Introduction – sleep and its association with substance use disorders
A disturbance of sleep continuity has effects on next day functioning and behavior. One such behavior is the use of psychoactive substances. Disturbed sleep is also a frequent complaint among persons using alcohol and illicit drugs. Further, sleep dysfunction in the context of substance misuse may contribute to increased severity of Substance Use Disorder (SUD), impaired quality of life, comorbid psychiatric complaints, suicidal behavior and psychosocial problems 1,2. This narrative review focuses on the identification and treatment of sleep disorders in persons with comorbid SUDs.
Assessment and diagnosis of substance use and sleep disorders
Substance use and SUD
Various aspects of substance use are relevant to sleep. Drugs can have an acute impact on sleep by either increasing or decreasing arousal. Pharmacologically-specific sleep-related withdrawal symptoms may occur upon cessation or reduction of heavy, sustained periods of substance use 3. Problematic patterns of substance use may also lead to distress, which may in turn impact sleep via non-pharmacological mechanisms. Commonly used substances in the context of sleep-related problems include alcohol, cocaine, cannabis (marijuana), opioids and sedative-hypnotic-anxiolytic medications.
Approach to the assessment of patients with sleep disorders
Patient complaints related to sleep most often consist of difficulty falling asleep, difficulty staying asleep, or impaired daytime functioning. Symptoms of impaired daytime functioning may include mood disturbance, fatigue, problems with concentration, or daytime sleepiness. The common sleep-related disorders evaluated in the context of substance use include the following:
insomnia,
circadian rhythm disorder-delayed sleep phase type, and
sleep-related breathing disorder (SRBD).
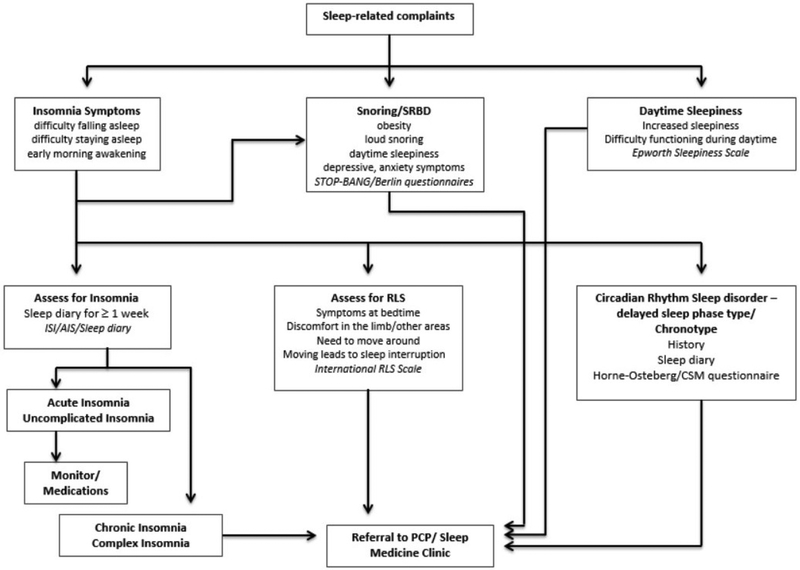
Figure 1 explains a strategy for screening patients in a clinical setting, especially in the context of a primary care setting when substance use is suspected or confirmed.
Figure 1.
Management of sleep-related symptoms in a patient with substance use disorder.
STOP-BANG questionnaire = a screening questionnaire for obstructive sleep apnea; AIS = Athens Insomnia Scale; CSM questionnaire = Composite Scale of Morningness questionnaire
Insomnia.
Insomnia is a disorder characterized by complaints of poor sleep continuity (i.e. difficulty falling asleep and/or staying asleep), early morning awakening, and impairment of daytime functioning 4. Insomnia may be assessed using a structured rating instrument such as the Insomnia Severity Index (ISI) or a sleep diary. The sleep diary should be prospectively completed for a week or more and yields multiple indices, see Table 1. Acute insomnia denotes a recent onset of insomnia, less than 3 months in duration and commonly precipitated by a psychosocial stressor, that may be treated with reassurance, close monitoring, or with medications. Acute insomnia is also common in the acute withdrawal phase from substances. However, most of the FDA-approved hypnotic medications such as temazepam or zolpidem may be contraindicated in patients with SUD. For those with chronic insomnia (≥ 3 months in duration), behavioral interventions such as Cognitive Behavioral Therapy for Insomnia (CBT-I) are the recommended first-line intervention. Insomnia comorbid with active substance use is optimally treated in a substance misuse program or primary care setting staffed by clinicians with experience in substance-related problems. In contrast, chronic insomnia in patients with remitted SUD are best treated by referral to a sleep medicine clinic (see Figure 1).
Table 1.
Commonly used terminologies in sleep medicine
| Acronym | Term | Description |
|---|---|---|
| SE | Sleep Efficiency (%) | The proportion of time spent sleeping through the night |
| NREM | NREM sleep | The initial stages of sleep (N1 + N2 + SWS); about 80% of sleep |
| N1 | Stage 1 sleep | Characterized by low eye movements, waves with low amplitude and, mostly 4-7 Hz frequency |
| N2 | Stage 2 sleep | The sleep stage that demonstrates sleep spindles and K complexes |
| N3/SWS | Slow Wave Sleep (stages 3 + 4) | The presence of high amplitude and low frequency (0.5-2Hz), ≥ 20% of the epoch |
| REM | Rapid Eye Movement sleep | Sleep with saw tooth waveforms, rapid eye movements and low muscle tone |
| SOL | Sleep Onset Latency (min) | Time from “lights out” until the onset of sleep in the reading pane |
| REM-L | REM Onset Latency (min) | Interval of time from onset of sleep to the appearance of the first epoch of REM sleep |
| S1 | Stage 1 (%) | The fraction of sleep that is spent in N1 (total Stage 1 sleep/TST × 100); usually about 4-5% |
| S2 | Stage 2 (%) | The fraction of sleep that is spent in N2 (total Stage 2 sleep/TST × 100); usually about 45-55% |
| SWS | Slow Wave Sleep (%) | The percentage of sleep that is spent in SWS (total SWS sleep/TST × 100); usually about 16-21% |
| REM | REM (%) | The percentage of sleep that is spent in REM (total REM sleep/TST × 100); usually about 20-25% |
| Apnea | (polysomnography or home sleep test) | A complete cessation of airflow for ≥ 10 seconds |
| Hypopnea | (polysomnography or home sleep test) | A partial cessation of airflow for ≥ 10 seconds, and, either a ≥ 4% drop in SpO2, or, an arousal (as seen on electroencephalogram) |
| AHI | Apnea Hypopnea Index (events/Hour) | Total number of apneas and hypopneas per hour of sleep, i.e. total number of apneas and hypopneas/TST |
| Advance | Phase Advance | Shift of the sleep cycle to an earlier time during a circadian period (24 hours) |
| Delay | Phase Delay | Shift of the sleep cycle to a later time during a circadian period (24 hours) |
| SRBD | Sleep Related Breathing Disorder | Abnormalities of respiration during sleep; include OSA, CSA, obesity hypoventilation and hypoventilation syndromes |
| OSA | Obstructive Sleep Apnea | A syndrome with symptoms (loud snoring and breathing interruptions)/cardio-metabolic syndrome + AHI ≥ 5 events/hr of sleep |
| CSA | Central Sleep Apnea | A syndrome with symptoms (sleepiness, insomnia, snoring and witnessed apneas)/A.Fibrillation/CHF + ≥ 5 central events/hr of sleep |
| CPAP | Continuous Positive Airway Pressure | A machine delivering air at a continuous pressure in order to prevent collapse of the air passage during sleep |
| BiPAP | Bi-level Positive Airway Pressure | A machine delivering air at two pressures; a higher pressure during inspiration and a lower pressure during expiration |
| ASV | Adaptive Servo-Ventilation | A specialized form of positive airway pressure support where the delivered pressure changes when respiratory events are detected |
| CBT-I | Cognitive Behavioral Therapy for Insomnia | A manualized behavioral treatment for insomnia, consisting of sleep restriction, stimulus control and cognitive therapy, for ≤ 8 weeks |
Data from American Academy of Sleep Medicine (AASM). The AASM Manual for the scoring of Sleep and Associated Events, 2007; and American Academy of Sleep Medicine (AASM), 2012. Available at: http://www.sleepnet.com/definition.html. Accessed Oct 28 2017.
Circadian Rhythm Sleep Disorder – delayed sleep phase type (CRSD-DSP)
Circadian rhythm sleep disorders are generated as a consequence of a mismatch between the individual’s internal (biological) rhythm and the required environmental schedule. CRSD-DSP is a particular subtype of circadian rhythm sleep disorders that is characterized by going to bed later in the night and awakening later in the morning. This later sleep-wake timing may interfere with daily activities and patients may present with complaints of insomnia, sleepiness and impaired daytime functioning. Circadian rhythm sleep disorders may be easily assessed in a clinic setting using sleep diaries, actigraphy or with the help of rating scales that evaluate the patient’s propensity for sleep at a particular time during the 24-hour period. Prior research has linked alcohol use with the blunting of circadian rhythms in healthy adults 5, and, alcohol use disorder (AUD) with insomnia and a nocturnal delay in the rise of melatonin level (a marker of circadian activity) 6,7.
Sleep-Related Breathing Disorder (SRBD)
SRBDs are characterized by disruption of sleep by respiratory events. An individual may be screened for SRBD either using an in-laboratory clinical polysomnogram or home sleep monitoring using a portable sleep monitor. OSA is condition characterized by loud snoring, breath interruptions and polysomnographic evidence of obstructive apneas and/or obstructive hypopneas, see Table 1. Central sleep apnea (CSA) syndrome may present similarly, but mostly consists of events with complete cessation of airflow along with cessation of thoracic and abdominal wall movements for ≥ 10 seconds. Figure 1 elaborates on ways to screen for SRBD in an outpatient setting. The management of SRBD requires referral to a sleep medicine service.
Limb Movement Disorders in Sleep
This category of sleep disorders includes Restless Leg Syndrome (RLS) and Periodic Limb Movement Disorder. A patient with RLS presents with “an urge to move the legs usually accompanied by or thought to be caused by uncomfortable and unpleasant sensation in the legs,” which in turn leads to difficulty falling asleep at bedtime 4. Patients in whom these disorders are suspected should also be referred to a sleep center.
It should be noted that an individual might have multiple comorbid sleep and/or substance use disorders. Complex case presentations may require co-treatment in substance use and sleep medicine clinics.
Alcohol and sleep disorders
Alcohol & Insomnia
Acute alcohol use has sedating effects, particularly on the descending limb of the blood-alcohol concentration curve, and is sometimes used for its sleep promoting effects. However, alcohol may disrupt sleep by interfering with homeostatic and circadian balance, disrupting local sleep mechanisms, and distorting the electrophysiology of sleep 1.
Risky alcohol use and AUD have been associated with subjective insomnia as well as objective sleep continuity disturbance. In those with AUD, the prevalence of insomnia ranges from 36-91% as compared to the 10% prevalence in the general population and is prevalent in all stages of alcohol use disorder 1. The prevalence of insomnia decreases once alcohol dependent individuals transition from active drinking to abstinence, with persisting insomnia possibly being a risk factor for relapse 1. However, in heavy drinkers with AUD, sleep dysfunction may persist up to 2 years into recovery in a subset of individuals and is a risk factor for relapse 8. Those who screen positive for AUD should be educated about the association of insomnia with AUD.
Behavioral and pharmacologic interventions for insomnia in AUD have been studied. Behavioral interventions have consistently demonstrated efficacy in treating insomnia among individuals with AUD 1. Cognitive behavioral therapy for insomnia (CBT-I) is the recommended first-line treatment for insomnia 9. Prior studies have demonstrated conflicting results with gabapentin and trazodone 1. Other drugs that have demonstrated an improvement in insomnia among alcohol-dependent patients have included acamprosate, agomelatine, and quetiapine 1.
If insomnia persists despite continued abstinence, patients should be referred to a sleep medicine clinic. If a behavioral sleep medicine specialist is unavailable in the sleep clinic, bibliotherapy, online sleep treatment programs, or evidence-based psychopharmacologic interventions should be considered, such as gabapentin or ramelteon.
Most of the currently approved hypnotic medications such as zolpidem or eszopiclone are contra-indicated in those with current AUD due to the potential risk of dependence and drug-drug interactions secondary to polypharmacy in this population. Therefore, gabapentin may be an alternative for those who continue to drink, although risks and benefits should be carefully reviewed.
Alcohol and SRBD
Alcohol relaxes the musculature of the upper airway and impairs protective arousal response during sleep. These effects may lead to an aggravation of snoring, fragmentation of sleep and worsening of preexisting SRBD. Treatment-seeking persons with AUD have a higher intensity of sleep disordered breathing during alcohol withdrawal and a high prevalence of SRBD 1. Continuous positive airway pressure (CPAP) is the first line treatment modality, although alternatives to CPAP are also available depending on severity.
Opioids and sleep disorders
Opioid prescriptions for chronic pain analgesia along with opioid morbidity have increased dramatically over the last two decades. Morasco et al. demonstrated that prescription opioid receipt and increasing opioid dose were associated with greater self-reported sleep disturbance among individuals with chronic pain, suggesting opioids worsen sleep beyond the disruptive sleep effects of pain 10. Insomnia and SRBD are the primary sleep disorders reported in those using opioids. Because pain and respiratory control are mediated by the endogenous opioid system, opioid-induced impairment in breathing must be a central concern of clinical evaluation in sleep-impaired persons.
Opioids and Insomnia
The majority of persons receiving opioids for chronic pain have insomnia, and approximately three-quarters of persons receiving methadone maintenance treatment (MMT) or buprenorphine for OUD have sleep complaints. Acute dosing of opioids for the treatment of pain appears to improve aspects of sleep quality11. But as use continues, there appears to be a significant association between prescription opioid dose and self-reported sleep dysfunction. The most frequent complaints include increased sleep latency and increased time awake after sleep onset12.
Among individuals with OUD, sleep gradually improves during the first 90 days after initiating treatment with buprenorphine/naltrexone 13. Factors contributing to persisting sleep complaints in opioid users are pain, depression14, benzodiazepine (BZD) use and cigarette smoking. It should be noted that use of medications in the BZD class is common in this population and is associated with longer sleep time estimates by patients than is demonstrated during sleep studies. Still, many opioid users will use BZDs to facilitate sleep15, increasing their risk for overdose, as both substances suppress respiration16,17.
CBT-I treatment has been shown to improve sleep-related outcomes18-20, including patients who had previously been prescribed medications for sleep21 and among patients whose impaired sleep was secondary to pain22. Trazodone has failed to show efficacy in one study 23, and no data exist on other medications like doxepin or suvorexant.
Opioids and SRBD
Opioid-induced impairment of breathing includes central depression of the respiratory rate, amplitude and reflex responses, reduced brain arousal, and upper airway dysfunction 24. SRBDs occur in the majority of chronic opioid users25. OSA was observed in 39% of 140 chronic pain patients taking opioids26, whereas the prevalence of OSA in the general population is estimated at 9% in women and 24% in men27. Risk factors for SRBD in patients with OUD include smoking, female gender and increased body weight 10. OSA in this population is likely due to opioid-induced reductions in airway muscle activation28. CSA may arise from depression of hypoxic and hypercapnic ventilatory drives, which are already reduced during sleep 24. This effect has been reported to occur in 0–60% of MMT patients 10 although it is rarely observed in the general population. CSA has been associated with methadone dose and concomitant benzodiazepine use, and with higher methadone blood concentration 10. Cases where CSA reverses after discontinuation of opioid treatment have been reported 10. Although very little information exists on SRBD associated with buprenorphine/naloxone, one study demonstrated incidence rates of 63% for mild, 16% for moderate and 17% for severe OSA 29.
Treatment recommendations for opioid-induced SRBD are limited by a lack of research on the topic. As a first step, the option of discontinuing opioids should be considered in the context of risks and benefits with this decision. When long-term treatment with opioid therapy is continued, and SRBD is identified, treatment options include CPAP, adaptive servo-ventilation (ASV), and bi-level spontaneous timed (ST) therapy 24. An alternative option is switching to an injectable formulation of depot-Naltrexone, a mu-opioid receptor antagonist.
Cannabis and sleep disorders
Acute cannabis or THC administration is associated with reduced latency to sleep onset, a decrease in REM sleep and an increase in Stage 3 sleep 30. The hypnotic properties of cannabis are often reported as a reason for use of cannabis, and may drive sustained use patterns among individuals with underlying sleep dysfunction 2. However, there is evidence of tolerance developing to the hypnotic effects of cannabis use, likely due to neurobiological changes in the endocannabioid system 30.
Daily cannabis users self-report greater sleep disturbance compared with non-users and less-frequent users, and treatment seeking cannabis users have high rates of disordered sleep 31,32. However, it remains unclear whether increased sleep problems among daily and treatment seeking cannabis users reflects a direct impact of heavy cannabis use or that the sleep dysfunction in this population is due to an underlying psychiatric or sleep pathology that predated, and possibly contributed to the development of Cannabis Use Disorder (CUD). Notably, one of the defining features of CUD is a cannabis withdrawal syndrome, in which sleep difficulty and an increase in vivid/strange dreams are hallmark features 33. Sleep difficulty typically lasts 2 to 3 weeks before returning to baseline levels, but the increase in vivid/strange dreams appears to persist indefinitely, suggesting that cannabis suppresses the recall or vividness of dreams rather than this being a true withdrawal effect 33.
Sleep difficulty during cannabis abstinence is a commonly reported barrier to cessation, with individuals reporting relapse to cannabis use, increased alcohol use, or use of sedative/hypnotic drugs to mitigate abstinence-induced sleep problems 33. Treatment-seeking cannabis users often exhibit clinically significant sleep dysfunction, and poor sleep at the beginning of treatment predicts relapse early in a quit attempt 32,34. Pilot studies suggest that behavioral and pharmacological interventions that can improve sleep during treatment for CUD may improve cannabis use outcomes35,36.
The medicinal use of cannabis in the treatment of pain, multiple sclerosis, or post-traumatic stress disorder (PTSD) can result in improved sleep. In some cases, the sleep improvement may contribute to better clinical outcomes and an improved quality of life. However, long-term use of cannabis or THC primarily as a hypnotic agent is not recommended due to the development of tolerance to its hypnotic properties, risk of long-term sleep disturbance and subsequent withdrawal symptoms on abrupt cessation, which can exacerbate symptoms of certain illnesses (e.g. PTSD).
Cocaine and its associated sleep disorders
Acute cocaine use increases arousal and binge use often occurs when the individual does not sleep. Persons with cocaine use disorder (CoUD) seldom seek help for insomnia, however recent studies that included objective measures indicate sleep dysfunction is common during cocaine withdrawal 33.
In a controlled trial of CoUD, modafinil (an FDA-approved medication for sleepiness in adequately treated obstructive sleep apnea, narcolepsy and circadian rhythm disorder-shift work) was superior to placebo in improving the total sleep time, stage 3 sleep and abstinence from cocaine 33. Other medications investigated for sleep continuity disturbance in individuals with CoUD have included lorazepam, tiagabine and mirtazapine. In a comparative efficacy trial of lorazepam and tiagabine, both drugs decreased sleep latency but tiagabine increased slow wave sleep in recently abstinent persons with CoUD.37 Mirtazapine, compared with placebo, transiently improved sleep onset latency (SOL) in depressed subjects with CoUD after 4 weeks of treatment, but didn’t reduce cocaine consumption38.
Sedative-hypnotic-anxiolytic drugs and sleep disorders
Sedative-hypnotic-anxiolytic medications include benzodiazepine (BZD) medications and newer non-BZD Z-drugs. Long-term use of BZDs may lead to dependence and characteristic withdrawal symptoms upon discontinuation that include, autonomic hyperactivity, insomnia, anxiety, and agitation. Similarly, reports also exist for abuse of non-BZD Z-drugs, though the rate of misuse of these medications is lower than for BZDs.
Benzodiazepine use has been associated with other substance use disorders including alcohol and opioid use, conditions that are also independently linked with insomnia symptoms39-41. Abrupt cessation of these medications has been associated with sleep complaints such as decreased total sleep time and a poor sleep quality. Among the sedative-hypnotic medications, the intensity of withdrawal-induced insomnia is higher in those using BZD class drugs as compared to the newer Z-drugs. In fact, withdrawal insomnia may be mild or non-existent after cessation of use of these Z-drugs. Sleep disturbances following abstinence from hypnotic medications typically improve over time during recovery, suggesting it is a true withdrawal effect42.
There is scant literature on the treatment of sleep problems in patients with sedative-hypnotic use disorder. Pregabalin has shown promise in decreasing insomnia and the use of benzodiazepines and to bolster abstinence43,44. In addition to medications, behavioral interventions such as relaxation therapy and CBT-I have demonstrated promise in treating the insomnia 45.
Summary and future directions
Sleep and SUDs are commonly comorbid conditions. Use of psychoactive substances often leads to the development of complaints of disturbed sleep, insomnia disorder or circadian rhythm sleep disorders. Abrupt cessation of substances of abuse commonly results in sleep disruption, which may be treated with behavioral or pharmacological interventions as a means of improving cessation attempt outcomes. Other sleep disorders, such as SRBD should be considered in the differential diagnosis for insomnia, especially in those with opioid use or AUD. When chronic insomnia or another intrinsic sleep disorder is suspected, a referral to the local sleep center is recommended. Chronic insomnia may be optimally treated with CBT-I. Acute insomnia or insomnia in the context of active substance use may be best treated in an addiction medicine or a primary care setting where the preliminary focus should be to target abstinence.
KEY POINTS.
Insomnia is linked with substance use and withdrawal.
Cognitive behavioral therapy for insomnia (CBT-I) has shown promise as an intervention for insomnia in individuals with alcohol and possibly other drug use disorders.
Sleep disordered breathing should be considered in the differential diagnosis of sleep maintenance insomnia, especially for patients misusing opioids and alcohol.
Abstinence from substance use should be recommended for those with short-term insomnia.
A referral to a sleep medicine clinic should be considered for insomnia disorder or other intrinsic sleep disorders, especially during abstinence.
SYNOPSIS.
Sleep and substance use disorders are disorders that commonly co-occur. Insomnia is commonly associated with use and withdrawal from substances. Circadian rhythm abnormalities are being increasingly linked with psychoactive substance use. Other sleep disorders such as sleep related breathing disorder should be considered in the differential diagnosis of insomnia especially in those with opioid use or alcohol use disorder. Insomnia that is brief or occurs in the context of active substance use is best treated by promoting abstinence. A referral to a sleep medicine clinic should be considered for those with chronic insomnia or when another intrinsic sleep disorder is suspected.
Acknowledgement
The editors wish to thank Deirdre A. Conroy, University of Michigan, and Bhanu Prakash Kolla, Mayo Clinic, Rochester, MN, for providing a critical review of this manuscript.
The study was supported by VA grant IK2CX000855 (S.C.), U01 DA031784 (R.G.V.), R01 DA034261, R01 NR015977 R34 DA032800 R21 DA031369 (M.D.S.). The content of this publication does not represent the views of the Department of Veterans Affairs or any other institution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
Dr. Chakravorty has received research support from AstraZeneca and Teva pharmaceuticals.
Dr. Vandrey is a paid consultant or serves on the advisory board of Zynerba Pharmaceuticals, Insys Therapeutics Inc., Battelle Memorial Institute, and several small U.S. businesses engaged in state medicinal cannabis programs.
Contributor Information
Subhajit Chakravorty, Perelman School of Medicine, 3900 Woodland Avenue, Philadelphia, PA 19104, USA.
Ryan Vandrey, Behavioral Pharmacology Research Unit, Johns Hopkins University School of Medicine, 5510 Nathan Shock Dr., Baltimore, MD 21224.
Sean He, School of Arts and Sciences, 116 College Hall/6377, University of Pennsylvania, Philadelphia, PA 19104.
Michael D. Stein, Health Law, Policy & Management, Boston University School of Public Health, 715 Albany Street, Boston, MA 02118.
References
- 1.Chakravorty S, Chaudhary NS, Brower KJ. Alcohol Dependence and Its Relationship With Insomnia and Other Sleep Disorders. Alcohol Clin Exp Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandrey R, Babson KA, Herrmann ES, Bonn-Miller MO. Interactions between disordered sleep, post-traumatic stress disorder, and substance use disorders. Int Rev Psychiatry. 2014;26(2):237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DSM-5. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 4.AASM. International Classification of Sleep Disorders - Third Edition. Darien, IL 60561; 2014. [Google Scholar]
- 5.Danel T, Libersa C, Touitou Y. The effect of alcohol consumption on the circadian control of human core body temperature is time dependent. American journal of physiology. Regulatory, integrative and comparative physiology. 2001;281(1):R52–55. [DOI] [PubMed] [Google Scholar]
- 6.Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biological psychiatry. 2003;54(12):1437–1443. [DOI] [PubMed] [Google Scholar]
- 7.Conroy DA, Hairston IS, Arnedt JT, Hoffmann RF, Armitage R, Brower KJ. Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiol Int. 2012;29(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brower KJ. Insomnia, alcoholism and relapse. Sleep medicine reviews. 2003;7(6):523–539. [DOI] [PubMed] [Google Scholar]
- 9.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 10.Hassamal S, Miotto K, Wang T, Saxon AJ. A narrative review: The effects of opioids on sleep disordered breathing in chronic pain patients and methadone maintained patients. The American journal on addictions. 2016;25(6):452–465. [DOI] [PubMed] [Google Scholar]
- 11.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2007;3(1):33–36. [PubMed] [Google Scholar]
- 12.Peles E, Schreiber S, Adelson M. Variables associated with perceived sleep disorders in methadone maintenance treatment (MMT) patients. Drug and alcohol dependence. 2006;82(2):103–110. [DOI] [PubMed] [Google Scholar]
- 13.Zheng WH, Wakim RJ, Geary RC, et al. Self-reported Sleep Improvement in Buprenorphine MAT (Medication Assisted Treatment) Population. Austin J Drug Abuse Addict. 2016;3(1). [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–1269. [DOI] [PubMed] [Google Scholar]
- 15.Stein MD, Kanabar M, Anderson BJ, Lembke A, Bailey GL. Reasons for Benzodiazepine Use Among Persons Seeking Opioid Detoxification. J Subst Abuse Treat. 2016;68:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Sangthong R, Chongsuvivatwong V, McNeil E, Li J. Lifetime multiple substance use pattern among heroin users before entering methadone maintenance treatment clinic in Yunnan, China. Drug Alcohol Rev. 2010;29(4):420–425. [DOI] [PubMed] [Google Scholar]
- 17.Stein MD, Herman DS, Bishop S, et al. Sleep disturbances among methadone maintained patients. J Subst Abuse Treat. 2004;26(3):175–180. [DOI] [PubMed] [Google Scholar]
- 18.Backhaus J, Hohagen F, Voderholzer U, Riemann D. Long-term effectiveness of a short-term cognitive-behavioral group treatment for primary insomnia. Eur Arch Psychiatry Clin Neurosci. 2001;251(1):35–41. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2003(1):CD003161. [DOI] [PubMed] [Google Scholar]
- 20.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(3):191–204. [DOI] [PubMed] [Google Scholar]
- 21.Dolan DC, Taylor DJ, Bramoweth AD, Rosenthal LD. Cognitive-behavioral therapy of insomnia: a clinical case series study of patients with co-morbid disorders and using hypnotic medications. Behav Res Ther. 2010;48(4):321–327. [DOI] [PubMed] [Google Scholar]
- 22.Currie SR, Wilson KG, Pontefract AJ, deLaplante L. Cognitive-behavioral treatment of insomnia secondary to chronic pain. J Consult Clin Psychol. 2000;68(3):407–416. [DOI] [PubMed] [Google Scholar]
- 23.Stein MD, Kurth ME, Sharkey KM, Anderson BJ, Corso RP, Millman RP. Trazodone for sleep disturbance during methadone maintenance: a double-blind, placebo-controlled trial. Drug and alcohol dependence. 2012;120(1-3):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Ryswyk E, Antic NA. Opioids and Sleep-Disordered Breathing. Chest. 2016;150(4):934–944. [DOI] [PubMed] [Google Scholar]
- 25.Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120(6):1273–1285. [DOI] [PubMed] [Google Scholar]
- 26.Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Med. 2008;9(4):425–432. [DOI] [PubMed] [Google Scholar]
- 27.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. [DOI] [PubMed] [Google Scholar]
- 28.Hajiha M, DuBord MA, Liu H, Horner RL. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J Physiol. 2009;587(Pt 11):2677–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farney RJ, McDonald AM, Boyle KM, et al. Sleep disordered breathing in patients receiving therapy with buprenorphine/naloxone. Eur Respir J. 2013;42(2):394–403. [DOI] [PubMed] [Google Scholar]
- 30.Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana. Sleep Med Rev. 2008;12(5):381–389. [DOI] [PubMed] [Google Scholar]
- 31.Conroy DA, Kurth ME, Strong DR, Brower KJ, Stein MD. Marijuana use patterns and sleep among community-based young adults. Journal of addictive diseases. 2016;35(2):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacek LR, Herrmann ES, Smith MT, Vandrey R. Sleep continuity, architecture and quality among treatment-seeking cannabis users: An in-home, unattended polysomnographic study. Exp Clin Psychopharmacol. 2017;25(4):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angarita GA, Emadi N, Hodges S, Morgan PT. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addict Sci Clin Pract. 2016;11(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babson KA, Boden MT, Bonn-Miller MO. The impact of perceived sleep quality and sleep efficiency/duration on cannabis use during a self-guided quit attempt. Addict Behav. 2013;38(11):2707–2713. [DOI] [PubMed] [Google Scholar]
- 35.Babson KA, Ramo DE, Baldini L, Vandrey R, Bonn-Miller MO. Mobile App-Delivered Cognitive Behavioral Therapy for Insomnia: Feasibility and Initial Efficacy Among Veterans With Cannabis Use Disorders. JMIR Res Protoc. 2015;4(3):e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan PT, Malison RT. Pilot study of lorazepam and tiagabine effects on sleep, motor learning, and impulsivity in cocaine abstinence. The American journal of drug and alcohol abuse. 2008;34(6):692–702. [DOI] [PubMed] [Google Scholar]
- 38.Afshar M, Knapp CM, Sarid-Segal O, et al. The efficacy of mirtazapine in the treatment of cocaine dependence with comorbid depression. The American journal of drug and alcohol abuse. 2012;38(2):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroll DS, Nieva HR, Barsky AJ, Linder JA. Benzodiazepines are Prescribed More Frequently to Patients Already at Risk for Benzodiazepine-Related Adverse Events in Primary Care. Journal of General Internal Medicine. 2016;31(9):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hackman DT, Greene MS, Fernandes TJ, Brown AM, Wright ER, Chambers RA. Prescription drug monitoring program inquiry in psychiatric assessment: detection of high rates of opioid prescribing to a dual diagnosis population. J Clin Psychiatry. 2014;75(7):750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manthey L, Lohbeck M, Giltay EJ, van Veena T, Zitman FG, Penninx BW. Correlates of benzodiazepine dependence in the Netherlands Study of Depression and Anxiety. Addiction. 2012;107(12):2173–2182. [DOI] [PubMed] [Google Scholar]
- 42.Lichstein KL. Behavioral intervention for special insomnia populations: Hypnotic-dependent insomnia and comorbid insomnia. Sleep Medicine. 2006;7(SUPPL. 1):S27–S31. [Google Scholar]
- 43.Oulis P, Konstantakopoulos G. Efficacy and safety of pregabalin in the treatment of alcohol and benzodiazepine dependence. Expert Opin Investig Drugs. 2012;21(7):1019–1029. [DOI] [PubMed] [Google Scholar]
- 44.Cho YW, Song ML. Effects of pregabalin in patients with hypnotic-dependent insomnia. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014;10(5):545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beaulieu-Bonneau S, Ivers H, Guay B, Morin CM. Long-Term Maintenance of Therapeutic Gains Associated With Cognitive-Behavioral Therapy for Insomnia Delivered Alone or Combined With Zolpidem. Sleep. 2017;40(3). [DOI] [PMC free article] [PubMed] [Google Scholar]