Abstract
Context
The Tehran Lipid and Glucose Study (TLGS) is a community-based study to reveal the frequency of non-communicable diseases (NCDs) in Tehran's population. This research consists of two main parts, a cross-sectional study on the prevalence of cardiovascular risk factors and a 20-year-ongoing prospective cohort study, which was initiated in 1999 in several phases with an approximate duration of 3.6 years, and is still ongoing. The aim of the present study is review the 20 year biochemical findings of the TLGS related to the NCDs in a large sample.
Methods
All articles on biochemical assessments derived from the TLGS from the earliest publications (2002) until 30 January 2018 were reviewed for their findings on different risk factors of NCDs.
Results
According to the TLGS findings high sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), homocysteine (Hcy), age, smoking, hypertension, and obesity were the most important risk factors of cardiovascular diseases (CVD). It was illustrated that in subjects with abdominal obesity, the hs-CRP and IL-6 serum levels were higher than in normal subjects. The most appropriate prognostic indexes and associations were for hs-CRP, IL-6, and Hcy with abdominal obesity, waist circumference, WHtR, and wrist circumference, respectively. Previous studies have demonstrated a direct relationship between obesity and serum levels of inflammatory factors.
Conclusions
According to the results of TLGS, serum levels of biochemical risk factors such as hs-CRP, IL-6, and Hcy could be beneficial in early diagnosis and effective treatment of cardiovascular, obesity and other metabolic diseases.
Keywords: TLGS, Iran, Biochemistry, Inflammatory Factors, Obesity, Metabolic Syndrome
1. Context
The highest prevalence and incidence of non-communicable disease (NCD) is related to cardiovascular, diabetes, obesity, and metabolic syndrome (MetS). MetS is a common clinical disorder characterized by the presence of three or more of five feature i.e. obesity, hyperglycemia, hypertension, low high density lipoprotein-cholesterol (HDL-C), and hypertriglyceridemia (1). MetS is a risk factor for cardiovascular disease (CVD), type 2 diabetes (T2D), and cancer. The main role of inflammation in the growth and progression of atherosclerosis has been documented, important roles are attributed to inflammatory cytokines such as interleukin-6 (IL-6) and high sensitivity C-reactive protein (hs-CRP) (2). Recently, in addition to a prognostic factor for the development and progression of CVD, homocysteine (Hcy) has been proposed as a risk factor. Hcy levels increase to 30% in patients with atherosclerosis and only 12% elevation than normal is related with a 3-fold rise in the risk of myocardial infarction (3, 4). Obesity is a public health problem worldwide in both poor and rich communities, and is associated with NCDs. Adipose tissue stores surplus triglycerols and acts as an endocrine organ by releasing adipokines that play roles in regulating appetite, insulin resistance, glucose and lipid metabolism, and inflammation; chronic inflammation or excessive response can lead to harmful effects (5).
TLGS is based on community being conducted to investigate the prevalence of NCDs and to provide population-based criteria aimed at reducing the prevalence or prevention of a growing risk factors for NCD. This research consists of two main parts, a cross-sectional study on the prevalence of cardiovascular risk factors and a 20-year-old prospective cohort study, conducted in several phases at intervals of duration of 3.6 years from 1999, and is still ongoing. Participants included 15005 citizens, > 3 years, from district 13 of Tehran classified by cluster sampling. This area based on population distribution it represents the general population of Tehran (6).
Among various related risk factors assessed this study only reviewed data on the biochemical risk factors of NCD. Although risk factors such as lipid components (triglycerides, HDL-C, LDL-C, total cholesterol), glucose, insulin, thyroid stimulating hormone, and thyroxine can be considered as biochemical or molecular risk factors, but focus of this article is on CRP, IL-6 and Hcy and their associations with anthropometric parameters. Visfatin as a product of visceral adipose tissue, considered as a helpful Insulin mimic hormone, and its associations with the mentioned risk factors has also been reported.
2. Methods
All articles contain biochemical, or IL-6 or Hcy or CRP and TLGS in their title in PubMed, SID, ScienceDirect, and Medline from the earliest publications (2002) until 30 January 2018 were reviewed for their findings on different risk factors of NCDs.
3. Results
3.1. Biochemical Risk Factors
3.1.1. C-Reactive Protein (CRP)
CRP is an acute phase reactant and an inflammatory marker (7). Acute phase reactants are created in hepatocytes and their production is regulated by cytokines such as TNF-α and IL-6; CRP, which is known as a sensitive and classical acute phase reactant, is a highly susceptible inflammatory systemic marker, and its serum levels rapidly rises in response to various motivations (8). CRP increase is associated with cardiovascular risk. Vessel inflammation or maybe the renin-angiotensin system plays an important role in hypertension, and CRP concentrations are significantly higher in subjects with hypertension (9). It has been shown that CRP levels increase in patients with high body mass index (BMI). The largest data set available on obesity related to CRP is the Third National Health and Nutrition Examination Survey of the US population conducted between 1988 and 1994. Obesity increased an odds ratio (OR) for having greater CRP (2.13 for men and 6.21 for women) after adjusting for other variables (10). Anthropometric measurements including height, weight, hip circumference, waist circumference (WC), wrist circumference, waist to hip ratio (WHR), and waist to height ratio (WHtR) were recorded in each phase of the study. Abdominal obesity was defined by cutoff WC ≥ 91 cm for women and ≥ 89 cm for men.
Hosseinzadeh-Attar et al. on a subsample of TLGS project in a well matched case control study (37 MetS and 37 matched controls, 46.35 ± 1.6 years), determined associations of anthropometric, biochemical profiles, and CRP with Visfatin. No significant association between CRP and Visfatin serum level found, but a significant correlation between CRP with BMI, WC and WHR was found. Visfatin level was significantly lower in patients with MetS (11). Zarkesh et al. on 365 individuals (160 MetS and 205 matched controls) revealed that the levels of hs-CRP were higher in MetS subjects; an interesting finding was a slow and significant rise in the hs-CRP levels in association with increasing numbers of MetS components. The best predictors for the level of hs-CRP in the MetS subjects were hip, WHtR, and height (12, 13). Associations between inflammatory factors and obesity in TLGS, in a cross sectional study (132 Men and 222 women, 46.1 ± 16.1 years) were assessed by Faam et al, and obtained data showed a higher level of hs-CRP in the abdominally obese group (14, 15).
In TLGS, 80 diabetic individuals were selected randomly, and compared with 73 participants who did not have diabetes in two phases as controls. Who had diabetes during the study were more obese (central and general) and had higher fasting and two hours’ glucose and insulin resistance, compared to the control group, their serum levels of fasting insulin, CRP, triglycerides, systolic and diastolic blood pressure (SBP and DBP), total cholesterol (TC) ratio to HDL-C was higher and HDL-C levels were lower. In addition, family history of diabetes was more common in them. The highest correlation was found between CRP and BMI (r = 0.51, P < 0.01) and WC (r = 0.45, P < 0.01). CRP was also associated with systolic and diastolic blood pressure, fasting blood sugar (FBS), HOMA-IR, fasting insulin, total cholesterol and triglycerides, but had no significant correlation with HDL-C and 2-hours blood glucose levels. CRP values were equally divided into three parts of the total population. Conditional logistic regression analysis showed that type 2 diabetes mellitus, CRP was predicted by an adjusted model with age (OR = 3.6; CI 95%: 1.5 - 8.2, P = 0.02). The OR of diabetes (model-2), after adjusting with age, systolic blood pressure (SBP), triglycerides, HDL-C was (OR = 2.5; CI 95%: 1.08 - 6.15, P = 0.03). In model 3, after adjusting for age, SBP, triglycerides, HDL-C, HOMA-IR, the OR significantly decreased to 0.8 (CI 95%: 0.2 - 2.8, P = 0.7) (16).
Studies on the relation of CRP and CVD are inadequate to white populations of North America and Europe indicating the need for data on the clinical worth of CRP amount must be confirmed in populations of different ethnic groups and ages. For this purpose, a nested case-control study was conducted on participants of TLGS (126 cases with CVD, 259 control, > 35 years). The cumulative incidence of cardiovascular disease was 1.96% in the studied population and the risk profile of cardiovascular disease (other than BMI) was more adverse than controls. The median levels of CRP were 1.74 mg/L (inter-quartile range (IQR): 0.76 - 3.19 mg/L) for cases and 0.94 mg/L (IQR: 0.52 - 2.25 mg/L) for controls (P < 0.001). Low correlations were detected between CRP and BMI (r = 0.34, P < 0.01), WHR (r = 0.22, P < 0.01), Framingham risk score (FRS) (r = 0.27, P < 0.01). Multivariate logistic regression analysis to obtain the OR of CVD associated with highest quadrant of CRP compared with its lowest quadrant was used in four models. In model one, CRP was the only variable entered, OR of CVD for individuals in the highest CRP quadrant in comparison with the lowest quadrant was (OR = 2.6; CI 95%: 1.4 - 5.1). In the second model, adjustment for the CVD family history, smoking and WHR was not significantly different (OR = 2.3; CI 95%: 1.1 - 4.6, P for trend = 0.02). However, further adjustment with cardiovascular risk factors led to a significant reduction of risk estimate in the third model, (OR = 0.8; CI 95%: 0.3 - 1.9, P for trend = 0.2) or FRS in the fourth model (OR = 1.4; CI 95%: 0.7 - 2.9, P for trend = 0.2). To examine whether CRP increases the predictive value of previous models based on the risk factors of a typical CVD or FRS, the area under the ROC curve (AUC) was calculated and compared for the probability of different logistic regression models with and without the inclusion of CRP. Since, the AUC of a model is its ability to correctly identify cases with and without CVD, it was shown that addition amount of CRP to a model containing conventional CVD risk factors, which in a clinical work, could be easily documented with medical history, physical activity and lipid profile measurement hardly changes the AUC (ΔAUC = 0.006, P = 0.2). The introduction of CRP into another model based on FRS did not significantly change the AUC of the model (ΔAUC = 0.013, P = 0.2). Results indicated that a model based on common risk factors had a better mixture of sensitivity (44%), specificity (92.8%), PPV (76.5%), and NPV (75.6%) than the same model after inclusion of CRP 41.8, 92, 74.2 and 74.3%. In the FRS-included model, adding CRP level slightly improved specificity and PPV, but reduced sensitivity and NPV.
3.1.2. Interluekin-6
IL-6 is a cytokine that have an important role in acute phase reactions, hematopoiesis, bone metabolism, inflammation, energy homeostasis regulation, cancer progression, activity of lipoprotein lipase inhibition, and appetite control/energy absorption at the hypothalamic level. IL-6 is independently associated with cardiovascular risk factors such as hypertension, BMI, and reduction of HDL-C (17, 18). In smokers, the risk of cardiovascular disease is higher. Smoking appears to increase IL-6 production which stimulates CRP production. Trials, including European Concerted Action on Thrombosis and Disabilities study (ECAT), have described an increase in CRP levels in smokers. The Multiple Risk Factor Interventional Trial (MRIFT) study in middle-aged men without CVD indicated that CRP elevation was associated with an increase in CVD mortality (19). In a well matched case control studies (37 MetS and 37 matched controls) by Hosseinzadeh-Attar et al. on a subsample of TLGS project, association of anthropometric, biochemical profiles, and IL-6 with Visfatin were studied. A significantly correlations between IL-6 with BMI, WC, and WHR were found, and no significant association between IL-6 and Visfatin serum level (11). Zarkesh et al. in a subsample of the TLGS, in a cross-sectional study, 365 individuals (160 MetS and 205 matched controls, 46.1 ± 16.1 years), found that levels of IL-6 in MetS subjects was higher, and a significant and gradual increase in the level of IL-6 in association with increasing numbers of components in the MetS group. A strong linear augmentation was observed in the IL-6 levels as the numbers of MetS components increased. In addition, good predictors for the level of IL-6 in the MetS subjects were hip, WHtR and height (12). A higher level of IL-6 in the abdominally obese group were reported by Faam et al. in an association study between inflammatory factors and obesity in TLGS, in the cross sectional study (132 Men and 222 women) (14).
3.1.3. Homocysteine
Hcy is a thiol-containing intermediate metabolite; which in population studies over the past two decades provide evidences on, to direct and independent linkage of that in plasma with the morbidity and mortality from atherosclerosis. Possible mechanisms that link Hcy to atherogenesis include prothrombotic and pro-inflammatory effects, increased oxidative stress, endothelial dysfunction and smooth muscle cell proliferation. With clinical observations after the initial studies, this factor was included in the list of cardiovascular risk factors (20). Since obesity is considered as a low-grade inflammatory disease, in a cross sectional study, Faam et al. examined the association between Hcy (an inflammatory marker) with obesity-related factors such as BMI, waist, hip in 352 adult TLGS participants (220 women and 132 men, aged ≥ 19) randomly enrolled from the population. Linear regression analysis was applied to examine the association between Hcy, anthropometric and biochemical factors. Abdominal obesity was observed in 199 (56.5%) individuals and the level of Hcy was higher in the abdominally obese, the wrist was predictor for Hcy in obese and hip and WHtR were the best predictors for Hcy in the normal group (14). In a similar cross-sectional study, Zarkesh et al. on MetS subjects with a matched control group in a subsample of the TLGS (160 MetS and 206 controls), aged > 19 years (mean of 46.1 ± 16.1 years), the levels of Hcy was higher in subjects with MetS. The best predictor for Hcy level in subjects with MetS was WHtR (12).
3.2. Summary findings of Biochemical factors in TLGS
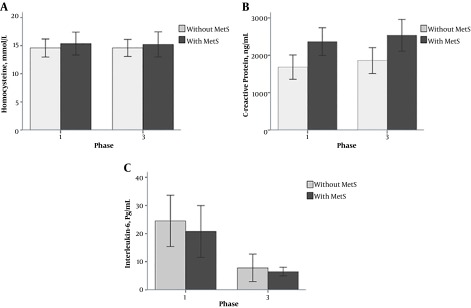
Previous studies in non-Iranian populations showed that the prevalence of obesity and its co-morbidities is increasing. To evaluate the level of inflammatory markers in subjects with and without abdominal obesity in Iranians, a cross-sectional study was designed on the basis of the TLGS. Of the 352 participants with an average age of 46.1 ± 16.1 years, 199 (56.5%) had abdominal obesity; in this group compared to those without abdominal study, mean of variables of obesity, lipid patterns and inflammatory factors, other than FBS and Hcy were higher. Based on Pearson correlation coefficient, there was a significant correlation between Hcy and height and wrist circumference in the group with abdominal obesity. The positive relationship of hs-CRP with BMI, waist circumference, hip circumference, WHtR, FBS and IL-6 and its negative correlation with height and wrist circumference were statistically significant. A positive correlation was shown between serum levels of IL-6 and WHtR and hs-CRP and a negative correlation was observed with height. In the normal group, a positive correlation was indicated between serum hs-CRP levels and age, BMI, WC, hip circumference, WHR, WHtR, SBP and DBP. Hcy and hs-CRP levels were negatively associated with hip circumference and height, respectively. Using the linear regression analysis, the most suitable predictive indexes for Hcy, hs-CRP, and IL-6 in the subjects with abdominal obesity, were wrist, WC and WHtR, respectively, while in the normal group, hip circumference and WHtR were the most appropriate predictors for Hcy and hs-CRP. In the normal group, since there was no correlated variable with IL-6, the predictive index could not be ascertained. Investigation of each inflammatory factors in the three groups of normal, abdominal or general obesity, and abdominal and general obesity groups revealed an increasing trend for hs-CRP and IL-6; hence after adjustment for age and sex in the linear regression analysis, an increase about 0.37 ng/mL (CI 95%: 0.24 - 0.48, P = 2.2 × 10-10) in the hs-CRP levels and 0.21 pg/mL (CI 95%: 0.10 - 0.33, P = 16 × 10-5) in the IL-6 levels was observed in the normal weight group compared to subjects with both general and abdominal obesity (Table 1). Comparison of hs-CRP, IL-6, and Hcy of phase 1 and 3 of TLGS in case and control groups was shown in Figure 1.
Table 1. Correlation Between Inflammatory Markers and Anthropometric/Biochemical Variables Among Subjects with and Without Abdominal Obesity.
| Variables | Hcy | hs-CRP | IL-6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AO | No AO | AO | No AO | AO | No AO | |||||||
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Sex | -0.211 | 0.003a | -0.227 | 0.005a | 0.168 | 0.018a | 0.112 | 0.169 | 0.087 | 0.221 | 0.092 | 0.258 |
| Age | -0.007 | 0.926 | 0.148 | 0.068 | 0.070 | 0.329 | 0.346 | 0.000a | 0.105 | 0.140 | 0.018 | 0.827 |
| Height | 0.200 | 0.005a | -0.111 | 0.177 | -0.181 | 0.010a | -0.209 | 0.010a | -0.139 | 0.050 | -0.035 | 0.665 |
| Weight | 0.081 | 0.256 | -0.020 | 0.806 | 0.480 | 0.505 | 0.112 | 0.169 | -0.010 | 0.890 | -0.097 | 0.232 |
| BMI | -0.107 | 0.133 | -0.117 | 0.149 | 0.204 | 0.004a | 0.288 | 0.000a | 0.137 | 0.053 | -0.061 | 0.452 |
| WC | -0.007 | 0.918 | -0.015 | 0.849 | 0.175 | 0.014a | 0.312 | 0.000a | 0.088 | 0.214 | 0.056 | 0.493 |
| Hip | -0.072 | 0.310 | -0.209 | 0.010a | 0.163 | 0.022a | 0.239 | 0.003a | 0.111 | 0.120 | 0.054 | 0.511 |
| Wrist | 0.225 | 0.001a | 0.159 | 0.490 | -0.139 | 0.050 | 0.094 | 0.247 | -0.065 | 0.358 | -0.126 | 0.121 |
| WHR | 0.010 | 0.161 | 0.152 | 0.060 | 0.004 | 0.959 | 0.166 | 0.040a | -0.043 | 0.545 | 0.020 | 0.803 |
| WHtR | -0.123 | 0.085 | -0.063 | 0.442 | 0.233 | 0.001a | 0.366 | 0.000a | 0.154 | 0.030a | 0.068 | 0.406 |
| FBS | -0.114 | 0.109 | 0.105 | 0.198 | 0.174 | 0.014a | 0.069 | 0.396 | 0.123 | 0.084 | -0.146 | 0.071 |
| TG b | -0.103 | 0.149 | 0.068 | 0.436 | 0.051 | 0.473 | 0.284 | 0.000a | -0.054 | 0.452 | -0.034 | 0.673 |
| TC | 0.099 | 0.163 | 0.085 | 0.297 | 0.150 | 0.035a | 0.403 | 0.000a | 0.002 | 0.980 | -0.032 | 0.694 |
| HDL-C | -0.155 | 0.028a | -0.033 | 0.683 | -0.009 | 0.897 | 0.109 | 0.181 | 0.064 | 0.372 | 0.078 | 0.335 |
| LDL-C | -0.006 | 0.930 | 0.750 | 0.359 | 0.156 | 0.029a | 0.345 | 0.000a | 0.013 | 0.859 | -0.056 | 0.489 |
| TG/HDL-C | 0.080 | 0.260 | 0.069 | 0.395 | 0.004 | 0.952 | -0.004 | 0.962 | -0.048 | 0.497 | -0.082 | 0.316 |
| TC/HDL-C | 0.049 | 0.491 | 0.109 | 0.182 | 0.098 | 0.167 | 0.229 | 0.004a | -0.037 | 0.605 | -0.094 | 0.247 |
| LDL-C/HDL-C | 0.118 | 0.099 | 0.098 | 0.226 | 0.115 | 0.110 | 0.220 | 0.006a | -0.028 | 0.696 | -0.105 | 0.197 |
| SBP | 0.002 | 0.975 | 0.091 | 0.270 | -0.004 | 0.958 | 0.190 | 0.020a | 0.123 | 0.085 | 0.007 | 0.932 |
| DBP b | 0.005 | 0.947 | 0.470 | 0.566 | -0.056 | 0.433 | 0.297 | 0.001a | 0.081 | 0.262 | -0.099 | 0.227 |
| Hcy b (mmol/L) | - | - | - | - | 0.158 | 0.414 | 0.021 | 0.797 | -0.007 | 0.921 | -0.103 | 0.207 |
| hs-CRP b (ng/mL) | 0.058 | 0.414 | 0.210 | 0.797 | - | - | - | - | 0.157 | 0.026a | 0.014 | 0.859 |
| IL-6 b (pg/mL) | -0.007 | 0.921 | -0.103 | 0.207 | 0.157 | 0.026a | 0.014 | 0.859 | - | - | - | - |
Abbreviations: AO, abdominal obesity; BMI, body mass index; DBP, diastolic blood pressure; FBS, fasting blood sugar; Hcy, homocysteine; HDL-C, high density lipoprotein cholesterol; hs-CRP, C-reactive protein; IL-6, interleukin-6; LDL-C, low density lipoprotein cholesterol; r, Pearson correlation (adjusted from ref. 6); SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WC, waist circumference; WHR, waist to hip ratio; WHtR, waist to height ratio.
a P < 0.05.
b Logarithmic transformation.
Figure 1. The mean levels of A, homocysteine; B, C-reactive protein; C, interleukin-6 in the subjects with and without MetS in phases 1 and 3 of the TLGS; Error bars: ± SE.
4. Discussion
In this study, association between abdominal obesity and inflammatory factors, it was found that the hs-CRP and IL-6 serum levels in patients with abdominal obesity were higher than in normal subjects. The most appropriate prognostic indexes for hs-CRP, IL-6, and Hcy were abdominal obesity, WC, WHtR, and wrist circumference, respectively. Previous studies have shown a direct relationship between obesity and serum levels of inflammatory factors. For example, a study by Stelzer et al. (21) indicated a positive correlation between IL-6 and the degree of overweight and obesity and suggested leptin and WHR to the best predictors of IL-6. Probably because the fact that abdominal fat tissue is one of the main sources of production of TNF-α and IL-6 cytokines, which, by stimulating the liver, increase the production of hs-CRP. The findings of a study on the association between hs-CRP and METS components showed that WC is the most appropriate indicator for predicting hs-CRP in the population under study. Other study by Vaya et al. (22) on Spanish adults showed that increased levels of Hcy were associated with abdominal obesity and insulin resistance. Contrary to these results, in the TLGS-related study, Hcy serum level was not correlated with an increase in obesity; an alteration which could be explained by variations in the ratio of adipose/muscle tissue in obese subjects and changes in the amount of oxidation of cells. However, this study emphasized that obesity, especially abdominal obesity, is a low-grade inflammatory disease.
In TLGS-dependent study, in subjects with and without MetS, inflammatory factors were compared in subjects with MetS and normal (after adjustment for age and sex) and a significant increase in the levels of hs-CRP, Hcy, and IL-6 was observed in subjects with MetS. It was also found that risk markers increased significantly in association with increasing numbers of MetS components. Multiple linear regression analysis showed that hip circumference and WHtR are the main independent variables in relation to the level of hs-CRP and IL-6 in subjects with MetS, respectively; a positive relationship between inflammatory factors of hs-CRP, IL-6, and WHtR and a negative association between Hcy and WHtR among the subjects with MetS was also found. WHtR is significantly correlated with all risk factors for obesity and MetS, and in longitudinal studies often acts better than BMI in predicting mortality and morbidity. In addition, WHtR can be more sensitive than WC due to adjustments for different statures and a negative correlation between height and certain metabolic risk factors in different populations. The Yusuf et al (23) in a case-control study conducted with < 300,000 participants, emphasized the importance of the WHtR parameter over BMI and WC for predicting MetS. The association between hip circumference and MetS can be explained by considering the effect of waist circumference. The risk of MetS attributed to the waist circumference might be underestimated, regardless of the effect of the hip circumference. WHtR and hips have already been shown to have an independent and opposite effects on metabolic risk factors. While WHtR has a positive correlation with health risk factors, the hip circumference is negatively correlated, which refers to the supportive effect of the hip circumcision, that is probably the result of a higher amount of lean mass in non-abdominal regions. Hence, for a given WHtR, the higher hip circumference does not indicate a health risk. Using these findings, one can find a way to use WHtR and hip circumference to predict metabolic disturbances. The components of MetS are individual and groups of risk factors for morbidity and mortality. People with MetS are twice as likely to die from cardiovascular disease and three times more likely to have a heart attack or stroke. The intra-abdominal fat plays a major role in cardiometabolic risks. Visceral fat is a metabolically active tissue that produces prothrombotic and pro-inflammatory cytokines. Furthermore, in the Jackson Heart Study, fatty liver and abdominal visceral adipose tissues were independent links of cardiometabolic risks (24).
In investigating the relationship between CRP and CVD, the effect of common CVD risk factors in various models with and without CRP in predicting the incidence of CVD, over a three year follow-up presented that CRP levels predict the incidence of CVD in middle aged Iranians, but not independently from the risk factors of traditional CVD commonly used in clinical settings. In other words, CRP measurements in middle-aged Iranian populations will not improve cardiovascular risk prediction (25). Several cohort studies have shown a high correlation for CRP with an increased CVD risk. In a meta-analysis performed by Danesh et al. (26) it was shown that after adjustment for established CVD risk factors, the adjusted combined multivariate OR for CHD was 1.58 (CI 95%: 1.48 - 1.68). Studies issued after this meta-analysis reported a similar relative risk for CRP, except in the study of Rotterdam's elderly population, where high CRP levels were not associated with an increased risk of cardiovascular disease; on the other hand, a moderate but significant correlation was observed between CRP levels and common risk factors of CVD which has also been observed in other studies. Hence, univariate dependence between CRP and CVD can be clarified by the correlation between CRP and other risk factors; CRP significantly contributed to the increased risk of CVD, only regarding status of smoking. This finding is similar to the recent American Heart Association's declaration that measurement of inflammatory factors may help recognize people who most likely benefit from changing their lifestyle. Like most other studies, adding CRP to AUC did not improve Framingham's risk function. These findings confirmed the results of the Rotterdam study that was conducted in an elderly population.
The results of the study on the role of CRP in predicting type 2 diabetes in a TLGS-dependent study showed that increased the levels of CRP was associated with an increased risk of increasing T2D, although this is independent of other diabetes risk factors such as FBS, familial history of diabetes, BMI, and HOMA-IR, so that the OR of having diabetes after adjustment for age of the highest tertile was 3.6 (CI 95%: 1.5 - 8.2, P = 0.001), after adjustment for FBS, familial history of diabetes, BMI, and HOMA-IR, this OR was significantly reduced (OR = 0.8; CI 95%: 0.2 - 2.8, P = 0.8). However, given that CRP had a strong association with BMI, FBS and HOMA-IR, the effect of CRP on prediction of diabetes after adjustment with these variables was not significant. In a study, Festa et al. (27) entitled “insulin resistance atherosclerosis study”; it was found that CRP was not significantly correlated with diabetes after adjusting for BMI and WC. In the MONICA cohort study, to investigate the association between CRP and incident diabetes mellitus among middle-aged men, the OR for diabetes after adjustment for age and survey, associated with the highest CRP quartile was 7.2 times higher than the lowest quartile, which was not statistically significant after adjustment for BMI, cigarette smoking, and SBP (28). There are also studies that reported a strong and independent relationship between CRP and type 2 diabetes, such as a case-control study of Hu et al. (29) in a large population (737 diabetics and 785 control subjects). In the women's health studies with 188 diabetics and 362 controls, by Pradhan et al. (30) CRP was introduced as a predictor of diabetes in women.
Acknowledgments
This work supported by Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The authors wish to acknowledge Ms. Niloofar Shiva for critical editing of English grammar and syntax of the manuscript.
Footnotes
Authors’ Contribution:Study concept and design: Mehdi Hedayati and Fereidoun Azizi; acquisition of data: Maryam Zarkesh and Bita Faam; analysis and interpretation of data: Maryam Sadat Daneshpour; drafting of the manuscript: Mehdi Hedayati, Marjan Zarif Yeganeh, and Sara Sheikholeslami; critical revision of the manuscript for important intellectual content: Fereidoun Azizi and Mehdi Hedayati; statistical analysis: Maryam Zarkesh and Bita Faam; study supervision: Fereidoun Azizi.
Conflict of Interests:The authors do not report any relevant conflict of interests.
Funding/Support:This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Mehdi Hedayati, Email: hedayati@endocrine.ac.ir.
Maryam Sadat Daneshpour, Email: daneshpour@endocrine.ac.ir.
Maryam Zarkesh, Email: zarkesh@endocrine.ac.ir.
Marjan Zarif Yeganeh, Email: yeganeh.marjan@gmail.com.
Sara Sheikholeslami, Email: sara.sheikholeslami@gmail.com.
Bita Faam, Email: bitafaam@yahoo.com.
Fereidoun Azizi, Email: azizi@endocrine.ac.ir.
References
- 1.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Tohidi M, Hadaegh F, Harati H, Azizi F. C-reactive protein in risk prediction of cardiovascular outcomes: Tehran lipid and glucose Study. Int J Cardiol. 2009;132(3):369–74. doi: 10.1016/j.ijcard.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 3.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agoston-Coldea L, Mocan T, Dobie L, Marginean A, Lupu S. The association between homocysteine level and metabolic syndrome in patients of prior myocardial infarction. Rom J Intern Med. 2010;48(2):151–8. [PubMed] [Google Scholar]
- 5.Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: An endocrine organ. Arch Med Sci. 2013;9(2):191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azizi F, Ghanbarian A, Momenan AA, Hadaegh F, Mirmiran P, Hedayati M, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran lipid and glucose study phase II. Trials. 2009;10:5. doi: 10.1186/1745-6215-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ferranti S, Rifai N. C-reactive protein and cardiovascular disease: A review of risk prediction and interventions. Clin Chim Acta. 2002;317(1-2):1–15. doi: 10.1016/S0009-8981(01)00797-5. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–7. doi: 10.1161/01.CIR.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 9.Heald AH, Anderson SG, Ivison F, Laing I, Gibson JM, Cruickshank K. C-reactive protein and the insulin-like growth factor (IGF)-system in relation to risk of cardiovascular disease in different ethnic groups. Atherosclerosis. 2003;170(1):79–86. doi: 10.1016/S0021-9150(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 10.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 11.Hosseinzadeh-Attar MJ, Golpaie A, Foroughi M, Hosseinpanah F, Zahediasl S, Azizi F. The relationship between visfatin and serum concentrations of C-reactive protein, interleukin 6 in patients with metabolic syndrome. J Endocrinol Invest. 2016;39(8):917–22. doi: 10.1007/s40618-016-0457-1. [DOI] [PubMed] [Google Scholar]
- 12.Zarkesh M, Faam B, Daneshpour MS, Azizi F, Hedayati M. The relationship between metabolic syndrome, cardiometabolic risk factors and inflammatory markers in a Tehranian population: The Tehran lipid and glucose study. Intern Med. 2012;51(24):3329–35. doi: 10.2169/internalmedicine.51.8475. [DOI] [PubMed] [Google Scholar]
- 13.Zarkesh M, Faam B, Daneshpour MS, Azizi F, Hedayati M. [Association between metabolic syndrome and hs-CRP, Hcy, and IL-6 levels.]. Iran J Diabet Metabol. 2013;12(6):564–73. [Google Scholar]
- 14.Faam B, Zarkesh M, Daneshpour MS, Azizi F, Hedayati M. The association between inflammatory markers and obesity-related factors in Tehranian adults: Tehran lipid and glucose study. Iran J Basic Med Sci. 2014;17(8):577–82. [PMC free article] [PubMed] [Google Scholar]
- 15.Faam B, Zarkesh M, Daneshpour MS, Azizi F, Hedayati M. [Association between abdominal obesity and HS-CRP, IL-6 and HCY in Tehranian adults: TLGS.]. Iran J Diabet Metabol. 2014;13(2):163–71. [Google Scholar]
- 16.Ebrahim M, Tohidi M, Hadaegh F, Azizi F. [Role of C-reactive protein in prediction of type 2 diabetes: Tehran lipid and glucose study.]. Iran J Endocrinol Metabol. 2008;10(1):11–6. [Google Scholar]
- 17.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu DC, Ma YF, Hur S, Li D, Rupert A, Scherzer R, et al. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive antiretroviral therapy. AIDS. 2016;30(13):2065–74. doi: 10.1097/QAD.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol. 2009;53(3):317–33. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Catena C, Colussi G, Nait F, Capobianco F, Sechi LA. Elevated homocysteine levels are associated with the metabolic syndrome and cardiovascular events in hypertensive patients. Am J Hypertens. 2015;28(7):943–50. doi: 10.1093/ajh/hpu248. [DOI] [PubMed] [Google Scholar]
- 21.Stelzer I, Zelzer S, Raggam RB, Pruller F, Truschnig-Wilders M, Meinitzer A, et al. Link between leptin and interleukin-6 levels in the initial phase of obesity related inflammation. Transl Res. 2012;159(2):118–24. doi: 10.1016/j.trsl.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Vaya A, Rivera L, Hernandez-Mijares A, de la Fuente M, Sola E, Romagnoli M, et al. Homocysteine levels in morbidly obese patients: Its association with waist circumference and insulin resistance. Clin Hemorheol Microcirc. 2012;52(1):49–56. doi: 10.3233/CH-2012-1544. [DOI] [PubMed] [Google Scholar]
- 23.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet. 2005;366(9497):1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: The Jackson heart study. Arterioscler Thromb Vasc Biol. 2011;31(11):2715–22. doi: 10.1161/ATVBAHA.111.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazemi-Saleh D, Koosha P, Sadeghi M, Sarrafzadegan N, Karbasi-Afshar R, Boshtam M, et al. Predictive role of adiponectin and high-sensitivity C-reactive protein for prediction of cardiovascular event in an Iranian cohort Study: The Isfahan cohort study. ARYA Atheroscler. 2016;12(3):132–7. [PMC free article] [PubMed] [Google Scholar]
- 26.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 27.Festa A, D'Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The insulin resistance atherosclerosis study. Kidney Int. 2000;58(4):1703–10. doi: 10.1046/j.1523-1755.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- 28.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: Results from the MONICA (monitoring trends and determinants in cardiovascular disease) augsburg cohort study, 1984 to 1992. Circulation. 1999;99(2):237–42. doi: 10.1161/01.CIR.99.2.237. [DOI] [PubMed] [Google Scholar]
- 29.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 30.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]