Abstract
Acute myocardial infarction (AMI) is a life-threatening event. Even with timely treatment, acute ischemic myocardial injury and ensuing ischemia reperfusion injury (IRI) can still be difficult issues to tackle. Apart from radiological and other auxiliary examinations, laboratory tests of applicable cardiac biomarkers are also necessary for early diagnosis and close monitoring of this disorder. Heart-type fatty acid binding protein (H-FABP), which mainly exists inside cardiomyocytes, has recently emerged as a potentially promising biomarker for myocardial injury. In this review we discuss the sensitivity and specificity of H-FABP in the assessment of myocardial injury and IRI, especially in the early stage, and its long-term prognostic value in comparison with other commonly used cardiac biomarkers, including myoglobin (Mb), cardiac troponin I (cTnI), creatine kinase MB (CK-MB), C-reactive protein (CRP), glycogen phosphorylase isoenzyme BB (GPBB), and high-sensitivity cardiac troponin T (hs-cTnT). The potential and value of combined application of H-FABP with other biomarkers are also discussed. Finally, the prospect of H-FABP is summarized; several technical issues are discussed to facilitate wider application of H-FABP in clinical practice.
Keywords: acute myocardial infarction, post-ischemic myocardial injury, ischemia reperfusion injury, heart-type fatty acid binding protein, cardiac biomarkers
Introduction
Coronary artery disease (CAD) is a type of cardiovascular disease that is common worldwide and can lead to severe adverse cardiac events in most cases. Impaired coronary perfusion (angina) is often triggered by exercise or psychological stress, and the symptoms usually last less than half an hour. However, complete occlusion of a coronary artery will lead to permanent damage of the underlying cardiac muscle if not treated promptly (myocardial infarction). These severe adverse cardiac events usually present as chest pain and shortness of breath. Diabetes, hypertension, smoking, hypercholesterolemia, and genetic makeup are all possible risk factors for CAD. Confirmation of diagnosis may require electrocardiography (ECG) and coronary angiography (CAG). Rarely, patients with CAD might have no symptoms at all. However, in many cases of coronary occlusion, patients will develop arrhythmia and progress to cardiac failure or even sudden death1. Those who manage to survive a myocardial infarction can still suffer from post-ischemic myocardial injury, with their cardiac function seriously compromised. The immediate and most important way to address myocardial infarction is to restore the blood supply to the ischemic heart via percutaneous coronary intervention (PCI) therapy, thrombolysis or coronary artery bypass grafting (CABG). Paradoxically, even timely restoration of coronary blood flow may cause further injury to the ischemic heart, a phenomenon that is termed ischemia reperfusion injury (IRI)2. In 2010, CAD alone was reported to cause more than 7 000 000 deaths globally, even with the advancement of biomedical research and modern technology3, representing a great burden on human health and, subsequently, on society. Previously, clinicians have mainly focused on the treatment of CAD with less effort made to efficiently reduce the incidence of relevant complications. Therefore, more attention will need to be paid to the prevention and early treatment of CAD-related complications.
Since it often takes several hours or even days from the presentation of relevant symptoms to the application of CAG in most patients, auxiliary examination methods, such as ECG, are normally arranged upon patients' visits and can play a vital role in the prediction and even early diagnosis of CAD during this crucial time interval. Additionally, detection of changes in the plasma levels of cardiac biomarkers is routinely used at the early stage of disease onset as it may be difficult to differentiate symptoms from other less important events such as gastroesophageal reflux and muscle strains. In clinical practice, these cardiac biomarkers are helpful for clinicians to make appropriate decisions before the final diagnosis is confirmed. However, as different biomarkers may vary greatly in terms of their respective sensitivity and specificity, it is necessary to continue to search for new types of cardiac biomarkers that can facilitate a faster prediction of immediate and also long-term post-ischemic myocardial injury.
Cardiomyocytes are rich in various proteins and enzymes, including myoglobin (Mb), troponin, creatine kinase (CK), lactic dehydrogenase (LDH), and creatine kinase MB (CK-MB). When cardiomyocytes are damaged, these proteins and enzymes can be released and distributed into the blood flow following cardiomyocyte necrosis and break-down. Hence, the elevation of certain relevant cardiac proteins and enzymes in plasma might indirectly signify myocardial injury and, to some extent, can even reflect its severity.
Currently, cardiac troponin I (cTnI), Mb, and CK-MB are the three biomarkers that are most often used in the hospital to assess the possibility of acute myocardial infarction (AMI) among suspected patients with typical symptoms, such as chest pain. cTnI is a highly specific biomarker for AMI; however, it is not very sensitive in the first hours after AMI. Mb, however, is rather sensitive and is elevated approximately within 3 h after AMI. However, its cardiac specificity is limited, as an injury to the striated muscles in other parts of the human body will also release Mb into blood and raise its plasma concentration. For decades, CK-MB has been considered the most widely accepted biomarker for myocardial injury worldwide. However, despite its popularity, CK-MB is not as sensitive as believed, particularly within 6 h after the onset of AMI. Although the combination of detection and close monitoring of these three markers has proven effective for diagnosing AMI, there is still an urgent need to look for new biomarkers with higher sensitivities and specificities, especially in the very early stage of myocardial injury.
Heart-type fatty acid binding protein (H-FABP) is a type of intra-cardiac protein that plays an essential part in the metabolism of fatty acid (FA) inside cardiomyocytes4. H-FABP was first reported to be a potential indicator for myocardial injury in 19885. In recent years, there has been increasing interest in the potential role of H-FABP as a biomarker for immediate myocardial injury and even for relatively long-term post-ischemic prognosis. Studies have shown that H-FABP is extraordinarily sensitive in the first hours after myocardial injury6 and during reperfusion injury7. The present study reviews the basic characteristics, potential roles, and relevant research studies regarding H-FABP to discuss whether it is suitable to serve as a sensitive and reliable biomarker for acute myocardial injury and, if possible, for long-term post-ischemic prognosis in comparison with other markers that are routinely used in clinical practice.
Post-ischemic myocardial injury/dysfunction
CAD affects millions of people throughout the world and is widely believed to be the most common cause of mortality worldwide8. As a result of the improvement in modern medicine and increased awareness of public health, there has been a trend of an apparent reduction in both the incidence and mortality of CAD in developed countries over the past decade9. By contrast, the situation in developing countries, unfortunately, is getting worse in that both the prevalence and mortality of CAD are continuously increasing10. Proper management of post-ischemic myocardial injury and relevant complications might help to reduce the incidence of adverse events among patients with CAD.
For patients with atherosclerosis, there is a high chance of AMI and development of post-ischemic myocardial complications. During the pathological process, the endothelium of an atherosclerotic coronary artery can become hardened, making its wall less elastic and thus limiting the volume of blood flow. Additionally, with the deposition of calcium phosphate, cholesterol, fatty acids and formation of foam cells transformed from macrophages, a typical plaque forms. As the plaque continues to grow, the lumen of the coronary artery becomes narrower, reducing the volume of blood flow, leading to myocardial ischemia. At this stage, patients can have angina pectoris and typical chest pain will present in most cases. Taking a rest and/or application of medications, such as nitroglycerin, are normally helpful to relieve symptoms. However, the condition will worsen as the plaque grows larger and the coronary artery lumen becomes extremely narrow. There is a very high chance of AMI when more than 75% of the coronary artery luminal area is blocked. However, AMI is still possible even when the plaque does not block 75% of the coronary artery luminal area as it can become unstable if there is necrosis and a rupture of the intima. Thrombus in the plaque can either cause local occlusion or detach from the original site and then travel as an embolus to cause an occlusion of other smaller segments of coronary arteries. When one or more of the branches of the coronary arteries are occluded, there will only be a tiny amount of or, in severe cases, no blood flow to nourish the corresponding part of the heart. Without enough oxygen and essential nutrients, the regional cardiomyocytes supplied by the occluded coronary artery will immediately suffer from ischemic injury. ATP production and cellular metabolic functions can be severely interrupted. As the ischemia continues, the injury to cardiomyocytes will become irreversible and massive cell death is inevitable, which is what occurs during AMI or a heart attack.
After the diagnosis of AMI is confirmed, PCI or CABG will be scheduled for patients to recanalize the occluded coronary artery. However, restoring blood perfusion to coronary circulation is often followed by IRI, a result of oxidative and inflammatory injuries caused by oxidative stress11, which results in further tissue damage of the infarcted heart. Therefore, attention needs to be paid to protecting patients from further injuries following revascularization. Moreover, patients with CAD and concomitant diabetes mellitus are even more vulnerable to myocardial IRI as they are less or not sensitive to cardioprotective interventions, such as ischemic pre- or post-conditioning, that are otherwise effective for patients without diabetes mellitus12,13,14. However, the exact mechanism underlying the above phenomenon has yet to be identified. There have been studies suggesting that a reduction in the level of adiponectin may be attributable to the loss of cardio-protection against IRI during hyperglycemia. Our recent study conducted on rats with type I diabetes mellitus revealed that hyperglycemia impaired the Adiponectin/Caveolin-3/STAT3 pathway and thus diminished ischemic post-conditioning protection15. However, there is still much work to be done to comprehensively clarify this issue. Given that scientific research, in an effort to identify an effective treatment for IRI, is still ongoing, the need to seek novel sensitive biomarkers to reflect or even predict the severity of IRI should not be neglected. This review, therefore, will also discuss the potential of H-FABP as a marker to evaluate IRI to facilitate corresponding management.
Commonly used myocardial markers
Mb, cTnI, and CK-MB are the most common biomarkers used to assess myocardial injury in hospitals. Other markers include C-reactive protein (CRP), glycogen phosphorylase isoenzyme BB (GPBB), and high-sensitivity cardiac troponin T (hs-cTnT). The sensitivity, specificity, and prognostic value of these biomarkers are presented and compared in Table 1.
Table 1.
Comparisons of several commonly used cardiac biomarkers.
| Mb | cTnI | CK-MB | CRP | GPBB | hs-cTnT | H-FABP | |
|---|---|---|---|---|---|---|---|
| High sensitivity | √ | √ | – | – | √ | √ | √ |
| High specificity | – | √ | – | – | – | – | √ |
| Good prognostic value | – | √ | – | – | – | √ | √ |
Mb: myoglobin; cTnI: cardiac troponin I; CK-MB: creatine kinase MB; CRP: C-reactive protein; GPBB: glycogen phosphorylase isoenzyme BB; hs-cTnT: high-sensitivity cardiac troponin T; H-FABP: heart-type fatty acid binding protein.
Mb
Mb is a type of protein that is detected inside muscle tissues and is capable of binding oxygen and iron and carrying them during metabolism16. Mb is mainly found in the myocardium and skeletal muscles. Therefore, the presence of Mb in the plasma might indicate a muscle injury. Since Mb has a relatively small molecular weight, it is rapidly released into the circulatory system after muscle damage. The plasma concentration of Mb can be elevated 2–4 h after AMI and can peak approximately 6 h after AMI17. Hence, Mb is regarded as one of the most sensitive biomarkers for AMI, especially at the early stage. However, its specificity can be disappointing in some situations, as Mb exists in all striated muscles. It is difficult to determine whether an increased Mb plasma concentration is the consequence of a myocardial injury or skeletal muscle injury. Elevation of the Mb level can also be seen in patients with impaired kidney function when the kidney fails to filter Mb efficiently18. As a result, an Mb test alone can only be treated as a screening method to rule out AMI. It is advised that Mb should be incorporated with other tests to diagnose AMI. For example, the combination of Mb and carbonic anhydrase III can make Mb a more specific biomarker for AMI even at an early stage19. Mb is also highly sensitive during post-ischemic reperfusion injury; a previous study showed that Mb can be used as an effective marker to identify the patency of a coronary artery following ischemic insult20. Researchers have attempted to use Mb as a prognostic marker for AMI21,22,23. However, the results were not satisfactory possibly because of the fast clearance of Mb from the plasma.
cTnI
cTnI is a small-sized molecule that exists in the heart muscle. It has a molecular weight of approximately 23.9 kDa. The plasma concentration of cTnI in normal human subjects is rather low or even undetectable. Consequently, cTnI has been the most specific biomarker for AMI during the last two decades. The sensitivity of cTnI is very high, though it is not released into blood as quickly as Mb during myocardial ischemia/reperfusion. Therefore, cTnI has been accepted by clinicians as the best biomarker, and to date, it is even better than the recognized golden biomarker CK-MB for diagnosing AMI. Myocardial injury is highly suspected if the plasma level of cTnI exceeds the upper limit. Irreversible heart muscle cell death is considered if the plasma concentration of cTnI continues to increase. However, the chance of AMI is small if the plasma level of cTnI remains negative for hours.
cTnI, but not other markers, has also been incorporated into new assays to diagnose unstable angina24 and identify relevant long-term adverse events25. Additional studies support the prognostic significance of cTnI for patients with acute coronary syndrome (ACS)26,27. One meta-analysis showed that high-sensitive troponin assays had a fairly good prognostic value for AMI as well28. Benamer et al 29 compared the prognostic significance between cTnI and CRP and found that the levels of plasma cTnI within 24 h after admission could independently forecast a patient's prognosis.
CK-MB
CK-MB is one of the three isoforms of the enzyme creatine kinase (CK). CK is not specific to AMI, as it exists in cerebral, myocardial, and skeletal tissues. In some cases with mild myocardial infarction, CK elevation might not be detected, indicating its lack of satisfactory sensitivity. Therefore, the CK test has been gradually abandoned and replaced by CK-MB because it is more specific in the heart muscle. Actually, CK-MB has already been recognized as the golden marker for AMI for the past two decades. The plasma concentration of CK-MB increases 4–6 h after AMI and reaches the highest level within 24 h. Usually, the CK-MB level will return to a normal range 48–72 h after the onset of AMI. As a biomarker, CK-MB is fast, economical, and efficient for AMI diagnosis and thus has been widely applied in clinical practice globally. Some studies also proved that CK-MB can be used to estimate the infarct size30,31,32 and even the left ventricular ejection fraction33. In addition, CK-MB can also serve as a marker for IRI. Numerous clinical trials have adopted CK-MB as an auxiliary examination for the assessment of reperfusion injury after restoration of coronary circulation34,35. In addition, CK-MB has been considered to have prognostic significance for post-ischemic mortality and long-term death36,37. However, Domanski et al 38 showed that troponin T is a better prognostic factor than CK-MB for long-term postoperative mortality after CABG.
However, CK-MB has some drawbacks when utilized as a marker for AMI. The specificity of CK-MB, though much better than that of CK, is still not favorable, as skeletal disorders can cause CK-MB elevation as well. Additionally, the sensitivity of CK-MB is compromised within 6 h after AMI, making it inappropriate as an early predictor. Studies also have demonstrated that CK-MB is a less eligible biomarker than cTnI for the diagnosis of minor myocardial injury39,40. Yet, CK-MB remains the most popular AMI biomarker despite the fact that new biomarkers are continuously reported.
CRP
It has been gradually acknowledged that the inflammatory response plays a key role in myocardial infarction41. CRP is a common marker for inflammation. It originates from the liver and is triggered by the cytokines that are secreted by adipocytes and macrophages. It is reasonable to correlate CRP to myocardial injury42. Circulating CRP has long been recognized as a promising indicator for adverse coronary events among patients with either stable or unstable angina pectoris43. CRP can also be an excellent prognostic factor for myocardial infarction. Mather et al 44 claimed that the post-operative CRP level 2 days after PCI can serve as the strongest biomarker to predict left ventricular remodeling. Liu et al 45 found that the combination of complementary and high-sensitivity CRP measurements after AMI can efficiently forecast left ventricle dysfunction. While CRP is valuable as a predictor for myocardial injury, consensus and a standardized criterion have yet to be reached for its wide application as a cardiac biomarker in the hospital.
GPBB
As an isoform of glycogen phosphorylase, GPBB has an essential role in the process of glycogenolysis. GPBB is mainly found in heart and brain tissues and is responsible for providing fuel for energy production during muscle contraction. During myocardial ischemia, GPBB accelerates glycogenolysis for anaerobic glycolysis. Additionally, GPBB can be detached from glycogen and released into the plasma when cell membrane permeability increases following myocardial ischemic insult. GPBB is very sensitive as its plasma concentration usually increases within 1–4 h after AMI, making it comparable to Mb in terms of sensitivity. Previously, large amounts of evidence showed that GPBB could be a promising biomarker for myocardial infarction46,47,48. However, a group of studies with negative findings have gradually been reported. Mion et al 49 and McCann et al 50 were both surprised to find that the combination of troponin and GPBB did not improve the sensitivity of troponin alone. In 2011, a large-scale clinical trial conducted by Keller et al 51 did not support the diagnostic value of GPBB as an early biomarker for AMI. Yet, GPBB remains a possible prognostic factor for patients with suspected acute coronary syndrome (ACS). Lillpopp et al 52 measured the GPBB levels in patients with chest pain upon admission and found that GPBB is valuable in improving the mid-term prognosis in these patients. Given that increasing evidence has denied the diagnostic value of GPBB for myocardial injury, further research is needed to assess its prognostic value.
hs-cTnT
Troponin T is one of the subtypes of troponins, and cardiac troponin T (cTnT) is a type of heart-specific structural protein that is found inside cardiomyocytes. Similar to cTnI, cTnT is released into plasma when there is a myocardial injury or cardiomyocyte death. With the advancement of technology, the detection of cTnT can be carried out using hypersensitive methods, ie, the hs-cTnT assay, to obtain even more sensitive results. The sensitivity of hs-cTnT is excellent as it is elevated and can be detected within 3 h after AMI. Thus, detection of hs-cTnT can help diagnose or exclude AMI at an early stage. In recent years, hs-cTnT has gained popularity in hospitals as a new cardiac biomarker. A multicenter study conducted from 2006 to 2008 confirmed the diagnostic value of hs-cTnT in the early diagnosis of AMI among patients with recent chest pain53. Moreover, hs-cTnT is capable of detecting micro infarcts or minimal necrosis with excellent diagnostic accuracy, eg, among patients with non-ST-segment elevation myocardial infarction, which cannot be detected by conventional assays54,55.
Although cTnT is heart tissue-specific, there are a few problems involving the specificity of hs-cTnT. It is reasonable that an increase in the sensitivity over the traditional cTnT assay will likely result in a decrease in its specificity. Thus, there might be greater false positive results in the diagnosis of AMI as the elevation of hs-cTnT is not necessarily relevant to ischemic causes56 or even cardiac causes57. Accordingly, an accurate diagnosis of AMI using hs-cTnT would also require comprehensive consideration of a patient's signs, symptoms, ECG, and other laboratory test results. However, the prognostic value of hs-cTnT is remarkable. Elevation of hs-cTnT among patients with stable or unstable angina is highly correlated to increased long-term mortality58. Reichlin et al 59 performed an international multicenter study from 2006 to 2009 and concluded that elevated hs-cTnT could be used to identify poor prognosis of patients with small AMI. Overall, hs-cTnT might be a favorable option with good cost in the management of AMI60.
H-FABP
General information
Fatty acid binding proteins (FABPs) are a group of proteins that are responsible for the transportation of fatty acids and lipophilic materials into or out of cells4. There are several types of tissue-specific FABPs, including liver-type, intestinal-type, heart-type, adipocyte-type, epidermal-type, ileal-type, brain-type, myelin-type, and testis-type FABPs4. Among them, heart-type FABP (H-FABP) is mainly found in the heart and has a molecular weight of 15 kDa. When there is an ischemic injury to the myocardium, H-FABP will rapidly be released into the circulatory system and then be eliminated by the kidney. Consequently, serum H-FABP measurement can, to some extent, reflect myocardial injury. Actually, H-FABP is so sensitive that it has already been utilized clinically as a marker to evaluate sub-clinical ischemia and to predict possible disease progresssion61. In addition, H-FABP is also apparently correlated to other clinical parameters in AMI patients, eg, leukocytosis and a significantly decreased ejection fraction62. These properties of H-FABP might help to understand the pathological process of AMI in a more comprehensive way and hopefully also help to guide the diagnosis as well as treatment. H-FABP is not only a sensitive marker for myocardial injury when it is over-expressed but it has also been shown to promote inflammation, growth and migration of vascular smooth muscle cells, and thus, it possibly plays a role in the pathophysiology of in-stent restenosis63. Therefore, it is reasonable and also valuable to investigate the diagnostic and prognostic potential of H-FABP.
Diagnostic property for AMI
H-FABP is highly sensitive to myocardial ischemic injury as it can be detected as early as 1 h after AMI. In 1992, Kleine et al 64 studied the release of H-FABP after AMI and found that its plasma concentration significantly exceeded the threshold level in less than three hours after AMI. In fact, H-FABP is actually more sensitive than most other cardiac biomarkers. Ishii et al 65 measured the levels of H-FABP and myoglobin within six hours after chest pain and found that H-FABP is much better than myoglobin in regard to both sensitivity and specificity for diagnosing AMI. Furthermore, Ecollan et al 6 tested the levels of H-FABP, CK-MB, Mb, and cTnI among patients with suspected AMI before hospital admission and found that H-FABP possesses significantly superior sensitivity to any of the other three markers. Therefore, the H-FABP test is preferably recommended as the first choice to diagnose or rule out AMI at an early stage, particularly within three hours after the onset of chest pain.
Additionally, H-FABP is fairly satisfactory in regard to specificity as it is relatively tissue-specific to the heart. A study by Pyati et al 66 revealed that both the sensitivity and specificity of H-FABP are better than those of CK-MB and Mb at 3 h and 3–6 h after a complaint of typical chest pain. Yet, the specificity of H-FABP is not as high as that of the troponin assay67. As a result, Vupputuri et al 68 proposed that a combined application of H-FABP at the early stage and cTnI at the late stage should be employed to achieve a perfect balance between sensitivity and specificity over the whole diagnostic window. Moreover, a patient's conditions can also be influential to the specificity of H-FABP. Garcia-Valdecasas et al 69 reported an unfavorable specificity of H-FABP for AMI, despite its excellent sensitivity and prognostic value. This unfavorable specificity was possibly explained by concomitant renal failure in many patients who were enrolled, disturbing the elimination of H-FABP and thus producing false positive results. Therefore, a combination of H-FABP with other cardiac biomarkers is highly recommended to achieve favorable diagnostic accuracy in clinical practice.
Marker for ischemia reperfusion injury
To effectively remove an occlusion of the coronary artery and re-perfuse the infarcted section of the heart, treatments such as PCI or CABG are normally requested. Yet, the restoration of coronary flow is inevitably followed by IRI and, subsequently, an increased level of cardiac markers, such as H-FABP70. Therefore, H-FABP can also serve as a marker for IRI and indirectly reflects the patency of the coronary artery and restoration of coronary flow.
As a result of its superior sensitivity to other markers, there have been studies that used H-FABP to evaluate the achievement of heart reperfusion after ischemic attack71,72. In 2000, Hayashida et al 73 compared the plasma concentrations of H-FABP, CK-MB, and troponin T in patients after CABG and concluded that H-FABP is a sensitive early biomarker for IRI. In clinical practice, the studies of Wong et al 74 and Huang et al 7 both included H-FABP as a marker to evaluate IRI in patients undergoing CABG surgery using cardiopulmonary bypass. Huang et al 7 also found H-FABP to be a more sensitive marker than cTnI and CK-MB for sensing post-ischemic myocardial reperfusion injury; thus, they proposed H-FABP to be an early predictor for peri-operative anesthetic cardio-protection against IRI.
Prognostic property
In addition to serving as a marker for AMI and IRI, H-FABP might also have prognostic characteristics. It is not unusual that, even after immediate and proper treatment, patients with AMI are likely to eventually have additional major cardiac events or even death. Since many of the common biomarkers we use do not have satisfactory prognostic value, it is of great importance to explore and employ other new assays to efficiently predict the outcomes of AMI patients. In 2005, Suzuki et al 75 proposed that H-FABP could be a biomarker that can independently predict adverse cardiac events within 30 days in patients with ACS. Then, in 2006, O'Donoghue et al 76 reported a study on a large cohort of 2287 patients and demonstrated that elevated H-FABP in the early hours was related to an increased chance of death and other adverse cardiac events among patients with ACS and that H-FABP alone might provide information for a risk evaluation for clinicians. Additionally, Ishii et al 77 claimed H-FABP to be a potentially independent prognostic factor within 6 months for patients with ACS, even superior to cTnT. Apart from its predictive value on long-term mortality, another prospective observational study also found that H-FABP was capable of identifying high-risk patients with adverse outcomes and that proper attention should be paid to these patients to improve their prognoses78. Furthermore, when used in combination with troponin, an H-FABP measurement could help with prediction of both long-term mortality and the chance for re-infarction in the presence of suspected ACS, even for low- to intermediate- risk subjects79. In addition, it is recommended that checking the H-FABP level only during a hospital stay is insufficient and that continuous follow up is needed. A recent study by Matsumoto et al 80 showed that an H-FABP measurement during the recovery stage of post-AMI patients could predict long-term mortality and the chance of readmission as a result of possible heart failure, even after discharge.
Conclusion
Prevention and early diagnosis are still the main principles in the management of AMI and require specific and sensitive biomarkers for the assessment of a post-ischemic myocardial injury. The present review suggests that H-FABP might be an ideal option based on the currently available evidence. It is highly sensitive and its plasma concentration increases even earlier than that of Mb after myocardial injury. The specificity of H-FABP is also sufficiently satisfactory, though slightly inferior to that of cTnI. As a result of its excellent sensitivity, it can also serve as a good marker for IRI. These properties of H-FABP could help clinicians to monitor AMI in a timely and close fashion and develop methods to reduce post-ischemic adverse effects. In addition, H-FABP is a potential prognostic indicator for long-term mortality. It should be noted, however, since the diagnostic window of H-FABP is slightly narrow and is eliminated quickly, the combination of H-FABP with other biomarkers, eg, H-FABP at the early stage and cTnI at the late stage of AMI is advised to achieve better diagnostic and prognostic significance.
Overall, the future prospect of H-FABP is rather promising (Figure 1). Yet, there are several issues to be addressed before it can be widely applied in hospitals. To promote fast and wide application, it is necessary to develop a sensitive assay to allow fast and convenient as well as economic measurements of H-FABP. In addition, consistent standardization needs to be settled internationally and a consensus should be reached regarding its threshold value as uniform diagnostic criterion. To achieve this, there is still an urgent need for more relevant research studies incorporating clinical trials in multiple centers before H-FABP is recognized as an accurate and reliable marker for immediate and long-term post-ischemic myocardial injury/dysfunction.
Figure 1.
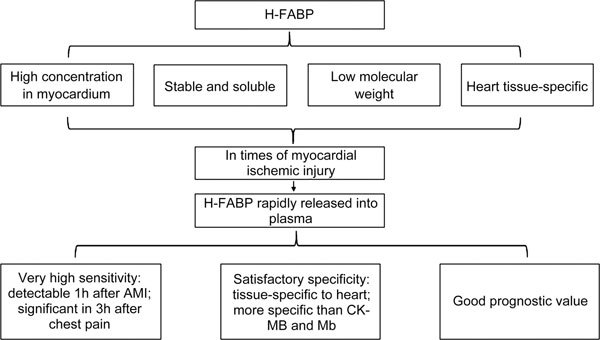
H-FABP as a potential cardiac biomarker.
Acknowledgements
The research work of the authors was supported by Research Grants Council (RGC)/GRF grants (17158616M and 17121315M).
Contributor Information
Michael G Irwin, Email: zyxia@hku.hk, Email: mgirwin@hku.hk.
Zhengyuan Xia, Email: zyxia@hku.hk, Email: mgirwin@hku.hk.
References
- 1.Mullasari AS, Balaji P, Khando T. Managing complications in acute myocardial infarction. J Assoc Physicians India. 2011;59:43–8. [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. 2006;47:39–48. doi: 10.1007/BF03194597. [DOI] [PubMed] [Google Scholar]
- 5.Glatz JF, van Bilsen M, Paulussen RJ, Veerkamp JH, van der Vusse GJ, Reneman RS. Release of fatty acid-binding protein from isolated rat heart subjected to ischemia and reperfusion or to the calcium paradox. Biochim Biophys Acta. 1988;961:148–52. doi: 10.1016/0005-2760(88)90141-5. [DOI] [PubMed] [Google Scholar]
- 6.Ecollan P, Collet JP, Boon G, Tanguy ML, Fievet ML, Haas R, et al. Pre-hospital detection of acute myocardial infarction with ultra-rapid human fatty acid-binding protein (H-FABP) immunoassay. Int J Cardiol. 2007;119:349–54. doi: 10.1016/j.ijcard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Huang Z, Zhong X, Irwin MG, Ji S, Wong GT, Liu Y, et al. Synergy of isoflurane preconditioning and propofol postconditioning reduces myocardial reperfusion injury in patients. Clin Sci (Lond) 2011;121:57–69. doi: 10.1042/CS20100435. [DOI] [PubMed] [Google Scholar]
- 8.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1459–544. [DOI] [PMC free article] [PubMed]
- 9.Puymirat E. Epidemiology of coronary artery disease. Rev Prat. 2015;65:317–20. [PubMed] [Google Scholar]
- 10.Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J. 2004;148:7–15. doi: 10.1016/j.ahj.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–66. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Oosterlinck W, Dresselaers T, Geldhof V, Nevelsteen I, Janssens S, Himmelreich U, et al. Diabetes mellitus and the metabolic syndrome do not abolish, but might reduce, the cardioprotective effect of ischemic postconditioning. J Thorac Cardiovasc Surg. 2013;145:1595–602. doi: 10.1016/j.jtcvs.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Yin X, Zheng Y, Zhai X, Zhao X, Cai L. Diabetic inhibition of preconditioning- and postconditioning-mediated myocardial protection against ischemia/reperfusion injury. Exp Diabetes Res. 2012;2012:198048. doi: 10.1155/2012/198048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lejay A, Fang F, John R, Van JA, Barr M, Thaveau F, et al. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J Mol Cell Cardiol. 2016;91:11–22. doi: 10.1016/j.yjmcc.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Yao W, Liu Z, Xu A, Huang Y, Ma XL, et al. Hyperglycemia abrogates ischemic postconditioning cardioprotection by impairing AdipoR1/Caveolin-3/STAT3 signaling in diabetic rats. Diabetes. 2016;65:942–55. doi: 10.2337/db15-0782. [DOI] [PubMed] [Google Scholar]
- 16.Ordway GA, Garry DJ. Myoglobin: an essential hemoprotein in striated muscle. J Exp Biol. 2004;207:3441–6. doi: 10.1242/jeb.01172. [DOI] [PubMed] [Google Scholar]
- 17.Apple FS. Creatine kinase isoforms and myoglobin: early detection of myocardial infarction and reperfusion. Coron Artery Dis. 1999;10:75–9. doi: 10.1097/00019501-199910001-00003. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Qin L, Wu T, Deng B, Sun Y, Hu D, et al. Elevated cardiac markers in chronic kidney disease as a consequence of hyperphosphatemia-induced cardiac myocyte injury. Med Sci Monit. 2014;20:2043–53. doi: 10.12659/MSM.890909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beuerle JR, Azzazy HM, Styba G, Duh SH, Christenson RH. Characteristics of myoglobin, carbonic anhydrase III and the myoglobin/carbonic anhydrase III ratio in trauma, exercise, and myocardial infarction patients. Clin Chim Acta. 2000;294:115–28. doi: 10.1016/S0009-8981(99)00261-2. [DOI] [PubMed] [Google Scholar]
- 20.Tanasijevic JM. Non-invasive assessment of coronary artery patency after thrombolysis using serum myoglobin measurements. Prilozi. 2007;28:5–11. [PubMed] [Google Scholar]
- 21.Roych C, Magioncalda A, Sanfelici D, Mattiauda C, Cavazzana C, Mellini M, et al. Diagnostic and prognostic value of radioimmunologic determination of myoglobin in myocardial infarction. Cardiologia. 1982;27:493–6. [PubMed] [Google Scholar]
- 22.Tashmatova A, Staroverov II, Titov VN, Ruda M. Blood myoglobin in the diagnosis and prognosis of acute myocardial infarct. Lab Delo. 1986;(3):145–9. [PubMed] [Google Scholar]
- 23.Venge P. The diagnostic and prognostic value of serum-myoglobin determinations in acute myocardial infarction. Ups J Med Sci. 1983;88:205–11. doi: 10.3109/03009738309178454. [DOI] [PubMed] [Google Scholar]
- 24.Wilson SR, Sabatine MS, Braunwald E, Sloan S, Murphy SA, Morrow DA. Detection of myocardial injury in patients with unstable angina using a novel nanoparticle cardiac troponin I assay: observations from the PROTECT-TIMI 30 Trial. Am Heart J. 2009;158:386–91. doi: 10.1016/j.ahj.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Tanasijevic MJ, Cannon CP, Antman EM. The role of cardiac troponin-I (cTnI) in risk stratification of patients with unstable coronary artery disease. Clin Cardiol. 1999;22:13–6. doi: 10.1002/clc.4960220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiwari RP, Jain A, Khan Z, Kohli V, Bharmal RN, Kartikeyan S, et al. Cardiac troponins I and T: molecular markers for early diagnosis, prognosis, and accurate triaging of patients with acute myocardial infarction. Mol Diagn Ther. 2012;16:371–81. doi: 10.1007/s40291-012-0011-6. [DOI] [PubMed] [Google Scholar]
- 27.Myint PK, Kwok CS, Bachmann MO, Stirling S, Shepstone L, Zaman MJ. Prognostic value of troponins in acute coronary syndrome depends upon patient age. Heart. 2014;100:1583–90. doi: 10.1136/heartjnl-2014-305533. [DOI] [PubMed] [Google Scholar]
- 28.Li WJ, Chen XM, Nie XY, Lin XX, Cheng YJ, Hu CH, et al. Early diagnostic and prognostic utility of high-sensitive troponin assays in acute myocardial infarction: a meta-analysis. Intern Med J. 2015;45:748–56. doi: 10.1111/imj.12642. [DOI] [PubMed] [Google Scholar]
- 29.Benamer H, Steg PG, Benessiano J, Vicaut E, Gaultier CJ, Boccara A, et al. Comparison of the prognostic value of C-reactive protein and troponin I in patients with unstable angina pectoris. Am J Cardiol. 1998;82:845–50. doi: 10.1016/S0002-9149(98)00490-1. [DOI] [PubMed] [Google Scholar]
- 30.Grande P, Naestoft J, Christiansen C. An easy and reliable estimation of acute myocardial infarct size from serum CK-MB measurements. Eur J Cardiol. 1980;11:71–7. [PubMed] [Google Scholar]
- 31.Roberts R, Ambos HD, Sobel BE. Estimation of infarct size with MB rather than total CK. Int J Cardiol. 1983;2:479–92. doi: 10.1016/0167-5273(83)90149-3. [DOI] [PubMed] [Google Scholar]
- 32.Mills JS, Mahaffey KW, Lokhnygina Y, Nicolau JC, Ruzyllo W, Adams PX, et al. Prediction of enzymatic infarct size in ST-segment elevation myocardial infarction. Coron Artery Dis. 2012;23:118–25. doi: 10.1097/MCA.0b013e32834e4f8f. [DOI] [PubMed] [Google Scholar]
- 33.Dohi T, Maehara A, Brener SJ, Genereux P, Gershlick AH, Mehran R, et al. Utility of peak creatine kinase-MB measurements in predicting myocardial infarct size, left ventricular dysfunction, and outcome after first anterior wall acute myocardial infarction (from the INFUSE-AMI trial) Am J Cardiol. 2015;115:563–70. doi: 10.1016/j.amjcard.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Luo Y, Pan YZ, Zeng C, Li GL, Lei XM, Liu Z, et al. Altered serum creatine kinase level and cardiac function in ischemia-reperfusion injury during percutaneous coronary intervention. Med Sci Monit. 2011;17:CR474–9. doi: 10.12659/MSM.881932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmberg FE, Ottas KA, Andreasen C, Perko MJ, Moller CH, Engstrom T, et al. Conditioning techniques and ischemic reperfusion injury in relation to on-pump cardiac surgery. Scand Cardiovasc J. 2014;48:241–8. doi: 10.3109/14017431.2014.923930. [DOI] [PubMed] [Google Scholar]
- 36.Bagai A, Schulte PJ, Granger CB, Mahaffey KW, Christenson RH, Bell G, et al. Prognostic implications of creatine kinase-MB measurements in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. Am Heart J. 2014;168:503–11. doi: 10.1016/j.ahj.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Lopes RD, Lokhnygina Y, Hasselblad V, Newby KL, Yow E, Granger CB, et al. Methods of creatine kinase-MB analysis to predict mortality in patients with myocardial infarction treated with reperfusion therapy. Trials. 2013;14:123. doi: 10.1186/1745-6215-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domanski MJ. Prognostic implications of troponin T and creatine kinase-MB elevation after coronary artery bypass grafting. Am Heart J. 2012;164:636–7. doi: 10.1016/j.ahj.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Saadeddin SM, Habbab MA, Sobki SH, Ferns GA. Biochemical detection of minor myocardial injury after elective, uncomplicated, successful percutaneous coronary intervention in patients with stable angina: clinical outcome. Ann Clin Biochem. 2002;39:392–7. doi: 10.1258/000456302760042155. [DOI] [PubMed] [Google Scholar]
- 40.Harris BM, Nageh T, Marsden JT, Thomas MR, Sherwood RA. Comparison of cardiac troponin T and I and CK-MB for the detection of minor myocardial damage during interventional cardiac procedures. Ann Clin Biochem. 2000;37:764–9. doi: 10.1258/0004563001900075. [DOI] [PubMed] [Google Scholar]
- 41.Melamed KH, Goldhaber SZ. Cardiology Patient Page: inflammation and myocardial infarction. Circulation. 2014;130:e334–6. doi: 10.1161/CIRCULATIONAHA.114.010614. [DOI] [PubMed] [Google Scholar]
- 42.Auer J, Berent R, Lassnig E, Eber B. C-reactive protein and coronary artery disease. Jpn Heart J. 2002;43:607–19. doi: 10.1536/jhj.43.607. [DOI] [PubMed] [Google Scholar]
- 43.Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349:462–6. doi: 10.1016/S0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 44.Mather AN, Fairbairn TA, Artis NJ, Greenwood JP, Plein S. Relationship of cardiac biomarkers and reversible and irreversible myocardial injury following acute myocardial infarction as determined by cardiovascular magnetic resonance. Int J Cardiol. 2013;166:458–64. doi: 10.1016/j.ijcard.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Liu D, Qi X, Li Q, Jia W, Wei L, Huang A, et al. Increased complements and high-sensitivity C-reactive protein predict heart failure in acute myocardial infarction. Biomed Rep. 2016;5:761–5. doi: 10.3892/br.2016.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krause EG, Rabitzsch G, Noll F, Mair J, Puschendorf B. Glycogen phosphorylase isoenzyme BB in diagnosis of myocardial ischaemic injury and infarction. Mol Cell Biochem. 1996;160–1:289–95. doi: 10.1007/BF00240061. [DOI] [PubMed] [Google Scholar]
- 47.Mair J. Progress in myocardial damage detection: new biochemical markers for clinicians. Crit Rev Clin Lab Sci. 1997;34:1–66. doi: 10.3109/10408369709038215. [DOI] [PubMed] [Google Scholar]
- 48.Rabitzsch G, Mair J, Lechleitner P, Noll F, Hofmann U, Krause EG, et al. Immunoenzymometric assay of human glycogen phosphorylase isoenzyme BB in diagnosis of ischemic myocardial injury. Clin Chem. 1995;41:966–78. [PubMed] [Google Scholar]
- 49.Mion MM, Novello E, Altinier S, Rocco S, Zaninotto M, Plebani M. Analytical and clinical performance of a fully automated cardiac multi-markers strategy based on protein biochip microarray technology. Clin Biochem. 2007;40:1245–51. doi: 10.1016/j.clinbiochem.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 50.McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008;29:2843–50. doi: 10.1093/eurheartj/ehn363. [DOI] [PubMed] [Google Scholar]
- 51.Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–93. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
- 52.Lillpopp L, Tzikas S, Ojeda F, Zeller T, Baldus S, Bickel C, et al. Prognostic information of glycogen phosphorylase isoenzyme BB in patients with suspected acute coronary syndrome. Am J Cardiol. 2012;110:1225–30. doi: 10.1016/j.amjcard.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 54.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–61. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 55.Giner-Caro JA, Caballero L, Casas-Pina T, Pastor-Perez F, Garrido-Bravo IP, Sanchez-Mas J, et al. High sensitive cardiac troponin T in the management of uncertain chest pain. Int J Cardiol. 2013;168:4422–3. doi: 10.1016/j.ijcard.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Durante A. The value of elevated high-sensitive troponin T. Am Heart J. 2011;161:e33. doi: 10.1016/j.ahj.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 57.Irfan A, Twerenbold R, Reiter M, Reichlin T, Stelzig C, Freese M, et al. Determinants of high-sensitivity troponin T among patients with a noncardiac cause of chest pain. Am J Med. 2012;125:491–8. doi: 10.1016/j.amjmed.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 58.Ndrepepa G, Braun S, Mehilli J, Birkmeier KA, Byrne RA, Ott I, et al. Prognostic value of sensitive troponin T in patients with stable and unstable angina and undetectable conventional troponin. Am Heart J. 2011;161:68–75. doi: 10.1016/j.ahj.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 59.Reichlin T, Twerenbold R, Reiter M, Steuer S, Bassetti S, Balmelli C, et al. Introduction of high-sensitivity troponin assays: impact on myocardial infarction incidence and prognosis. Am J Med. 2012;125:1205–13. doi: 10.1016/j.amjmed.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 60.Vaidya A, Severens JL, Bongaerts BW, Cleutjens KB, Nelemans PJ, Hofstra L, et al. High-sensitive troponin T assay for the diagnosis of acute myocardial infarction: an economic evaluation. BMC Cardiovasc Disord. 2014;14:77. doi: 10.1186/1471-2261-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jirak P, Fejzic D, Paar V, Wernly B, Pistulli R, Rohm I, et al. Influences of Ivabradine treatment on serum levels of cardiac biomarkers sST2, GDF-15, suPAR and H-FABP in patients with chronic heart failure. Acta Pharmacol Sin 2017. doi: 10.1038/aps.2017.167. [DOI] [PMC free article] [PubMed]
- 62.Schernthaner C, Lichtenauer M, Wernly B, Paar V, Pistulli R, Rohm I, et al. Multibiomarker analysis in patients with acute myocardial infarction. Eur J Clin Invest. 2017;47:638–48. doi: 10.1111/eci.12785. [DOI] [PubMed] [Google Scholar]
- 63.Chen K, Chen QJ, Wang LJ, Liu ZH, Zhang Q, Yang K, et al. Increment of HFABP level in coronary artery in-stent restenosis segments in diabetic and nondiabetic minipigs: HFABP overexpression promotes multiple pathway-related inflammation, growth and migration in human vascular smooth muscle cells. J Vasc Res. 2016;53:27–38. doi: 10.1159/000446652. [DOI] [PubMed] [Google Scholar]
- 64.Kleine AH, Glatz JF, Van Nieuwenhoven FA, Van der Vusse GJ. Release of heart fatty acid-binding protein into plasma after acute myocardial infarction in man. Mol Cell Biochem. 1992;116:155–62. doi: 10.1007/BF01270583. [DOI] [PubMed] [Google Scholar]
- 65.Ishii J, Wang JH, Naruse H, Taga S, Kinoshita M, Kurokawa H, et al. Serum concentrations of myoglobin vs human heart-type cytoplasmic fatty acid-binding protein in early detection of acute myocardial infarction. Clin Chem. 1997;43:1372–8. [PubMed] [Google Scholar]
- 66.Pyati AK, Devaranavadagi BB, Sajjannar SL, Nikam SV, Shannawaz M, Sudharani Heart-type fatty acid binding protein: a better cardiac biomarker than CK-MB and myoglobin in the early diagnosis of acute myocardial infarction. J Clin Diagn Res. 2015;9:BC08–11. doi: 10.7860/JCDR/2015/15132.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kagawa Y, Toyofuku M, Masaoka Y, Muraoka Y, Okimoto T, Otsuka M, et al. Comparison of heart-type fatty acid binding protein and sensitive troponin for the diagnosis of early acute myocardial infarction. Int J Cardiol. 2013;166:347–51. doi: 10.1016/j.ijcard.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 68.Vupputuri A, Sekhar S, Krishnan S, Venugopal K, Natarajan KU. Heart-type fatty acid-binding protein (H-FABP) as an early diagnostic biomarker in patients with acute chest pain. Indian Heart J. 2015;67:538–42. doi: 10.1016/j.ihj.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Valdecasas S, Ruiz-Alvarez MJ, Garcia De Tena J, De Pablo R, Huerta I, Barrionuevo M, et al. Diagnostic and prognostic value of heart-type fatty acid-binding protein in the early hours of acute myocardial infarction. Acta?Cardiol. 2011;66:315–21. doi: 10.1080/ac.66.3.2114131. [DOI] [PubMed] [Google Scholar]
- 70.Ozdemir M, Durakoglugil E, Gulbahar O, Turkoglu S, Sancak B, Pasaoglu H, et al. Heart fatty acid binding protein and myoglobin after reperfusion of acute myocardial infarction. Acta?Cardiol. 2007;62:473–8. doi: 10.2143/AC.62.5.2023410. [DOI] [PubMed] [Google Scholar]
- 71.Das DK, Barua PK, Jones RM. Release of fatty acid-binding protein from ischemic-reperfused rat heart and its prevention by mepacrine. Biochim Biophys Acta. 1991;1073:394–401. doi: 10.1016/0304-4165(91)90148-A. [DOI] [PubMed] [Google Scholar]
- 72.de Lemos JA, Antman EM, Morrow DA, Llevadot J, Giugliano RP, Coulter SA, et al. Heart-type fatty acid binding protein as a marker of reperfusion after thrombolytic therapy. Clin Chim Acta. 2000;298:85–97. doi: 10.1016/S0009-8981(00)00259-X. [DOI] [PubMed] [Google Scholar]
- 73.Hayashida N, Chihara S, Akasu K, Oda T, Tayama E, Kai E, et al. Plasma and urinary levels of heart fatty acid-binding protein in patients undergoing cardiac surgery. Jpn Circ J. 2000;64:18–22. doi: 10.1253/jcj.64.18. [DOI] [PubMed] [Google Scholar]
- 74.Wong GT, Huang Z, Ji S, Irwin MG. Remifentanil reduces the release of biochemical markers of myocardial damage after coronary artery bypass surgery: a randomized trial. J Cardiothorac Vasc Anesth. 2010;24:790–6. doi: 10.1053/j.jvca.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki M, Hori S, Noma S, Kobayashi K. Prognostic value of a qualitative test for heart-type fatty acid-binding protein in patients with acute coronary syndrome. Int Heart J. 2005;46:601–6. doi: 10.1536/ihj.46.601. [DOI] [PubMed] [Google Scholar]
- 76.O'Donoghue M, de Lemos JA, Morrow DA, Murphy SA, Buros JL, Cannon CP, et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114:550–7. doi: 10.1161/CIRCULATIONAHA.106.641936. [DOI] [PubMed] [Google Scholar]
- 77.Ishii J, Ozaki Y, Lu J, Kitagawa F, Kuno T, Nakano T, et al. Prognostic value of serum concentration of heart-type fatty acid-binding protein relative to cardiac troponin T on admission in the early hours of acute coronary syndrome. Clin Chem. 2005;51:1397–404. doi: 10.1373/clinchem.2004.047662. [DOI] [PubMed] [Google Scholar]
- 78.Kilcullen N, Viswanathan K, Das R, Morrell C, Farrin A, Barth JH, et al. Heart-type fatty acid-binding protein predicts long-term mortality after acute coronary syndrome and identifies high-risk patients across the range of troponin values. J Am Coll Cardiol. 2007;50:2061–7. doi: 10.1016/j.jacc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 79.Viswanathan K, Kilcullen N, Morrell C, Thistlethwaite SJ, Sivananthan MU, Hassan TB, et al. Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. 2010;55:2590–8. doi: 10.1016/j.jacc.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 80.Matsumoto S, Nakatani D, Sakata Y, Suna S, Shimizu M, Usami M, et al. Elevated serum heart-type fatty acid-binding protein in the convalescent stage predicts long-term outcome in patients surviving acute myocardial infarction. Circ J. 2013;77:1026–32. doi: 10.1253/circj.CJ-12-0999. [DOI] [PubMed] [Google Scholar]


