Abstract
Chronic heart failure (CHF) represents a major cause of hospitalization and death. Recent evidence shows that novel biomarkers such as soluble suppression of tumorigenicity (sST2), growth-differentiation factor-15 (GDF-15), soluble urokinase plasminogen activator receptor (suPAR) and heart-type fatty acid binding protein (H-FABP) are correlated with inflammatory and ischemic responses in CHF patients. In this study we examined the effects of Ivabradine that inhibited the hyperpolarization-activated cyclic nucleotide-gated channel (HCN channel, also called funny current If), thereby leading to selective heart rate reduction and improved myocardial oxygen supply on the cardiac biomarkers sST2, GDF-15, suPAR and H-FABP in 50 CHF patients at the University Hospital of Jena. Patients were divided into three groups based on the etiology of CHF: dilated cardiomyopathy (DCM, n=20), ischemic cardiomyopathy (ICM, n=20) and hypertensive cardiomyopathy (HCM, n=10). The patients were administered Ivabradine (5 mg, bid for 3 months, and 7.5 mg bid for further 3 months). Analyses of cardiovascular biomarkers were performed at baseline as well as at 3- and 6-month follow-ups. At 6-month follow-up, GDF-15 levels were significantly reduced compared to baseline levels (P=0.0215), indicating a reduction in the progress of cardiac remodeling. H-FABP concentration was significantly lower in DCM patients compared to ICM (1.89 vs 3.24 μg/mL) and HCM patients (1.89 vs 3.80 μg/mL), and decreased over the 6-month follow-up (P=0.0151). suPAR median levels remained elevated, implying major ongoing inflammatory processes. As shown by significant decreases in GDF-15 and H-FABP levels, a reduction in ventricular remodeling and sub-clinical ischemia could be assumed. However, markers of hemodynamic stress (sST2) and inflammation (suPAR) showed no change or progression after 6 months of Ivabradine treatment in CHF patients. Further studies are necessary to validate the clinical applicability of these novel cardiovascular biomarkers.
Keywords: heart failure, cardiomyopathy, Ivabradine, heart rate, biomarker, sST2, GDF-15, suPAR, H-FABP
Introduction
Heart failure (HF) is considered a leading cause of hospitalization and death with a prevalence of 1%–2% of the adult population in first-world countries1,2. With an increasing prevalence in elderly patients, heart failure is responsible for a growing rate of morbidity and mortality worldwide, representing a public health challenge with huge expenses for the healthcare system1,2,3.
According to current ESC Guidelines, heart failure can be divided into three subgroups depending on left ventricular ejection fraction: HF with reduced ejection fraction (HFrEF; LVEF <40%, “systolic“), HF with mid-range ejection fraction (HFmrEF; LVEF 40%–49%) and HF with preserved ejection fraction (HFpEF; LVEF ≥ 50%, “diastolic” HF),4.
Heart failure was shown to be associated with inflammatory processes mediated by pro-inflammatory cytokines including IL-1, IL, 4, IL-6, IL-18, high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor alpha (TNF-alpha), and FAS ligand5,6. Higher levels of these inflammatory biomarkers induce heart muscle remodeling, endothelial dysfunction and ventricular fibrosis6,7. The degree of inflammation plays an important role in the progression of heart failure and has been reported to correlate with disease severity, resulting in increased mortality rates8.
In recent years, novel biomarkers such as soluble suppression of tumorigenicity (sST2)9, growth-differentiation factor-15 (GDF-15), soluble urokinase plasminogen activator receptor (suPAR), and heart-type fatty acid binding protein (H-FABP) have been shown in several studies to correlate with inflammatory and ischemic responses in patients suffering from heart failure10,11,12,13.
sST2 represents a marker of inflammation as well as hemodynamic stress and cardiomyocyte strain. It has two isoforms, a membrane-bound receptor (ST2L) with IL-33 as the functional ligand and a soluble form (sST2)14. GDF-15 increases in response to hypoxia, oxidative stress, and inflammation as well as coronary artery disease and is also involved in the regulation of apoptotic pathways and remodeling15. H-FABP is an early marker of myocardial ischemia and cardiomyocyte damage16. Furthermore, recent studies demonstrated a significant increase in H-FABP levels in heart failure patients17. suPAR is a marker of immune system and pro-inflammatory activity and is associated with systemic inflammatory response syndrome (SIRS), malignancies and cardiovascular disease18,19.
A high resting heart rate is of highest prognostic relevance in heart failure and is associated with rapid disease progression, resulting in increased rates of morbidity and mortality in patients with reduced ejection fraction20,21,22. Ivabradine has been evaluated as being one of the most promising new therapeutic options for heart rate reduction20,22 and specifically inhibits the hyperpolarization-activated cyclic nucleotide-gated funny current channel (If; located in pacemaker cells of the sinoatrial node), thereby leading to a reduction in heart rate20,23. In comparison to beta-blockers, Ivabradine has no effect on myocardial contractility, atrioventricular or ventricular conduction24,25 and has been proven to reduce cardiovascular mortality and hospitalization due to heart failure26,27.
In this study, we aimed to investigate the pharmacodynamic effects of Ivabradine on four novel cardiovascular biomarkers (namely, the suppression of tumorigenicity (sST2), growth-differentiation factor-15 (GDF-15), soluble urokinase plasminogen activator receptor (suPAR), and heart-type fatty acid binding protein (H-FABP)) in patients suffering from ischemic cardiomyopathy (ICM), dilated cardiomyopathy (DCM) and hypertensive cardiomyopathy (HCM).
Materials and methods
In total, 50 patients were included in this study. Recruitment of patients was conducted at the HF outpatient clinic at the University Hospital Jena, Germany. All patients included in our study had stable CHF for over 1 year and were under optimal medical therapy. Moreover, informed consent was obtained from all patients participating in the study. The study protocol was approved by the local ethics committee and was conducted in accordance with the “Declaration of Helsinki” (1964).
The inclusion criteria were as follows: (i) age >18 years; (ii) sinus rhythm; (iii) heart rate >75 bpm; (iv) left ventricular systolic dysfunction (LV-EF <50%); (v) ischemic, dilated, or hypertensive stable CHF >12 months; and (vi) standard heart failure therapy with beta-blockers, ACE-inhibitors, and aldosterone-receptor antagonists (NYHA III-IV) at adequate dosages.
Exclusion criteria were chosen based on potential interference with our analyses and were defined as follows: (i) recent cardiac decompensation (<3 months); (ii) recent acute coronary syndrome (<3 months); (iii) acute or chronic infections; (iv) malignancies; (v) autoimmune diseases; (vi) hyperthyroidism; (vii) medication with immunosuppressive agents; and (viii) unknown etiology of CHF.
The included patients were divided into three subgroups depending on the etiology of CHF. Groups were defined as (1) ischemic cardiomyopathy (ICM; CAD was proven by coronary angiography); (2) dilated cardiomyopathy (DCM; angiographically excluded CAD, occasionally proven by biopsy, no history of hypertension); and (3) hypertensive cardiomyopathy (HCM; angiographically excluded CAD, LV hypertrophy, history of hypertension). In our study cohort, DCM and ICM were diagnosed in 20 patients and HCM was diagnosed in 10 patients.
The total duration of the study was 6 months. Following the baseline visit, two follow-ups were performed at 3 and 6 months at the outpatient clinic, and adverse events, clinical course of heart failure and vital parameters as well as biomarker levels were evaluated. Furthermore, laboratory samples were drawn and analyzed. All laboratory values were obtained from the standard in-hospital laboratory. Biomarker levels were obtained using ELISA (see Laboratory analysis).
Of the 50 enrolled patients, 17 had to be withdrawn from the study due to following side effects: dizziness/nausea, 4; CHF worsening, 4; eczema, 4; headache/stomach pain, 1; weakness, 1; noncompliance, 1; pacemaker implantation, 1; and diarrhea, 1.
At the baseline visit, Ivabradine was started on a dosage of 2× 5 mg per day. At the 3-month follow-up, the dosage was adjusted to 2× 7.5 mg if heart rate remained above 60 bpm. In patients with a heart rate below 60 bpm, a dosage of 2 × 5 mg per day was maintained. After the 6-month follow-up, the study was ended.
Laboratory analysis
Standard clinical laboratory parameters were measured and analyzed at the Department of Clinical Chemistry (University Hospital Jena). The measurements included brain natriuretic peptide (BNP; pg/mL), interleukin 6 (IL-6; ng/L), C-reactive protein (CRP, mg/L), troponin I (TNI; μg/L), creatinine (mg/dL), urea (mg/dL), sodium (mg/dL) and hemoglobin (mg/dL). Serum concentrations of sST2, GDF-15, suPAR, and H-FABP were quantified using a commercially available ELISA kit (DuoSet ELISA, DY523B, DY957, DY807 and DY1678, R&D Systems, USA). ELISA assays were performed in accordance with the instructions supplied by the manufacturer at baseline and at 3- and 6-month follow-ups.
In brief, serum samples and standard proteins were added to multiwell plates (Nunc Maxisorp plates, VWR International, Austria) that were coated with the respective capture antibody, and the plates were incubated for two hours. The plates were then washed with washing buffer (Tween 20, Sigma Aldrich, USA and phosphate-buffered saline solution). Then, a biotin-labeled antibody was added to each well, and the plate was incubated for another two hours. The ELISA plates were washed once again, and a streptavidin-labeled horseradish peroxidase solution was added. After adding tetramethylbenzidine (TMB; Sigma Aldrich, USA) a color reaction was achieved and was then stopped by H2SO4. The optical density was measured at 450 nm using an ELISA plate-reader (iMark Microplate Absorbance Reader, Bio-Rad Laboratories, Austria). Intra-assay precision and inter-assay precision were recorded as follows: sST2: 4.4%–5.6%, 5.4%–7.1%; GDF-15: 1.8%–2.8%, 5.1%–5.9%; suPAR: 2.1%–7.5%, 4.7%–6%; and H-FABP: 0.3%–4.7%, 1.3%–17.4%, respectively.
Statistical analysis
All statistical analyses were performed using GraphPad-Prism software (GraphPad Software, La Jolla, CA, USA) and SPSS (22.0, SPSS Inc, USA). Normally distributed parameters were assessed using the Kolmogorov-Smirnov test and are presented as the mean±standard error of the mean; all other values are shown as median±interquartile range in the figures and as median±minimum and maximum in the tables. Means were compared between groups using Student`s t-test or the Mann-U test. Changes in biomarker levels over the follow-up period were compared using the Wilcoxon matched-pairs test. Correlation analysis was assessed using Spearman's rank correlation coefficient. NYHA stages were compared using the Kruskal-Wallis test with Dunn's post-test for multiple comparisons. A value of P<0.05 was considered statistically significant.
Results
Baseline characteristics
The effects of Ivabradine treatment on vital parameters have been previously described by Rohm et al28. The study design and the time points of clinical follow-ups and biomarker analyses of the present study are shown in Figure 1. Baseline characteristics are shown in Table 1. Patients suffering ICM received diuretic therapy less often than patients suffering DCM and HCM (P=0.08) and were affected more frequently by syncope (P=0.09). There were no significant differences in NYHA stage and cardiovascular risk factors between the three groups at baseline. After 6 months of Ivabradine treatment, symptom improvement was seen, as shown by a decrease in NYHA functional class (from a median of 2.25 to 2.0, P<0.001); in addition, for the subgroups, a trend towards a lower NYHA stage was evident (from 2.0 to 1.5 in DCM patients (P=0.12), from 2.75 to 2.0 in ICM patients (P=0.06), and from 3.0 to 2.0 in HCM patients (P=0.06).
Figure 1.
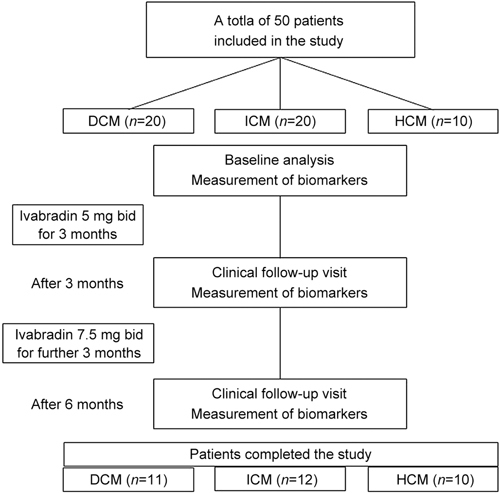
Study design and time points of clinical follow-ups and biomarker analyses.
Table 1.
Patient demographics and characteristics at baseline.
| DCM | ICM | HCM | Overall cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Min | Max | Median | Min | Max | Median | Min | Max | Median | Min | Max | P-value | |
| sST2 | 4288.5 | 2294.0 | 7172.8 | 3746.8 | 1735.1 | 6530.2 | 3843.0 | 3019.5 | 13207.4 | 4126.8 | 1735.1 | 13207.4 | .817 |
| H-FABP | 2.7 | 1.3 | 387.0 | 1.8 | 0.0 | 3.1 | 2.1 | 1.4 | 7.4 | 2.1 | 0.0 | 387.0 | .090 |
| GDF-15 | 732.4 | 594.3 | 118257.0 | 884.0 | 338.3 | 2074.6 | 711.1 | 695.3 | 1887.1 | 768.5 | 338.3 | 118257.0 | .965 |
| suPAR | 2275.2 | 1505.0 | 3294.5 | 2069.6 | 1053.2 | 3647.9 | 1671.2 | 1422.7 | 3137.1 | 2136.6 | 1053.2 | 3647.9 | .373 |
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||||||
| Heart rate (bpm) | 79.9 | 2.3 | 78.9 | 1.5 | 81.3 | 1.8 | 79.7 | 1.1 | .725 | ||||
| Blood pressure (systolic) | 123.0 | 5.0 | 150.7 | 6.3 | 130.7 | 7.7 | 137.4 | 4.3 | .007 | ||||
| Blood pressure (diastolic) | 82.0 | 3.6 | 87.4 | 2.8 | 89.8 | 4.6 | 86.0 | 2.0 | .333 | ||||
| QRS length (ms) | 118.9 | 8.0 | 119.6 | 9.1 | 131.6 | 17.2 | 121.9 | 5.8 | .698 | ||||
| EF (%) | 32.5 | 3.0 | 34.2 | 2.2 | 30.0 | 4.8 | 32.8 | 1.7 | .673 | ||||
| LVEDD (mm) | 60.8 | 2.4 | 61.6 | 3.4 | 62.0 | 3.8 | 61.4 | 1.9 | .974 | ||||
| LVESD (mm) | 49.2 | 3.3 | 50.1 | 3.8 | 54.2 | 5.8 | 50.6 | 2.3 | .730 | ||||
| LVESV (mL) | 102.9 | 16.0 | 102.4 | 9.9 | 133.7 | 20.6 | 109.5 | 8.3 | .310 | ||||
| Pulmonary arterial pressure (PAP) | 14.9 | 4.7 | 14.8 | 6.9 | 22.4 | 7.5 | 16.5 | 3.7 | .716 | ||||
| BNP (pg/mL) | 320.1 | 149.9 | 217.5 | 85.5 | 578.3 | 330.3 | 320.4 | 88.8 | .353 | ||||
| IL-6 (pg/mL) | 7.1 | 2.3 | 5.2 | 1.1 | 12.2 | 5.0 | 7.2 | 1.4 | .174 | ||||
| hsCRP (mg/L) | 6.0 | 1.4 | 4.1 | 1.2 | 14.2 | 4.5 | 6.6 | 1.2 | .009 | ||||
| Troponin I (ng/mL) | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | .477 | ||||
| Creatinine (μmol/L) | 97.8 | 5.4 | 109.0 | 9.0 | 120.8 | 12.9 | 107.1 | 5.2 | .288 | ||||
| BUN (mmol/L) | 7.8 | 0.9 | 9.0 | 1.3 | 11.3 | 2.7 | 8.9 | 0.8 | .356 | ||||
| Sodium (mmol/L) | 139.3 | 0.8 | 139.9 | 0.6 | 139.8 | 0.9 | 139.7 | 0.4 | .843 | ||||
| Hemoglobin (mmol/L) | 8.8 | 0.2 | 9.3 | 0.2 | 8.8 | 0.4 | 9.0 | 0.1 | .204 | ||||
| Male sex | 83% | 87% | 83% | 85% | 0.97 | ||||||||
| Diabetes mellitus type 2 | 42% | 67% | 40% | 53% | 0.35 | ||||||||
| Arterial Hypertension | 58% | 80% | 100% | 76% | 0.13 | ||||||||
| Smoking | 25% | 53% | 20% | 38% | 0.22 | ||||||||
| Hyperlipidemia | 50% | 67% | 80% | 63% | 0.46 | ||||||||
| Adipositas | 73% | 67% | 50% | 66% | 0.64 | ||||||||
| Betablocker | 100% | 100% | 100% | 100% | |||||||||
| ACEI/AT1 | 100% | 100% | 100% | 100% | |||||||||
| Diuretics | 100% | 73% | 100% | 88% | 0.08 | ||||||||
| Aldosterone antagonists | 67% | 73% | 40% | 66% | 0.40 | ||||||||
| NYHA stages | |||||||||||||
| I | 8% | 0% | 0% | 3% | 0.41 | ||||||||
| II | 50% | 47% | 33% | 45% | 0.79 | ||||||||
| III | 42% | 47% | 67% | 48% | 0.60 | ||||||||
| IV | 0% | 0% | 0% | 0% | |||||||||
Heart rate analysis
An overall heart rate reduction was observed from 79.7±2.6 beats per minute (bpm) to 64.4±4.0 bpm after 6 months (P<0.001). The lowest heart rates at follow-up were found in patients with DCM (59.8±2.2 bpm) compared to ICM (68.4±3.2 bpm) and HCM patients (65.4±2.1 bpm) (P<0.01 for all subgroups). The effects of Ivabradine treatment on NYHA stage, heart rate, ejection fraction (EF) and BNP levels are shown in Supplementary Figure S1.
Biomarker levels – baseline values and at 3-month follow-up
In the per-protocol-analysis (n=33), the baseline levels of biomarkers showed no significant differences between the three groups. At the 3-month follow-up, a general trend toward higher biomarker levels for sST2, GDF-15, suPAR and H-FABP was observed; however, no significant differences were observed compared to baseline levels. Furthermore, there were no significant differences in biomarker levels between ICM, DCM, and HCM patients at the 3-month follow-up.
Biomarker levels – 6-month follow-up
At the six-month follow-up, the median concentration of GDF-15 in the total cohort was significantly lower compared to baseline levels (baseline, 768.5 μg/mL vs 6 months, 567.5 μg/mL; P=0.0215). No significant differences were observed between ICM, DCM, and HCM patients. The median concentration of H-FABP also decreased significantly in the total cohort (from 2.1 to 1.6 ng/mL, P=0.0151; Figure 2). However, for all the other biomarkers tested, no significant differences were found between DCM, ICM, and HCM patients. Median suPAR levels were significantly elevated at the 6-month follow-up (from 2137 to 3178 pg/mL, P=0.009), and there were no significant differences between the groups with differing CHF etiology. Median levels of sST2 did not significantly differ from baseline levels. In addition, no significant differences in sST2 levels were observed between the ICM, DCM, and HCM patients.
Figure 2.
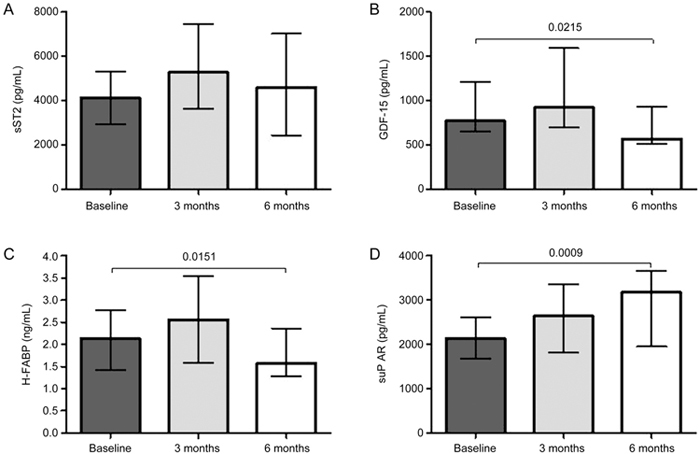
Per-protocol-analysis of novel cardiovascular biomarkers in CHF patients. sST2 levels did not significantly change (A). GDF-15 and H-FABP levels decreased significantly after 6 months of treatment with Ivabradine (B, C). suPAR concentrations increased over the follow-up period (D).
For BNP, IL-6 and hsCRP, no significant differences were found at the 6-month follow-up compared to baseline values. BNP levels were 113 pg/mL at baseline and 132 pg/mL after 6 months, IL-6 levels were 4.0 ng/L at baseline and 3.7 ng/L after 6 months, and hsCRP levels were 4.2 mg/L at the start of the study and 3.2 mg/L after completion (P=0.65, P=0.61 and P=0.25, respectively). Among the tested novel cardiovascular biomarkers, only GDF-15 was correlated with BNP (r=0.46, P=0.0088). No significant correlations were found for H-FABP, sST2 or suPAR with BNP, IL-6 or hsCRP.
Intention to treat analysis
A similar response was evident in the intention to treat analysis (total n=50). GDF-15 levels decreased significantly after 6 months of Ivabradine treatment (from 637 to 510 pg/mL, P=0.0231). H-FABP concentrations decreased only in trend (from 2.2 to 1.9 ng/mL, P=0.21). suPAR levels increased over the follow-up period of 6 months (from 2231 to 2549 pg/mL, P=0.0025). sST2 levels showed no significant decrease in the intention to treat analysis (from 4051 to 3648 pg/mL, P=0.87) (Figure 3).
Figure 3.
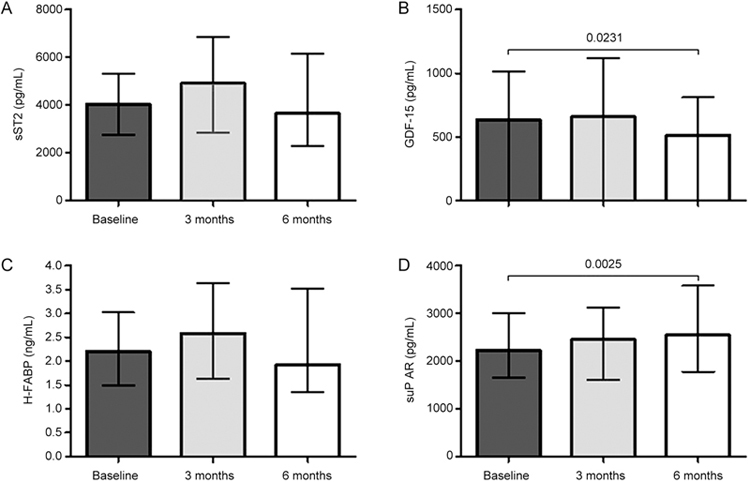
Intention to treat analysis. sST2 levels showed no significant changes (A). GDF-15 levels decreased significantly after 6 months (B), but H-FABP levels decreased marginally (C). suPAR concentrations increased after 6 months (D).
Correlation analysis of biomarkers and risk factors
Concerning cardiovascular risk factors, all four biomarkers were tested for their association with smoking, angina pectoris, arterial hypertension and DM type II (Table 2). A correlation between smoking and sST2 levels was observed (P=0.04). Besides, no correlations between mentioned cardiovascular risk factors and biomarker levels were evident.
Table 2.
Biomarker levels in different subgroups of patients with heart failure (pg/mL for sST2, GDF-15 and uPAR, ng/mL for H-FABP).
| Non smoking | Smoking | ||||||
| Median | Min | Max | Median | Min | Max | P-value | |
| sST2 | 3404 | 1742 | 6348 | 5116 | 1735 | 13207 | 0.04 |
| H-FABP | 2.18 | 0.00 | 367.00 | 2.22 | 0.86 | 387.00 | 0.92 |
| GDF-15 | 739 | 338 | 2045 | 921 | 604 | 118257 | 0.24 |
| suPAR | 2184 | 1053 | 3648 | 2176 | 1372 | 3396 | 0.77 |
| No angina pectoris | Angina pectoris | ||||||
| Median | Min | Max | Median | Min | Max | P-value | |
| sST2 | 3843 | 1735 | 13207 | 3837 | 2755 | 4919 | 0.93 |
| H-FABP | 2.29 | 0.00 | 387.00 | 1.99 | 1.31 | 2.67 | 0.64 |
| GDF-15 | 761 | 338 | 2075 | 675 | 515 | 836 | 0.40 |
| suPAR | 2137 | 1053 | 3294 | 2330 | 1264 | 3396 | 0.93 |
| No arterial hypertension | Arterial hypertension | ||||||
| Median | Min | Max | Median | Min | Max | P-value | |
| sST2 | 4803 | 1735 | 6530 | 3843 | 1742 | 13207 | 0.44 |
| H-FABP | 2.24 | 0.86 | 367.00 | 2.07 | 0.00 | 387.00 | 0.44 |
| GDF-15 | 1108 | 632 | 118257 | 761 | 338 | 2075 | 0.18 |
| suPAR | 2137 | 1505 | 3294 | 2137 | 1053 | 3648 | 0.93 |
| No type 2 diabetes | Type 2 diabetes | ||||||
| Median | Min | Max. | Median | Min | Max | P-value | |
| sST2 | 4065 | 1735 | 13207 | 4094 | 2258 | 7173 | .80 |
| H-FABP | 2.33 | 0.86 | 367.00 | 2.10 | 0.00 | 387.00 | .44 |
| GDF-15 | 887 | 594 | 2045 | 802 | 338 | 118257 | .69 |
| suPAR | 2079 | 1372 | 3156 | 2314 | 1053 | 3648 | .41 |
Discussion
A high resting heart rate is of highest prognostic relevance in heart failure patients and is associated with increased morbidity and mortality in patients with reduced ejection fraction20,21,22. Myocardial oxygen consumption and energy demand increase due to higher resting heart rates while myocardial perfusion decreases due to a shortening of diastole, resulting in a decrease in oxygen supply despite the increased myocardial strain29,30. The consequences are a rapid disease progression leading to a significant worsening of heart failure symptoms and, consequently, NYHA functional class. Therefore, in recent years, numerous studies have examined new pharmacological treatments for heart rate reduction, with Ivabradine being one of the most promising new therapeutic options. In several prior studies, a beneficial effect of Ivabradine on morbidity and mortality was shown in heart failure patients26,31. While these effects have mainly been attributed to a reduction in heart rate and an improvement in cardiac output and oxygen consumption, more recent studies have proposed additional pleiotropic effects of Ivabradine, especially on inflammatory and ischemic processes28,32. According to Sargento et al, the NT-pro BNP reduction triggered by short-term Ivabradine treatment is correlated closely with the heart rate reduction in patients suffering from systolic heart failure33; however, Ordu et al reported no correlation between heart rate variation and NT-pro BNP34. The role of biomarkers in treating heart failure patients has been the subject of intensive investigations in recent years, representing a new possibility for prognosis and the evaluation of therapy success. In particular, sST2 has entered clinical practice and has been included in the current heart failure guidelines of the AHA35. Therefore, we aimed to investigate the correlation of Ivabradine treatment in heart failure patients (ICM, DCM and HCM) with the levels of four novel cardiovascular biomarkers (sST2, GDF-15, suPAR and H-FABP).
In our study population, there were no significant differences in NYHA stage and cardiovascular risk factors between ICM, DCM, and HCM patients at baseline, representing a homogenous patient population. Confirming former studies, our analysis showed that Ivabradine treatment led to a significant decrease in resting heart rate in all patients with CHF, regardless of disease etiology20.
Baseline levels of biomarkers showed no significant differences between the three groups, indicating etiology-independent changes in CHF. After 3 months, no significant differences in the biomarker levels for sST2, GDF-15, suPAR, and H-FABP were evident. Biomarker levels even tended to be higher after 3 months of Ivabradine treatment. However, considering the time necessary for cardioprotective alterations and response, a period of three months may be too short to find a measurable impact of Ivabradine treatment on disease progression and significant changes in biomarker levels. Furthermore, no significant differences in biomarker levels were observed between the three HF groups after three months. Hence, the susceptibility for Ivabradine-induced changes in biomarkers is assumed to be similar among the ICM, DCM, and HCM patients.
After six months of Ivabradine treatment, significant decreases in GDF-15 and H-FABP levels were evident. Because GDF-15 represents a marker of oxidative stress and inflammation, GDF-15 downregulation indicates that the cardioprotective effect occurs through anti-inflammatory and anti-oxidative effects36. Because inflammation and ischemia are leading contributors to disease progression in HF, a beneficial effect of Ivabradine in addition to its bradycardic effect in CHF could be assumed37,38.
In contrast, H-FABP represents an ischemic marker that is susceptible to very early ischemic myocardial damage39. A decrease in H-FABP levels is considered a marker for improved myocardial perfusion and reduced subclinical myocardial ischemia39. However, H-FABP downregulation can be mainly attributed to the heart rate-reducing effect of Ivabradine. Oxygen consumption is reduced by a decrease in heart rate, while myocardial perfusion is increased through prolonged diastole, thus optimizing myocardial blood supply.
Surprisingly, levels of sST2 showed no significant changes at the six-month follow-up in our study. sST2 is a marker of hemodynamic stress and inflammation as well as myocardial strain40. As hemodynamic stress and myocardial strain are improved by Ivabradine treatment due to its heart rate-reducing effects, a decrease of sST2 levels was expected. Additionally, previous studies also described an inhibiting effect of Ivabradine on the secretion of pro-inflammatory cytokines such as IL-6 and TNF-alpha, which trigger the secretion of sST2, thus suggesting an additional anti-inflammatory effect of Ivabradine28. However, the absence of the progression of sST2 levels observed in our study indicates that at least a mild anti-inflammatory effect occurred, most likely due to Ivabradine treatment. Furthermore, levels of sST2 decreased after 6 months when compared to those at the 3-month follow-up, suggesting a stronger effect of Ivabradine on sST2 levels in long term treatment.
Levels of suPAR were increased after 3 months, reaching statistical significance at the 6-month follow-up. suPAR is involved in various processes, such as tissue remodeling, wound healing, and the development of cancer and metastasis13,19. Levels of suPAR are often elevated in these processes and mainly facilitate proteolysis. Therefore, in CHF-patients, a correlation between suPAR upregulation and cardiac remodeling and fibrosis can be assumed. As a result, elevated suPAR levels after six months are primarily interpreted as a marker of ongoing cardiac remodeling and disease progression in our study population. Interestingly, ICM patients showed the strongest correlation with elevated suPAR levels, again suggesting an involvement in cardiac remodeling. Furthermore, it appears that the secretion of suPAR is not influenced by Ivabradine treatment. However, due to our relatively short treatment window and the chronic and complex processes involved in cardiac remodeling, it is possible that suPAR levels may have shown a late response to bradycardic treatment that was not observable by our study.
Limitations
This study was conducted as a retrospective and single-center study. Furthermore, the number of patients in our study population was quite small, resulting in a reduced statistical power. There were only two follow-ups, covering 6 months of therapy; this period is relatively short considering the time necessary for cardioprotective alterations and biomarker response. Moreover, the study was not double-blinded, which might have influenced the patients' subjective perceptions of their symptoms.
Conclusion
In our current study, we observed an anti-inflammatory effect of Ivabradine as represented by a decrease of GDF-15 levels and the absence of an increase in sST2 levels. Considering the pathophysiology of CHF, this result is highly relevant for future therapy. Moreover, we observed an impact of Ivabradine on subclinical ischemia in HF as represented by a decrease in H-FABP levels. However, suPAR levels remained high in our study, most likely indicating ongoing cardiac remodeling even 6 months after Ivabradine therapy. Therefore, we conclude that Ivabradine has an anti-inflammatory and anti-ischemic potential in short-term use in addition to its bradycardic effects, thereby targeting different pathophysiological mechanisms in CHF. Nevertheless, more prospective studies are needed to further evaluate the effect of Ivabradine on inflammatory and ischemic processes as well as on cardiac remodeling, especially for long-term treatment.
Electronic supplementary material
Effects of Ivabradine treatment on NYHA stage, heart rate, ejection fraction (EF) and BNP levels.
Electronic supplementary material
Supplementary Information is available on the website of Acta Pharmacologica Sinica. Molecular Psychiatry website 10.1038/aps.2017.167
References
- 1.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure: The Rotterdam Study. Eur Heart J. 2004;25:1614–9. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 3.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–46. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lekavich CL, Barksdale DJ, Neelon V, Wu JR. Heart failure preserved ejection fraction (HFpEF): an integrated and strategic review. Heart Fail Rev. 2015;20:643–53. doi: 10.1007/s10741-015-9506-7. [DOI] [PubMed] [Google Scholar]
- 5.Oikonomou E, Tousoulis D, Siasos G, Zaromitidou M, Papavassiliou AG, Stefanadis C. The role of inflammation in heart failure: new therapeutic approaches. Hellenic J Cardiol. 2011;52:30–40. [PubMed] [Google Scholar]
- 6.Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35. doi: 10.1159/000338166. [DOI] [PubMed] [Google Scholar]
- 7.Peng H, Sarwar Z, Yang XP, Peterson EL, Xu J, Janic B, et al. Profibrotic role for interleukin-4 in cardiac remodeling and dysfunction. Hypertension. 2015;66:582–9. doi: 10.1161/HYPERTENSIONAHA.115.05627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev. 2010;15:331–41. doi: 10.1007/s10741-009-9140-3. [DOI] [PubMed] [Google Scholar]
- 9.Dieplinger B, Mueller T. Soluble ST2 in heart failure. Clin Chim Acta. 2015;443:57–70. doi: 10.1016/j.cca.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Andersson C, Enserro D, Sullivan L, Wang TJ. Januzzi JL Jr, Benjamin EJ, et al. Relations of circulating GDF-15, soluble ST2, and troponin-I concentrations with vascular function in the community: The Framingham Heart Study. Atherosclerosis. 2016;248:245–51. doi: 10.1016/j.atherosclerosis.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouleur AC. Which biomarkers do clinicians need for diagnosis and management of heart failure with reduced ejection fraction? Clin Chim Acta. 2015;443:9–16. doi: 10.1016/j.cca.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Reiter M, Twerenbold R, Reichlin T, Mueller M, Hoeller R, Moehring B, et al. Heart-type fatty acid-binding protein in the early diagnosis of acute myocardial infarction. Heart. 2013;99:708–714. doi: 10.1136/heartjnl-2012-303325. [DOI] [PubMed] [Google Scholar]
- 13.Eugen-Olsen J, Andersen O, Linneberg A, Ladelund S, Hansen TW, Langkilde A, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. doi: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 14.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wollert KC, Kempf T. Growth differentiation factor 15 in heart failure: an update. Curr Heart Fail Rep. 2012;9:337–45. doi: 10.1007/s11897-012-0113-9. [DOI] [PubMed] [Google Scholar]
- 16.Al-Hadi HA, Fox KA. Cardiac markers in the early diagnosis and management of patients with acute coronary syndrome. Sultan Qaboos Univ Med J. 2009;9:231–46. [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtenauer M, Jirak P, Wernly B, Paar V, Rohm I, Jung C, et al. A comparative analysis of novel cardiovascular biomarkers in patients with chronic heart failure. Eur J Intern Med. 2017;44:31–8. doi: 10.1016/j.ejim.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157–72. doi: 10.1155/2009/504294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sehestedt T, Lyngbak S, Eugen-Olsen J, Jeppesen J, Andersen O, Hansen TW, et al. Soluble urokinase plasminogen activator receptor is associated with subclinical organ damage and cardiovascular events. Atherosclerosis. 2011;216:237–43. doi: 10.1016/j.atherosclerosis.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 20.Muller-Werdan U, Stockl G, Werdan K. Advances in the management of heart failure: the role of ivabradine. Vasc Health Risk Manag. 2016;12:453–70. doi: 10.2147/VHRM.S90383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–21. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 22.Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–94. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 23.Borer JS, Deedwania PC, Kim JB, Bohm M. Benefits of heart rate slowing with ivabradine in patients with systolic heart failure and coronary artery disease. Am J Cardiol. 2016;118:1948–53. doi: 10.1016/j.amjcard.2016.08.089. [DOI] [PubMed] [Google Scholar]
- 24.DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106:434–46. doi: 10.1161/CIRCRESAHA.109.208041. [DOI] [PubMed] [Google Scholar]
- 25.de Silva R, Fox KM. Angina: Ivabradine for treatment of stable angina pectoris. Nat Rev Cardiol. 2009;6:329–30. doi: 10.1038/nrcardio.2009.47. [DOI] [PubMed] [Google Scholar]
- 26.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–85. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 27.Beautiful Study Group, Ferrari R, Ford I, Fox K, Steg PG, Tendera M. The BEAUTIFUL study: randomized trial of ivabradine in patients with stable coronary artery disease and left ventricular systolic dysfunction - baseline characteristics of the study population. Cardiology 2008; 110: 271–82. [DOI] [PubMed]
- 28.Rohm I, Kretzschmar D, Pistulli R, Franz M, Schulze PC, Stumpf C, et al. Impact of ivabradine on inflammatory markers in chronic heart failure. J Immunol Res. 2016;2016:6949320. doi: 10.1155/2016/6949320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardehali A, Ports TA. Myocardial oxygen supply and demand. Chest. 1990;98:699–705. doi: 10.1378/chest.98.3.699. [DOI] [PubMed] [Google Scholar]
- 30.van den Wijngaard JP, Kolyva C, Siebes M, Dankelman J, van Gemert MJ, Piek JJ, et al. Model prediction of subendocardial perfusion of the coronary circulation in the presence of an epicardial coronary artery stenosis. Med Biol Eng Comput. 2008;46:421–32. doi: 10.1007/s11517-008-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Psotka MA, Teerlink JR. Ivabradine: role in the chronic heart failure armamentarium. Circulation. 2016;133:2066–75. doi: 10.1161/CIRCULATIONAHA.115.018094. [DOI] [PubMed] [Google Scholar]
- 32.Dominguez-Rodriguez A, Consuegra-Sanchez L, Blanco-Palacios G, Abreu-Gonzalez P, Sanchez-Grande A, Bosa-Ojeda F, et al. Anti-inflammatory effects of ivabradine in patients with acute coronary syndrome: a pilot study. Int J Cardiol. 2012;158:160–2. doi: 10.1016/j.ijcard.2012.04.076. [DOI] [PubMed] [Google Scholar]
- 33.Sargento L, Satendra M, Longo S, Lousada N, Palma dos Reis R. Early NT-proBNP decrease with ivabradine in ambulatory patients with systolic heart failure. Clin Cardiol. 2013;36:677–82. doi: 10.1002/clc.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ordu S, Yildiz BS, Alihanoglu YI, Ozsoy A, Tosun M, Evrengul H, et al. Effects of ivabradine therapy on heart failure biomarkers. Cardiol J. 2015;22:501–9. doi: 10.5603/CJ.a2015.0012. [DOI] [PubMed] [Google Scholar]
- 35.Yancy CW, Jessup M, Bozkurt B, Butler J. Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Kempf T, Wollert KC. Growth-differentiation factor-15 in heart failure. Heart Fail Clin. 2009;5:537–47. doi: 10.1016/j.hfc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Briasoulis A, Androulakis E, Christophides T, Tousoulis D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail Rev. 2016;21:169–76. doi: 10.1007/s10741-016-9533-z. [DOI] [PubMed] [Google Scholar]
- 38.Carson P, Wertheimer J, Miller A, O'Connor CM, Pina IL, Selzman C, et al. The STICH trial (Surgical Treatment for Ischemic Heart Failure): mode-of-death results. JACC Heart Fail. 2013;1:400–8. doi: 10.1016/j.jchf.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niizeki T, Takeishi Y, Arimoto T, Takabatake N, Nozaki N, Hirono O, et al. Heart-type fatty acid-binding protein is more sensitive than troponin T to detect the ongoing myocardial damage in chronic heart failure patients. J Card Fail. 2007;13:120–7. doi: 10.1016/j.cardfail.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Ciccone MM, Cortese F, Gesualdo M, Riccardi R, Di Nunzio D, Moncelli M, et al. A novel cardiac bio-marker: ST2: a review. Molecules. 2013;18:15314–28. doi: 10.3390/molecules181215314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of Ivabradine treatment on NYHA stage, heart rate, ejection fraction (EF) and BNP levels.


