DNA damage, perhaps coupled with a specific apoptosis deficiency in the spleen.
In an epidemiological study of relatives of NBS patients we have previously reported an increased tumour risk for heterozygotes [4]. Similarly, in many, but not all reports, heterozygous NBN mutations have been found more frequently amongst cancer patients than in the general population [36–38]. Examining mice heterozygous for a null mutation, Nbnins6, indicated an increased tumour occurrence without loss of the remaining wild type allele [8]. Similarly, the same Nbnins6 mutation on a mammary tumour prone MMTV-neu background lead to the development of highly metastatic mammary tumours [34]. We have examined here the mutation frequency in mice heterozygous for a null mutation in Nbn and although we find no evidence for an increased basal mutation rate, we do see a shift in mutation spectrum in comparison to wild type mice. This shift, a reduction in the proportion of complex mutations and an increase in base transitions, is practically identical to the spectrum of the humanised mice in vivo and very similar to that of Nbn null mutant fibroblasts in vitro. This finding supports our concept of NBN as a haploinsufficient tumour suppressor gene [10] and suggests furthermore that the failure to repair spontaneous DNA lesions is a major contributor to the increased cancer risk not only in NBS patients but probably also in heterozygous carriers.
Supplementary Material
Acknowledgments
The authors are grateful to Bettina Ueberle, Klinik fuer Dermatologie und Allergologie, Universitaetsklinikum Ulm, for instruction in the lacZ assay and Brigitte Köttgen, Institut für Klinische Chemie und Pathochemie, Charité Berlin for instruction in the comet assay. We thank Susanne Rothe for mouse genotyping, Vittoria von Petersdorff for assistance in DNA sequencing and Moonsook Lee for assistance with the lacZ assay. This work was supported by the Deutsche Forschungsgemeinschaft (grant number DI 505/3–1 to M.D.) and NIH to J.V.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.mrfmmm.2014.07.001.
References
- [1].Varon R, Vissinga C, Platzer M, Cerosaletti KM, Chrzanowska KH, Saar K, Beckmann G, Seemanova E, Cooper PR, Nowak NJ, Stumm M, Weemaes CM, Gatti RA, Wilson RK, Digweed M, Rosenthal A, Sperling K, Concannon P, Reis A, Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome, Cell 93 (1998) 467–476. [DOI] [PubMed] [Google Scholar]
- [2].Chrzanowska KH, Gregorek H, Dembowska-Baginska B, Kalina MA, Dig-weed M, Nijmegen breakage syndrome (NBS), Orphanet. J. Rare Dis 7 (2012) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chrzanowska KH, Digweed M, Sperling K, Seemanova E, DNA-repair deficiency and cancer lessons from lymphoma, in: Hereditary Tumors, Wiley-VCH Verlag GmbH & Co. KGaA, 2009, pp. 377–391. [Google Scholar]
- [4].Seemanova E, Jarolim P, Seeman P, Varon R, Digweed M, Swift M, Sperling K, Cancer risk of heterozygotes with the NBN founder mutation, J. Natl. Cancer Inst 99 (2007) 1875–1880. [DOI] [PubMed] [Google Scholar]
- [5].Maser RS, Zinkel R, Petrini JH, An alternative mode of translation permits production of a variant NBS1 protein from the common Nijmegen breakage syndrome allele, Nat. Genet 27 (2001) 417–421. [DOI] [PubMed] [Google Scholar]
- [6].Demuth I, Frappart PO, Hildebrand G, Melchers A, Lobitz S, Stockl L, Varon R, Herceg Z, Sperling K, Wang ZQ, Digweed M, An inducible null mutant murine model of Nijmegen breakage syndrome proves the essential function of NBS1 in chromosomal stability and cell viability, Hum. Mol. Genet 13 (2004) 2385–2397. [DOI] [PubMed] [Google Scholar]
- [7].Difilippantonio S, Celeste A, Fernandez-Capetillo O, Chen HT, Reina San Martin B, Van Laethem F, Yang YP, Petukhova GV, Eckhaus M, Feigenbaum L, Manova K, Kruhlak M, Camerini-Otero RD, Sharan S, Nussenzweig M, Nussenzweig A, Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models, Nat. Cell Biol 7 (2005) 675–685. [DOI] [PubMed] [Google Scholar]
- [8].Dumon-Jones V, Frappart PO, Tong WM, Sajithlal G, Hulla W, Schmid G, Herceg Z, Digweed M, Wang ZQ, Nbn heterozygosity renders mice susceptible to tumor formation and ionizing radiation-induced tumorigenesis, Cancer Res 63 (2003) 7263–7269. [PubMed] [Google Scholar]
- [9].Zhu J, Petersen S, Tessarollo L, Nussenzweig A, Targeted disruption of the Nijmegen breakage syndrome gene NBS1 leads to early embryonic lethality in mice, Curr. Biol 11 (2001) 105–109. [DOI] [PubMed] [Google Scholar]
- [10].Demuth I, Digweed M, The clinical manifestation of a defective response to DNA double-strand breaks as exemplified by Nijmegen breakage syndrome, Oncogene 26 (2007) 7792–7798. [DOI] [PubMed] [Google Scholar]
- [11].Gossen JA, Martus HJ, Wei JY, Vijg J, Spontaneous and X-ray-induced deletion mutations in a LacZ plasmid-based transgenic mouse model, Mutat. Res 331 (1995) 89–97. [DOI] [PubMed] [Google Scholar]
- [12].Dolle ME, Martus HJ, Gossen JA, Boerrigter ME, Vijg J, Evaluation of a plasmid-based transgenic mouse model for detecting in vivo mutations, Muta-genesis 11 (1996) 111–118. [DOI] [PubMed] [Google Scholar]
- [13].Dolle ME, Snyder WK, van Orsouw NJ, Vijg J, Background mutations and polymorphisms in lacZ-plasmid transgenic mice, Environ. Mol. Mutagen 34 (1999) 112–120. [PubMed] [Google Scholar]
- [14].Garcia AM, Derventzi A, Busuttil R, Calder RB, Perez E Jr., Chadwell L, Dolle ME, Lundell M, Vijg J, A model system for analyzing somatic mutations in Drosophila melanogaster, Nat. Methods 4 (2007) 401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Speit G, Rothfuss A, The comet assay: a sensitive genotoxicity test for the detection of DNA damage and repair, Methods Mol. Biol 920 (2012) 79–90. [DOI] [PubMed] [Google Scholar]
- [16].Konca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Gozdz S, Koza Z, Wojcik A, A cross-platform public domain PC image-analysis program for the comet assay, Mutat. Res 534 (2003) 15–20. [DOI] [PubMed] [Google Scholar]
- [17].Collins AR, Dobson VL, Dusinska M, Kennedy G, Stetina R, The comet assay: what can it really tell us? Mutat. Res 375 (1997) 183–193. [DOI] [PubMed] [Google Scholar]
- [18].Seemanova E, Passarge E, Beneskova D, Houstek J, Kasal P, Sevcikova M, Familial microcephaly with normal intelligence, immunodeficiency, and risk for lymphoreticular malignancies: a new autosomal recessive disorder, Am. J. Med. Genet 20 (1985) 639–648. [DOI] [PubMed] [Google Scholar]
- [19].Busuttil RA, Munoz DP, Garcia AM, Rodier F, Kim WH, Suh Y, Hasty P, Campisi J, Vijg J, Effect of Ku80 deficiency on mutation frequencies and spectra at a LacZ reporter locus in mouse tissues and cells, PLoS One 3 (2008) e3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Busuttil RA, Lin Q, Stambrook PJ, Kucherlapati R, Vijg J, Mutation frequencies and spectra in DNA polymerase eta-deficient mice, Cancer Res 68 (2008) 2081–2084. [DOI] [PubMed] [Google Scholar]
- [21].Mark SC, Sandercock LE, Luchman HA, Baross A, Edelmann W, Jirik FR, Elevated mutant frequencies and predominance of G:C to A:T transition mutations in Msh6(–/–) small intestinal epithelium, Oncogene 21 (2002) 7126–7130. [DOI] [PubMed] [Google Scholar]
- [22].Kraakman-van der Zwet M, Overkamp WJ, Friedl AA, Klein B, Verhaegh GW, Jaspers NG, Midro AT, Eckardt-Schupp F, Lohman PH, Zdzienicka MZ, Immortalization and characterization of Nijmegen Breakage syndrome fibro-blasts, Mutat. Res 434 (1999) 17–27. [DOI] [PubMed] [Google Scholar]
- [23].Kasten-Pisula U, Tastan H, Dikomey E, Huge differences in cellular radiosensitivity due to only very small variations in double-strand break repair capacity, Int. J. Radiat. Biol 81 (2005) 409–419. [DOI] [PubMed] [Google Scholar]
- [24].Yuan J, Yeasky TM, Rhee MC, Glazer PM, Frequent T:A→G:C transversions in X-irradiated mouse cells, Carcinogenesis 16 (1995) 83–88. [DOI] [PubMed] [Google Scholar]
- [25].Kimura H, Higuchi H, Iyehara-Ogawa H, Kato T, Sequence analysis of X-ray-induced mutations occurring in a cDNA of the human hprt gene integrated into mammalian chromosomal DNA, Radiat. Res 134 (1993) 202–208. [PubMed] [Google Scholar]
- [26].Kino K, Sugiyama H, Possible cause of G-C→C-G transversion mutation by guanine oxidation product, imidazolone, Chem. Biol 8 (2001) 369–378. [DOI] [PubMed] [Google Scholar]
- [27].Lehnert S, Damage to DNA by ionizing radiation, in: Biomolecular Action of Ionizing Radiation, Taylor & Francis, 2007. [Google Scholar]
- [28].Frijhoff AF, Krul CA, de Vries A, Kelders MC, Weeda G, van Steeg H, Baan RA, Influence of nucleotide excision repair on N-hydroxy-2-acetylaminofluorene-induced mutagenesis studied in lambda lacZ-transgenic mice, Environ. Mol. Mutagen 31 (1998) 41–47. [DOI] [PubMed] [Google Scholar]
- [29].Baross-Francis A, Makhani N, Liskay RM, Jirik FR, Elevated mutant frequencies and increased C:G→T:A transitions in Mlh1−/−versus Pms2−/− murine small intestinal epithelial cells, Oncogene 20 (2001) 619–625. [DOI] [PubMed] [Google Scholar]
- [30].Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J, BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures, Genes Dev 14 (2000) 927–939. [PMC free article] [PubMed] [Google Scholar]
- [31].Mirzoeva OK, Kawaguchi T, Pieper RO, The Mre11/Rad50/Nbs1 complex interacts with the mismatch repair system and contributes to temozolomide-induced G2 arrest and cytotoxicity, Mol. Cancer Ther 5 (2006) 2757–2766. [DOI] [PubMed] [Google Scholar]
- [32].Schroering AG, Williams KJ, Rapid induction of chromatin-associated DNA mismatch repair proteins after MNNG treatment, DNA Repair (Amst.) 7 (2008) 951–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sagan D, Mortl S, Muller I, Eckardt-Schupp F, Eichholtz-Wirth H, Enhanced CD95-mediated apoptosis contributes to radiation hypersensitivity of NBS lymphoblasts, Apoptosis 12 (2007) 753–767. [DOI] [PubMed] [Google Scholar]
- [34].Wan R, Crowe DL, Haploinsufficiency of the Nijmegen breakage syndrome 1 gene increases mammary tumor latency and metastasis, Int. J. Oncol 41 (2012) 345–352. [DOI] [PubMed] [Google Scholar]
- [35].Frappart PO, Tong WM, Demuth I, Radovanovic I, Herceg Z, Aguzzi A, Digweed M, Wang ZQ, An essential function for NBS1 in the prevention of ataxia and cerebellar defects, Nat. Med 11 (2005) 538–544. [DOI] [PubMed] [Google Scholar]
- [36].Bogdanova N, Feshchenko S, Schurmann P, Waltes R, Wieland B, Hillemanns P, Rogov YI, Dammann O, Bremer M, Karstens JH, Sohn C, Varon R, Dork T, Nijmegen Breakage Syndrome mutations and risk of breast cancer, Int. J. Cancer 122 (2008) 802–806. [DOI] [PubMed] [Google Scholar]
- [37].Steffen J, Varon R, Mosor M, Maneva G, Maurer M, Stumm M, Nowakowska D, Rubach M, Kosakowska E, Ruka W, Nowecki Z, Rutkowski P, Demkow T, Sadowska M, Bidzinski M, Gawrychowski K, Sperling K, Increased cancer risk of heterozygotes with NBS1 germline mutations in Poland, Int. J. Cancer 111 (2004) 67–71. [DOI] [PubMed] [Google Scholar]
- [38].Mosor M, Ziolkowska I, Pernak-Schwarz M, Januszkiewicz-Lewandowska D, Nowak J, Association of the heterozygous germline I171V mutation of the NBS1 gene with childhood acute lymphoblastic leukemia, Leukemia 20 (2006) 1454–1456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.