Abstract
Vascular endothelial cell senescence is a leading cause of age-associated and vascular diseases. Mammalian target of rapamycin complex 2 (mTORC2) is a conserved serine/threonine (Ser/Thr) protein kinase that plays an important regulatory role in various cellular processes. However, its impact on endothelial senescence remains controversial. In this study we investigated the role and molecular mechanisms of mTORC2 in endothelial senescence. A replicative senescence model and H2O2-induced premature senescence model were established in primary cultured human umbilical vein endothelial cells (HUVECs). In these senescence models, the formation and activation of mTORC2 were significantly increased, evidenced by the increases in binding of Rictor (the essential component of mTORC2) to mTOR, phosphorylation of mTOR at Ser2481 and phosphorylation of Akt (the effector of mTORC2) at Ser473. Knockdown of Rictor or treatment with the Akt inhibitor MK-2206 attenuated senescence-associated β-galactosidase (β-gal) staining and expression of p53 and p21 proteins in the senescent endothelial cells, suggesting that mTORC2/Akt facilitates endothelial senescence. The effect of mTORC2/Akt on endothelial senescence was due to suppression of nuclear factor erythroid 2-related factor 2 (Nrf2) at the transcriptional level, since knockdown of Rictor reversed the reduction of Nrf2 mRNA expression in endothelial senescence. Furthermore, mTORC2 suppressed the expression of Nrf2 via the Akt/GSK-3β/C/EBPα signaling pathway. These results suggest that the mTORC2/Akt/GSK-3β/C/EBPα/Nrf2 signaling pathway is involved in both replicative and inducible endothelial senescence. The deleterious role of mTORC2 in endothelial cell senescence suggests therapeutic strategies (targeting mTORC2) for aging-associated diseases and vascular diseases.
keywords: mTORC2, vascular endothelium, senescence, Akt, Nrf2
Introduction
Vascular endothelial cells play a pivotal role in regulating vascular hemostasis, vascular tone and permeability, angiogenesis, and inflammation [1]. In various physiological and pathophysiological processes, including aging, hypertension, atherosclerosis, and diabetes, endothelial cells develop accelerated senescence and become dysfunctional [2, 3]. These senescent endothelial cells are characterized by increased expression of senescence-associated β-galactosidase (SA-β-gal); cell cycle arrest, which is reflected as augmented expression of cyclin-dependent kinase inhibitors, such as p53 and p21 [4]; and telomere attrition and reduced telomerase activity [5, 6]. Endothelial senescence and dysfunction promote the progression of vascular diseases, such as atherosclerosis, ultimately raising the cardiovascular risk [7–9]. Thus, therapies that target endothelial cell senescence have important clinical implications [10].
Target of rapamycin (TOR) is a highly conserved protein kinase that senses and integrates environmental and intracellular nutrients and growth factor signals to regulate basic cellular and organismal responses, such as cell growth, proliferation, and apoptosis [11]. Mammalian TOR (mTOR) exists as two structurally and functionally distinct protein complexes: mTORC1 and mTORC2. mTORC1, which contains mTOR, Raptor (a regulatory protein associated with mTOR), and mammalian lethal with Sec13 protein 8 (mLST8, also known as GßL), is sensitive to the immunosuppressive and anticancer drug rapamycin [12–14]. By contrast, mTOR interacts with mLST8 and Rictor (a rapamycin-insensitive companion of mTOR) and forms the rapamycin-insensitive mTORC2 [15, 16]. In addition to their structures, these two complexes differ in their functions as they regulate various downstream targets. mTORC1 mainly phosphorylates p70S6 kinase 1 (S6K1) and eIF4E-binding protein (4EBP), which stimulate transcription, ribosome biogenesis, translation initiation, nutrient uptake, and autophagy in response to the abundance and quality of available nutrients [17]. mTORC2 phosphorylates several members of the protein kinase (PK)A/PKG/PKC (AGC) family, among which Akt is the most important substrate due to its key role in insulin/PI3K signaling [11, 15]. Since it was first discovered in 2004 [16], the role of mTORC2 in various aspect of cellular processes, such as cytoskeleton rearrangement, glucose metabolism, and cell migration [11, 15, 18], has drawn increasing attention.
A growing list of evidence suggests that mTOR signaling has a critical impact on regulating lifespan and cellular senescence [19–21]. Most studies focus on the effect of the mTORC1/S6K1 signaling pathway on senescence, as inhibition of mTORC1 or deletion of the mTORC1 substrate S6K1 increases lifespan [19–21]. mTORC1/S6K1 can activate the RAS signal to upregulate the cell cycle repressors p53, p21 and p16, ultimately contributing to cell senescence [22–24]. In endothelial senescence, mTORC1–S6K1 signaling plays a causative role in endothelial nitric oxide synthase uncoupling [25] and coagulation factor tissue factor expression [26, 27]. Unlike mTORC1, whose role in endothelial senescence has been well documented, the regulatory mechanisms and functions of mTORC2 are less well characterized. According to limited published reports, mTORC2 appears to play a controversial role in endothelial cell senescence. Activation of the mTORC2/Akt signaling pathway by 14,15-epoxyeicosatrienoic acid delayed endothelial senescence and recovered age-induced endothelial dysfunction [18]. By contrast, activation of mTORC2/Akt by arginase 2 was reported to accelerate endothelial cell senescence and atherosclerotic plaque formation through activating mTORC1-RPS6KB1/S6K1 and thus suppressing endothelial autophagy [28]. Likewise, long-term activation of mTORC2/Akt signaling by diet-induced obesity leads to vascular senescence [29]. In summary, the exact role of mTORC2 in endothelial senescence has not been fully explored and remains poorly understood.
The present study aimed to investigate the regulatory role and mechanisms of the mTORC2/Akt signaling pathway in stress-induced premature endothelial senescence (SIPS) and replicative endothelial senescence, and to elucidate the possibility that strategies targeting the mTORC2/Akt signaling pathway might influence endothelial senescence and suggest therapeutic potential for vascular diseases.
Materials and methods
Cell culture
Human umbilical cords were obtained from the Maternity Department at the First Affiliated Hospital of Sun Yat-sen University. Human umbilical vein endothelial cells (HUVECs) were isolated by digesting human umbilical veins using trypsin and were then maintained in endothelial cell medium (ECM) with 5% fetal bovine serum (Sciencell, San Diego, CA, USA) at 37 °C in a humidified 5% CO2 incubator. For treatment, cells were seeded on plates with medium containing 20% ECM and 80% Medium 199. Cells from four to eight passages were used for H2O2-induced cell senescence experiments, while cells at passage 3 (P3) and passage 11 (P11) were, respectively, utilized as young groups and senescence groups for the replicative senescence experiment. The Akt inhibitor MK-2206 2HCl was purchased from Selleck Chemicals (Houston, TX, USA).
SA-β-gal assay
To assess the SA-β-gal activity of cells, a β-Galactosidase Staining Kit (Beyotime, Haimen, China) was used. Cells were washed three times with warm phosphate-buffered saline, fixed with fixative solution, and finally incubated for 12–24 h with the staining solution mix at 37 °C. The SA-β-gal activity of HUVECs was measured according to the percentage of stained cells and the total cell number counted under a microscope.
Western blot analysis
Cell samples were extracted by radio-immunoprecipitation assay buffer and phenylmethanesulfonyl fluoride and then quantified using a BCA protein assay kit (Thermo Fisher, Rockford, IL, USA). Protein samples with equal loading quantities were separated by SDS-polyacrylamide gel electrophoresis and then blotted onto polyvinylidene difluoride membranes. The membrane was blocked with 5% skimmed milk for 1–2 h at room temperature, after which it was incubated with primary antibodies overnight at 4 °C and with horseradish peroxidase-conjugated secondary antibodies for 1.5 h at room temperature. Finally, chemiluminescence reagents were used. Blots were visualized by a GE ImageQuant LAS 4000 mini and analyzed by ImageJ software (National Institutes of Health, USA).
Antibodies against mTOR, p-mTOR (Ser2481), Rictor, Akt, p-Akt (Ser473), GSK-3β, C/EBPα, and p-C/EBPα (Thr222/226) were purchased from Cell Signaling Technology (Boston, MA, USA). An antibody against p-GSK-3β (Ser9) was purchased from Bioworld Technology (St. Louis Park, MN, USA). The antibody against Nrf2 was purchased from Santa Cruz (Dallas, TX, USA). Antibodies against p21, p53, and β-actin were from Proteintech Group (Chicago, IL, USA).
Immunoprecipitation
Cells were lysed by immunoprecipitation (IP) lysis buffer, and 300 µg of proteins was incubated with the mTOR antibody (1:50) overnight at 4 °C with rotation, while the control groups were incubated with rabbit IgG (0.2 μL). Then, Protein A-Sepharose beads were combined with the antibody and/or IgG and incubated for 4 h at 4 °C with rotation. Proteins combined with the antibody were released from beads by wash buffers. Then, Western blotting was performed to detect whether the binding of mTOR and Rictor was altered in treatment groups compared to control groups.
Small-interfering RNAs and transient transfection
Small-interfering RNAs (siRNAs) for the negative control (nonspecific siRNA), Rictor, Nrf2, and C/EBPα were purchased from RIBBIO (RIBBIO, Guangzhou, China). HUVECs were first seeded in 60 mm plates for 24 h, and then, transfections were performed when cells were 50–60% confluent. The final concentration of siRNA was 50 nM, except when noted otherwise. After 5 h, the transfection medium was removed, and 2 mL of 20% ECM was added to support cell growth. After further treatment, cells were harvested for distinct experiments.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from HUVECs using TRIzol reagent (Takara Biotechnology, Dalian, China), and cDNA was synthesized from 1 μg of total RNA in a reverse-transcribing reaction (Thermo RevertAid First Strand cDNA Synthesis Kit). The mRNA expression levels of each sample reflected according to the cycle time (Ct) values were determined using a SYBR-Green Quantitative PCR kit (TOYOBO, Japan) with an iCycler iQ system (iCycler, Bio-Rad, Hercules, CA, USA). Human-specific primers for Nrf2, C/EBPα, and Rictor were synthesized by Sangon (Shanghai, China). GADPH served as an endogenous control.
Statistics analysis
All experiments were performed at least three independent times. Differences between two groups were assessed by Student’s t test. Differences among three or more than three groups were evaluated by one-way analysis of variance with the Bonferroni post hoc test. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). P < 0.05 was considered statistically significant.
Results
Integrity and activity of mTORC2 were elevated in endothelial senescence
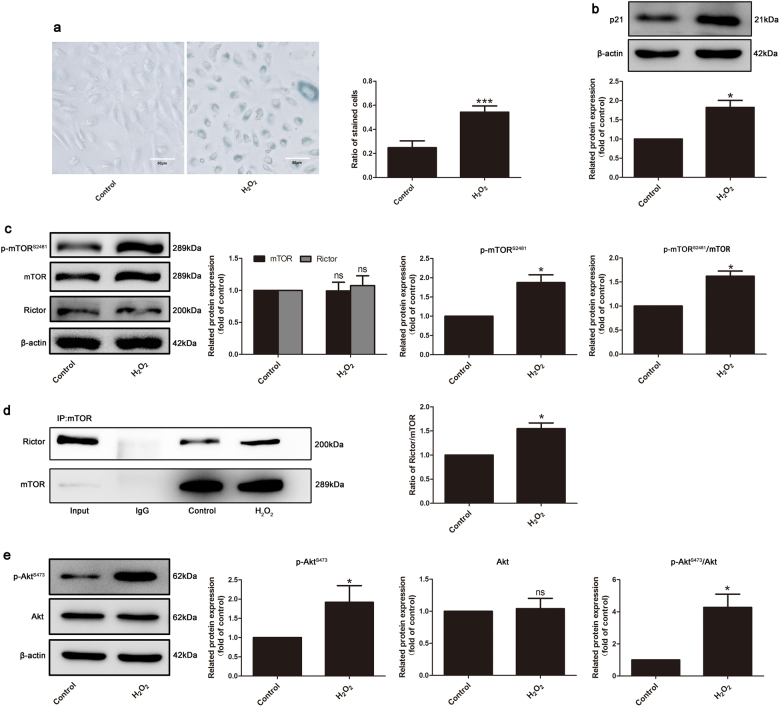
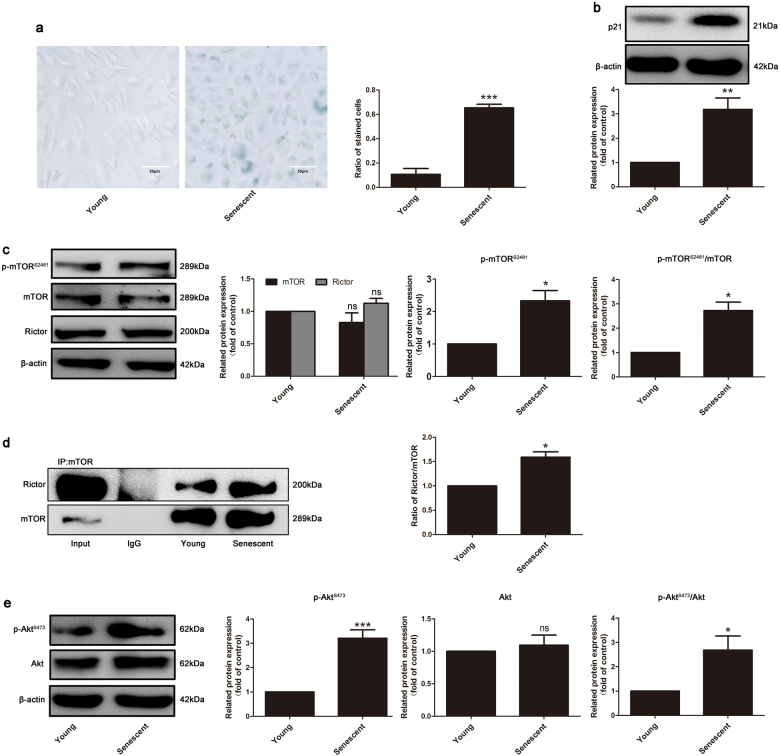
To determine the exact role of mTORC2 in endothelial cell replicative senescence and SIPS, we first established two senescent endothelial cell models. Primary cultured HUVECs at the eleventh passage (P11) were used as replicative senescent endothelial cells, while P3 cells were used as the young control. SIPS was induced by a 100 μM H2O2 treatment for 1 h, followed by incubation with complete culture medium for 2 days. As shown in Figs. 1a, b and 2a, b, the ratio of SA-β-gal-positive cells and protein expression of p21, typical markers of aging endothelial cells, were both increased in these two models. In these two senescent endothelial cell models, mTORC2 complex integrity was detected by co-IP. Notably, the interactions of mTOR and Rictor were significantly exaggerated in both senescent models (Figs. 1d and 2d), without altering their own expression (Figs. 1c and 2c), suggesting that the formation of mTORC2 is augmented in endothelial senescence. In addition, phosphorylation of mTOR Ser2481, a marker of intact mTORC2 [30], was also increased compared to that of the control groups (Figs. 1c and 2c). Furthermore, phosphorylation of Akt on Ser473, the downstream effector of mTORC2 as a serine kinase, was upregulated (Figs. 1e and 2e). These observations suggest that the formation and activation of mTORC2 are enhanced during endothelial cell replicative senescence and SIPS.
Fig. 1.
Integrity and activity of mTORC2 were elevated in stress-induced senescence. HUVECs (P4–P6) were incubated with 100 μM H2O2 for 1 h to cause stress-induced senescence and subsequently cultured in complete medium for 48 h before harvest. a The control group and H2O2-induced senescent group were subjected to β-gal staining and b immunoblotting to determine the p21 protein level, n = 3. c Western blotting analysis for the detection of expression of mTOR, Rictor, and p-mTOR-S2481, n = 4. d Co-immunoprecipitation was performed to detect the interactions between mTOR and Rictor, n = 3. e Detection of the expression of p-Akt-S473 and Akt by Western blotting, n = 4. β-Actin served as the loading control. The bar graphs show the expression levels. Data are shown as the means ± S.E.M. *P < 0.05, ***P < 0.001 vs. control group
Fig. 2.
Integrity and activity of mTORC2 were elevated in HUVEC replicative senescence. a The young HUVECs (passage 3) and replicative senescent HUVECs (passage 11) were subjected to β-gal staining and b immunoblotting to determine the p21 protein level, n = 5. c Western blotting analysis for the detection of expression of mTOR, Rictor, and p-mTOR-S2481, n = 3. d Co-immunoprecipitation was performed to detect the interaction between mTOR and Rictor, n = 3. e Detection of expression of p-Akt-S473 and Akt by Western blotting, n = 5. β-Actin served as the loading control. The bar graphs show the expression levels. Data are shown as the means ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group
Repression of mTORC2 formation alleviated endothelial senescence
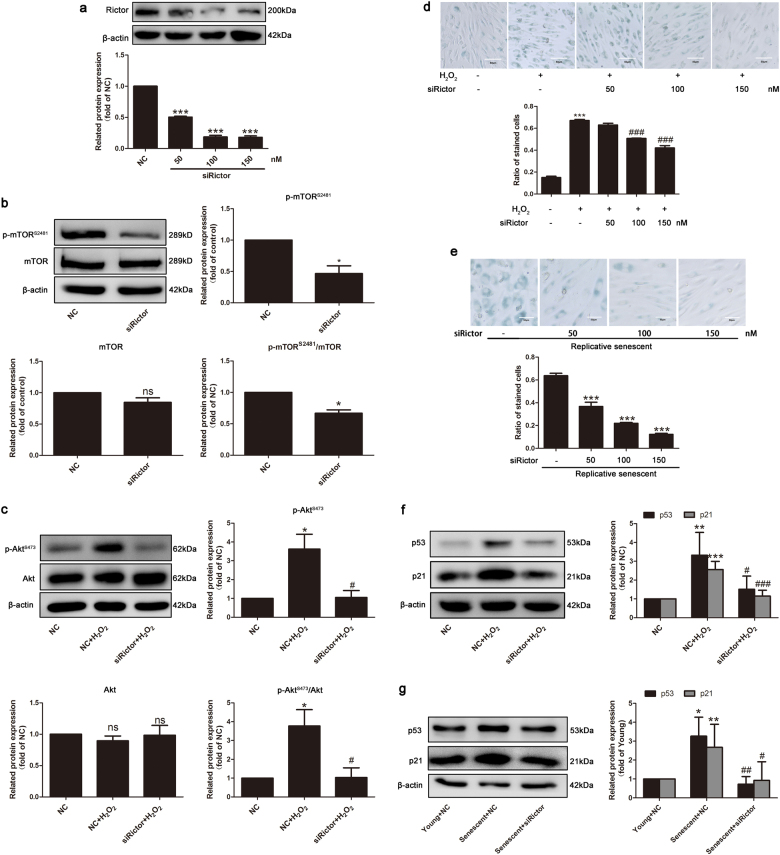
Next, the effect of mTORC2 on endothelial senescence was investigated. Since mTORC2 is insensitive to acute rapamycin treatment and specific inhibitors of mTORC2 are currently not commercially available, siRNA interference of Rictor was used to repress the formation of mTORC2 and to block its activity. The inhibitory efficiency of Rictor siRNA in Rictor expression was tested by Western blotting. As shown in Fig. 3a, Rictor siRNA at concentrations above 50 nM effectively decreased the protein expression of Rictor. Cells transfected with Rictor siRNA exhibited a decrease of mTOR phosphorylation at Ser2481, accompanied by decreased Rictor protein expression (Fig. 3b). As the downstream signaling pathway of mTORC2 activation, the phosphorylation level of Akt on Ser473 was reduced by Rictor depletion, whereas the total expression of Akt was not altered (Fig. 3c). Importantly, transfection of Rictor siRNA ameliorated endothelial senescence induced by H2O2 or replicative senescence in a dose-dependent manner, judging from the results of SA-β-gal staining and the expression of endothelial cell senescent markers p53 and p21 (Fig. 3d–g). Taken together, these observations indicate that decreases of the mTORC2 integrity and activity by Rictor knockdown might alleviate endothelial senescence.
Fig. 3.
Repression of mTORC2 formation alleviated endothelial senescence. a HUVECs were transfected with different concentrations of Rictor siRNA or the negative control (NC, nonspecific siRNA) for 48 h; then, cells were harvested for Western blotting analysis for the detection of Rictor, n = 3. b HUVECs transfected with 100 nM Rictor siRNA were harvested for Western blotting analysis for the detection of mTOR and p-mTOR-S2481, n = 3. c HUVECs carrying NC siRNA or Rictor siRNA were grown with or without H2O2, and the p-Akt and Akt protein levels were detected, n = 3. d HUVECs transfected with different concentrations of Rictor siRNA were incubated with or without H2O2 and then subjected to β-gal staining, n = 3. e Replicative senescent HUVECs transfected with different concentrations of Rictor siRNA were subjected to β-gal staining, n = 3. f HUVECs carrying NC siRNA or Rictor siRNA were grown with or without H2O2, and the p53 and p21 protein levels were detected, n = 3–6. g Young and replicative senescent HUVECs were transfected with Rictor siRNA, and the p53 and p21 protein levels were detected, n = 3. β-Actin served as the loading control. The bar graphs show the expression levels. Data are shown as the means ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NC group or young group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. NC plus H2O2 group or NC plus senescent group
Inhibition of Akt prevented endothelial senescence
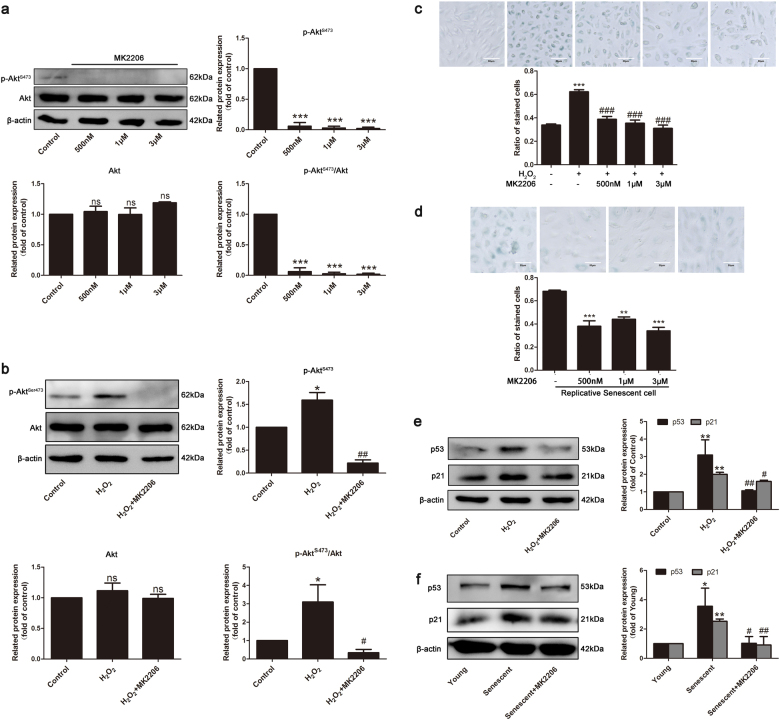
Because mTORC2 directly activates Akt through phosphorylation at Ser473 [31], the effect of mTORC2 on endothelial senescence was further evaluated using a specific inhibitor of Akt, MK-2206, to block the downstream signal of mTORC2. First, the inhibitory efficiency of MK-2206 in Akt activity was tested. MK-2206 at concentrations above 500 nM effectively diminished the phosphorylation level of Akt at Ser473 (Fig. 4a). Additionally, MK-2206 treatment reversed Akt activation induced by H2O2 without affecting its total expression (Fig. 4b). These observations imply that MK-2206 could abolish mTORC2/Akt signaling. Second, the effects of MK-2206 on replicative endothelial senescence and SISP were investigated. MK-2206 at concentrations of 500 nM, 1 μM, or 3 μM significantly decreased the percentage of SA-β-gal-positive cells in both H2O2-treated cells and cells at passage 11 (Fig. 4c, d). Meanwhile, MK-2206 treatment downregulated the p53 and p21 protein levels in both senescent endothelial cell models (Fig. 4e, f). Thus, inhibition of mTORC2/Akt protects HUVECs against replicative senescence and SISP.
Fig. 4.
Inhibition of Akt prevented endothelial senescence. a HUVECs were treated with 500 nM, 1 μM, or 3 μM of the p-Akt-S473 inhibitor MK-2206 for 24 h and then harvested for Western blotting analysis for the detection of p-Akt-S473 and Akt, n = 3. b H2O2-induced HUVECs were treated with MK-2206 (1 μM) for 24 h. Cells were harvested for the detection of the expression of p-Akt-S473 and Akt by Western blotting, n = 4. c H2O2-induced HUVECs treated with different concentrations of MK-2206 were subjected to β-gal staining, n = 3. d Replicative senescent HUVECs treated with different concentrations of MK-2206 were subjected to β-gal staining, n = 3. e H2O2-induced HUVECs were treated with MK-2206 (1 μM) for 24 h. Cells were harvested for the detection of the expression of p53 and p21 by Western blotting, n = 3. f Replicative senescent HUVECs were treated with MK-2206 (1 μM) for 24 h. Cells were harvested for the detection of the expression of p53 and p21 by Western blotting, n = 3. β-Actin served as the loading control. The bar graphs show the expression levels. Data are shown as the means ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NC group or young group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. NC plus H2O2 group or NC plus senescent group
Nrf2 was involved in the regulation of mTORC2 in endothelial senescence
It is still unclear how mTORC2/Akt leads to endothelial senescence. Accumulating evidence indicates that oxidative stress is one of the leading causes of cellular senescence [4], and Nrf2, the pivotal cell defense response against oxidative stress, plays an essential role in preventing endothelial senescence [32]. It was hypothesized that Nrf2 may be involved in the regulation of mTORC2 in endothelial cell senescence. The changes of Nrf2 in endothelial senescence were first studied. The present results demonstrated that the mRNA and protein expression of Nrf2 were significantly reduced in senescent HUVECs induced by H2O2 or culture replication (Fig. 5a–c). Notably, when these senescent cells were transfected with si-Rictor to reduce the integrity of mTORC2, the decrease of Nrf2 expression was reversed (Fig. 5a–c). These observations thus suggest that mTORC2 might be involved in the regulation of Nrf2 transcription. Moreover, Nrf2 depletion significantly reversed the decrease of p21 expression induced by Rictor knockdown in H2O2-induced SIPS (Fig. 5d). These data suggest that mTORC2 triggers endothelial senescence through suppressing Nrf2 transcription.
Fig. 5.
Nrf2 was involved in the regulation of mTORC2 in endothelial senescence. HUVECs were exposed to H2O2 and co-incubated with Rictor siRNA or negative control siRNA (NC, nonspecific siRNA). Then, Western blot analysis was performed to determine the a protein and b mRNA expression of Nrf2, n = 4. c Young and replicative senescent HUVECs were transfected with Rictor siRNA or NC siRNA, and mRNA expression of Nrf2 was detected by qRT-PCR, n = 3. d HUVECs were transfected with NC siRNA or Rictor siRNA with or without siNrf2, followed by incubation with H2O2, and the protein expression of p53 and p21 was detected by Western blotting, n = 4. β-Actin served as the loading control. The bar graphs show the expression levels. Data are shown as the means ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NC group or young group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. NC plus H2O2 group or NC plus senescent group; ξP < 0.05 vs. si-Rictor plus H2O2 treatment group
mTORC2 attenuated the transcription of Nrf2 in an Akt-GSK-3β-C/EBPα-dependent manner
To further explore the mechanisms by which mTORC2 regulates the transcription level of Nrf2, the transcription factor upstream of Nrf2 was predicted using the AliBaba2.1 website (http://gene-regulation.com/pub/programs/alibaba2/index.html). As shown by the predicted results, numerous potential transcriptional factors were identified, including C/EBPα, AP-1, c-Jun, and Oct-1 (Supplementary information). Further experiments were conducted to investigate the potential role of these transcriptional factors involved in the regulation of mTORC2 in Nrf2 transcription by repressing mTORC2 through Rictor knockdown. According to our results, Rictor depletion recovered the attenuation of phosphorylated C/EBPα (Thr222/226) in HUVECs induced by H2O2, whereas the total expression of C/EBPα was unchanged (Fig. 6a). In addition, upregulation of Nrf2 mRNA by Rictor deficiency was markedly overcome by C/EBPα depletion (Fig. 6b). Therefore, these findings indicate that C/EBPα is involved in the downstream signaling of mTORC2 and participates in the regulation of Nrf2 transcription.
Fig. 6.
mTORC2 attenuated the transcription of Nrf2 in an Akt-GSK-3β-C/EBPα-dependent manner. a HUVECs carrying negative control siRNA (NC, nonspecific siRNA) or Rictor siRNA were grown with or without H2O2 for 48 h, and cells were harvested for Western blotting analysis for the detection of the expression of C/EBPα and p-C/EBPα-T222/226, n = 3. b HUVECs were transfected with NC siRNA or Rictor siRNA with or without siC/EBPα, followed by incubation with H2O2; the mRNA expression of p21 was detected by qRT-PCR, n = 4. c HUVECs carrying NC or Rictor siRNA were grown with or without H2O2 for 48 h, and cells were harvested for Western blotting analysis for detection of GSK-3β and p-GSK-3β-S9, n = 3. β-Actin served as the loading control. The bar graphs show the expression levels. Data are shown as the means ± S.E.M. *P < 0.05, **P < 0.01 vs. NC group; #P < 0.05, ##P < 0.01 vs. NC plus H2O2 treatment group; ξP < 0.05 vs. si-Rictor plus H2O2 treatment group
Since the transcriptional activity of C/EBPα is activated through its phosphorylation at Thr222 and Thr226 by GSK-3β [33, 34] and the activity of GSK-3β is inhibited when it is phosphorylated by Akt [35], the activity of GSK-3β was studied in Rictor-silenced cells. Phosphorylation of GSK-3β, which indicates inhibition of GSK-3β, was enhanced by H2O2 treatment, but was reduced in Rictor gene-silenced cells (Fig. 6c). These observations thus suggest that Rictor depletion may increase the activity of GSK-3β, subsequently enhancing the transcriptional activity of C/EBPα to increase Nrf2 transcription.
Taken together, these results raise the possibility that mTORC2 suppresses the mRNA level of Nrf2 via the mTORC2/Akt/GSK-3β/C/EBPα axis, which contributes to the development of endothelial cell senescence.
Discussion
In recent years, mTORC2 has attracted extensive attention because it participates in various cellular processes, including cytoskeletal remodeling, cell metabolism, ion transportation, and cell migration [36]. mTORC2 has been identified as a vital kinase that modules the phosphorylation of several members of the AGC (PKA/PKG/PKC) family of protein kinases [37]. The most important role of mTORC2 is the phosphorylation and activation of Akt, a key effector of insulin/PI3K signaling [31], which promotes cell survival, proliferation, and growth through the phosphorylation of several key substrates, such as the FoxO1/3a transcription factors and metabolic regulator GSK-3β [11]. Currently, the role of mTORC2/Akt in cellular senescence, in particular, vascular endothelial cell senescence, which is associated with the development of various vascular diseases, is still undetermined. Thus, the present study was designed to investigate the regulatory role of the mTORC2/Akt signaling pathway in endothelial senescence. During both inducible senescence and replicative senescence in HUVECs, the assembly of mTORC2, which is characterized by the binding of Rictor to mTOR, was observed to be increased. Because mTORC1 and mTORC2 contain differentially phosphorylated mTOR and intact mTORC2 is necessary for mTOR phosphorylation at Ser2481, phosphor-Ser2481 is regarded to be a marker of intact mTORC2 [30]. Our observations showed that phosphorylation of mTOR at Ser2481 was augmented in the senescent endothelial cell models, further confirming that mTORC2 formation is enhanced in endothelial senescence. Moreover, the phosphorylation level of Akt on Ser473 was augmented in senescent HUVECs, indicating that mTORC2 is activated during endothelial senescence. In line with the previous findings that mTORC2 exhibits age-associated activation in various fasted male mouse tissues, including the liver, muscle, and heart [38], these results provide evidence that activation of mTORC2 is involved in cellular senescence and age-dependent disorders.
The present findings demonstrate that activation of the mTORC2/Akt signaling pathway plays a causative role in both inducible and replicative endothelial senescence. This conclusion is supported by the observations that disruption of Rictor, the essential component of mTORC2, or treatment with the Akt inhibitor MK-2206 significantly reduced SA-β-gal staining and downregulated the expression of the cyclin-dependent kinase inhibitors p53 and p21 in senescent HUVECs induced by H2O2 or culture replication. These results are consistent with previous studies showing that activation of mTORC2 by arginase 2 prompts endothelial senescence and leads to the formation of an atherosclerotic plaque [28] and that Akt activation accelerates endothelial cell senescence through a p53-/p21-dependent pathway [39, 40], confirming that activation of mTORC2/Akt facilitates the development of endothelial senescence. However, the present findings diverge from previous observations indicating that mTORC2/Akt activation by 14,15-epoxyeicosatrienoic acid delays endothelial senescence [18]. It is possible that these discrepancies are due to differences in cell types and experimental settings. The present results do not permit further speculation on the reasons for these apparent discrepancies.
Our study also attempted to explore the mechanisms by which mTORC2/Akt leads to endothelial senescence. Oxidative stress is the most important mediator of endothelial senescence [4]. Accumulating evidence has suggested that the increase in intracellular reactive oxygen species (ROS) by Akt activation is an important cause of cellular senescence [41, 42]. Thus, the involvement of the redox balance regulator Nrf2 in mTORC2/Akt activation was investigated. Nrf2 has been recognized as a pivotal participant in pro-longevity signaling pathways due to its capacity of upregulating ROS-scavenging proteins and antioxidant enzymes [43]. Activation of the Nrf2-dependent cellular antioxidant defense system prevents senescence [44–46], whereas inhibition of Nrf2 significantly promotes cellular senescence [47–49], demonstrating the protective role of Nrf2 against senescence. The expression and activity of Nrf2 have been observed to be reduced during aging [46, 50, 51]. Likewise, our work also showed that the mRNA and protein levels of Nrf2 were decreased in endothelial senescence. Of note, depletion of Rictor reversed the reduction of Nrf2 mRNA expression in both inducible senescence and replicative senescence, indicating that Nrf2 is involved in the regulation of mTORC2 signaling in endothelial senescence. However, Rictor knockdown did not alter either the phosphorylation level or nuclear translocation of Nrf2 (data not shown). Therefore, our study suggests that mTORC2 facilitates the repression of Nrf2 at the transcription level rather than affecting its transcriptional activity during endothelial senescence. Furthermore, Nrf2 deficiency reversed the inhibitory effect of Rictor silencing on endothelial senescence, confirming that mTORC2 regulates endothelial senescence via Nrf2.
To further explore the molecular mechanisms by which mTORC2/Akt regulates Nrf2, potential transcriptional factors responsible for Nrf2 transcription were predicted by AliBaba2.1. Among all of the predicted transcriptional factors, C/EBPα was identified to be involved in the regulation of mTORC2/Akt in Nrf2 transcription. This conclusion is based on the following observations: the activity of C/EBPα, reflected by its phosphorylation at Thr222 and Thr226, was attenuated in H2O2-induced endothelial senescence, but was reversed when Rictor was silenced, and C/EBPα depletion reversed the upregulation of Nrf2 mRNA by Rictor knockdown. We further showed that GSK-3β, which activates C/EBPα through phosphorylating it at Thr222/226 [33, 34], was activated in Rictor-silenced cells. Because Akt is able to directly phosphorylate GSK-3β to inhibit its activity [35], it is obvious that GSK-3β serves as the downstream signal of mTORC2/Akt and the upstream signal responsible for C/EBPα transactivation. Thus, these findings support the hypothesis that the Akt/GSK-3β/C/EBPα signaling pathway is indispensable for the regulation of mTORC2 in Nrf2 transcription.
Of note, mTORC2 has also been reported to be an upstream activator of mTORC1 [11], which triggers endothelial senescence through upregulation of cyclin-dependent kinase inhibitors and suppression of autophagy [23, 24, 28]. It seems possible that the deleterious role of mTORC2 in endothelial senescence is also attributed to the activation of mTORC1. However, our observations showed that knockdown of Rictor did not alter the expression of Raptor or the phosphorylation of 4E-BP1 (the effector of mTORC1) (Supplementary Figure S1). Thus, these results indicate that Rictor deficiency does not disturb the activity of mTORC1 and that the involvement of mTORC1 could be eliminated from the regulatory effect of mTORC2 on endothelial senescence. Moreover, treatment with rapamycin, the mTORC1 inhibitor, did not influence the mRNA level of Nrf2 (Supplementary Figure S2), further suggesting that the suppression of Nrf2 expression by the mTORC2/Akt/GSK-3β/C/EBPα signaling pathway is independent of mTORC1.
As a limitation, the present study only investigated the regulatory role of mTORC2/Akt in endothelial cell senescence in vitro. It would be interesting and critical to explore whether suppression of mTORC2/Akt signaling pathway can rejuvenate aged blood vessels in vivo. In fact, previous findings demonstrate that inhibition of mTORC2/Akt by chorionic treatment with rapamycin or a genetic knockout of Akt prevents vascular senescence in a diet-induced obesity mouse model [29]. Further studies using aged animal models or cardiovascular disease models should be performed to unmask the exact role of mTORC2/Akt in the development of endothelial senescence.
In conclusion, the present study provides evidence that the formation and activity of mTORC2 are increased in both inducible and replicative endothelial senescence and that the activation of the mTORC2/Akt signaling pathway facilitates the development of endothelial senescence. Regulation of mTORC2 in endothelial senescence is at least partially due to the suppression of Nrf2 expression at the transcriptional level via the Akt/GSK-3β/C/EBPα signaling pathway. Strategies targeting inhibition of mTORC2 might ameliorate endothelial senescence and suggest therapeutic potential for aging-associated diseases and vascular diseases.
Electronic supplementary material
Acknowledgements
The study is supported by the National Natural Science Foundation of China (No. 81400359, 81473205, and 81673433), Guangdong Natural Science Foundation (S2013040014102), Specialized Research Fund for the Doctoral Program of Higher Education of China (20130171120048), Training Program for Young Teachers from Sun Yat-sen University (No.15ykpy03), and the National Engineering and Technology Research Center for New Drug Druggability Evaluation (Seed Program of Guangdong Province), and funding from Guangdong Provincial Engineering Laboratory of Druggability and New Drugs Evaluation, and Guangzhou Key Laboratory of Druggability Assessment for Biologically Active Compounds.
Author contributions
P.-q.L. and Z.-m.L. designed the research; H.-w.Y., H.-l.H., W.-w.L., C.-m.D., and X.-y.C. performed the research; H.-w.Y., H.-l.H., W.-w.L., L.-p.W., Q.L., and Z.-q.L. analyzed the data; H.-w.Y. and Z.-m.L. wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Pei-qing Liu, Email: liupq@mail.sysu.edu.cn.
Zhuo-ming Li, Phone: +86 20 39943026, Email: lizhm5@mail.sysu.edu.cn.
Electronic supplementary material
The online version of this article (10.1038/s41401-018-0079-6) contains supplementary material, which is available to authorized users.
References
- 1.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 2.Luna C, Alique M, Navalmoral E, Noci MV, Bohorquez-Magro L, Carracedo J, et al. Aging-associated oxidized albumin promotes cellular senescence and endothelial damage. Clin Interv Aging. 2016;11:225–36. doi: 10.2147/CIA.S91453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89:122–35. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol (1985) 2009;106:326–32. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–8. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–4. doi: 10.1161/01.CIR.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 7.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol. 2005;40:634–42. doi: 10.1016/j.exger.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 9.Favero G, Paganelli C, Buffoli B, Rodella LF, Rezzani R. Endothelium and its alterations in cardiovascular diseases: life style intervention. Biomed Res Int. 2014;2014:801896. doi: 10.1155/2014/801896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naylor RM, Baker DJ, van Deursen JM. Senescent cells: a novel therapeutic target for aging and age-related diseases. Clin Pharmacol Ther. 2013;93:105–16. doi: 10.1038/clpt.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–76. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/S0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/S1097-2765(03)00114-X. [DOI] [PubMed] [Google Scholar]
- 14.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. doi: 10.1016/S0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 15.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 16.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–26. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- 18.Yang C, Pan S, Yan S, Li Z, Yang J, Wang Y, et al. Inhibitory effect of 14,15-EET on endothelial senescence through activation of mTOR complex 2/Akt signaling pathways. Int J Biochem Cell Biol. 2014;50:93–100. doi: 10.1016/j.biocel.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–77. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–6. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 21.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–4. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Astle MV, Hannan KM, Ng PY, Lee RS, George AJ, Hsu AK, et al. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene. 2012;31:1949–62. doi: 10.1038/onc.2011.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yentrapalli R, Azimzadeh O, Sriharshan A, Malinowsky K, Merl J, Wojcik A, et al. The PI3K/Akt/mTOR pathway is implicated in the premature senescence of primary human endothelial cells exposed to chronic radiation. PLoS ONE. 2013;8:e70024. doi: 10.1371/journal.pone.0070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ming XF, Montani JP, Yang Z. Perspectives of targeting mTORC1-S6K1 in cardiovascular aging. Front Physiol. 2012;3:5. doi: 10.3389/fphys.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajapakse AG, Yepuri G, Carvas JM, Stein S, Matter CM, Scerri I, et al. Hyperactive S6K1 mediates oxidative stress and endothelial dysfunction in aging: inhibition by resveratrol. PLoS ONE. 2011;6:e19237. doi: 10.1371/journal.pone.0019237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camici GG, Steffel J, Amanovic I, Breitenstein A, Baldinger J, Keller S, et al. Rapamycin promotes arterial thrombosis in vivo: implications for everolimus and zotarolimus eluting stents. Eur Heart J. 2010;31:236–42. doi: 10.1093/eurheartj/ehp259. [DOI] [PubMed] [Google Scholar]
- 27.Ming XF, Rajapakse AG, Carvas JM, Ruffieux J, Yang Z. Opposing and uncoupling effects of mTOR and S6K1 in the regulation of endothelial tissue factor expression. FEBS Lett. 2010;584:135–40. doi: 10.1016/j.febslet.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Y, Yepuri G, Forbiteh M, Yu Y, Montani JP, Yang Z, et al. ARG2 impairs endothelial autophagy through regulation of MTOR and PRKAA/AMPK signaling in advanced atherosclerosis. Autophagy. 2014;10:2223–38. doi: 10.4161/15548627.2014.981789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal. 2009;2:ra11. doi: 10.1126/scisignal.2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–7. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 32.Bachschmid MM, Schildknecht S, Matsui R, Zee R, Haeussler D, Cohen RA, et al. Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med. 2013;45:17–36. doi: 10.3109/07853890.2011.645498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross SE, Erickson RL, Hemati N, MacDougald OA. Glycogen synthase kinase 3 is an insulin-regulated C/EBPalpha kinase. Mol Cell Biol. 1999;19:8433–41. doi: 10.1128/MCB.19.12.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datta J, Majumder S, Kutay H, Motiwala T, Frankel W, Costa R, et al. Metallothionein expression is suppressed in primary human hepatocellular carcinomas and is mediated through inactivation of CCAAT/enhancer binding protein alpha by phosphatidylinositol 3-kinase signaling cascade. Cancer Res. 2007;67:2736–46. doi: 10.1158/0008-5472.CAN-06-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–31. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–71. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 37.Gaubitz C, Prouteau M, Kusmider B, Loewith R. TORC2 structure and function. Trends Biochem Sci. 2016;41:532–45. doi: 10.1016/j.tibs.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Baar EL, Carbajal KA, Ong IM, Lamming DW. Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell. 2016;15:155–66. doi: 10.1111/acel.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–20. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minamino T, Miyauchi H, Tateno K, Kunieda T, Komuro I. Akt-induced cellular senescence: implication for human disease. Cell Cycle. 2004;3:449–51. doi: 10.4161/cc.3.4.819. [DOI] [PubMed] [Google Scholar]
- 41.Kim YY, Jee HJ, Um JH, Kim YM, Bae SS, Yun J. Cooperation between p21 and Akt is required for p53-dependent cellular senescence. Aging Cell. 2017;16:1094–103. doi: 10.1111/acel.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–70. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Asp Med. 2011;32:234–46. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Senthil KKJ, Gokila VM, Wang SY. Activation of Nrf2-mediated anti-oxidant genes by antrodin C prevents hyperglycemia-induced senescence and apoptosis in human endothelial cells. Oncotarget. 2017;8:96568–87. doi: 10.18632/oncotarget.19951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, et al. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal. 2013;18:1906–19. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 46.Kapeta S, Chondrogianni N, Gonos ES. Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts. J Biol Chem. 2010;285:8171–84. doi: 10.1074/jbc.M109.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volonte D, Liu Z, Musille PM, Stoppani E, Wakabayashi N, Di YP, et al. Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol Biol Cell. 2013;24:1852–62. doi: 10.1091/mbc.e12-09-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jodar L, Mercken EM, Ariza J, Younts C, Gonzalez-Reyes JA, Alcain FJ, et al. Genetic deletion of Nrf2 promotes immortalization and decreases life span of murine embryonic fibroblasts. J Gerontol A Biol Sci Med Sci. 2011;66:247–56. doi: 10.1093/gerona/glq181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, et al. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–9. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duan W, Zhang R, Guo Y, Jiang Y, Huang Y, Jiang H, et al. Nrf2 activity is lost in the spinal cord and its astrocytes of aged mice. Vitr Cell Dev Biol Anim. 2009;45:388–97. doi: 10.1007/s11626-009-9194-5. [DOI] [PubMed] [Google Scholar]
- 51.Shih PH, Yen GC. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8:71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.