Abstract
Zinc is an essential nutrient for human health and has anti-oxidative stress and anti-inflammatory functions. The association between zinc deficiency and the development of cardiovascular diseases (CVDs) has been supported by numerous studies. Supplementing zinc can reduce the risk of atherosclerosis and protect against myocardial infarction and ischemia/reperfusion injury. In this review we summarize the evidence in the literature, to consolidate the current knowledge on the dysregulation of zinc homeostasis in CVDs, and to explore the significant roles of the zinc homeostasis-regulatory proteins in cardiac physiology and pathophysiology. Moreover, this review also deliberates on the potential diagnostic and prognostic implications of zinc/zinc homeostasis-associated molecules (ZIP, ZnT, and MTs) in CVDs.
Keywords: zinc homeostasis, zinc transporter (ZnT), Zrt, Irt-like protein (ZIP), metallothionein (MT), cardiovascular diseases, atherosclerosis, inflammation, ROS
Introduction
The micronutrient zinc is essential to all living organisms and participates in numerous biochemical pathways in human cells. The human body mass contains 2–3 g of zinc, and approximately 57% and 29% of total body zinc exist in skeletal muscle and bone, respectively; heart and blood plasma are known to contain 0.4% and 0.1% of body zinc1,2. Inadequate intake, decreased absorption, or increased loss of zinc all can result in zinc deficiency. In experiment animals, severe zinc deficient diet led to 30% loss of body zinc3. In human body, severe zinc deficiency has serious impact, such as growth retardation, anemia, and neuronal dysfunctions; marginal zinc deficiency is associated with a broad spectrum of pathological conditions, especially aged-related ones including cardiovascular diseases (CVDs)4. Based on the estimated prevalence of zinc deficiency, the population with suboptimal zinc status ranges from 4% to 73% in different countries, and zinc deficiency may affect up to two billion people worldwide5.
Zinc carries out its multiple biological functions in several aspects. First, it is required by more than 300 enzymes for their catalytic activation, thus participating in various enzymatic and metabolic cellular processes in human body. Secondly, zinc binds to over 2500 proteins, equivalent to 10% of total human proteome, and maintains the structural integrity for many of them. For example, nitric oxide synthase (NOS), a family of metalloenzymes involved in blood pressure regulation and cardiovascular and renal functions, uses zinc for its structural stabilization6. This category also includes transcription factors, the DNA-binding proteins with zinc finger domains, copper/zinc superoxide dismutase, and various proteins involved in DNA repair. Thirdly, zinc as a metal ion may also directly regulate kinases, phosphatases, or channel activities4,7. Collectively, zinc has a critical role in maintaining human health, especially in terms of anti-oxidative stress and anti-inflammation8.
It remains to be a challenge to clearly determine zinc deficiency, especially marginal zinc deficiency. First of all, the common deficient signs of zinc nutrient are “multiple nonspecific general shifts in metabolism”, including chronic oxidative stress and inflammation9. Secondly, zinc exists in various forms, and the changes in its concentration are dynamic. The plasma zinc turns over rapidly to supply different tissues and is replenished daily from diet. The intracellular zinc exists in three forms: tightly bound to proteins, loosely associated with protein or ligands, and unbound labile ions. Thirdly, the currently used indicators for zinc status are far from reliable. The widely accepted methods to evaluate zinc status and identify the risk of zinc deficiency are measuring plasma zinc concentration and monitoring dietary zinc intake, which neglect individual variability, poor sensitivity, and non-specificity9. There have been some attempts to adopt zinc status assessment with several combined indicators. A recent study identified the correlation of higher erythrocyte zinc concentration and urinary zinc excretion in population with metabolic syndrome, suggesting the importance of using several zinc indicators as a more reliable biomarker to better understand the relationship between the zinc nutrient and pathophysiological conditions10.
CVD is a blanket term for a number of linked pathologies, including coronary artery and peripheral artery diseases and cerebrovascular diseases. It is a leading cause of death globally, accounting for 31% of human mortality. Many of these diseases are related to the process of atherosclerosis. It causes myocardial infarction (MI), stroke, and ischemia/reperfusion (I/R) injury. Other types of CVDs contain atrial fibrillation (AF), chronic heart failure (CHF), and diabetic cardiovascular complications. Dyslipidemia, smoking, hypertension, and diabetes mellitus are well-known risk factors for these CVDs11. Many of these risk factors for CVDs are known to cause oxidative stress and induce inflammation.
Given the importance of zinc in anti-oxidative stress and anti-inflammation, it is not surprising to see that accumulating evidence has emerged, suggesting that zinc deficiency is associated with the development of CVDs, especially atherosclerosis, and zinc supplementation can protect against MI and I/R injury. It would be of importance to establish the association between zinc status and various CVDs. The aim of this review is to summarize the evidence in the literature, to consolidate the current knowledge on such association, and to explore the significant roles of the zinc homeostasis-regulatory proteins in cardiac physiology and CVDs. Moreover, this review also deliberates about the potential prognostic implications of zinc/zinc homeostasis-associated molecules in CVDs.
Antioxidant effects of zinc and redox signaling
Oxidative stress is a key risk factor contributing to the development and progression of CVDs. Oxidative stress represents the status of imbalance between the production of free radicals and the ability of cells to detoxify, thus increasing oxidative damage to DNA, proteins, and lipids. Existing as a divalent cation in biological systems, although not redox active under physiological conditions, zinc is involved in multiple events regulating cell oxidant/antioxidant balance as an antioxidant or a signaling molecule12.
Many studies have shown an inverse-relationship between zinc availability and cellular oxidative stress. For example, zinc deficiency increases the production of reactive oxygen species (ROS) in various cells such as mouse 3T3 cells, rat C6 glioma cells, human fibroblasts, neuronal and epithelial cells13. In dietary zinc deficient animals, vascular smooth muscle cells (VSMCs) were also demonstrated to contain increased oxidative stress measured by fluorescent indicator 2′,7′-dichlorofluorescin diacetate (DCFDA)14. Moreover, a randomized controlled trial of zinc supplementation in elderly human subjects showed that this population had higher oxidative stress and lower plasma zinc levels than young adults; and the plasma lipid peroxidation markers were significantly decreased by zinc supplementation compared to the placebo group15.
Since zinc is not redox active, the antioxidant function of zinc is through some indirect mechanisms of action (the mechanisms are reviewed elsewhere13,16,17,18). In mammalian cells, the main redox active metals, including copper and iron, catalyze the production of ROS and reactive nitrogen species (RNS), which are capable of oxidizing cellular components, such as lipid bilayer19. Zinc competes with these redox active metals for negative charges in lipid bilayer; and as such, it protects the cell membrane from lipid oxidation20. Zinc is also able to interact with thiol or sulfhydryl groups in proteins and peptides, to reduce the reactivity of sulfhydryl groups. This stabilization prevents intramolecular disulfide formation, and thus zinc functions as an antioxidant to protect these molecules from oxidation18. In addition, zinc can accelerate the synthesis of metallothioneins (MTs), a family of cysteine-rich protein, via activating the zinc-sensing Metal regulatory Transcription Factor 1 (MTF-1)21. Since MTs are abundant in the cytosol, they provide more thiol groups and act as direct oxidant scavengers. Zinc also protects cells from oxidative stress by increasing glutathione (GSH) biosynthesis which is responsible for the maintenance of cellular redox state22.
Zinc additionally serves as a cofactor in antioxidant enzymes such as superoxide dismutase 1 (SOD1) that scavenges superoxide anions23. Although zinc is tightly incorporated in SOD1 and unlikely spontaneously released from the protein just because of changes in zinc status, there has been evidence to show that zinc supplementation affected SOD1 protein stability and protected mice bearing unstable SOD mutant gene24. Upon zinc depletion, SOD1 mutants underwent conformational changes due to zinc dissociation, which resulted in oxidative stress and endoplasmic reticulum (ER) stress25. The excess oxidative stress caused by dysfunction of SOD1 or SOD3 can also react with nitric oxide (NO) to form RNS, which in turn oxidize endothelial nitric oxide synthase (eNOS) and uncouple functional eNOS dimers. Accumulating evidence has demonstrated that the eNOS dimer uncoupling increases pathologic ROS production and decreases NO synthesis by the endothelium26. Alternatively, superoxide could directly quench NO and thus reduce its bioavailability without affecting eNOS expression or activity in rat hearts27. Since NO is a key molecule controlling vasodilation, zinc deficiency-caused stress and reduction of NO are considered to play an important role in the pathogenesis of atherosclerosis and diabetic complications28, which will be discussed in detail in the following sections.
In another aspect of function, zinc participates in the redox signaling network by interacting with proteins. Oxidative stress can trigger zinc release from the binding sites, leading to conformational changes of these proteins. For example, Korichneva et al demonstrated that zinc was released from protein kinase C (PKC) during activation by ROS and was thus involved in the myocardial redox signaling network12,29.
Anti-inflammatory function of zinc
Inflammation is another major risk factor contributing to the pathological process of CVDs. Zinc has been well known to be essential for the normal function of the immune system in both innate and adaptive immunity responding to pathogen or tissue damage. In general, zinc deficiency results in the impairment of antibody-mediated and cell-mediated immune response; however, it also causes increased auto-reactivity likely due to damaged immune tolerance and/or inefficient removal of autoreactive T-cells. In addition, zinc deficiency contributes to chronic inflammatory conditions, where no inducer appears to be required. These conditions are common and of particular interest since they coincide with and are likely to contribute to CVDs, type 2 diabetes (T2DM), and other chronic diseases30.
Two transcription factors serve as key modulators in the inflammatory response pathways: nuclear factor kappa-B (NF-κB) and hypoxia-inducible factor-1 alpha (HIF-1α). Zinc status regulates both30,31,32. NF-κB mediates the central inflammatory signaling cascade33. In unstimulated cells, NF-κB heterodimer (p65/p50) is sequestered in the cytosol through the interactions with a class of inhibitory proteins called IκB (IκBα, -β, and -ε). Many stimuli induce NF-κB activation, such as tumor necrosis factor-α (TNF-α), interleukin-1(IL-1), activators of PKC, ionizing radiation, and oxidants. These signals cause phosphorylation and subsequent degradation of IκB proteins via the ubiquitination-proteasome pathway, and thereby, free NF-κB can enter the nucleus and induce the expression of targeted genes. NF-κB-regulated genes contain many inflammatory cytokines, such as IL-1, IL-6, TNF-α, lymphotoxin, and interferon-γ (IFN-γ). It is notable that IL-1 and TNF-α work to activate NF-κB, therefore creating a feedback loop. Dietary zinc deficiency or intracellular zinc deprivation has been shown to result in increased activation of NF-κB and NF-κB-regulated inflammatory cytokine expression in cultured cells, animal models, and humans30,34. Supplementation of zinc could suppress NF-κB activation and NF-κB-regulated inflammatory cytokine release likely through inhibiting the phosphorylation and degradation of IκB.
As a master regulator in hypoxia and ischemia, HIF-1 not only can rapidly respond to hypoxia, but also is activated by several non-hypoxic stimuli, such as inflammatory mediators or cytokines35. Since HIF-1 generally supports cell survival and the production of pro-inflammatory cytokines, it in turn further promotes angiogenesis, vascular remodeling, glucose metabolism, and redox homeostasis. Zinc has been shown to block hypoxia-stimulated HIF-1α nuclear translocation and subsequently disrupting HIF-1 heterodimerization as well as to induce HIF-1α proteasomal degradation32. On the other hand, zinc preconditioning can induce expression of HIF genes including HIF-1α in renal cells36. Therefore, zinc status may have different effects on HIF-1α in different cells at different inflammatory stages.
In brief, zinc deficiency affects the generation of inflammatory cytokines, including IL-1, IL-6, TNF-α, and IFN-γ37. These zinc status-involved inflammatory responses often commingle with their antioxidant effects. For instance, zinc deficiency induced-oxidative stress results in the reduction of NO in epithelium; and NO, as an endogenous signaling molecule, has anti-inflammatory effects under normal physiological condition; and zinc is further involved in immune responses by cytokine-activated macrophages, which release NO in high concentrations.
Zinc transporters and MTs in vascular and heart cells
Intracellular zinc homeostasis is tightly regulated by two known classes of zinc transporters and MTs. Zrt, Irt-like protein (ZIP), encoded by solute carrier family 39 gene (SLC39A), functions in the uptake of zinc into the cytoplasm of cell from either the extracellular space or from intracellular organelles, such as endoplasmic reticulum, Golgi apparatus, and mitochondria38. Zinc transporter (ZnT), encoded by solute carrier family 30 gene (SLC30A), moves zinc from the cytoplasm to the outside of the cell or into the lumen of intracellular organelles39. There are 14 ZIPs and 10 ZnTs identified in human with differential tissue-specific expression.
As a family of highly conserved, low-molecular-weight, thiol-rich protein, MT binds zinc with high affinity and gathers about 5%–15% of the cytosolic zinc pool40. MT is genetically polymorphous with subfamilies and 12 isoforms. Each molecule of MT can incorporate up to 7 zinc ions or other divalent metals. In addition to its metal storage function, MTs can also work as zinc acceptors and donors to exchange the metal ion with other proteins in the cells via its reversible dissociation of zinc ions and oxidoreduction of the cysteine sulfur donors in zinc/thiolate clusters. Since the sulfur donors coordinating zinc in MTs are redox reactive, oxidation of the sulfur donors leads to the release of zinc, which contributes to overall intracellular zinc signaling. In addition, the interaction of MT with NO also controls the release of zinc and the availability of zinc to other apoenzymes involved in NO signaling.
Although the association study between altered zinc homeostasis and CVDs is emerging, the exact member(s) of the zinc transporters and isoform(s) of MTs expressed in normal vascular and heart cells are not very clear, let alone their biological roles in governing the overall zinc homeostasis under pathophysiological conditions41. Owing to the recent effort on Human Protein Atlas projects using a quantitative transcriptomics analysis (RNA-Seq) and antibody-based proteomics approach, we were able to extract data from these publicly available databases and compare the relative expression levels of the 24 zinc transporters and 12 MTs in heart tissues and vascular endothelial cells42,43. As shown in Figure 1A, SLC39A1, SLC39A7, SLC39A13, SLC39A14, and SLC30A9, which encode ZIP1, ZIP7, ZIP13, ZIP14, and ZnT9, respectively, are markedly abundant in human heart muscle tissues. Interestingly, these genes were also found to be abundant in rat ventricular myocytes although with slight variations in the zinc transporter profiles of rat and human myocardium44. In two human immortalized endothelial cell lines, ZIP1, ZIP13, and ZnT5 are the dominant zinc transporters expressed (Figure 1B and 2). Different from heart and endothelial cells, smooth muscle cells contain abundant ZIP6 in addition to ZIP1 and ZIP13 as zinc influx transporters, while ZnT1, ZnT5, and ZnT9 are abundant as zinc efflux transporters (Figure 1C)45. It has been reported that ZIP1 and ZIP14 localize to plasma membrane in rat cardiomyocytes, whereas ZIP7 and ZIP13 in the ER, Golgi, or cytoplasmic vesicles. The differences in their localization imply the characteristic roles of these zinc transporters in transferring zinc from either extracellular environment or lumen of intracellular compartments to the cell cytosol. ZIP14 is known to mobilize diverse metals including zinc, iron, and manganese. The precise function and metal specificity of ZIP14 in myocyte, however, has not been explored. ZnT9 is the most highly expressed in both human and rat hearts, suggesting its common role in the export of zinc out of myocytes.
Figure 1.
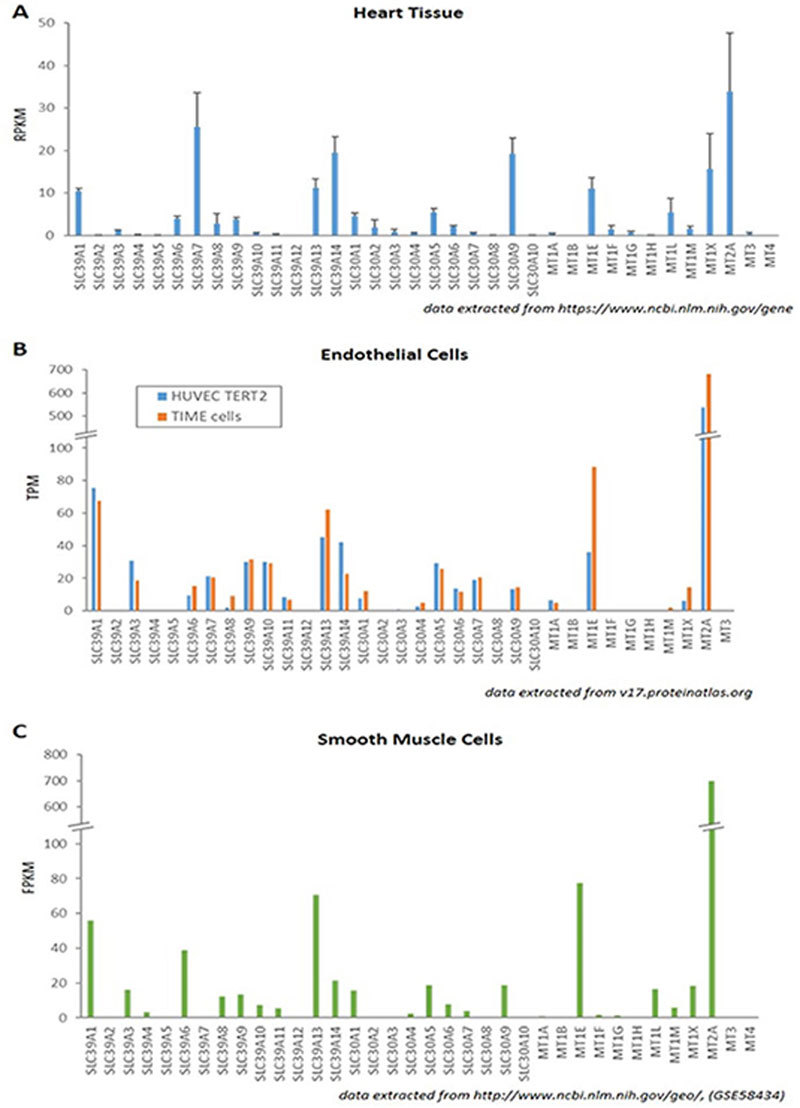
Expression levels of zinc transporters and MTs in heart and vascular cells. Data are extracted from publically available RNA-seq databases. (A) ZIPs, ZnTs, and MTs expression in human heart muscle tissue. Each gene's reads per kilobase per million reads placed (RPKM) was downloaded and examined for normal heart tissues. Error bars represent a standard deviation from RPKM values in four tissue samples. (B) ZIPs, ZnTs, and MTs expression in human endothelial cell lines. Transcript Per Million (TPM) of each gene was searched in RNA-seq data in Cell atlas section in Human Protein Atlas. Two bars in each gene represent HUVEC TERT2 (blue) and TIME cells (orange), respectively. (C) ZIPs, ZnTs, and MTs expression in human bronchial smooth muscle cells. Fragments per kilobase of transcript per million mapped reads (FPKM) value for each gene was obtained from the “Control Baseline” group in dataset GSE58434 at the Gene Expression Omnibus.
Figure 2.
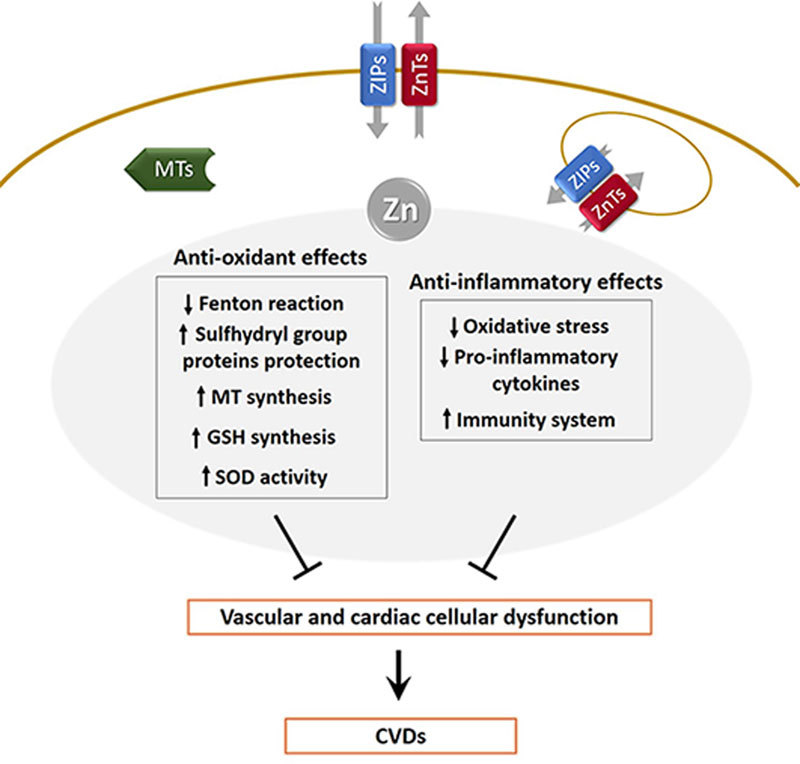
Schematic summary of key regulatory roles of zinc in CVDs. ZIPs and ZnTs are simply depicted to be localized in the cell plasma membrane and intracellular organelle membrane. MTs represents metallothionein proteins.
In regard to MTs, their basal levels are usually very low, although they may vary depending upon the type of tissue or in response to aging or diseases. MT2A is the most abundant one in heart, smooth muscle, and endothelial cells, whereas MT1E and MT1X are with less abundance but still significantly expressed, suggesting their important functions in cardiovascular physiology. Accordingly, the polymorphisms of these genes are likely associated with various CVDs, which will be discussed in a later section separately.
Zinc and atherosclerosis
Atherosclerosis is the major cause of CVDs. It is characterized by plaque formation within the vessel wall of arteries, extensive apoptosis/necrosis, and fibrosis of surrounding tissues. Once the vulnerable plaque is raptured, the thrombotic vessels, especially small diameter vessels such as coronary arteries, can be occluded. Consequent heart attacks or strokes often result in sudden death or chronic heart failure/brain damage. The etiology of atherosclerosis has been extensively studied, and accumulating evidence demonstrates complex atherogenic process regulated by many factors from the metabolic, vascular, and immune systems. One of key characteristic traits of atherosclerosis is the increased oxidative stress, which results in endothelial damage/dysfunction, disturbed NO and NF-κB-related signaling, and the oxidative modification of low-density lipoprotein (LDL). As discussed earlier, zinc is involved in all these aspects mainly through its antioxidant and anti-inflammatory functions46. It is thus not a surprise to see that zinc regulates atherosclerotic process and zinc deficiency may be a strong risk factor for atherosclerosis.
Animal and in vitro evidence
Zinc deficiency has been shown to increase endothelial cell apoptosis and amplify the detrimental effects of oxidized LDL in atherosclerosis47. The nutrient deficiency can also activate the NOD-like receptor (NLR) family and pyrin domain-containing 3 (NLRP3) inflammasome and induce IL-1β secretion in high fat diet-induced atherosclerosis animal model48, whereas zinc supplementation can inhibit NLRP3 inflammasome activation through its antioxidant effects in an in vitro study49. Furthermore, Beattie et al studied suboptimal dietary zinc intake in a mouse model of atherosclerosis and found that zinc deficiency promoted vascular inflammation and arterial plaque formation34. Later, they also provided evidence to show that marginal dietary zinc deficiency in vivo induced vascular smooth muscle cell apoptosis; and long-term zinc deprivation, on the contrary, accelerated rat vascular smooth muscle cell proliferation through down-regulating c-Jun N-terminal kinase 1/2 (JNK1/2) expression in mitogen-activated protein kinase (MAPK) signaling pathway14,50.
These in vitro and animal studies provide strong evidence to link reduced zinc intake with increased plasma lipoprotein levels, increased smooth muscle remodeling, increased vascular inflammation, and plaques formation in mice consuming dietary zinc levels resembling human Western-type diet during the progression of atherosclerosis.
Human studies
Epidemiological evidence suggests that the progression of atherosclerosis is modified by nutritional factors including zinc51. A number of studies reported that zinc deficiency could contribute to atherosclerosis52 and there was an inverse association between atherosclerosis and serum zinc levels, or rather the serum zinc/24-h urine zinc loss ratio53,54. According to the Biomarkers of Nutrition for Development (BOND) project, the dietary zinc intake record is recommended as one of the indicators to evaluate zinc levels in the human body9. Using carotid intima-media thickness (CIMT) as a valuable marker, the subclinical atherosclerosis was measured and explored for its relationship with the dietary zinc intake55. In the middle-aged and elderly populations, the low zinc intake group showed a higher CIMT than the high zinc intake group. The inverse correlation of zinc intake and atherosclerosis risk was also reported in another cross-sectional study56. Lower consumption of dietary zinc was associated with low high-density lipoprotein (HDL) cholesterol levels as well as an increased prevalence of coronary artery disease. However, the association between zinc intake and the risk of atherosclerosis or other CVDs has not yet been confirmed by cohort or randomized clinical studies. Iowa Women's Health Study (IWHS) showed no significant inverse association between zinc intake and the mortality of CVDs including ischemic heart disease and stroke in overall study population, although it was the case in a high alcohol-intake group57. A very recent meta-analysis of randomized primary prevention trials also failed to show significant beneficial effects of the nutrient on CVDs58. It is worthwhile to point out that IWHS and these primary prevention trials used a combined supplement with multiple nutrients containing a relatively low dose of zinc (≤ 20 mg/d). Therefore, more well-designed studies with zinc supplementation are required to reveal the exact contribution of the zinc nutrient in CVDs. Many factors should be included into consideration, such as the interaction between zinc and other nutrients, and the actual bioavailability of zinc which is in a wide variety depending on one's genetic backgrounds and dietary patterns.
Zinc status in CVDs
The zinc level in heart tissues was found to be positively correlated with ejection fraction in human and distinctly localized to the sarcomere I-band59,60. Zinc also affects Ca2+ signaling that regulates versatile cellular responses in cardiomyocytes61. However, the exact function of zinc ions in normal heart physiology remains largely unknown. The changes in zinc status have been reported in various CVDs.
Hypertension
Loss of zinc homeostasis can be both the cause and effect of hypertension. A large amount of data from both clinical and animal studies has confirmed the altered distribution of zinc between the extracellular and intracellular spaces as well as tissue distribution in arterial hypertension62. Earlier studies found that there was a negative correlation between arterial blood pressure and zinc content in the serum and higher zinc concentration in the red blood cells in patients suffering from primary arterial hypertension. The concentrations of zinc were lower in the scalp hair and blood, but higher in the urine samples of hypertensive patients63. In a strain of spontaneously hypertensive rat, high zinc levels in erythrocytes and heart muscles were found, and zinc deficient diet could facilitate the development of hypertension likely through oxidative stress64. The detailed mechanisms underlying the association of zinc status and hypertension can be found elsewhere47. In pulmonary artery hypertension which is caused by chronic hypoxia and often leads to right ventricular hypertrophy, several recent studies demonstrated a significant elevation of intracellular labile zinc levels in both pulmonary endothelial cells and vascular smooth muscle cells65,66; however, the source for the labile zinc appeared to be different in these cells: zinc released from MTs upon NO activation and zinc influx through ZIP12, respectively.
Myocardial infarction
It has long been known that serum zinc level falls after acute tissue injury including MI. An earlier study showed that patients with MI showed a highly significant reduction in serum zinc within the first three days, compared to control or borderline groups67. The minimum serum zinc level and the peak value of serum enzymes, and also some other clinical MI estimators of prognosis are nicely correlated. Thus, it has been suggested that serum zinc is a reliable diagnostic indicator for acute MI, and the extent of the fall has prognostic implications. In a meta-analysis with 2886 subjects from 41 case-control studies, the authors revealed that the subjects with MI had lower zinc levels in serum and hair than healthy controls68. Moreover, a recent study using zinc quartiles also demonstrated a significant inverse correlation between serum zinc levels and serum creatine kinase (CK), CK-MB, and cardiac troponin T levels69. This study further showed the prevalence rate of MIs decreased with increasing zinc quartiles. Taken together, all data consistently suggest that serum zinc may work together with other cardiac markers as prognostic markers for MI.
In addition to its risk evaluation value, zinc also has a cardioprotective role for MI, at least well demonstrated in animal studies. In a MI dog model generated by left coronary artery branch occlusion, administration of zinc sulphate (10 mg/kg) hours before coronary occlusion significantly reduced the infarct size by almost half70. In two Langendorff perfused rat heart studies, administrating zinc with zinc ionophore pyrithione during reperfusion and maintaining high levels of cytosolic zinc by postconditioning greatly improved myocardial recovery after I/R to almost 100%, and decreased arrhythmias and infarct sizes more than two-fold71,72. This protective function of zinc is likely through preservation of PKC isoforms, and/or through reperfusion injury salvage kinase (RISK) pathway.
Atrial fibrillation
It is the common sustained cardiac arrhythmia associated with substantially increased morbidity and mortality. Many cases of AF occur in patients who have just received cardiac surgery73. A recent report demonstrated an inverse association between the plasma zinc concentrations during recovery period and the occurrence of postoperative AF in patients undergoing coronary artery bypass grafting73. Along with significant lower zinc concentrations at postoperative days 1 and 3 in AF patients, the patient group experiencing postoperative AF showed a slower recovery rate back to preoperative zinc level, compared to the group without AF. In a prospective study of 78 patients with clinically advanced congestive heart failure, serum zinc and copper levels did not show significant differences between patients with AF and those with sinus rhythm74. But both of these patient groups, compared to the healthy control group, showed significant hypozincemia (low blood zinc) and a decreased zinc/copper ratio in their serums.
Congestive heart failure
CHF is a progressive systemic illness accompanying with oxidative stress in multiple tissues and pro-inflammatory phenotypes. A disturbed homeostasis of micronutrients such as calcium, magnesium, zinc, selenium, and vitamin D, may contribute to the appearance of oxidative stress and to compromised endogenous anti-oxidative defenses to combat CHF75. Several studies showed an association between zinc deficiency and heart failure, such as in children with CHF and in patients with dilated cardiomyopathy76,77. Long-term treatment of multiple micronutrient supplements including magnesium, zinc, copper, selenium, and vitamins, improved left ventricular volumes, left ventricular ejection fraction, and quality of life in CHF patients.
Irrespective of the etiologic origins of CHF, hypozincemia has been widely reported in patients with this disease77. While it is pointed out that low serum zinc is attributed to multiple factors, such as activation of the renin-angiotensin-aldosterone system, CHF medications, and pathophysiological progress of CHF77, it is still unclear how much of the factors contribute to the hypozincemia in patients. For example, thiazide diuretics, which are widely used to treat hypertension by increasing urine flow, cause urinary zinc loss46. Other medications for CHF, such as angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and diuretic furosemide, can also lead to zinc deficiency46. Other underlying mechanisms for hypozincemia in CHF include decreased dietary zinc intake and/or impaired absorption in the small intestine, and increased zinc redistribution into the liver, muscle, and bone for storage75.
Zinc transporters and MTs in CVDs
While zinc status itself is associated with many types of CVDs, zinc transporters and MTs may also play important roles in the pathological progress of these diseases. Table 1 is an attempt to summarize up-to-date known, although far from complete, zinc transporters and MTs involved.
Table 1.
Zinc transporters and MTs in CVDs.
| Molecule | Changes/Data | Diseases | Ref. |
|---|---|---|---|
| ZIP2 | Expression required for effects of postconditioning | I/R injury (cardioprotective effect of postconditioning) | 72 |
| ZIP6 | Increase in RNA (1.3-1.7 fold; microarray, qRT-PCR) | Endothelial cell senescence (vascular pathology) | 103 |
| ZIP7 | Increase in RNA (1.3 fold; microarray) | Endothelial cell senescence | 103 |
| ZIP12 | Increase in RNA, protein(qRT-PCR, IHC) | Pulmonary hypertension (by hypoxia) | 66 |
| ZIP13 | Increase in RNA (1.5 fold; microarray) | Endothelial cell senescence | 103 |
| ZnT1 | Increase in RNA (2.5-3 fold; microarray, qRT-PCR) | Endothelial cell senescence | 103 |
| Increase in protein (WB) | AF | 81 | |
| Increase in protein (WB) | I/R injury (hypoxia in cardiomyocytes) | 44 | |
| Manipulation of expression adversely affects I/R injury | I/R injury (cardiomyocytes) | 79 | |
| ZnT2 | Increase in protein (WB) | I/R injury (hypoxia/reoxygeneration in cardiomyocytes) | 44 |
| ZnT3 | Decrease in RNA (RT-PCR) | Vascular smooth muscle cell senescence (by angiotensin II) | 104 |
| ZnT5 | Increase in protein (WB) | I/R injury (hypoxia/reoxygeneration in cardiomyocytes) | 44 |
| ZnT6 | Decrease in RNA (1.4 fold; microarray) | Endothelial cell senescence | 103 |
| ZnT7 | cis-expression quantitative trait locus mapped | Atherosclerosis (high plasma TMAO level) | 78 |
| ZnT8 | rs200185429 (stop gained, decreased expression) | T2DM (reduction in risk) | 95 |
| rs587777582 (frame shift, decreased expression) | T2DM (reduction in risk) | 95 | |
| rs13266634 (missense) | T2DM | 105 | |
| Identified as autoantigen | T1DM | 91 | |
| ZnT10 | Decrease in RNA(RT-PCR) | Vascular smooth muscle cell senescence (by angiotensin II) | 104 |
| MT-1A | rs8052394 (missense) | T2DM | 106 |
| MT-1E | Increase in RNA (1.2 fold; microarray) | T2DM | 107 |
| MT-1M | Increase in RNA (1.2 fold; microarray) | T2DM | 107 |
| MT-1X | Increase in RNA (1.2 fold; microarray) | T2DM | 107 |
| MT-2A | rs10636 (3′-UTR variant) | Atherosclerosis; carotid artery stenosis, coronary heart disease | 83, 84, 85, 86 |
| rs1610216 (upstream variant) | T2DM with atherosclerosis | 84 | |
| Increase in RNA (1.3 fold; microarray) | T2DM | 107 |
ZnTs and ZIPs
There has been no report so far to show any direct link between zinc transporters and atherosclerosis. However, some recent evidence suggests such direction. Increased serum trimethylamine N-oxide (TMAO), the product of gut microbiome and hepatic-mediated metabolism of dietary choline and L-carnitine, has recently been identified as a novel risk factor for the development of atherosclerosis in mice and humans78. A genome-wide association study identified a locus for TMAO levels on chromosome 3 that co-localized with a highly significant cis-expression quantitative trait locus for ZnT7 encoding gene, suggesting ZnT7 as a candidate gene responsible for the association signal with atherosclerosis. Future characterization on the specific role of ZnT7 in atherosclerosis will be required to address the question.
The first report revealing the roles of zinc transporters in ischemic heart diseases was from an in vitro study using adult mouse atrial cardiomyocyte HL-1 cells79. Beharier et al demonstrated that ZnT1 overexpression markedly decreased both lactate dehydrogenase (LDH) release and caspase activation following myocardial I/R, whereas knockdown of endogenous ZnT1 enhanced these I/R-induced activities. The cardioprotective function of ZnT1 is likely through its ability to interact with Raf-1 kinase, which regulates the extracellular signal-regulated kinase (ERK) signaling pathway. It was recently reported that hypoxia increased the expression of ZnT1, but reoxygenation significantly increased the expression of ZnT2 and ZnT5, while ZnT9 expression remained unchanged in myocardial I/R44. Interestingly, ZnT9 is more abundant, and ZnT1, ZnT2 and ZnT5 are much less abundant in the heart (Figure 1A). These data suggest that ZnT9 may be important to maintain normal cellular function of cardiomyocytes while the other ZnTs are involved in cellular response to hypoxia and reoxygenation.
Similar to ZnTs 1, ZnT2, and ZnT5, ZIP2 appears to have very low expression in heart tissues, smooth muscle cells, and endothelial cells (Figure 1). But it may respond to I/R injury in cardiomyocytes. A recent report showed that knockdown of ZIP2 partially attenuated the protective effect of postconditioning on I/R injury in cardiac cells, pointing out that a normal level of ZIP2 is required for the protection of postconditioning72. Since zinc deprivation alters multiple zinc transporters' expression at the mRNA level80, we speculate that other zinc transporters may be involved in myocardial I/R as well.
Beharier et al reported that acute rapid pacing of the rat atria increased the expression of ZnT1 that acted as an inhibitor of L-type calcium channel, in cultured cardiomyocytes or intact heart79. Overexpression of ZnT1 decreased arrhythmogenic Ca2+ signals, while knockdown of ZnT1 enhanced the signals. Later, this group further demonstrated the increased ZnT1 expression in the atria of patients with AF81. Collectively, these data suggest that ZnT1 is a contributing factor for atrial tachycardia remodeling in patients with persistent AF.
Metallothioneins
The expression of MTs is dynamically regulated by oxidative stress and cellular zinc level82. MTs are perhaps more readily responsive to pathological changes in atherosclerosis than zinc transporters are. It has been well known that the persistence of oxidative stress and chronic pro-inflammatory cytokines exist in atherosclerosis. Indeed, MT gene expression is up-regulated accompanying with the reduced zinc ion availability in peripheral blood mononuclear cells although those MT proteins may be in a less functional form as an antioxidant protein83. Furthermore, several novel MT polymorphisms have been identified in pathological process of atherosclerosis83,84,85,86. Giacconi et al reported that the polymorphism at −209 A/G locus of MT2A gene (NCBI rs1610216) not only affects hyperglycemia and glycosylated hemoglobin in diabetic-atherosclerotic patients, but also is associated with ischemic cardiomyopathy84. Another polymorphism of MT2A at +838 C/G (NCBI rs10636) in 3′-UTR region affects the susceptibility of atherosclerosis and carotid artery disease83.
Despite some earlier in vitro studies, the first evidence in intact animals suggesting MT's cardioprotective role in myocardia I/R injury was shown in a cardiac-specific MT-overexpressing transgenic mouse model87. Kang et al demonstrated that overexpression of MTs improved the recovery of cardiac contractile function and reduced infarct size in I/R injured heart. Later studies further revealed that this protective function of MTs was achieved by suppressing oxidative stress and inhibiting apoptosis in cardiomyocytes.
ZnT8 and MTs in diabetic cardiovascular complications
Diabetes is classified as a chronic metabolic disease with two distinct forms: type 1 and type 2. Type 1 is an autoimmune disease that destroys the insulin-producing pancreatic islet β-cells; and type 2 accounts for >90% of all diabetes cases with adult-onset, although more and more cases are diagnosed in younger people. Both types of diabetes act as a major risk factor for CVDs, with 50% of diabetes-associated deaths resulting from cardiovascular complications88. Zinc has been known to play a key role in diabetes for more than 80 years. Back in the 1930s, it was already discovered that the pancreas of diabetic patients contained only half the amount of zinc compared to a healthy pancreas. Since then, a large amount of evidence has revealed the hormone-like functions of zinc ions in islet biology, the biochemistry of zinc in insulin synthesis, secretion and storage as well as the maintenance of the conformational integrity of insulin89.
Transporting zinc into the insulin secretory granules of β-cells is mainly through ZnT890. Due to the importance of zinc for normal physiology of β-cells in healthy pancreas, any dysfunction of ZnT8 could result in serious consequences in insulin homeostasis. It has been discovered that autoantibodies against ZnT8 are major forms of autoantibodies in type 1 diabetes (T1DM)91, which is associated with antibody-mediated β-cell dysfunction and cytotoxicity. The mutations and polymorphism in ZnT8 have been linked to T2DM. Overexpression of ZnT8 increased insulin secretion in response to hyperglycemia in INS-1 insulin-secreting β-cells92. On the other hand, the β cell-specific ZnT8-null mice displayed impaired morphology of β-cell granules and insulin processing as well as reduced first phase insulin secretion93. A non-synonymous single nucleotide polymorphism (SNP) (rs13266634 C>T) in ZnT8 is also associated with T2DM. This polymorphism contains an amino acid switch, where arginine at position 325 is replaced by tryptophan. This SNP disrupts the function of ZnT8 to transport zinc ions into the secretion granules where insulin is matured and formed to be zinc-bound hexameric structure94. Moreover, a recent study reported the loss-of-function mutations in SLC30A8 (ZnT8) gene locus from the sequencing and genotyping results of ∼150 000 individuals95. These loss-of-function mutations, interestingly, appear to protect human against T2DM. While the exact mechanism requires further investigation, these data nevertheless suggest that SNPs or mutations of ZnT8 may have prognostic value, and the targeted manipulation of the transporter may be a therapeutic strategy in diabetes prevention.
While ZnT8 is involved in diabetes through its role to transport zinc, dysfunction of MTs contributes to the pathology of diabetes mainly because of the reduced antioxidant and decreased radical species scavenging properties96. This notion has been well supported by many studies in diabetic animals. Overexpression of MT has been shown to reduce oxidative stress and DNA damage in β cells of streptozotocin-induced diabetic mice, whereas knockout of MT1 and MT2 resulted in mild obesity and hyperleptinemia in mice97,98. In human subjects, the expression of MT can be significantly induced by zinc supplementation, and such induction reduces diabetic vascular complications99. In correspondence with this, T2DM patients with lower MT levels exhibited to be more susceptible to oxidative stress and hyperglycemia100. Similar as in atherosclerosis, some SNPs of MTs have also been associated with cardiovascular complications in T2DM. For example, the C+ carriers of MT1A polymorphism +647 A/C (rs11640851) in T2DM patients displayed worse glycemic control, reduced zinc release from MT and higher MT production in PBMCs, suggesting the possible involvement of MT in diabetic cardiovascular complications101. More details for MTs polymorphisms can be found in a recent comprehensive review article102.
Conclusion and future directions
Dysregulation of zinc metabolism is clearly associated with many CVDs. Since zinc concentration itself is not a good biomarker4, searching for a reliable, sensitive and specific biomarker to assess zinc status has been a research focus. One recently emerging method is the assessment of the expression of zinc transporters and/or zinc-binding proteins, such as MTs in circulating blood cells108. The advantage of using circulating blood cells, such as leukocytes, PBMC and erythrocytes, is their availability in routine clinical visits. Among a few tested zinc homeostasis pertinent proteins, eg, ZIP1, ZnT1, and MT, the expression of MT in leukocytes appears to be a preferred biomarker to assess zinc status, since it decreases in response to zinc depletion and increases in response to zinc supplementation in a dose-dependent manner. The changes in MT expression, moreover, respond to elevated amounts of dietary zinc at the earliest time point measured (2 days)108.
In addition to zinc status, the alteration in zinc transporters and MTs may have diagnostic and prognostic implications for CVDs directly (Table 1). Recent research in this area enthusiastically recognizes extracellular vesicles (EVs) from human liquid biopsy as intriguing sources to differentiate disease from healthy states109,110. These EV-based disease markers may be detected prior to the onset of symptoms, making them promising candidates for early stage of CVDs109. As heterogeneous populations of phospholipid bilayer-enclosed vesicles that are secreted into the extracellular space, EVs of endothelial or cardiac tissue origin have been found to contribute to CVDs110. Interestingly, some recent studies determined that the endothelial cell-derived exosomes indeed contained MT proteins responding to cellular stress conditions and EVs from various cells or human samples exhibited the presence of zinc transporters as well111. We do not yet understand the pathophysiological roles of these zinc transporters or MTs in EVs in CVDs. In order to fully examine the diagnostic and prognostic values of zinc transporters and MTs, future investigation on the association between EVs containing a specific zinc transporter(s) or MT(s) and CVDs is required.
Abbreviations
Cardiovascular disease (CVD); nitric oxide synthase (NOS); endothelial nitric oxide synthase (eNOS); reactive oxygen species (ROS); reactive nitrogen species (RNS); myocardial infarction (MI): vascular smooth muscle cells (VSMCs); metallothionein (MT); protein kinase C (PKC); glutathione (GSH); superoxide dismutase (SOD); endoplasmic reticulum (ER); nitric oxide (NO); type 2 diabetes (T2DM); type 1 diabetes (T1DM); nuclear factor kappa-B (NF-κB); hypoxia-inducible factor-1 alpha (HIF-1α); NF-κB light polypeptide gene enhancer in B-cells inhibitor (IκB); tumor necrosis factor- α (TNF-α); interleukin (IL); interferon (IFN); Zrt, Irt-like protein (ZIP); solute carrier family 39 (SLC39A); zinc transporter (ZnT); solute carrier family 30 (SLC30A); low-density lipoprotein (LDL); NOD-like receptor (NLR); NLR Family Pyrin Domain Containing 3(NLRP3); c-Jun N-terminal kinase 1/2 (JNK1/2); mitogen-activated protein kinase (MAPK); carotid intima-media thickness (CIMT); high-density lipoprotein (HDL); creatine kinase (CK); reperfusion injury salvage kinases (RISK); atrial fibrillation (AF); congestive heart failure (CHF); trimethylamine N-oxide (TMAO); lactate dehydrogenase (LDH); ischemia/reperfusion (I/R); extracellular signal-regulated kinase (ERK); single nucleotide polymorphism (SNP); untranslated region (UTR)
References
- 1.King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130:1360S–6S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 2.Jackson MJ. Physiology of Zinc: general aspects. In: Mills CF, Editor. Zinc in human biology. London: Springer; 1989. p 1–14.
- 3.Jackson MJ, Jones DA, Edwards RH. Tissue zinc levels as an index of body zinc status. Clin Physiol. 1982;2:333–43. doi: 10.1111/j.1475-097x.1982.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 4.Pan Z, Choi S, Ouadid-Ahidouch H, Yang JM, Beattie JH, Korichneva I. Zinc transporters and dysregulated channels in cancers. Front Biosci (Landmark Ed) 2017;22:623–43. doi: 10.2741/4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad AS. Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol. 2012;26:66–9. doi: 10.1016/j.jtemb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Hemmens B, Goessler W, Schmidt K, Mayer B. Role of bound zinc in dimer stabilization but not enzyme activity of neuronal nitric-oxide synthase. J Biol Chem. 2000;275:35786–91. doi: 10.1074/jbc.M005976200. [DOI] [PubMed] [Google Scholar]
- 7.Choi S, Cui C, Luo Y, Kim SH, Ko JK, Huo X, et al. Selective inhibitory effects of zinc on cell proliferation in esophageal squamous cell carcinoma through Orai1. FASEB J. 2018;32:404–16. doi: 10.1096/fj.201700227RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008;43:370–7. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 9.King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, et al. Biomarkers of nutrition for development (BOND)-Zinc review. J Nutr. 2016;pii:jn220079. doi: 10.3945/jn.115.220079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freitas EP, Cunha AT, Aquino SL, Pedrosa LF, Lima SC, Lima JG, et al. Zinc status biomarkers and cardiometabolic risk factors in metabolic syndrome: a case control study. Nutrients 2017; 9: pii: E175. doi: 10.3390/nu9020175. [DOI] [PMC free article] [PubMed]
- 11.Lorber D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2014;7:169–83. doi: 10.2147/DMSO.S61438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korichneva I. Zinc dynamics in the myocardial redox signaling network. Antioxid Redox Signal. 2006;8:1707–21. doi: 10.1089/ars.2006.8.1707. [DOI] [PubMed] [Google Scholar]
- 13.Eide DJ. The oxidative stress of zinc deficiency. Metallomics. 2011;3:1124–9. doi: 10.1039/c1mt00064k. [DOI] [PubMed] [Google Scholar]
- 14.Allen-Redpath K, Ou O, Beattie JH, Kwun IS, Feldmann J, Nixon GF. Marginal dietary zinc deficiency in vivo induces vascular smooth muscle cell apoptosis in large arteries. Cardiovasc Res. 2013;99:525–34. doi: 10.1093/cvr/cvt114. [DOI] [PubMed] [Google Scholar]
- 15.Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–44. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 16.Oteiza PI. Zinc and the modulation of redox homeostasis. Free Radic Biol Med. 2012;53:1748–59. doi: 10.1016/j.freeradbiomed.2012.08.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marreiro DD, Cruz KJ, Morais JB, Beserra JB, Severo JS, de Oliveira AR. Zinc and oxidative stress: current mechanisms. Antioxidants (Basel) 2017; 6. pii: E24. doi: 10.3390/antiox6020024. [DOI] [PMC free article] [PubMed]
- 18.Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med. 1990;8:281–91. doi: 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- 19.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–36. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 20.Zago MP, Oteiza PI. The antioxidant properties of zinc: interactions with iron and antioxidants. Free Radic Biol Med. 2001;31:266–74. doi: 10.1016/s0891-5849(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 21.Westin G, Schaffner W. A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J. 1988;7:3763–70. doi: 10.1002/j.1460-2075.1988.tb03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortese MM, Suschek CV, Wetzel W, Kroncke KD, Kolb-Bachofen V. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med. 2008;44:2002–12. doi: 10.1016/j.freeradbiomed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Li HT, Jiao M, Chen J, Liang Y. Roles of zinc and copper in modulating the oxidative refolding of bovine copper, zinc superoxide dismutase. Acta Biochim Biophys Sin (Shanghai) 2010;42:183–94. doi: 10.1093/abbs/gmq005. [DOI] [PubMed] [Google Scholar]
- 24.Ermilova IP, Ermilov VB, Levy M, Ho E, Pereira C, Beckman JS. Protection by dietary zinc in ALS mutant G93A SOD transgenic mice. Neurosci Lett. 2005;379:42–6. doi: 10.1016/j.neulet.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 25.Homma K, Fujisawa T, Tsuburaya N, Yamaguchi N, Kadowaki H, Takeda K, et al. SOD1 as a molecular switch for initiating the homeostatic ER stress response under zinc deficiency. Mol Cell. 2013;52:75–86. doi: 10.1016/j.molcel.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 26.Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164:213–23. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paolocci N, Biondi R, Bettini M, Lee CI, Berlowitz CO, Rossi R, et al. Oxygen radical-mediated reduction in basal and agonist-evoked NO release in isolated rat heart. J Mol Cell Cardiol. 2001;33:671–9. doi: 10.1006/jmcc.2000.1334. [DOI] [PubMed] [Google Scholar]
- 28.Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Role of endoplasmic reticulum stress in atherosclerosis and diabetic macrovascular complications. Biomed Res Int. 2014;2014:610140. doi: 10.1155/2014/610140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korichneva I, Hoyos B, Chua R, Levi E, Hammerling U. Zinc release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J Biol Chem. 2002;277:44327–31. doi: 10.1074/jbc.M205634200. [DOI] [PubMed] [Google Scholar]
- 30.Wong CP, Ho E. Zinc and its role in age-related inflammation and immune dysfunction. Mol Nutr Food Res. 2012;56:77–87. doi: 10.1002/mnfr.201100511. [DOI] [PubMed] [Google Scholar]
- 31.Bao S, Liu MJ, Lee B, Besecker B, Lai JP, Guttridge DC, et al. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2010;298:L744–54. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nardinocchi L, Pantisano V, Puca R, Porru M, Aiello A, Grasselli A, et al. Zinc downregulates HIF-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS One. 2010;5:e15048. doi: 10.1371/journal.pone.0015048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beattie JH, Gordon MJ, Duthie SJ, McNeil CJ, Horgan GW, Nixon GF, et al. Suboptimal dietary zinc intake promotes vascular inflammation and atherogenesis in a mouse model of atherosclerosis. Mol Nutr Food Res. 2012;56:1097–105. doi: 10.1002/mnfr.201100776. [DOI] [PubMed] [Google Scholar]
- 35.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–28. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao K, Sethi K, Ischia J, Gibson L, Galea L, Xiao L, et al. Protective effect of zinc preconditioning against renal ischemia reperfusion injury is dose dependent. PLoS One. 2017;12:e0180028. doi: 10.1371/journal.pone.0180028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4:676–94. doi: 10.3390/nu4070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong J, Eide DJ. The SLC39 family of zinc transporters. Mol Aspects Med. 2013;34:612–9. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S, Bird AJ. Zinc'ing sensibly: controlling zinc homeostasis at the transcriptional level. Metallomics. 2014;6:1198–215. doi: 10.1039/c4mt00064a. [DOI] [PubMed] [Google Scholar]
- 40.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627–47. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–76. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 42.Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, et al. A subcellular map of the human proteome. Science 2017; 356. pii: eaal3321. doi: 10.1126/science.aal3321. [DOI] [PubMed]
- 44.Bodiga VL, Thokala S, Kovur SM, Bodiga S. Zinc dyshomeostasis in cardiomyocytes after acute hypoxia/reoxygenation. Biol Trace Elem Res. 2017;179:117–29. doi: 10.1007/s12011-017-0957-7. [DOI] [PubMed] [Google Scholar]
- 45.Himes BE, Koziol-White C, Johnson M, Nikolos C, Jester W, Klanderman B, et al. Vitamin D modulates expression of the airway smooth muscle transcriptome in fatal asthma. PLoS One. 2015;10:e0134057. doi: 10.1371/journal.pone.0134057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Little PJ, Bhattacharya R, Moreyra AE, Korichneva IL. Zinc and cardiovascular disease. Nutrition. 2010;26:1050–7. doi: 10.1016/j.nut.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Foster M, Samman S. Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid Redox Signal. 2010;13:1549–73. doi: 10.1089/ars.2010.3111. [DOI] [PubMed] [Google Scholar]
- 48.Summersgill H, England H, Lopez-Castejon G, Lawrence CB, Luheshi NM, Pahle J, et al. Zinc depletion regulates the processing and secretion of IL-1beta. Cell Death Dis. 2014;5:e1040. doi: 10.1038/cddis.2013.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan Y, Zhang XL, Yang LN, Wang J, Hu Y, Bian AS, et al. Zinc inhibits high glucose-induced NLRP3 inflammasome activation in human peritoneal mesothelial cells. Mol Med Rep. 2017;16:5195–202. doi: 10.3892/mmr.2017.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alcantara EH, Shin MY, Feldmann J, Nixon GF, Beattie JH, Kwun IS. Long-term zinc deprivation accelerates rat vascular smooth muscle cell proliferation involving the down-regulation of JNK1/2 expression in MAPK signaling. Atherosclerosis. 2013;228:46–52. doi: 10.1016/j.atherosclerosis.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 51.Kritchevsky D. Diet and atherosclerosis. J Nutr Health Aging. 2001;5:155–9. [PubMed] [Google Scholar]
- 52.Beattie JH, Kwun IS. Is zinc deficiency a risk factor for atherosclerosis? Br J Nutr. 2004;91:177–81. doi: 10.1079/BJN20031072. [DOI] [PubMed] [Google Scholar]
- 53.Atsumi T, Numano F. Blood zinc levels in patients with arteriosclerosis obliterans, thromboangiitis obliterans and Takayasu's disease. Jpn Heart J. 1975;16:664–9. doi: 10.1536/ihj.16.664. [DOI] [PubMed] [Google Scholar]
- 54.Giannoglou GD, Konstantinou DM, Kovatsi L, Chatzizisis YS, Mikhailidis DP. Association of reduced zinc status with angiographically severe coronary atherosclerosis: a pilot study. Angiology. 2010;61:449–55. doi: 10.1177/0003319710366702. [DOI] [PubMed] [Google Scholar]
- 55.Yang YJ, Choi BY, Chun BY, Kweon SS, Lee YH, Park PS, et al. Dietary zinc intake is inversely related to subclinical atherosclerosis measured by carotid intima-media thickness. Br J Nutr. 2010;104:1202–11. doi: 10.1017/S0007114510001893. [DOI] [PubMed] [Google Scholar]
- 56.Singh RB, Niaz MA, Rastogi SS, Bajaj S, Zhang GL, Zhu SM. Current zinc intake and risk of diabetes and coronary artery disease and factors associated with insulin resistance in rural and urban populations of north India. J Am Coll Nutr. 1998;17:564–70. doi: 10.1080/07315724.1998.10718804. [DOI] [PubMed] [Google Scholar]
- 57.Lee DH, Folsom AR, Jacobs DR. Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: the Iowa Women's Health Study. Am J Clin Nutr. 2005;81:787–91. doi: 10.1093/ajcn/81.4.787. [DOI] [PubMed] [Google Scholar]
- 58.Schwingshackl L, Boeing H, Stelmach-Mardas M, Gottschald M, Dietrich S, Hoffmann G, et al. Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a systematic review and meta-analysis of primary prevention trials. Adv Nutr. 2017;8:27–39. doi: 10.3945/an.116.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oster O, Dahm M, Oelert H. Element concentrations (selenium, copper, zinc, iron, magnesium, potassium, phosphorous) in heart tissue of patients with coronary heart disease correlated with physiological parameters of the heart. Eur Heart J. 1993;14:770–4. doi: 10.1093/eurheartj/14.6.770. [DOI] [PubMed] [Google Scholar]
- 60.Palmer BM, Vogt S, Chen Z, Lachapelle RR, Lewinter MM. Intracellular distributions of essential elements in cardiomyocytes. J Struct Biol. 2006;155:12–21. doi: 10.1016/j.jsb.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 61.Yi T, Vick JS, Vecchio MJ, Begin KJ, Bell SP, Delay RJ, et al. Identifying cellular mechanisms of zinc-induced relaxation in isolated cardiomyocytes. Am J Physiol Heart Circ Physiol. 2013;305:H706–15. doi: 10.1152/ajpheart.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tubek S. Selected zinc metabolism parameters in women with arterial hypotension. Biol Trace Elem Res. 2007;116:73–9. doi: 10.1007/BF02685920. [DOI] [PubMed] [Google Scholar]
- 63.Afridi HI, Kazi TG, Talpur FN, Kazi A, Arain SS, Arain SA, et al. Interaction between essential elements selenium and zinc with cadmium and mercury in samples from hypertensive patients. Biol Trace Elem Res. 2014;160:185–96. doi: 10.1007/s12011-014-0048-y. [DOI] [PubMed] [Google Scholar]
- 64.Sato M, Yanagisawa H, Nojima Y, Tamura J, Wada O. Zn deficiency aggravates hypertension in spontaneously hypertensive rats: possible role of Cu/Zn-superoxide dismutase. Clin Exp Hypertens. 2002;24:355–70. doi: 10.1081/ceh-120004797. [DOI] [PubMed] [Google Scholar]
- 65.Bernal PJ, Leelavanichkul K, Bauer E, Cao R, Wilson A, Wasserloos KJ, et al. Nitric-oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circ Res. 2008;102:1575–83. doi: 10.1161/CIRCRESAHA.108.171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao L, Oliver E, Maratou K, Atanur SS, Dubois OD, Cotroneo E, et al. The zinc transporter ZIP12 regulates the pulmonary vascular response to chronic hypoxia. Nature. 2015;524:356–60. doi: 10.1038/nature14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Low WI, Ikram H. Plasma zinc in acute myocardial infarction. Diagnostic and prognostic implications. Br Heart J. 1976;38:1339–42. doi: 10.1136/hrt.38.12.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu B, Cai ZQ, Zhou YM. Deficient zinc levels and myocardial infarction: association between deficient zinc levels and myocardial infarction: a meta-analysis. Biol Trace Elem Res. 2015;165:41–50. doi: 10.1007/s12011-015-0244-4. [DOI] [PubMed] [Google Scholar]
- 69.Huang L, Teng T, Zhao J, Bian B, Yao W, Yu X, et al. The relationship between serum zinc levels, cardiac markers and the risk of acute myocardial infarction by zinc quartiles. Heart Lung Circ 2018; 27: 66–72. doi: 10.1016/j.hlc.2017.01.022. [DOI] [PubMed]
- 70.Lal A. Effect of zinc sulphate on infarct size in experimental myocardial infarction in dogs. Indian J Med Res. 1991;94:316–9. [PubMed] [Google Scholar]
- 71.Karagulova G, Yue Y, Moreyra A, Boutjdir M, Korichneva I. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J Pharmacol Exp Ther. 2007;321:517–25. doi: 10.1124/jpet.107.119644. [DOI] [PubMed] [Google Scholar]
- 72.Xu Z, Kim S, Huh J. Zinc plays a critical role in the cardioprotective effect of postconditioning by enhancing the activation of the RISK pathway in rat hearts. J Mol Cell Cardiol. 2014;66:12–7. doi: 10.1016/j.yjmcc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 73.Yan YQ, Zou LJ. Relation between zinc, copper, and magnesium concentrations following cardiopulmonary bypass and postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. Biol Trace Elem Res. 2012;148:148–53. doi: 10.1007/s12011-012-9356-2. [DOI] [PubMed] [Google Scholar]
- 74.Ghaemian A, Salehifar E, Jalalian R, Ghasemi F, Azizi S, Masoumi S, et al. Zinc and copper levels in severe heart failure and the effects of atrial fibrillation on the zinc and copper status. Biol Trace Elem Res. 2011;143:1239–46. doi: 10.1007/s12011-011-8956-6. [DOI] [PubMed] [Google Scholar]
- 75.Kamalov G, Holewinski JP, Bhattacharya SK, Ahokas RA, Sun Y, Gerling IC, et al. Nutrient dyshomeostasis in congestive heart failure. Am J Med Sci. 2009;338:28–33. doi: 10.1097/MAJ.0b013e3181aaee63. [DOI] [PubMed] [Google Scholar]
- 76.Cohen N, Golik A. Zinc balance and medications commonly used in the management of heart failure. Heart Fail Rev. 2006;11:19–24. doi: 10.1007/s10741-006-9189-1. [DOI] [PubMed] [Google Scholar]
- 77.Efeovbokhan N, Bhattacharya SK, Ahokas RA, Sun Y, Guntaka RV, Gerling IC, et al. Zinc and the prooxidant heart failure phenotype. J Cardiovasc Pharmacol. 2014;64:393–400. doi: 10.1097/FJC.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hartiala J, Bennett BJ, Tang WH, Wang Z, Stewart AF, Roberts R, et al. Comparative genome-wide association studies in mice and humans for trimethylamine N-oxide, a proatherogenic metabolite of choline and L-carnitine. Arterioscler Thromb Vasc Biol. 2014;34:1307–13. doi: 10.1161/ATVBAHA.114.303252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beharier O, Dror S, Levy S, Kahn J, Mor M, Etzion S, et al. ZnT-1 protects HL-1 cells from simulated ischemia-reperfusion through activation of Ras-ERK signaling. J Mol Med (Berl) 2012;90:127–38. doi: 10.1007/s00109-011-0845-0. [DOI] [PubMed] [Google Scholar]
- 80.Cousins RJ, Blanchard RK, Popp MP, Liu L, Cao J, Moore JB, et al. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc Natl Acad Sci U S A. 2003;100:6952–7. doi: 10.1073/pnas.0732111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Etzion Y, Ganiel A, Beharier O, Shalev A, Novack V, Volvich L, et al. Correlation between atrial ZnT-1 expression and atrial fibrillation in humans: a pilot study. J Cardiovasc Electrophysiol. 2008;19:157–64. doi: 10.1111/j.1540-8167.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 82.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 83.Giacconi R, Caruso C, Malavolta M, Lio D, Balistreri CR, Scola L, et al. Pro-inflammatory genetic background and zinc status in old atherosclerotic subjects. Ageing Res Rev. 2008;7:306–18. doi: 10.1016/j.arr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 84.Giacconi R, Cipriano C, Muti E, Costarelli L, Maurizio C, Saba V, et al. Novel -209A/G MT2A polymorphism in old patients with type 2 diabetes and atherosclerosis: relationship with inflammation (IL-6) and zinc. Biogerontology. 2005;6:407–13. doi: 10.1007/s10522-005-4907-y. [DOI] [PubMed] [Google Scholar]
- 85.Giacconi R, Muti E, Malavolta M, Cipriano C, Costarelli L, Bernardini G, et al. The +838 C/G MT2A polymorphism, metals, and the inflammatory/immune response in carotid artery stenosis in elderly people. Mol Med. 2007;13:388–95. doi: 10.2119/2007-00045.Giacconi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang XY, Sun JH, Ke HY, Chen YJ, Xu M, Luo GH. Metallothionein 2A genetic polymorphism and its correlation to coronary heart disease. Eur Rev Med Pharmacol Sci. 2014;18:3747–53. [PubMed] [Google Scholar]
- 87.Kang YJ, Li G, Saari JT. Metallothionein inhibits ischemia-reperfusion injury in mouse heart. Am J Physiol. 1999;276:H993–7. doi: 10.1152/ajpheart.1999.276.3.H993. [DOI] [PubMed] [Google Scholar]
- 88.DiSilvestro RA. Zinc in relation to diabetes and oxidative disease. J Nutr. 2000;130:1509S–11S. doi: 10.1093/jn/130.5.1509S. [DOI] [PubMed] [Google Scholar]
- 89.Vallee BL. Biochemistry, physiology and pathology of zinc. Physiol Rev. 1959;39:443–90. doi: 10.1152/physrev.1959.39.3.443. [DOI] [PubMed] [Google Scholar]
- 90.Shan Z, Bao W, Zhang Y, Rong Y, Wang X, Jin Y, et al. Interactions between zinc transporter-8 gene (SLC30A8) and plasma zinc concentrations for impaired glucose regulation and type 2 diabetes. Diabetes. 2014;63:1796–803. doi: 10.2337/db13-0606. [DOI] [PubMed] [Google Scholar]
- 91.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:17040–5. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci. 2006;119:4199–206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 93.Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, et al. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53:1656–68. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–83. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Flannick J, Thorleifsson G, Beer NL, Jacobs SB, Grarup N, Burtt NP, et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet. 2014;46:357–63. doi: 10.1038/ng.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maret W. Redox biochemistry of mammalian metallothioneins. J Biol Inorg Chem. 2011;16:1079–86. doi: 10.1007/s00775-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 97.Chen H, Carlson EC, Pellet L, Moritz JT, Epstein PN. Overexpression of metallothionein in pancreatic beta-cells reduces streptozotocin-induced DNA damage and diabetes. Diabetes. 2001;50:2040–6. doi: 10.2337/diabetes.50.9.2040. [DOI] [PubMed] [Google Scholar]
- 98.Beattie JH, Wood AM, Newman AM, Bremner I, Choo KH, Michalska AE, et al. Obesity and hyperleptinemia in metallothionein (-I and -II) null mice. Proc Natl Acad Sci U S A. 1998;95:358–63. doi: 10.1073/pnas.95.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, et al. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544–54. doi: 10.1161/CIRCULATIONAHA.105.537894. [DOI] [PubMed] [Google Scholar]
- 100.Maret W, Krezel A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol Med. 2007;13:371–5. doi: 10.2119/2007-00036.Maret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giacconi R, Bonfigli AR, Testa R, Sirolla C, Cipriano C, Marra M, et al. +647 A/C and +1245 MT1A polymorphisms in the susceptibility of diabetes mellitus and cardiovascular complications. Mol Genet Metab. 2008;94:98–104. doi: 10.1016/j.ymgme.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Raudenska M, Gumulec J, Podlaha O, Sztalmachova M, Babula P, Eckschlager T, et al. Metallothionein polymorphisms in pathological processes. Metallomics. 2014;6:55–68. doi: 10.1039/c3mt00132f. [DOI] [PubMed] [Google Scholar]
- 103.Malavolta M, Costarelli L, Giacconi R, Basso A, Piacenza F, Pierpaoli E, et al. Changes in Zn homeostasis during long term culture of primary endothelial cells and effects of Zn on endothelial cell senescence. Exp Gerontol. 2017;99:35–45. doi: 10.1016/j.exger.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Patrushev N, Seidel-Rogol B, Salazar G. Angiotensin II requires zinc and downregulation of the zinc transporters ZnT3 and ZnT10 to induce senescence of vascular smooth muscle cells. PLoS One. 2012;7:e33211. doi: 10.1371/journal.pone.0033211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–5. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 106.Yang L, Li H, Yu T, Zhao H, Cherian MG, Cai L, et al. Polymorphisms in metallothionein-1 and -2 genes associated with the risk of type 2 diabetes mellitus and its complications. Am J Physiol Endocrinol Metab. 2008;294:E987–92. doi: 10.1152/ajpendo.90234.2008. [DOI] [PubMed] [Google Scholar]
- 107.Marselli L, Thorne J, Dahiya S, Sgroi DC, Sharma A, Bonner-Weir S, et al. Gene expression profiles of Beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One. 2010;5:e11499. doi: 10.1371/journal.pone.0011499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hennigar SR, Kelley AM, McClung JP. Metallothionein and Zinc transporter expression in circulating human blood cells as biomarkers of Zinc status: a systematic review. Adv Nutr. 2016;7:735–46. doi: 10.3945/an.116.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jansen F, Nickenig G, Werner N. Extracellular vesicles in cardiovascular disease: potential applications in diagnosis, prognosis, and epidemiology. Circ Res. 2017;120:1649–57. doi: 10.1161/CIRCRESAHA.117.310752. [DOI] [PubMed] [Google Scholar]
- 110.Hromada C, Muhleder S, Grillari J, Redl H, Holnthoner W. Endothelial extracellular vesicles-promises and challenges. Front Physiol. 2017;8:275. doi: 10.3389/fphys.2017.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles 2012; 1. [DOI] [PMC free article] [PubMed]


