Abstract
Defensins play an essential role in innate immunity. In this study, a novel recombinant β-defensin that targets the epidermal growth factor receptor (EGFR) was designed and prepared. The EGFR-targeting β-defensin consists of an EGF-derived oligopeptide (Ec), a β-defensin-1 peptide (hBD1) and a lidamycin-derived apoprotein (LDP), which serves as the “scaffold” for the fusion protein (Ec-LDP-hBD1). Ec-LDP-hBD1 effectively bound to EGFR highly expressed human epidermoid carcinoma A431 cells. The cytotoxicity of Ec-LDP-hBD1 to EGFR highly expressed A431 cells was more potent than that to EGFR low-expressed human lung carcinoma A549 and H460 cells (the IC50 values in A431, A549, and H460 cells were 1.8 ± 0.55, 11.9 ± 0.51, and 5.19 ± 1.21 μmol/L, respectively); in addition, the cytotoxicity of Ec-LDP-hBD1 was much stronger than that of Ec-LDP and hBD1. Moreover, Ec-LDP-hBD1 suppressed cancer cell proliferation and induced mitochondria-mediated apoptosis. Its in vivo anticancer action was evaluated in athymic mice with A431 and H460 xenografts. The mice were administered Ec-LDP-hBD1 (5, 10 mg/kg, i.v.) two times with a weekly interval. Administration of Ec-LDP-hBD1 markedly inhibited the tumor growth without significant body weight changes. The in vivo imaging further revealed that Ec-LDP-hBD1 had a tumor-specific distribution with a clear image of localization. The results demonstrate that the novel recombinant EGFR-targeting β-defensin Ec-LDP-hBD1 displays both selectivity and enhanced cytotoxicity against relevant cancer cells by inducing mitochondria-mediated apoptosis and exhibits high therapeutic efficacy against the EGFR-expressed carcinoma xenograft. This novel format of β-defensin, which induces mitochondrial-mediated apoptosis, may play an active role in EGFR-targeting cancer therapy.
Keywords: defensin, recombinant protein, EGFR-targeting, lidamycin apoprotein, mitochondria-mediated apoptosis, human epidermoid carcinoma A431 cells, human lung carcinoma A549 cells, human lung carcinoma H460 cells
Introduction
Human defensins, which serve as important mediators of the innate immune system, are small cationic peptides produced by epithelial cells and neutrophils and occur mainly in two genetically distinct forms, α- and β-defensins [1]. Through neutralizing bacterial toxins or disrupting the cytoplasmic membrane, defensins may kill microbial pathogens [2]. Additionally, they may also be involved in adaptive immunity by serving as chemo-attractants and activators of the immune cells [3, 4]. Previous studies have shown that human defensins have antibacterial activity, antiviral activity and tumor cell cytotoxicity [5]. Compared with traditional chemotherapeutics, defensins would potentially have a therapeutic advantage in that it is difficult for cancer cells to develop drug resistance; in addition, defensins show no immunogenicity and are resistant to proteolysis [6–8].
Human β-defensin-1 (hBD1) is constitutively expressed in a number of epithelial cells and can kill bacteria, fungi, viruses, and tumor cells [9–11]. Studies revealed that hBD1 expression was significantly lower in tumors compared with that in normal tissues [12, 13]. In vitro studies have shown that hBD1 could kill tumor cells by a unique mechanism that involves membrane lysis and DNA damage [14, 15]. It induces cytolysis and caspase-mediated apoptosis activity against tumor cells that are thought to either destabilize the cellular membrane or cause the formation of open pores that allow vital biomolecules to leak out of the cell [12, 16]. Consequently, the cells will undergo apoptosis and death. The drastic destruction effect of defensin on the cellular membrane may be what makes it more difficult for cancer cells to develop resistance. Furthermore, gene therapy with human β-defensin-1 can inhibit tumor growth in renal carcinoma and prostate cancer [8, 10, 13]. Recently, the plant defensin NaD1 and the human β-defensin 3 (hBD3) were both shown to induce tumor cell lysis by directly binding to the plasma membrane phosphoinositide, phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) [14, 15]. These data not only identify an evolutionary conservation of defensin structure and function for lipid binding but also suggest that the cell membrane binding effect of defensins could be exploited for the development of novel therapeutics.
Lidamycin (LDM, also called C-1027) is an antitumor antibiotic with extremely potent cytotoxicity [17]. The LDM molecule consists of an active enediyne chromophore (AE, 843 Da), which is responsible for its highly potent cytotoxicity, and a noncovalently bound apoprotein (LDP, 10, 500 Da) that provides a hydrophobic domain for stabilizing and protecting the enediyne chromophore [18]. Previous tissue microarray studies have shown that LDP could bind to various human tumors with significantly different affinity than the corresponding normal tissues; in addition, LDP has a moderate antitumor efficacy. It is of interest that LDP can be used as a scaffold to construct recombinant novel multifunctional proteins [18, 19].
The overexpression of epidermal growth factor receptor (EGFR) has been observed in many human tumors, and the receptor has been found to be an important validated molecular target for the development of targeted therapeutics [20–22]. As shown, an oligopeptide (Ec) derived from epidermal growth factor (EGF) can bind to EGFR-overexpressed cancer cells and evidently serve as the guiding molecule in preparation of EGFR-targeted fusion proteins. Considering the fact that human β-defensin is active in mediating cytolysis of tumor cells, it may consequently play a critical role in suppressing tumor growth. The present study aims to construct a recombinant β-defensin-based EGFR-targeting protein Ec-LDP-hBD1. In this recombinant protein molecule, Ec is the EGFR-binding oligopeptide, the apoprotein LDP serves as the “scaffold”, and hBD1 acts as the effector agent. Furthermore, the recombinant protein Ec-LDP-hBD1 was determined and evaluated for its EGFR-targeting capability, antitumor efficacy and related mechanism of action.
Materials and methods
Cell culture
Human epidermoid carcinoma A431 cells and lung carcinoma A549 and H460 cells were cultured as reported previously [23, 24]. Human cancer cell lines A431, A549, and H460 were purchased from the American Type Culture Collection (ATCC). These cell lines were tested using STR analysis by the China Center for Type Culture Collection (CCTCC) in 2013. The human lung cell line PG-BE1 was a gift in kind from Professor Hong-Wei He (Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College).
Construction of pET-Ec-ldp-hBD1
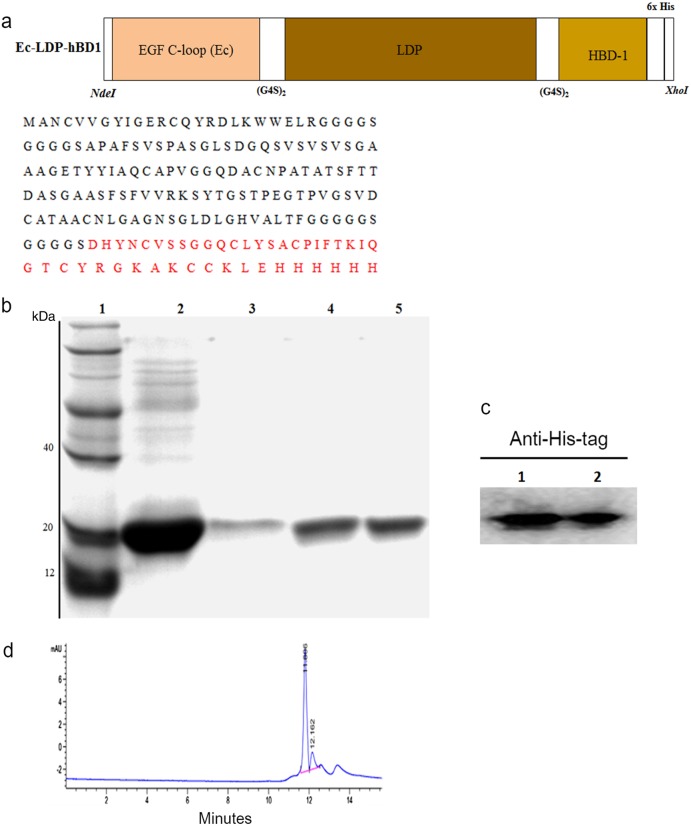
As shown in Fig. 1a, the full gene of the recombinant protein Ec-LDP-hBD1 (from 5′ to 3′) consisted of a C-loop in EGF (the 22 amino acids of EGF COOH terminal; [21, 22]), apoprotein LDP (110 amino acids; [18]), and hBD1 (36 amino acids; [13]). Two G4S linkers were inserted separately into the spaces between the coding sequences of the EGF C-loop and LDP and that of LDP and hBD1. After PCR and the DNA cloning process, the resultant 546-bp fragment was digested by NdeI/XhoI and inserted into a pET30a expression vector to generate plasmid pET-Ec-ldp-hBD1.
Fig. 1.
Construction and characterization of the recombinant EGFR-targeting β-defensin Ec-LDP-hBD1. a Diagram of the NdeI/XhoI gene fragments encoding for the protein Ec-LDP-hBD1 (top) and the amino acid sequence of Ec-LDP-hBD1 (bottom). b Expression analysis of the protein Ec-LDP-hBD1 by 12% SDS-PAGE. Lane 1, molecular weight marker; Lane 2, the first step of purified protein Ec-LDP-hBD1 by His Trap affinity columns; Lane 3, the second step of purified protein Ec-LDP-hBD1 by His Trap affinity columns; Lane 4, the third step of purified protein Ec-LDP-hBD1 by His Trap affinity columns; Lane 5, the fourth step of purified protein Ec-LDP-hBD1 by His Trap affinity columns. c Western blot detection of the fusion protein Ec-LDP-hBD1 using mouse anti-His-tag monoclonal antibody (1/1000 dilution) and HRP-conjugated goat anti-mouse IgG (1/2000 dilution). Lanes 1 and 2, western blot detection of the fusion proteins Ec-LDP-hBD1. d HPLC analysis of protein Ec-LDP-hBD1 purity
Expression and purification of the fusion proteins
Expression plasmid pET-Ec-ldp-hBD1 was transformed into Escherichia coli strain BL21 (DE3) star (Novagen, Merk, China). After overnight culture, bacteria were grown at 37 °C until the OD600 reached 1.0. Gene expression was induced with the addition of IPTG at 1 mmol/L concentration at 37 °C for 8 h. The proteins yielded through a C-terminal 6×His-tag were purified by affinity chromatography (HisTrap HP, GE Healthcare) according to the manufacturer’s instructions. The purified proteins were refolded via stepwise dialysis as reported [25], and the purity of the protein Ec-LDP-hBD1 was analyzed by high-performance liquid chromatography (HPLC) on an S3000 column (Tosoh). The Ec-LDP and LDP proteins were produced as reported in our previous study [18, 21].
Binding specificity with cancer cells
The binding activity of Ec-LDP-hBD1 was detected by ELISA as described previously [25]. Briefly, serial dilutions of refolded Ec-LDP-hBD1 in PBS were added into 1% BSA−PBS pre-coated plates, incubated and washed. The plate was then incubated with anti-His-tag HRP-conjugate and washed. Thereafter, 3.3′5.5′-tetramethylbenzidine was used as the chromogen for the color development, and absorbance values at 450 nm were measured on a microplate reader (Bio-Rad).
The binding specificity of the protein Ec-LDP-hBD1 to A431, A549, and H460 cells was evaluated with a fluorescence microscope. This assay was performed as previously reported [20, 26]. The cells were then overlaid with mouse anti-His-Tag monoclonal antibodies. After a final washing step with PBS, the slides were mounted with Tetramethylrhodamine (TRITC)-conjugated goat anti-mouse antibody and then restained with propidium iodide (PI); the fluorescence images were captured by a fluorescence microscope. To quantitatively compare the binding affinity of each protein sample to target cancer cells, we used a fluorescence-activated cell sorting (FACS) – based saturation binding assay. The data were analyzed with the Prism 5.0 software (GraphPad Software).
In vivo fluorescence imaging
A431 cells were inoculated into the right armpit of athymic mice. After 10 d, DyLight 680 antibody-labeled Ec-LDP-hBD1 (20 mg/kg) was intravenously injected into the xenograft-bearing mice (n = 3). Then, the mice were anesthetized by inhalation of isoflurane, and the images were observed with the Xenogen Ivis 200 system and recorded with the built-in camera (Caliper Life Sciences).
Cell viability assay
For the kit-8 assay, cells were plated in 96-well plates at 2×103 cells per well. The cells were then serum-starved for 8 h and further incubated with various concentrations of Ec-LDP-hBD1, Ec-LDP, LDP, or HBD-1 for 24 h at 37 °C. After adding 10 μL CCK-8 solution (Dojindo, Kumamoto, Japan) to 100 μL of culture media, optical density was measured at 450 nm.
Transmission electron microscopy observation
Transmission electron microscopy (TEM) was used to observe the ultrastructural changes in the cells treated with different protein samples. Harvested cells were immediately fixed in 2.5% glutaraldehyde, then rinsed in PBS, and embedded in 4% agarose. After fixation in 1% osmium tetroxide, the specimens were dehydrated using alcohol and embedded in pure epoxy resin. Ultrathin sections were prepared with the Leica Ultracut UCT slicer (Leica EM UC6, Germany), stained with uranyl acetate and lead citrate, and analyzed using TEM (Tecnai spirit 120kv).
Flow cytometric analysis
Flow cytometry was used to analyze the influence of the recombinant proteins on A431 cells. A total of 5×105 A431 cells cultured on six-well plates were treated with different concentrations of Ec-LDP-hBD1, Ec-LDP, and HBD-1 for 24 h. The cells were collected, resuspended in annexin V-FITC and PI, and analyzed on a BD Calibur flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA).
Western blot analysis
A431, A549, and H460 cells were pelleted and lysed. Western blotting was used to detect the expression levels of the protein EGFR with the corresponding antibodies. H520 cells were plated into 100-mm dishes at the density of 5×105 and grown to 70–80% confluence, after which the cells were washed three times in PBS and cultured for 12 h in serum-free medium. Cells were incubated with Ec-LDP(AE)-DF (0.1, 0.5, or 1 nmol/L) at 37 °C for 24 h, followed by stimulation with EGF (Abcam, 50 ng/mL), or both at 37 °C for 30 min. Twenty micrograms of each total protein were applied on 12% SDS-PAGE and then electroblotted onto polyvinylidene difluoride membranes (Millipore). The membranes were incubated with 3% BSA for 1 h at room temperature before incubation overnight at 4 °C with primary antibodies (diluted 1:1000, Cell Signaling Technology). Then the membranes were incubated with secondary HRP-conjugated antibodies (1:2000 dilution; Beijing Zhongshan Jinqiao Biotechnology, China) for 1 h after washing three times with TBST buffer. The specifc bands were visualized with the Immobilon Western Chemiluminescent HRP Substrate kit (Millipore) and captured by AI600 imaging system (GECorp.).
Animal experiments
Antitumor experiments were carried out using A431 and H460 xenograft models. Female athymic mice (BALB/c, nu/nu) were purchased from the Institute for Experimental Animals, Chinese Academy of Medical Sciences and Peking Union Medical College. The study protocols were designed in accordance with the regulations of Good Laboratory Practice for Non-Clinical Laboratory Studies of Drugs issued by the National Scientific and Technologic Committee of the People’s Republic of China. The treatment and use of animals during the study was approved by the Animal Ethics Committee of the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College (permission number: c1-2013-0108). Epidermoid carcinoma A431 cells and lung cancer H460 cells were suspended in sterile saline (1×107 cells/mL) and 200 μL of the suspension was inoculated subcutaneously into the right armpit of athymic mouse. After 2 weeks, tumors in donor animals were aseptically dissected and mechanically minced. Tumor pieces from the excised tissue blocks (2 mm3 in size) were transplanted (subcutaneously) into the athymic mice by a trocar needle. When the tumors reached approximately 100 mm3 in size, the mice were divided into groups (n = 6) and treated with different doses of tested sample proteins, delivered in a 200 μL volume of sterile saline, by intravenous injection into the tail vein. Each mouse received a total of two injections with a weekly interval. One group of mice received the sample proteins intravenously with physiological saline as a control. Tumor volumes were measured as described previously [20].
Statistical analyses
Statistical analyses were performed using either the SPSS software, version 17.0 (SPSS Inc.) or with GraphPad Prism 5.0 (GraphPad Software, Inc.). The unpaired Student’s t test or ANOVA test was used to perform a statistical comparison between two groups. The threshold of significance was set at P < 0.05.
Results
Construction, preparation and characterization of the recombinant EGFR-targeting β-defensin Ec-LDP-hBD1
The construction of the gene encoding the protein Ec-LDP-hBD1 is shown in Fig. 1a. The DNA fragments encoding for the targeting oligopeptide Ec (EGF c-coop), LDP (apoprotein of lidamycin), and hBD1 (β-defensin 1) were obtained by genetic engineering. The primary determination of protein expression was carried out via analysis by SDS-PAGE and western blotting; these results are presented in Fig. 1b, c. The purity of the protein Ec-LDP-hBD1 isolated by molecular sieve approached 90% as assessed by HPLC (Fig. 1d). As determined by MALDI-TOF-MS analysis, the molecular mass of Ec-LDP-hBD1 was approximately 19.7 kDa.
Binding affinity of the protein Ec-LDP-hBD1 to cancer cells
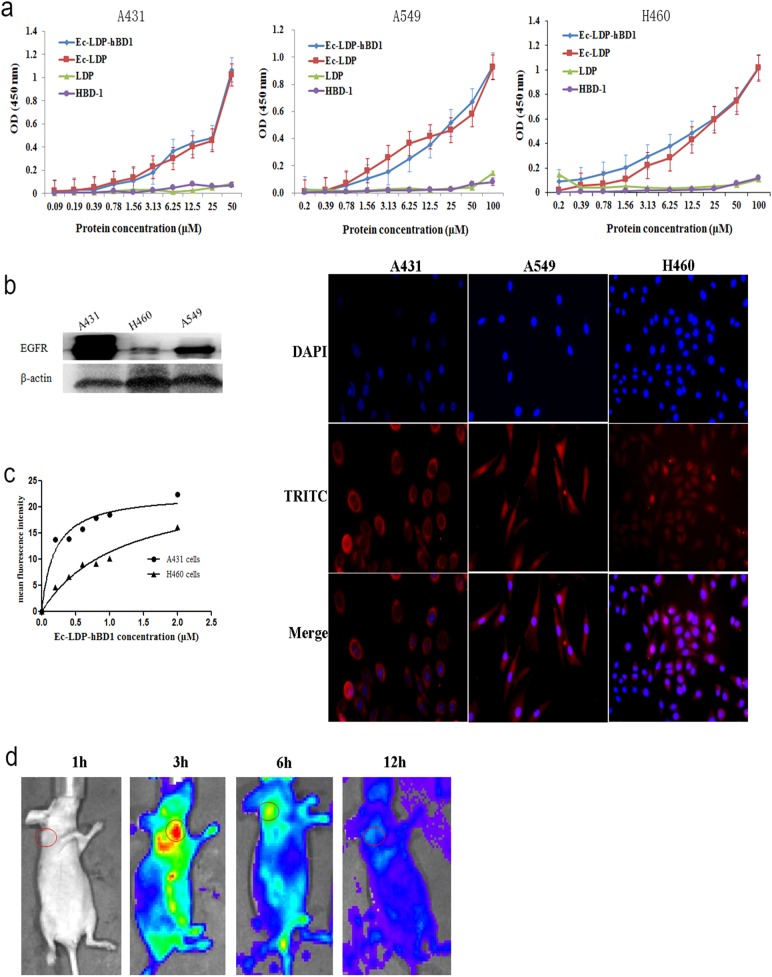
To assess the specificity, the binding of Ec-LDP-hBD1 to different tumor cells was determined by ELISA assay. As expected, Ec-LDP-hBD1 bound to human EGFR (Fig. 2a). The binding specificity of Ec-LDP-hBD1 with cancer cells was also characterized by immunofluorescence. The expression of EGFR on different carcinoma cell lines was analyzed by western blot. We found that Ec-LDP-hBD1 protein bound specifically to the EGFR-expressing cancer cells (Fig. 2b); however, LDP and hBD1 were without binding capability (Fig. 2a and Fig. S1). FACS analysis revealed that Ec-LDP-hBD1 bound strongly to the EGFR-overexpressed A431 cells; by contrast, it bound less to the EGFR low-expressed H460 cells (Fig. 2c).
Fig. 2.
Binding affinity of the protein Ec-LDP-hBD1 in vitro and the optical imaging in vivo. a Binding affinity of Ec-LDP-hBD1 with A431, A549, and H460 cells determined by ELISA. Points are means from triplicate measurements. b Expression of EGFR on different carcinoma cell lines analyzed by western blotting and immunofluorescence assay measuring binding affinity of Ec-LDP-hBD1 to A431, A459, and H460 cells. Red fluorescence located around the cells stained with anti-His-tag antibody (TRITC-conjugated second) as well as 4′,6-diamidino-2-phenylindole (DAPI). c Binding affinity of the protein Ec-LDP-hBD1 to A431 and H460 cells. Following FACS, the mean fluorescence intensity was plotted vs. protein concentration. d Optical imaging in living athymic mice bearing A431 xenografts treated with DyLight 680 antibody-labeled Ec-LDP-hBD1. The images were collected at different times after i.v. injection of DyLight 680 antibody-labeled Ec-LDP-hBD1. Red circles, the tumor areas
In vivo fluorescence imaging
An optical in vivo imaging system was used to evaluate the tissue distribution and tumor targeting capability of Ec-LDP-hBD1. The images of DyLight 680 antibody-labeled Ec-LDP-hBD1 showed fast tumor localization and accumulation in the epidermoid carcinoma A431 xenografts (Fig. 2d), yielding images with good contrast as early as 3 h after injection.
Cytotoxicity of the protein Ec-LDP-hBD1 to cancer cells
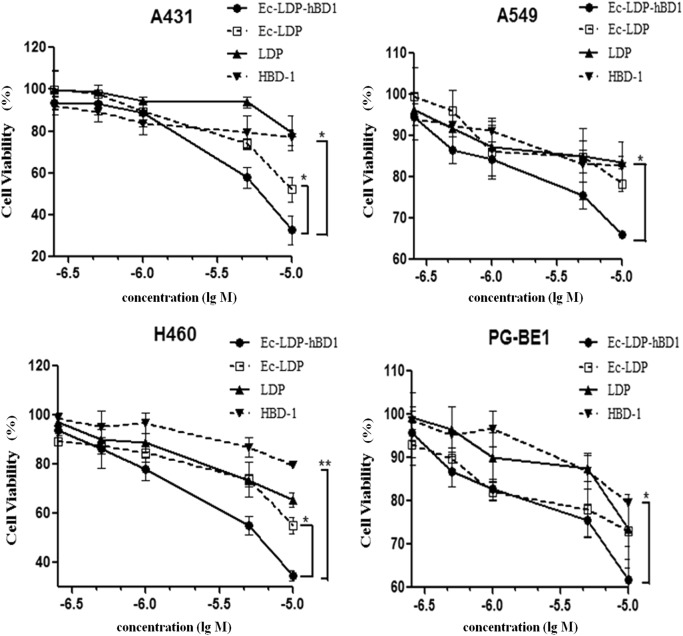
The cytotoxicity of Ec-LDP-hBD1 was tested with CCK-8 or MTT assay in four human carcinoma cell lines including a human epidermoid carcinoma cell line, A431, and the three human lung carcinoma cell lines, A549, H460, and PG-BE1 (Fig. 3 and Table 1). The recombinant protein Ec-LDP and free hBD-1 were also tested for comparison. By CCK-8 assay (Fig. 3), Ec-LDP-hBD1 markedly inhibited cancer cell proliferation. Compared among equivalent concentrations, Ec-LDP-hBD1 was more potent than free hBD-1, suggesting that the potency of β-defensin was increased in cancer cells by tethering with Ec-LDP. In addition, Ec-LDP-hBD1 was more potent than Ec-LDP and LDP. By CCK-8 assay, as presented in Table 1, Ec-LDP-hBD1 inhibited the viability of A431, A549, H460, and PG-BE1 cells in a concentration-dependent manner; the IC50 values were 1.8 ± 0.55, 11.9 ± 0.51, 5.19 ± 1.21, and 31.3 ± 0.49 μM, respectively. Meanwhile, the IC50 values of free hBD-1 for all four cell lines were >100 μM. In terms of IC50 values, EGFR overexpressing A431 cells were more sensitive to Ec-LDP-hBD1 than EGFR low-expressing A549 and H460 cells; furthermore, Ec-LDP-hBD1 was more potent than Ec-LDP and free hBD-1 in equivalent concentrations (P < 0.05), implying that the enhanced cytotoxic potency of Ec-LDP-hBD1 can be attributed to the EGFR-targeted effect in the EGFR overexpression A431 cells.
Fig. 3.
Cytotoxicity assessment of Ec-LDP-hBD1 to A549 and H460 cells. The growth inhibition of A431, A549, H460, and PG-BEI cells was indicated by absorbance in experimental groups compared with the control group. Data represent mean ± SD of three replicates, *P < 0.05
Table 1.
The IC50 values of Ec-LDP-hBD1 and various agents for four cancer cell lines determined by CCK-8 assay
| Cell lines | IC50 (μM) | |||
|---|---|---|---|---|
| A431 | A549 | H460 | PG-BE1 | |
| HBD-1 | >100 | >100 | >100 | >100 |
| Ec-LDP-hBD1 | 1.8 | 11.9 | 5.19 | 31.3 |
| Ec-LDP | 10.1 | 26.4 | 15 | 49.7 |
| LDP | 21.2 | 166.7 | 29.7 | 66.2 |
| Mitomycin C | 17.5 | 87.4 | 48.3 | 69.3 |
In vivo therapeutic efficacy of Ec-LDP-hBD1
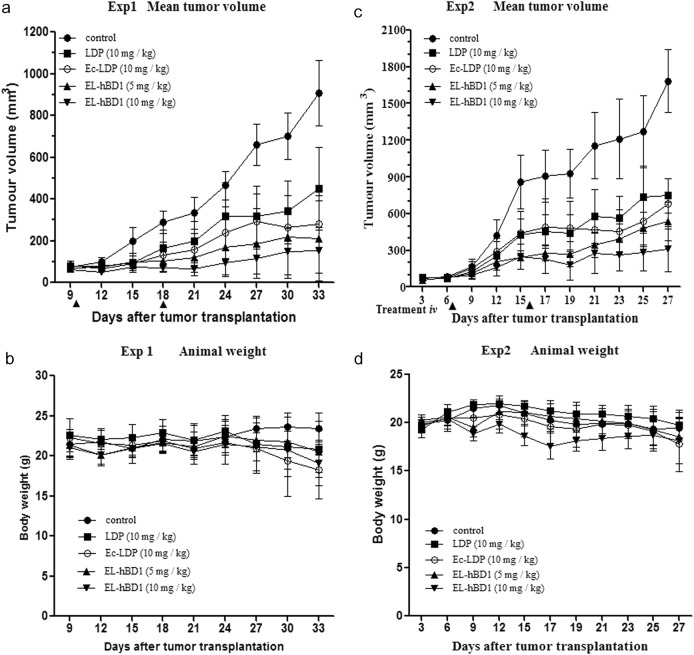
The therapeutic efficacy of Ec-LDP-hBD1 was evaluated on human epidermoid carcinoma A431 and lung carcinoma H460 xenografts in athymic mice. For comparison, LDP and Ec-LDP were separately tested in these experiments. In Experiment 1, epidermoid carcinoma A431 xenograft-bearing athymic mice were treated with the tested proteins by an intravenous route, once a week, for a total of two injections. As shown, Ec-LDP-hBD1 (5 mg/kg, 10 mg/kg) markedly suppressed the growth of tumors; notably, it was more effective than Ec-LDP (10 mg/kg) and LDP (10 mg/kg) in suppressing tumor growth (P < 0.05) (Fig. 4a, Exp1). At the end of the experiment, the tumors were excised, and tumor growth inhibition rates (%) were determined. Ec-LDP-hBD1 at doses of 5 and 10 mg/kg inhibited the growth of A431 xenograft by 70.6 and 88.1%, respectively. There were no deaths and no significant body weight changes in mice of all treatment groups, indicating that the administered doses were well tolerated (Fig. 4b). The therapeutic effects on the tumors were further confirmed by histopathological examination.
Fig. 4.
In vivo efficacy of Ec-LDP-hBD1. Experiment 1 (Exp 1), athymic mice bearing human epidermoid carcinoma A431 xenografts were treated with LDP, Ec-LDP, and Ec-LDP-hBD1 intravenously at different doses (n = 6). Mean tumor volumes (a) and mean body weights (b) of mice in each group are shown. Arrows, the day of injection (days 10 and 18). Experiment 2 (Exp 2), athymic mice bearing H460 xenografts. Mean tumor volumes (c) and mean body weights (d) of mice in each group are shown. Arrows, the day of injection (days 7 and 16). *P < 0.05, #P < 0.05
In Experiment II, therapeutic experiments were performed with lung carcinoma H460 xenograft (relatively low EGFR expression) in athymic mice. As shown in Fig. 4c Exp2, Ec-LDP-hBD1 suppressed the growth of H460 xenograft by 56.4 and 80.9% at doses of 5 and 10 mg/kg, respectively, while Ec-LDP suppressed growth by 53.7% at a dose of 10 mg/kg. There were no significant body weight changes (Fig. 4d, Exp2) and no deaths during the observation period. Compared between dosage levels of 5 and 10 mg/kg, Ec-LDP-hBD1 exerted higher inhibition rates in A431 xenograft than that in H460 xenograft.
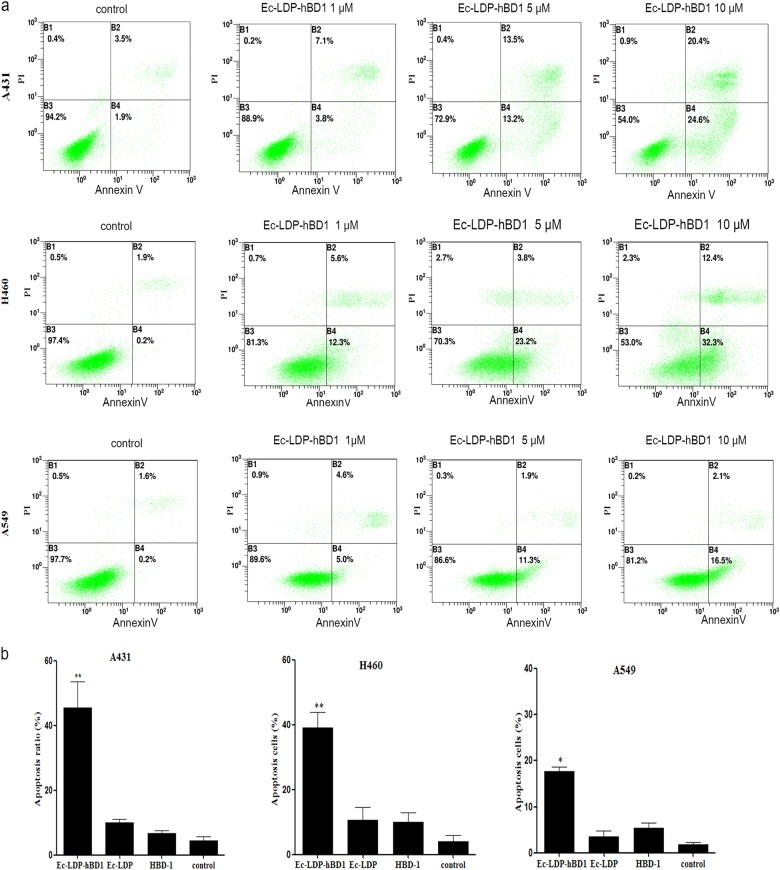
Induction of apoptosis by Ec-LDP-hBD1
Flow cytometry analysis indicated that the ratios of early-stage and late-stage apoptosis of A431 cells treated with 5 and 10 μM Ec-LDP-hBD1 were 26.7 and 45.0%, respectively (Fig. 5), more effective than those treated with Ec-LDP (Fig. S1). The ratios of early-stage and late-stage apoptosis of H460 and A549 cells treated with 8 μM Ec-LDP-hBD1 were 44.7 and 18.6% (Fig. 5), respectively, far more effective than those treated respectively with Ec-LDP and HBD-1 (P < 0.05). As shown in Fig. 5b, EC-LDP and hBD1 had little effect on the expression levels of mitochondrial pathway proteins in A431, A549, and H460 cells.
Fig. 5.
Induction of apoptosis by the fusion protein Ec-LDP-hBD1. a Flow cytometry analysis of apoptosis in A431, H460, and A549 cells treated with Ec-LDP-hBD1. b Rates of apoptotic cells, mean ± SD apoptosis. The experiments were repeated at least three times. *P < 0.05, **P < 0.01
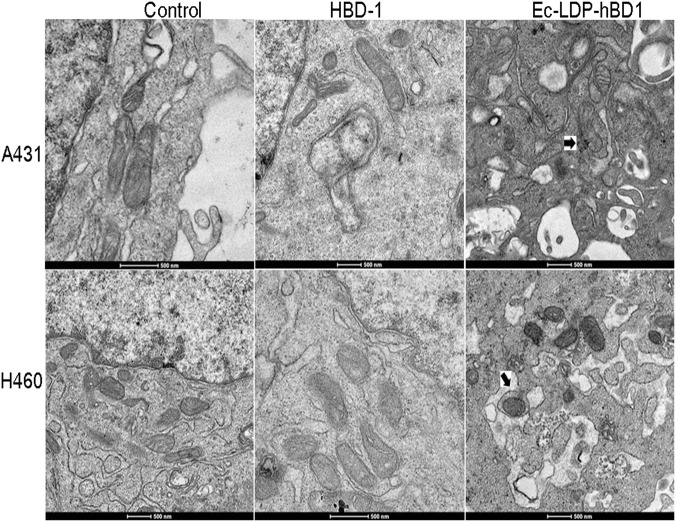
Induction of mitochondrial changes by Ec-LDP-hBD1
Mitochondrial changes induced by Ec-LDP-hBD1 were observed with TEM. As shown in Fig. 6, marked mitochondrial changes were observed in Ec-LDP-hBD1-treated cells, including mitochondrial swelling, vacuole formation, cristae segregation, fusion, and disruption. By contrast, no mitochondrial changes were detected in the untreated control cells.
Fig. 6.
Effects of Ec-LDP-hBD1 on ultrastructure and mitochondrial membrane potential in human A431 and H460 carcinoma cells. Morphological observation with transmission electron microscopy in A431 and H460 cell treated with 5 μM Ec-LDP-hBD1 and the control. Upper row: scale bar, 2 μm; under row: scale bar, 500 nm. As shown, mitochondrial changes were found in the treated cells. The changes are indicated using arrows
Discussion
Defensins play an important role in innate immunity. The potential use of defensins in cancer therapy has received much attention. A variety of defensins have been investigated; however, the therapeutic efficacy against cancer in experimental in vivo models have been limited [27]. Preparing new formats of defensins with the attributes of molecular targeted drugs would be an efficient way to increase the therapeutic efficacy. Targeted therapy encompasses a wide variety of strategies, such as antibody therapeutics and targeting peptides [28]. Targeting peptides that recognize particular cell-surface receptors have been shown to increase drug specificity with reduced systemic drug toxicity. In the present study, the epidermal growth factor (EGF)-derived oligopeptide Ec was employed as the EGFR-targeting molecule [22] for preparing the defensin-based protein. In the newly generated protein Ec-LDP-hBD1, in addition to Ec as the targeting oligopeptide, the β-defensin moiety hBD1 acts as the “effector” agent, while the apoprotein LDP serves as the “scaffold” for the recombinant protein (Fig. 1a). As shown, this genetically engineered protein, Ec-LDP-hBD1, bound to EGFR highly expressed tumor cells (Fig. 2). This protein selectively bound to the cellular membrane receptor. Moreover, the proliferation capability of A431, A549, H460, and PE-BG1 cells was markedly inhibited by Ec-LDP-hBD1, while little effect was found with treatment using the Ec-LDP protein, indicating that the integrated defensin hBD1 molecule played an active role in the observed inhibition (Fig. 3). The study further determined that EGFR-overexpressed tumor cells were more sensitive to Ec-LDP-hBD1 than the EGFR low-expressed tumor cells, indicating that this EGFR-targeting β-defensin displayed a selective cytotoxicity. Notably, the free β-defensin HBD-1 only displayed weak cytotoxicity to tumor cells; by contrast, Ec-LDP-hBD1 exerted much more potent cytotoxicity to the EGFR-overexpressed A431 cells. When compared in terms of IC50 values, there appeared to be a greater than 50-fold increase in the potency of cytotoxicity (Fig. 3). This outcome provides evidence that this EGFR-targeting β-defensin, a novel format of recombinant fusion protein, has been endowed with both selectivity and enhanced cytotoxic potency. Evidently, once the defensin molecule is integrated into the fusion protein, it can display a drastic escalation of cytotoxic potency. By in vitro imaging, the labeled Ec-LDP-hBD1 showed a clear tumor localization, indicating a specific biodistribution. In the athymic mice, the fusion protein Ec-LDP-hBD1 markedly suppressed the EGFR highly expressed carcinoma A431 (Fig. 4a). At the tolerated dose of 10 mg/kg, i.v. ×2, tumor growth was inhibited by 88.1%, implying that the use of the EGFR-targeting β-defensin could accomplish significant therapeutic efficacy (Fig. 4a).
As reported, human defensins contain an oncolytic motif that binds to the cell membrane and then disrupts the cytoplasmic and/or mitochondrial membranes, causing irreversible cell damage [9, 12, 14]. In addition, β-defensin HBD-1 can induce the permeation and swelling of mitochondria via mitochondrial membrane disruption, which causes the release of cytochrome C and leads to apoptosis [7, 16, 29]. With higher magnification, marked mitochondrial changes were observed in Ec-LDP-hBD1-treated cells, including visible mitochondrial swelling, vacuole formation, and the fusion and disruption of cristae (Fig. 6a). The present study revealed that Ec-LDP-hBD1 can induce apoptosis in A431, A549, and H460 cancer cells (Figs. 5a and 6b). As shown, the hBD1 had little influence on the mitochondria in A431 cells (Fig. 5b). These results collectively suggest that, once selectively internalized into the target relevant A431 cells, Ec-LDP-hBD1 could trigger mitochondria-mediated apoptosis.
In summary, a novel format of defensin that targets EGFR highly expressed cancer cells was designed and generated. The recombinant EGFR-targeting β-defensin Ec-LDP-hBD1 consists of an EGF-derived oligopeptide (Ec), a β-defensin-1 peptide (hBD1), and a lidamycin-derived apoprotein (LDP) that serves as the “scaffold” for the fusion protein. As shown, Ec-LDP-hBD1 selectively bound to EGFR highly expressed cancer cells, and its cytotoxicity was more potent than that of free β-defensin. Ec-LDP-hBD1 suppressed cancer cell proliferation and induced mitochondria-mediated apoptosis. Notably, Ec-LDP-hBD1 exerted potent therapeutic efficacy against the growth of human epidermoid carcinoma A431 and lung carcinoma H460 xenografts in athymic mice at well-tolerated doses. By in vivo imaging, Ec-LDP-hBD1 displayed a tumor-specific distribution with a clear image of localizations. To the best of our knowledge, this is the first report on the generation of an EGFR-targeting β-defensin and the evaluation of its highly therapeutic efficacy. This study suggests that the recombinant EGFR-targeting β-defensin Ec-LDP-hBD1 could be a promising agent in EGFR-targeting cancer therapy.
Electronic supplementary material
Acknowledgements
This study was financially supported by “Significant New Drug Development” Major Science and Technology Development Projects of China (No. 2014ZX09201042-003); High-Tech Research and Development Program of Shandong Province (2014GSF118022); National Natural Science Foundation of China (No. 81502691 and No. 81573246); and the Natural Science Foundation of Shandong Province (ZR2015HM040).
Author contributions
Wen-juan Liu and Yong-su Zhen designed the research; Wen-juan Liu, Xiu-jun Liu (animal experiments), Yi Li (animal experiments), Sheng-hua Zhang (In vivo fluorescence imaging) and Liang Li (HPLC) performed the experiments; Jia-lin Wang and Qing-fang Miao analyzed the data; Wen-juan Liu and Jian Xu wrote the manuscript. An author may list more than one contribution, and more than one author may have contributed to the same aspect.
Competing interests
The authors declare no competing interests.
Contributor Information
Wen-Juan Liu, Email: 122liuwenjuan@163.com.
Yong-Su Zhen, Email: zhenys@imb.pumc.edu.cn.
Electronic supplementary material
The online version of this article 10.1038/s41401-018-0069-8 contains supplementary material, which is available to authorized users.
References
- 1.Jarczak J, Kosciuczuk EM, Lisowski P, Strzalkowska N, Jozwik A, Horbanczuk J, et al. Defensins: natural component of human innate immunity. Hum Immunol. 2013;74:1069–79. doi: 10.1016/j.humimm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Suarez-Carmona M, Hubert P, Delvenne P, Herfs M. Defensins: “Simple” antimicrobial peptides or broad-spectrum molecules? Cytokine Growth Factor Rev. 2015;26:361–70. doi: 10.1016/j.cytogfr.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Ulm H, Wilmes M, Shai Y, Sahl HG. Antimicrobial host defensins-specific antibiotic activities and innate defense modulation. Front Immunol. 2012;3:249. doi: 10.3389/fimmu.2012.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cederlund A, Gudmundsson GH, Agerberth B. Antimicrobial peptides important in innate immunity. FEBS J. 2011;278:3942–51. doi: 10.1111/j.1742-4658.2011.08302.x. [DOI] [PubMed] [Google Scholar]
- 5.Du Y, Shang BY, Sheng WJ, Zhang SH, Li Y, Miao QF, et al. A recombinantly tailored beta-defensin that displays intensive macropinocytosis-mediated uptake exerting potent efficacy against K-Ras mutant pancreatic cancer. Oncotarget. 2016;7:58418–34. doi: 10.18632/oncotarget.11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly CJ, Glover LE, Campbell EL, Kominsky DJ, Ehrentraut SF, Bowers BE, et al. Fundamental role for HIF-1alpha in constitutive expression of human beta defensin-1. Mucosal Immunol. 2013;6:1110–18. doi: 10.1038/mi.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winter J, Pantelis A, Reich R, Martini M, Kraus D, Jepsen S, et al. Human beta-defensin-1, -2, and -3 exhibit opposite effects on oral squamous cell carcinoma cell proliferation. Cancer Invest. 2011;29:196–201. doi: 10.3109/07357907.2010.543210. [DOI] [PubMed] [Google Scholar]
- 8.Sun CQ, Arnold R, Fernandez-Golarz C, Parrish AB, Almekinder T, He J, et al. Human beta-defensin-1, a potential chromosome 8p tumor suppressor: control of transcription and induction of apoptosis in renal cell carcinoma. Cancer Res. 2006;66:8542–49. doi: 10.1158/0008-5472.CAN-06-0294. [DOI] [PubMed] [Google Scholar]
- 9.Gan W, Schneidman D, Zhang N, Ma B, Nussinov R. Probing oligomerized conformations of defensin in the membrane. Methods Mol Biol. 2017;1529:353–62. doi: 10.1007/978-1-4939-6637-0_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose SK, Gibson W, Bullard RS, Donald CD. PAX2 oncogene negatively regulates the expression of the host defense peptide human beta defensin-1 in prostate cancer. Mol Immunol. 2009;46:1140–48. doi: 10.1016/j.molimm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papo N, Shai Y. Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci. 2005;62:784–90. doi: 10.1007/s00018-005-4560-2. [DOI] [PubMed] [Google Scholar]
- 12.Schink KO, Tan KW, Stenmark H. Phosphoinositides in control of membrane dynamics. Annu Rev Cell Dev Biol. 2016;32:143–71. doi: 10.1146/annurev-cellbio-111315-125349. [DOI] [PubMed] [Google Scholar]
- 13.Bullard RS, Gibson W, Bose SK, Belgrave JK, Eaddy AC, Wright CJ, et al. Functional analysis of the host defense peptide Human Beta Defensin-1: new insight into its potential role in cancer. Mol Immunol. 2008;45:839–48. doi: 10.1016/j.molimm.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan TK, Lay FT, Poon IK, Hinds MG, Kvansakul M, Hulett MD. Human beta-defensin 3 contains an oncolytic motif that binds PI(4,5)P2 to mediate tumour cell permeabilisation. Oncotarget. 2016;7:2054–69. doi: 10.18632/oncotarget.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baxter AA, Richter V, Lay FT, Poon IK, Adda CG, Veneer PK, et al. The tomato defensin TPP3 binds phosphatidylinositol (4,5)-bisphosphate via a conserved dimeric cationic grip conformation to mediate cell lysis. Mol Cell Biol. 2015;35:1964–78. doi: 10.1128/MCB.00282-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagaoka I, Niyonsaba F, Tsutsumi-Ishii Y, Tamura H, Hirata M. Evaluation of the effect of human beta-defensins on neutrophil apoptosis. Int Immunol. 2008;20:543–53. doi: 10.1093/intimm/dxn012. [DOI] [PubMed] [Google Scholar]
- 17.Shao RG, Zhen YS. Enediyne anticancer antibiotic lidamycin: chemistry, biology and pharmacology. Anticancer Agents Med Chem. 2008;8:123–31. doi: 10.2174/187152008783497055. [DOI] [PubMed] [Google Scholar]
- 18.Ru Q, Shang BY, Miao QF, Li L, Wu SY, Gao RJ, et al. A cell penetrating peptide-integrated and enediyne-energized fusion protein shows potent antitumor activity. Eur J Pharm Sci. 2012;47:781–89. doi: 10.1016/j.ejps.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Liu XJ, Li L, Zhang SH, Li Y, Gao RJ, et al. An engineered TIMP2-based and enediyne-integrated fusion protein for targeting MMP-14 shows potent antitumor efficacy. Oncotarget. 2015;6:26322–34. doi: 10.18632/oncotarget.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Du Y, Liu XJ, Zhu BY, Zhang SH, Li L, et al. Recombinant EGFR/MMP-2 bi-targeted fusion protein markedly binding to non-small-cell lung carcinoma and exerting potent therapeutic efficacy. Pharmacol Res. 2017;126:66–76. doi: 10.1016/j.phrs.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Guo XF, Zhu XF, Shang Y, Zhang SH, Zhen YS. A bispecific enediyne-energized fusion protein containing ligand-based and antibody-based oligopeptides against epidermal growth factor receptor and human epidermal growth factor receptor 2 shows potent antitumor activity. Clin Cancer Res. 2010;16:2085–94. doi: 10.1158/1078-0432.CCR-09-2699. [DOI] [PubMed] [Google Scholar]
- 22.van de Poll ML, van Vugt MJ, Lenferink AE, van Zoelen EJ. Identification of the minimal requirements for binding to the human epidermal growth factor (EGF) receptor using chimeras of human EGF and an EGF repeat of Drosophila Notch. J Biol Chem. 1998;273:16075–81. doi: 10.1074/jbc.273.26.16075. [DOI] [PubMed] [Google Scholar]
- 23.Liu WJ, Liu XJ, Li L, Li Y, Zhang SH, Zhen YS. Tuftsin-based, EGFR-targeting fusion protein and its enediyne-energized analog show high antitumor efficacy associated with CD47 down-regulation. Cancer Immunol Immunother. 2014;63:1261–72. doi: 10.1007/s00262-014-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo XF, Zhu XF, Yang WC, Zhang SH, Zhen YS. An EGFR/HER2-bispecific and enediyne-energized fusion protein shows high efficacy against esophageal cancer. PLoS ONE. 2014;9:e92986. doi: 10.1371/journal.pone.0092986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong G, Zhang S, Li Y, Liu X, Gao R, Miao Q, et al. A tandem scFv-based fusion protein and its enediyne-energized analogue show intensified therapeutic efficacy against lung carcinoma xenograft in athymic mice. Cancer Lett. 2010;295:124–33. doi: 10.1016/j.canlet.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Du Y, Liu WJ, Li L, Li Y, Wang XF, et al. Intensive fibrosarcoma-binding capability of the reconstituted analog and its antitumor activity. Drug Deliv. 2018;25:102–11. doi: 10.1080/10717544.2017.1410261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Droin N, Hendra JB, Ducoroy P, Solary E. Human defensins as cancer biomarkers and antitumour molecules. J Proteom. 2009;72:918–27. doi: 10.1016/j.jprot.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Sun YW, Xu J, Zhou J, Liu WJ. Targeted drugs for systemic therapy of lung cancer with brain metastases. Oncotarget. 2018;9:5459–72. doi: 10.18632/oncotarget.23616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen R, Banik B, Pathak RK, Kumar A, Kolishetti N, Dhar S. Nanotechnology inspired tools for mitochondrial dysfunction related diseases. Adv Drug Deliv Rev. 2016;99:52–69. doi: 10.1016/j.addr.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.