Abstract
Leptin, an adipokine that is implicated in the control of food intake via appetite suppression, may also stimulate oxidative stress, inflammation, thrombosis, arterial stiffness, angiogenesis and atherogenesis. These leptin-induced effects may predispose to the development of cardiovascular diseases. In the present review we discuss the evidence linking leptin levels with the presence, severity and/or prognosis of both coronary artery disease and non-cardiac vascular diseases such as stroke, carotid artery disease, peripheral artery disease (PAD) and abdominal aortic aneurysms (AAA) as well as with chronic kidney disease (CKD) and type 2 diabetes mellitus (T2DM). Leptin levels have been positively associated with the presence, severity, extent and lesion complexity of coronary atherosclerosis as well as with the presence, severity and poor clinical outcomes of both ischemic and hemorrhagic strokes. But conflicting results also exist. Furthermore, leptin was reported to independently predict common carotid intima-media thickness and carotid plaque instability. A link between hyperleptinemia and PAD has been reported, whereas limited data were available on the potential association between leptin and AAA. Elevated leptin concentrations have also been related to CKD incidence and progression as well as with insulin resistance, T2DM, micro- and macrovascular diabetic complications. Statins and antidiabetic drugs (including sitagliptin, metformin, pioglitazone, liraglutide and empagliflozin) may affect leptin levels. Further research is needed to establish the potential use (if any) of leptin as a therapeutic target in these diseases.
Keywords: leptin, coronary heart disease, stroke, peripheral artery disease, carotid artery disease, chronic kidney disease, abdominal aortic aneurysms, obesity
Introduction
Leptin is a hormone mainly secreted by adipocytes that is involved in the control of food intake via its action on the hypothalamus, leading to the suppression of appetite1. Therefore, leptin is an “anorexigenic” hormone. However, obesity is characterized by hyperleptinemia due to the development of leptin resistance2.
Apart from obesity, hyperleptinemia has been also associated with hypertension and insulin resistance3,4,5,6,7. The peripheral actions of leptin include stimulation of inflammatory reaction, oxidative stress, atherogenesis and thrombosis, thus promoting endothelial dysfunction, arterial stiffness, development and vulnerability of atherosclerotic plaques8,9,10. Furthermore, leptin regulates bone homeostasis, reproduction and angiogenesis11. Based on these leptin-induced effects, the role of leptin on the presence, severity and prognosis of both cardiac and non-cardiac vascular diseases are being investigated. Of note, chronic kidney disease (CKD) is characterized by increased cardiovascular (CV) risk12,13,14; leptin metabolism is also being evaluated in CKD patients. It should be stated that leptin may have a “protective” role against adiposity or pancreatic damage as shown in animal studies15,16. Furthermore, leptin administration (within the subphysiological to physiological range) was shown to reduce atherosclerotic lesions in low-density lipoprotein receptor (LDLR) knockout mice deficient in leptin17. These effects were mainly attributed to improvements in hypercholesterolemia and liver steatosis, as well as upregulation of adiponectin mRNA expression in the adipose tissue, connected with increases in circulating adiponectin levels.
Apart from leptin, other adipokines such as adiponectin, resistin and visfatin may be involved in the pathogenesis of CV and metabolic diseases. In this context, adiponectin was shown to affect insulin resistance, atherosclerosis, inflammation and oxidative stress pathways18,19,20. Hypoadiponectinemia has been linked to increased CV risk and T2DM21,22,23,24, although conflicting results exist25,26,27. With regard to CKD, adiponectin levels are elevated and may predict disease progression28,29. Resistin and visfatin have also been implicated in insulin resistance, T2DM, CKD and CV disease30,31,32.
In the present narrative review, the associations of leptin levels with coronary heart disease (CHD) and non-cardiac vascular diseases including stroke, carotid artery disease, peripheral artery disease (PAD) and abdominal aortic aneurysms (AAA) are discussed. The links between leptin concentrations and CKD progression and complications are also commented. Finally, the potential use of leptin as a therapeutic target is reviewed according to available data.
Leptin and CHD
It has been reported that CHD patients have higher leptin levels compared with controls33,34,35, as also supported by a meta-analysis36. Serum leptin concentrations are increased after myocardial infarction (MI)37; percutaneous coronary intervention can also raise its levels38. Six weeks of exercise prevented the increase of leptin concentrations in CHD patients following an acute MI39. However, conflicting results exist40,41 with a recent meta-analysis reporting no relationship between leptin levels and the risk of CHD42.
In CHD patients, elevated leptin levels were significantly associated with an increased risk of cardiac death, acute coronary syndrome, non-fatal MI, stroke and hospitalization for congestive heart failure (HF)43,44. However, in another study, increased leptin levels and body mass index (BMI) were predictors of a better prognosis in CHD patients45. Likewise, an inverse association between leptin concentrations and incidence of adverse events was observed in patients suffering an acute MI46 as well as between leptin levels and CV morbidity and mortality in patients with stable CHD47. Furthermore, leptin concentrations may affect thrombolytic therapy (TT) outcomes as high leptin levels on admission within 6 h after an acute MI were related to reduced TT efficacy48. Of note, leptin correlated inversely with mortality in men with HF and positively with mortality in men without HF49. In the same study, overweight/obese men with HF had a lower mortality risk compared with normal weight ones; however, adjustment for leptin eliminated this association, possibly reflecting cachexia49.
Elevated serum leptin concentrations and certain leptin gene polymorphisms have been related to the presence and severity of HF with normal ejection fraction in CHD patients50. HF patients also have higher leptin levels than controls51 in both cases with preserved and reduced ejection fraction52. In such patients, gender differences exist with women having higher leptin concentrations than men53. Furthermore, a direct association between leptin levels and HF progression (i.e. cardiac dysfunction) has also been reported54. Leptin correlated with epicardial fat thickness in HF patients55. Of note, HF patients with cardiac cachexia had lower leptin levels compared with HF patients without cachexia56.
Leptin to insulin ratio was linked to CHD severity assessed by the Gensini score in female CHD patients57. Similarly, higher serum leptin levels were significantly related to increasing number of stenotic coronary arteries and arterial stiffness in CHD patients58. Interestingly, plasma leptin concentrations were higher in patients with stable angina than in controls and were even higher in patients with unstable angina59. The presence, severity, extent and lesion complexity of coronary atherosclerosis has been associated with higher leptin levels in CHD patients60. Certain leptin gene polymorphisms were shown to independently predict the presence for coronary atherosclerosis61,62,63.
Leptin may affect cardiac remodelling, metabolism and contractile function64. In this context, leptin levels were shown to correlate directly with left ventricular (LV) relative wall thickness, LV end diastolic diameter and impaired LV diastolic function in patients with CHD65,66. Furthermore, in such patients, soluble leptin receptor and leptin content in epicardial adipocytes was higher by 56.9% and 28.6% than in subcutaneous adipocytes67. In another study, leptin expression was increased in the perivascular adipose tissue, leading to inflammation, fibrosis and vascularization, in patients undergoing coronary artery bypass surgery68. In this context, leptin levels were positively associated with the concentrations of myeloperoxidase and C reactive protein (inflammatory markers) as well as with raised factor VII activity in CHD patients (but not in healthy controls)69,70,71. Leptin gene expression was increased in the epicardial, paracardial and subcutaneous adipose tissue in CHD patients with metabolic syndrome (MetS)72. Leptin was also previously reported to enhance platelet activation in CHD patients73 as well as the calcification of vascular cells in vitro by exerting pro-osteogenic differentiation effects74. Furthermore, leptin may directly affect coronary endothelial cells by increasing the expression of tissue factor and cellular adhesion molecules75. Apart from atherosclerosis, leptin can also enhance insulin resistance in CHD patients76,77.
Statins can decrease leptin concentrations in CHD patients78,79. Whether this statin-induced effect is involved in the atheroprotective properties of statins should be elucidated in future studies. Apart from statins, several other drugs including hypoglycemic, antihypertensive and antiobesity agents were shown to affect leptin levels80,81. Leptin may be a target candidate for therapeutic intervention.
Overall, hyperleptinemia has been linked to the presence and severity of CHD and HF. Statins and other drugs may reduce leptin concentrations. It follows that in such patients, the selection of leptin-lowering therapies may contribute to minimizing their CV risk. However, there is a need for more evidence.
Leptin and stroke
Elevated leptin levels have been reported to predict stroke risk in both genders, even independently of traditional CV risk factors82,83,84, as supported by a meta-analysis36. However, conflicting results exist with earlier meta-analyses85,86,87 and a recent meta-analysis42 reporting the absence of any significant association between leptin and stroke risk. Furthermore, in elderly individuals (mean age=79 years; 62% women) from the Framingham Original Cohort (n=757) followed-up for 10 years, an inverse association was observed between the risk of both ischemic stroke and first-ever all-stroke with leptin concentrations in individuals with the highest waist to hip ratio88. However, in another prospective study with 3411 elderly men (aged 60–79 years), followed-up for 9 years, higher leptin levels correlated with an increased stroke risk89. Furthermore, in a substudy of the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, higher baseline leptin levels were protective of CV events (including strokes), especially in normal weight, overweight and obese patients (but not in severely obese ones)90. Ischemic stroke subtypes may affect the association between leptin and stroke91.
Gender differences exist in stroke patients with women having higher leptin levels than men92. Certain gene polymorphisms of leptin receptors have been related to an increased risk of stroke as reported in previous studies and a recent meta-analysis93,94. Furthermore, elevated leptin concentrations have been associated with worse cognitive, neurological and functional outcomes95,96.
It has been suggested that leptin may be involved in the neuroendocrine abnormalities that occur after stroke such as the regulation of cortisol axis and vascular tone97. Furthermore, leptin can stimulate endothelial dysfunction, angiogenesis, oxidative stress, platelet aggregation and atherothrombosis, thus triggering vascular stiffness as well as inflammatory and atherosclerotic responses97,98,99. Of note, leptin, apart from crossing the blood brain barrier, is also synthesized in the brain100.
In states of leptin resistance such as obesity and type 2 diabetes mellitus (T2DM)101,102, leptin action is decreased in the brain parenchyma and vessels, despite its elevated levels in the plasma and cerebrospinal fluid103. It has been hypothesized that in such cases, lowering leptin concentrations may predispose to vascular events due to the loss of protection from oxidative damage and lipotoxicity in non-adipose tissues induced by leptin103. Nevertheless, further research is required to establish the pathophysiological mechanisms of leptin metabolism in the brain.
Both animal and in vitro studies have shown that leptin administration (intraperitoneal or intracerebral) at the acute phase of stroke exerted neuroprotective properties against ischemic stroke via leptin receptors104,105,106. However, when leptin was delivered 10 days after experimental stroke, no effect on functional outcomes was observed despite the induced neurogenesis and angiogenesis107. Based on these findings, it has been suggested that using leptin in tandem with tissue plasminogen activator (tPA) might be a promising approach to improve stroke outcomes100. This combination treatment may extend the efficacy of tPA and decrease reperfusion injury. However, in one study with patients with acute ischemic stroke, increased plasma leptin levels were associated with larger infarct volume following tPA treatment108. Further human studies are needed to elucidate whether leptin co-administration with tPA may be clinically useful in acute ischemic stroke patients.
With regard to hemorrhagic strokes, elevated plasma leptin concentrations have been associated with increased severity and poor clinical outcomes, predicting early neurological deterioration, hematoma growth and functionality following intracerebral hemorrhage due to both hypertension and aneurysms109,110,111,112.
Overall, hyperleptinemia has been linked to the presence and severity of both ischemic and hemorrhagic strokes. Experimental studies have reported that leptin administration at the acute phase of stroke may improve outcomes. However, human trials are needed to establish the therapeutic role of leptin, if any, in such patients.
Leptin and carotid artery disease
In obese patients, leptin was an independent predictor of common carotid intima-media thickness (cIMT)113. A similar association has also been reported in healthy individuals of both genders114, obese children115 and patients with psoriasis116,117. Furthermore, the presence of carotid plaques correlated with hyperleptinemia in patients with systemic lupus erythematosus (SLE)118. Of note, both psoriasis and SLE have been linked to increased CV risk119,120,121,122. In contrast, data from the community Carotid Atherosclerosis Progression Study did not support any association between leptin levels and cIMT123. Leptin to adiponectin ratio has also been linked to cIMT124.
With regard to the severity of carotid disease, high leptin concentrations were related to features of plaque instability in patients scheduled for carotid endarterectomy125. It has been shown that leptin was locally overproduced in the macrophages and smooth muscle cells of the carotid plaques in symptomatic compared with asymptomatic patients, thus potentially contributing to lesion instability via paracrine or autocrine effects126. Furthermore, leptin receptor gene was overexpressed in advanced carotid atherosclerotic lesions127. However, a previous study reported lower leptin concentrations in symptomatic carotid artery disease patients compared with asymptomatic ones128. Of note, genistein (an isoflavone) was shown to attenuate neointima formation that was induced by leptin in a rat carotid artery injury model129.
Overall, hyperleptinemia was associated with increased cIMT and carotid plaque instability. However, further evidence is needed to evaluate the clinical implications of these associations.
Leptin and PAD
A link between hyperleptinemia and PAD has been reported130. In this context, higher leptin levels predicted PAD in hypertensive patients131. In another study among PAD patients, diabetic women with CHD had greater leptin concentrations than their non-diabetic counterparts132. Furthermore, gender differences were observed in African Americans PAD patients, with women having higher leptin levels than men133. Overall, more studies are required to further explore the role of leptin in PAD development.
Leptin and AAA
There are limited data on any potential link between leptin and AAA. The Health in Men study (involving 12 203 men 65 to 83 years screened with ultrasound; 875 had an AAA ≥30 mm) reported no association between serum leptin levels and AAA134. However, there is evidence that leptin is synthesized locally in the wall of AAA in humans135. Furthermore, animal studies showed that leptin accelerated the growth of both AAA and ascending aortic aneurysms135,136. Further research is needed to elucidate the relationship between leptin and aortic aneurysms.
Leptin and CKD
A link between increased plasma leptin concentrations and CKD has been reported possibly due to a reduced renal clearance137,138,139,140. In this context, leptin has been recognised as an “uremic toxin”, being involved in both the progression of renal disease (via pro-hypertensive and pro-fibrotic effects) and the development of CKD-related complications (such as chronic inflammation, protein energy wasting, cachexia, bone and CV disorders)137. Leptin levels are elevated not only in the earlier stages of CKD but also in patients on hemodialysis or peritoneal dialysis, as well as in kidney transplant recipients141,142,143,144. Interestingly, leptin concentrations gradually increased with severity of CKD, from stage 1-2 to stage 3-4 and, finally stage 5145. In chronic hemodialysis patients, elevated serum leptin levels correlated with increasing age, female gender, obesity and good nutritional status146. In hemodialysis patients, fistula maturation failure rate was higher in those patients at the highest leptin tertile, independently of gender, age, obesity and diabetes147. Furthermore, certain leptin gene polymorphisms correlated with obesity and survival in peritoneal dialysis patients148. It has been reported that leptin concentrations may decrease with time in chronic hemodialysis patients (n=101; follow-up=24 months), but this change seems not to affect body composition or nutritional status149.
In CKD patients, plasma leptin levels have been inversely associated with GFR and directly associated with urinary albumin levels as well as age and obesity markers (BMI and waist circumference)150,151. Similarly, in kidney transplant recipients, serum leptin concentrations negatively correlated with GFR and positively with BMI152. However, in these patients, elevated leptin levels were associated with a lower risk of all-cause mortality and death with a functioning graft, whereas the risk of graft loss was higher in patients with low serum leptin concentrations152. A similar association between low serum leptin levels and increased all-cause death has been reported in hemodialysis patients153,154. It was suggested that this may not be related to CV mortality153. Interestingly, adiposity may affect the relationship between leptin and mortality (both total and CV)155. In this context, leptin levels were directly associated with risk for CV and all-cause death in hemodialysis patients with an increased waist circumference (and not in those with smaller waist circumference)155.
Epicardial adiposity has been linked to increased CV risk156,157. In this context, increased epicardial fat was associated with a higher risk for CV events in CKD patients; a direct association between epicardial fat and leptin levels was also observed158. Likewise, increased visceral adiposity has been associated with elevated leptin concentrations in non-dialysis dependent CKD patients159.
Apart from obesity, hyperleptinemia has been related to metabolic disorders such as MetS and non-alcoholic fatty liver disease (NAFLD)160,161,162,163, which are associated with increased CV risk164,165,166,167,168. MetS and NAFLD may co-exist with CKD169,170. In this context, elevated leptin levels correlated with the presence of MetS in CKD patients171. Furthermore, in CKD men, hyperleptinemia was related to hypogonadism172, a disorder that has been linked to CV morbidity and mortality173,174.
In CKD patients, lifestyle modifications, including diet and exercise, led to weight loss and a decrease in leptin levels at 12 weeks175. Relative interdialytic weight gain, a predictor of long-term adverse CV outcomes, was inversely related to leptin concentrations in chronic hemodialysis patients176. Of note, in vitamin D deficient patients with end-stage renal disease (ESRD), vitamin D supplementation significantly reduced serum leptin concentrations177. However, the association between leptin and vitamin D supplementation remains unclear178.
Overall, hyperleptinemia has been linked to the presence, severity and progression of CKD. In contrast, an inverse association between leptin levels and all-cause mortality was reported in hemodialysis patients and kidney transplant recipients. However, the role of leptin in the treatment of CKD patients has not been established yet.
Leptin and T2DM
Elevated leptin levels are associated with insulin resistance and T2DM development179. In T2DM, a link between high leptin concentrations and increased CV risk, as well as the presence of microvascular complications and cardiac autonomic dysfunction, has also been reported180,181,182,183. In this context, leptin concentrations correlated with the presence and severity of silent MI as well as with carotid atherosclerosis (assessed by cIMT) in T2DM patients184,185. Furthermore, obesity, hypertension, MetS and endothelial dysfunction are more frequent in T2DM patients with increased leptin levels186,187,188. Of note, leptin was shown to decrease after an oral fat tolerance meal in both T2DM patients and healthy individuals189. Certain leptin gene polymorphisms have also been related to T2DM presence190,191,192,193. Leptin replacement therapy has been reported to improve muscle and liver insulin resistance in patients with lipodystrophy as well as to suppress liver gluconeogenesis, lipolysis and fasting hyperglycemia in animal diabetic models194.
Among dipeptidyl peptidase-4 (DPP-4) inhibitors, data exist only for sitagliptin which was shown to reduce serum leptin levels in both animal and human studies195,196,197. Metformin can also decrease leptin concentrations in T2DM patients198,199 and upregulate the expression of leptin receptors in the liver of mice200. Improvement in leptin hypothalamic sensitivity was reported in relation to metformin therapy201,202. Furthermore, in vitro studies showed that metformin reduced leptin-related reactive oxygen species production, smooth muscle cell proliferation and matrix metalloproteinase-2 expression203. Of note, metformin was reported to decrease leptin concentrations in women with polycystic ovary syndrome (PCOS) in a meta-analysis204. Pioglitazone therapy may lower leptin levels205, although conflicting results exist206.
Reductions in leptin concentrations were observed following treatment with liraglutide (a glucagon-like peptide-1 receptor agonist, GLP-1 RA) in PCOS women207. Furthermore, in vitro studies showed that liraglutide improved endothelial dysfunction and reversed leptin resistance208, whereas in animal models, liraglutide and leptin co-administration suppressed food intake and reduced weight loss209. However, data in T2DM patients are lacking. It should be noted that liraglutide has been approved for the treatment of both T2DM (at a dose up to 1.8 mg) and obesity (at the dose of 3 mg)210. Furthermore, liraglutide may lower the risk of CV morbidity and mortality211,212 as also shown in the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial213. Based on these results, liraglutide was approved for CV benefit by the US Food and Drug Administration (FDA)214. Whether leptin is implicated in these liraglutide-induced CV effects remains to be established in future trials.
With regard to the other commercially available GLP-1RAs, there are no data on leptin for both lixisenatide and dulaglutide. Limited evidence exists for exenatide; when leptin was co-administered with exenatide, body weight and food intake were decreased and hyperglycemia was improved to a greater extent than either monotherapy215. Of note, both lixisenatide and exenatide did not affect CV morbidity and mortality in the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial216 and the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) study217, respectively. Regarding dulaglutide, its CV outcome clinical trial, the Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) study, is still ongoing218.
Empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, was shown to reduce plasma leptin concentrations in an animal study219. No data exist for dapagliflozin and canagliflozin in relation to leptin. Of note, SGLT2 inhibitors have been reported to beneficially affect CV risk and renal function220,221. In this context, both empagliflozin and canagliflozin significantly decreased the composite endpoint of CV morbidity and mortality as well as hospitalization for HF in T2DM patients with established CV disease [in the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME) trial]222 and at elevated CV risk [in the Canagliflozin Cardiovascular Assessment Study (CANVAS) program]223, respectively. However, only empagliflozin was shown to significantly reduce CV and total mortality, whereas only canagliflozin therapy was associated with a significantly increased risk of amputations and bone fractures. Empagliflozin has been approved by the FDA for lowering the risk of CV death in T2DM patients with established CV disease224. Regarding dapagliflozin, its CV outcome clinical trial, the Dapagliflozin Effect on CardiovascuLAR Events (DECLARE) trial, is still ongoing225. Whether leptin is involved in any of the observed effects of these drugs remains to be elucidated in future trials.
Overall, hyperleptinemia has been linked to the presence of insulin resistance, T2DM and diabetic vascular complications. There are antidiabetic drugs that can lower leptin levels, including metformin, pioglitazone, sitagliptin, liraglutide and empagliflozin, although the clinical implications, if any, of this drug effect have not been clarified yet.
The abovementioned associations of leptin with cardiometabolic and non-cardiac vascular diseases may be, at least partly, explained by the pathophysiological mechanisms affected by leptin that predispose to these diseases, including vascular inflammation, oxidative stress, endothelial dysfunction, cardiac remodelling and insulin resistance226 (Figure 1).
Figure 1.
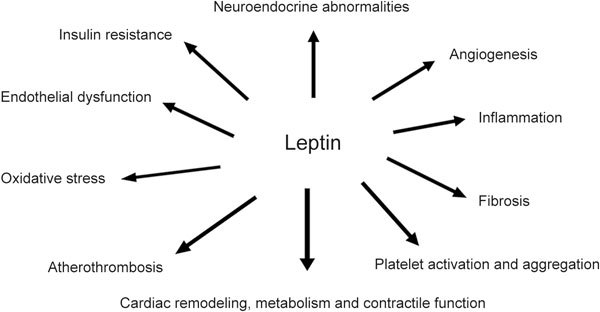
Pathophysiological mechanisms affected by leptin.
Overall, the presence, severity and extent of CHD have been associated with leptin levels. Elevated leptin concentrations were also related to the presence, severity and poor clinical outcomes of both ischemic and hemorrhagic strokes. However, conflicting results also exist. Furthermore, carotid atherosclerosis (assessed by common carotid intima-media thickness and carotid plaque instability) was linked to hyperleptinemia. Elevated leptin levels have also been related to CKD incidence and progression as well as with insulin resistance, T2DM, micro- and macrovascular diabetic complications. Limited data exist with regard to the associations of leptin with PAD and AAA. Further evidence is needed to elucidate the clinical implications of these associations.
Statins and antidiabetic drugs such as sitagliptin, metformin, pioglitazone, liraglutide and empagliflozin were shown to reduce leptin levels. Whether these drug-induced effects may affect clinical practice remains to be elucidated in the future. Table 1 summarizes the associations of leptin levels with cardiac and non-cardiac vascular diseases, CKD and T2DM.
Table 1.
Associations of leptin levels with cardiac and non-cardiac vascular diseases, chronic kidney disease and type 2 diabetes mellitus.
| Diseases | Leptin levels |
|---|---|
| CHD | Increased leptin levels have been associated with the presence and severity of CHD and HF, as well as with CV morbidity and mortality in CHD patients. |
| Stroke | Increased leptin levels have been associated with the presence and severity of both ischemic and hemorrhagic strokes. |
| Carotid artery disease | Increased leptin levels have been associated with the presence and severity of carotid artery disease. |
| PAD | Increased leptin levels have been associated with the presence of hypertension and T2DM in PAD patients. |
| AAA | Increased leptin levels have been associated with accelerated growth of AAA and ascending aortic aneurysms. |
| CKD | Increased leptin levels have been associated with the presence, severity and progression of CKD. |
| T2DM | Increased leptin levels have been associated with the development of T2DM as well as with micro- and macrovascular diabetic complications. |
CHD: coronary heart disease; PAD: peripheral artery disease; AAA: abdominal aortic aneurysm; CKD: chronic kidney disease; T2DM: type 2 diabetes mellitus; HF: heart failure; CV: cardiovascular
Conclusions
There is evidence linking leptin levels with the presence, severity and/or prognosis of CHD, stroke, PAD, carotid artery disease, CKD and T2DM. Leptin promotes inflammation, thrombosis, arteriosclerosis, angiogenesis and atherosclerosis. Lifestyle measures and several drugs, including statins and antidiabetic drugs, may affect its levels. Further research is needed to establish leptin as a potential therapeutic target.
Declaration of interest
Niki KATSIKI has given talks, attended conferences and participated in trials sponsored by Amgen, Angelini, Astra Zeneca, Boehringer Ingelheim, MSD, Novartis, NovoNordisk, Sanofi and WinMedica. Dimitri P MIKHAILIDIS has given talks and attended conferences sponsored by MSD, AstraZeneca and Libytec. Maciej BANACH declares advisory boards fees from Abbott Vascular, Amgen, Daichi Sankyo, Esperion, Lilly, MSD, Resverlogix, Sanofi-Aventis, Speakers Bureau from Abbott/Mylan, Abbott Vascular, Actavis, Akcea, Amgen, Biofarm, KRKA, MSD, Sanofi-Aventis, and Valeant and grants from Valeant, and Sanofi-Aventis.
References
- 1.Mechanick JI, Zhao S, Garvey WT. Leptin, An Adipokine With Central Importance in the Global Obesity Problem. Glob Heart. 2017 Dec 13. [Epub ahead of print] [DOI] [PubMed]
- 2.Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF. Leptin resistance in obesity: an epigenetic landscape. Life Sci. 2015;140:57–63. doi: 10.1016/j.lfs.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Bell BB, Rahmouni K. Leptin as a Mediator of Obesity-Induced Hypertension. Curr Obes Rep. 2016;5:397–404. doi: 10.1007/s13679-016-0231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stepien M, Stepien A, Wlazel RN, Paradowski M, Rizzo M, Banach M, et al. Predictors of insulin resistance in patients with obesity: a pilot study. Angiology. 2014;65:22–30. doi: 10.1177/0003319712468291. [DOI] [PubMed] [Google Scholar]
- 5.Stepien M, Stepien A, Banach M, Wlazel RN, Paradowski M, Rizzo M, et al. New obesity indices and adipokines in normotensive patients and patients with hypertension: comparative pilot analysis. Angiology. 2014;65:333–42. doi: 10.1177/0003319713485807. [DOI] [PubMed] [Google Scholar]
- 6.Stępień M, Wlazeł RN, Paradowski M, Banach M, Rysz M, Misztal M, et al. Serum concentrations of adiponectin, leptin, resistin, ghrelin and insulin and their association with obesity indices in obese normo- and hypertensive patients - pilot study. Arch Med Sci. 2012;8:431–6. doi: 10.5114/aoms.2012.29397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stepien M, Rosniak-Bak K, Paradowski M, Misztal M, Kujawski K, Banach M, et al. Waist circumference, ghrelin and selected adipose tissue-derived adipokines as predictors of insulin resistance in obese patients: preliminary results. Med Sci Monit. 2011;17:PR13–18. doi: 10.12659/MSM.882030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Pérez A, Vilariño-García T, Fernández-Riejos P, Martín-González J, Segura-Egea JJ, Sánchez-Margalet V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017;35:71–84. doi: 10.1016/j.cytogfr.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Liberale L, Bonaventura A, Vecchiè A, Matteo C, Dallegri F, Montecucco F, et al. The role of adipocytokines in coronary atherosclerosis. Curr Atheroscler Rep. 2017;19:10. doi: 10.1007/s11883-017-0644-3. [DOI] [PubMed] [Google Scholar]
- 10.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Tahergorabi Z, Khazaei M. Leptin and its cardiovascular effects: Focus on angiogenesis. Adv Biomed Res. 2015;4:79. doi: 10.4103/2277-9175.156526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Athyros VG, Katsiki N, Karagiannis A, Mikhailidis DP. Editorial: should chronic kidney disease be considered as a coronary heart disease equivalent? Curr Vasc Pharmacol. 2012;10:374–7. doi: 10.2174/157016112799959422. [DOI] [PubMed] [Google Scholar]
- 13.Athyros VG, Katsiki N, Karagiannis A, Mikhailidis DP. Stage of chronic kidney disease and severity of coronary heart disease manifestation. Expert Opin Pharmacother. 2012;13:457–60. doi: 10.1517/14656566.2012.661716. [DOI] [PubMed] [Google Scholar]
- 14.Said S, Hernandez GT. The link between chronic kidney disease and cardiovascular disease. J Nephropathol. 2014;3:99–104. doi: 10.12860/jnp.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terauchi Y, Matsui J, Kamon J, Yamauchi T, Kubota N, Komeda K, et al. Increased serum leptin protects from adiposity despite the increased glucose uptake in white adipose tissue in mice lacking p85alpha phosphoinositide 3-kinase. Diabetes. 2004;53:2261–70. doi: 10.2337/diabetes.53.9.2261. [DOI] [PubMed] [Google Scholar]
- 16.Jaworek J, Bonior J, Pierzchalski P, Tomaszewska R, Stachura J, Sendur R, et al. Leptin protects the pancreas from damage induced by caerulein overstimulation by modulating cytokine production. Pancreatology. 2002;2:89–99. doi: 10.1159/000055897. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann A, Ebert T, Klöting N, Dokas J, Jeromin F, Jessnitzer B, et al. Leptin dose-dependently decreases atherosclerosis by attenuation of hypercholesterolemia and induction of adiponectin. Biochim Biophys Acta. 2016;1862:113–20. doi: 10.1016/j.bbadis.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Kollia C, Antonopoulos AS, Siasos G, Konsola T, Oikonomou E, Gouliopoulos N, et al. Associations between adiponectin gene variability, pro-inflammatory and angiogenetic markers: implications for microvascular disease development in type 2 diabetes mellitus? Curr Vasc Pharmacol 2018 Jan 7. [Epub ahead of print] [DOI] [PubMed]
- 19.Katsiki N, Mantzoros C, Mikhailidis DP. Adiponectin, lipids and atherosclerosis. Curr Opin Lipidol. 2017;28:347–54. doi: 10.1097/MOL.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 20.Koleva DI, Orbetzova MM, Nikolova JG, Deneva TI. Pathophysiological role of adiponectin, leptin and asymmetric dimethylarginine in the process of atherosclerosis. Folia Med (Plovdiv) 2016;58:234–40. doi: 10.1515/folmed-2016-0039. [DOI] [PubMed] [Google Scholar]
- 21.Kishida K, Funahashi T, Shimomura I. Molecular mechanisms of diabetes and atherosclerosis: role of adiponectin. Endocr Metab Immune Disord Drug Targets. 2012;12:118–31. doi: 10.2174/187153012800493468. [DOI] [PubMed] [Google Scholar]
- 22.Katsiki N, Yovos JG, Gotzamani-Psarrakou A, Karamitsos DT. Adipokines and vascular risk in type 2 diabetes mellitus. Angiology. 2011;62:601–4. doi: 10.1177/0003319711409201. [DOI] [PubMed] [Google Scholar]
- 23.Gasbarrino K, Gorgui J, Nauche B, Côté R, Daskalopoulou SS. Circulating adiponectin and carotid intima-media thickness: A systematic review and meta-analysis. Metabolism. 2016;65:968–86. doi: 10.1016/j.metabol.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Mo X, Hao Y, Huang J, Lu X, Cao J, Gu D. Adiponectin levels and risk of coronary heart disease: a meta-analysis of prospective studies. Am J Med Sci. 2013;345:455–61. doi: 10.1097/MAJ.0b013e318262dbef. [DOI] [PubMed] [Google Scholar]
- 25.Gorgui J, Gasbarrino K, Georgakis MK, Karalexi MA, Nauche B, Petridou ET, et al. Circulating adiponectin levels in relation to carotid atherosclerotic plaque presence, ischemic stroke risk, and mortality: A systematic review and meta-analyses. Metabolism. 2017;69:51–66. doi: 10.1016/j.metabol.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez AJ, Nunes Vdos S, Mastronardi CA, Neeman T, Paz-Filho GJ. Association between circulating adipocytokine concentrations and microvascular complications in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of controlled cross-sectional studies. J Diabetes Complications. 2016;30:357–67. doi: 10.1016/j.jdiacomp.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Arregui M, Buijsse B, Fritsche A, di Giuseppe R, Schulze MB, Westphal S, et al. Adiponectin and risk of stroke: prospective study and meta-analysis. Stroke. 2014;45:10–7. doi: 10.1161/STROKEAHA.113.001851. [DOI] [PubMed] [Google Scholar]
- 28.Heidari M, Nasri P, Nasri H. Adiponectin and chronic kidney disease; a review on recent findings. J Nephropharmacol. 2015;4:63–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Markaki A, Psylinakis E, Spyridaki A. Adiponectin and end-stage renal disease. Hormones (Athens) 2016;15:345–54. doi: 10.14310/horm.2002.1698. [DOI] [PubMed] [Google Scholar]
- 30.Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Briffa JF, McAinch AJ, Poronnik P, Hryciw DH. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol Renal Physiol. 2013;305:F1629–36. doi: 10.1152/ajprenal.00263.2013. [DOI] [PubMed] [Google Scholar]
- 32.Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622–32. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montazerifar F, Bolouri A, Paghalea RS, Mahani MK, Karajibani M. Obesity, serum resistin and leptin levels linked to coronary artery disease. Arq Bras Cardiol. 2016;107:348–53. doi: 10.5935/abc.20160134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanker J, Rao VS, Ravindran V, Dhanalakshmi B, Hebbagodi S, Kakkar VV. Relationship of adiponectin and leptin to coronary artery disease, classical cardiovascular risk factors and atherothrombotic biomarkers in the IARS cohort. Thromb Haemost. 2012;108:769–80. doi: 10.1160/TH12-04-0263. [DOI] [PubMed] [Google Scholar]
- 35.Taneli F, Yegane S, Ulman C, Tikiz H, Bilge AR, Ari Z, et al. Increased serum leptin concentrations in patients with chronic stable angina pectoris and ST-elevated myocardial infarction. Angiology. 2006;57:267–72. doi: 10.1177/000331970605700302. [DOI] [PubMed] [Google Scholar]
- 36.Zeng R, Xu CH, Xu YN, Wang YL, Wang M. Association of leptin levels with pathogenetic risk of coronary heart disease and stroke: a meta-analysis. Arq Bras Endocrinol Metabol. 2014;58:817–23. doi: 10.1590/0004-2730000003390. [DOI] [PubMed] [Google Scholar]
- 37.Khafaji HA, Bener AB, Rizk NM, Al Suwaidi J. Elevated serum leptin levels in patients with acute myocardial infarction; correlation with coronary angiographic and echocardiographic findings. BMC Res Notes. 2012;5:262. doi: 10.1186/1756-0500-5-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azar RR, Sarkis A, Salameh E, Gannagé-Yared MH, Amm-Azar M, Badaoui G, et al. Percutaneous coronary intervention increases leptin and decreases adiponectin levels. Clin Endocrinol (Oxf). 2006;65:712–6. doi: 10.1111/j.1365-2265.2006.02654.x. [DOI] [PubMed] [Google Scholar]
- 39.Kosydar-Piechna M, Bilińska M, Janas J, Piotrowicz R. Influence of exercise training on leptin levels in patients with stable coronary artery disease: A pilot study. Cardiol J. 2010;17:477–81. [PubMed] [Google Scholar]
- 40.Poulakou MV, Paraskevas KI, Wilson MR, Iliopoulos DC, Tsigris C, Mikhailidis DP, Perrea D. Apolipoprotein J and leptin levels in patients with coronary heart disease. In Vivo. 2008;22:537–42. [PubMed] [Google Scholar]
- 41.Hoefle G, Saely CH, Risch L, Rein P, Koch L, Schmid F, et al. Leptin, leptin soluble receptor and coronary atherosclerosis. Eur J Clin Invest. 2007;37:629–36. doi: 10.1111/j.1365-2362.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- 42.Yang H, Guo W, Li J, Cao S, Zhang J, Pan J, et al. Leptin concentration and risk of coronary heart disease and stroke: A systematic review and meta-analysis. PLoS One. 2017;12:e0166360. doi: 10.1371/journal.pone.0166360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puurunen VP, Kiviniemi A, Lepojärvi S, Piira OP, Hedberg P, Junttila J, et al. Leptin predicts short-term major adverse cardiac events in patients with coronary artery disease. Ann Med. 2017;49:448–54. doi: 10.1080/07853890.2017.1301678. [DOI] [PubMed] [Google Scholar]
- 44.Bickel C, Schnabel RB, Zeller T, Lackner KJ, Rupprecht HJ, Blankenberg S, et al. Predictors of leptin concentration and association with cardiovascular risk in patients with coronary artery disease: results from the AtheroGene study. Biomarkers. 2017;22:210–8. doi: 10.3109/1354750X.2015.1130745. [DOI] [PubMed] [Google Scholar]
- 45.Simiti LA, Todor I, Stoia MA, Goidescu CM, Anton FP, Farcas AD. Better prognosis in overweight/obese coronary heart disease patients with high plasma levels of leptin. Clujul Med. 2016;89:65–71. doi: 10.15386/cjmed-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morita Y, Maeda K, Kondo T, Ishii H, Matsudaira K, Okumura N, et al; Nagoya Acute Myocardial Infarction Study (NAMIS) Group. Impact of adiponectin and leptin on long-term adverse events in Japanese patients with acute myocardial infarction. Results from the Nagoya Acute Myocardial Infarction Study (NAMIS). Circ J 2013; 77: 2778–85. [DOI] [PubMed]
- 47.Ku IA, Farzaneh-Far R, Vittinghoff E, Zhang MH, Na B, Whooley MA. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: the Heart and Soul Study. Atherosclerosis. 2011;217:503–8. doi: 10.1016/j.atherosclerosis.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amasyali B, Aytemir K, Kose S, Kilic A, Abali G, Iyisoy A, et al. Admission plasma leptin level strongly correlates with the success of thrombolytic therapy in patients with acute myocardial infarction. Angiology. 2006;57:671–80. doi: 10.1177/0003319706295204. [DOI] [PubMed] [Google Scholar]
- 49.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Papacosta O, Sattar N. The obesity paradox in men with coronary heart disease and heart failure: the role of muscle mass and leptin. Int J Cardiol. 2014;171:49–55. doi: 10.1016/j.ijcard.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abd El-Aziz TA, Mohamed RH, Mohamed RH, Pasha HF. Leptin, leptin gene and leptin receptor gene polymorphism in heart failure with preserved ejection fraction. Heart Vessels. 2012;27:271–9. doi: 10.1007/s00380-011-0152-2. [DOI] [PubMed] [Google Scholar]
- 51.Straburzyńska-Migaj E, Gwizdała A, Siniawski A, Ochotny R, Grajek S. Leptin and inflammation in patients with chronic heart failure. Kardiol Pol. 2010;68:1243–7. [PubMed] [Google Scholar]
- 52.Faxén UL, Hage C, Andreasson A, Donal E, Daubert JC, Linde C, et al. HFpEF and HFrEF exhibit different phenotypes as assessed by leptin and adiponectin. Int J Cardiol. 2017;228:709–16. doi: 10.1016/j.ijcard.2016.11.194. [DOI] [PubMed] [Google Scholar]
- 53.Cundrle I, Somers VK, Singh P, Johnson BD, Scott CG, Olson LJ. Sex differences in leptin modulate ventilation in heart failure. Heart Lung. 2017;46:187–91. doi: 10.1016/j.hrtlng.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Bobbert P, Jenke A, Bobbert T, Kühl U, Rauch U, Lassner D, et al. High leptin and resistin expression in chronic heart failure: adverse outcome in patients with dilated and inflammatory cardiomyopathy. Eur J Heart Fail. 2012;14:1265–75. doi: 10.1093/eurjhf/hfs111. [DOI] [PubMed] [Google Scholar]
- 55.Karayannis G, Giamouzis G, Tziolas N, Georgoulias P, Skoularigis J, Mikhailidis DP, et al. Association between epicardial fat thickness and weight homeostasis hormones in patients with noncachectic heart failure. Angiology. 2013;64:173–80. doi: 10.1177/0003319712447978. [DOI] [PubMed] [Google Scholar]
- 56.Barbosa-Ferreira JM, Fernandes F, Dabarian A, Mady C. Leptin in heart failure. Expert Opin Med Diagn. 2013;7:113–7. doi: 10.1517/17530059.2013.735229. [DOI] [PubMed] [Google Scholar]
- 57.Gumanova NG, Gavrilova NE, Chernushevich OI, Kots AY, Metelskaya VA. Ratios of leptin to insulin and adiponectin to endothelin are sex-dependently associated with extent of coronary atherosclerosis. Biomarkers. 2017;22:239–45. doi: 10.1080/1354750X.2016.1201539. [DOI] [PubMed] [Google Scholar]
- 58.Tsai JP, Wang JH, Chen ML, Yang CF, Chen YC, Hsu BG. Association of serum leptin levels with central arterial stiffness in coronary artery disease patients. BMC Cardiovasc Disord. 2016;16:80. doi: 10.1186/s12872-016-0268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basati G, Razavi AE, Abdi S, Sarrafzedegan N. Association of plasma leptin, homocysteine and nitric oxide levels with the presence and unstability of coronary artery disease. Biomark Med. 2014;8:405–12. doi: 10.2217/bmm.13.131. [DOI] [PubMed] [Google Scholar]
- 60.Hasan-Ali H, Abd El-Mottaleb NA, Hamed HB, Abd-Elsayed A. Serum adiponectin and leptin as predictors of the presence and degree of coronary atherosclerosis. Coron Artery Dis. 2011;22:264–9. doi: 10.1097/MCA.0b013e3283452431. [DOI] [PubMed] [Google Scholar]
- 61.An BQ, Lu LL, Yuan C, Xin YN, Xuan SY. Leptin receptor gene polymorphisms and the risk of non-alcoholic fatty liver disease and coronary atherosclerosis in the Chinese Han population. Hepat Mon. 2016;16:e35055. doi: 10.5812/hepatmon.35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li XL, Sui JQ, Lu LL, Zhang NN, Xu X, Dong QY, et al. Gene polymorphisms associated with non-alcoholic fatty liver disease and coronary artery disease: a concise review. Lipids Health Dis. 2016;15:53. doi: 10.1186/s12944-016-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin SG, Chen GL, Yang SL, Zhao MY. Gene-gene interactions among CX3CL1, LEPR and IL-6 related to coronary artery disease in Chinese Han population. Int J Clin Exp Pathol. 2015;8:5968–73. [PMC free article] [PubMed] [Google Scholar]
- 64.Feijóo-Bandín S, Portolés M, Roselló-Lletí E, Rivera M, González-Juanatey JR, Lago F. 20 years of leptin: Role of leptin in cardiomyocyte physiology and physiopathology. Life Sci. 2015;140:10–8. doi: 10.1016/j.lfs.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 65.Puurunen VP, Lepojärvi ES, Piira OP, Hedberg P, Junttila MJ, Ukkola O, et al. High plasma leptin levels are associated with impaired diastolic function in patients with coronary artery disease. Peptides. 2016;84:17–21. doi: 10.1016/j.peptides.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Farcaş AD, Rusu A, Stoia MA, Vida-Simiti LA. Plasma leptin, but not resistin, TNF-α and adiponectin, is associated with echocardiographic parameters of cardiac remodeling in patients with coronary artery disease. Cytokine. 2018;103:46–9. doi: 10.1016/j.cyto.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Gruzdeva OV, Akbasheva OE, Dyleva YA, Antonova LV, Matveeva VG, Uchasova EG, et al. Adipokine and cytokine profiles of epicardial and subcutaneous adipose tissue in patients with coronary heart disease. Bull Exp Biol Med. 2017;163:608–11. doi: 10.1007/s10517-017-3860-5. [DOI] [PubMed] [Google Scholar]
- 68.Drosos I, Chalikias G, Pavlaki M, Kareli D, Epitropou G, Bougioukas G, et al. Differences between perivascular adipose tissue surrounding the heart and the internal mammary artery: possible role for the leptin-inflammation-fibrosis-hypoxia axis. Clin Res Cardiol. 2016;105:887–900. doi: 10.1007/s00392-016-0996-7. [DOI] [PubMed] [Google Scholar]
- 69.Basati G, Emami Razavi A, Abdi S, Sarrafzadegan N. Association between adipokine and myeloperoxidase levels in patients with coronary artery disease. Acta Med Iran. 2015;53:25–9. [PubMed] [Google Scholar]
- 70.Lodh M, Goswami B, Parida A, Patra S, Saxena A. Assessment of serum leptin, pregnancy-associated plasma protein A and CRP levels as indicators of plaque vulnerability in patients with acute coronary syndrome. Cardiovasc J Afr. 2012;23:330–5. doi: 10.5830/CVJA-2012-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karatela RA, Sainani GS. Interrelationships of factor VII activity and plasma leptin with insulin resistance in coronary heart disease. Atherosclerosis. 2010;209:235–40. doi: 10.1016/j.atherosclerosis.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 72.Gormez S, Demirkan A, Atalar F, Caynak B, Erdim R, Sozer V, et al. Adipose tissue gene expression of adiponectin, tumor necrosis factor-α and leptin in metabolic syndrome patients with coronary artery disease. Intern Med. 2011;50:805–10. doi: 10.2169/internalmedicine.50.4753. [DOI] [PubMed] [Google Scholar]
- 73.Bigalke B, Stellos K, Geisler T, Seizer P, Mozes V, Gawaz M. High plasma levels of adipocytokines are associated with platelet activation in patients with coronary artery disease. Platelets. 2010;21:11–9. doi: 10.3109/09537100903377584. [DOI] [PubMed] [Google Scholar]
- 74.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–60. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 75.Cirillo P, Angri V, De Rosa S, Calì G, Petrillo G, Maresca F, et al. Pro-atherothrombotic effects of leptin in human coronary endothelial cells. Thromb Haemost. 2010;103:1065–75. doi: 10.1160/TH09-06-0392. [DOI] [PubMed] [Google Scholar]
- 76.Syed Ikmal SI, Zaman Huri H, Vethakkan SR, Wan Ahmad WA. Potential biomarkers of insulin resistance and atherosclerosis in type 2 diabetes mellitus patients with coronary artery disease. Int J Endocrinol. 2013;2013:698567. doi: 10.1155/2013/698567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sainani GS, Karatela RA. Plasma leptin in insulin-resistant and insulin-nonresistant coronary artery disease and its association with cardio-metabolic risk factors among Asian Indians. Metab Syndr Relat Disord. 2009;7:335–40. doi: 10.1089/met.2008.0097. [DOI] [PubMed] [Google Scholar]
- 78.Takahashi Y, Satoh M, Tabuchi T, Nakamura M. Prospective, randomized, single-blind comparison of effects of 6 months' treatment with atorvastatin versus pravastatin on leptin and angiogenic factors in patients with coronary artery disease. Heart Vessels. 2012;27:337–43. doi: 10.1007/s00380-011-0156-y. [DOI] [PubMed] [Google Scholar]
- 79.Sun YM, Li J, Luan Y, Wang LF. Effect of statin therapy on leptin levels in patients with coronary heart disease. Peptides. 2010;31:1205–7. doi: 10.1016/j.peptides.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 80.Katsiki N, Mikhailidis DP, Gotzamani-Psarrakou A, Yovos JG, Karamitsos D. Effect of various treatments on leptin, adiponectin, ghrelin and neuropeptide Y in patients with type 2 diabetes mellitus. Expert Opin Ther Targets. 2011;15:401–20. doi: 10.1517/14728222.2011.553609. [DOI] [PubMed] [Google Scholar]
- 81.Katsiki N, Mikhailidis DP, Gotzamani-Psarrakou A, Didangelos TP, Yovos JG, Karamitsos DT. Effects of improving glycemic control with insulin on leptin, adiponectin, ghrelin and neuropeptide Y levels in patients with type 2 diabetes mellitus: a pilot study. Open Cardiovasc Med J. 2011;5:136–47. doi: 10.2174/1874192401105010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, Butler KR, Buxbaum SG, Sung JH, Campbell BW, Taylor HA. Leptinemia and and its association with stroke and coronary heart disease in the Jackson Heart Study. Clin Endocrinol (Oxf) 2010;72:32–7. doi: 10.1111/j.1365-2265.2009.03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Söderberg S, Stegmayr B, Stenlund H, Sjöström LG, Agren A, Johansson L, et al. Leptin, but not adiponectin, predicts stroke in males. J Intern Med. 2004;256:128–36. doi: 10.1111/j.1365-2796.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 84.Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, Gami AS, Sert Kuniyoshi FH, Wolk R, et al. Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am J Cardiol. 2007;100:234–9. doi: 10.1016/j.amjcard.2007.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bidulescu A, Liu J, Chen Z, Hickson DA, Musani SK, Samdarshi TE, et al. Associations of adiponectin and leptin with incident coronary heart disease and ischemic stroke in African Americans: the Jackson heart study. Front Public Health. 2013;1:16. doi: 10.3389/fpubh.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prugger C, Luc G, Haas B, Arveiler D, Machez E, Ferrieres J, et al; PRIME Study Group. Adipocytokines and the risk of ischemic stroke: the PRIME Study. Ann Neurol 2012; 71: 478–86. [DOI] [PubMed]
- 87.Rajpathak SN, Kaplan RC, Wassertheil-Smoller S, Cushman M, Rohan TE, McGinn AP, et al. Resistin, but not adiponectin and leptin, is associated with the risk of ischemic stroke among postmenopausal women: results from the Women's Health Initiative. Stroke. 2011;42:1813–20. doi: 10.1161/STROKEAHA.110.607853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saber H, Himali JJ, Shoamanesh A, Beiser A, Pikula A, Harris TB, et al. Serum leptin levels and the risk of stroke: the Framingham Study. Stroke. 2015;46:2881–5. doi: 10.1161/STROKEAHA.115.009463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Adiposity, adipokines, and risk of incident stroke in older men. Stroke. 2013;44:3–8. doi: 10.1161/STROKEAHA.112.670331. [DOI] [PubMed] [Google Scholar]
- 90.Wolk R, Bertolet M, Singh P, Brooks MM, Pratley RE, Frye RL, et al; BARI 2D Study Group. Prognostic value of adipokines in predicting cardiovascular outcome: explaining the obesity paradox. Mayo Clin Proc 2016; 91: 858–66. [DOI] [PMC free article] [PubMed]
- 91.Kim BJ, Lee SH, Ryu WS, Kim CK, Yoon BW. Adipocytokines and ischemic stroke: differential associations between stroke subtypes. J Neurol Sci. 2012;312:117–22. doi: 10.1016/j.jns.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 92.Kantorova E, Chomova M, Kurca E, Sivak S, Zelenak K, Kučera P, et al. Leptin, adiponectin and ghrelin, new potential mediators of ischemic stroke. Neuro Endocrinol Lett. 2011;32:716–21. [PubMed] [Google Scholar]
- 93.Wu L, Sun D. Leptin receptor gene polymorphism and the risk of cardiovascular disease: a systemic review and Meta-analysis. Int J Environ Res Public Health 2017; 14. pii: E375. doi: 10.3390/ijerph14040375. [DOI] [PMC free article] [PubMed]
- 94.Tang H, Zhang Z, Li ZK, Lin J, Fang DZ. Association of leptin receptor gene polymorphisms with genetic susceptibility to ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24:2128–33. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 95.Lee JY, Lim OK, Lee JK, Park Y, Kim C, Yoon JW, et al. The association between serum leptin levels and post-stroke depression: a retrospective clinical study. Ann Rehabil Med. 2015;39:786–92. doi: 10.5535/arm.2015.39.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carbone F, Burger F, Roversi G, Tamborino C, Casetta I, Seraceni S, et al. Leptin/adiponectin ratio predicts poststroke neurological outcome. Eur J Clin Invest. 2015;45:1184–91. doi: 10.1111/eci.12538. [DOI] [PubMed] [Google Scholar]
- 97.Opatrilova R, Caprnda M, Kubatka P, Valentova V, Uramova S, Nosal V, et al. Adipokines in neurovascular diseases. Biomed Pharmacother. 2017;98:424–32. doi: 10.1016/j.biopha.2017.12.074. [DOI] [PubMed] [Google Scholar]
- 98.Gairolla J, Kler R, Modi M, Khurana D. Leptin and adiponectin: pathophysiological role and possible therapeutic target of inflammation in ischemic stroke. Rev Neurosci. 2017;28:295–306. doi: 10.1515/revneuro-2016-0055. [DOI] [PubMed] [Google Scholar]
- 99.Windham BG, Griswold ME, Farasat SM, Ling SM, Carlson O, Egan JM, et al. Influence of leptin, adiponectin, and resistin on the association between abdominal adiposity and arterial stiffness. Am J Hypertens. 2010;23:501–7. doi: 10.1038/ajh.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Signore AP, Zhang F, Weng Z, Gao Y, Chen J. Leptin neuroprotection in the CNS: mechanisms and therapeutic potentials. J Neurochem. 2008;106:1977–90. doi: 10.1111/j.1471-4159.2008.05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Engin A. Diet-induced obesity and the mechanism of leptin resistance. Adv Exp Med Biol. 2017;960:381–97. doi: 10.1007/978-3-319-48382-5_16. [DOI] [PubMed] [Google Scholar]
- 102.Van Doorn C, Macht VA, Grillo CA, Reagan LP. Leptin resistance and hippocampal behavioral deficits. Physiol Behav. 2017;176:207–13. doi: 10.1016/j.physbeh.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Letra L, Sena C. Cerebrovascular disease: consequences of obesity-induced endothelial dysfunction. Adv Neurobiol. 2017;19:163–89. doi: 10.1007/978-3-319-63260-5_7. [DOI] [PubMed] [Google Scholar]
- 104.Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–36. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- 105.Avraham Y, Davidi N, Lassri V, Vorobiev L, Kabesa M, Dayan M, et al. Leptin induces neuroprotection neurogenesis and angiogenesis after stroke. Curr Neurovasc Res. 2011;8:313–22. doi: 10.2174/156720211798120954. [DOI] [PubMed] [Google Scholar]
- 106.Valerio A, Dossena M, Bertolotti P, Boroni F, Sarnico I, Faraco G, et al. Leptin is induced in the ischemic cerebral cortex and exerts neuroprotection through NF-kappaB/c-Rel-dependent transcription. Stroke. 2009;40:610–7. doi: 10.1161/STROKEAHA.108.528588. [DOI] [PubMed] [Google Scholar]
- 107.Avraham Y, Dayan M, Lassri V, Vorobiev L, Davidi N, Chernoguz D, et al. Delayed leptin administration after stroke induces neurogenesis and angiogenesis. J Neurosci Res. 2013;91:187–95. doi: 10.1002/jnr.23147. [DOI] [PubMed] [Google Scholar]
- 108.Calleja AI, Cortijo E, García-Bermejo P, Reyes J, Bermejo JF, Muñoz MF, et al. Blood biomarkers of insulin resistance in acute stroke patients treated with intravenous thrombolysis: temporal profile and prognostic value. J Diab Res Clin Metab. 2013;2:2. [Google Scholar]
- 109.Du Q, Yang DB, Shen YF, Yu WH, Zhang ZY, Zhu Q, et al. Plasma leptin level predicts hematoma growth and early neurological deterioration after acute intracerebral hemorrhage. Peptides. 2013;45:35–9. doi: 10.1016/j.peptides.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 110.Zhao QJ, Sun M, Zhang XG, Wang LX. Relationship between serum leptin levels and clinical outcomes of hypertensive intracerebral hemorrhage. Clin Exp Hypertens. 2012;34:161–4. doi: 10.3109/10641963.2011.561902. [DOI] [PubMed] [Google Scholar]
- 111.Fan XF, Chen ZH, Huang Q, Dai WM, Jie YQ, Yu GF, et al. Leptin as a marker for severity and prognosis of aneurysmal subarachnoid hemorrhage. Peptides. 2013;48:70–4. doi: 10.1016/j.peptides.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 112.Zhang X, Lu XM, Huang LF, Li X. Prognostic value of leptin: 6-month outcome in patients with intracerebral hemorrhage. Peptides. 2013;43:133–6. doi: 10.1016/j.peptides.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 113.Csongrádi É, Káplár M, Nagy B, Koch CA, Juhász A, Bajnok L, et al. Adipokines as atherothrombotic risk factors in obese subjects: associations with haemostatic markers and common carotid wall thickness. Nutr Metab Cardiovasc Dis. 2017;27:571–80. doi: 10.1016/j.numecd.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 114.Ciccone M, Vettor R, Pannacciulli N, Minenna A, Bellacicco M, Rizzon P, et al. Plasma leptin is independently associated with the intima-media thickness of the common carotid artery. Int J Obes Relat Metab Disord. 2001;25:805–10. doi: 10.1038/sj.ijo.0801623. [DOI] [PubMed] [Google Scholar]
- 115.Stroescu R, Bizerea T, Doroş G, Marazan M, Lesovici M, Mãrginean O. Correlation between adipokines and carotid intima media thickness in a group of obese Romanian children: is small for gestational age status an independent factor for cardiovascular risk? Arch Endocrinol Metab. 2017;61:14–20. doi: 10.1590/2359-3997000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Robati RM, Partovi-Kia M, Haghighatkhah HR, Younespour S, Abdollahimajd F. Increased serum leptin and resistin levels and increased carotid intima-media wall thickness in patients with psoriasis: is psoriasis associated with atherosclerosis? J Am Acad Dermatol. 2014;71:642–8. doi: 10.1016/j.jaad.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 117.Asha K, Sharma SB, Singal A, Aggarwal A. Association of carotid intima-media thickness with leptin and apoliprotein B/apoliprotein A-I ratio reveals imminent predictors of subclinical atherosclerosis in psoriasis patients. Acta Medica (Hradec Kralove) 2014;57:21–7. doi: 10.14712/18059694.2014.4. [DOI] [PubMed] [Google Scholar]
- 118.McMahon M, Skaggs BJ, Grossman JM, Sahakian L, Fitzgerald J, Wong WK, et al. A panel of biomarkers is associated with increased risk of the presence and progression of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:130–9. doi: 10.1002/art.38204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dattilo G, Borgia F, Guarneri C, Casale M, Bitto R, Morabito C, et al. Cardiovascular risk in psoriasis: current state of the art. Curr Vasc Pharmacol 2017 Nov 16. [Epub ahead of print] [DOI] [PubMed]
- 120.Doumas M, Katsiki N, Papademetriou V. Psoriasis and cardiovascular disease: two sides of the same coin? Angiology. 2018;69:5–9. doi: 10.1177/0003319717702303. [DOI] [PubMed] [Google Scholar]
- 121.Croca S, Rahman A. Atherosclerosis in systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2017;31:364–72. doi: 10.1016/j.berh.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 122.Lewandowski LB, Kaplan MJ. Update on cardiovascular disease in lupus. Curr Opin Rheumatol. 2016;28:468–76. doi: 10.1097/BOR.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bevan S, Meidtner K, Lorenz M, Sitzer M, Grant PJ, Markus HS. Adiponectin level as a consequence of genetic variation, but not leptin level or leptin: adiponectin ratio, is a risk factor for carotid intima-media thickness. Stroke. 2011;42:1510–4. doi: 10.1161/STROKEAHA.110.602375. [DOI] [PubMed] [Google Scholar]
- 124.Norata GD, Raselli S, Grigore L, Garlaschelli K, Dozio E, Magni P, et al. Leptin:adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke. 2007;38:2844–6. doi: 10.1161/STROKEAHA.107.485540. [DOI] [PubMed] [Google Scholar]
- 125.Gasbarrino K, Mantzoros C, Gorgui J, Veinot JP, Lai C, Daskalopoulou SS. Circulating chemerin is associated with carotid plaque instability, whereas resistin is related to cerebrovascular symptomatology. Arterioscler Thromb Vasc Biol. 2016;36:1670–8. doi: 10.1161/ATVBAHA.115.306741. [DOI] [PubMed] [Google Scholar]
- 126.Schneiderman J, Schaefer K, Kolodgie FD, Savion N, Kotev-Emeth S, Dardik R, et al. Leptin locally synthesized in carotid atherosclerotic plaques could be associated with lesion instability and cerebral emboli. J Am Heart Assoc. 2012;1:e001727. doi: 10.1161/JAHA.112.001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schneiderman J, Simon AJ, Schroeter MR, Flugelman MY, Konstantinides S, Schaefer K. Leptin receptor is elevated in carotid plaques from neurologically symptomatic patients and positively correlated with augmented macrophage density. J Vasc Surg. 2008;48:1146–55. doi: 10.1016/j.jvs.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 128.Bountouris I, Paraskevas KI, Koutouzis M, Tzavara V, Nikolaou N, Nomikos A, et al. Serum leptin levels in patients undergoing carotid endarterectomy: a pilot study. Angiology. 2009;60:698–704. doi: 10.1177/0003319709350133. [DOI] [PubMed] [Google Scholar]
- 129.Tsai YC, Leu SY, Peng YJ, Lee YM, Hsu CH, Chou SC, et al. Genistein suppresses leptin-induced proliferation and migration of vascular smooth muscle cells and neointima formation. J Cell Mol Med. 2017;21:422–31. doi: 10.1111/jcmm.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gherman CD, Mironiuc AI. Evaluation of serum adipokines in peripheral arterial occlusive disease. Mediators Inflamm. 2012;2012:257808. doi: 10.1155/2012/257808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang IC, Chang CC, Hsu BG, Lee CJ, Wang JH. Association of hyperleptinemia with peripheral arterial disease in hypertensive patients. Ci Ji Yi Xue Za Zhi. 2017;29:148–53. doi: 10.4103/tcmj.tcmj_56_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gardner AW, Parker DE, Montgomery PS, Sosnowska D, Casanegra AI, Ungvari Z, et al. Influence of diabetes on ambulation and inflammation in men and women with symptomatic peripheral artery disease. J Clin Transl Endocrinol. 2015;2:137–43. doi: 10.1016/j.jcte.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gardner AW, Parker DE, Montgomery PS, Sosnowska D, Casanegra AI, Ungvari Z, et al. Gender and racial differences in endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. J Vasc Surg. 2015;61:1249–57. doi: 10.1016/j.jvs.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Golledge J, Clancy P, Jamrozik K, Norman PE. Obesity, adipokines, and abdominal aortic aneurysm: health in men study. Circulation. 2007;116:2275–9. doi: 10.1161/CIRCULATIONAHA.107.717926. [DOI] [PubMed] [Google Scholar]
- 135.Tao M, Yu P, Nguyen BT, Mizrahi B, Savion N, Kolodgie FD, et al. Locally applied leptin induces regional aortic wall degeneration preceding aneurysm formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2013;33:311–20. doi: 10.1161/ATVBAHA.112.300543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ben-Zvi D, Savion N, Kolodgie F, Simon A, Fisch S, Schäfer K, et al. Local application of leptin antagonist attenuates angiotensin ii-induced ascending aortic aneurysm and cardiac remodeling. J Am Heart Assoc 2016; 5. [DOI] [PMC free article] [PubMed]
- 137.Alix PM, Guebre-Egziabher F, Soulage CO. Leptin as an uremic toxin: deleterious role of leptin in chronic kidney disease. Biochimie. 2014;105:12–21. doi: 10.1016/j.biochi.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 138.Zhang J, Wang N. Leptin in chronic kidney disease: a link between hematopoiesis, bone metabolism, and nutrition. Int Urol Nephrol. 2014;46:1169–74. doi: 10.1007/s11255-013-0623-8. [DOI] [PubMed] [Google Scholar]
- 139.Korolczuk A, Dudka J. Increased risk of cardiovascular complications in chronic kidney disease: a possible role of leptin. Curr Pharm Des. 2014;20:666–74. doi: 10.2174/13816128113199990013. [DOI] [PubMed] [Google Scholar]
- 140.Stępień M, Stępień A, Wlazeł RN, Paradowski M, Banach M, Rysz M, et al. Obesity indices and adipokines in non-diabetic obese patients with early stages of chronic kidney disease. Med Sci Monit. 2013;19:1063–72. doi: 10.12659/MSM.889390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ocak N, Dirican M, Ersoy A, Sarandol E. Adiponectin, leptin, nitric oxide, and C-reactive protein levels in kidney transplant recipients: comparison with the hemodialysis and chronic renal failure. Ren Fail. 2016;38:1639–46. doi: 10.1080/0886022X.2016.1229965. [DOI] [PubMed] [Google Scholar]
- 142.Tsai JP, Lee MC, Chen YC, Ho GJ, Shih MH, Hsu BG. Hyperleptinemia is a risk factor for the development of central arterial stiffness in kidney transplant patients. Transplant Proc. 2015;47:1825–30. doi: 10.1016/j.transproceed.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 143.Jiang S, Song K, Feng S, Shi YB. Association between serum leptin levels and peritoneal dialysis: A meta-analysis. Exp Ther Med. 2015;10:300–8. doi: 10.3892/etm.2015.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Malyszko J, Wolczynski S, Mysliwiec M. Adiponectin, leptin and thyroid hormones in patients with chronic renal failure and on renal replacement therapy: are they related? Nephrol Dial Transplant. 2006;21:145–52. doi: 10.1093/ndt/gfi081. [DOI] [PubMed] [Google Scholar]
- 145.Ambarkar M, Pemmaraju SV, Gouroju S, Manohar SM, Bitla AR, Yajamanam N, et al. Adipokines and their relation to endothelial dysfunction in patients with chronic kidney disease. J Clin Diagn Res. 2016;10:BC04–8. doi: 10.7860/JCDR/2016/15867.7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kara E, Ahbap E, Sahutoglu T, Sakaci T, Basturk T, Koc Y, et al. Elevated serum leptin levels are associated with good nutritional status in non-obese chronic hemodialysis patients. Clin Nephrol. 2015;83:147–53. doi: 10.5414/CN108409. [DOI] [PubMed] [Google Scholar]
- 147.Kim JK, Choi SR, Lee WY, Park MJ, Lee HS, Song YR, et al. Leptin, pre-existing vascular disease, and increased arteriovenous fistula maturation failure in dialysis patients. J Vasc Surg. 2016;64:402–10. doi: 10.1016/j.jvs.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 148.Cao L, Mou S, Fang W, Qi C, Chang X, Gu L, et al. Correlational studies on insulin resistance and leptin gene polymorphisms in peritoneal dialysis patients. Iran J Basic Med Sci. 2015;18:878–86. [PMC free article] [PubMed] [Google Scholar]
- 149.Beberashvili I, Sinuani I, Azar A, Yasur H, Feldman L, Averbukh Z, et al. Longitudinal study of leptin levels in chronic hemodialysis patients. Nutr J. 2011;10:68. doi: 10.1186/1475-2891-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.de Oliveira RB, Liabeuf S, Okazaki H, Lenglet A, Desjardins L, Lemke HD, et al; European Uremic Toxin Work Group (EUTox). The clinical impact of plasma leptin levels in a cohort of chronic kidney disease patients. Clin Kidney J 2013; 6: 63–70. [DOI] [PMC free article] [PubMed]
- 151.Mills KT, Hamm LL, Alper AB, Miller C, Hudaihed A, Balamuthusamy S, et al. Circulating adipocytokines and chronic kidney disease. PLoS One. 2013;8:e76902. doi: 10.1371/journal.pone.0076902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Molnar MZ, Nagy K, Remport A, Gaipov A, Fülöp T, Czira ME, et al. Association between serum leptin level and mortality in kidney transplant recipients. J Ren Nutr. 2017;27:53–61. doi: 10.1053/j.jrn.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 153.Bian X, Liu N, Bai Y, Zheng L, He P, Su X, et al. Association of leptin with mortality in patients on maintenance hemodialysis: a prospective study. Iran J Kidney Dis. 2014;8:314–20. [PubMed] [Google Scholar]
- 154.Scholze A, Rattensperger D, Zidek W, Tepel M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity (Silver Spring) 2007;15:1617–22. doi: 10.1038/oby.2007.191. [DOI] [PubMed] [Google Scholar]
- 155.Zoccali C, Postorino M, Marino C, Pizzini P, Cutrupi S, Tripepi G; CREDIT Working Group. Waist circumference modifies the relationship between the adipose tissue cytokines leptin and adiponectin and all-cause and cardiovascular mortality in haemodialysis patients. J Intern Med 2011; 269: 172–81. [DOI] [PubMed]
- 156.Katsiki N, Athyros VG, Mikhailidis DP. Abnormal peri-organ or intra-organ fat (APIFat) deposition: an underestimated predictor of vascular risk? Curr Vasc Pharmacol. 2016;14:432–41. doi: 10.2174/1570161114666160722112738. [DOI] [PubMed] [Google Scholar]
- 157.Katsiki N, Mikhailidis DP, Wierzbicki AS. Epicardial fat and vascular risk: a narrative review. Curr Opin Cardiol. 2013;28:458–63. doi: 10.1097/HCO.0b013e3283605fba. [DOI] [PubMed] [Google Scholar]
- 158.Cordeiro AC, Amparo FC, Oliveira MA, Amodeo C, Smanio P, Pinto IM, et al. Epicardial fat accumulation, cardiometabolic profile and cardiovascular events in patients with stages 3-5 chronic kidney disease. J Intern Med. 2015;278:77–87. doi: 10.1111/joim.12344. [DOI] [PubMed] [Google Scholar]
- 159.Cordeiro AC, Qureshi AR, Lindholm B, Amparo FC, Tito-Paladino-Filho A, Perini M, et al. Visceral fat and coronary artery calcification in patients with chronic kidney disease. Nephrol Dial Transplant. 2013;28 Suppl 4:iv152–9. doi: 10.1093/ndt/gft250. [DOI] [PubMed] [Google Scholar]
- 160.Paspala I, Katsiki N, Kapoukranidou D, Mikhailidis DP, Tsiligiroglou-Fachantidou A. The role of psychobiological and neuroendocrine mechanisms in appetite regulation and obesity. Open Cardiovasc Med J. 2012;6:147–55. doi: 10.2174/1874192401206010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Patel SB, Reams GP, Spear RM, Freeman RH, Villarreal D. Leptin: linking obesity, the metabolic syndrome, and cardiovascular disease. Curr Hypertens Rep. 2008;10:131–7. doi: 10.1007/s11906-008-0025-y. [DOI] [PubMed] [Google Scholar]
- 162.Polyzos SA, Aronis KN, Kountouras J, Raptis DD, Vasiloglou MF, Mantzoros CS. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia. 2016;59:30–43. doi: 10.1007/s00125-015-3769-3. [DOI] [PubMed] [Google Scholar]
- 163.Canale MP, Manca di Villahermosa S, Martino G, Rovella V, Noce A, De Lorenzo A, et al. Obesity-related metabolic syndrome: mechanisms of sympathetic overactivity. Int J Endocrinol. 2013;2013:865965. doi: 10.1155/2013/865965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Athyros VG, Tziomalos K, Katsiki N, Doumas M, Karagiannis A, Mikhailidis DP. Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: An update. World J Gastroenterol. 2015;21:6820–34. doi: 10.3748/wjg.v21.i22.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Katsiki N, Athyros VG, Karagiannis A, Wierzbicki AS, Mikhailidis DP. Should we expand the concept of coronary heart disease equivalents? Curr Opin Cardiol. 2014;29:389–95. doi: 10.1097/HCO.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 166.Katsiki N, Imprialos K, Vlachopoulos C. Editorial: Arterial Stiffness, Central haemodynamics and non-alcoholic fatty liver disease: links with cardiovascular risk and effects of drug treatment. Curr Vasc Pharmacol. 2018;16:401–4. doi: 10.2174/1570161116666171205105402. [DOI] [PubMed] [Google Scholar]
- 167.Katsiki N, Perez-Martinez P, Anagnostis P, Mikhailidis DP, Karagiannis A. Is nonalcoholic fatty liver disease indeed the hepatic manifestation of metabolic syndrome? Curr Vasc Pharmacol. 2018;16:219–27. doi: 10.2174/1570161115666170621075619. [DOI] [PubMed] [Google Scholar]
- 168.Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism. 2016;65:1109–23. doi: 10.1016/j.metabol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 169.Machado MV, Gonçalves S, Carepa F, Coutinho J, Costa A, Cortez-Pinto H. Impaired renal function in morbid obese patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:241–8. doi: 10.1111/j.1478-3231.2011.02623.x. [DOI] [PubMed] [Google Scholar]
- 170.Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP. Metabolic syndrome and non-cardiac vascular diseases: an update from human studies. Curr Pharm Des. 2014;20:4944–52. doi: 10.2174/1381612819666131206100750. [DOI] [PubMed] [Google Scholar]
- 171.Tsai JP, Tsai CC, Liu HM, Lee CJ, Liou HH, Hsu BG. Hyperleptinaemia positively correlated with metabolic syndrome in hemodialysis patients. Eur J Intern Med. 2011;22:e105–9. doi: 10.1016/j.ejim.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 172.Cobo G, Cordeiro AC, Amparo FC, Amodeo C, Lindholm B, Carrero JJ. Visceral adipose tissue and leptin hyperproduction are associated with hypogonadism in men with chronic kidney disease. J Ren Nutr. 2017;27:243–8. doi: 10.1053/j.jrn.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 173.Kloner RA, Carson C, Dobs A, Kopecky S, Mohler ER. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545–57. doi: 10.1016/j.jacc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 174.Corona G, Rastrelli G, Monami M, Guay A, Buvat J, Sforza A, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 175.Navaneethan SD, Fealy CE, Scelsi AC, Arrigain S, Malin SK, Kirwan JP. A trial of lifestyle modification on cardiopulmonary, inflammatory, and metabolic effects among obese with chronic kidney disease. Am J Nephrol. 2015;42:274–81. doi: 10.1159/000441155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ahbap E, Sakaci T, Kara E, Sahutoglu T, Koc Y, Basturk T, et al. Relationship between relative interdialytic weight gain and serum leptin levels, nutrition, and inflammation in chronic hemodialysis patients. Clin Nephrol. 2015;83:154–60. doi: 10.5414/cn108450. [DOI] [PubMed] [Google Scholar]
- 177.Naini AE, Vahdat S, Hedaiati ZP, Shahzeidi S, Pezeshki AH, Nasri H. The effect of vitamin D administration on serum leptin and adiponectin levels in end-stage renal disease patients on hemodialysis with vitamin D deficiency: A placebo-controlled double-blind clinical trial. J Res Med Sci. 2016;21:1. doi: 10.4103/1735-1995.175144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dinca M, Serban MC, Sahebkar A, Mikhailidis DP, Toth PP, Martin SS, et al; for Lipid Blood Pressure Meta-analysis Collaboration LBPMC Group. Does vitamin D supplementation alter plasma adipokines concentrations? A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 2016; 107: 360–71.
- 179.Andrade-Oliveira V, Câmara NO, Moraes-Vieira PM. Adipokines as drug targets in diabetes and underlying disturbances. J Diabetes Res. 2015;2015:681612. doi: 10.1155/2015/681612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kurajoh M, Koyama H, Kadoya M, Naka M, Miyoshi A, Kanzaki A, et al. Plasma leptin level is associated with cardiac autonomic dysfunction in patients with type 2 diabetes: HSCAA study. Cardiovasc Diabetol. 2015;14:117. doi: 10.1186/s12933-015-0280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vavruch C, Länne T, Fredrikson M, Lindström T, Östgren CJ, Nystrom FH. Serum leptin levels are independently related to the incidence of ischemic heart disease in a prospective study of patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:62. doi: 10.1186/s12933-015-0208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rodríguez AJ, Nunes Vdos S, Mastronardi CA, Neeman T, Paz-Filho GJ. Association between circulating adipocytokine concentrations and microvascular complications in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of controlled cross-sectional studies. J Diabetes Complications. 2016;30:357–67. doi: 10.1016/j.jdiacomp.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 183.Katsiki N, Yovos JG, Gotzamani-Psarrakou A, Karamitsos DT. Adipokines and vascular risk in type 2 diabetes mellitus. Angiology. 2011;62:601–4. doi: 10.1177/0003319711409201. [DOI] [PubMed] [Google Scholar]
- 184.Guzel S, Seven A, Kocaoglu A, Ilk B, Guzel EC, Saracoglu GV, et al. Osteoprotegerin, leptin and IL-6: association with silent myocardial ischemia in type 2 diabetes mellitus. Diab Vasc Dis Res. 2013;10:25–31. doi: 10.1177/1479164112440815. [DOI] [PubMed] [Google Scholar]
- 185.Yamazaki Y, Emoto M, Morioka T, Kawano N, Lee E, Urata H, et al. Clinical impact of the leptin to soluble leptin receptor ratio on subclinical carotid atherosclerosis in patients with type 2 diabetes. J Atheroscler Thromb. 2013;20:186–94. doi: 10.5551/jat.14662. [DOI] [PubMed] [Google Scholar]
- 186.Liu X, Li X, Li C, Gong C, Liu S, Shi Y. Study on regulation of adipokines on body fat distribution and its correlation with metabolic syndrome in type 2 diabetes mellitus. Minerva Endocrinol 2017 Dec 4. [DOI] [PubMed]
- 187.Asakawa H, Tokunaga K, Kawakami F. Relationship of leptin level with metabolic disorders and hypertension in Japanese type 2 diabetes mellitus patients. J Diabetes Complications. 2001;15:57–62. doi: 10.1016/s1056-8727(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 188.Morioka T, Emoto M, Yamazaki Y, Kawano N, Imamura S, Numaguchi R, et al. Leptin is associated with vascular endothelial function in overweight patients with type 2 diabetes. Cardiovasc Diabetol. 2014;13:10. doi: 10.1186/1475-2840-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Iraklianou S, Melidonis A, Tournis S, Konstandelou E, Tsatsoulis A, Elissaf M, et al. Postprandial leptin responses after an oral fat tolerance test: differences in type 2 diabetes. Diabetes Care. 2001;24:1299–301. doi: 10.2337/diacare.24.7.1299-a. [DOI] [PubMed] [Google Scholar]
- 190.Zhang L, Qin Y, Liang D, Li L, Liang Y, Chen L, et al. Association of polymorphisms in LEPR with type 2 diabetes and related metabolic traits in a Chinese population. Lipids Health Dis. 2018;17:2. doi: 10.1186/s12944-017-0644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Motawi T, Salman T, Shaker O, Abdelhamid A. Association of polymorphism in adiponectin (+45 T/G) and leptin (-2548 G/A) genes with type 2 diabetes mellitus in male Egyptians. Arch Med Sci. 2015;11:937–44. doi: 10.5114/aoms.2015.54848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Su S, Zhang C, Zhang F, Li H, Yang X, Tang X. The association between leptin receptor gene polymorphisms and type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;121:49–58. doi: 10.1016/j.diabres.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 193.Yang MM, Wang J, Fan JJ, Ng TK, Sun DJ, Guo X, et al. Variations in the obesity gene “LEPR” contribute to risk of type 2 diabetes mellitus: evidence from a meta-analysis. J Diabetes Res. 2016;2016:5412084. doi: 10.1155/2016/5412084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Perry RJ, Petersen KF, Shulman GI. Pleotropic effects of leptin to reverse insulin resistance and diabetic ketoacidosis. Diabetologia. 2016;59:933–7. doi: 10.1007/s00125-016-3909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saad MI, Kamel MA, Hanafi MY. Modulation of adipocytokines production and serum nefa level by metformin, glimepiride, and sitagliptin in HFD/STZ diabetic rats. Biochem Res Int. 2015;2015:138134. doi: 10.1155/2015/138134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wu QM, Ni HX, Lu X. Changes of adipocytokine expression after diabetic rats received sitagliptin and the molecular mechanism. Asian Pac J Trop Med. 2016;9:893–7. doi: 10.1016/j.apjtm.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 197.Li S, Li H, Wang R, Zhang JP. The effect of sitagliptin on obese patients with insulin treatment-induced diabetes mellitus. Eur Rev Med Pharmacol Sci. 2017;21:3490–5. [PubMed] [Google Scholar]
- 198.Farooq R, Amin S, Hayat Bhat M, Malik R, Wani HA, Majid S. Type 2 diabetes and metabolic syndrome - adipokine levels and effect of drugs. Gynecol Endocrinol. 2017;33:75–8. doi: 10.1080/09513590.2016.1207165. [DOI] [PubMed] [Google Scholar]
- 199.Nar A, Gedik O. The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Acta Diabetol. 2009;46:113–8. doi: 10.1007/s00592-008-0067-2. [DOI] [PubMed] [Google Scholar]
- 200.Tang X, Li J, Xiang W, Cui Y, Xie B, Wang X, et al. Metformin increases hepatic leptin receptor and decreases steatosis in mice. J Endocrinol. 2016;230:227–37. doi: 10.1530/JOE-16-0142. [DOI] [PubMed] [Google Scholar]
- 201.Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323–9. doi: 10.1097/MED.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 202.Kim YW, Kim JY, Park YH, Park SY, Won KC, Choi KH, et al. Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes. 2006;55:716–24. doi: 10.2337/diabetes.55.03.06.db05-0917. [DOI] [PubMed] [Google Scholar]
- 203.Li L, Mamputu JC, Wiernsperger N, Renier G. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes. 2005;54:2227–34. doi: 10.2337/diabetes.54.7.2227. [DOI] [PubMed] [Google Scholar]
- 204.Kong W, Niu X, Zeng T, Lu M, Chen L. Impact of treatment with metformin on adipocytokines in patients with polycystic ovary syndrome: a Meta-Analysis. PLoS One. 2015;10:e0140565. doi: 10.1371/journal.pone.0140565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Negrotto L, Farez MF, Correale J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. 2016;73:520–8. doi: 10.1001/jamaneurol.2015.4807. [DOI] [PubMed] [Google Scholar]
- 206.Miyazaki Y, DeFronzo RA. Rosiglitazone and pioglitazone similarly improve insulin sensitivity and secretion, glucose tolerance and adipocytokines in type 2 diabetic patients. Diabetes Obes Metab. 2008;10:1204–11. doi: 10.1111/j.1463-1326.2008.00880.x. [DOI] [PubMed] [Google Scholar]
- 207.Frøssing S, Nylander M, Chabanova E, Frystyk J, Holst JJ, Kistorp C, et al. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: A randomized clinical trial. Diabetes Obes Metab. 2018;20:215–8. doi: 10.1111/dom.13053. [DOI] [PubMed] [Google Scholar]
- 208.Li N, Zhao Y, Yue Y, Chen L, Yao Z, Niu W. Liraglutide ameliorates palmitate-induced endothelial dysfunction through activating AMPK and reversing leptin resistance. Biochem Biophys Res Commun. 2016;478:46–52. doi: 10.1016/j.bbrc.2016.07.095. [DOI] [PubMed] [Google Scholar]
- 209.Kanoski SE, Ong ZY, Fortin SM, Schlessinger ES, Grill HJ. Liraglutide, leptin and their combined effects on feeding: additive intake reduction through common intracellular signalling mechanisms. Diabetes Obes Metab. 2015;17:285–93. doi: 10.1111/dom.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Katsiki N, Christou GA, Kiortsis DN. Liraglutide and cardiometabolic effects: more than just another antiobesity drug? Curr Vasc Pharmacol. 2016;14:76–9. doi: 10.2174/157016111401151126161741. [DOI] [PubMed] [Google Scholar]
- 211.Athyros VG, Katsiki N, Tentolouris N. Editorial: do some glucagon-like-peptide-1 receptor agonists (GLP-1 RA) reduce macrovascular complications of type 2 diabetes mellitus a commentary on the liraglutide effect and action in diabetes: evaluation of Cardiovascular Outcome Results (LEADER) Trial. Curr Vasc Pharmacol. 2016;14:469–73. doi: 10.2174/1570161114666160909161537. [DOI] [PubMed] [Google Scholar]
- 212.Katsiki N, Purrello F, Tsioufis C, Mikhailidis DP. Cardiovascular disease prevention strategies for type 2 diabetes mellitus. Expert Opin Pharmacother. 2017;18:1243–60. doi: 10.1080/14656566.2017.1351946. [DOI] [PubMed] [Google Scholar]
- 213.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al; LEADER steering committee; leader trial investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–22. [DOI] [PMC free article] [PubMed]
- 214.American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 2018; 41: S73–S85. [DOI] [PubMed]
- 215.Sakai T, Kusakabe T, Ebihara K, Aotani D, Yamamoto-Kataoka S, Zhao M, et al. Leptin restores the insulinotropic effect of exenatide in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and high-fat diet. Am J Physiol Endocrinol Metab 2014; 307: E712–9. [DOI] [PubMed]
- 216.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373: 2247–57. [DOI] [PubMed]
- 217.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–39. [DOI] [PMC free article] [PubMed]
- 218.https://clinicaltrials.gov/ct2/show/NCT01394952 Last accessed 15 February 2018
- 219.Vickers SP, Cheetham SC, Headland KR, Dickinson K, Grempler R, Mayoux E, et al. Combination of the sodium-glucose cotransporter-2 inhibitor empagliflozin with orlistat or sibutramine further improves the body-weight reduction and glucose homeostasis of obese rats fed a cafeteria diet. Diabetes Metab Syndr Obes. 2014;7:265–75. doi: 10.2147/DMSO.S58786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Katsiki N, Mikhailidis DP, Theodorakis MJ. Sodium-glucose cotransporter 2 inhibitors (SGLT2i): their role in cardiometabolic risk management. Curr Pharm Des. 2017;23:1522–32. doi: 10.2174/1381612823666170113152742. [DOI] [PubMed] [Google Scholar]
- 221.Katsiki N, Athyros VG, Mikhailidis DP. Cardiovascular effects of sodium-glucose cotransporter 2 inhibitors: multiple actions. Curr Med Res Opin. 2016;32:1513–4. doi: 10.1080/03007995.2016.1201465. [DOI] [PubMed] [Google Scholar]
- 222.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–28. [DOI] [PubMed]
- 223.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–57. [DOI] [PubMed]
- 224.https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm Last accessed 15 February 2018
- 225.https://clinicaltrials.gov/ct2/show/NCT01730534 Last accessed 15 February 2018
- 226.Hou N, Luo JD. Leptin and cardiovascular diseases. Clin Exp Pharmacol Physiol. 2011;38:905–13. doi: 10.1111/j.1440-1681.2011.05619.x. [DOI] [PubMed] [Google Scholar]


