Abstract
Cognitive impairment (CI), a debilitating and pervasive feature of multiple sclerosis (MS), is correlated with hippocampal atrophy. Findings from postmortem MS hippocampi indicate that expression of genes involved in both excitatory and inhibitory neurotransmission are altered in MS, and although deficits in excitatory neurotransmission have been reported in the MS model experimental autoimmune encephalomyelitis (EAE), the functional consequence of altered inhibitory neurotransmission remains poorly understood. In this study, we used electrophysiological and biochemical techniques to examine inhibitory neurotransmission in the CA1 region of the hippocampus in EAE. We find that tonic, GABAergic inhibition is enhanced in CA1 pyramidal cells from EAE mice. Although plasma membrane expression of the GABA transporter GAT-3 was decreased in the EAE hippocampus, an increased surface expression of α5 subunit-containing GABAA receptors appears to be primarily responsible for the increase in tonic inhibition during EAE. Enhanced tonic inhibition during EAE was associated with decreased CA1 pyramidal cell excitability and inhibition of α5 subunit-containing GABAA receptors with the negative allosteric modulator L-655,708 enhanced pyramidal cell excitability in EAE mice. Together, our results suggest that altered GABAergic neurotransmission may underlie deficits in hippocampal-dependent cognitive function in EAE and MS.
Keywords: Multiple sclerosis, experimental autoimmune encephalomyelitis, hippocampus, inhibitory synaptic transmission, tonic inhibition, intrinsic excitability, long-term potentiation
Introduction
Cognitive impairment (CI) is a debilitating feature of multiple sclerosis (MS) that presents in both relapsing remitting and progressive MS subtypes (Ruano et al., 2016), and is associated with reduced quality of life due to impacts on employment and social engagement (Rao et al., 1991). Brain magnetic resonance imaging studies show atrophy within the hippocampus, disproportionately affecting the CA1 region (Sicotte et al., 2008). Importantly, both total and regional hippocampal atrophy (Sicotte et al., 2008, Longoni et al., 2015) are correlated with impairments in visuospatial/verbal learning, one of the primary cognitive domains affected by MS (Litvan et al., 1988, Denney et al., 2005, Ruano et al., 2016, for reviews see Chiaravalloti and DeLuca 2008, Guimaraes and Sa 2012). Therefore, the vulnerability of the hippocampus and CA1 subregion to pathologic processes during MS may contribute to the clinical presentation of CI.
Although the molecular mechanisms underlying hippocampal dysfunction in MS are unclear, findings from postmortem MS hippocampi indicate a downregulation of genes involved in excitatory synaptic transmission, including NMDA receptor (NMDAR) and AMPA receptor subunits, but an upregulation of genes involved in inhibitory synaptic transmission, including α5, β3, and γ2 subunits of GABAA receptors (Dutta et al., 2011). Consistent with these findings, alterations in both excitatory (Centonze et al., 2009, Ziehn et al., 2012, Grasselli et al., 2013, Mandolesi et al., 2013) and phasic forms of inhibitory synaptic transmission (Rossi et al., 2011, Mandolesi et al., 2012, Nistico et al., 2013), have been reported in the MS model experimental autoimmune encephalomyelitis (EAE). Interestingly, α5/β3/γ2 subunit-containing GABAA receptors give rise to an extrasynaptic pool of GABAA receptors that mediate a distinct form of inhibitory transmission, called tonic inhibition, in hippocampal CA1 pyramidal cells (Caraiscos et al., 2004; Zarnowska et al., 2015). Although studies of inhibitory synaptic dysfunction in MS have focused on alterations in phasic forms of inhibition (Mandolesi et al., 2015), changes in GABAA receptor subunit expression that occur in MS suggest that tonic forms of inhibition might be altered as well. By regulating neuronal excitability, tonic inhibition not only influences neuronal information processing (Mitchell and Silver 2003, Chadderton et al., 2004, Pavlov et al., 2009), but also opposes the induction of activity-dependent forms of synaptic plasticity involved in memory formation, such as long-term potentiation (LTP) (Martin et al., 2010, Whissell et al., 2013). Furthermore, pharmacological inhibition of α5 subunit-containing GABAARs (α5-GABAARs) improves performance on hippocampus-dependent learning tasks (Atack et al., 2006; Etherington et al., 2017), and α5 subunit null mutant mice exhibit enhanced spatial learning (Collinson et al., 2002). Therefore, an increase in tonic inhibition might contribute to deficits in LTP (Kim et al., 2012; Di Filippo et al., 2013; Novkovic et al., 2015; Di Filippo et al., 2016; Planche et al., 2016), as well as learning and memory (Ziehn et al., 2010; Acharjee et al., 2013; Novkovic et al., 2015; Di Filippo et al., 2016), observed during EAE.
To determine whether alterations in GABAergic inhibition might contribute to disruption of hippocampal function in MS, we examined phasic and tonic inhibition in EAE. We find that both forms of inhibition are significantly enhanced in hippocampal CA1 pyramidal cells during EAE. We then examined how changes in plasma membrane levels of α5-GABAARs and GABA transporters might contribute to enhanced tonic inhibition in EAE and explored the functional effects of enhanced tonic inhibition on pyramidal cell intrinsic excitability and LTP induction.
Experimental Procedures
All experimental protocols used in this study were approved by UCLA’s Chancellor’s Animal Research Committee and are consistent with PHS Policy on Humane Care and Use of Laboratory Animals.
Active EAE induction
Active EAE was induced in C57BL/6 mice (Jackson labs) using myelin oligodendrocyte glycoprotein (MOG) amino acids 35–55 peptide (MOG35–55), as described (Itoh et al., 2018). Briefly, female mice at 8–15 weeks of age underwent subcutaneous injection of MOG35–55 (200 μg/animal, Mimotopes) emulsified in Complete Freund’s Adjuvant, supplemented with Mycobacterium Tuberculosis H37Ra (200 μg/animal, Difco Laboratories), over two sites drained by left inguinal and auxiliary lymph nodes on day 0 (0.1 ml/mouse). A booster immunization was applied subcutaneously on day 7 over contralateral lymph nodes. Pertussis toxin (500 ng/mouse) (List Biological Laboratories) was injected intraperitoneally on days 0 and 2. Animals were monitored daily and scored based on a standard EAE 0–5 scale scoring system: 0, healthy; 1, complete loss of tail tonicity; 2, loss of righting reflex; 3, partial paralysis; 4, complete paralysis of one or both hind limbs; and 5, premoribund state. Whole-cell voltage-clamp recordings of phasic and tonic inhibitory transmission were performed 21–42 days post-induction (DPI). The induction of LTP by theta pulse stimulation was examined at both early (16–29 DPI) and later (starting at 40 DPI) time points post EAE induction. Whole-cell current-clamp recordings were used to examine CA1 pyramidal cell intrinsic excitability starting at 42 DPI and tissues for western blotting were collected at 40 DPI.
Inhibitory Synaptic Transmission:
Mice were deeply anesthetized with isofluorane, decapitated, and the brain was rapidly removed and placed into ice-cold, oxygenated (bubbled with 100% O2) cutting solution containing 135 mM N-methyl-D-glucamine, 10 mM D-glucose, 4.0 mM MgCl2, 0.5 mM CaCl2, 1.0 mM KCl, 1.2 mM KH2PO4, and 26 mM HEPES (pH = 7.4). Coronal brain slices (320 μm thick) containing the dorsal hippocampus and striatum were cut using a Campden 7000SMZ-2 Vibratome. Slices were then maintained in an interface-slice type chamber perfused at 2–3 ml/min with a warm (30 °C), oxygenated (95% 02/5% CO2) artificial cerebrospinal fluid (ACSF) containing 124 mM NaCl, 4.4 mM KCl, 25 mM NaHC03, 1.0 mM NaH2P04, 2.0 mM CaCl2, 1.2 mM MgS04, and 10 mM glucose (pH = 7.4). Slices were allowed to recover for at least 1 hour prior to electrophysiological recordings. For each experiment, slices were transferred to a submerged-slice type recording chamber (Warner Instruments) perfused with warm (30 °C), oxygenated ACSF and individual cells were visualized using infrared differential interference contrast microscopy (Zeiss Axio Examiner.D1 fitted with 40x water-immersion objective) and a CMOS camera (QImaging Rolera Bolt). Low-resistance electrodes (3–6 MΩ) filled with an internal solution containing 120 mM CsMeSO4, 10 mM CsCl, 5 mM TEA-Cl, 1.5 mM MgCl2, 10 mM HEPES, 0.1 mM EGTA, 2 mM Na-ATP, 0.5 mM Na-GTP, and 5 mM QX-314 (pH = 7.3, osmolarity = 275–285 mOsm) were used to record spontaneous inhibitory postsynaptic currents (sIPSCs) in CA1 pyramidal cells or medium spiny neurons in the striatum. Whole-cell voltage-clamp recordings were performed using a Multiclamp 700B amplifier and pClamp 10 (Molecular Devices) and series resistance and whole-cell capacitance were automatically compensated. Recordings were discontinued if series resistance increased by >20%. Pyramidal cells were voltage- clamped at the reversal potential for excitatory postsynaptic currents (+10 mV) to record sIPSCs. Bath application of 100 μM SR-95531 (Caymen Chemical) blocked sIPSCs, confirming that inhibitory synaptic currents were adequately isolated using this approach. Data analysis was performed using Clampfit (Molecular Devices). The EVAN LabView-based program developed by the Moody laboratory at UCLA was used for the detection, and measurement of peak amplitudes, 10–90% rise times, weighted decay time constants, and frequency of sIPSCs.
Tonic inhibition was measured by the change in the holding current needed to voltage-clamp cells at +10 mV induced by bath application of ≥ 100 μM SR-95531. In our initial experiments examining tonic inhibition in CA1 pyramidal cells (Fig 2A,B), slices were continuously bathed in ACSF containing 5.0 μM GABA to enhance tonic inhibitory currents. All other experiments were performed without supplemental GABA in the ACSF. In experiments examining the effects of the GABAAR modulators L-655,708 and DS2 (Tocris) or the GABA transporter inhibitor nipecotic acid (Tocris) on tonic inhibition, drugs were bath applied until changes in holding current needed to clamp cells at +10 mV reached steady state and SR-95531 was then bath applied. The current needed to clamp cells in the presence of SR-95531 was then subtracted from the amplitude of the holding current before and after application of L-655,708, DS2, or nipecotic acid to determine drug effects on tonic inhibition.
Figure 2.
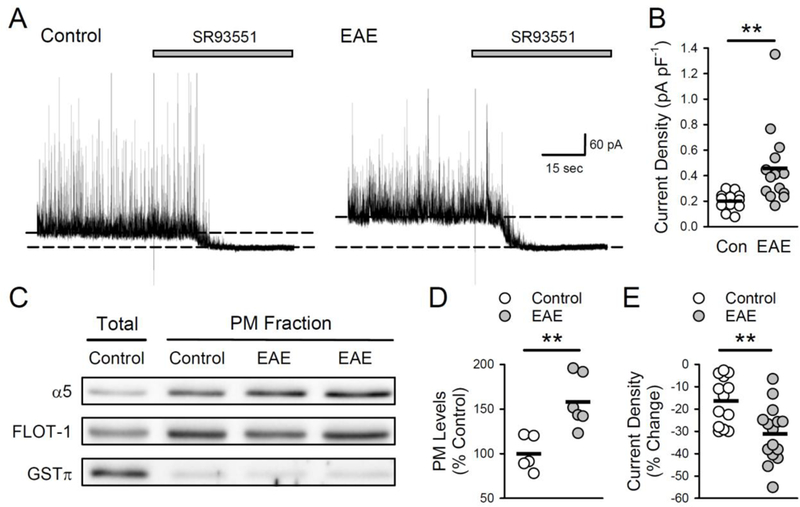
Tonic inhibition and plasma membrane levels of α5 GABAAR subunits are increased in EAE. A, Examples of tonic inhibition in cells from control and EAE mice (slices were continuously bathed in ACSF containing 5.0 μM GABA). Tonic inhibition is revealed by the change in the holding current needed to voltage-clamp cell at +10 mV (indicated by the dashed lines) before and after blocking GABAA receptors with bath application of SR93551 (100 μM, indicated by the bar). B, Tonic inhibition is significantly enhanced in CA1 pyramidal cells from EAE mice. Tonic inhibitory current density was 0.2 ± 0.02 pA/pF in control cells (n = 12 cells from 4 mice) and 0.46 ± 0.08 pA/pF in EAE cells (n = 14 cells from 4 mice, t(24) = 2.845, **p = 8.93×10−3). Plot shows results from all cells, bars indicate mean values. C, Immunoblots showing total and plasma membrane (PM) levels of α5 subunit levels in samples prepared from control and EAE hippocampi. Comparison of total and plasma membrane fractions shows enrichment in the membrane fraction while GSTπ levels show extent of cytosolic protein contamination in membrane fractions. Levels of α5 subunits were normalized to FLOT-1 levels. D, Plot shows results from all experiments, bars indicate mean values. Plasma membrane levels of α5 GABAAR subunits in hippocampal homogenates prepared from EAE mice (n = 6) were 157.9 ± 12.0% of levels in homogenates from control animals (n = 5, t(9) = 3.753, **p = 0.005). E, A 10 minute bath application of L-655,708 (5 μM) reduced tonic inhibition to a greater extend in pyramidal cells from EAE mice compared to controls. Plot shows results from all cells, bars indicate mean values. Tonic inhibition was reduced by 31.2 ± 3.3% in cells from EAE mice (n = 15 cells from 3 mice) compared to 16.3 ± 3.0% in control cells (n = 13 cells from 3 mice, t(26) = 3.284, **p = 2.93×10−3).
Intrinsic Excitability:
Intrinsic excitability was examined using 400 μm thick dorsal hippocampal slices prepared using previously described techniques (Babiec et al., 2017) and maintained at 30 °C in interface-type chambers perfused with ACSF at 2–3 mL/min. Slices were transferred to a submerged-slice recording chamber perfused with ACSF and whole-cell current-clamp recordings from CA1 pyramidal cells were performed using patch electrodes (4–8 MΩ) containing 122.5 mM K-gluconate, 17.5 mM KCl, 10 mM HEPES, 0.2 mM EGTA, 10 mM Na2-phosphocreatine, 4 mM Mg-ATP, 0.3 mM Na-GTP (pH = 7.3, 290 mOsm). The number of action potentials elicited by 500 millisecond depolarizing current pulses (50 – 150 pA) was used to determine neuronal excitability and the peak change in membrane potential elicited by a 500 millisecond long hyperpolarizing current pulse (−100 pA) was used to measure membrane input resistance. Reported membrane potentials have not been corrected for an estimated liquid junction potential of 13.4 mV.
LTP Induction Protocol:
Standard extracellular recording techniques (Babiec et al., 2017) were used to examine LTP in slices maintained in interface-slice recording chambers. In these experiments, a glass microelectrode filled with ACSF (resistance = 5 – 10 MΩ) was placed in stratum radiatum of the CA1 region to record field excitatory postsynaptic potentials (fEPSPs) evoked by single pulses of presynaptic fiber stimulation (0.2 msec in duration, stimulation rate = 0.02 Hz) delivered by a bipolar stimulating electrode placed in stratum radiatum. At the start of each experiment, the intensity of presynaptic fiber stimulation was varied to determine the maximum fEPSP amplitude and the stimulation strength was then set to evoke fEPSPs that were 50% of maximum. After a stable baseline recording of 20 minutes, LTP was induced by a 150 pulse train of theta- frequency stimulation (single stimulation pulses delivered at 5 Hz). fEPSPs were recorded for 45 minutes post-LTP induction. fEPSP slopes were normalized to the pre-5 Hz stimulation baseline and fEPSP slopes averaged over the final 5 minutes were used for statistical comparisons.
Plasma membrane protein isolation:
Dorsal portions of the hippocampus were dissected free from rest of the brain and immediately frozen on dry ice. Frozen hippocampi were pulverized using a mechanical tissue grinder, and further processed for either plasma membrane or whole lysate fractions. Plasma membrane fractions were prepared using a commercially available plasma membrane protein extraction kit (Abcam, ab65400) according to manufacturer’s instructions. Briefly, pulverized tissue was resuspended in homogenization buffer containing 1:500 protease inhibitor cocktail, and homogenized using a glass Dounce homogenizer. Homogenates were centrifuged for 10 min at 700 rcf to remove debris and the nuclear fraction. The supernatants were then centrifuged for 30 min at 10,000 rcf, to separate the cytosol fraction from total cellular membrane protein. The pellets from this spin were resuspended in upper phase and lower phase solutions provided in the kit. The upper phase, containing plasma membrane proteins, was purified after three, 5 min centrifugation steps at 1000 rcf. The purified upper phase was then diluted in water (5x), and centrifuged for 10 min at 13,500 rpm. The resulting pellet was resuspended in modified RIPA (mRIPA) buffer, containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, 10 mM EGTA, 10 mM EDTA, 25 mM sodium pyrophosphate, 10 μM cantharidin, and cocktails of both protease (Sigma- Aldrich) and protein phosphatase inhibitors (Phosphatase Inhibitor Cocktails 2 and 3 from Sigma-Aldrich). For whole lysate protein isolation, mechanically pulverized hippocampi were resuspended in mRIPA buffer and briefly homogenized with a handheld sonicator. Protein concentrations were determined by Bradford assay.
Western Blotting:
Whole hippocampal lysates and plasma membrane protein fractions were diluted in 2X loading buffer (National Diagnostics) and denatured by boiling for 5 minutes. Samples containing 10 μg of protein were separated by SDS-PAGE, using 4% and 12% acrylamide stacking and resolving gels, respectively. Proteins were transferred onto a 0.2 μm nitrocellulose membrane and blocked for 1 hour at room temperature in 4% nonfat dry milk dissolved in Tris-buffered saline containing 0.05% Tween-20 (TBST). Nitrocellulose membranes were incubated overnight at 4°C in milk/TBST containing the antibodies: anti-α5-GABAAR (Aviva Systems Biology, RRID:AB_2046033), anti-GAT3 (Abcam, RRID:AB_304437), anti-GAT1 (Abcam, RRID:AB_2189971), anti-Flotillin-1 (Santa Cruz Biotechnology, RRID:AB_2106567), and anti-GSTπ (Enzo Life Science, RRID:AB_2039146). Blots were then washed three times in TBST and incubated for 3 hours at room-temperature in milk/TSBT containing HRP-conjugated secondary antibodies (1:2000, GE Healthcare catalog no. NA934V, NA931V). To image blots, membranes were immersed in ProtoGlow ECL (National Diagnostics), and immunoreactive bands were detected using a 12-bit cooled CCD camera and Image Lab software (Bio-Rad). Densitometry analysis was performed using ImageJ (NIH). For quantification, density values from α5-GABAAR, GAT1, and GAT3 bands were normalized to values for either the membrane-associated protein flotillin-1, for plasma membrane fractions, or βIII tubulin (Millipore, RRID:AB_309804), for whole lysate proteins, to control for loading variations. Levels of α5-GABAAR and GAT1/3 were expressed as a percentage of control. To estimate molecular weights, MagicMark XP (Thermo Fisher Scientific) standard lanes were imaged together with sample lanes. To confirm enrichment of plasma membrane proteins, dorsal hippocampal lysates containing total protein from controls were run together with plasma membrane fractions during Western immunoblotting. In addition, immunoblots were probed with a glutathione S-transferase π (GSTπ) antibody to confirm the absence of significant cytosolic protein contamination in plasma membrane fractions.
Statistical Analyses
All values are reported as mean ± SEM. Student’s t tests as well as two-way and three-way ANOVAs (followed by Bonferroni t-tests for specific comparisons) were used to determine statistical significance. All statistical analyses were performed using Prism 6 (GraphPad Software) or SigmaPlot (SPSS).
Results
Phasic inhibitory synaptic transmission in CA1 pyramidal cells is enhanced in EAE
Previous studies have found that the frequency of sIPSCs in striatal medium spiny neurons (Rossi et al., 2011), cerebellar Purkinje cells (Mandolesi et al., 2012), and hippocampal CA1 pyramidal cells (Nistico et al., 2013) is significantly reduced during early stages of EAE (20–25 DPI). Thus, to determine how inhibitory transmission in hippocampal CA1 region is altered at later stages of EAE, we first examined sIPSCs in CA1 pyramidal cells from healthy, age-matched control mice and EAE mice 21–42 days post-induction (Fig. 1A,B). Strikingly, at these time points post-EAE induction, sIPSC frequency was significantly higher in CA1 pyramidal cells from EAE mice (Fig. 1B,C) There was no difference, however, in the amplitude, rise time, or decay time course of sIPSCs in cells from EAE and control mice (Fig. 1C). Although our findings, along with those of Nistico et al (2013), suggest that phasic inhibition in the hippocampal CA1 region undergoes distinct changes during EAE, phasic inhibitory synaptic transmission in striatal medium spiny neurons is significantly reduced at both early (20 DPI) and later stages of EAE (up to 90 DPI) (Rossi et al., 2011). Thus, the increase in sIPSC frequency in CA1 pyramidal cells during EAE suggested that there might be regional differences in how inhibitory synaptic transmission is altered in EAE. To examine this possibility we next recorded sIPSCs in striatal medium spiny neurons from both EAE (21–42 DPI) and control mice. Consistent with previous findings (Rossi et al., 2011), the frequency of sIPSCs was significantly reduced in medium spiny neurons from EAE mice (Fig 1D,E). Together, these results suggest that there are significant time and brain region-dependent effects of EAE on phasic inhibitory synaptic transmission.
Figure 1.
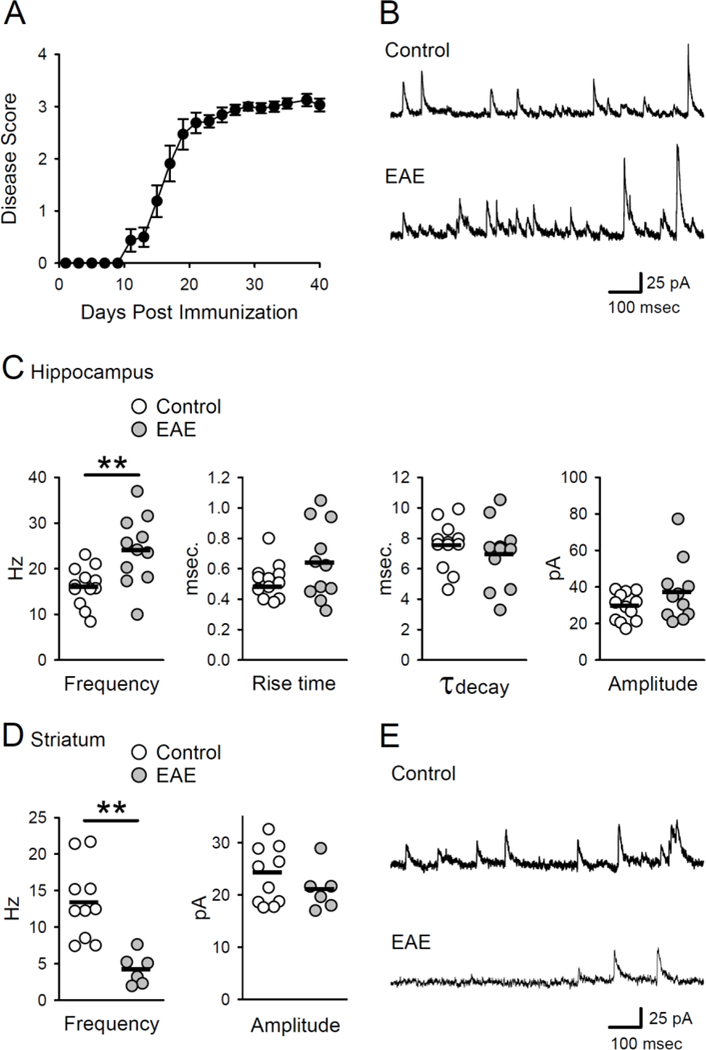
Phasic inhibition is altered in EAE. A, Disease scores for EAE animals used in experiments investigating phasic and tonic inhibition (N = 17). Disease scores were averaged across all animals, yielding a mean disease score per group. B, Examples of sIPSCs in CA1 pyramidal cells in slices from control and EAE mice. C, Frequency, amplitude, rise time, and decay time of sIPSCs in cells from healthy control and EAE mice. Plots show results from all cells, bars indicate mean values. The frequency of sIPSCs in cells from EAE mice (24 ± 2.3 Hz, n = 12 cells from 4 mice) was significantly higher than the sIPSC frequency in cells from controls (16.2 ± 1.2 Hz, n = 11 cells from 3 mice, t(21) = 3.139, **p = 4.96 × 10−3). Peak sIPSC amplitudes were 29.2 ± 2.2 pA in control cells and 37.3 ± 5.1 pA in EAE cells (t(21) = 1.503, p = 0.148). Rise times (1090% of peak amplitude) were 0.52 ± 0.03 msec in control cells and 0.64 ± 0.08 msec in EAE cells (t(21) = 1.503, p = 0.148). Decay time constants derived from single exponential fits to the decaying phase of sIPSCs were 7.5 ± 0.5 msec in control cells and 6.9 ± 0.7 msec in EAE cells (t(21) = 0.767, p = 0.45). D, Frequency and amplitude of sIPSCs in striatal medium spiny neurons. The frequency of sIPSCs in cells from EAE mice (4.2 ± 0.9 Hz, n = 6 cells from 3 mice) was significantly reduced compared to control cells (13.4 ± 1.6 Hz, n = 10 cells from 3 mice, t( 14) = 4.11, **p = 0.001). Peak sIPSC amplitudes were 23.7 ± 1.8 pA in control cells and 21.0 ± 1.7 pA in cells from EAE mice (t(14) = 0.96, p = 0.353). E, Examples of sIPSCs in medium spiny neurons in slices from control and EAE mice.
Increased surface expression of α5-GABAARs contributes to enhanced tonic inhibition in EAE
To examine whether tonic inhibition is altered during EAE, we measured the decrease in the steady-state current needed to voltage-clamp CA1 pyramidal cells at +10 mV induced by the GABAA receptor antagonist SR-93551 (gabazine) (Fig. 2A). As shown in figure 2B, tonic inhibition in cells from EAE mice was approximately 2-fold larger compared to control cells. Tonic inhibition in CA1 pyramidal cells is primarily mediated by α5 subunit-containing receptors (Caraiscos et al., 2004; Zarnowska et al., 2015). Thus, to determine whether differences in the expression of extrasynaptic GABAARs might contribute to the enhanced tonic inhibition in EAE, we measured plasma membrane levels of α5 subunits in hippocampal samples from control and EAE mice. Interestingly, plasma membrane levels of α5 subunits were significantly increased in EAE compared to normal (Fig 2C,D). In a separate series of experiments, we found that total levels of GABAAR α5 subunit expression were also significantly elevated in hippocampal lysates prepared from EAE mice (levels in samples from EAE mice were 131 ± 11% of control, n = 5 EAE and 6 control mice, t(9) = 2.39, p = 0.04). This suggests that that the increased plasma membrane levels of α5 subunits is due, at least in part, to increased total levels of α5 subunit protein. To test whether enhanced α5- GABAARs expression is responsible for increased tonic inhibition during EAE, we examined the effects of L-655,708, an inverse-agonist of the α5 subunit-containing GABAARs (Quirk et al., 1996) on tonic inhibition in CA1 pyramidal cells from EAE and control mice. Following bath application of L-655,708 (5 μM) tonic inhibition was reduced to a greater extent in cells from EAE mice than from control mice (Fig 2E). Moreover, in the presence of L-655,708, tonic inhibition in cells from EAE mice was not significantly different from control cells (t(26) = 1.792, p = 0.08). Because δ subunit- containing GABAARs also contribute to tonic inhibition in CA1 neurons (Scimemi et al., 2005), we examined the effects of DS2, a positive allosteric modulator of 5 subunit- containing GABAARs (Wafford et al., 2009) on tonic inhibition in EAE and control cells. The increase in tonic inhibition produced by bath application of DS2 (2 μM) was the same in control cells (26.1 ± 3.8%, n = 14 cells from 3 mice) and cells from EAE mice (22.5 ± 4.0%, n = 11 cells from 3 mice, t(23) = 0.653, p = 0.52). Together, these results indicate that an increase in plasma membrane expression of α5 subunit-containing GABAARs has an important role in enhanced tonic inhibition during EAE.
Plasma membrane expression of the GABA transporter GAT-3 is reduced in EAE
The GABA transporters GAT-1 and GAT-3 are the primary neuronal and astrocytic GABA transporters, in hippocampal pyramidal cells (Chembrowski et al., 2016) and astrocytes (Chai et al., 2017). Moreover, both of these transporters are thought to have important roles in regulating tonic inhibition by controlling extracellular levels of GABA (Nusser and Mody, 2002; Jensen et al., 2003; Semyanov et al., 2003; Kersante et al., 2013). In contrast, the GAT-2 GABA transporter is not expressed in hippocampal pyramidal cells or astrocytes (Durkin et al., 1995; Evans et al., 1996; Cembrowski et al., 2016; Chai et al., 2017). Thus, to investigate whether deficits in GABA uptake might also contribute to enhanced tonic inhibition during EAE, we examined whether the expression of GAT-1 and/or GAT-3 is altered during EAE. Both plasma membrane (Fig. 3A) and total GAT-1 levels in whole hippocampal lysates from EAE mice were not significantly different from controls (total levels in lysates from EAE mice were 129 ± 14.3% of control, n = 6 EAE and 6 control mice, t(10) = 1.437, p = 0.181). In contrast, GAT-3 plasma membrane expression was significantly decreased in the EAE hippocampus (Fig 3B). There was no difference, however, in total levels of GAT-3 in lysates from EAE and control mice (levels were 96 ± 6.7% of control in EAE lysates, n = 6 EAE and 6 control mice, t(10) = 0.371, p = 0.718). This suggests that the decrease in plasma membrane GAT-3 levels during EAE is due to an alteration in GAT- 3 membrane trafficking. To address the functional significance of decreased GAT-3 membrane expression during EAE, we next determined whether the increase in tonic inhibition induced by GAT-1 and GAT-3 inhibitor nipecotic acid (Pandit et al., 2013) was blunted in CA1 pyramidal cells from EAE mice (Fig 3C). As shown in figure 3D, bath application of nipecotic acid (10 μM) induced a robust increase in tonic inhibition in both control and EAE cells. Though this finding does not rule out a potential role for deficits in GABA transporter function in the enhancement of tonic inhibition in EAE, it does suggest that an increase in α5 subunit-containing GABAAR is likely to have a more important role.
Figure 3.
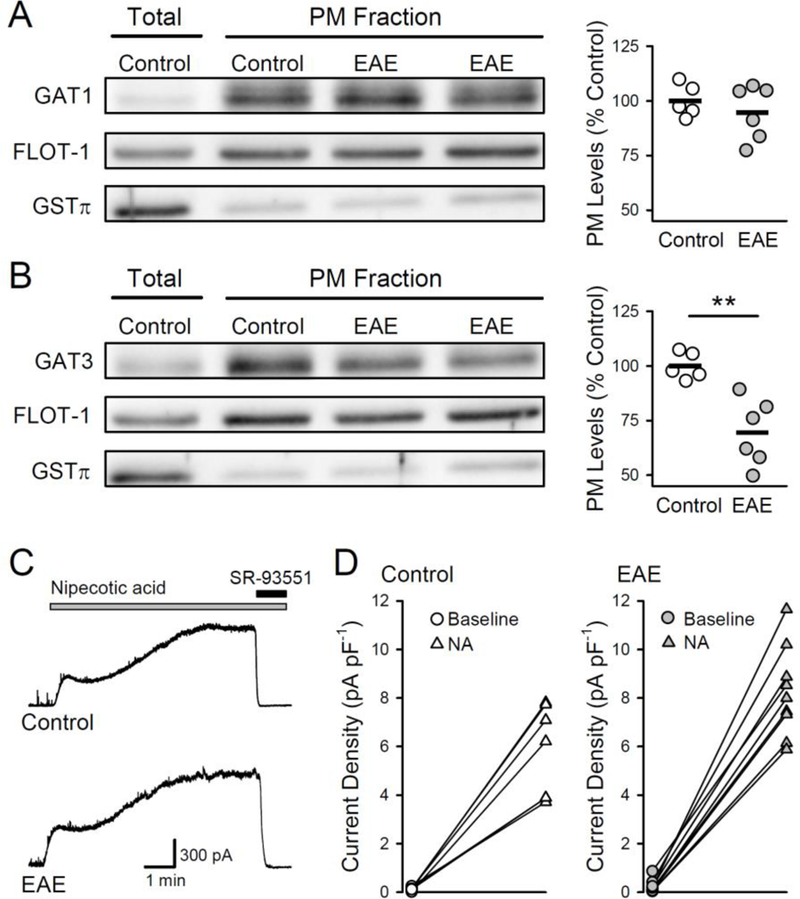
Plasma membrane levels of the GABA transporter GAT-3 are reduced in EAE. A,B, Plasma membrane (PM) levels of GAT-1 (A) and GAT-3 (B) in hippocampal samples from control (n = 5) and EAE mice (n = 6). GAT-1 and GAT-3 levels were normalized to levels of plasma membrane protein FLOT-1. Total protein levels in hippocampal lysates were used to confirm enrichment in the plasma membrane fraction, and GSTπ levels were used to monitor the extent of cytosolic contamination. Plots show results from all experiments, bars indicate mean values. Although plasma membrane levels of GAT-1 were not altered (levels were 95 ± 5% of control, t(9) = 0.827, p = 0.43), membrane levels of GAT-3 were significantly reduced in samples from EAE mice (levels were 69 ± 6% of control, (t(9) = 4.192, **p = 0.002). C, Examples of outward currents induced by bath application of the GAT-1/3 inhibitor nipecotic acid (10 μM, indicated by the bar) in CA1 pyramidal cells from control and EAE mice (Vhold = +10 mV). The contribution of GABAARs to the outward current (tonic inhibition) was determined by bath application of 100 μM SR-93551 at the end of the recording (indicated by the bar). D, Increase in tonic inhibition induced by nipecotic acid (NA) in CA1 pyramidal cells from control (n = 7 cells from 2 mice) and EAE mice (n = 9 cells from 2 mice).
Decreased CA1 pyramidal cell excitability and LTP deficits in EAE
Tonic inhibition acts as a form of shunting inhibition that can potently reduce neuronal excitability. Thus, to begin to explore the impact of enhanced tonic inhibition in EAE on hippocampal function we first examined whether intrinsic excitability of CA1 pyramidal cells is reduced in EAE. Although resting membrane potentials in cells from control and EAE mice were similar (Fig. 4A), membrane input resistance was significantly lower in cells from EAE mice (Fig. 4B). Consistent with previous findings (Novkovic et al., 2015), there was also a robust, right-shift in action potential firing-to- current injection curves in cells from EAE mice, indicating a significant decrease in intrinsic excitability (Fig. 4C,D). To determine whether the decrease in pyramidal cell excitability in EAE is due to enhanced tonic inhibition, we next examined resting membrane potential, input resistance, and excitability in cells continuously bathed in ACSF containing 5.0 μM L-655,708. Although L-655,708 had no effect on resting membrane potential (Fig. 4E), membrane input resistance in cells from control and EAE mice were no longer significantly different (Fig. 4F). Moreover, L-655,708 significantly enhanced depolarization-induced action potential firing in cells from EAE mice but had no effect on excitability in cells from control mice (Fig 4.G,H), indicating that the decrease in CA1 pyramidal cell excitability in EAE is due to enhanced tonic inhibition.
Figure 4.
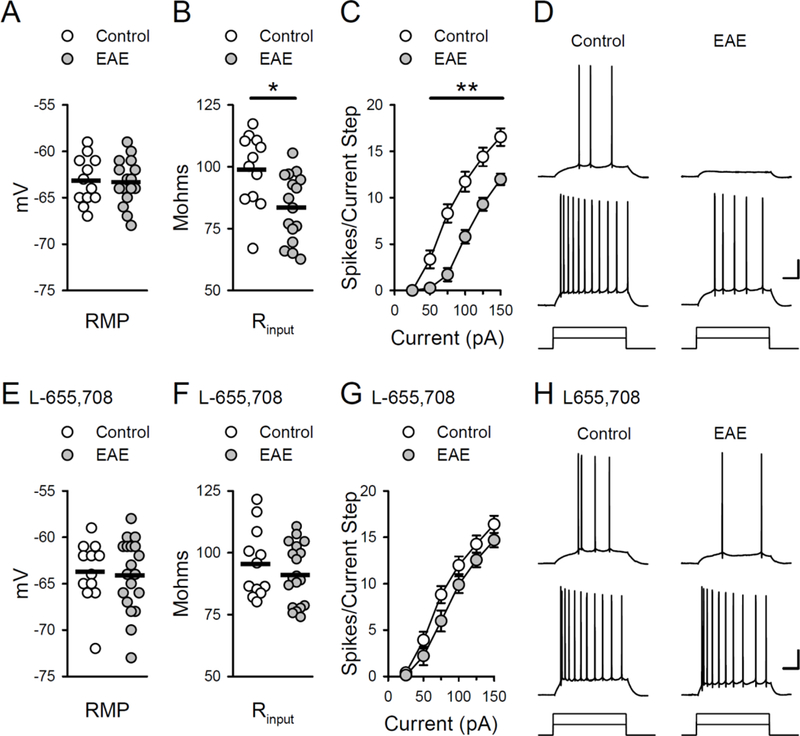
Enhanced tonic inhibition reduces CA1 pyramidal cell excitability in EAE. A, Resting membrane potential (RMP) in cells from EAE and control mice. Resting membrane potentials were −63.3 ± 0.6 mV in 16 cells from 7 EAE mice and −63.2 ± 0.7 mV in 12 cells from 5 control mice. B, Membrane input resistance (Rin) was significantly lower in cells from EAE mice (83.6 ± 3.4 MΩ) compared to controls (98.9 ± 4 .2 MΩ, *p < 0.01, Bonferroni t-test comparison). Plots in A and B show results from all cells, bars indicate mean values. C, Action potential firing induced by somatic current injections is significantly reduced in cells from EAE mice (n = 17 cells from 7 EAE mice and 12 cells from 5 control mice, **p < 0.001, Bonferroni t-test comparison). D, Traces show examples of action potentials elicited by 50 and 100 pA current injections in cells from control and EAE mice. Calibration: 20 mV, 100 ms. E, Resting membrane potentials in cells from EAE and control mice in the presence of 5.0 μM L-655,708. Resting membrane potentials were −63.8 ± 0.9 mV in 12 cells from 5 control mice and −64.2 ± 0.9 mV in 19 cells from 6 EAE mice. A two-way ANOVA analysis of resting membrane potentials in cells from EAE and control mice in the presence and absence (panel A) of L-655,708 revealed no effect of group (EAE vs. control), F(1,55) = 0.086, p = 0.77, or L- 655,708, F(1,55) = 0.652, p = 0.423, and no group x L-655,708 interaction, F(1,55) = 0.156, p = 0.903. F, Membrane input resistance in cells from EAE and control mice in the presence of L-655,708. Input resistance was 91.1 ± 2.8 MΩ in cells from EAE mice and 95.5 ± 3.9 MΩ in cells from control mice. A two-way ANOVA analysis of membrane input resistance in cells from EAE and control mice in the presence and absence (panel B) of L-655,708 revealed a significant difference between cells from EAE and control mice, F(1,55) = 7.582, p = 0.0008. Post-hoc Bonferroni t-tests: cells from EAE vs. control mice in the absence of L-655,708, t = 2.971, p = 0.004; cells from EAE vs. control mice in the presence of L-655,708, t = 0.887, p = 0.379. Plots in E and F show results from all cells, bars indicate mean values. G, Depolarization-induced action potential firing in cells from control and EAE mice in the presence of L-655,708 (n = 13 cells from 5 control mice and 19 cells from 6 EAE mice). A three-way ANOVA analysis of the action potential firing-to-current injection curves in the presence and absence (panel C) of L-655,708 revealed a significant effect of group (EAE vs. control) F(1,342) = 81.705, p < 0.001), L-655,708 (F(1,342) = 25.081, p < 0.001), and a significant group x L-655,708 interaction (F(1,342) = 20.521, p < 0.001). Post-hoc Bonferroni t-tests: EAE vs. control cells in the absence of L-655,708, t = 9.38; p < 0.001), L-655,708 had no effect on excitability in control cells (t = 0.307, p = 0.759) but significantly enhanced excitability in cells from EAE mice (t = 7.441, p < 0.001). H, Traces show examples of action potential firing in response to 50 and 100 pA current injections in cells from control an d EAE mice in the presence of L-655,708. Calibration: 20 mV and 100 ms.
Because enhanced inhibition and decreased excitability can oppose the postsynaptic depolarization needed for activation of NMDARs and LTP induction, we next examined whether enhanced tonic inhibition is responsible for LTP deficits seen in EAE. Compared to slices obtained from healthy controls, the induction of LTP by a brief train of theta-pulse stimulation (150 pulses at 5 Hz) was significantly reduced in slices obtained from EAE mice at both early and later time points post-EAE induction (16–29 and > 40 days, respectively) (Fig 5A,B). However, reducing tonic inhibition with L- 655,708 failed to enhance LTP induction in slices obtained from mice >40 day post-EAE induction (Fig. 5B), a time point post-EAE induction where L-655,708 strongly enhances CA1 pyramidal cell excitability (Fig. 4G,H). L-655,708 also had no effect on theta-pulse stimulation-induced LTP induction in slices obtained from healthy control mice (in the presence of L-655,708, fEPSPs in were potentiated to 143 ± 7% of baseline, n = 7 slices from 5 mice, t(16) = 0.595, p = 0.56 compared to vehicle controls). Thus, the LTP deficits that occur during EAE are not simply due to changes in tonic inhibition but instead likely involve additional pathophysiological changes in excitatory synaptic function.
Figure 5.
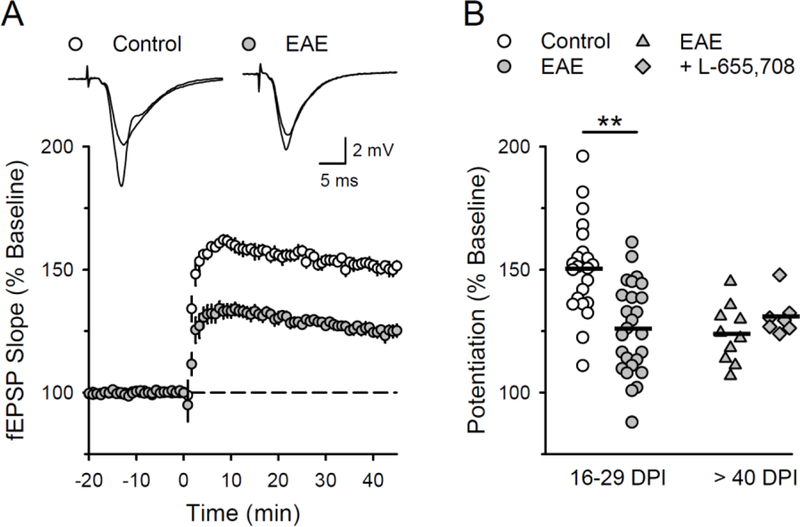
LTP is impaired in the CA1 region of the hippocampus during EAE. A, LTP was induced by theta-frequency stimulation (150 presynaptic fiber stimulation pulses at 5 Hz, delivered at time = 0) in the CA1 region of hippocampal slices obtained from healthy control and EAE mice (16 to 29 days post EAE induction). Traces show superimposed fEPSPs recorded during baseline and 45 minutes post-LTP induction. B, Plot shows results from all LTP experiments, bars indicate mean values. In hippocampal slices obtained from EAE mice 16–29 days post induction (DPI), fEPSPs were potentiated to 125 ± 4% of baseline in slices from EAE mice (26 slices from 7 mice) and to 150 ± 4% of baseline in slices from healthy controls (22 slices from 5 mice, t(46) = 4.498, **p = 4.64 × 10−5). In experiments where hippocampal slices from EAE mice (>40 days post induction) were continuously bath in ACSF containing 5.0 μM L-655,708 fEPSPs were potentiated to 131 ± 3% of baseline (n = 7 slices from 5 mice) compared to 124 ± 4% of baseline in vehicle control experiments (0.1% DMSO, n = 10 slices from in 7 mice, t(15) = 1.39, p = 0.185).
DISCUSSION
In this study, we assessed the role of GABAergic transmission in modifying hippocampal function in EAE. We found that CA1 pyramidal cells exhibit both enhanced phasic and tonic currents during EAE. Although decreased surface expression of the GAT-3 GABA transporter may contribute, our results indicate that enhanced tonic inhibition during EAE is mediated primarily by increased surface expression of α5- GABAARs. Importantly, tonic inhibition regulates neuronal excitability and thus can strongly influence information transmission through neural circuits (Semyanov et al., 2004). For example, in the cerebellar cortex, GABAA-mediated tonic inhibition is the main inhibitory influence regulating the transfer of information at mossy fiber synapses onto granule cells, and is thought to have a crucial role in the sparse coding of sensory information in the cerebellar cortex (Hamann et al., 2002, Chadderton et al., 2004). Tonic inhibition also regulates the excitability of hippocampal pyramidal cells (Glykys and Mody, 2006, Bonin et al., 2007, Pavlov et al., 2009, Groen et al., 2014). Thus, alterations in network activity and information processing due to increased tonic inhibition and decreased neuronal excitability may contribute to cognitive disability in MS.
Although our study is the first to identify changes in tonic inhibition in EAE, previous studies have found that phasic inhibitory synaptic transmission is also altered in EAE (Rossi et al., 2011, Mandolesi et al., 2012, Nistico et al., 2013). Interestingly, although sIPSPC frequency in CA1 pyramidal cells is reduced relatively soon after EAE induction (Nisticò et al., 2013), we find that the frequency of sIPSCs is enhanced at later time points post-EAE induction. Although differences in EAE induction protocols might contribute to this difference, our results, along with those of Nisticò et al. (2013), suggest that phasic inhibitory synaptic transmission in the hippocampus undergoes distinct, time-dependent changes during the course of EAE. In contrast to the changes in phasic inhibitory synaptic transmission that occur in hippocampal CA1 pyramidal cells during EAE, sIPSC frequency is strongly reduced in striatal medium spiny neurons at both early and later time points post-EAE induction (Rossi et al., 2011). Thus, EAE may lead to brain region-specific alterations in phasic inhibition, perhaps due to region and/or cell type-specific effects on expression of distinct GABAAR subtypes. Indeed, there are brain region differences in EAE-induced changes in astrocyte gene expression (Itoh et al., 2018). Notably, loss of parvalbumin (PV) positive inhibitory interneurons occurs in both MS (Clements et al., 2008) and EAE (Ziehn et al., 2010; Rossi et al., 2011; Nisticò et al., 2013). Although loss of PV-positive interneurons is thought to underlie the reduction in sIPSC frequency in medium spiny neurons (Rossi et al., 2011), we observed an increase is sIPSC frequency in CA1 pyramidal cells even though the number of PV-positive interneurons is also reduced in hippocampal CA1 region during EAE (Ziehn et al., 2010; Nisticò et al., 2013). Thus, the mechanisms responsible for increased sIPSCs in CA1 pyramidal cells during EAE are unclear. It is interesting, however, that PV-positive, basket and bistratified interneurons in the CA1 region form inhibitory synaptic connections onto both pyramidal cells and other inhibitory neurons (Bezaire and Soltesz, 2013). For example, nearly 30% of all inhibitory synapses onto PV-positive interneurons in the CA1 region arises from other, PV-positive cells (Gulyás et al., 1999, for review see Hu et al., 2014). Thus, the increase in sIPSC frequency we observed during EAE may reflect disinhibition of inhibitory interneuron networks due to partial loss of PV-positive interneurons.
Tonic inhibition in hippocampal CA1 pyramidal cells is primarily mediated by α5β3γ2 subunit-containing GABAARs (Caraiscos et al., 2004; Zarnowska et al., 2015), although α5β2 subunit-containing receptors may also be involved (Ju et al., 2009). Thus, although our results are consistent with the notion that up-regulation of α5β3γ2 subunit-containing receptors has an important role in the increase in tonic inhibition in EAE, additional experiments are needed to better define how the expression of β3/2 and γ2 subunits changes during EAE. The molecular mechanisms responsible for increases in α5-GABAARs during EAE are also unclear. However, our finding that α5- GABAAR-mediated tonic inhibition is enhanced in the hippocampal CA1 during EAE parallels other studies suggesting that inflammatory molecules acting on GABAARs can effect cell excitability, excitatory neurotransmission and synaptic plasticity. For example, the pro-inflammatory cytokine interleukin-1beta (IL-1β) induces a rapid increase in GABAAR surface expression in hippocampal neurons (Serantes et al., 2006) and mediates the increase in α5-GABAARs surface expression and tonic inhibition in CA1 pyramidal cells following systemic inflammation induced by the bacterial endotoxin lipopolysaccharide (Wang et al., 2012). Although other inflammatory cytokines, such as tumor necrosis factor alpha and interleukin 6, can regulate inhibitory synaptic transmission (Stellwagen et al., 2005, Garcia-Oscos et al., 2012), these cytokines have little, if any, effect on tonic inhibition in cultured hippocampal neurons (Wang et al., 2012). Interestingly, extrasynaptic α5-GABAAR expression and tonic inhibition are also strongly increased in dentate gyrus granule cells in the 5xFAD model of Alzheimer’s disease (Wu et al., 2014). Moreover, enhanced α5-GABAAR-mediated tonic inhibition in cortical pyramidal neurons found within the peri-infarct region contributes to reduced cortical plasticity and motor recovery in a rodent stroke model (Clarkson et al., 2010). Together with these findings, our results suggest that enhanced α5-GABAAR-mediated tonic inhibition may be a common pathological response that occurs during neurodegeneration and brain injury.
Because GABAergic inhibition potently regulates the induction of LTP at glutamatergic synapses (Wigstrom and Gustafsson, 1983, Davies et al., 1991, Arima- Yoshida et al., 2011), we investigated if the enhanced tonic inhibition in EAE leads to LTP deficits in the hippocampal CA1 region. Consistent with previous findings showing that LTP is reduced in EAE (Kim do et al., 2012, Di Filippo et al., 2013, Novkovic et al., 2015), the induction of LTP by a theta-frequency stimulation protocol was significantly reduced in hippocampal slices from EAE mice. However, reducing tonic inhibition with L-655,708 had little effect on theta frequency stimulation-induced LTP. This suggests that the LTP deficits that occur during EAE are not simply due to enhanced tonic inhibition but instead are more likely to reflect alterations in the NMDAR-dependent signaling pathways involved in the induction and/or maintenance of LTP. Notably, there is considerable controversy regarding how LTP induction is altered in EAE. Although the deficits in theta-frequency stimulation-induced LTP observed in our experiments are consistent with deficits in high-frequency stimulation-induced LTP seen in previous studies (Kim et al., 2012, Di Filippo et al., 2013, Novkovic et al., 2015), other studies have found either no change (Ziehn et al., 2010; Prochnow et al., 2013) or even an enhancement of LTP in EAE (Nisticò et al., 2013). A number of experimental variables, such as differences in the pattern of synaptic stimulation used to induce LTP and methods for EAE induction, might contribute to these disparate findings. Interestingly, although we find significant deficits in the induction of LTP by relatively modest theta pulse stimulation protocols at early time points following EAE induction, recent findings indicate that the induction of LTP by stronger, high-frequency synaptic stimulation in the hippocampal CA1 region becomes progressively impaired as disease progresses (Novkovic et al., 2015). Moreover, LTP induction in the dentate gyrus is impaired early in EAE when high-frequency stimulation-induced LTP in CA1 is intact, suggesting that hippocampal dysfunctions may extend through the trisynaptic loop as the disease progresses (Planche et al., 2016). These findings parallel the clinical observation that cognitive deficits accumulate with aging and progression of other MS disabilities (Ruano et al., 2016). Thus, future studies examining how deficits in the induction of LTP by different patterns of synaptic stimulation and how alterations in tonic inhibition evolve with time during EAE should provide important insights into the synaptic and molecular mechanisms underlying CI in MS.
Highlights.
Tonic GABAergic inhibition in the hippocampal CA1 region is enhanced in experimental autoimmune encephalomyelitis (EAE).
Plasma membrane expression of α5-GABAA receptors that mediate tonic inhibition in CA1 pyramidal cells is enhanced in EAE.
EAE is also associated with decreased membrane expression of the GABA transporter GAT-3.
Enhanced tonic inhibition does not appear to underlie deficits in LTP that occur in EAE.
Alterations in both inhibitory synaptic transmission and LTP may importantly contribute to cognitive impairment in MS.
Acknowledgments:
We thank Voskuhl lab members Noriko Itoh, Heaveen Ahdi, Valerie Vessels, and Darian Nitin Mangu for assistance with animal care. We also thank Drs. Ryan Guglietta and Walter Babiec for technical advice. This work was supported by a grant from the Conrad N. Hilton Foundation (#20150232), the California Community Foundation (#BAPP-15–118094), and the Tom Sherak MS Hope Foundation to RRV. LGK was supported by the UCLA Laboratory of Neuroendocrinology (LNE) training grant (5T32HD007228) and by the National Institute on Aging of the National Institutes of Health (F31AG051381). Author contributions: L.G.K, W.W., S.A.J., R.R.V., and T.J.O. designed research; L.G.K., W.W., S.A.J., and T.J.O. performed research; L.G.K, W.W., S.A.J., and T.J.O. analyzed data; L.G.K., W.W., S.A.J., R.R.V., and T.J.O. wrote the paper.
Abbreviations:
- ACSF
artificial cerebrospinal fluid
- CI
cognitive impairment
- DPI
days post-induction
- EAE
experimental autoimmune encephalomyelitis
- fEPSP
field excitatory postsynaptic potential
- IPSC
inhibitory postsynaptic current
- LTP
long-term potentiation
- MS
multiple sclerosis
- NMDAR
NMDA receptor
- PM
plasma membrane
- sIPSC
spontaneous inhibitory postsynaptic current
Footnotes
Conflict of Interest: The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Acharjee S, Nayani N, Tsutsui M, Hill MN, Ousman SS, Pittman QJ (2013) Altered cognitive-emotional behavior in early experimental autoimmune encephalitis--cytokine and hormonal correlates. Brain Behav Immun 33:164–172. [DOI] [PubMed] [Google Scholar]
- Arima-Yoshida F, Watabe AM, Manabe T (2011) The mechanisms of the strong inhibitory modulation of long-term potentiation in the rat dentate gyrus. Eur J Neurosci 33:1637–1646. [DOI] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR (2006) L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5- containing GABAA receptors. Neuropharmacology 51:1023–1029. [DOI] [PubMed] [Google Scholar]
- Babiec WE, Jami SA, Guglietta R, Chen PB, O’Dell TJ (2017) Differential regulation of NMDA receptor-mediated transmission by SK channels underlies dorsal-ventral differences in dynamic of Schaffer collateral synaptic function. J Neurosci 37:1950–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezaire MJ, Soltesz I (2013) Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus 23:751–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Martin LJ, MacDonald JF, Orser BA (2007) α5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol 98:2244–2254. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA (2004) Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101:3662–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cembrowski MS, Wang L, Sugino K, Shields BC, Spruston N (2016) Hipposeq: a comprehensive RNA-seq database of gene expression in hippocampal principal neurons. Elife 5: e14997. doi: 10.7554/eLife.14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, Musella A, D’Amelio M, Cavallucci V, Martorana A, Bergamaschi A, Cencioni MT, Diamantini A, Butti E, Comi G, Bernardi G, Cecconi F, Battistini L, Furlan R, Martino G (2009) Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci 29:3442–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M (2004) Integration of quanta in cerebellar granule cells during sensory processing. Nature 428:856–860. [DOI] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, Coppola G, Khakh BS (2017) Neural Circuit- Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95:531–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151. [DOI] [PubMed] [Google Scholar]
- Clements RJ, McDonough J, Freeman EJ. (2008) Distribution of parvalbumin and calretinin immunoreactive interneurons in motor cortex from multiple sclerosis post-mortem tissue. Exp Brain Res 187:459–65. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST (2010) Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 468:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW (2002) Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci 22:5572–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL (1991) GABA au toreceptors regulate the induction of LTP. Nature 349:609–611. [DOI] [PubMed] [Google Scholar]
- Denney DR, Sworowski LA, Lynch SG (2005) Cognitive impairment in three subtypes of multiple sclerosis. Arch Clin Neuropsychol 20:967–981. [DOI] [PubMed] [Google Scholar]
- Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampa C, Costa C, Tantucci M, Zianni E, Boraso M, Siliquini S, de lure A, Ghiglieri V, Colcelli E, Baker D, Sarchielli P, Fusco FR, Di Luca M, Calabresi P (2013) Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol Dis 52:229–236. [DOI] [PubMed] [Google Scholar]
- Di Filippo M, de lure A, Giampa C, Chiasserini D, Tozzi A, Orvietani PL, Ghiglieri V, Tantucci M, Durante V, Quiroga-Varela A, Mancini A, Costa C, Sarchielli P, Fusco FR, Calabresi P (2016) Persistent activation of microglia and NADPH oxidase drive hippocampal dysfunction in experimental multiple sclerosis. Sci Reports 6: 20926; doi: 10.1038/srep20926(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL (1995) Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Brain Res Mol Brain Res. 33:7–21. [DOI] [PubMed] [Google Scholar]
- Dutta R, Chang A, Doud MK, Kidd GJ, Ribaudo MV, Young EA, Fox RJ, Staugaitis SM, Trapp BD (2011) Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol 69:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington LA, Mihalik, Pálvölgyi A, Ling I, Pallagi K, Kertész S, Varga P, Gunn BG, Brown AR, Livesey MR, Monteiro O, Belelli D, Barkoczy J, Spedding M, Gacsályi I, Antoni FA, Lambert JJ (2017) Selective inhibition of extra-synaptic α5-GABAA receptors by S44819, a new therapeutic agent. Neuropharmacology 125:353–364. [DOI] [PubMed] [Google Scholar]
- Evans JE, Frostholm A, Rotter A (1996) Embryonic and postnatal expression of four gamma-aminobutyric acid transporter mRNAs in the mouse brain and leptomeninges. J Comp Neurol 376:431–446. [DOI] [PubMed] [Google Scholar]
- Garcia-Oscos F, Salgado H, Hall S, Thomas F, Farmer GE, Bermeo J, Galindo LC, Ramirez RD, D’Mello S, Rose-John S, Atzori M (2012) The stress-induced cytokine interleukin-6 decreases the inhibition/excitation ratio in the rat temporal cortex via trans-signaling. Biol Psychiatry 71:574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I (2006) Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit-deficient mice. J Neurophysiol 95:2796–2807. [DOI] [PubMed] [Google Scholar]
- Grasselli G, Rossi S, Musella A, Gentile A, Loizzo S, Muzio L, Di Sanza C, Errico F, Musumeci G, Haji N, Fresegna D, Sepman H, De Chiara V, Furlan R, Martino G, Usiello A, Mandolesi G, Centonze D (2013) Abnormal NMDA receptor function exacerbates experimental autoimmune encephalomyelitis. Br J Pharmacol 168:502–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen MR, Paulsen O, Perez-Garci E, Nevian T, Wortel J, Dekker MP, Mansvelder HD, van Ooyen A, Meredith RM (2014) Development of dendritic tonic GABAergic inhibition regulates excitability and plasticity in CA1 pyramidal neurons. J Neurophysiol 112:287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes J, Sa MJ (2012) Cognitive dysfunction in multiple sclerosis. Front Neurol 3:74. doi: 10.3389/fneur.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, Megais M, Emri Z, Freund T (1999) Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci 19:10082–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D (2002) Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron 33:625–633. [DOI] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P (2014) Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345:1255263 DOI: 10.1126/science. [DOI] [PubMed] [Google Scholar]
- Itoh N, Itoh Y, Tassoni A, Ren E, Kaito M, Ohno A, Ao Y, Farkhondeh V, Johnsonbaugh H, Burda J, Sofroniew MV, Voskuhl RR (2018) Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proc Natl Acad Sci USA 115: E302–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I (2003) GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol 90:2690–2701. [DOI] [PubMed] [Google Scholar]
- Ju YH, Guzzo A, Chiu MW, Taylor P, Moran MF, Gurd JW, MacDonald JF, Orser BA (2009) Distinct properties of murine α5 gamma-aminobutyric acid type a receptors revealed by biochemical fractionation and mass spectroscopy. J Neurosci Res 87:1737–47. [DOI] [PubMed] [Google Scholar]
- Kersante F, Rowley SC, Pavlov I, Gutierrez-Mecinas M, Semyanov A, Reul JM, Walker MC, Linthorst AC (2013) A functional role for both gamma-aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J Physiol 591:2429–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim do Y, Hao J, Liu R, Turner G, Shi FD, Rho JM (2012) Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PloS one 7:e35476 https://doi.org/10.1371/journal.pone.0035476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V, Maguire J (2014) The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits 8:3. doi: 10.3389/fncir.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Grafman J, Vendrell P, Martinez JM (1988) Slowed information processing in multiple sclerosis. Arch Neurol 45:281–285. [DOI] [PubMed] [Google Scholar]
- Longoni G, Rocca MA, Pagani E, Riccitelli GC, Colombo B, Rodegher M, Falini A, Comi G, Filippi M (2015) Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct Funct 220:435–444. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Gentile A, Musella A, Fresegna D, De Vito F, Bullitta S, Sepman H, Marfia GA, Centonze D (2015) Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat Rev Neurol 11:711 −724. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Grasselli G, Musella A, Gentile A, Musumeci G, Sepman H, Haji N, Fresegna D, Bernardi G, Centonze D (2012) GABAergic signaling and connectivity on Purkinje cells are impaired in experimental autoimmune encephalomyelitis . Neurobiol Dis 46:414–424. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Musella A, Gentile A, Grasselli G, Haji N, Sepman H, Fresegna D, Bullitta S, De Vito F, Musumeci G, Di Sanza C, Strata P, Centonze D (2013) Interleukin-1beta alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J Neurosci 33:12105–12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Zurek AA, MacDonald JF, Roder JC, Jackson MF, Orser BA (2010) α5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J Neurosci 30:5269–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA (2003) Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38:433–445. [DOI] [PubMed] [Google Scholar]
- Nistico R, Mango D, Mandolesi G, Piccinin S, Berretta N, Pignatelli M, Feligioni M, Musella A, Gentile A, Mori F, Bernardi G, Nicoletti F, Mercuri NB, Centonze D (2013) Inflammation subverts hippocampal synaptic plasticity in experimental multiple sclerosis. PloS One 8:e54666 https://doi.org/10.1371/journal.pone.0054666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novkovic T, Shchyglo O, Gold R, Manahan-Vaughan D (2015) Hippocampal function is compromised in an animal model of multiple sclerosis. Neuroscience 309:100–112. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I (2002) Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87:2624–2628. [DOI] [PubMed] [Google Scholar]
- Pandit S, Jeong JA, Jo JY, Cho HS, Kim DW, Kim JM, Ryu PD, Lee SY, Kim HW, Jeon BH, Park JB (2013) Dual mechanisms diminishing tonic GABAA inhibition of dentate gyrus granule cells in Noda epileptic rats. J Neurophysiol 110:95–102. [DOI] [PubMed] [Google Scholar]
- Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC (2009) Outwardly rectifying tonically active GABAA receptors in pyramidal cells modulate neuronal offset, not gain. J Neurosci 29:15341–15350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planche V, Panatier A, Hiba B, Ducourneau EG, Raffard G, Dubourdieu N, Maitre M, Leste-Lasserre T, Brochet B, Dousset V, Desmedt A, Oliet SH, Tourdias T (2016) Selective dentate gyrus disruption causes memory impairment at the early stage of experimental multiple sclerosis. Brain Behav Immun 60:240–254. [DOI] [PubMed] [Google Scholar]
- Prochnow N, Gold R, Haghikia A (2013) An electrophysiologic approach to quantify impaired synaptic transmission and plasticity in experimental autoimmune encephalomyelitis. J Neuroimmunol 264:48–53. [DOI] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM (1996) [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the α5 subunit. Neuropharmacology 35:1331–1335. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F (1991) Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 41:692–696. [DOI] [PubMed] [Google Scholar]
- Rossi S, Muzio L, De Chiara V, Grasselli G, Musella A, Musumeci G, Mand olesi G, De Ceglia R, Maida S, Biffi E, Pedrocchi A, Menegon A, Bernardi G, Furlan R, Martino G, Centonze D (2011) Impaired striatal GABA transmission in experimental autoimmune encephalomyelitis. Brain Behav Immun 25:947–956. [DOI] [PubMed] [Google Scholar]
- Ruano L, Portaccio E, Goretti B, Niccolai C, Severo M, Patti F, Cilia S, Gallo P, Grossi P, Ghezzi A, Roscio M, Mattioli F, Stampatori C, Trojano M, Viterbo RG, Amato MP (2016) Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler J doi: 10.1177/1352458516674367 [DOI] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC (2005) Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci 25:10016–10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM (2003) GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci 6:484–490. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA (2004) Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci 27:262–269. [DOI] [PubMed] [Google Scholar]
- Serantes R, Arnalich F, Figueroa M, Salinas M, Andres-Mateos E, Codoceo R, Renart J, Matute C, Cavada C, Cuadrado A, Montiel C (2006) Interleukin-1β enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: relevance to sepsis-associated encephalopathy. J Biol Chem 281:14632–14643. [DOI] [PubMed] [Google Scholar]
- Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, Bookheimer SY (2008) Regional hippocampal atrophy in multiple sclerosis. Brain 131:1134–1141. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC (2005) Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci 25:3219–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ (2009) Novel compounds selectively enhance δ subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology 56:182–189. [DOI] [PubMed] [Google Scholar]
- Wang DS, Zurek AA, Lecker I, Yu J, Abramian AM, Avramescu S, Davies PA, Moss SJ, Lu WY, Orser BA (2012) Memory deficits induced by inflammation are regulated by α5- subunit-containing GABAA receptors. Cell Rep 2:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whissell PD, Eng D, Lecker I, Martin LJ, Wang DS, Orser BA (2013) Acutely increasing δGABAA receptor activity impairs memory and inhibits synaptic plasticity in the hippocampus. Front Neural Circuits 7:146. doi: 10.3389/fncir.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B (1983) Heterosynaptic modulation of homosynaptic long- lasting potentiation in the hippocampal slice. Acta Physiol Scand 119:455–458. [DOI] [PubMed] [Google Scholar]
- Wu Z, Guo Z, Gearing M, Chen G (2014) Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s disease model. Nature Commun 5:4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowska ED, Rodgers FC, Oh I, Rau V, Lor C, Laha KT, Jurd R, Rudolph U, Eger EI 2nd, Pearce RA (2015) Etomidate blocks LTP and impairs learning bu t does not enhance tonic inhibition in mice carrying the N265M point mutation in the β3 subunit of the GABAA receptor. Neuropharmacology 93:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehn MO, Avedisian AA, Dervin SM, Umeda EA, O’Dell TJ, Voskuhl RR (2012) Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J Neurosci 32:12312–12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR (2010) Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest 90:774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]


