Abstract
The pathologic interpretation of gut biopsies in hematopoietic cell transplant recipients to assess graft-versus-disease (GVHD) is well accepted and supplements clinical and endoscopic findings. However, the histologic activity grading of GVHD is controversial, with attempts to predict prognosis or response to treatment largely unsuccessful. GVHD is being diagnosed earlier in its course, raising the possibility that the pathologic grading system can be profitably modified. We have developed a histologic activity grading system designed to replace the commonly used modified-Lerner grading systems. Our system stratifies the low-level Lerner grade I category into 4 activity grade categories, based on the average frequency of apoptotic cells. The results are expressed as ordinal categories, GVHD of minimal, mild, moderate, severe histologic activity, or severe histologic activity with destruction (activity grades 1–5). In a retrospective study, 87 consecutive cases with 201 post-transplant specimens (median 48 days, range 18–1479 days) of stomach, duodenum, and colorectum, which had been activity graded at the time of the original diagnosis, were studied. Most of the biopsies diagnosed as GVHD were low grade—minimal (11%) or mild (71%) histologic activity. We hypothesized that the higher activity grades should be associated with more therapeutic intervention. The odds of increased therapy in the combined all-site specimens were increased as the activity grade increased [odds ratio (OR) = 2.9 (1.9–4.5), p=<.0001]. Thus, our grading system was validated. To investigate whether the activity grade was associated with therapy within the formerly undivided Lerner grade I category, the analysis was restricted to these 174 all-site specimens. The validation result was similar [OR = 3.1 (1.3–7.2), p=.009]. This result interestingly suggests that there is useful information hidden in the Lerner grade I category which could potentially guide immediately actionable treatment decisions. This histologic activity grade system has been in use at our institution for over 2 years, with good acceptance.
Graphical abstract
Introduction
Although gut-biopsies are typically standard of care for diagnosing GVHD following bone-marrow transplantation, there remain controversies and multiple unsettled issues concerning their interpretation and clinical utility [1–4]. Most histologic studies use the traditional Lerner grading schema or modifications of it [5–9]. Such histopathologic grading systems have generally been ineffective at predicating survival [10–12] or steroid resistance [11], especially in the early stages of GVHD. Indeed, there is general agreement only that high grade histologic lesions portend a poor outcome [2,7,10–14]. Survival is best predicted by a combination of clinical and endoscopic findings [4,15–18], sometimes including biomarkers [19–22], and sometimes usefully supplemented by histopathologic data [10,11]. At the early or mild stages of GVHD, clinical and endoscopic assessment grades show little correlation with the histopathologic grade [2,11,12,23–26]. Perhaps we have to rethink what information can be deduced from histologic examination. The need exists for a simple histologic grading system for gut biopsies that can be used to inform early diagnosis and guide intervention, with the ultimate goal of preventing progression.
The NIH 2015 consensus document recommends investigating grading schemes based on the degree of apoptotic activity independent of the stage of crypt or mucosal destruction [2]. Here, we investigate a GVHD activity grading system which separates changes usually encompassed within the lowest diagnostic grade of GVHD. We hypothesize that such a system could be applicable to all biopsies in which GVHD is in the differential diagnosis. We describe the development of a pathology-defined system, centered on the frequency of apoptotic cells. We tested this system, evaluating a large number of consecutive biopsies of the stomach, duodenum, and colon-rectum for possible GVHD. Validation of our system was not through predictions of drug resistance or survival, but through a prediction of therapeutic intervention, intended to render the histologic activity grade findings immediately relevant and actionable.
Methods
Material studied
The study was conducted with approval of the Institutional Review Board. Ninety-three consecutive, post-transplant, pathology cases with specimens of stomach, duodenum and/or colorectum which had been originally interpreted the primary author (DM) were identified. The cases originated from the Fred Hutchinson Cancer Research Center (FHCRC) transplant pathology service, the University of Washington Medical Center (UWMC), Children’s Hospital Medical Center (CHMC), and the Seattle Cancer Care Alliance (SCCA). The specimens had been obtained between August 21, 2014 and October 26, 2015, and had been diagnosed and reported using the pathologic activity grading system described herein. The patients all had symptoms that could be attributed to GVHD, and for which GVHD was in the clinical differential diagnosis. GVHD is evaluated for clinical purposes using modified Przepiorka criteria [16], described in Leisenring et al. [18], which does not employ input from pathology. Patients were usually aggressively followed and biopsied early, if deemed clinically important. Biopsies were obtained via upper and/or lower endoscopy, as clinically indicated. None of the biopsies were performed as part of a procurement protocol. In accord with the hypothesis that all GVHD evaluations could be activity graded, the cases were not further selected, and included, for example, patients with biopsies at multiple times, prior treatment for GVHD, drug-resistant GVHD, suspected or known acute GVHD, and suspected or known chronic GVHD. The pathologists and clinicians specialize in hematopoietic cell transplantation and have been working together for many years, with frequent communication regarding cases.
No pre-endoscopic preparation was used, as sodium phosphate or other preparations may produce apoptotic cells in colon biopsies [27]. Two cases with specific non-GVHD diagnoses of the biopsies were censored—one with a diagnosis of severe CMV enteritis, and the other with only a stomach biopsy, diagnosed as chronic active gastritis. Three additional cases were censored due to non-actionable GI pathology results because of concurrent conditions—a perforated small intestine, MRSA sepsis, and severe skin GVHD that had already informed treatment. There was one additional case censored due to the absence of treatment records. Thus, 87 pathology cases were considered for analysis. They encompassed a total of 201 separate “specimens”, each specimen consisting of one or more biopsy tissue fragments from the stomach, duodenum, or colorectum (Supplemental Table 1).
There were 74 patients, with 10 biopsied twice and 1 biopsied 4 times. The age median was 49, range 1 to 73. There were 23 patients with AML, 3 CML (1 blast crisis, 1 lymphoid blast crisis), 12 ALL, 7 MDS, 7 MPN, 1 CLL, 3 multiple myeloma, 7 NHL, 1 CHL, 1 CMML, 1 non-hematopoietic neoplasm (NHN), and 8 non-neoplastic diseases (NND). Cases were a median of 48 days post-transplant, ranging from 18 to 1479, with 64 <100 days and 23 ≥100 days.
Biopsies were performed with standard adult or pediatric endoscopy tools and immediately fixed in neutral buffered formalin. Tissues were processed in the usual fashion and embedded in paraffin, with each paraffin block prepared from each submitted vial, each with one or multiple tissue fragments, and embedded in groups of 1–8 as received. Sections were cut at 4μm, with multiple sections on each of 2 slides, typically 8 sections per slide if space allowed, and stained with hematoxylin and eosin.
Criteria for apoptotic enterocyte
The identification of an apoptotic cell was made per the NIH 2005 and NIH 2015 consensus documents, and illustrated by Ponec et al., Washington and Jagasia, and Kreft et al. [1,2,5,12,28] A summary of our pathologic classification criteria, in tabular form, is presented in Supplemental Table 2. In summary, the classical appearance of an apoptotic cell was with condensed nuclear chromatin appearing as a clamshell, ring, or fragments, and eosinophilic red nucleoplasm, with cytoplasmic eosinophilia and shrinkage. A single membrane-bound nuclear fragment, appearing as a subnuclear-size body with both condensed chromatin and associated eosinophilic nucleoplasm, was considered an apoptotic cell. Apoptotic cells also presented as multiply fragmented nuclei appearing as 2 or more nuclear fragments, and sometimes as several small fragments appearing as dust. Phagocytized nuclear debris was accepted. Occasionally, apoptotic cells were predominantly located adjacent to a lymphoid aggregate in the colon; they were considered to be due to GVHD [9]. Only apoptosis in the epithelial cell layer of glands within the lamina propria was considered. Apoptosis in the superficial plate was not considered, as it is a normal feature. In accord with Kreft, a single isolated nuclear fragment was not considered an apoptotic cell, as it may represent a fragment of a lymphocyte. A single eosinophilic fragment was considered of uncertain provenance, and not an apoptotic cell. Isolated condensation of nuclear chromatin, cell shrinkage, or cell ballooning was not considered sufficient for the diagnosis of apoptosis. G-cells in the stomach may show cytoplasmic clearing. Adenomatous tissue was not considered, as it may contain apoptotic cells.
The enumeration of apoptotic cells may be subject to variation. For purposes of enumeration if multiple nuclear fragments could be encompassed in a circle or elongated oval as depicted in Kreft et al., only one apoptotic cell is counted [28]. If two adjacent or nearby nuclear fragments with eosinophilic cytoplasm could conceivably have been derived from a single cell, only one apoptotic cell is counted. It is recognized that clouds of nuclear fragments might appear in successive sections and be counted twice, apoptotic cells may be present which do not meet criteria and may not be counted, or two or more adjacent apoptotic cells may not be fully enumerated using these criteria.
Apoptotic index
GVHD may present as a focal disease on all scales, with some individual glands, groups of glands, or gross regions of organs being heavily affected, with other similar regions being lightly affected or unaffected [2]. We addressed inhomogeneity by using the arithmetic mean. Each slide was interpreted as to diagnosis, and if GVHD was considered, apoptotic cells enumerated. The basic measure of histologic activity is the apoptotic index expressed as apoptotic cells per tissue cross section. Typically, there were 16 cross sections of each tissue fragment. The apoptotic cells proved conveniently enumerated with a hematology differential counting device. For each pathology case, each specimen (stomach, duodenum or colorectum) had an apoptotic index calculated as the arithmetic average of total apoptotic cells per total tissue cross sections, regardless of whether these sections were present on one or more paraffin blocks.
Enumeration of apoptotic cells was sometimes challenging due to factors beyond our control. Tissues were generally oriented longitudinally; however, infrequent sections were oriented tangentially, resulting in the occurrence of considerable variation, as apoptotic cells may be concentrated in particular levels of glands [12]. No effort was made to correct for this cause of variance. Each tissue fragment was about 2–4 mm long, and contained about 20 glands or crypts. If markedly different, i.e., less than 10 or more than 40 glands or crypts, the denominator was adjusted as appropriate. Sometimes long strips of biopsy tissue, small pediatric biopsies, or poorly oriented tissue, necessitated this adjustment. This adjustment occurred on 25% of the blocks and was usually minimal. No distinction was made for various parts of the colon, sigmoid, and rectum, with the sigmoid and/or rectum virtually always present. All counts and calculations were performed without knowledge of the clinical treatment result.
Activity Grading
Our activity grade system expands on the Sale modification of the Lerner grading system [6,8,9]. Criteria for Sale-Lerner grade I, in modern nomenclature, requires apoptotic epithelial cells, and tolerates neutrophilic abscesses and focal crypt atrophy. The higher Sale-Lerner grades II, III, and IV may involve “exploding crypts”, apoptotic abscesses, degenerating crypts, or more severe changes. The characterization is largely reflective of Kreft et al., and may be different from some other systems in that mere loss of crypts is considered nonspecific and not evidence of activity or destruction [28] (Supplemental Table 2). There are no pathologic changes specific for chronic GVHD of the stomach, duodenum, or colorectum, as that is a clinical determination [1,2,5]. However, there are pathologic indications of duration, such as collagen deposition in the lamina propria and regenerative changes of crypts, that are often present in chronic GHVD, and these are noted in the resulting diagnosis.
Validation
For validation of the activity grading system, we hypothesized that the higher activity grades should be associated with more intensive therapeutic intervention. Kreisel et al. used a similar approach with endoscopic grading [25]. The patient records were reviewed to determine whether there was a temporally associated i) a reduction in therapy, ii) no change in therapy, or iii) initiation or increase in therapy. In almost all cases the electronic medical record made for an unambiguous determination of the associated therapeutic change. Occasional cases were perceived to be clinically certain GVHD, and the therapeutic intervention was made up to 3 days prior to the biopsy. The data is included in Supplemental Table 1, and the two ambiguous cases delineated. Therapeutic change was defined as a change in medication (or dose) of systemic steroids (prednisone or dexamethasone), topical steroids (beclomethasone, budesonide), calcineurin inhibitor (cyclosporine or tacrolimus), sirolimus, ATG, or mycophenolate. The exchange of calcineurin inhibitors to avoid toxicity or the replacement of mycophenolate mofetil with mycophenolic acid were not considered a change. The therapeutic change is expressed on an ordinal scale of 3 units with outcomes assigned integer values 1 unit apart.
Statistics
Activity grade of the 3 sites were compared to each other pairwise using Spearman’s rank correlation and testing the null hypotheses that the estimated correlation coefficient (ρ) is equal to zero. The primary validation analysis examined the association between site-specific activity grade from all specimens and therapeutic change, using ordinal logistic regression, with 3 ordered outcomes (reduction in therapy, no change in therapy, increase in therapy) parameterized as −1, 0, and +1. In addition, all sites were combined together and generalized estimating equations were used with a logistic link function, thus allowing the multiple sites within a single patient to be appropriately considered as a “cluster” which was important in the estimate of the variance. For these purposes, the outcome was the occurrence of “increase in therapy” (i.e., reduction and no change in therapy were combined and treated as no increase in therapy). Activity grade was modeled as a continuous linear variable. All confidence intervals reflect 95% confidence.
Results
Activity grading
A GVHD activity grading system for stomach and intestinal biopsies was developed based only on the histology information interpreted from biopsies. This was conceived as expanding and stratifying the lowest grade Lerner category and compressing the higher grades. Other clinical and laboratory information was considered in assessing whether GVHD was present or not, but once it was determined to be possibly GVHD or likely GVHD (per NIH 2015) [2,29], a histological activity grade was assigned. The activity grade was based on the average frequency of apoptotic cells per section. Criteria and methodology are summarized in the Methods. The cutoffs for the activity grades were determined entirely empirically. The goal was to set cutoffs so that low grade activity (minimal or mild) would appear to not necessarily demand intervention, while high grade activity (moderate or severe) would reflect an apoptotic intensity that did not appear sustainable if continued unabated and would appear to demand therapeutic intervention (Supplemental Table 3). The cutoffs for the activity grades were developed in consultation with a gastroenterologist (GS) on previous sets of cases, and underwent a few iterations before settling on those presented in Table 1. An activity grade of 0 reflects no GVHD, a normal biopsy, or a nonspecifically inflamed biopsy, for example. Activity grade scores of 1–5 are diagnosed, respectively, as GVHD with minimal, mild, moderate, or severe histologic activity, and severe histologic activity with destruction. Activity grades 1,2,3, and 4 are intended to be an expansion and stratification of Lerner grade I. Activity grade 5 is intended to represent a compression of Lerner grades II, III, and IV.
Table 1.
Activity grade criteria for GVHD of the gut.
| Activity Grade* | Diagnostic Nomenclature | Apoptotic cells per section | Lerner Grade |
|---|---|---|---|
| 0 | No diagnostic alteration/Nonspecific changes/Nonspecific inflammation | < 0.07 | 0 |
| 1 | GVHD of minimal histologic activity | ≥ 0.07 – < 0.25 | I |
| 2 | GVHD of mild histologic activity | ≥ 0.25 – < 4 | I |
| 3 | GVHD of moderate histologic activity | ≥ 4 – < 25 | I |
| 4 | GVHD of severe histologic activity | ≥ 25 | I |
| 5 | GVHD of severe histologic activity, with destruction** | ≥ 25 | II, III, IV |
Categories are based on the arithmetical average of the number of apoptotic cells per biopsy cross-section, with approximately 20 crypts/glands per cross-section, typically evaluating 16 tissue sections.
With apoptotic abscesses, exploding crypts, ulceration, denudation
The mild histologic activity category (activity grade 2) is large, encompassing a 16-fold difference of the apoptotic index, whereas the moderate category encompasses only a 6-fold difference. Nevertheless, the relationship between each successive category proved approximately geometric, showing a similar ratio of apoptotic cell frequency (Figure 1). Hence, the solid line is approximately linear when apoptotic cells are plotted on a logarithmic scale.
Figure 1.
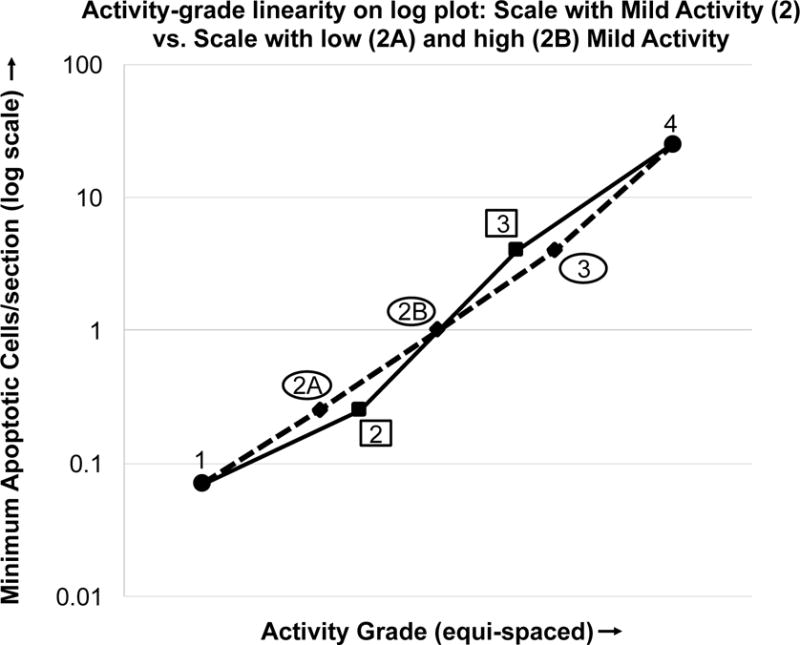
Apoptotic cells per section (plotted on a log scale) vs. activity grade. (solid line, ■) Scale comprising grades 1, 2, 3 and 4. (dotted line, ◆) Scale comprises grades 1, 2A, 2B, 3 and 4. The former 2 is divided into low (2A) and high (2B). This shows the grading scale with 2A and 2B with more equally spaced ratios, appearing as more of a straight line on the log plot.
There were three specimens exhibiting destruction, with relatively few apoptotic cells (Supplemental Table 1). These are diagnosed as the activity grade observed “with destruction”. For purposes of this analysis, they are classified as though destruction was not present. Correcting for destruction did not significantly change the results.
Descriptive statistics
The activity grade distribution all specimens is presented as a stacked bar graph, with the activity grades converted to ordinal numbers 0–5 (N=201) (Figure 2). Most of the GVHD cases were low grade—minimal or mild histologic activity—comprising 11% and 71% of biopsies with GVHD, respectively. The mean activity grade for specimens from all sites was 1.9, with 1.7, 2.3, and 1.9 for specimens from the stomach, duodenum, and colorectum, respectively. The duodenum site exhibits the highest activity grade in our cases, still demonstrated if the data is normalized by restricting cases to those with specimens from all 3 sites, 1.9, 2.3, and 2.0 respectively (N=105).
Figure 2.
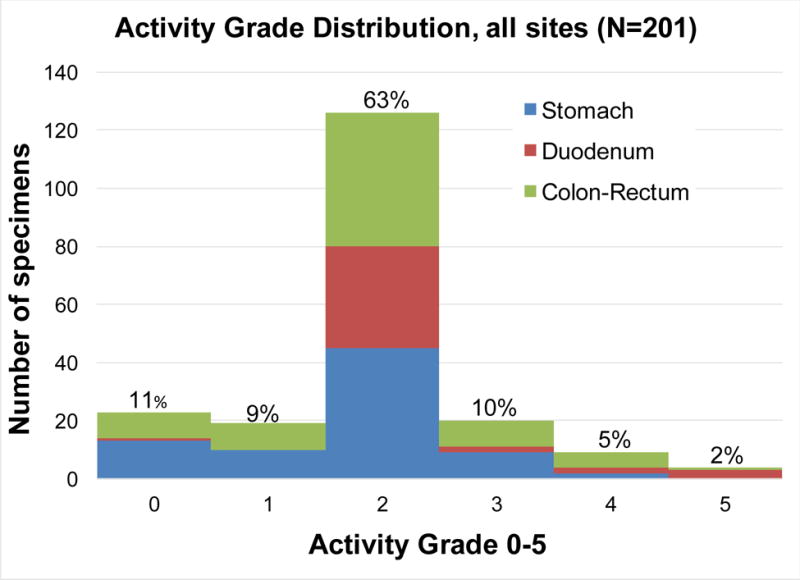
Distribution of activity grades found in all specimens.
Site associations
Each site (stomach, duodenum, and colorectum) was compared to one another for possible similarity of the GVHD activity grades. The activity grade was compared for sites pairwise, using those cases for which concurrent specimens were taken (Supplemental Figure 4). There was a fair correlation between the activity scores of the colorectum and the other two sites (colorectum and stomach, ρ=0.44, p=.0001; colorectum and duodenum, ρ=0.49, p=.002). The stomach and duodenum were less well correlated (ρ=0.19, p=.23).
Validation
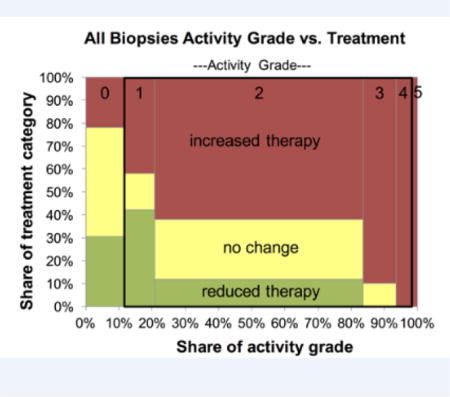
To validate the activity grading system, we hypothesized that the higher activity grades should be associated with more intensive therapeutic intervention. The treatment category is expressed on an ordinal scale with reduced therapy, no change, and increased (or initiated) therapy, assigned values 1 unit apart. There were 14 cases with a reduction in therapy, 20 with no change in therapy, and 53 with an increase in therapy. Site-specific results are presented in Supplemental Table 5, where the results show that as activity score increases, the odds of an increase in treatment are increased and the magnitude of these odds ratios are on the same order of magnitude across the three sites. Based on these results, we also combined all site-specific activity grades and looked at the association between activity grade and the probability of increase in therapy (for these purposes, reduction and no change are combined and classified as no increase; see Statistics section). The activity score vs. therapy change results for the aggregation of all sites are presented in a Marimekko chart (Figure 3). The widths of the bars for each activity grade category, and the areas (which also reflect the treatment category), are proportional to the numbers of specimens. The odds of increased therapy are increased as the combined activity grade increases [odds ratio (OR) = 2.9 (1.9–4.5), p=<.0001].
Figure 3.
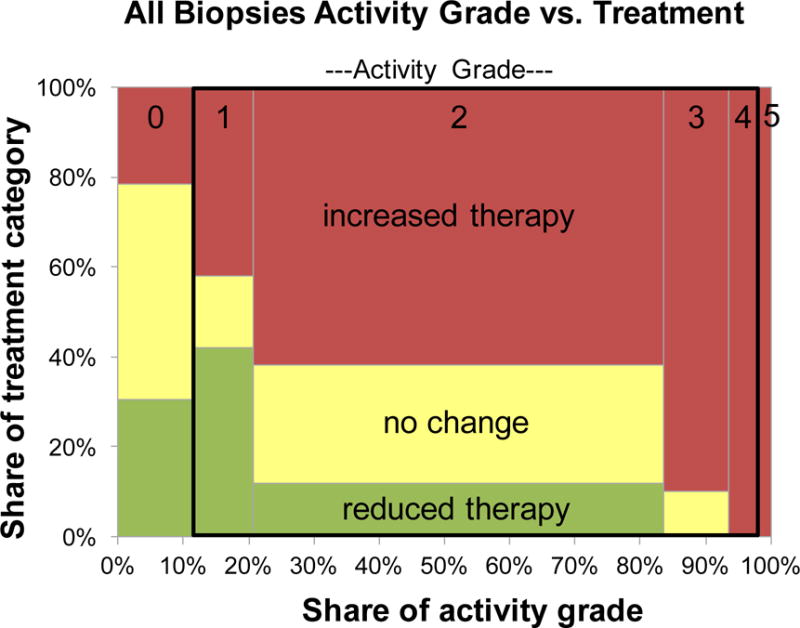
Marimekko chart showing relation between activity grade and change in therapy for all specimen sites, and within the black rectangular outline, the subset of specimens with activity grades 1, 2, 3, and 4. The area reflects number of specimens in each activity grade or therapeutic change category.
The activity grades with apoptosis alone (grade 1, 2, 3, and 4) correspond to a single Lerner grade I, in the often-used grading system of Lerner and its modifications. To assess whether this activity grade distinction provides a measurable benefit, the data were reanalyzed considering only these cases. The site-specific results are contained in Supplemental Table 5, and the pictorial of specimens with activity grades 1, 2, 3, and 4 is depicted in Figure 3 as the portion of the plot within the black rectangular outline. Using the combined scores, logistic regression shows an increased odds of need for increased therapy as activity grade increases [OR = 3.1 (1.3–7.2), p=.009]. These results are similar to the whole data set, and interestingly suggest that there is actionable information embedded solely in low-level differences of activity grades.
Further refinement of activity grade
The apoptotic cell frequency cutoffs for the activity grades were determined empirically, as previously noted. The result was that activity grade 2, GVHD of mild histologic activity, encompassed a wide range of apoptotic cell frequencies, a 16-fold span, and furthermore, comprised most of our specimens, 63%. This raises the question of whether grade 2 could be usefully divided into 2 parts, with low-mild (0.25 to <1.0 apoptotic cells/section), and high-mild (1.0 to <4.0 apoptotic cells/section) histologic activity, for convenience denoted as 2A and 2B, respectively. The resulting cutoffs would follow an even more consistent apoptotic cell frequency ratio between grades, depicted in Figure 1, with the dotted line showing a more nearly linear appearance between all activity grades [Grades 1, 2A, 2B, 3 and 4 exhibit an apoptotic index ratio of 3.6, 4.0, 4.0, and 6.3, respectively (rather than 3.6, 16, and 6.3)]. All biopsies classified as grade 2 were further inspected and recategorized as 2A or 2B. Validation was attempted by testing whether the 2B category was associated with more increased therapy (vs. no change or reduction) than 2A. Fifty-nine percent (45/76) of 2A specimens were associated with an increase in therapy compared to 66% (33/50) of the 2B patients. The difference was small and not statistically significant (Fisher exact test). Thus, we have no data to support splitting the mild histologic activity category into low and high segments.
Discussion
Prompt diagnosis and initiation of therapy is essential for proper control of GVHD [14,30]. Pathologic assessment, although part of standard care, typically relies on histologic criteria originally designed for diagnosis of late stage GVHD, making it of limited utility for diagnosing GVHD at its early stages. Our focus has been on developing a histology-based GVHD activity grading system using histologic apoptotic activity to address the relatively mild activity changes of GVHD in situations of acute or chronic GVHD. We aim to replace the Lerner grading system for gut biopsies which has been in use—in various highly modified forms—for over 40 years. The Lerner system was the result of a description of autopsy findings in patients with severe GVHD in the early days of transplantation [8]. As a result, it is heavily weighted towards severe GVHD. A variety of modifications have been developed, generally with small differences in the details of what is included in Grade I [5,7,9,31]. Due to treatment advances, the intensity of GVHD as diagnosed has been decreasing over time [32,33]. Intervention is desired long before the diffuse crypt dropout and apoptotic abscesses of Lerner Grade II. Lerner grades II, III, and IV are considered a failure to adequately control GVHD, either because of failure to timely apply available treatment or resistance to such treatment, and may require extraordinary therapeutic measures. We have endeavored to develop a grading system at the lower levels of GVHD that provides meaningful actionable information.
In our system, the activity grades 1–4 are encompassed within Lerner grade I (presuming that activity grade 1 would be classified as GVHD at all). Our activity grade 5 (GVHD of severe histologic activity, with tissue destruction) would be graded as Lerner II, III, or IV, depending on the degree of destruction, which may include, for example, exploding crypts, apoptotic abscess, diffuse crypt dropout, or complete denudation.
Restricting the analysis to those biopsies included in the single Lerner grade I category, we have shown that stratifying the biopsies on the basis of apoptotic index correlates with therapy decisions, validating our approach. The implication is that the single Lerner grade for all these biopsies is too broad. The argument is recognized to be somewhat circular, as the clinicians may have had the benefit of our activity grade before deciding on treatment decisions. This is inherent in the study design. However, our clinicians are universally well-acquainted with GVHD and are experienced at taking all information into account regarding the treatment decision. They are well aware of the historical failure of low-level pathologic involvement to correlate to clinical symptoms or endoscopic appearance [1,2,11,12,26,34–37]. It was assumed that treatment would depend on the combined clinical, endoscopic, and histological assessment, as has long been recommended [2,4].
The use of apoptotic cells to diagnose early stage GVHD is nothing new. However, agreement on the minimal diagnostic criteria remains an issue. Normal colorectal biopsies, evaluating 100 crypts, contain no apoptotic cells about 75% of the time, and show a mean of 0.2 apoptotic cells per 20 crypts [38]. This suggests overlap with our activity grade of GVHD of minimal histologic activity for somewhat less than 25% of biopsies. Nguyen at al. found a single apoptotic cell in all the sections was sufficient to indicate GVHD in some cases [39]. Many pathologists feel that an acceptable sensitivity and specificity can be obtained with >1 to 2 apoptotic bodies per tissue fragment (Fred Hutchinson criterion), or the slightly more restrictive criterion which demands at least a single apoptotic cell in each individual tissue fragment (University of Michigan criterion) [1]. These criteria are roughly in accord with our lowest activity grade, of minimal histologic activity. Lin, et al. suggested a criterion of 7 apoptotic cells per 10 contiguous crypts in the most affected part of the colon biopsy [40]. This system is not directly comparable to ours, but appears roughly to correspond to moderate histologic activity under our system. However, the system is insensitive, with Gomez et al. reporting in the subset specimens restricted to Lerner grade 1, colorectal GVHD was detected with a sensitivity of 59% [41]. Both Lin and Gomez note that as few as a single apoptotic cell may reflect GVHD, in accord with Nguyen, and suggest a new Lerner indeterminate category to encompass the lower levels of apoptotic activity. We accommodate the resulting false negatives with our grades of minimal and, possibly, mild histologic activity. Our system is expressly designed to be sensitive at the possible detriment to specificity.
We recognize that the ultimate clinical assessment involves patient data in its totality, considering the duration post-transplant, clinical signs and symptoms, endoscopic appearance, and possibly previous GVHD, chronic GVHD, GVHD treatment, type of treatment, refractory GVHD, and biomarkers [2,4,5,11,20,21,42]. The diagnosis of early stage GVHD is rarely “black and white”—clinicians are frequently required to make diagnoses based on a spectrum of qualitative observations which can frequently be conflicting or complicated by confounding situations. Apoptosis-based approaches, for example, are confounded by a number of commonly used drugs may be associated with variable intensities of apoptosis [2]. Among these drugs are proton pump inhibitors (PPIs) [43], mycophenolate mofetil [44–47], non-steroidal anti-inflammatory drugs (NSAIDs) [48], and sodium phosphate bowel preparation [27]. Some infections may be associated with apoptosis, notably cytomegalovirus (CMV) [2,49,50]. However, the success of the validation provides evidence that our grading system nevertheless provides actionable information, in spite of these often-difficult situations and confounders.
Our data show that there may be broad utility in activity grading. For example, it could potentially inform treatment options at both the initial evaluation, and at follow-up evaluations involving response to previous treatment. Since our study included all situations, it has no ability to distinguish differences in outcome of such subsets.
We have obtained data from 3 sites, the stomach, duodenum, and colorectum. Although the activity grade comparison was not a primary objective in our study, the finding of comparable duodenal activity and some between-site correlation is well supported by others [12,23,25,26,37,51–54], and is consistent with our samples being typical.
Our activity grading system is consistent with the NIH 2015 nomenclature requirements of no GVHD, possible GVHD, and likely GVHD [2]. However, it should be noted that “possible GVHD” diagnostic category proposed by NIH 2015 is distinct from our activity grade 1 of “GVHD of minimal histologic activity.” “Possible GVHD” is diagnosed when there are other significant possibilities for the etiology of the lesion, and is not necessarily reflective of a low quantity of apoptotic cells. The Mount Sinai acute GVHD International Consortium encourages biopsies whenever possible, and provides a rubric for the incorporation of pathology data into the clinical assessment, with various degrees of clinico-pathologic certainty [4]. Although our activity grading system is not specifically incorporated into the International Consortium recommendations, their categorization of pathology findings as unequivocal, probable, or possible, is highly compatible with our activity grading, but the specific crosswalk awaits future studies.
We considered a possible modification of our activity grading system with the division of the broad activity grade 2 category (mild) into 2 categories, low (2A) and high (2B), delivering a more similar ratio of apoptotic indexes (Figure 1). This possibility was tested, but failed to produce a significant difference in outcome, as measured by change in therapy. The validation methodology, however, provides a potential structure for future studies with a larger or more restricted patient sample.
Additional modification of the grading system could be considered. Changes indicating long duration were sometimes present, such as increased collagen in the lamina propria, misshapen and regenerative glands including nubbin-like crypts or foveoli, sometimes with atypical epithelial cells. In most cases these sections did not show destruction, and the activity grading was straight-forward, if a sufficient number of glands were available. In a few cases these sections showed evidence of destruction, but the apoptotic activity was graded as mild or moderate. They do not have any ordinal counterpart in our system, and were diagnosed descriptively, for example, “GHVD of mild histologic activity with destruction, regenerative changes and focal denudation”. These cases raise the question of whether GVHD might be more appropriately evaluated as grade and stage, similar to the Batts and Ludwig grading of chronic hepatitis [55]. The grade would be informed by the apoptotic index, and the stage, reflecting a measure on duration and destruction, would be informed by indicators such as glandular atypia and regenerative change, as well as tissue damage. Chronic GVHD might be addressable by such a system.
Reproducibility was not directly measured in the present effort, but the interpretation was retrospective, and therefore blind. We recognize that our apoptotic index may be more reproducible between cases if the apoptotic index is evaluated per crypt, rather than per section. In this case, it is suggested that 20 foveoli/crypts be equivalent to one tissue section as the denominator. This would obviate any adjustment for small or large tissue sections, but may suffer issues with regard to tangential orientation.
Our activity grading and validation rubric may inform future studies. Possibilities include: subset analyses such as first biopsies in the less than day-84 post-transplant period, large-scale retrospective analyses on biopsies that have not been diagnosed with an activity grade, or more classical gold-standard outcome analyses.
We have developed a grading system designed to provide short term actionable information, presented in Table 1. It is recognized that, “the diagnosis of GVHD relies upon synthesis of clinical, histologic, and laboratory findings” [5]. Our activity grade levels are designed to reflect some of the pertinent histologic details, such as “with crypt dropout”, or “with ulceration”, with the intent of therapeutic guidance. Because an apoptotic cell exists for only one to several hours in a tissue section [56,57] the presence of a small number of apoptotic cells reflects a continuous loss. Hence our activity grade is based on the observation that the histology reflects a “snapshot in time of a complex and dynamic process” that includes duration, therapy, concurrent etiologies, and location [1,2]. Our grading system, expanded and stratified at the lower levels, was shown to add value, when compared against the traditional Lerner grading, when validation is interpreted as reflecting treatment decisions. Our activity grading proposal does not, however, alter the essential necessity of non-pathological information in the diagnosis of GVHD. All the inaccuracies inherent in the diagnosis of GVHD are inherent in the activity grade. While we show correlation of activity grade to therapy, we offer no new insight as to whether the therapy will work, which is the widely-sought goal. Our activity grading system is designed to only reflect ongoing damage in the short term, and therefore be immediately actionable. It has been well accepted for over 2 years at our institution.
Supplementary Material
Highlights.
GVHD Activity grading system based on average apoptotic cell frequency.
Lerner grade I category expanded and stratified into 4 parts.
Goal to provide immediately actionable information from histology.
Validation shows activity grade is associated with therapeutic increase.
There is actionable information hidden within Lerner grade I.
Acknowledgments
We thank Amanda Moklebust and Kristin Shimp for technical services, and Petri Muhlhauser for data services. Supported, in part, by National Institutes of Health grant NCI P30 CA015704. Salary support was provided by the Clinical Research Division of Fred Hutchinson Cancer Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have financial or personal relationships to disclose.
References
- 1.Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. Histopathologic Diagnosis of Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biology of Blood and Marrow Transplantation. 2006;12:31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Shulman HM, Cardona DM, Greenson JK, Hingorani S, Horn T, Huber E, et al. NIH Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. The 2014 Pathology Working Group Report. Biology of Blood and Marrow Transplantation. 2015;21:589–603. doi: 10.1016/j.bbmt.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong NACS. Gastrointestinal pathology in transplant patients. Histopathology. 2014;66:467–79. doi: 10.1111/his.12542. [DOI] [PubMed] [Google Scholar]
- 4.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biology of Blood and Marrow Transplantation. 2016;22:4–10. doi: 10.1016/j.bbmt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Washington K, Jagasia M. Pathology of graft-versus-host disease in the gastrointestinal tract. Human Pathology. 2009;40:909–17. doi: 10.1016/j.humpath.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Epstein RJ, McDonald GB, Sale GE, Shulman HM, Thomas ED. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology. 1980;78:764–71. [PubMed] [Google Scholar]
- 7.Melson J, Jakate S, Fung H, Arai S, Keshavarzian A. Crypt loss is a marker of clinical severity of acute gastrointestinal graft-versus-host disease. Am J Hematol. 2007;82:881–6. doi: 10.1002/ajh.20976. [DOI] [PubMed] [Google Scholar]
- 8.Lerner KG, Kao GF, Storb R, Buckner CD, Clift RA, Thomas ED. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant Proc. 1974;6:367–71. [PubMed] [Google Scholar]
- 9.Sale GE, Shulman HM, McDonald GB, Thomas ED. Gastrointestinal graft-versus-host disease in man. A clinicopathologic study of the rectal biopsy. Am J Surg Pathol. 1979;3:291–9. doi: 10.1097/00000478-197908000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Castilla-Llorente C, Martin PJ, McDonald GB, Storer BE, Appelbaum FR, Deeg HJ, et al. Prognostic factors and outcomes of severe gastrointestinal GVHD after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2014;49:966–71. doi: 10.1038/bmt.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham J, Janin A, Gornet J-M, de Latour RP, Robin M, Xhaard A, et al. Clinical severity scores in gastrointestinal graft-versus-host disease. Transplantation. 2014;97:965–71. doi: 10.1097/01.TP.0000438209.50089.60. [DOI] [PubMed] [Google Scholar]
- 12.Ponec RJ, Hackman RC, McDonald GB. Endoscopic and histologic diagnosis of intestinal graft-versus-host disease after marrow transplantation. Gastrointestinal Endoscopy. 1999;49:612–21. doi: 10.1016/S0016-5107(99)70390-1. [DOI] [PubMed] [Google Scholar]
- 13.Socie G. Prognostic value of apoptotic cells and infiltrating neutrophils in graft-versus-host disease of the gastrointestinal tract in humans: TNF and Fas expression. Blood. 2004;103:50–7. doi: 10.1182/blood-2003-03-0909. [DOI] [PubMed] [Google Scholar]
- 14.McDonald GB. How I treat acute graft-versus-host disease of the gastrointestinal tract and the liver. Blood. 2016 doi: 10.1182/blood-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisdorf DJ, Hurd D, Carter S, Howe C, Jensen LA, Wagner J, et al. Prospective grading of graft-versus-host disease after unrelated donor marrow transplantation: a grading algorithm versus blinded expert panel review. Biology of Blood and Marrow Transplantation. 2003;9:512–8. doi: 10.1016/S1083-8791(03)00162-9. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. doi: 10.1097/pcc.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 17.Glucksberg HR, Storb R, Fefer A, Buckner CD. Clinical Manifestatiopns of Graft-versus-Host Disease in Human Recipients of Marrow From HL-A-Matched Sibling Donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Leisenring WM, Martin PJ, Petersdorf EW, Regan AE, Aboulhosn N, Stern JM, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108:749–55. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malard F, Mohty M. New insight for the diagnosis of gastrointestinal acute graft-versus-host disease. Mediators Inflamm. 2014;2014:701013–3. doi: 10.1155/2014/701013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali AM, Dipersio JF, Schroeder MA. The Role of Biomarkers in the Diagnosis and Risk Stratification of Acute Graft-versus-Host Disease: A Systematic Review. Biology of Blood and Marrow Transplantation. 2016;22:1552–64. doi: 10.1016/j.bbmt.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paczesny S. Discovery and validation of graft-versus-host disease biomarkers. Blood. 2013;121:585–94. doi: 10.1182/blood-2012-08-355990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara JLM, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–8. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross WA, Ghosh S, Dekovich AA, Liu S, Ayers GD, Cleary KR, et al. Endoscopic Biopsy Diagnosis of Acute Gastrointestinal Graft-Versus-Host Disease: Rectosigmoid Biopsies Are More Sensitive Than Upper Gastrointestinal Biopsies. Am J Gastroenterol. 2008;103:982–9. doi: 10.1111/j.1572-0241.2007.01639.x. [DOI] [PubMed] [Google Scholar]
- 24.Patey-Mariaud de Serre N, Reijasse D, Verkarre V, Canioni D, Colomb V, Haddad E, et al. Chronic intestinal graft-versus-host disease: clinical, histological and immunohistochemical analysis of 17 children. Bone Marrow Transplant. 2002;29:223–30. doi: 10.1038/sj.bmt.1703329. [DOI] [PubMed] [Google Scholar]
- 25.Kreisel W, Dahlberg M, Bertz H, Harder J, Potthoff K, Deibert P, et al. Endoscopic diagnosis of acute intestinal GVHD following allogeneic hematopoietic SCT: a retrospective analysis in 175 patients. Bone Marrow Transplant. 2012;47:430–8. doi: 10.1038/bmt.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson B, Salzman D, Steinhauer J, Lazenby AJ, Wilcox CM. Prospective endoscopic evaluation for gastrointestinal graft-versus-host disease: determination of the best diagnostic approach. Bone Marrow Transplant. 2006;38:371–6. doi: 10.1038/sj.bmt.1705453. [DOI] [PubMed] [Google Scholar]
- 27.Driman DK, Preiksaitis HG. Colorectal inflammation and increased cell proliferation associated with oral sodium phosphate bowel preparation solution. Human Pathology. 1998;29:972–8. doi: 10.1016/s0046-8177(98)90203-9. [DOI] [PubMed] [Google Scholar]
- 28.Kreft A, Mottok A, Mesteri I, Cardona DM, Janin A, Kühl AA, et al. Consensus diagnostic histopathological criteria for acute gastrointestinal graft versus host disease improve interobserver reproducibility. Virchows Arch. 2015;467:255–63. doi: 10.1007/s00428-015-1803-y. [DOI] [PubMed] [Google Scholar]
- 29.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biology of Blood and Marrow Transplantation. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109:4119–26. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sale GE. Pathology and recent pathogenetic studies in human graft-versus-host disease. Surv Synth Pathol Res. 1984;3:235–53. doi: 10.1159/000156929. [DOI] [PubMed] [Google Scholar]
- 32.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin PJ, McDonald GB, Sanders JE, Anasetti C, Appelbaum FR, Deeg HJ, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2004;10:320–7. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Correa M, Poonawala A, Abraham SC, Wu TT, Zahurak M, Vogelsang G, et al. Endoscopic findings predict the histologic diagnosis in gastrointestinal graft-versus-host disease. Endoscopy. 2002;34:808–13. doi: 10.1055/s-2002-34257. [DOI] [PubMed] [Google Scholar]
- 35.Yeh S-P, Liao Y-M, Hsu C-H, Chen C-L, Shen Y-C, Hsueh C-T, et al. Gastric Bleeding Due to Graft-vs-Host Disease. Am J Clin Pathol. 2004;122:919–25. doi: 10.1309/23DAL9F6P74XWJHL. [DOI] [PubMed] [Google Scholar]
- 36.Cheung DY, Kim JI, Kim SS, Sung HY, Cho S-H, Park S-H, et al. Endoscopic Evaluation in Gastrointestinal Graft-Versus-Host Disease: Comparisons with Histological Findings. Dig Dis Sci. 2008;53:2947–54. doi: 10.1007/s10620-008-0262-6. [DOI] [PubMed] [Google Scholar]
- 37.Roy J, Snover D, Weisdorf S, Mulvahill A, Filipovich A, Weisdorf D. Simultaneous upper and lower endoscopic biopsy in the diagnosis of intestinal graft-versus-host disease. Transplantation. 1991;51:642–6. doi: 10.1097/00007890-199103000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Lee FD. Importance of apoptosis in the histopathology of drug related lesions in the large intestine. Journal of Clinical Pathology. 1993;46:118–22. doi: 10.1136/jcp.46.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen CV, Kastenberg DM, Choudhary C, Katz LC, DiMarino A, Palazzo JP. Is Single-Cell Apoptosis Sufficient for the Diagnosis of Graft-Versus-Host Disease in the Colon? Dig Dis Sci. 2007;53:747–56. doi: 10.1007/s10620-007-9904-3. [DOI] [PubMed] [Google Scholar]
- 40.Lin J, Fan R, Zhao Z, Cummings OW, Chen S. Is the presence of 6 or fewer crypt apoptotic bodies sufficient for diagnosis of graft versus host disease? A decade of experience at a single institution. Am J Surg Pathol. 2013;37:539–47. doi: 10.1097/PAS.0b013e318272c62a. [DOI] [PubMed] [Google Scholar]
- 41.Gomez AJ, Arai S, Higgins JP, Kambham N. Clinicopathologic Threshold of Acute Colorectal Graft-versus-Host Disease. Arch Pathol Lab Med. 2016;140:570–7. doi: 10.5858/arpa.2015-0187-OA. [DOI] [PubMed] [Google Scholar]
- 42.McDonald GB, Tabellini L, Storer BE, Lawler RL, Martin PJ, Hansen JA. Plasma biomarkers of acute GVHD and nonrelapse mortality: predictive value of measurements before GVHD onset and treatment. Blood. 2015;126:113–20. doi: 10.1182/blood-2015-03-636753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch DC, Wirth PS, Goldenring JR, Ness E, Jagasia M, Washington K. Gastric graft-versus-host disease revisited: does proton pump inhibitor therapy affect endoscopic gastric biopsy interpretation? Am J Surg Pathol. 2006;30:444–9. doi: 10.1097/00000478-200604000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Papadimitriou JC, Cangro CB, Lustberg A, Khaled A, Nogueira J, Wiland A, et al. Histologic Features of Mycophenolate Mofetil-Related Colitis: A Graft-Versus-Host Disease-Like Pattern. Int J Surg Pathol. 2003;11:295–302. doi: 10.1177/106689690301100406. [DOI] [PubMed] [Google Scholar]
- 45.Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol. 2008;32:1367–72. doi: 10.1097/pas.0b013e31816bf3fe. [DOI] [PubMed] [Google Scholar]
- 46.Star KV, Ho VT, Wang HH, Odze RD. Histologic features in colon biopsies can discriminate mycophenolate from GVHD-induced colitis. Am J Surg Pathol. 2013;37:1319–28. doi: 10.1097/PAS.0b013e31829ab1ef. [DOI] [PubMed] [Google Scholar]
- 47.Liapis G, Boletis J, Skalioti C, Bamias G, Tsimaratou K, Patsouris E, et al. Histological spectrum of mycophenolate mofetil-related colitis: association with apoptosis. Histopathology. 2013:n/a–n/a. doi: 10.1111/his.12222. [DOI] [PubMed] [Google Scholar]
- 48.Akashi M, Ando T, Hamashima T, Yoshita H, Nanjo S, Mihara H, et al. Multiple Colon Ulcers with Typical Small Intestinal Lesions Induced by Non-Steroidal Anti-Inflammatory Drugs. Intern Med. 2015;54:1995–9. doi: 10.2169/internalmedicine.54.3919. [DOI] [PubMed] [Google Scholar]
- 49.Snover DC. Graft-versus-host disease of the gastrointestinal tract. Am J Surg Pathol. 1990;14(Suppl 1):101–8. [PubMed] [Google Scholar]
- 50.Washington K, Bentley RC, Green A. Gastric graft-versus-host disease: a blinded histologic study. Am J Surg Pathol. 1997;21:1037–46. doi: 10.1097/00000478-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Aslanian H, Chander B, Robert M, Cooper D, Proctor D, Seropian S, et al. Prospective Evaluation of Acute Graft-Versus-Host Disease. Dig Dis Sci. 2011;57:720–5. doi: 10.1007/s10620-011-1938-x. [DOI] [PubMed] [Google Scholar]
- 52.Sultan M, Ramprasad J, Jensen MK, Margolis D, Werlin S. Endoscopic Diagnosis of Pediatric Acute Gastrointestinal Graft-Versus-Host Disease. J Pediatr Gastroenterol Nutr. 2012;55:417–20. doi: 10.1097/MPG.0b013e31825048eb. [DOI] [PubMed] [Google Scholar]
- 53.Ma C, Maluf HM, Liu T-C. Acute graft-versus-host disease is more prevalent and severe in the lower than the upper gastrointestinal tract. Human Pathology. 2015;46:1480–7. doi: 10.1016/j.humpath.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Ip S, Marquez V, Schaeffer DF, Donnellan F. Sensitivities of Biopsy Sites in the Endoscopic Evaluation of Graft-Versus-Host Disease: Retrospective Review from a Tertiary Center. Dig Dis Sci. 2016;61:2351–6. doi: 10.1007/s10620-016-4142-1. [DOI] [PubMed] [Google Scholar]
- 55.Batts KP, Ludwig J. An Update on Terminology and Reporting. Am J Surg Pathol. 1995;19:1409–17. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. Journal of Cell Science. 1994;107(Pt 12):3569–77. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 57.Saisho Y, Manesso E, Gurlo T, Huang CJ, Toffolo GM, Cobelli C, et al. Development of factors to convert frequency to rate for -cell replication and apoptosis quantified by time-lapse video microscopy and immunohistochemistry. AJP: Endocrinology and Metabolism. 2008;296:E89–E96. doi: 10.1152/ajpendo.90697.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


