Abstract
Salvianolic acid A (SAA) is a minor phenolic carboxylic acid extracted from Salviae miltiorrhizae Bunge (Danshen). SAA exhibits a variety of pharmacological activities, such as antioxidative, anti-thrombotic, neuroprotective, and anti-fibrotic effects, as well as protection from myocardial ischemia and prevention of diabetes and other diseases. Furthermore, SAA has shown renal-protective effects in doxorubicin-induced nephropathy. However, there has been limited research regarding the effects of SAA and underlying mechanisms in chronic kidney disease (CKD). Here, we examined the effects and molecular mechanisms of SAA in an established animal model of 5/6 nephrectomized (5/6Nx) rats. The rats were injected with SAA (2.5, 5, and 10 mg/kg per day, intraperitoneally (ip)) for 28 days. SAA dose-dependently lowered the levels of urine protein, blood urea nitrogen, serum creatinine, plasma total cholesterol, and plasma triglycerides in 5/6Nx rats. Histological examination revealed that SAA dose-dependently attenuated renal pathological lesions, evidenced by reduced renal tubulointerstitial fibrosis by decreasing the expression levels of tumor growth factor-β1 and α-smooth muscle actin in 5/6Nx rats. Moreover, SAA dose-dependently inhibited the activation of nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinase (MAPK) signaling pathways, subsequently attenuating the secretion of tumor necrosis factor-α and interleukin-1β and inhibiting the expression of monocyte chemotactic protein-1, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in kidneys of 5/6Nx rats. The above results were consistent with those obtained in lipopolysaccharide-induced HK-2 cells in vitro (a recognized in vitro inflammatory model). In conclusion, our results demonstrated that SAA effectively attenuates kidney injury in 5/6Nx rats. The therapeutic effects of SAA on kidney injury can be attributed to its anti-inflammatory activities through inhibition of the activation of the NF-κB and p38 MAPK signaling pathways.
Keywords: salvianolic acid A, chronic kidney disease; inflammation, NF-κB, p38 MAPK, 5/6 nephrectomized rat, LPS-induced HK-2 cell
Introduction
Chronic kidney disease (CKD) is an increasing public health concern that harms the health and life of many individuals [1–3]. In a 2010 Global Burden of Disease study, CKD rose from the 27th greatest cause of the total number of global deaths in 1990 (age-standardized annual death rate of 15.7 per 100,000) to the alarming rank of 18th (with an annual death rate of 16.3 per 100,000 [4]). According to a systematic analysis of the 2015 Global Burden of Disease Study, the mortality and disability-adjusted life-years burden of CKD increased due to low glomerular filtration rates from 2005 to 2015 [5]. Two important features of CKD, inflammation and oxidative stress, can drive the progression of CKD and lead to cardiovascular and other body system complications [6, 7]. Moreover, another key feature of CKD is renal fibrosis, which is the final pathological stage of all progressive renal diseases [8, 9]. As a model of CKD, 5/6 nephrectomized (5/6Nx) rats have been frequently used as to study the progression of CKD [10–12]. Lipopolysaccharide (LPS), a bacterial endotoxin, can trigger inflammatory responses by increasing the synthesis and release of pro-inflammatory cytokines [13]. HK-2 cells stimulated in vitro with LPS have been used to characterize the anti-inflammatory effects of various compounds in the kidney [14, 15].
Nuclear factor-κB (NF-κB), a transcription factor that is widely expressed in a variety of cells and tissues, can quickly respond to various inflammatory stimuli. NF-κB can be regulated by the activation of IκB kinase and mitogen-activated protein (MAP) kinase pathways [16]. IκB kinase-β (IKKβ) can phosphorylate the IκB protein following proteasomal degradation, allowing NF-κB to translocate into the nucleus and initiate pro-inflammatory gene transcription [17]. Several pro-inflammatory cytokines and chemokines, including adhesion molecules such as tumor necrosis factor-α (TNF-α) and monocyte chemotactic protein-1 (MCP-1, CCL2), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) are associated with the regulation of most inflammatory processes, which are collectively regulated by NF-κB [18, 19]. Previous studies have shown that NF-κB is activated in human renal disease and various experimental models of renal inflammatory disease [13, 20, 21].
It has been reported that the activation of p38 mitogen-activated protein kinase (MAPK), a member of the family of MAPKs, plays a key role in kidney inflammation [22, 23]. The activation of p38 MAPK can promote the production of pro-inflammatory cytokines and regulate cellular apoptosis during renal injury. Furthermore, the activation of p38 MAPK in glomerular cells has been correlated with segmental proliferative necrotic lesions and the degree of interstitial inflammation [22, 24].
Salvianolic acid A (SAA) is a minor phenolic carboxylic acid extracted from Salviae miltiorrhizae Bunge (Danshen). As one of the major active components of this plant, SAA exhibits multiple pharmacological activities, such as antioxidative, anti-thrombotic, neuroprotective, and anti-fibrotic effects, as well as protection from myocardial ischemia and prevention of diabetes and other diseases [25]. Furthermore, SAA has been reported to attenuate inflammation through inhibition of the activation of NF-κB in cells [26]. It has been reported that SAA shows beneficial renal-protective effects in doxorubicin-induced nephropathy through its pharmacological activities, including anti-inflammatory and antioxidative effects and ameliorating effects for podocyte injury, among other beneficial properties [27].
However, to date there has been limited research regarding the effects and molecular mechanisms of action of SAA in CKD. Here, we evaluated the effects and molecular mechanisms of SAA in an established animal model of 5/6Nx rats and further confirmed these in vivo results in LPS-treated HK-2 cells in vitro.
Materials and methods
Materials
SAA (purity ≧98% by high-performance liquid chromatography) was provided by the laboratory of Jin-hui Wang (Shenyang Pharmaceutical University). The antibodies used in this study included anti-p-IKKα/β (#2697, CST), anti-IKKβ (#8943, CST), anti-p-NF-κB p65 (#3033, CST), anti-NF-κB p65 (#8242, CST), anti-p-IκBα (#2859, CST), anti-IκBα (#4814, CST), anti-p-p38 MAPK (#4511, CST), anti-p38 MAPK (#8690, CST), anti-α-SMA (ab5694, Abcam), anti-MCP-1 (ab7202, Abcam), anti-tumor growth factor-β1 (TGF-β1) (BA0290, Boster), anti-ICAM-1 (#60299-1-lg, Proteintech), anti-VCAM-1 (#11444-1-AP, Proteintech), and rhodamine (tetramethylrhodamine (TRITC))-conjugated goat anti-rabbit IgG (H + L) (SA00007-2, Proteintech). Anti-rabbit and anti-mouse secondary antibodies were also purchased from CST.
In vivo experiments
Animals and experimental design
Adult male Sprague–Dawley (SD) rats (body weight 200–250 g) purchased from the Center for Experimental Animals of Shenyang Pharmaceutical University were used in the present study. Rats were housed in an environmentally controlled room under a 12 h light–dark cycle and given standard rodent chow and tap water ad libitum. Rats were acclimated to handling before randomization and then divided into five groups: Group 1 was the 5/6Nx group (n = 10), in which the upper and lower poles of the left kidney were removed in addition to the whole right kidney; Groups 2–4 were the SAA-treated 5/6Nx groups, injected with 2.5, 5, and 10 mg/kg SAA (5/6Nx + SAA (2.5, 5, and 10 mg/kg), respectively, intraperitoneally (ip); n = 10); and Group 5 served as the control group (n = 10) and were subjected to a sham operation. Surgery was performed under chloral hydrate solution anesthesia (350 mg/kg, ip). All animal experiments were conducted per the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as well as the policies of our university authority.
Functional studies
After 28 days of treatment, 24 h urine samples were collected from the rats using metabolic cages for the measurement of urinary protein levels. Then, blood samples were collected from orbital venous plexi. Urinary protein, blood urea nitrogen (BUN), serum creatinine (SCr), plasma triglycerides (TGs), and plasma total cholesterol (TC) levels were measured using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China).
Histological examination
All rats were sacrificed with intraperitoneal injection of chloral hydrate (350 mg/kg), and the kidney tissues were rapidly removed (with kidney tissue isolated from adipose tissue). Renal tissues were fixed in 10% formalin, dehydrated with ethanol, rendered transparent with xylene, embedded in liquid paraffin, and sliced with an automatic slicing machine. Sections (4 µm thick) were stained with hematoxylin and eosin and Masson’s trichrome. The blue area in the tubulointerstitium was selected as the positive area in sections stained with Masson’s trichrome. The severity of tubulointerstitial fibrosis was graded as follows: (1) <10% fibrosis; (2) 10%–25% fibrosis; (3) 25%–50% fibrosis; (4) 50%–75% fibrosis; and (5) more than 75% fibrosis [28]. The tubulointerstitial fibrosis indexes (scores) of 10 random fields in each group were counted.
Immunohistochemical analysis
For immunohistochemical staining, the sections were incubated with primary antibodies against MCP-1, TGF-β1, or α-SMA (1:200) overnight at 4 °C. After washing three times with phosphate-buffered saline (PBS), sections were incubated with a secondary antibody for 30 min at 37 °C. The slides were washed with PBS three times, and diaminobenzidine substrate was then used to reveal positive staining and hematoxylin was used to counterstain nuclei. The positive area (brown area in the tubulointerstitium (TGFβ1, αSMA) or view (MCP-1)) in 10 random fields in each group of immunohistochemical sections was quantified using Image-Pro-Plus software as the integral optical density of TGFβ1, αSMA, and MCP-1.
Pro-inflammatory cytokine assays
The levels of plasma and renal TNF-α and IL-1β in 5/6Nx rats were measured using commercially available ELISA kits (Boster Biological Technology Co Ltd, Wuhan, China) according to the manufacturer’s instructions. Renal tissue was homogenized in 10 mmol/L Tris-HCl-buffered solution (pH 7.4, 1 mmol/L EDTA, 2 mol/L NaCl, 0.01% Tween-80, 1 mmol/L phenylmethylsulfonyl fluoride), and then centrifuged at 12,000 × g for 15 min at 4 °C [29]. The supernatant was subsequently used for cytosine assays.
Protein extraction and Western blot analysis
Kidney tissues were lysed in RIPA lysis buffer and homogenized on ice, followed by centrifugation at 12,000 × g for 15 min at 4 °C. The protein concentration was measured using a BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China). Protein samples were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels for separation, electro-transferred onto polyvinylidene difluoride (PVDF) membranes, and then blocked with 5% skim milk in Tris-buffered saline for 2 h at room temperature. Membranes were incubated with primary antibodies (anti-p-IKKα/β, anti-IKKβ, anti-p-NF-κB p65, anti-NF-κB p65, anti-p-IκBα, anti-IκBα, anti-p-p38 MAPK, and anti-p38 MAPK, 1:1000; anti-ICAM-1 and VCAM-1, 1:500) overnight at 4 °C, and then incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies (diluted 1:5000). The membranes were visualized using enhanced chemiluminescence (ECL) agents and developed by autoluminography.
In vitro experiments
Cell culture
Renal proximal tubule epithelial cells (HK-2 cells) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). HK-2 cells were cultured in Dulbecco's modified Eagle's medium/F12 media supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in a humidified incubator containing 5% CO2. These cells were treated with 5 µg/mL LPS in the presence or absence of SAA at the indicated concentrations for the indicated periods. SAA was dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO in media was <0.1% in all groups.
MTT assay for cell viability
Cell viability was measured using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT) assay. HK-2 cells were seeded in 96-well plates at 104 cells pe rwell. After 24 h incubation, cells were pretreated with or without various concentrations of SAA for 2 h, and then co-incubated with or without LPS (5 µg/mL) for 24 h. Later, the supernatant was replaced with serum-free medium; 20 µL of MTT (5 mg/mL) was added to each well and incubated for another 4 h, followed by removal of the supernatant. One hundred microliters of DMSO was added to dissolve the formazan. The solution absorbance at λ = 490 nm was measured using a microplate reader.
Pro-inflammatory cytokine assays
HK-2 cells were seeded in 6-well plates at 2 × 105 cells per well. After 24 h incubation, cells were pretreated with or without various concentrations of SAA (e.g., 3, 10, and 30 μmol/L) for 2 h, and then co-incubated with or without LPS (5 µg/mL) for 24 h. The levels of TNF-α and IL-1β in cell culture supernatants were measured using commercially available ELISA kits according to the manufacturer’s instructions.
Protein extraction and Western blot analysis
HK-2 cells were pretreated with or without various concentrations of SAA (3, 10, and 30 μmol/L) for 2 h following co-incubation with or without LPS (5 µg/mL) for various times (see Results section), before harvesting the cells. Next, the cells were lysed in RIPA lysis buffer and homogenized on ice, followed by centrifugation at 12,000 × g for 15 min at 4 °C. The protein concentration was measured using a BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China). Protein samples were loaded onto 10% SDS-PAGE gels for separation, electro-transferred onto PVDF membranes, and then blocked with 5% skim milk in Tris-buffered saline for 2 h at room temperature. Membranes were incubated with primary antibodies (anti-p-IKKα/β, anti-IKKβ, anti-p-NF-κB p65, anti-NF-κB p65, anti-p-IκBα, anti-IκBα, anti-p-p38 MAPK, anti-p38 MAPK, 1:1000; anti-ICAM-1 and VCAM-1, 1:500) overnight at 4 °C, and then incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies. The membranes were visualized using ECL agents and developed by autoluminography.
Immunofluorescent staining
HK-2 cells were plated on chamber slides in 24-well plates at 1 × 104 cells per well. After 24 h incubation, cells were pretreated with or without SAA (30 μmol/L) for 2 h, and then co-incubated with or without LPS (5 µg/mL) for 1.5 h. Cells were fixed with 4% paraformaldehyde for 30 min and then incubated with 0.3% Triton X-100 for 30 min. Then, cells were blocked with 5% normal goat serum for 60 min. The cells were incubated with a primary anti-NF-κB p65 antibody (1:100) overnight at 4 °C, followed by incubation with a rhodamine (TRITC)-conjugated goat anti-rabbit IgG (H + L) antibody for 2 h at room temperature in the dark. The cell nuclei were stained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (Beyotime Institute of Biotechnology, Shanghai, China), and the NF-κB p65 subunit was observed with a fluorescence microscope (Nikon, Japan).
Statistical analysis
All data are expressed as the means ± SD and analyzed by one-way analysis of variance (ANOVA) (SPSS version 17.0 software; SPSS Inc., Chicago, IL, USA). Quantitative data were tested for homogeneity of variance. If the variances were homogeneous, analysis of variance (ANOVA) was performed followed by the post hoc least significant difference test for multiple comparisons. If the variances were not homogeneous, a one-way ANOVA followed by the post hoc Dunnett’s T3 test was used for multiple comparisons. A Pvalue of <0.05 was considered significant.
Results
Biochemical parameters in experimental animals
All rats were subjected to 5/6 nephrectomy to establish the 5/6Nx rat model. The levels of urine protein, BUN, SCr, plasma TC, and plasma TGs were tested in the 5/6Nx rats. The results showed that the levels of urine protein, BUN, SCr, plasma TC, and plasma TGs were significantly increased compared to the control group, which suggests that CKD was successfully established in our rat model. Treatment with SAA resulted in dose-dependent reductions in the levels of urine protein, BUN, SCr, plasma TC and plasma TGs (Table 1) compared to the untreated 5/6Nx group, suggesting that SAA plays an important role in this model.
Table 1.
The effects of SAA on biochemical parameters in rats subjected to 5/6Nx
| Groups | Urine protein (mg/24 h) | BUN (mmol/L) | SCr (μmol/L) | TC (mmol/L) | TG (mmol/L) |
|---|---|---|---|---|---|
| Control | 14 ± 1.8 | 14.9 ± 2.1 | 40.6 ± 4.5 | 1.8 ± 0.66 | 0.73 ± 0.14 |
| 5/6Nx | 88.1 ± 13.9** | 56.5 ± 7.4** | 88.5 ± 15.7** | 5.1 ± 1.62** | 1.79 ± 0.18** |
| 5/6Nx+SAA (2.5 mg/kg) | 64.7 ± 16.4 | 44.2 ± 4.3# | 71.9 ± 8.6 | 3.48 ± 1.08 | 1.24 ± 0.18## |
| 5/6Nx+SAA (5 mg/kg) | 53.4 ± 8.3## | 32.7 ± 4## | 57.6 ± 6.7# | 3.19 ± 0.34# | 0.93 ± 0.11## |
| 5/6Nx+SAA (10 mg/kg) | 41.1 ± 18.3## | 27.7 ± 5.5## | 52 ± 13.7# | 2.37 ± 0.51## | 0.83 ± 0.11## |
Data are expressed as the means ± SD (n = 6, all samples were randomly selected from a pool of 10 rats, and then these samples were analyzed). **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. 5/6Nx group
BUN blood urea nitrogen, SCr serum creatinine, TG triglyceride, TC total cholesterol, SAA salvianolic acid A, 5/6Nx 5/6 nephrectomized
SAA treatment improves renal pathological lesions and tubulointerstitial fibrosis, and reduces the levels of chemokines and pro-inflammatory cytokines in 5/6Nx rats
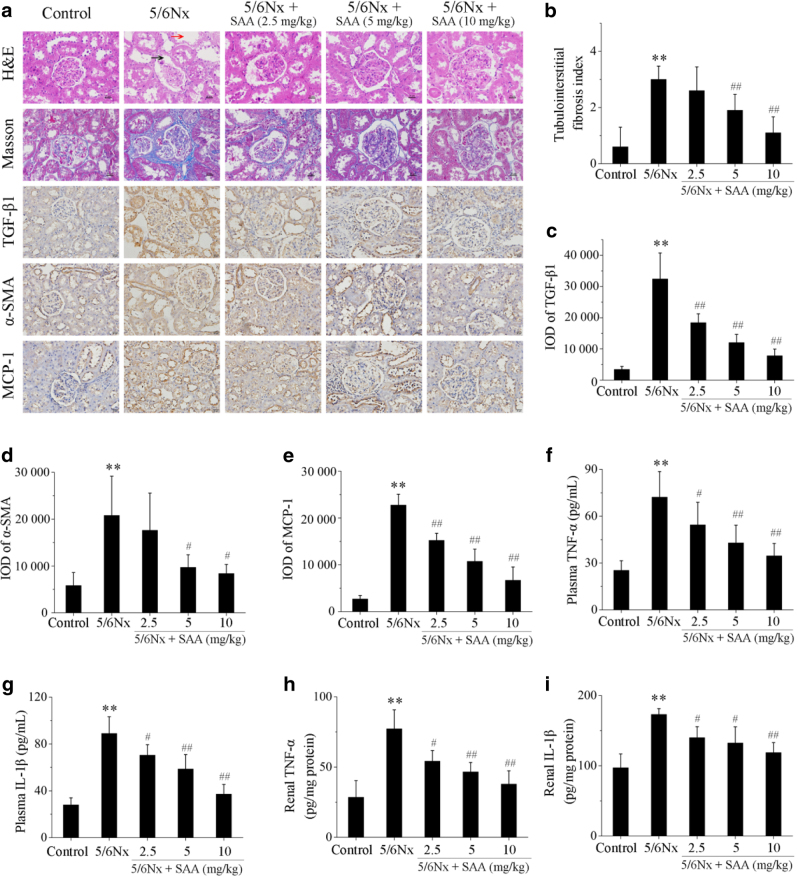
Histopathological changes in the renal sections of treated and untreated 5/6Nx rats were examined to evaluate the effects of SAA on renal injury. Renal histology samples were normal in the control group in contrast to the kidneys of the 5/6Nx rats, which exhibited histopathological changes in the renal tissues characterized by dilation of the renal capsule cavity and tubular dilatation (Fig. 1a). However, treatment with SAA resulted in remarkable improvement of the pathological lesions in a dose-dependent manner (Fig. 1a).
Fig. 1.
The effects of SAA on histopathological changes, tubulointerstitial fibrosis, and the levels of chemokines and pro-inflammatory cytokines in 5/6Nx rats. Renal histopathological changes and tubulointerstitial fibrosis were examined by hematoxylin and eosin (H&E) staining and Masson’s trichrome staining in renal sections (magnification: ×400). The black arrow indicates dilation of the renal capsule cavity; the red arrow indicates tubular dilation by H&E staining. The blue area in the tubulointerstitium was selected as a positive area by Masson’s trichrome staining. The expression of TGF-β1, α-SMA, and MCP-1 was visualized in renal sections using immunohistochemistry (magnification: ×400). The brown area in the tubulointerstitium was selected as a positive area of TGF-β1 and α-SMA staining; the brown area in the section was selected as a positive area of MCP-1 staining in immunohistochemistry analysis. The levels of the plasma and renal pro-inflammatory cytokines TNF-α and IL-1β in each group were measured by ELISA. a Renal sections with H&E staining, Masson’s trichrome staining, or immunohistochemical staining; b semiquantitative measurements of tubulointerstitial areas; integral optical density (IOD) of c TGF-β1; d α-SMA; and e MCP-1; f plasma levels of TNF-α; g plasma levels of IL-1β; h levels of renal TNF-α; and i levels of renal IL-1β. Data are expressed as the means ± SD (n = 10). **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. 5/6Nx group
Fibrosis of the tubulointerstitial area was examined by Masson’s trichrome staining (Fig. 1a). Renal histology was normal in all rats in the control group. However, 5/6Nx rats showed a significant increase in renal tubulointerstial fibrosis (blue areas) compared to rats in the control group (Fig. 1a, b). Treatment with SAA resulted in a significant attenuation of renal tubulointerstial fibrosis compared to rats of the 5/6Nx group, in a dose-dependent manner (Fig. 1a, b). Two key proteins were detected in the progression of renal fibrosis, TGF-β1 and α-SMA, using immunohistochemistry. There was only very weak staining of TGF-β1 and α-SMA in renal tubulointerstitial sections in control rats, which indicates an inferior role of the expression of TGF-β1 and α-SMA in normal kidney function (Fig. 1a). However, the levels of both of these proteins were significantly increased in 5/6Nx rats. Administration of SAA significantly decreased the expression of TGF-β1 and α-SMA in renal tubulointerstitial sections in 5/6Nx rats in a dose-dependent manner (Fig. 1a, c, d).
The levels of MCP-1, which plays a key role in other renal inflammatory diseases [19], were detected in renal sections of 5/6Nx rats by immunohistochemistry staining and were significantly elevated compared to the relatively low expression of MCP-1 in sections from controls rats. Treatment with SAA significantly attenuated the expression of MCP-1 in a dose-dependent manner (Fig. 1a, e). To investigate other pro-inflammatory cytokines in 5/6Nx rats, we measured TNF-α and IL-1β levels in plasma and renal tissues by enzyme-linked immunosorbent assay (ELISA), which were significantly increased in 5/6Nx rats compared to the control group. Administration of SAA significantly reduced the plasma and renal levels of TNF-α and IL-1β in 5/6Nx rats in a dose-dependent manner (Fig. 1f–i).
SAA inhibits activation of the NF-κB and p38 MAPK signaling pathways in 5/6Nx rats
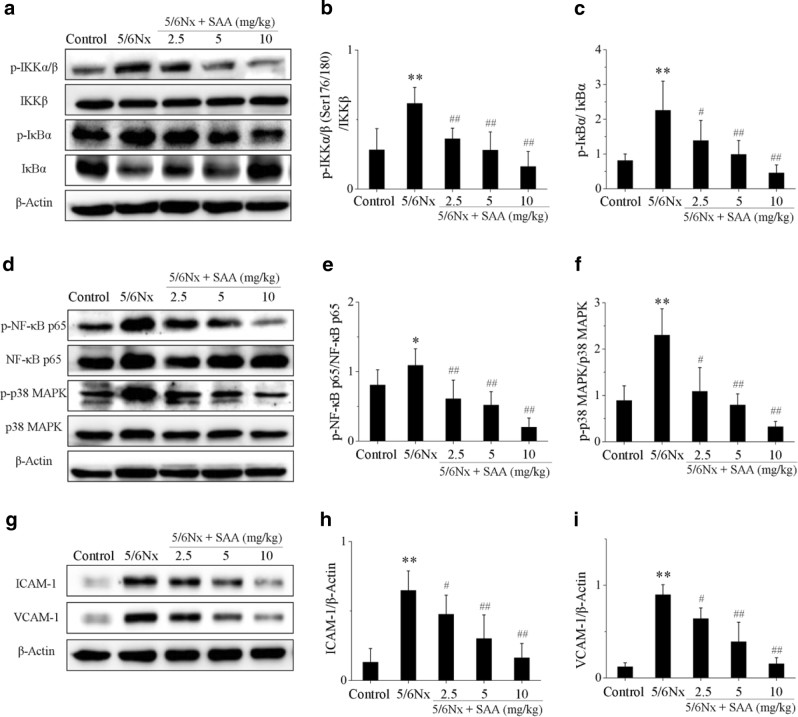
Several studies have suggested that the NF-κB and p38 MAPK signaling pathways are closely associated with kidney inflammation [13, 20–23]. Thus, levels of major proteins of the NF-κB and p38 MAPK signaling pathways were measured during CKD. The protein levels of p-IKKα/β, p-IκBα, p-NF-κB p65, and p-p38 MAPK in 5/6Nx rats were significantly increased compared to control rats, whereas the expression levels of the IκBα protein were decreased, which suggests that the NF-κB and p38 MAPK signaling pathways were activated during renal inflammation. Treatment with SAA significantly attenuated the expression of p-IKKα/β, p-IκBα, p-NF-κB p65, and p-p38 MAPK proteins compared to the 5/6Nx group and increased the expression of the IκBα protein in a dose-dependent manner, which suggests that SAA inhibits activation of the NF-κB and p38 MAPK signaling pathways (Fig. 2).
Fig. 2.
The effects of SAA on the NF-κB and p38 MAPK signaling pathways in 5/6Nx rats. a The protein expression levels of p-IKKα/β, IKKβ, p-IκBα, and IκBα proteins were analyzed by Western blot; b p-IKKα/β protein expression relative to IKKβ protein levels; c p-IκBα protein expression relative to IκBα protein levels; d the protein expression levels of p-NF-κB p65, NF-κB p65, p-p38 MAPK, and p38 MAPK proteins were analyzed by Western blot; e p-NF-κB p65 protein expression relative to NF-κB p65 protein levels; f p-p38 MAPK protein expression relative to p38 MAPK protein levels; g the protein expression levels of ICAM-1 and VCAM-1 proteins were analyzed by Western blot; h ICAM-1 protein expression relative to β-actin protein expression; i VCAM-1 protein expression relative to β-actin protein expression. Data are expressed as the means ± SD (n = 6, all samples were randomly selected from a pool of 10 rats, and then these samples were analyzed). *P < 0.05, **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. 5/6Nx group
ICAM-1 and VCAM-1 have been implicated in most inflammatory processes, and both of these proteins are regulated by NF-κB. Both protein levels were significantly increased in 5/6Nx rats compared to the control rat group. Treatment with SAA significantly decreased the expression levels of the ICAM-1 and VCAM-1 proteins compared to levels in the 5/6Nx group at different concentrations of the compound (Fig. 2g–i).
SAA improves cell viability and attenuates the secretion of pro-inflammatory cytokines in LPS-induced HK-2 cells
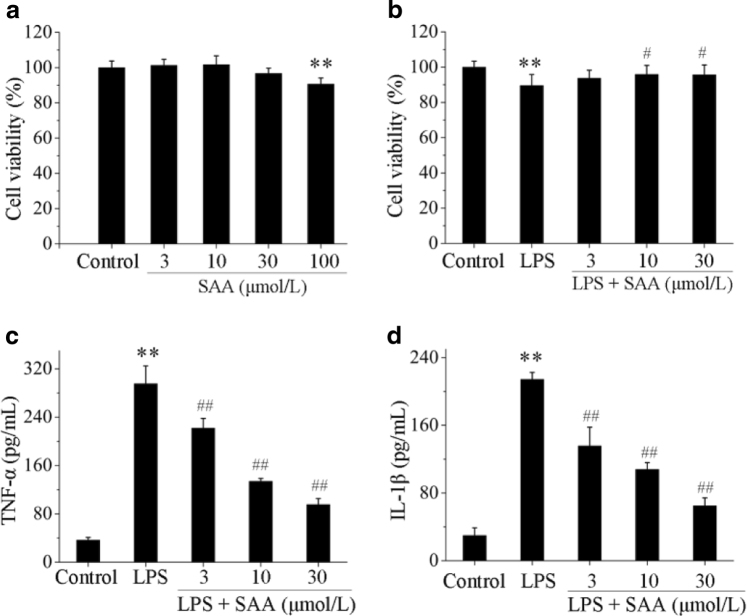
To further confirm the effects of SAA that we observed in vivo, we next determined the effects of SAA in an in vitro model of kidney inflammation (LPS-induced HK-2 cells). SAA did not inhibit the viability of HK-2 cells up to a concentration of 30 μmol/L. At a concentration of 100 μmol/L, SAA was cytotoxic to HK-2 cells (Fig. 3a). Therefore, we chose a concentration of 30 μmol/L SAA for our in vitro model. Even though LPS showed marked cytotoxicity to cells, treatment with SAA significantly enhanced the cell viability in LPS-induced HK-2 cells (Fig. 3b).
Fig. 3.
The effects of SAA on HK-2 cell viability and TNF-α and IL-1β levels in LPS-induced HK-2 cells. Cells were pretreated with or without various concentrations of SAA for 2 h, and then co-incubated with or without LPS (5 µg/mL) for 24 h. Cell viability was assessed by MTT assay. Effects of SAA on HK-2 cell viability (a) and effects of SAA on LPS-induced HK-2 cell viability (b). The levels of the pro-inflammatory cytokines TNF-α and IL-1β in each group were measured by ELISA. TNF-α (c) and IL-1β (d) levels in LPS-induced HK-2 cells. Data are expressed as the means ± SD (n = 3). **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. LPS group
The secretion of pro-inflammatory cytokines in LPS-induced HK-2 cells was also examined. The levels of TNF-α and IL-1β in cell culture supernatants stimulated with LPS (5 μg/mL) significantly increased compared to the untreated control group, which indicates that the model of inflammation was successfully established in vitro.
Administration of SAA significantly attenuated the secretion of TNF-α and IL-1β in LPS-induced HK-2 cells in a concentration-dependent manner (Fig. 3c, d).
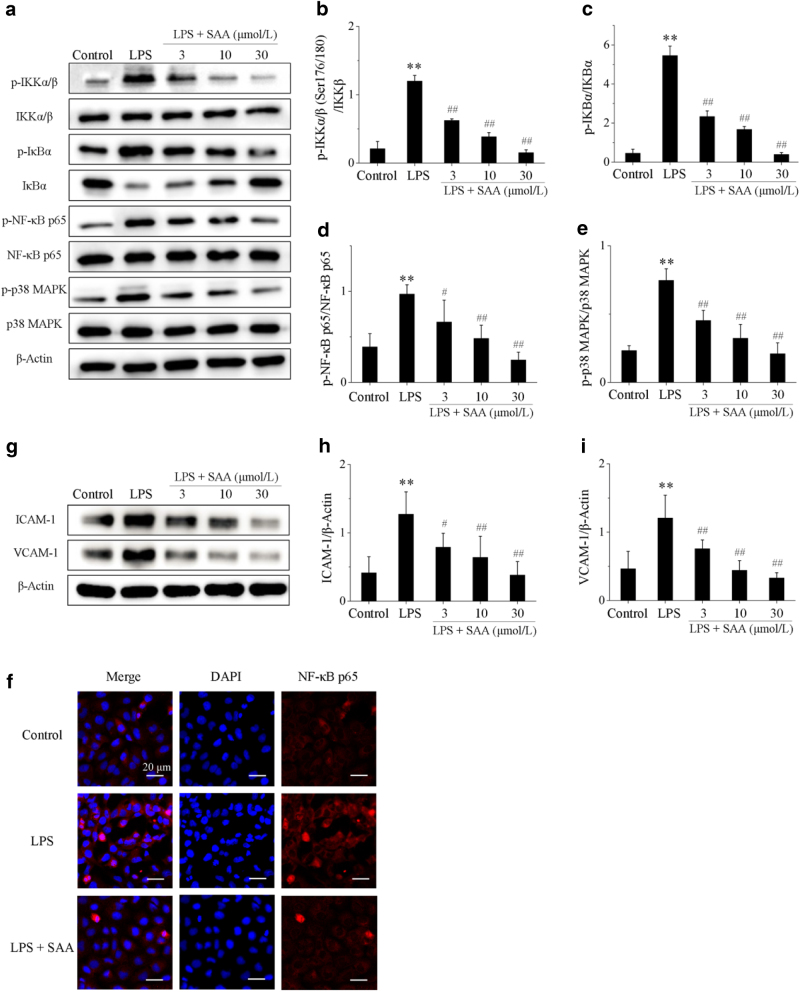
SAA inhibits activation of the NF-κB and p38 MAPK signaling pathways in LPS-induced HK-2 cells
Major proteins of the NF-κB and p38 MAPK signaling pathways were also analyzed in LPS-induced HK-2 cells. Cells were first stimulated with LPS for 1.5 h, and the results showed that p-IKKα/β, p-IκBα, p-NF-κB p65, and p-p38 MAPK protein levels in LPS-stimulated HK-2 cells were significantly increased compared to LPS-untreated control cells, while the protein expression of IκBα was decreased after LPS treatment. This suggests that NF-κB and p38 MAPK signaling pathways are activated. Treatment with SAA significantly decreased the expression of p-IKKα/β, p-IκBα, p-NF-κB p65, and p-p38 MAPK proteins compared to HK-2 cells stimulated with LPS, and increased the expression of the IκBα protein to varying degrees depending on the concentration of SAA (Fig. 4). NF-κB p65 was mainly localized in the cytosol of HK-2 cells in the control group, while stimulation with LPS remarkably promoted NF-κB p65 translocation into the nucleus. Treatment with SAA dramatically lowered the overall translocation of NF-κB p65 into the nuclei (Fig. 4f).
Fig. 4.
The effects of SAA on the NF-κB and p38 MAPK signaling pathways in LPS-induced HK-2 cells. Cells were pretreated with or without various concentrations of SAA (3, 10, and 30 μmol/L) for 2 h, and then stimulated with or without LPS (5 µg/mL) for 1.5 or 24 h. a The protein expression levels of p-IKKα/β, IKKβ, p-IκBα, IκBα, p-NF-κB p65, NF-κB p65, p-p38 MAPK, and p38 MAPK proteins were analyzed by Western blot; b p-IKKα/β protein expression relative to IKKβ protein expression; c p-IκBα protein expression relative to IκBα protein expression; d p-NF-κB p65 protein expression relative to NF-κB p65 protein expression; e p-p38 MAPK protein expression relative to p38 MAPK protein expression; f the effect of 30 μmol/L SAA on NF-κB translocation in LPS-induced HK-2 cells (magnification: ×600); g the protein expression levels of ICAM-1 and VCAM-1 proteins were analyzed by Western blot; h ICAM-1 protein expression relative to β-actin protein expression; i VCAM-1 protein expression relative to β-actin protein expression. Data are expressed as the means ± SD (n = 3). **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. LPS group
Similarly, the expression levels of ICAM-1 and VCAM-1 proteins in LPS-induced HK-2 cells were measured. ICAM-1 and VCAM-1 protein levels in the LPS group were significantly increased compared to the control group. Treatment with SAA significantly decreased the expression of ICAM-1 and VCAM-1 proteins compared to the LPS-treated control group in a concentration-dependent manner (Fig. 4g–i). These results are collectively consistent with the results obtained using the in vivo 5/6Nx rat model.
Discussion
In this study, we used the 5/6 nephrectomy method to generate an experimental model of CKD, which was characterized by high levels of proteinuria and BUN and SCr (three important parameters of renal function) [30, 31] in association with increased oxidative stress and inflammation [6]. Moreover, the concentration of plasma TGs and TCs are commonly elevated in experimental animal models of chronic renal failure [32]. The 5/6 nephrectomy model shows similar pathological changes as those observed in patients with CKD, such as the accumulation of extracellular matrix in glomeruli and in the renal interstitium [33]. Thus, this model is frequently used in the field to study the influence of pharmacological and additional factors on morphological and functional changes in the kidney [34]. As a water-soluble component of Danshen, which is used regularly in traditional Chinese medicine, SAA exhibits multiple pharmacological activities in vitro and in vivo and has renoprotective effects [26, 27]. Moreover, SAA can effectively improve microcirculation and ameliorate blood stasis [35]. In the present study, our results show that SAA decreased the levels of urine protein, BUN, SCr, TC, and TG and improved renal pathology in 5/6Nx rats, suggesting that SAA might have a beneficial effect on kidney injury (Table 1 and Fig. 1).
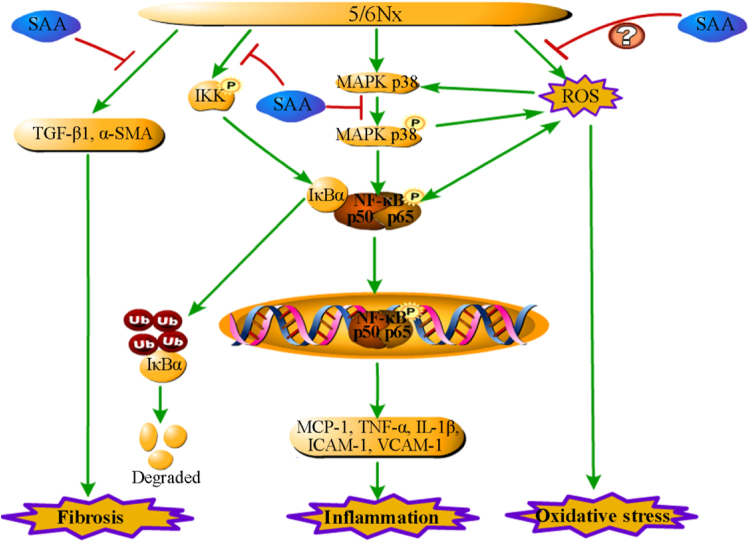
In the development and progression of CKD, chronic inflammation and oxidative stress play a key role; these are closely associated with the activation of the NF-κB signaling pathway [36, 37]. As previously shown, chronic systemic inflammation and oxidative stress are key factors in the development of kidney disease [7], and inhibiting inflammation may therefore be an effective strategy for treating renal diseases. SAA has been shown to exhibit strong anti-inflammatory activities [26]. In the current study, we investigated these effects in more depth and demonstrated that SAA inhibits inflammation by decreasing the activation of the NF-κB signaling pathway, and thereby affecting the expression of a series of downstream proteins (Fig. 5). As our results show, SAA treatment significantly attenuated the protein expression levels of p-IKKα/β, p-IκBα, and p-NF-κB p65 and increased the protein expression of IκBα in 5/6Nx rats (Fig. 2). The same results were observed with HK-2 cells stimulated with LPS (Fig. 4). NF-κB is a pivotal transcription factor that controls the expression of many inflammatory genes, including TNF-α, IL-1β, MCP-1, ICAM-1, and VCAM-1, which all play a pivotal role in the pathogenesis of CKD [19, 36]. Previous studies have shown that circulating and renal levels of pro-inflammatory cytokines and markers such as TNF-α and IL-1β were elevated in CKD, and this is significantly associated with a higher risk of mortality [38–41]. TNF-α can activate the NF-κB signaling pathway, which in turn can increase the expression of many other inflammatory cytokines and subsequently exacerbate the inflammatory response in CKD [42, 43]. TNF-α, IL-1β, and MCP-1 are secreted and trigger an increased inflammatory response. Our results suggest that SAA lowers the expression levels of plasma and renal TNF-α and IL-1β and renal MCP-1 in 5/6Nx rats (Fig. 1), and the same results were confirmed in LPS-induced HK-2 cells in vitro (Fig. 3). ICAM-1 and VCAM-1 are two adhesion molecules that promote leukocyte adherence to the endothelium of blood vessels to allow leukocytes to migrate into tissues at sites of injury, where they initiate inflammatory responses as part of the immune response [44, 45]. In this study, we have demonstrated that SAA inhibits the expression of ICAM-1 and VCAM-1 in 5/6Nx rats (Fig. 2), and these results were confirmed in LPS-induced HK-2 cells in vitro (Fig. 4). Our results indicate that SAA reduces inflammation by inhibiting the activation of the NF-κB signaling pathway and subsequently lowering the expression of its downstream proteins.
Fig. 5.
A schematic illustration of SAA attenuation of kidney injury and inflammation in 5/6Nx rats. SAA improves kidney injury and inflammation through inhibition of the NF-κB and p38 MAPK signaling pathways in 5/6Nx rats
We additionally examined the p38 MAPK signaling pathway upstream of NF-κB. The activation of MAPK signaling pathways can induce the apoptosis of renal proximal tubule epithelial cells and promote inflammation and renal injury [46–48]. Several traditional Chinese monomer medicines and their extracts (such as Emodin and Berberine) and several traditional Chinese medicine compounds, such as Yishen Huoxue decoction, can attenuate inflammatory reactions in renal tissues through regulation of the p38 MAPK signaling pathway, and thus reduce glomerular and renal interstitial inflammatory injury [49]. In the current study, we demonstrated that SAA could inhibit both the activation of the p38 MAPK signaling pathway in vivo and in vitro (Figs. 2 and 4).
Tubulointerstitial fibrosis is recognized as a common pathological feature of CKD and is characterized by excessive deposition of extracellular matrix [33, 50]. As a multifunctional cytokine, TGF-β1 exhibits multiple biological properties, including cell differentiation, proliferation, apoptosis, autophagy, and production of extracellular matrix, among others [51]. Studies have shown that TGF-β1 plays a key role in the progression of renal fibrosis [50] and has pro-inflammatory and profibrogenic effects in HK-2 cells [52]. Wang et al. [33] previously reported that the expression of TGF-β1 was increased in 5/6Nx rats. Moreover, in a unilateral ureteral occlusion model of mice, apelin significantly attenuates renal fibrosis through suppression of the expression of TGF-β1 and α-SMA and maintenance of the expression of E-cadherin and laminin [33]. In this study, SAA improved tubulointerstitial fibrosis and reduced the expression of TGF-β1 and α-SMA in 5/6Nx rats, which demonstrates that SAA possesses anti-fibrotic activity (Fig. 1).
In conclusion, our results have demonstrated for the first time that SAA can partially protect against kidney injury in 5/6Nx rats. As shown in Fig. 5, one of the key mechanisms underlying the therapeutic effects of SAA in kidney injury is primarily related to its anti-inflammatory functions. SAA attenuated the production of inflammatory cytokines, chemokines, and adhesion molecules by inhibiting the activation of the NF-κB and p38 MAPK signaling pathways in 5/6Nx rats and LPS-stimulated HK-2 cells. As a minor phenolic acid, the anti-oxidant properties of SAA may be another potential mechanism contributing to its beneficial role in kidney injury. The anti-oxidant and anti-fibrotic effects of SAA in CKD will be studied in future studies. In sum, the novel results in this study provide a pharmacological foundation for exciting future studies of SAA in the treatment of CKD.
Acknowledgements
This study was supported by the Department of Science and Technology of Liaoning Province (No. 201501053), the Major Science and Technology Project for “Significant New Drug Creation” (No. 2017ZX09305005), and the Innovation Team of Shenyang Pharmaceutical University.
Author contributions
H-fZ designed the study, performed the experiments, and wrote the manuscript; ZZ and J-hW designed the study, contributed materials, and revised the manuscript; Y-lW, CG, Y-tG, JH, and J-hW supported several experiments and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jia-hong Wang, Phone: +86-024-23986549, Email: jiahong_wang_sy@163.com.
Zhou Zhang, Phone: +86-024-23986549, Email: zzhouzhang@163.com.
References
- 1.Drawz P, Rahman M. Chronic kidney disease. Ann Intern Med. 2015;162:ITC1–16. doi: 10.7326/AITC201506020. [DOI] [PubMed] [Google Scholar]
- 2.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–72. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 3.Alsahli M, Gerich JE. Hypoglycemia, chronic kidney disease, and diabetes mellitus. Mayo Clin Proc. 2014;89:1564–71. doi: 10.1016/j.mayocp.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2017;388:1659–724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–41. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cachofeiro Victoria, Goicochea Marian, de Vinuesa Soledad García, Oubiña Pilar, Lahera Vicente, Luño José. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney International. 2008;74:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- 8.Lin X, Zha Y, Zeng XZ, Dong R, Wang QH, Wang DT. Role of the Wnt/beta-catenin signaling pathway in inducing apoptosis and renal fibrosis in 5/6-nephrectomized rats. Mol Med Rep. 2017;15:3575–82. doi: 10.3892/mmr.2017.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawson J, Elliott J, Wheeler-Jones C, Syme H, Jepson R. Renal fibrosis in feline chronic kidney disease: known mediators and mechanisms of injury. Vet J. 2015;203:18–26. doi: 10.1016/j.tvjl.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Tapia E, Franco M, Sanchez-Lozada LG, Soto V, Avila-Casado C, Santamaria J, et al. Mycophenolate mofetil prevents arteriolopathy and renal injury in subtotal ablation despite persistent hypertension. Kidney Int. 2003;63:994–1002. doi: 10.1046/j.1523-1755.2003.00811.x. [DOI] [PubMed] [Google Scholar]
- 11.Tapia E, Sanchez-Lozada LG, Soto V, Manrique AM, Ortiz-Vega KM, Santamaria J, et al. Sildenafil treatment prevents glomerular hypertension and hyperfiltration in rats with renal ablation. Kidney Blood Press Res. 2012;35:273–80. doi: 10.1159/000334952. [DOI] [PubMed] [Google Scholar]
- 12.Aminzadeh MA, Reisman SA, Vaziri ND, Khazaeli M, Yuan J, Meyer CJ. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica. 2014;44:570–8. doi: 10.3109/00498254.2013.852705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu C, Qi D, Sun JF, Li P, Fan HY. Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-kappaB activities. Sci Rep-UK. 2015;5:11822. doi: 10.1038/srep11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang JY, Shin HS. Effects of 1,7-substituted methylxanthine derivatives on LPS-stimulated expression of cytokines and chemokines in Raw 264.7 and HK-2 cells. J Microbiol Biotechnol. 2015;25:296–301. doi: 10.4014/jmb.1410.10044. [DOI] [PubMed] [Google Scholar]
- 15.Quoilin C, Mouithys-Mickalad A, Duranteau J, Gallez B, Hoebeke M. Endotoxin-induced basal respiration alterations of renal HK-2 cells: a sign of pathologic metabolism down-regulation. Biochem Biophys Res Commun. 2012;423:350–4. doi: 10.1016/j.bbrc.2012.05.128. [DOI] [PubMed] [Google Scholar]
- 16.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–67. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–74. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 18.Nam NH. Naturally occurring NF-kappaB inhibitors. Mini-Rev Med Chem. 2006;6:945–51. doi: 10.2174/138955706777934937. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Wan DY, Li JS, Chen H, Huang K, Zheng L, et al. Histone acetyltransferase PCAF regulates inflammatory molecules in the development of renal injury. Epigenetics-US. 2015;10:62–72. doi: 10.4161/15592294.2014.990780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Sun SC. NF-kappaB in inflammation and renal diseases. Cell Biosci. 2015;5:63. doi: 10.1186/s13578-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahu BD, Tatireddy S, Koneru M, Borkar RM, Kumar JM, Kuncha M, et al. Naringin ameliorates gentamicin-induced nephrotoxicity and associated mitochondrial dysfunction, apoptosis and inflammation in rats: possible mechanism of nephroprotection. Toxicol Appl Pharmacol. 2014;277:8–20. doi: 10.1016/j.taap.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Stambe C, Nikolic-paterson DJ, Hill PA, Dowling J, Atkins RC. p38 mitogen-activated protein kinase activation and cell localization in human glomerulonephritis: correlation with renal injury. J Am Soc Nephrol. 2004;15:326–36. doi: 10.1097/01.ASN.0000108520.63445.E0. [DOI] [PubMed] [Google Scholar]
- 23.Peng L, Li J, Xu YX, Wang YT, Du H, Shao JQ, et al. The protective effect of beraprost sodium on diabetic nephropathy by inhibiting inflammation and p38 MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic rats. Int J Endocrinol. 2016;2016:1690474. doi: 10.1155/2016/1690474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams JL, Badger AM, Kumar S, Lee JC. p38 MAP kinase: molecular target for the inhibition of pro-inflammatory cytokines. Prog Med Chem. 2001;38:1–60. doi: 10.1016/S0079-6468(08)70091-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Zhang W, Zhao Y, Yang X, Fang L, Wang S, et al. Research progress of salvianolic acid A. China J Chin Mater Med. 2011;36:2603–9. [PubMed] [Google Scholar]
- 26.Oh KS, Oh BK, Mun J, Seo HW, Lee BH. Salvianolic acid A suppress lipopolysaccharide-induced NF-kappaB signaling pathway by targeting IKKbeta. Int Immunopharmacol. 2011;11:1901–6. doi: 10.1016/j.intimp.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Fan HY, Yang MY, Qi D, Zhang ZK, Zhu L, Shang-Guan XX, et al. Salvianolic acid A as a multifunctional agent ameliorates doxorubicin-induced nephropathy in rats. Sci Rep-UK. 2015;5:12273. doi: 10.1038/srep12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toba H, Tojo C, Wang J, Noda K, Kobara M, Nakata T. Telmisartan inhibits vascular dysfunction and inflammation via activation of peroxisome proliferator-activated receptor-gamma in subtotal nephrectomized rat. Eur J Pharmacol. 2012;685:91–8. doi: 10.1016/j.ejphar.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Tzeng TF, Liou SS, Chang CJ, Liu IM. Zerumbone, a tropical ginger sesquiterpene, ameliorates streptozotocin-induced diabetic nephropathy in rats by reducing the hyperglycemia-induced inflammatory response. Nutr Metab. 2013;10:64. doi: 10.1186/1743-7075-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandilands EA, Dhaun N, Dear JW, Webb DJ. Measurement of renal function in patients with chronic kidney disease. Br J Clin Pharmacol. 2013;76:504–15. doi: 10.1111/bcp.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–21. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 32.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Ren. 2006;290:262–72. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 33.Wang DT, Huang RH, Cheng X, Zhang ZH, Yang YJ, Lin X. Tanshinone IIA attenuates renal fibrosis and inflammation via altering expression of TGF-beta/Smad and NF-kappaB signaling pathway in 5/6 nephrectomized rats. Int Immunopharmacol. 2015;26:4–12. doi: 10.1016/j.intimp.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 34.Kujal P, Vernerová Z. 5/6 nephrectomy as an experimental model of chronic renal failure and adaptation to reduced nephron number. Cesk Fysiol. 2008;57:104–9. [PubMed] [Google Scholar]
- 35.Fan HY, Fu FH, Yang MY, Xu H, Zhang AH, Liu K. Antiplatelet and antithrombotic activities of salvianolic acid A. Thromb Res. 2010;126:17–22. doi: 10.1016/j.thromres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Impellizzeri D, Esposito E, Attley J, Cuzzocrea S. Targeting inflammation: new therapeutic approaches in chronic kidney disease (CKD) Pharmacol Res. 2014;81:91–102. doi: 10.1016/j.phrs.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Wang YQ, Wang B, Du F, Su XS, Sun GP, Zhou GY, et al. Epigallocatechin-3-gallate attenuates oxidative stress and inflammation in obstructive nephropathy via NF-κB and Nrf2/HO-1 signalling pathway regulation. Basic Clin Pharmacol. 2015;117:164–72. doi: 10.1111/bcpt.12383. [DOI] [PubMed] [Google Scholar]
- 38.Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol. 2009;24:1445–52. doi: 10.1007/s00467-008-1046-0. [DOI] [PubMed] [Google Scholar]
- 39.Pereira BJG, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA. Plasma levels of IL-1β, TNFα and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int. 1994;45:890–6. doi: 10.1038/ki.1994.117. [DOI] [PubMed] [Google Scholar]
- 40.Kondo M, Tahara A, Hayashi K, Abe M, Inami H, Ishikawa T, et al. Renoprotective effects of novel interleukin-1 receptor-associated kinase 4 inhibitor AS2444697 through anti-inflammatory action in 5/6 nephrectomized rats. N-S Arch Pharmacol. 2014;387:909–19. doi: 10.1007/s00210-014-1023-z. [DOI] [PubMed] [Google Scholar]
- 41.Ortega LM, Fornoni A. Role of cytokines in the pathogenesis of acute and chronic kidney disease, glomerulonephritis, and end-stage kidney disease. J Interf Cytokine Med Res. 2017;2:49–62. [Google Scholar]
- 42.Lee HH, Cho YI, Kim SY, Yoon YE, Kim KS, Hong SJ, et al. TNF-α-induced inflammation stimulates apolipoprotein-A4 via activation of TNFR2 and NF-kappaB signaling in kidney tubular cells. Sci Rep-UK. 2017;7:8856. doi: 10.1038/s41598-017-08785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degaspari S, Tzanno-Martins CB, Fujihara CK, Zatz R, Branco-Martins JP, Viel TA, et al. Altered KLOTHO and NF-kappaB-TNF-α signaling are correlated with nephrectomy-Induced cognitive impairment in rats. PLoS ONE. 2015;10:e0125271. doi: 10.1371/journal.pone.0125271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones SC, Banks RE, Haidar A, Gearing AJH, Hemingway IK, Ibbotson SH, et al. Adhesion molecules in inflammatory bowel. Gut. 1995;36:724–30. doi: 10.1136/gut.36.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank PG, Lisanti MP. ICAM-1: role in inflammation and in the regulation of vascular permeability. Am J Physiol Heart C. 2008;295:926–7. doi: 10.1152/ajpheart.00779.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alcorn JF, Velden JVD, Brown AL, McElhinney B, Irvin CG, Janssen-Heininger YMW. c-Jun N-terminal kinase 1 is required for the development of pulmonary fibrosis. Am J Resp Cell Mol. 2009;40:422–32. doi: 10.1165/rcmb.2008-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao YJ, Zhang YM, Wang N, He LC. Antioxidant effect of imperatorin from Angelica dahurica in hypertension via inhibiting NADPH oxidase activation and MAPK pathway. J Am Soc Hypertens. 2014;8:527–36. doi: 10.1016/j.jash.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Lin YL, Lee TF, Huang YJ, Huang YT. Antiproliferative effect of salvianolic acid A on rat hepatic stellate cells. J Pharm Pharmacol. 2006;58:933–9. doi: 10.1211/jpp.58.7.0008. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Q, Wan YG, Wang CJ, Wei QX, Chen HL, Meng XJ, et al. Regulatory mechanism of p38 MAPK signaling pathway on renal tissue inflammation in chronic kidney disease and interventional effect of traditional Chinese medicine. China J Chin Mater Med. 2012;37:1700–4 (Chinese). [PubMed]
- 50.Meng XM, Tang PMK, Li J, Lan HY. TGF-beta/Smad signaling in renal fibrosis. Front Physiol. 2015;6:82. doi: 10.3389/fphys.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meng XM, Chung AC, Lan HY. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin Sci. 2013;124:243–54. doi: 10.1042/CS20120252. [DOI] [PubMed] [Google Scholar]
- 52.Wang WM, Zhang HD, Jin YM, Zhu BB, Chen N. PPAR-γ agonists inhibit TGF-beta1-induced chemokine expression in human tubular epithelial cells. Acta Pharmacol Sin. 2009;30:107–12. doi: 10.1038/aps.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]