Abstract
High titers of pathogenic autoantibodies are a hallmark of many autoimmune diseases. However, much remains unknown about the self-reactive plasma cells that are key mediators of disease. We propose a model in which the varying efficacy of precursor B cell depletion for the treatment of humoral autoimmunity can be explained by differences in the relative contributions of pathogenic antibodies by short-lived vs. long-lived plasma cells. Beyond therapeutic considerations, this model suggests that we can infer the cellular source of disease-associated autoantibodies by the durability of serum titers following B cell depletion. Data from clinical trials and animal models across different autoimmune diseases may provide useful insights into the lifespan, lifestyle and fate of autoreactive plasma cells.
Introduction
In multiple autoimmune disorders, high-titer autoantibodies can predict disease onset, serve as biomarkers for diagnosis, or directly promote disease pathogenesis [1,2]. Despite these important roles for autoantibodies in human disease, the biology of plasma cells that are the source of serum immunoglobulin remains poorly understood. During an adaptive immune response to foreign antigen, specific antibodies are produced by both short- and long-lived plasma cell subpopulations [3]. Multiple lines of evidence suggest that similar activation pathways underlie autoimmune pathogenesis. However, since autoreactive plasma cells are rare cells residing in inaccessible locations within the bone marrow, secondary lymphoid organs and inflamed tissues, direct study of plasma cell biology in human autoimmunity is technically challenging.
Over the past two decades, a number of B cell depleting therapies have been trialed in human autoimmunity. The most well-studied agent, rituximab (Rituxan), is a humanized monoclonal antibody binding CD20, a B cell surface marker first expressed at the late pre-B cell stage of bone marrow development, maintained throughout peripheral B cell maturation, and downregulated during differentiation into antibody-secreting cells (ASC). Since CD20 expression is lost during plasma cell maturation, treatment with rituximab or related B cell depletion therapies is not predicted to directly target mature plasma cells [3,4]. Rather, these therapies likely impact circulating autoantibody titers by either eliminating autoreactive B cells that are the precursors of pathogenic plasma cells and/or by directly targeting recently generated plasmablasts which can retain low-level CD20 expression [5–7]. Based on these observations, we propose a model in which the impact of B cell ablation on autoantibody titers can be used to infer the characteristics of self-reactive plasma cells in individual diseases. Importantly, therapeutic benefits in B cell depletion frequently precede reductions in autoantibody titers, suggesting that loss of B cell antigen presentation and/or cytokine production contributes to clinical efficacy [2]. However, rather than an exhaustive review of clinical trials of B cell depletion in autoimmunity, in the current manuscript we will focus specifically on the impact of B cell targeting on serum autoantibody titers. As models of distinct mechanisms in autoimmunity, we will highlight data from clinical trials in pemphigus vulgaris, Sjögren’s syndrome and systemic lupus erythematosus (SLE); three diseases that we believe exemplify the differential contributions of short- and long-lived plasma cells in autoimmune pathogenesis.
Overlapping contributions of short- and long-lived plasma cells to humoral immunity
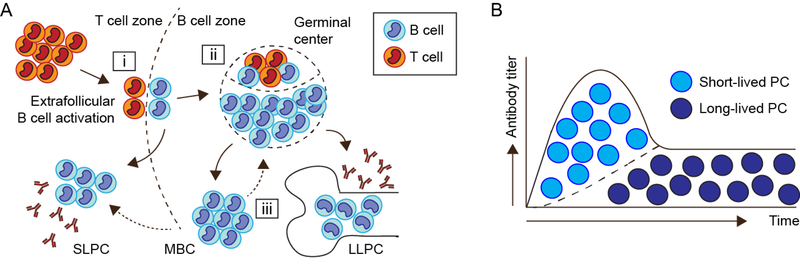
During a humoral immune response, antigen-specific B cells differentiate into memory B cells and antibody-producing plasma cells. Memory B cells are antigen-experienced B cells that remain quiescent for prolonged periods before rapid secondary response to antigen rechallenge. In contrast, plasma cells are effector B cells which serve as the source for both protective and pathogenic antibodies. Functionally, plasma cells can be divided into two subsets based on survival kinetics and location: a short-lived population thought to be generated predominantly via extrafollicular B cell activation and to reside in the splenic red pulp or lymph node medullary cords; and long-lived plasma cells (LLPC) that are primarily germinal center (GC)-derived and traffic to bone marrow survival niches [3]. Although considered separately here, short- and long-lived plasma cells are generated concurrently during a T-dependent immune response. After initial antigen challenge, rapid extrafollicular plasma cell responses are followed by the generation of GC-derived, affinity-matured LLPCs, thereby providing overlapping humoral protection from infectious challenge (Figure 1).
Figure 1. T cell-dependent humoral immune response:
(A) (i) After antigen exposure, antigen-specific B cells and CD4+ T cells migrate to the T cell:B cell border. These intial cognate interactions promote B cell proliferation and facilitate the rapid differentiation of short-lived plasma cells/plasmablasts which are the source for early, low-affinity protective antibody titers. (ii) Subsequently, continued B cell:T cell co-stimulatory and cytokine crosstalk drives T follicular helper (Tfh) cell differentiation and the formation of germinal centers (GC). Within the GC, iterative rounds of B cell somatic hypermutation and affinity maturation ultimately results in the formation of high-affinity memory B cells (MBC) and plasma cells, a subset of which are able to engraft into the long-lived bone marrow compartment. (iii) Of particular relevance to autoimmunity, MBC exhibit lower thresholds for antigen-dependent activation, resulting in either GC re-entry or rapid differentiation into antibody-producing plasma cells. (B) Theoretical model indicating the relative contributions of short- vs. long-lived plasma cells to serum antibody titers over time. The initial increase in antibody titers following T-dependent antigen exposure is believed to be produced predominantly by SLPCs, with LLPC being responsible for the long-term maintenance of protective antibody levels.
Notably, the paradigm that long-term maintenance of protective antibody titers depends on a small population of LLPCs is a relatively new one. Until twenty years ago, the humoral component of immune memory was believed to be supplied by terminally differentiated plasma cells that had not been observed to survive longer than a week. Since the half-life of an antibody in serum is ~28 days, long-term antibody titers were attributed to the continual homeostatic and/or antigen-driven differentiation of memory B cells into short-lived plasma cells (SLPC). However, in 1998, Slifka et al. demonstrated long-term maintenance of virus-specific antibodies despite memory B cell depletion, establishing the existence of LLPCs maintained without replenishment from the memory B cell pool [8]. A recent study from the same group reported durable anti-tetanus titers in rhesus macaques despite aggressive B cell depletion, with a subset of anti-tetanus plasma cells exhibiting incorporation of BrdU administered at the time of immunization a decade prior [9]. In humans, phenotypic characterization of bone-marrow antibody-secreting cells (ASCs) indicated that durable anti-vaccine titers are produced by analogous plasma cells with a CD19-CD38+CD138+ surface phenotype [4]. Consistent with these data, protective antibody titers were maintained for >6 months in patients undergoing anti-CD19 chimeric antigen receptor (CAR) T cell therapy for B cell malignancy, despite the complete and persistent ablation of CD19+ B cells induced by this therapy [10]. Thus, human and animal data confirm that plasma cells can be maintained for decades without ongoing cellular division, with the theoretical lifespan of LLPCs exceeding the life of the host.
Distinct contributions of short- and long-lived plasma cells to humoral autoimmunity
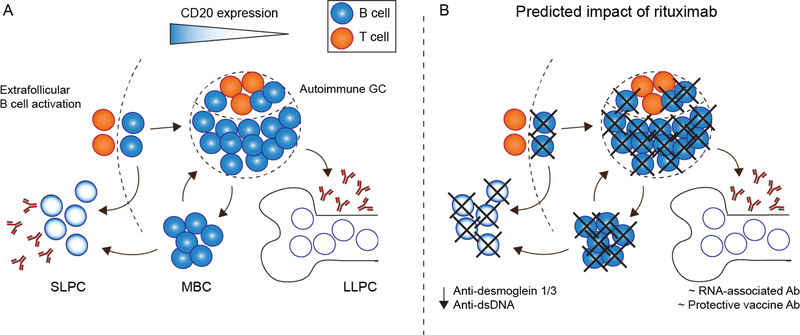
While parallel short- and long-lived antibody responses evolved to resist infection, similar processes theoretically underlie humoral autoimmunity. However, whereas the timing of antigen exposure can be identified after infection or vaccination, humoral autoimmunity occurs stochastically and antigen exposure could be considered continuous. Thus, serum autoantibodies might theoretically be maintained by pathogenic LLPCs, or by the continuous extrafollicular production of autoreactive SLPCs in a persistent inflammatory milieu. This distinction is of clinical importance, since SLPCs may be sensitive to anti-proliferative immunosuppression or depletion of B cell precursors, whereas LLPCs largely resist such treatment [5,11,12]. However, uncovering the relative contribution of each plasma cell population in specific autoimmune diseases is challenging. Secondary lymphoid organs and bone marrow niches, where SLPC and LLPCs are believed to reside, require invasive procedures to access, and plasma cell function is difficult to model ex vivo [3,13]. Moreover, the autoimmune state is associated with greater total numbers of plasma cells, made possible by expansion of plasma cell niches in the spleen and at peripheral sites of inflammation [11]. These observations align with a model in which LLPC populations are regulated chiefly by available space in a niche [14,15], but suggest that only studying a particular compartment may miss important PC populations in autoimmunity. Ultimately, the best definition of a long-lived plasma cell may be a functional one: the presence of stable antibody titers for many months after complete depletion of B cell precursors (Figure 2).
Figure 2. Theoretical impact of anti-CD20 B cell depletion on plasma cell subsets:
(A) During humoral autoimmunity, autoantibodies might be derived from either short- or longlived plasma cell subsets. In this model, the degree of surface CD320 expression is indicated by a blue gradient, including: CD20+ naïve B cells, GC B cells and MBC; CD20low/neg short-lived plasmablasts/plasma cells; and, CD20neg LLPC. (B) Based on surface CD20 expression, rituximab treatment is predicted to spare CD20neg LLPC, but deplete SLPC via either direct CD20 targeting or indirectly via ablation of CD20+ B cell precursors. Thus, protective vaccine titers and RNA-associated autoantibodies are preserved following B cell depletion, whereas SLPC-derived anti-desmoglein 1/3 and anti-dsDNA autoantibodies decline.
Pemphigus: a model for SLPC-driven disease
Pemphigus vulgaris, and the related disorder pemphigus foliaceus, are chronic blistering diseases of the skin and mucous membranes driven by IgG4 autoantibodies targeting epidermal desmoglein proteins [16]. Notably, anti-desmoglein antibodies are both necessary and sufficient for disease pathogenesis, as evidenced by high sensitivity and specificity of autoantibody titers in pemphigus diagnosis [17], and the development of neonatal pemphigus following placental transfer of maternal anti-desmoglein antibodies [18]. Interestingly, animal studies have demonstrated that adoptive transfer of anti-desmoglein Fab fragments alone can drive blister formation, indicating that disease results from structural disruption of epidermal integrity without the need for Fc-dependent immune activation [19]. These insights drove initial attempts in the use of rituximab as salvage therapy in patients with corticosteroid-refractory pemphigus. In early trials in subjects with severe, treatment-resistant disease, clinical remissions occurred in the majority of patients within three months [20,21]. Subsequently, a larger prospective open label trial demonstrated that rituximab plus short-course prednisone resulted in ~90% complete remission, which correlate with the rapid reduction in anti-desmoglein antibodies [22]. In addition to improving the management of this life-threatening disease, these trials also provide insight into the pathogenesis of pemphigus. Since CD20 expression is lost during plasma cell differentiation, pathogenic anti-desmoglein antibodies are likely derived from continuously generated SLPCs [23]. Consequently, rituximab-mediated depletion of autoreactive CD20+ B cell precursors and/or the direct targeting of pathogenic CD20low plasmablasts is sufficient for treatment of pemphigus.
Sjögren’s syndrome: rituximab-resistant autoantibodies implicate LLPCs in disease pathogenesis
Sjögren’s syndrome is an autoimmune condition characterized by exocrine dysfunction of the lacrimal and salivary glands [24]. Disease development is associated with the infiltration of activated B and CD4+ T cells into inflamed glands. In addition, plasma cells are frequently identified in salivary glands of Sjögren’s patients, in keeping with expansion of plasma cell survival niches outside the BM in other inflammatory conditions [25]. Although autoantibodies have not been directly implicated in disease pathogenesis, Sjögren’s syndrome is characterized by autoantibodies targeting Ro/SSA and La/SSB autoantigens. For this reason, rituximab has been studied in several clinical trials in Sjögren’s syndrome, with limited clinical utility [26–29]. While the impact of rituximab on serum autoantibody titers is an infrequent clinical endpoint in Sjögren’s trials, available data indicate that anti-CD20 B cell depletion does not affect anti-SSA and –SSB titers [30–32]. Notably, this failure to reduce anti-SSA titers was observed despite the apparent depletion of CD19+ B cells in both peripheral blood and in salivary gland biopsies [32]. Thus, these data support the idea that the majority of SSA-reactive ASCs in Sjögren’s syndrome are likely long-lived, capable of surviving for >5 months in the absence of regeneration from CD20+ memory B cell precursors.
SLE – paradigm for contribution of both SLPCs and LLPCs to autoantibody repertoire
In contrast to the above data implying that pemphigus vulgaris and Sjögren’s syndrome exhibit distinct contributions of short- vs. long-lived plasma cells, respectively, significant complexity underlies the autoantibody repertoire in systemic lupus erythematosus (SLE). Lupus is a chronic and clinically heterogeneous systemic autoimmune disease characterized by the near universal presence of class-switched autoantibodies against a wide-range of antinuclear antigens. While anti-nuclear antibodies (ANA) are likely insufficient for disease development (since ANA development can predate clinical symptoms by years [33]), autoantibodies contribute to several lupus clinical manifestations, including autoimmune hemolytic anemia and immune complex glomerulonephritis [34]. Importantly, animal models and human clinical studies have indicated that both extra-follicular and GC-dependent B cell activation pathways likely contribute to autoreactive B cell activation in SLE. For example, spontaneous GCs are observed in murine lupus models and in human clinical samples, and ANA from lupus patients can exhibit extensive somatic hypermutation (SHM) implicating a GC origin [35–37]. In contrast, in MRL.Faslpr lupusprone mice, spontaneous activation of autoreactive B cells occurs via a predominantly extrafollicular pathway, resulting in GC-independent SHM [38]. These animal models correspond with human studies that show an expansion of circulating plasmablasts in SLE [39,40], with a subset of these plasmablasts exhibiting reduced mutation frequency and prominent clonality that can persist for months [41]. Cumulatively, these data implicate both short- and long-lived plasma cells as likely contributors to the autoantibody repertoire in SLE. Consistent with this model, results from clinical trials have indicated that anti-dsDNA titers decrease following B cell depletion, whereas autoantibodies against RNA-associated nuclear antigens remain unchanged, consistent with production by short- and long-lived plasma cells, respectively [42–45]. Thus, although large randomized controlled trials of rituximab in SLE (EXPLORER [45] and LUNAR [46]) failed to meet their clinical endpoints, data from these and other B cell depletion studies highlight the complexity of SLE and have informed our understanding of lupus biology.
Challenges with inferring the contributions of short vs. long-lived PCs in autoimmunity
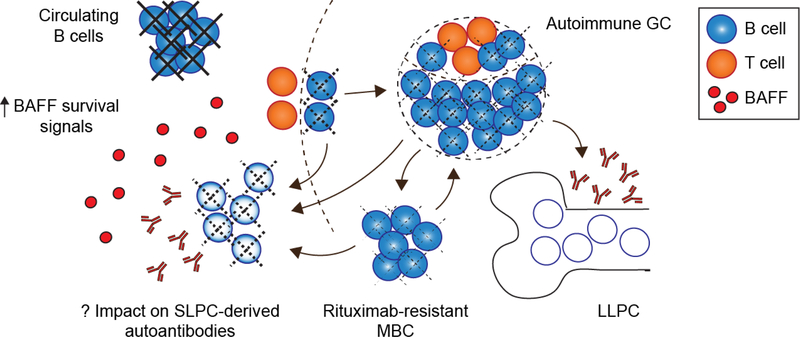
An important caveat to our proposed model is that our ability to infer the plasma cell subset producing specific autoantibodies requires complete ablation of peripheral B cell precursors following B cell targeting. In human clinical trials, rituximab results in the rapid depletion of circulating CD20+ B cells that is maintained for >3 months in the majority of subjects. However, resident B cells in the tissues and secondary lymphoid organs are less efficiently cleared, as evidenced by the long-term persistence of splenic vaccinia virus-specific memory B cells and robust amnestic tetanus toxoid immunization responses following rituximab treatment [47–49]. Independent of autoimmunity, inflammatory conditions are also associated with a shortened therapeutic half-life and reduced efficacy of CD20-depletion in mouse models [50]. Consistent with these data, presumably-autoreactive B cells can persist in synovial membranes in rheumatoid arthritis and the salivary glands in Sjögren’s syndrome following rituximab treatment [51,52].
Among the humoral autoimmune diseases, lupus B cells are particularly resistant to therapeutic depletion. For example, the Shlomchik group reported that splenic B cells resist anti-CD20 ablation in independent models of murine lupus, with longer treatment required for equivalent B cell depletion as control mice [53]. In addition, distinct B cell subsets within the spleen and lymph nodes exhibited variable sensitivity to anti-CD20 antibodies. In murine models, peritoneal B1 B cells, GC B cells and to a lesser degree marginal zone B cells resist B cell ablation [5356]; findings of particular relevance to autoimmunity since these B cell subpopulations have been identified as potential sources of pathogenic ASCs [57–59]. Moreover, SLE is characterized by increased serum titers of the B cell survival cytokine B-cell-activating factor of the tumor-necrosis-factor family (BAFF), which promotes resistance to B cell depletion [60,61]. A recent study reported that increased serum BAFF following B cell depletion also extended the survival of splenic plasma cells in both wild-type and lupus prone mice; findings which correlated with plasma cell maturation and the adoption of a “long-lived” gene expression profile [62].
Together, these data may explain the incomplete clinical efficacy of B cell depletion in autoimmunity, and suggest caution in attributing a cellular source to distinct autoantibody specificities. Although anti-desmoglein autoantibodies in pemphigus are almost certainly derived from SLPCs, it is currently not clear whether apparently “rituximab resistant” autoantibody specificities in SLE are derived from true LLPCs. Given incomplete B cell ablation, pathogenic SLPCs might continue to be generated from residual memory B cells. Alternatively, increased serum BAFF levels following peripheral B cell depletion may result in the maturation and/or long-term survival of self-reactive plasma cells otherwise destined to be short-lived (Figure 3). In this context, recent results from a clinical trial of combined rituximab and BAFF inhibition (belimumab, Benlysta) for severe lupus nephritis are particularly interesting [63]. In this study, anti-dsDNA and RNA-associated autoantibody titers were both reduced by ~50%, whereas titers of LLPC-derived vaccine antibodies remained unchanged. While this trial included relatively few patients, these data suggest that SLPCs and LLPCs contribute to both DNA- and RNA-reactive autoantibodies in SLE, with increased serum BAFF contributing to the differential maintenance of autoreactive plasma cells or memory B cell precursors [64]. Results from a large ongoing randomized trial in lupus nephritis comparing rituximab and cyclophosphamide with and without belimumab (CALIBRATE; NCT02260934) should be particularly informative in this regard.
Figure 3. Incomplete anti-CD20 B cell depletion and elevated serum BAFF complicates the interpretation of B cell depletion trials:
Despite near-complete ablation of circulating B cells, autoreactive GC B cells and MBC (as well as MZ and B1 B cells, not shown) are relatively resistant to rituximab-mediated depletion (dashed lines indicate partial ablation). Thus, persistent serum autoantibodies following rituximab treatment might be produced by pathogenic SLPCs continuously generated from MBC, GC and/or other resistant B cell subsets. Moreover, increased serum BAFF levels following B cell depletion may promote the long-term survival of SLPC in autoimmunity.
Finally, the careful study of clinical data from anti-CD19 CAR T cell trials is likely to yield additional insights into autoimmune plasma cell biology. The surface marker CD19, targeted by recently approved CAR T cell therapies for B cell malignancy, is also expressed by nonmalignant B cell subsets in a manner largely overlapping with rituximab’s cognate antigen, CD20 [65]. Evidence of striking anti-tumor efficacy in subjects where rituximab had previously failed to control disease suggests that anti-CD19 CAR T cells are likely more potent and/or penetrant in the depletion of endogenous B cells. Consistent with this model, patients achieving long-term remission following anti-CD19 CAR T cell transfer exhibit profound B cell aplasia that can persist for years after a single treatment [10]. Thus, antibody titers maintained long-term under these conditions may reasonably be ascribed to LLPC, while declining antibody specificities likely derive from SLPC or from CD19+ ASC populations that have been observed in human vaccination studies [66]. One caveat is that current CAR T cell protocols require lymphodepleting conditioning, with treatment provided as salvage therapy following multiple rounds of cytotoxic chemotherapy and/or rituximab treatment. Additionally, the malignancy itself may perturb the dynamics of hematopoiesis, B cell development, and the bone marrow microenvironment. Nevertheless, the small amount of existing data on antibody titers in antiCD19 CAR T cell-treated patients suggests that LLPC are strikingly resilient. In patients with profound B cell aplasia extending >180 days, both vaccine-induced anti-body titers to measles and HSV1/2 titers arising from pre-treatment natural infection have been found to be maintained with remarkable stability [10].
Significant toxicity and expense are likely to prohibit the near-term application of CAR T cell therapy in autoimmunity. However, since patients with humoral autoimmune diseases, including SLE, rheumatoid arthritis and Sjögren’s syndrome, are at increased risk of developing malignancy, in particular B cell-lineage lymphomas [67–70], anti-CD19 CAR T cells will inevitably be offered to subjects with pre-existing autoantibodies. Thus, the measurement of autoantibody titers following CAR T cell treatment has the potential to greatly expand our understanding of plasma cell biology, to add insights into the long-term effects of B cell aplasia, and to stimulate new approaches to therapy for patients with autoimmunity.
Concluding remarks
Careful analysis of both animal models and human clinical trials of B cell depletion has resulted in many insights into the biology of autoreactive plasma cells. However, important questions remain regarding the cellular identity, plasticity and durability of autoantibody-secreting cells. First, what are the mechanisms that drive the formation of short- vs. long-lived plasma cells in autoimmunity? What disease-specific B cell activation pathways or tolerance mechanisms prevent the establishment of pathogenic LLPCs in pemphigus, but not in Sjögren’s syndrome or SLE? What role do BAFF-mediated survival signals play in the long-term persistence of autoantibody titers in systemic autoimmunity? Finally, would a more robust depletion of tissue-resident B cells, for example using anti-CD19 CAR T cells or bi-specific antibodies [65,71], result in greater therapeutic efficacy in humoral autoimmunity?
Ultimately, while targeting B cell precursors has given immunologists important information about the cellular sources of circulating autoantibodies, direct plasma cell depletion may be required for effective treatment of antibody-mediated disease. Many of the features that enable LLPC to provide stable, life-long protective antibody titers also render plasma cells resistant to therapeutic ablation. However, recent advances in the treatment for multiple myeloma, a plasma cell malignancy, hold the promise of more effective tools in the near future [72,73]. As agents for the selective depletion of malignant plasma cells improve in potency and safety, their use will likely extend to autoimmunity, providing new options for treatment and advancing our understanding of disease mechanisms [74,75].
Highlights:
Pathogenic autoantibodies can be derived from short- or long-lived plasma cells
Therapeutic B cell depletion is a common treatment approach in humoral autoimmunity
B cell ablation strategies target surface markers absent on mature plasma cells
Distinct autoimmune diseases exhibit variable clinical responses to B cell depletion
Autoantibody titers after B cell depletion correspond with plasma cell lifespan
Acknowledgements:
This work was supported by NIAID of the National Institutes of Health under award number: T32GM007266 (University of Washington Medical Scientist Training Program, MH); DP3-DK111802 (DJR); R21AI123818 (DJR); and, K08AI112993 (SWJ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support provided by the Children’s Guild Association Endowed Chair in Pediatric Immunology (DJR); by the Benaroya Family Gift Fund (DJR); by the American College of Rheumatology (ACR) Rheumatology Research Foundation (RRF) Career Development K Supplement (SWJ); by the Arthritis National Research Foundation (ANRF) Eng Tan Scholar Award (SWJ); and, by a Novel Research Grant from the Lupus Research Alliance (SWJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Suurmond J, Diamond B: Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest 2015, 125:2194–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawlings DJ, Metzler G, Wray-Dutra M, Jackson SW: Altered B cell signalling in autoimmunity. Nat Rev Immunol 2017, 17:421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM: The generation of antibody-secreting plasma cells. Nat Rev Immunol 2015, 15:160–171. [DOI] [PubMed] [Google Scholar]
- 4.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, Popova L,Kaminiski D, Fucile CF, Albizua I, et al. : Long-Lived Plasma Cells Are Contained within the CD19(−)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity 2015, 43:132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang H, Benoist C, Mathis D: Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A 2010, 107:4658–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF: Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol 2008, 180:361–371. [DOI] [PubMed] [Google Scholar]
- 7.Ellebedy AH, Jackson KJ, Kissick HT, Nakaya HI, Davis CW, Roskin KM, McElroy AK,Oshansky CM, Elbein R, Thomas S, et al. : Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol 2016, 17:1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slifka MK, Antia R, Whitmire JK, Ahmed R: Humoral immunity due to long-lived plasma cells. Immunity 1998, 8:363–372. [DOI] [PubMed] [Google Scholar]
- 9.Hammarlund E, Thomas A, Amanna IJ, Holden LA, Slayden OD, Park B, Gao L, Slifka MK: Plasma cell survival in the absence of B cell memory. Nat Commun 2017, 8:1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhoj VG, Arhontoulis D, Wertheim G, Capobianchi J, Callahan CA, Ellebrecht CT, Obstfeld AE, Lacey SF, Melenhorst JJ, Nazimuddin F, et al. : Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 2016, 128:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F,Manz RA: Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med 2004, 199:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahevas M, Michel M, Weill JC, Reynaud CA: Long-lived plasma cells in autoimmunity: lessons from B-cell depleting therapy. Front Immunol 2013, 4:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reismann D, Stefanowski J, Gunther R, Rakhymzhan A, Matthys R, Nutzi R, Zehentmeier S, Schmidt-Bleek K, Petkau G, Chang HD, et al. : Longitudinal intravital imaging of the femoral bone marrow reveals plasticity within marrow vasculature. Nat Commun 2017, 8:2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiepe F, Dorner T, Hauser AE, Hoyer BF, Mei H, Radbruch A: Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol 2011, 7:170–178. [DOI] [PubMed] [Google Scholar]
- 15.Wilmore JR, Allman D: Here, There, and Anywhere? Arguments for and against the Physical Plasma Cell Survival Niche. J Immunol 2017, 199:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ran NA, Payne AS: Rituximab therapy in pemphigus and other autoantibody-mediated diseases. F1000Res 2017, 6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt E, Dahnrich C, Rosemann A, Probst C, Komorowski L, Saschenbrecker S, Schlumberger W, Stocker W, Hashimoto T, Brocker EB, et al. : Novel ELISA systems for antibodies to desmoglein 1 and 3: correlation of disease activity with serum autoantibody levels in individual pemphigus patients. Exp Dermatol 2010, 19:458–463. [DOI] [PubMed] [Google Scholar]
- 18.Moncada B, Kettelsen S, Hernandez-Moctezuma JL, Ramirez F: Neonatal pemphigus vulgaris: role of passively transferred pemphigus antibodies. Br J Dermatol 1982, 106:465–467. [DOI] [PubMed] [Google Scholar]
- 19.Rock B, Labib RS, Diaz LA: Monovalent Fab’ immunoglobulin fragments from endemic pemphigus foliaceus autoantibodies reproduce the human disease in neonatal Balb/c mice. J Clin Invest 1990, 85:296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed AR, Spigelman Z, Cavacini LA, Posner MR: Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med 2006, 355:1772–1779. [DOI] [PubMed] [Google Scholar]
- 21.Joly P, Mouquet H, Roujeau JC, D’Incan M, Gilbert D, Jacquot S, Gougeon ML, Bedane C,Muller R, Dreno B, et al. : A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med 2007, 357:545–552. [DOI] [PubMed] [Google Scholar]
- 22.Joly P, Maho-Vaillant M, Prost-Squarcioni C, Hebert V, Houivet E, Calbo S, Caillot F,Golinski ML, Labeille B, Picard-Dahan C, et al. : First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet 2017, 389:2031–2040. [DOI] [PubMed] [Google Scholar]
- 23.Mouquet H, Musette P, Gougeon ML, Jacquot S, Lemercier B, Lim A, Gilbert D, Dutot I,Roujeau JC, D’Incan M, et al. : B-cell depletion immunotherapy in pemphigus: effects on cellular and humoral immune responses. J Invest Dermatol 2008, 128:2859–2869. [DOI] [PubMed] [Google Scholar]
- 24.Mariette X, Criswell LA: Primary Sjogren’s Syndrome. N Engl J Med 2018, 378:931–939. [DOI] [PubMed] [Google Scholar]
- 25.Szyszko EA, Brokstad KA, Oijordsbakken G, Jonsson MV, Jonsson R, Skarstein K: Salivary glands of primary Sjogren’s syndrome patients express factors vital for plasma cell survival. Arthritis Res Ther 2011, 13:R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijer JM, Meiners PM, Vissink A, Spijkervet FK, Abdulahad W, Kamminga N, Brouwer E, Kallenberg CG, Bootsma H: Effectiveness of rituximab treatment in primary Sjogren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2010, 62:960–968. [DOI] [PubMed] [Google Scholar]
- 27.Dass S, Bowman SJ, Vital EM, Ikeda K, Pease CT, Hamburger J, Richards A, Rauz S,Emery P: Reduction of fatigue in Sjogren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis 2008, 67:1541–1544. [DOI] [PubMed] [Google Scholar]
- 28.Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, Berthelot JM, Perdriger A, Puechal X,Le Guern V, Sibilia J, Gottenberg JE, Chiche L, et al. : Treatment of primary Sjogren syndrome with rituximab: a randomized trial. Ann Intern Med 2014, 160:233–242. [DOI] [PubMed] [Google Scholar]
- 29.Bowman SJ, Everett CC, O’Dwyer JL, Emery P, Pitzalis C, Ng WF, Pease CT, Price EJ, Sutcliffe N, Gendi NST, et al. : Randomized Controlled Trial of Rituximab and Cost-Effectiveness Analysis in Treating Fatigue and Oral Dryness in Primary Sjogren’s Syndrome. Arthritis Rheumatol 2017, 69:1440–1450. [DOI] [PubMed] [Google Scholar]
- 30.Carubbi F, Cipriani P, Marrelli A, Benedetto P, Ruscitti P, Berardicurti O, Pantano I, Liakouli V, Alvaro S, Alunno A, et al. : Efficacy and safety of rituximab treatment in early primary Sjogren’s syndrome: a prospective, multi-center, follow-up study. Arthritis Res Ther 2013, 15:R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St Clair EW, Levesque MC, Prak ET, Vivino FB, Alappatt CJ, Spychala ME, Wedgwood J, McNamara J, Moser Sivils KL, Fisher L, et al. : Rituximab therapy for primary Sjogren’s syndrome: an open-label clinical trial and mechanistic analysis. Arthritis Rheum 2013, 65:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devauchelle-Pensec V, Pennec Y, Morvan J, Pers JO, Daridon C, Jousse-Joulin S, Roudaut A, Jamin C, Renaudineau Y, Roue IQ, et al. : Improvement of Sjogren’s syndrome after two infusions of rituximab (anti-CD20). Arthritis Rheum 2007, 57:310–317. [DOI] [PubMed] [Google Scholar]
- 33.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB: Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003, 349:1526–1533. [DOI] [PubMed] [Google Scholar]
- 34.Tsokos GC: Systemic lupus erythematosus. N Engl J Med 2011, 365:2110–2121. [DOI] [PubMed] [Google Scholar]
- 35.Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH: The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A 2005, 102:9258–9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, et al. : In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 2011, 186:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson SW, Kolhatkar NS, Rawlings DJ: B cells take the front seat: dysregulated B cell signals orchestrate loss of tolerance and autoantibody production. Curr Opin Immunol 2015, 33C:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.William J, Euler C, Christensen S, Shlomchik MJ: Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 2002, 297:2066–2070. [DOI] [PubMed] [Google Scholar]
- 39.Jacobi AM, Odendahl M, Reiter K, Bruns A, Burmester GR, Radbruch A, Valet G, Lipsky PE, Dorner T: Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum 2003, 48:1332–1342. [DOI] [PubMed] [Google Scholar]
- 40.Banchereau R, Hong S, Cantarel B, Baldwin N, Baisch J, Edens M, Cepika AM, Acs P, Turner J, Anguiano E, et al. : Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell 2016, 165:551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, Schieferl S, Hom J, Jenks S, Feldman RJ, et al. : Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 2015, 16:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tew GW, Rabbee N, Wolslegel K, Hsieh HJ, Monroe JG, Behrens TW, Brunetta PG, Keir ME: Baseline autoantibody profiles predict normalization of complement and anti-dsDNA autoantibody levels following rituximab treatment in systemic lupus erythematosus. Lupus 2010, 19:146–157. [DOI] [PubMed] [Google Scholar]
- 43.Md Yusof MY, Shaw D, El-Sherbiny YM, Dunn E, Rawstron AC, Emery P, Vital EM: Predicting and managing primary and secondary non-response to rituximab using B-cell biomarkers in systemic lupus erythematosus. Ann Rheum Dis 2017, 76:1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu TY, Ng KP, Cambridge G, Leandro MJ, Edwards JC, Ehrenstein M, Isenberg DA: A retrospective seven-year analysis of the use of B cell depletion therapy in systemic lupus erythematosus at University College London Hospital: the first fifty patients. Arthritis Rheum 2009, 61:482–487. [DOI] [PubMed] [Google Scholar]
- 45.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh HJ, et al. : Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010, 62:222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, et al. : Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012, 64:1215–1226. [DOI] [PubMed] [Google Scholar]
- 47.Mamani-Matsuda M, Cosma A, Weller S, Faili A, Staib C, Garcon L, Hermine O, Beyne-Rauzy O, Fieschi C, Pers JO, et al. : The human spleen is a major reservoir for long-lived vaccinia virus-specific memory B cells. Blood 2008, 111:4653–4659. [DOI] [PubMed] [Google Scholar]
- 48.Kamburova EG, Koenen HJ, Borgman KJ, ten Berge IJ, Joosten I, Hilbrands LB: A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am J Transplant 2013, 13:1503–1511. [DOI] [PubMed] [Google Scholar]
- 49.Bingham CO 3rd, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, Trzaskoma B, Martin F, Agarwal S, Kelman A: Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum 2010, 62:64–74. [DOI] [PubMed] [Google Scholar]
- 50.Laws LH, Parker CE, Cherala G, Koguchi Y, Waisman A, Slifka MK, Oberbarnscheidt MH,Obhrai JS, Yeung MY, Riella LV: Inflammation Causes Resistance to Anti-CD20-Mediated B Cell Depletion. Am J Transplant 2016, 16:3139–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teng YK, Levarht EW, Toes RE, Huizinga TW, van Laar JM: Residual inflammation after rituximab treatment is associated with sustained synovial plasma cell infiltration and enhanced B cell repopulation. Ann Rheum Dis 2009, 68:1011–1016. [DOI] [PubMed] [Google Scholar]
- 52.Pijpe J, Meijer JM, Bootsma H, van der Wal JE, Spijkervet FK, Kallenberg CG, Vissink A, Ihrler S: Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjogren’s syndrome. Arthritis Rheum 2009, 60:3251–3256. [DOI] [PubMed] [Google Scholar]
- 53.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ: Depletion of B cells in murine lupus: efficacy and resistance. J Immunol 2007, 179:3351–3361. [DOI] [PubMed] [Google Scholar]
- 54.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, et al. : Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 2005, 174:817–826. [DOI] [PubMed] [Google Scholar]
- 55.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, et al. : Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum 2010, 62:2443–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamaguchi Y, Uchida J, Cain DW, Venturi GM, Poe JC, Haas KM, Tedder TF: The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. J Immunol 2005, 174:4389–4399. [DOI] [PubMed] [Google Scholar]
- 57.Fairfax KA, Tsantikos E, Figgett WA, Vincent FB, Quah PS, LePage M, Hibbs ML, Mackay F: BAFF-driven autoimmunity requires CD19 expression. J Autoimmun 2015, 62:1–10. [DOI] [PubMed] [Google Scholar]
- 58.Vinuesa CG, Sanz I, Cook MC: Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol 2009, 9:845–857. [DOI] [PubMed] [Google Scholar]
- 59.Sang A, Zheng YY, Morel L: Contributions of B cells to lupus pathogenesis. Mol Immunol 2014, 62:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin WY, Gong Q, Seshasayee D, Lin Z, Ou Q, Ye S, Suto E, Shu J, Lee WP, Lee CW, et al. : Anti-BR3 antibodies: a new class of B-cell immunotherapy combining cellular depletion and survival blockade. Blood 2007, 110:3959–3967. [DOI] [PubMed] [Google Scholar]
- 61.Lin W, Seshasayee D, Lee WP, Caplazi P, McVay S, Suto E, Nguyen A, Lin Z, Sun Y,DeForge L, et al. : Dual B cell immunotherapy is superior to individual anti-CD20 depletion or BAFF blockade in murine models of spontaneous or accelerated lupus. Arthritis Rheumatol 2015, 67:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thai LH, Le Gallou S, Robbins A, Crickx E, Fadeev T, Zhou Z, Cagnard N, Megret J, Bole C, Weill JC, et al. : BAFF and CD4(+) T cells are major survival factors for long-lived splenic plasma cells in a B-cell-depletion context. Blood 2018, 131:1545–1555. [DOI] [PubMed] [Google Scholar]
- 63.Kraaij T, Kamerling SWA, de Rooij ENM, van Daele PLA, Bredewold OW, Bakker JA, Bajema IM, Scherer HU, Toes REM, Huizinga TJW, et al. : The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun 2018, 91:45–54. [DOI] [PubMed] [Google Scholar]
- 64.Nagel A, Podstawa E, Eickmann M, Muller HH, Hertl M, Eming R: Rituximab mediates a strong elevation of B-cell-activating factor associated with increased pathogen-specific IgG but not autoantibodies in pemphigus vulgaris. J Invest Dermatol 2009, 129:2202–2210. [DOI] [PubMed] [Google Scholar]
- 65.Salter AI, Pont MJ, Riddell SR: Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood 2018, 131:2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arumugakani G, Stephenson SJ, Newton DJ, Rawstron A, Emery P, Doody GM, McGonagle D, Tooze RM: Early Emergence of CD19-Negative Human Antibody-Secreting Cells at the Plasmablast to Plasma Cell Transition. J Immunol 2017, 198:4618–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zintzaras E, Voulgarelis M, Moutsopoulos HM: The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med 2005, 165:2337–2344. [DOI] [PubMed] [Google Scholar]
- 68.Cao L, Tong H, Xu G, Liu P, Meng H, Wang J, Zhao X, Tang Y, Jin J: Systemic lupus erythematous and malignancy risk: a meta-analysis. PLoS One 2015, 10:e0122964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S: Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther 2015, 17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang Y, Yang Z, Qin B, Zhong R: Primary Sjogren’s syndrome and malignancy risk: a systematic review and meta-analysis. Ann Rheum Dis 2014, 73:1151–1156. [DOI] [PubMed] [Google Scholar]
- 71.Velasquez MP, Bonifant CL, Gottschalk S: Redirecting T cells to hematological malignancies with bispecific antibodies. Blood 2018, 131:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, et al. : T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol 2018:JCO2018778084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boussi L, Niesvizky R: Advances in immunotherapy in multiple myeloma. Curr Opin Oncol 2017, 29:460–466. [DOI] [PubMed] [Google Scholar]
- 74.Schrezenmeier E, Jayne D, Dorner T: Targeting B Cells and Plasma Cells in Glomerular Diseases: Translational Perspectives. J Am Soc Nephrol 2018, 29:741–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hiepe F, Radbruch A: Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat Rev Nephrol 2016, 12:232–240. [DOI] [PubMed] [Google Scholar]