Abstract
Synthetic monorhamnolipids differ from biologically produced material because they are produced as single congeners, depending on the β-hydroxyalkanoic acid used during synthesis. Each congener is produced as one of four possible diastereomers resulting from two chiral centers at the carbinols of the lipid tails [(R,R), (R,S), (S,R) and (S,S)]. We compare the biodegradability (CO2 respirometry), acute toxicity (Microtox assay), embryo toxicity (Zebrafish assay), and cytotoxicity (xCELLigence and MTS assays) of synthetic rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoate (Rha-C10-C10) monorhamnolipids against biosynthesized monorhamnolipid mixtures (bio-mRL). All Rha-C10-C10 diastereomers and bio-mRL were inherently biodegradable ranging from 34 to 92% mineralized. The Microtox assay showed all Rha-C10-C10 diastereomers and bio-mRL are slightly toxic according to the US EPA ecotoxicity categories with 5 min EC50 values ranging from 39.6 to 87.5 μM. The zebrafish assay showed that of 22 developmental endpoints tested, only mortality was observed at 120 hours post fertilization; all Rha-C10-C10 diastereomers and bio-mRL caused significant mortality at 640 μM, except the Rha-C10-C10 (R,R) which showed no developmental effects. xCELLigence and MTS showed IC50 values ranging from 103.4 to 191.1 μM for human lung cell line H1299 after 72 h exposure. These data provide key information regarding Rha-C10-C10 diastereomers that is pertinent when considering potential applications.
Keywords: Rhamnolipid, biodegradation, toxicity, stereochemistry, biosurfactant
Graphical Abstract
1. Introduction
The addition of surfactants to enhance remediation of contaminated sites can improve remediation time and completeness [1]. However, use of surfactant amendments must be carefully considered to ensure the treatment does not cause environmental harm or a secondary contamination event, due to toxicity of the surfactant itself [2]. The use of bio-based and biogenic surfactants as amendments in remediation applications is attracting increased interest because they can be less toxic and more biodegradable than synthetic compounds [1].
One family of biological surfactants that has been studied extensively with respect to potential environmental applications is the rhamnolipids, which are produced primarily by Pseudomonas aeruginosa strains. Rhamnolipids are considered “green” materials; they are a renewable resource and generally accepted as more biodegradable and less toxic than currently used synthetic surfactants [3]. Rhamnolipids have been examined as remediation agents in numerous environmental applications to increase the solubility and biodegradation of hydrocarbons [4–6], mobilize metals [7–11], decrease metal toxicity [12], modify cellular characteristics and behavior [13], improve oil recovery [14], and for biocidal activity [15, 16]. Despite these potential applications, the technical and economic hurdles of biologically producing rhamnolipids has limited the scale-up and use of these materials for remediation and other applications [17, 18].
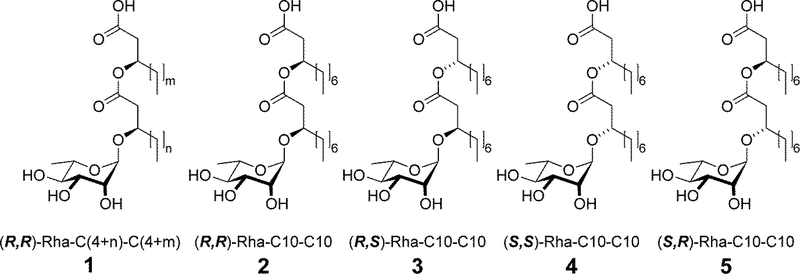
The generalized structure of biosynthesized rhamnolipid is a lipid unit, (R,R)-β-hydroxyalkanoyl-β-hydroxyalkanoic acids (two esterified β-hydroxy fatty acids) of variable chain lengths (C6-C14), that is trans-1,2-O-glycosylated by a mono- or disaccharide formed from L-rhamnopyranosyl units [19–23]. Biosynthetic rhamnolipids are thus a congener mixture of (R,R) rhamnolipids with more than 60 congeners reported [23].
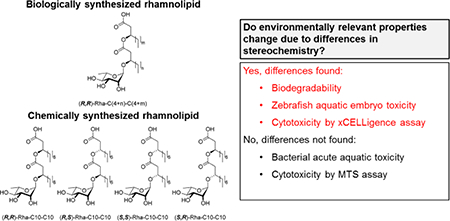
Recent advances in glycosylation research have enabled synthetic production of rhamnolipid surfactants as an alternative to biological production. The chemical synthesis has high green indices and uses minimal steps and renewable resources [24, 25]. There are two major differences between synthetically and biologically produced rhamnolipids. First, the synthetic methodology can produce a single congener with specific carbon chain lengths whereas biosynthesized rhamnolipids are congener mixtures. Secondly, during biosynthesis the stereochemical orientation at the secondary carbinol groups of the lipid tails is controlled by stereospecific enzymes such that biosynthesized congeners have (R) as the absolute configuration. In contrast, the chemical synthesis produces four diastereomers, (R,R), (R,S), (S,S), and (S,R) (Fig. 1), in roughly equimolar amounts without additional tedious separation steps at various points along the synthetic process. The (R,S), (S,S), and (S,R) diastereomers represent novel molecules which have not been assessed for toxicity, biodegradability, or useful applications. Yet, it is well-known that different chiral centers in small organic molecules can alter toxicity [26], degradability, and transformation products [27–29].
Figure 1.
Structures of bio-mRL (1) and synthetic (2–5) monorhamnolipids utilized in this study. The varying chain lengths of bio-mRL are represented by ‘m’ and ‘n’ values which vary from 4 to 12.
The objective of this study is to examine the biodegradability, eco-, and cyto-toxicity characteristics of synthetic rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoate (Rha-C10-C10) monorhamnolipid diastereomers relative to a biosynthetic monorhamnolipid congener mixture. Rha-C10-C10 was selected because it is the most abundant congener (typically 75–85%) produced by Pseudomonas aeruginosa ATCC 9027, the model strain used in this study [30]. Biodegradability was assessed by CO2 evolution (mineralization) in biometer flasks. Toxicity was assessed using the Microtox bacterial acute toxicity assay, the zebrafish developmental toxicity assay, and two human cell cytotoxicity assays: xCELLigence Real-Time Cell Analysis and MTS. The synthetic diastereomers were examined individually and as a mixture and were compared to a congener mixture of biosynthetic monorhamnolipid, hereafter referred to as bio-mRL.
2. Experimental
2.1. Monorhamnolipids
All assays tested the bio-mRL, the Rha-C10-C10 diastereomers, and their mixture, except for the human cell cytoxicity assays which excluded the synthetic mixture. All monorhamnolipid solutions were made in ultrapure water (≥18 MΩ·cm) using a molecular weight of 504 g mol−1 and pH adjusted to 7.0 except where otherwise noted.
2.1.1. Biosynthesized Rhamnolipid
Bio-mRL Production.
Bio-mRL was produced by Pseudomonas aeruginosa ATCC 9027 which was obtained from the American Type Culture Collection (ATTC). This strain has been previously shown to only produce monorhamnolipid congeners [4, 31]. The bio-mRL was produced, harvested, and purified as previously described in detail [11]. Bio-mRL purity was assessed by high performance liquid chromatography [31], and characteristics are provided in Table 1. Critical micelle concentration (CMC), minimum surface tension, and molecular area for this bio-mRL have been previously discussed [32].
Table 1.
Characteristics of the rhamnolipid treatments
| Monorhamnolipid Treatment | Purity | CMCa | Minimum Surface Tensiona | Areaa | Congener Mixture | Mineralizationb |
|---|---|---|---|---|---|---|
| (%) | (μM) | (mN m−1) | (Å2 molecule−1) | (%) | ||
| Bio-mRLc | >99 | 201 ± 12 | 29.0 ± 0.5 | 85.8 ± 1.9 | Yes | 92 2 |
| Rha-C10-C10 Mixtured | >95 | 277 ± 21 | 27.9 ± 0.3 | 73 ± 2 | No | 69 2 |
| Rha-C10-C10 (R,S)d | 99 | 79 ± 3 | 27.4 ± 0.2 | 82 ± 1 | No | 60 1 |
| Rha-C10-C10 (R,R)d | 87 | 270 ± 77 | 28.1 ± 0.2 | 119 ± 10 | No | 731, 692 |
| Rha-C10-C10 (S,S)d | 88 | 201 ± 51 | 29.5 ± 0.2 | 94 ± 8 | No | 34 1 |
| Rha-C10-C10 (S,R)d | 92 | 180 ± 24 | 28.5 ± 0.2 | 105 ± 4 | No | 51 1 |
Measured at pH 8
Mineralization values were determined using two experiments as indicated by the superscript next to each value.
= experiment 1 (Fig. 2A)
= experiment 2 (Fig. 2B). Rha-C10-C10 R,R was evaluated in both experiments.
CMC, minimum surface tension, and molecular area data from Eismin et al. [32].
CMC, minimum surface tension, and molecular area data from Palos Pacheco et al. [25].
2.1.2. Synthetic Monorhamnolipids
Synthetic Rha-C10-C10 diastereomers were synthesized as recently described [25]. Their production involves diastereomeric chromatographic enrichment via enantiomer-diastereomer conversion, and therefore, small amounts of other diastereomers with similar retention are unavoidably present [25]. Diastereomer purity was assessed by HPLC and is reported in Table 1 in terms of total % monorhamnolipid in each sample. Subsequent NMR analysis also showed the presence of small amounts of water due to the hygroscopic character of these molecules. Trace amounts of the bismuth catalyst used in the synthetic process may also be present. These diastereomeric Rha-C10-C10 monorhamnolipids were used in biodegradability and toxicity tests without further purification. Differences in characteristics for these diastereomers, including CMC, minimum surface tension, and molecular area, have been previously discussed [25].
2.2. Biodegradability Testing
Mineralization of bio-mRL and Rha-C10-C10 diastereomers was measured by quantitation of CO2 as described previously with the following specifications [33]. Each experiment was performed in triplicate sealed biometer flasks (Corning Life Sciences, Corning, NY) and included a control treatment, also in triplicate, without added monorhamnolipid. The microbial inoculum was aeration basin mixed liquor suspended solids (MLSS) from the Tres Rios Water Reclamation Facility (Pima County, AZ). The MLSS was kept aerated and used within 4 h of collection. Before addition to flasks, the MLSS was allowed to settle in a graduated cylinder until the apparent volume of solids became constant. The supernatant was decanted and reserved. The total reaction volume in each flask was 25 mL consisting of 2 mL settled MLSS flocculate, 15 mL MLSS supernatant, 6 mL EPA minimal salts medium [34], and 2 mL monorhamnolipid (5 mg mL−1) solution in ultrapure water (final concentration 400 mg L−1). Control flasks received 2 mL ultrapure water with no monorhamnolipid. Flasks were shaken on a rotary shaker at 25 °C for 30 d and assayed for CO2 evolution every 24 h for the first 6 d and then every 48 h thereafter.
Carbon dioxide produced in the flasks was captured in 10 mL of 0.1 M KOH placed into the biometer flask’s sidearm through the formation of K2CO3(aq). The amount of CO2 produced was measured by titration; the sidearm solution was removed and mixed with phenolphthalein indicator, 1 mL of 1 M BaCl2, and 5 mL of water used to wash the sidearm. This solution was titrated with 0.05 M HCl. The K2CO3(aq) reacts with BaCl2(aq) to form BaCO3(s) which prevents the release and recapture of CO2 by unreacted −OH during titration. All reagents were made with ultrapure water purged of CO2 for 20 min with nitrogen gas sparging.
Mineralization of the monorhamnolipid treatments was measured as percent mineralization which was calculated using equation 1:
| (1) |
where CO2 mRL is the moles of CO2 from mineralized monorhamnolipid— monorhamnolipid transformed in to CO2, H2O, and cell mass—and CmRL is moles of carbon added as monorhamnolipid (based on MW 504 g mol−1). A monorhamnolipidfree control was used to quantify CO2 production from mineralization of carbon in the MLSS solids and supernatant. Thus, CO2 mRL was calculated using equation 2:
| (2) |
Where CO2 treatment is moles of CO2 produced for each treatment and CO2 control is the average moles of CO2 measured in the monorhamnolipid-free controls.
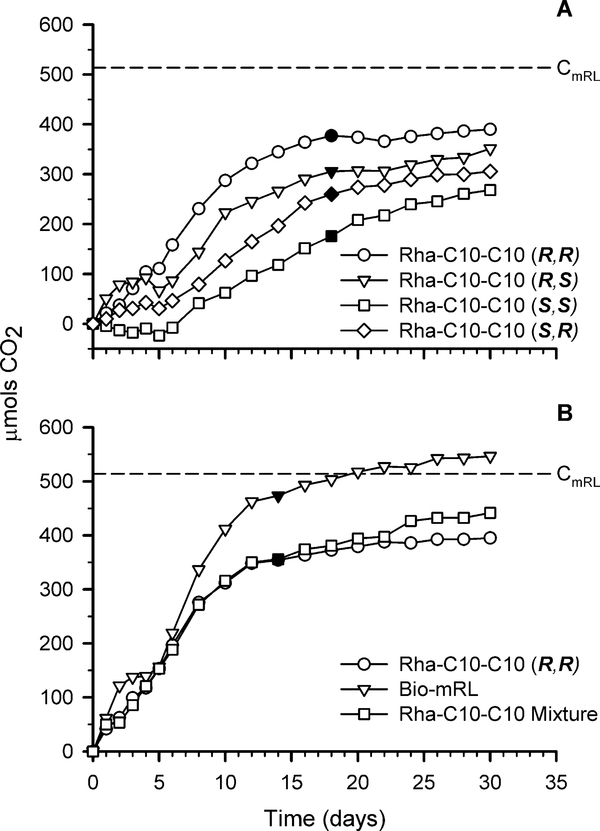
Mineralization was calculated at the estimated transition from exponential phase to stationary phase (filled symbols in Fig. 2). It is assumed that CO2 production measured after this transition point is due to endogenous decay (decay and degradation of new cells produced) and this CO2 was not included in calculations for estimated monorhamnolipid biodegradation.
Figure 2.
CO2 production due to mineralization of (A) Rha-C10-C10 (R,R), (R,S), (S,S), and (S,R) and (B) Rha-C10-C10 (R,R), Rha-C10-C10 mixture, and bio-mRL. CmRL represents the moles of carbon added as monorhamnolipid. The filled symbols indicate the estimated transition from exponential to stationary phase at which point mineralization was calculated. Increases in CO2 after this time are attributed to endogenous decay. Error bars represent the standard deviation.
2.3. Microtox Acute Toxicity Assay
Bacterial acute toxicity of the surfactants was assessed using a Microtox Model 500 analyzer (Modern Water Inc., New Castle, DE, USA). The Microtox assay assesses toxicity by determining the effective concentration (EC50) at which a toxicant reduces bioluminescence of the marine bacterium Aliivibrio fischeri by 50% relative to a toxicantfree control. Reagents were sourced from Modern Water Inc. (New Castle, DE). Triplicate experiments with monorhamnolipid solutions were tested as previously described [35]. In brief, a 2 mM monorhamnolipid stock solution was diluted to adjust the osmotic strength using the Microtox osmotic adjustment solution as instructed by the manufacturer. The adjusted solution was then 1:1 serially diluted using the Microtox diluent to create 9 monorhamnolipid concentrations (3.5–889 μM). Microtox diluent was used as the toxicant free control. Initial bioluminescence was measured then the toxicant solutions were mixed with the bacterium, and bioluminescence was measured at 5, 15, and 30 min to determine the EC50 for each exposure time point.
2.4. Zebrafish Toxicity Assay
Monorhamnolipid toxicity during embryogenesis was investigated using Zebrafish (Danio rerio). Embryos with intact chorions were exposed to the monorhamnolipid treatments from 6 to 120 h post-fertilization (hpf), following the protocol outlined in Truong et al. [36] Briefly, at 6 hpf, embryos were placed manually in 96-well plates containing embryo medium (90 μL) consisting of (in mg L−1): NaCl (875.0), KCl (37.5), CaCl2•H2O (145.0), KH2PO4 (20.5), Na2HPO4 (7.1), and MgSO4•7H2O (4.9). Monorhamnolipid samples were added (10 μL) in the concentration range 0.064–640 μM, and 32 replicates were run for each concentration and controls. All wells were normalized to 0.64% dimethylsulfoxide (DMSO) to enhance monorhamnolipid solubility.
The 96-well plates were covered in foil and incubated at 28 °C in the dark. At 24 and 120 hpf, development abnormality and mortality assessments were performed [36]. The endpoints evaluated at 24 hpf included mortality, developmental delay, spontaneous movement, and notochord appearance. At 120 hpf endpoints evaluated included mortality, notochord appearance, yolk sac edema, pericardial edema, and abnormalities in body axis, eye, snout, jaw, optic vesicle, brain, somite, pectoral fin, caudal fin, pigment, circulation, truncated body, swim bladder, and touch response. Endpoint scoring and statistical analyses were performed in R software as described previously [36].
2.5. Human Cell Cytotoxicity Assays
2.5.1. Human Cell Culture
H1299, a human lung cell line, was purchased from ATCC. Cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1× Antibiotic-Antimycotic (ThermoFisher). Cells were seeded at a density of 125,000 cells into T75 cm2 tissue culture flasks. Fresh media was added every 2 d, and after approximately 5 d of culture, when cells had reached 70–80% confluence, a subculture was prepared by washing the cells with Dulbecco’s phosphate-buffered saline, incubating the cells with 0.25% trypsin/2.21 mM EDTA at 37 °C for 6 min, and then inactivating the trypsin with an equal volume of culture medium. Cells were pelleted at 230 x g for 5 min at room temperature, resuspended in fresh growth medium, and counted using a Scepter 2.0 Handheld Automated Cell Counter (MilliporeSigma).
2.5.2. Preparation of Monorhamnolipid Solutions
All synthesized surfactants were suspended in DMEM containing 10% FBS at a concentration of 5 mg mL−1 prior to assays. Stocks were made in sufficient volume to complete multiple tests of each toxicity assay to limit exposure of compounds to room air. Stocks were stored at 4 °C and warmed to 37 °C immediately prior to use in cytotoxicity assays.
2.5.3. xCELLigence Real-Time Cell Analysis (RTCA) Cytotoxicity Assay
H1299 cells were plated at a density of 2000 cells per well in a volume of 120 μL per well in an E-Plate 96 (Acea Biosciences, San Diego, CA). All plates were analyzed in an xCELLigence RTCA MP system (Acea BioSciences), while being maintained in a cell culture incubator at 37 °C and 5% CO2. After plating, cells were cultured for 24 h, with measurements taken at 15 min intervals to measure Cell Index, a measure of electrical impedance across well electrodes resulting from cell adherence. Cells were then administered serial dilutions (0.002–1984 μM) of respective monorhamnolipid in a volume of 30 μL per well, with 8 replicate wells per dose. Plates were returned to the xCELLigence and the cytotoxicity of the monorhamnolipid was evaluated over 72 h with measurements taken at 15 min intervals. Inhibitory concentration 50, IC50, values were determined using the DRC (area-under-curve in a time period vs concentration) function of the RTCA Software 2.0 (Acea Biosciences). Analysis was performed based on the mean value of the replicate wells. The timeframe chosen for analysis in each experiment was between the first sweep taken following addition of compounds and the last sweep of each assay. All compounds were tested with 3 biological replicates, unless otherwise indicated.
2.5.4. MTS Cytotoxicity Assay
H1299 cells were plated in 96-well plates at a density of 4000 cells per well with 120 μL of medium per well and allowed to grow for 24 h. Cells were then administered serial dilutions (0.002–1984 μM) of respective monorhamnolipid in a volume of 30 μL per well and 6 wells per dose. Plates were incubated for 72 h following this addition. Cytotoxicity was assessed using the CellTiter 96 AQueous Non-Radioactive Cell Proliferation MTS (3(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) Assay (Promega, Madison, WI) by adding 20 μL of MTS assay reagent (2 mg mL−1 MTS and 42 μg mL−1 phenazine methosulfate) to each well. Plates were incubated for 90 min and absorbance was read at 490 nm in a Synergy HTX Multi-Mode Plate Reader (BioTek, Winooski, VT). IC50 values were determined with Prism 6.0 software (GraphPad, San Diego, CA) using the non-linear curve fitting of the log concentration of inhibitor vs. response with variable slope (four parameters). The number of independent replicate experiments for each treatment is given in Table 2.
Table 2.
Monorhamnolipid toxicity (μM) as measured by Microtox (effective concentration 50, EC50), xCELLigence (inhibitory concentration, IC50), and MTS (IC50) assays.
| Monorhamnolipid Treatment | Microtox EC50 |
xCELLigence IC50 |
MTS IC50 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 mina (n=3)b | 15 mina (n=3)b | 30 mina (n=3)b | 72 ha (n=3)b | 72 ha | nb | |||||
| Bio-mRL | 80.3 ± 10.3 | A | 51.2 ± 8.0 | AB | 45.6 ± 4.9 | A | 132.9 ± 6.8 | A | 165.6 ± 49.4 | 5 |
| Rha-C10-C10 Diastereomer Mixture | 39.6 ± 10.4 | B | 31.3 ± 7.3 | B | 31.7 ± 6.0 | A | ||||
| Rha-C10-C10 (R,S) | 87.5 ± 14.5 | A | 58.2 ± 13.9 | A | 56.4 ± 15.2 | A | 191.1 ± 17.5 | B | 189.5 | 2 |
| Rha-C10-C10 (R,R) | 78.7 ± 4.0 | A | 53.8 ± 3.3 | AB | 47.3 ± 1.2 | A | 122.1 ± 9.1 | A | 129.7 ± 1.1 | 5 |
| Rha-C10-C10 (S,S) | 64.3 ± 8.5 | AB | 42.6 ± 4.4 | AB | 38.8 ± 3.7 | A | 108.2 ± 5.3 | A | 125.1 ± 18.6 | 7 |
| Rha-C10-C10 (S,R) | 61.9 ± 4.7 | AB | 39.6 ± 2.9 | AB | 36.6 ± 2.9 | A | 111.3 ± 6.2 | A | 103.4 ± 31.9 | 4 |
Values reported are the means ± the standard deviations of the mean. Significant treatment differences were determined by one-way ANOVA using the Tukey–Kramer HSD test to compare means (α = 0.05). Each column was tested independently. Levels not connected by same letter are significantly different.
n = number of replicates performed
3. Results and Discussion
3.1. Biodegradation Assay
The Environmental Protection Agency classifies chemicals as “readily biodegradable” when the chemicals have passed specified screening tests for ultimate biodegradability. Because the screening tests are stringent, the readily biodegradable classification allows one to assume readily biodegradable compounds will rapidly and completely biodegrade in aquatic, aerobic environments [34]. Usually a series of ready biodegradability tests are performed, one of which is measurement of CO2 to evaluate mineralization. In order to be considered readily biodegradable, the measured CO2 must be >60% of the theoretical CO2 (equal to CmRL) within a 10-day window which begins when measured CO2 surpasses 10% of theoretical CO2; the 10-day window must fall within a 28-day study [37].
Mineralization of the monorhamnolipids was evident for all treatments tested with percentages ranging from 34 to 92% (Fig. 2, Table 1). A limited number of biometer flasks allowed only four treatments and a control to be assayed in each experiment. Therefore two experiments were performed: experiment 1 compared mineralization of the Rha-C10-C10 diastereomers (Fig. 2A) and experiment 2 compared the bio-mRL, Rha-C10-C10 (R,R), and the Rha-C10-C10 mixture (Fig. 2B). Bio-mRL, Rha-C10-C10 (R,R), the mixture, and Rha-C10-C10 (S,R) can all be considered readily biodegradable with 60% or greater mineralization. Interestingly, bio-mRL had the highest level of mineralization and Rha-C10-C10 (R,R) was next. These two treatments have the same stereo-orientations which suggests the (R,R) orientation may better facilitate interaction of degrading enzymes with the substrate. Although the diastereomer mixture was readily biodegradable (69% mineralization) it is noted that Rha-C10-C10 (S,R) (51% mineralization) and Rha-C10-C10 (S,S) (34% mineralization) did not meet the readily biodegradable criterion for this test. Unlike bio-mRL and Rha-C10-C10 (R,R), Rha-C10C10 (S,R) and Rha-C10-C10 (S,S) have not been previously experienced in the environment, and their biodegradation may be enhanced as environmental and/or test organisms become acclimatized to these molecules.
Taken together, these results suggest that the Rha-C10-C10 diastereomers are biodegradable, but further testing under less ideal conditions is required to fully understand the fate of these materials in the environmental. For example, though the COREXIT anionic surfactant bis-(2-ethylhexyl) sulfosuccinate (DOSS) was biodegradable in laboratory settings [38], it was found to persist in deep-sea sediment and coral communities 6 months after and on surrounding beaches nearly 4 years after application to an oil spill in the Gulf of Mexico [39].
3.2. Toxicity of synthetic monorhamnolipids
To determine the risk associated with environmental amendments, a suite of tests are used to develop an ecological effect characterization that describes a substance’s toxicity. Characterization tests study substances in the short-term (acute) and long-term (chronic) using a variety of species and measures, e.g., mortality, growth effects, behavioral effects, effect duration, recovery potential, bioaccumulation, etc. [40]. In this study, we used two aquatic toxicity assays—Microtox and Zebrafish—and two human cell cytotoxicity assays—xCELLigence and MTS—to examine monorhamnolipid toxicity.
3.2.1. Microtox Acute Toxicity Assay
The Microtox assay measures the acute toxicity of a test compound to aquatic bacteria using the model bacterium Aliivibrio fischeri by determining the EC50 values where the bacterium’s bioluminescence is reduced by half. Results for the rhamnolipids tested in this study revealed EC50 values ranging from 39.6 to 87.5 uM at 5 min and 31.7 to 56.4 uM at 30 min (Table 2). Over time, the bio-mRL, Rha-C10-C10 (R,R), (S,S), and (S,R), had significantly increased (α=0.05) toxicity with extended exposure from 5 to 15 min while there was no increase for Rha-C10-C10 (R,S) or the diastereomer mixture (data not shown). There was no significant change in toxicity between the 15 and 30 min exposure times for any of the treatments (data not shown).
Among treatments, a comparison of EC50 values at each time point tested shows that the Rha-C10-C10 (R,S), (R,R), and bio-mRL treatments were generally least toxic and the diastereomer mix was most toxic (Table 2). Statistical analysis of these results shows that the diastereomer mixture was significantly (α = 0.05) more toxic than RhaC10-C10 (R,S) at 5 and 15 min exposure times and more toxic than the bio-mRL and the (R,R), diastereomer at 5 min (Table 2). There was no significant difference between the bio-mRL and individual diastereomers at any time point. There were no significant differences at the 30 min exposure time.
Based on EC50 values, the monorhamnolipids can be categorized into one of five EPA ecotoxicity categories for aquatic organism acute exposure concentrations: very highly toxic (<0.1 mg L−1), highly toxic (0.1–1 mg L−1), moderately toxic (>1–10 mg L−1), slightly toxic (>10–100 mg L−1), and practically nontoxic (>100 mg L−1) [40]. All of the monorhamnolipids tested fall into the slightly toxic category (supplementary Table S1), and the EC50 values are consistent with literature values of 50 and 13 mg L−1 for unspecified rhamnolipid mixtures obtained from Pseudomonas spp. [41]. Though Table 2 shows there were a few statistical differences between the monorhamnolipids tested, the classification of all the treatments into the same EPA ecotoxicity category shows there is no practical difference among them. The statistically significant increase in toxicity between the 5 and 15 min exposures for the bio-mRL, Rha-C10-C10 (R,R), (S,S), and (S,R) treatments, however, may demonstrate different activities between the rhamnolipids. We hypothesize the increase in toxicity across the 5 and 15 min exposures indicates the time required for these rhamnolipids to fully penetrate into the cell is longer than those rhamnolipid treatments without significant differences ((R,S) and the diastereomer mixture).
3.2.2. Zebrafish Toxicity Assay
The zebrafish (Danio rerio) is a well-accepted vertebrate animal model for the in vivo testing of chemical bioactivity. This test was selected because zebrafish have a short generation time, rapid development, and short life cycle during which toxicological effects can be observed [36].
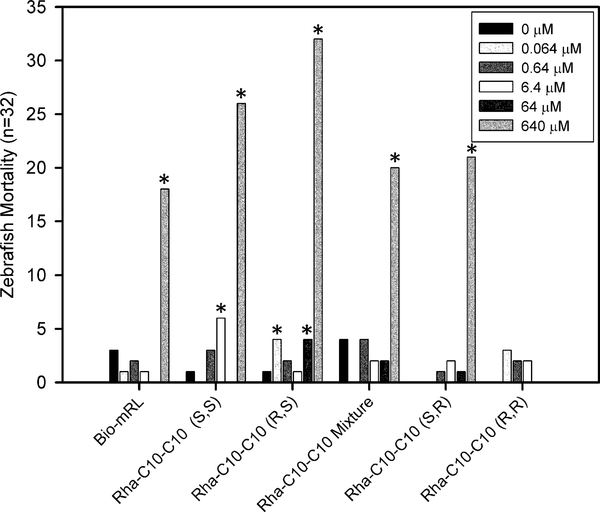
In this study, 22 developmental and potential neurotoxic chemical effects were screened. Mortality was the only developmental endpoint associated with exposure to monorhamnolipids in the concentration range tested. All monorhamnolipids, except Rha-C10-C10 (R,R), were associated with significant mortality at the highest concentration tested, 640 μM, relative to the no monorhamnolipid control (Fig. 3). The most toxic was Rha-C10-C10 (R,S), with 100% mortality (32 out of 32), followed by (S,S), with 81% mortality. The diastereomer mixture, Rha-C10-C10 (S,R), and the bio-mRL were associated with similar mortality incidences, ranging from 56 to 66%, while Rha-C10-C10 (R,R) was not associated with mortality.
Figure 3.
Zebrafish mortality (n = 32) at 120 hpf for monorhamnolipid treatments from 0 to 640 μM. Stars indicate a significant difference in mortality compared to the monorhamnolipid-free control.
Significant mortality was also observed in some cases for exposure to lower concentrations: 6.4 μM Rha-C10-C10 (S,S) (19% ), 64 μM (R,S) (13%), and, unexpectedly, 0.064 (R,S) (13%). Mortality was observed after the 24 hpf assessments but prior to hatching at 48 hpf (supplementary Figure S1), suggesting that the toxic monorhamnolipids crossed the chorion membrane. The 640 μM concentration is relatively high and likely not of environmental relevance except perhaps before dilution in areas where a concentrated amendment might be applied. This high concentration was included to guarantee a detection of a threshold for high toxicity in this study. The lower, more environmentally relevant concentrations were far less toxic and suggest that rhamnolipids have a low developmental hazard potential.
These results are similar to the results of Johann et al. [42] who observed 48 hpf zebrafish mortality but no sub-lethal morphology changes. The concentration at which mortality was observed differed between the two studies. Johann et al. reported 100% mortality at concentrations of ≥100 mg L−1 (−200 μM) monorhamnolipids from a recombinant strain of Pseudomonas putida. In this study, less than 100% mortality was found for all the treatments, except Rha-C10-C10 (R,S), at the highest rhamnolipid concentration of 323 mg L−1 (640 μM); bio-mRL resulted in only 56% mortality at this concentration. The toxicity difference between the studies is likely the result of the variable length fatty-acids present in the congener mixtures produced by P. putida and P. aeruginosa. Indeed, in a review of anionic and nonionic surfactant toxicity, Lechuga et al. [43] found that chain length of the hydrophobic moiety controlled the relative toxicity of surfactants. For example, Microtox data for the alkyl sulfonates C8, C10, C12, and C14 molecules yielded 30 min EC50 values of 265, 33, 19, and 66 mg L−1, respectively. Data for the analogous alkyl sulfates were 35.1, 7.0, 0.98, and 34.8 mg L−1, respectively [44]. From these series, chain length was clearly a large contributor to aquatic toxicity with maximum toxicities in the C10–12 range. This suggests that it may be possible to moderate the acute toxicity of the Rha-C10-C10 by shortening the fatty acid moiety to C8 or lengthening it to a C14 when selecting a β-hydroxy fatty acid’s length during rhamnolipid synthesis.
3.2.3. Human Cell Cytotoxicity Assays
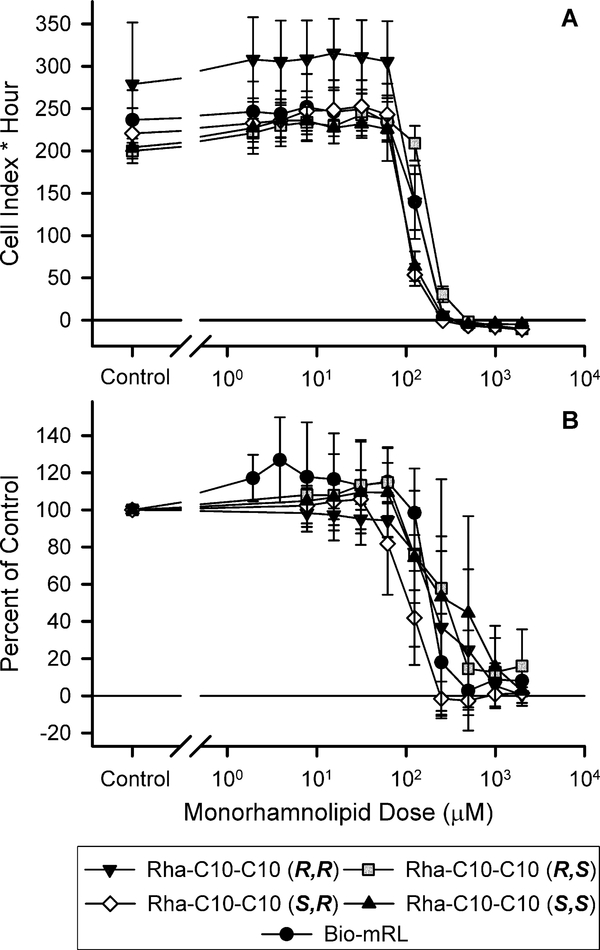
To compare the relative effects of synthetic rhamnolipid diastereomers in human cells, H1299 lung cells were exposed to increasing monorhamnolipid concentrations in the xCELLIGENCE and MTS assays (Fig. 4). The xCELLigence assay measures growth inhibition utilizing cell culture plates with gold electrodes embedded in the bottom of the wells in which the cells are plated. As cells adhere to the bottom of the wells and divide, they disrupt the electrical current flowing through the electrodes, which can be measured in real time as electrical impedance. This impedance is proportional to the number of cells present and is reported using a unitless parameter called Cell Index. The MTS assay provides a relative measure of the presence of viable cells. Viable cells contain active enzymes that convert MTS to a formazan crystal product, which can be measured at 490 nm in a spectrophotometer. Cell toxicity results in a loss of viability and a decrease in 490 nm formazan product produced relative to control.
Figure 4.
Growth inhibition curves for monorhamnolipids as measured by the xCELLigence RTCA (A) or MTS assay (B). Error bars indicate the standard error.
Growth inhibition was observed within the dose range of the tested compounds using the xCELLigence assay (Fig. 4A). IC50 values ranged from 108.2 to 191.1 μM after 72 h (Table 2). Rha-C10-C10 (R,S) was the least toxic treatment and the only treatment to be significantly different from any of the other monorhamnolipid treatments. The three highest monorhamnolipid concentrations tested 1984, 992, and 496 μM exhibited complete growth inhibition as indicated by an immediate loss of Cell Index in the RTCA analysis. This result indicates immediate loss of cellular contact with the bottom of the culture plates which can be interpreted as cytotoxicity of the respective compounds. MTS assay results followed the same pattern as the xCELLigence assay with IC50 values ranging from 103.4 to 189.5 μM (Fig. 4B, Table 2), however, statistical analysis could not be performed because test compound quantity limitations only allowed for two independent experimental replicates for Rha-C10-C10 (R,S).
Akiyode et al. [45] used a comparable MTT cytotoxicity assay to study rhamnolipid effects on MCF-7 human breast cancer cells and found 72 h IC50 values of 33.08 and 30.05 mg L−1 for two commercially available rhamnolipid mixtures. These values are below the range reported for both the MTS and xCELLigence assays herein (52.1–96.3 mg L−1, supplementary Table S1) though they are of a generally similar magnitude. The difference could be due to sensitivities of the different cell lines to rhamnolipids or differences in the rhamnolipid type and congener mixture or both. Akiyode et al. [45] also reports that their commercial mixtures contain a mixture of mono- and di-rhamnolipids with a high relative abundance of Rha2-C10-C12 and Rha2-C12-C10 congeners [45]. Dirhamnolipids have been shown to be more toxic than monorhamnolipids to MCF-7 cells by MTT assay [46]. A second study using the MTT assay reports no inhibitory activity against human small cell lung cancer cells (NCIH187) at the highest dirhamnolipid concentration tested (50 mg L−1), which is also consistent with the results of this study [47].
At the three highest monorhamnolipid concentrations tested (1984, 992, and 496 μM), the xCELLigence assay indicated complete inhibition and thus clear cytotoxicity (Fig. 4A). The MTS data at these three concentrations, however, still showed measurable enzymatic activity in the 1984 μM treatments ranging from 1–16% activity of the monorhamnolipid-free control (Fig. 4B). This may indicate that monorhamnolipids do not have complete cytotoxicity at these concentrations and that the complete loss of Cell Index in the xCELLigence assay is the result of both cell disintegration and whole cell release from the well surface. In either case, these high concentrations were tested to determine the threshold for cytotoxicity and likely have limited practical relevance as noted above for the zebrafish assay.
4. Conclusion
Novel rhamnolipids with altered stereochemistry or congener makeup, may have different properties. This study shows that such differences resulted in measurable changes in biodegradation, zebrafish toxicity, and xCELLigence human lung cell toxicity; no significant differences were detected for acute aquatic prokaryotic toxicity using the Microtox assay or for the MTS human cell cytotoxicity assay. This work lays a foundation for understanding the environmental compatibility of novel synthetic monorhamnolipid diastereomers and provides pertinent information when considering potential applications for use either individually or as a mixture. To further demonstrate the marketability and utility of these synthetic monorhamnolipids, future work should evaluate the cost effectiveness of synthetic versus biosynthetic monorhamnolipids, especially in regard to understanding the costs of scaled-up production.
Supplementary Material
Highlights.
Synthetic monorhamnolipid production is congener specific yielding four diastereomers
All four Rha-C10-C10 diastereomers were inherently biodegradable.
Monorhamnolipids were EPA ecotoxicity category ‘slightly toxic’ by Microtox assay.
Zebrafish mortality was observed for all monorhamnolipids except Rha-C10-C10 (R,R).
Cytotoxicity IC50 values ranged from 103.4 to 191.1 μM for human lung cell.
Acknowledgements
This work was supported by a National Science Foundation (NSF) Graduate Research Fellowship Grant (DGE-1143953, 7/15/2011–6/30/2016) to DEH and a NSF Networks for Sustainable Molecular Design and Synthesis Grant (CHE-1339597) co-funded with the Environmental Protection Agency. Zebrafish toxicity tests were supported by the 2014 NIEHS KC Donnelly Externship to CIO and the University of Arizona Superfund Program Training Core (NIEHS grant P42 ES0940). SWM was supported as a NIEHS Trainee in Toxicology of Human Diseases (NIEHS grant T32 ES007091). RPP was supported by fellowships from Consejo Nacional de Ciencia y Tecnología and Secretaría de Educación Pública of México.
Footnotes
Two authors of this paper (RMM, JEP) have equity ownership in GlycoSurf, which is developing products related to the research being reported. The terms of this arrangement have been reviewed and approved by the University of Arizona in accordance with its policy on objectivity in research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Shah A, Shahzad S, Munir A, Nadagouda MN, Khan GS, Shams DF, Dionysiou DD, Rana UA, Micelles as soil and water decontamination agents, Chem. Rev 116 (2016) 6042–6074. [DOI] [PubMed] [Google Scholar]
- [2].Rebello S, Asok AK, Mundayoor S, Jisha M, Surfactants: toxicity, remediation and green surfactants, Environ. Chem. Lett 12 (2014) 275–287. [Google Scholar]
- [3].Pacwa-Płociniczak M, Płaza GA, Piotrowska-Seget Z, Cameotra SS, Environmental applications of biosurfactants: recent advances., Int. J. Mol. Sci 12 (2011) 633–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang Y, Miller R, Effect of a Pseudomonas rhamnolipid biosurfactant on cell hydrophobicity and biodegradation of octadecane, Appl. Environ. Microbiol 60 (1994) 2101–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang Y, Miller R, Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant), Appl. Environ. Microbiol 58 (1992) 3276–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang Y, Miller R, Effect of rhamnolipid (biosurfactant) structure on solubilization and biodegradation of n-alkanes, Appl. Environ. Microbiol 61 (1995) 2247–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ochoa-Loza FJ, Artiola JF, Maier RM, Stability constants for the complexation of various metals with a rhamnolipid biosurfactant, J. Environ. Qual 30 (2001) 479–485. [DOI] [PubMed] [Google Scholar]
- [8].Herman DC, Artiola JF, Miller RM, Removal of cadmium, lead, and zinc from soil by a rhamnolipid biosurfactant, Environ. Sci. Technol 29 (1995) 2280–2285. [DOI] [PubMed] [Google Scholar]
- [9].Tan H, Champion JT, Artiola JF, Brusseau ML, Miller RM, Complexation of cadmium by a rhamnolipid biosurfactant, Environ. Sci. Technol 28 (1994) 2402–2406. [DOI] [PubMed] [Google Scholar]
- [10].Schalnat TA, Metal complexation and interfacial behavior of the microbially-produced surfactant monorhamnolipid by Pseudomonas aeruginosa ATCC 9027, PhD Dissertation, University of Arizona, 2012. [Google Scholar]
- [11].Hogan DE, Curry JE, Pemberton JE, Maier RM, Rhamnolipid biosurfactant complexation of rare earth elements, J. Hazard. Mater 340 (2017) 171–178. [DOI] [PubMed] [Google Scholar]
- [12].Sandrin TR, Chech AM, Maier RM, A rhamnolipid biosurfactant reduces cadmium toxicity during naphthalene biodegradation, Appl. Environ. Microbiol 66 (2000) 4585–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Van Hamme JD, Singh A, Ward OP, Physiological aspects - part 1 in a series of papers devoted to surfactants in microbiology and biotechnology, Biotechnol. Adv 24 (2006) 604–620. [DOI] [PubMed] [Google Scholar]
- [14].Singh A, Van Hamme JD, Ward OP, Surfactants in microbiology and biotechnology: part 2. application aspects, Biotechnol. Adv 25 (2007) 99–121. [DOI] [PubMed] [Google Scholar]
- [15].Stanghellini ME, Miller RM, Biosurfactants: their identity and potential efficacy in the biological control of zoosporic plant pathogens, Plant disease. 81 (1997) 4–12. [DOI] [PubMed] [Google Scholar]
- [16].Magalhaes L, Nitschke M, Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin, Food Control. 29 (2013) 138–142. [Google Scholar]
- [17].Abdel-Mawgoud AM, Hausmann R, Lépine F, Müller MM, Déziel E, Rhamnolipids: detection, analysis, biosynthesis, genetic regulation, and bioengineering of production, in: Soberón-Chávez G (Ed.), Biosurfactants: from genes to applications, Springer, Berlin, Heidelberg, 2011, pp. 13–55. [Google Scholar]
- [18].Mulligan CN, Environmental applications for biosurfactants, Environ. Pollut 133 (2005) 183–198. [DOI] [PubMed] [Google Scholar]
- [19].Schenk T, Breitschwerdt A, Kessels G, Schuphan I, Schimdt B, A biosynthetic route to [14C] labelled rhamnolipids, J. Label. Compd. Radiopharm 39 (1997) 705–710. [Google Scholar]
- [20].Bauer J, Brandenburg K, Zähringer U, Rademann J, Chemical synthesis of a glycolipid library by a solid phase strategy allows elucidation of the structural specificity of immunostimulation by rhamnolipids, Chem. Eur. J 12 (2006) 7116–7124. [DOI] [PubMed] [Google Scholar]
- [21].Andra J, Rademann J, Howe J, Koch M, Heine H, Zahringer U, Brandenburg K, Endotoxin-like properties of a rhamnolipid exotoxin from Burkholderia (Pseudomonas) plantarii: immune cell stimulation and biophysical characterization, Biol. Chem 387 (2006) 301–310. [DOI] [PubMed] [Google Scholar]
- [22].Abdel-Mawgoud AM, Lepine F, Deziel E, A stereospecific pathway diverts betaoxidation intermediates to the biosynthesis of rhamnolipid biosurfactants, Chem. Biol 21 (2014) 156–164. [DOI] [PubMed] [Google Scholar]
- [23].Abdel-Mawgoud AM, Lepine F, Deziel E, Rhamnolipids: diversity of structures, microbial origins and roles, Appl. Microbiol. Biotechnol 86 (2010) 1323–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Coss CS, Minimally competent lewis acid catalysts. General methods for the synthesis and separation of diastereomeric mixtures of monorhamnolipids of Pseudomonas aeruginosa with peracetate glycoside donors, PhD Dissertation, University of Arizona, 2012. [Google Scholar]
- [25].Palos Pacheco R, Eismin RJ, Coss CS, Wang H, Maier RM, Polt R, Pemberton JE, Synthesis and Characterization of Four Diastereomers of Monorhamnolipids, J. Am. Chem. Soc 139 (2017) 5125–5132. [DOI] [PubMed] [Google Scholar]
- [26].Liu W, Gan JJ, Qin S, Separation and aquatic toxicity of enantiomers of synthetic pyrethroid insecticides, Chirality. 17 (2005) S127–S133. [DOI] [PubMed] [Google Scholar]
- [27].Wong CS, Environmental fate processes and biochemical transformations of chiral emerging organic pollutants, Anal. and Bioanal. Chem 386 (2006) 544–558. [DOI] [PubMed] [Google Scholar]
- [28].Mueller TA, Kohler H-E, Chirality of pollutants - effects on metabolism and fate, Appl. Microbiol. Biotechnol 64 (2004) 300–316. [DOI] [PubMed] [Google Scholar]
- [29].Smith SW, Chiral toxicology: it's the same thing...only different, Toxicol. Sci 110 (2009) 4–30. [DOI] [PubMed] [Google Scholar]
- [30].Zhang L, Pemberton JE, Maier RM, Effect of fatty acid substrate chain length on Pseudomonas aeruginosa ATCC 9027 monorhamnolipid yield and congener distribution, Process Biochem. 49 (2014) 989–995. [Google Scholar]
- [31].Lebron-Paler A, Pemberton JE, Becker BA, Otto WH, Larive CK, Maier RM, Determination of the acid dissociation constant of the biosurfactant monorhamnolipid in aqueous solution by potentiometric and spectroscopic methods, Anal. Chem 78 (2006) 7649–7658. [DOI] [PubMed] [Google Scholar]
- [32].Eismin RJ, Munusamy E, Kegel LL, Hogan DE, Maier RM, Schwartz SD, Pemberton JE, Evolution of aggregate structure in solutions of anionic monorhamnolipids: experimental and computational results, Langmuir. 33 (2017) 7412–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bartha R, Pramer D, Features of a flask and method for measuring the persistence and biological effects of pesticides in soil, Soil Sci. 100 (1965) 68–70. [Google Scholar]
- [34].U.S. Environmental Protection Agency, Fate, transport and transformation test guidelines: OPPTS 835.3140 ready biodegradability-- CO2 in sealed vessels (headspace test). EPA 712-C-08–001, 2008.
- [35].Bulich A, Isenberg D, Use of the luminescent bacterial system for the rapid assessment of aquatic toxicity, ISA Trans. 20 (1981) 29–33. [PubMed] [Google Scholar]
- [36].Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL, Multidimensional in vivo hazard assessment using zebrafish, Toxicol. Sci 137 (2014) 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Organization for Economic Co-operation and Development, OECD Test no. 301: ready biodegradability, 1992.
- [38].Campo P, Venosa AD, Suidan MT, Biodegradability of corexit 9500 and dispersed south Louisiana crude oil at 5 and 25 C, Environ. Sci. Technol 47 (2013) 1960–1967. [DOI] [PubMed] [Google Scholar]
- [39].White HK, Lyons SL, Harrison SJ, Findley DM, Liu Y, Kujawinski EB, Long-term persistence of dispersants following the Deepwater Horizon oil spill, Environ. Sci. & Technol. Lett 1 (2014) 295–299. [Google Scholar]
- [40].U.S. Environmental Protection Agency, Technical overview of ecological risk assessment - analysis phase: ecological effects characterization. 2018. https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/technicaloverview-ecological-risk-assessment-0
- [41].Klosowska-Chomiczewska I, Medrzycka K, Karpenko E, Biosurfactants— biodegradability, toxicity, efficiency in comparison with synthetic surfactants, in: Plaza E, Levlin E (Eds.), Proc. of the Polish-Swedish-Ukrainian Seminar “Research and Application of New Technologies in Wastewater Treatment and Municipal Solid Waste Disposal in Ukraine, Sweden, and Poland; ”, Krakow, 2011, pp. 1–9. [Google Scholar]
- [42].Johann S, Seiler T, Tiso T, Bluhm K, Blank LM, Hollert H, Mechanism-specific and whole-organism ecotoxicity of mono-rhamnolipids, Sci. Total Environ 548 (2016) 155–163. [DOI] [PubMed] [Google Scholar]
- [43].Lechuga M, Fernández-Serrano M, Jurado E, Núñez-Olea J, Ríos F, Acute toxicity of anionic and non-ionic surfactants to aquatic organisms, Ecotoxicol. Environ. Saf 125 (2016) 1–8. [DOI] [PubMed] [Google Scholar]
- [44].Leal JS, Gonzalez J, Comelles F, Campos E, Ciganda T, Biodegradability and toxicity of anionic surfactants, Acta Hydrochim. Hydrobiol 19 (1991) 703–709. [Google Scholar]
- [45].Akiyode O, George D, Getti G, Boateng J, Systematic comparison of the functional physico-chemical characteristics and biocidal activity of microbial derived biosurfactants on blood-derived and breast cancer cells, J. Colloid Interface Sci 479 (2016) 221–233. [DOI] [PubMed] [Google Scholar]
- [46].Zhao J, Wu Y, Alfred A, Xin X, Yan S, Chemical structures and biological activities of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa M14808, J. Chem. Pharm. Res 5 (2013) 177–182. [Google Scholar]
- [47].Thanomsub B, Pumeechockchai W, Limtrakul A, Arunrattiyakorn P, Petchleelaha W, Nitoda T, Kanzaki H, Chemical structures and biological activities of rhamnolipids produced by Pseudomonas aeruginosa B189 isolated from milk factory waste, Bioresour. Technol 97 (2006) 2457–2461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.