Abstract
Curcumin is a widely researched and utilized natural product used for a variety of ailments including as a gastrointestinal aide and an anticancer agent. Curcumin however suffers from poor bioavailability. A strategy to circumvent poor bioavailability is to administer with an adjuvant or by synthetic modification. Herein we demonstrate the incorporation of curcumin into a self-degradable polymer by condensation with N,N′-di-Boc-L-cystine. The polymer is made self-degradable upon deprotection of the cystine amines. Degradation is confirmed by thermogravimetric analysis and differential scanning calorimetry. Curcumin retains its anti-cancer activity within the polymer showing activity against HT29 human colon cancer cells and DU-145 prostate cancer cells. The self-degrading polymer showed enhanced activity against HT29 cells compared to that of curcumin.
Graphical abstract
INTRODUCTION
Curcumin, a diarylheptanoid ingredient in the spice turmeric, is well-studied for its medicinal benefits.1 Turmeric is widely used as a home remedy for gastrointestinal2 and skin ailments3. Studies have shown it to possess anti-bacterial4, anti-viral5 and anti-cancer6 properties. The hydroxyls of this polyphenol are responsible for its antioxidant properties while the methoxy groups are key to its anti-inflammatory and anti-proliferative properties.6 Curcumin inhibits tumor growth by both the activation of cell death pathways and the inhibition of proliferation pathways.6 Cancer cell death is attained through regulation of multiple cell signaling pathways including caspase activation pathway7 (caspase-8, 3, 9), tumor suppressor pathway8 (p53, p21), direct damage to DNA9 and the generation of reactive oxidative species10. Curcumin is attractive as a chemotherapeutic because it shows success against a wide variety of cancers including several carcinoma and sarcoma cancers.
Curcumin shows strong intrinsic activity against therapeutic targets, however a drawback to its utilization has been low bioavailability.11 After oral administration, low levels of curcumin are found in blood serum and limited tissue distribution. This is attributed to poor absorption, rapid metabolism, and rapid systemic elimination of curcumin. Strategies have been developed that improve the bioavailability of curcumin that include administering with an adjuvant like piperine that inhibits glucuronidation.12 Delivery of curcumin by encapsulation within micelles13, liposomes14 and nanoparticles15,16 are also successful strategies to improve bioavailability.
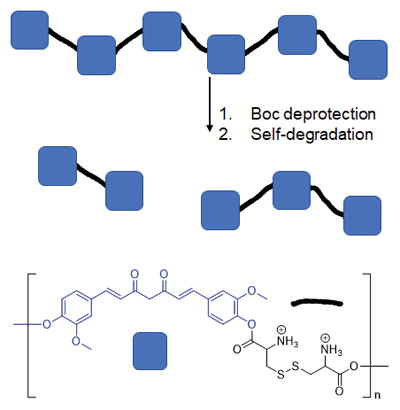
Another approach to improve bioavailability is the synthetic modification of curcumin. Modification at the phenolic positions has been used to improve water solubility and reduce glucouronidation. Numerous curcumin bioconjugates have been developed including modifications with amino acids, fatty acids and folic acid.17–19 Curcumin has also been incorporated into biodegradable polymer chains, with polymer showing greater cytotoxicity than that of curcumin.20 We have developed a biodegradable polymer by condensing curcumin with the dipeptide cystine. The strategy for the delivery of curcumin from the polymer is to incorporate cleavable ester and disulfide bonds in the polymer chain. Ester bonds are cleaved by hydrolysis while disulfide bonds are redox reactive and converted to thiols in the reducing environment of cells. Curcumin was made into a linear polymer by condensation with t-butyl carbamate (Boc) protected cystine via carbodiimide coupling. Then de-protection with trifluoroacetic acid produced the cationic polymer. Cationic polymers are useful for gene delivery or nanoparticle synthesis for drug delivery.21,22 The revealed amino groups can initiate self-degradation by reacting with the ester linkages along the polymer backbone. Herein we describe the synthesis and stability of this polymer in addition to its activity against HT29 human colon cancer cells and DU-145 prostate cancer cells.
EXPERIMENTAL
Materials
All chemicals were used as is. Curcumin, dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), and trifluoroacetic acid (TFA) were purchased from Acros Organics. N,N′-di-Boc-L-cystine ((Boc-Cys-OH)2) was purchased from Sigma Aldrich. Dextran (40 KDa) was obtained from TCI America. For NMR measurements, a Bruker 300 Ultrashield instrument was used. Gel Permeation Chromatography (GPC) results were obtained at the New Jersey Center for Biomaterials. Two PLgel columns 103 and 105 Å were used in series (Polymer Laboratories, Amherst, MA). Dimethylformamide with 0.1% TFA at a flow rate of 0.8 mL/min was used as the mobile phase. Calibration was obtained using polystyrene standards (Waters Corporation, Milford, MA) of molecular weights 523000, 204500, 96000, 30230 and 7200 g/mol.
Boc-Cys-Cur Polymer Synthesis
The dipeptide (Boc-Cys-OH)2 (0.9 mmol) was dissolved in 50 mL tetrahydrofuran along with DCC (2 mmol) and NHS (2 mmol). The solution was stirred for 1 h and the formation of dicyclohexyl urea (DCU) observed. Curcumin (0.9 mmol) was then added and the solution stirred for 24 h. The mixture was then filtered to remove DCU and concentrated to approximated 10 mL under reduced pressure. A precipitate was obtained by adding diethyl ether. The precipitate was collected filtration and washed with diethyl ether and water. This resulted in 550 mg of a foamy yellow orange solid. 1H NMR (300 MHz, CDCl3) δ (ppm) = 7.6 (d, 1H), 7.1 (dd, 1H), 7.0 (d, 1H), 6.9 (d, 1H), 6.5 (d, 1H), 5.8 (s, 1H), 5.4 (d, 1H), 4.5 (m, 1H), 3.9 (s, 3H), 3.1 (d, 2H), 1.4 (s, 9H).
Cys-Cur Polymer Synthesis
Boc-Cys-Cur
Polymer (550 mg) was dissolved in 5 mL of CH2Cl2 and cooled in an ice bath. The solution immediately became dark red when 5 mL of TFA was added slowly. The mixture was stirred for 2 h and then precipitated by adding diethyl ether. The precipitate was collected by filtration and washed with ethyl acetate. The product (266 mg) was collected as a hygroscopic yellow solid. 1H NMR (300 MHz, acetone-d6) δ (ppm) = 7.6 (1H), 7.5 (1H), 7.3 (1H), 7.2 (1H), 6.9 (1H), 6.7 (1H), 6.0 (1H), 4.3 (3H), 3.9 (3H), 3.6 (2H), 3.3 (1H).
Thermal analysis
The thermal stability of the Boc-Cys-Cur and Cys-Cur was investigated using thermogravimetric analysis (TG) coupled with differential thermal analysis (DTA, S2 EXSTAR6000, TG/DTA6200, Seiko, Japan), and differential scanning calorimetry (DSC, TA-Q-20 New Castle, DE). The TG-DTA measurements were carried out in platinum crucibles under flowing helium (100mL/min) or air (100mL/min) at a heating rate of 5°/min in the temperature range of 50 to 600°C. The DSC analysis was performed in aluminum crucibles under nitrogen flow (50mL/min) using a heating rate of 5°C/min in the temperature range of −50 to 400°C.
Degradation Study
Degradation studies were performed on a Prominence HPLC system (Shimadzu Corporation, Japan) with a TSKgel α-M column (Tosoh Bioscience, Japan). Dimethylformamide at a flow rate of 0.5 mL/min was used as the mobile phase. The polymer Cys-Cur (2.5 mg) was dissolved in a dimethyl sulfoxide and 100 μL of this solution added to 1 mL of pH 7.4 Tris Buffer. The degradation of the buffered solution then followed over the period of 24 h.
Cell cultures
HT29 (p53positive) human colon cancer cells and DU-145 prostate cancer cells were purchased from the American Type Culture Collection (ATCC), maintained per ATCC protocols, and utilized within 6 months of thawing each vial. All cell lines were cultured in humidified atmosphere at 37°C with 5% CO2, and media (DU145 cells) were replaced every alternate day.
Growth inhibitory activity by MTT assay
The MTT assay was used to determine the sensitivity of HT29 and DU-145 cells to the agents. Cells were seeded at 1 × 103 cells/well in 96-well plates and allowed to attach for 24 h. The medium was replaced with fresh medium containing dimethyl sulfoxide (control) or compound. The cells were treated for 96 h. Cell growth and viable cell numbers were monitored by 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) staining as previously described.23 Micromolar concentrations were calculated using the molar mass of the polymer repeating unit (Boc-Cys-Cur MW 788 g/mol and Cys-Cur MW 816 g/mol).
Western blot analysis
Protein extracts were prepared with RIPA buffer containing a mixture of protease inhibitors as described.23 Briefly, 50 μg of protein was applied to a 12% SDS/PAGE and transferred to nitrocellulose membranes. Membranes were incubated with Odyssey blocking buffer (Li-Cor) prior to incubation with polyclonal or monoclonal antibodies to phospho-JNK, p53, caspase3, or β-actin overnight at 4°C. Goat anti-rabbit IgG (HþL) 800 CW and/or goat anti-mouse (HþL) was applied for 60 minutes at room temperature (1:25,000, LI-COR) prior to washing with 1X Phosphate Buffered Saline Tween-20 (PBS-T). Visualization and quantification were carried out with the LI-COR OdysseyH scanner and software (LI-COR Biosciences).
RESULTS AND DISCUSSION
Polymer Synthesis
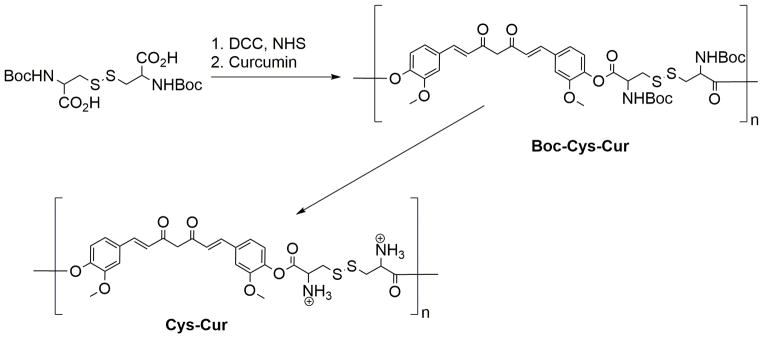
Described herein is the development of a degradable polyamine based on curcumin, a polyphenol cancer therapeutic and wound healing agent. The strategy for the delivery of curcumin from the polymer was to incorporate cleavable ester and disulfide bonds in the polymer chain. Ester bonds can be cleaved by hydrolysis or self-degradation if amines are present into the polymer. Disulfide bonds are often utilized as a drug delivery mechanism for polymers because they are redox reactive and cleaved to form thiols in the reducing environment of cells. Curcumin was incorporated into a linear polymer by condensation with Boc-protected cystine via carbodiimide coupling. Initial coupling was attempted with N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride and 4-dimethylaminopyridine. However the NMR showed the presence of a resilient impurity which was deduced to be the result of side reactions of the O-acyl isourea intermediate.24 This was presumed to occur because of the poor nucleophilicity of curcumin’s phenols. This problem was circumvented by converting the carboxylic acids of (Boc-Cys-OH)2 to reactive esters by the in situ carbodiimide coupling with NHS, followed by condensation with curcumin. This produced polymer Boc-Cys-Cur which showed solubility in a wide range of organic solvents and was insoluble in water. The Boc protected polymer Boc-Cys-Cur was characterized by GPC and the Mn and Mw were found to be 70,401 and 86,233 g/mol respectively with a polydispersity of 1.2 when the condensation polymerization is run for 24 hours. If the polymerization is increased to 48 hours, the polydispersity increases to 3.2 with the Mn and Mw increasing to 287,000 and 933,00 g/mol. This increase in molecular weight with reaction time agrees with the kinetics of a step-growth polymerization where high molecular weight is achieved near the end of the polymerization.25 The Boc protecting groups were removed with a 50% solution of trifluoroacetic acid and CH2Cl2 to produce the cationic Cys-Cur polymer which was soluble in water. The reaction time was limited to 2 h, as prolonged times saw significant degradation of the product when characterized by NMR (Figure 2a). Complete removal of the Boc protecting groups was not achieved as with increased deprotection reaction time came the drawback of degradation of the product. At reaction times greater than 2 h there was improved removal of the Boc group but this increased hard to remove impurities and the eventual degradation of the material to unrecognizable byproducts.
Figure 2.
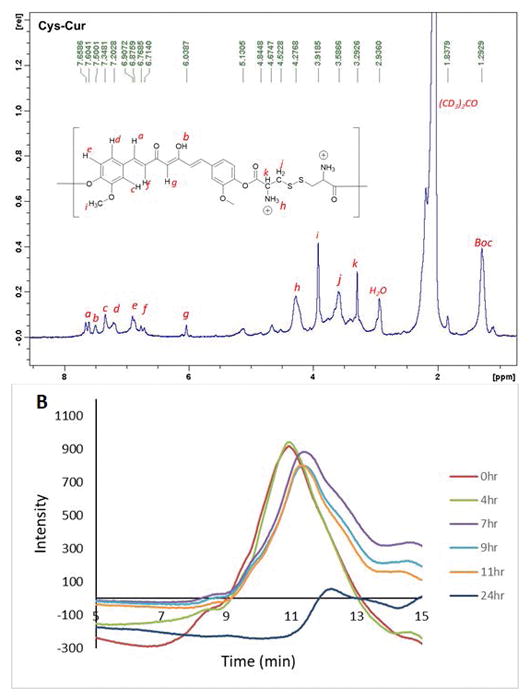
a) 1H NMR of Cys-Cur and b) degradation of Cys-Cur in pH 7.4 Tris buffer followed by GPC.
Degradation Studies
The polymer Cys-Cur has primary amines within the polymer chain that are proposed to enable self-degradation of the polymer backbone by cleavage of the esters. To examine this, Cys-Cur was suspended in pH 7.4 Tris buffer to simulate physiological conditions and monitored by GPC (Figure 2b). Little change was observed after the first 4 hours of incubation in buffer. At 7 hours a significant increase in the polymer’s elution time was observed. This increase indicates the polymer size has decreased, confirming that self-degradation is occurring. At 24 h of incubation in buffer the polymer peak has almost completely disappeared and it can be concluded that the polymer has been completely degraded.
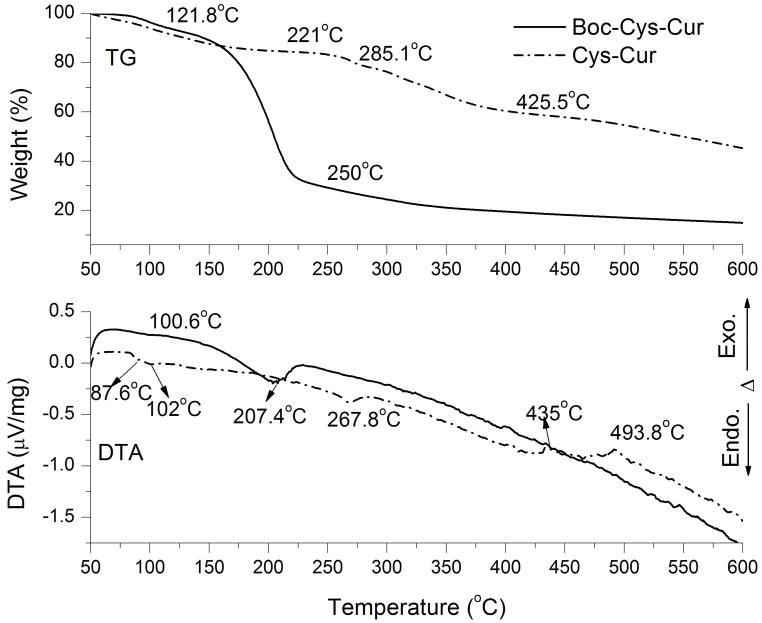
Figure 3 displays the TG and DTA curves of the Boc-Cys-Cur and Cys-Cur samples recorded in the inert atmosphere of helium. The TG curve of Boc-Cys-Cur sample shows two steps of decomposition. The first step is between 83 and 121.8°C and is accompanied on the DTA curve by an endothermic effect with a minimum at ~100.6°C which can be assigned to evaporation of traces of water. In the second step of decomposition, between 145.1 and 250°C, ~60% of the weight of the sample is lost. This second step presents a secondary endothermal effect with a minimum at 207.4°C on the DTA curve. This step can be associated with breaking of a part of the Boc-Cys-Cur organic polymer and elimination of a volatile component with high molecular mass. This is likely removal of the Boc protecting group which has been shown to be thermolytically labile which could be removed with heating to 185°C.26 In contrast, the TG curve of the Cys-Cur shows that this compound starts to lose mass immediately as the heating starts at 50°C. This first step of decomposition is located between 50 and 195°C and is responsible of 15% of the weight loss of the Cys-Cur. On the DTA curve this first step of decomposition displays two endothermic effects at 87.6°C and 102°C. The second step of decomposition for Cys-Cur is positioned between 221 and 285.1°C. This presents and endothermic effect at 267.8°C on the DTA curve which can be assigned to a partial pyrolysis of the Cys-Cur molecule. The third step of decomposition occurs between 285.1 and 425.5°C. Finally, the forth step of decomposition can be identified between 425.5 and 600°C on the TG curve and is associated with two small exothermic effects at 435°C and at 493.8°C, respectively.
Figure 3.
The TG-DTA analysis of Boc-Cys-Cur and Cys-Cur, recorded under He atmosphere.
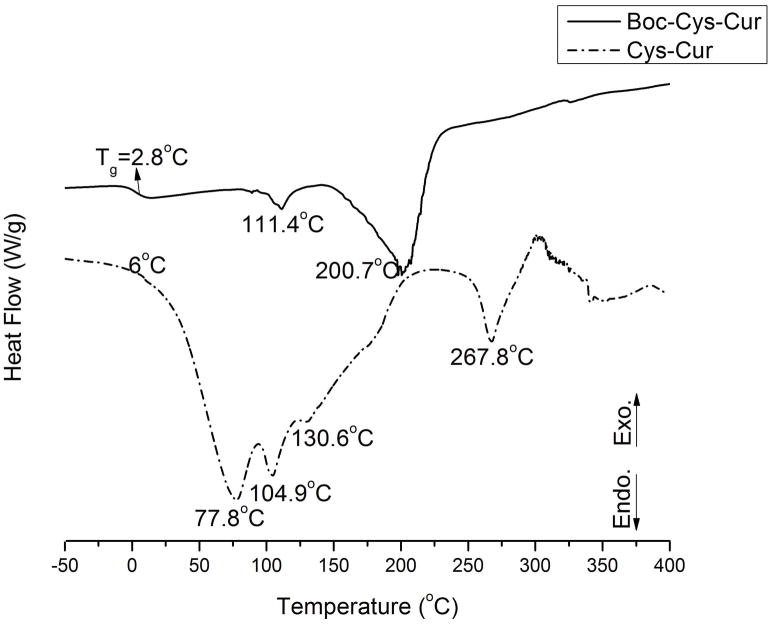
Figure 4 presents the DSC curves of the Boc-Cys-Cur and Cys-Cur. For the Boc-Cys-Cur a glass transition was identified at 2.8°C. The glass transition is generally considered as the interval of transition between glassy and liquid states. This can be also associated to some macroscopic properties of the polymers, such as viscosity and/or mechanical properties. The DSC curves present two endothermic effect at 111.4°C and 200.7°C. Those two effects are in good agreement with those identified on the DTA curve. These effects can be associated with water elimination and pyrolysis and/or breaking chemical bonds inside of the Boc-Cys-Cur polymer. On the other hand, Cys-Cur does not show any glass transitions. This starts to decompose at ~6°C. This is presumed to be the self-degradation of the polymer by reaction of the cystine amines with the ester linkages in the polymer backbone. The DSC analysis, which is more sensitive than DTA, shows that main decomposition is taking place at very low temperatures. There were identified three endothermic effects at 77.8°C, 104.9°C and 130.6°C which are assigned to the decomposition of Cys-Cur polymer. Another endothermic effect was identified at 267.8°C which can be linked with the endothermic effect identified at 226.8°C on the DTA curve.
Figure 4.
The DSC curves of Boc-Cys-Cur and Cys-Cur.
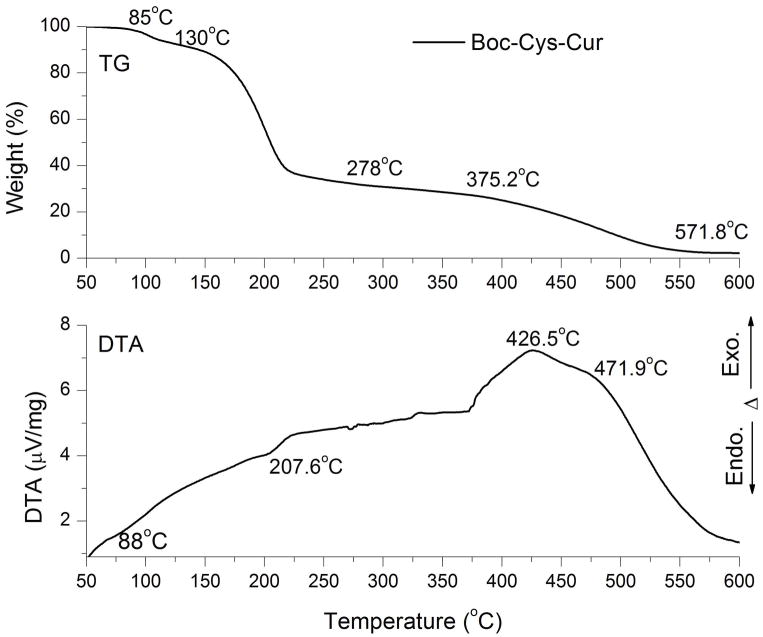
Based on the TG-DTA and DSC analyses it was identified that the Boc-Cys-Cur present a higher thermal stability than the Cys-Cur which starts to degrade at ~6°C in the inert atmosphere. The thermal stability in air of the more stable polymer Boc-Cys-Cur was monitored also. The TG-DTA curves recorded in air for the Boc-Cys-Cur are presented in Figure 5. The TG curve presents 3 steps of decomposition. The first step of decomposition is between 85 and 130°C and shows an endothermic minimum at 88°C on the DTA curve and was assigned to the solvent elimination. The second step is almost identical to the second step of the decomposition in the inert atmosphere. This step occurs between 130°C and 278°C and the weight loss ~60%. This step presents an endothermic effect at 207.6°C. It can be concluded that in both cases this is due to thermolysis of the Boc group. The third step of decomposition is taking place between 375.2°C and 571.2°C and is responsible of ~ 26.5% of the weight loss. This step displays two exothermic effects on the DTA curve with the maxima at 426.5°C and 471.9°C, respectively. These exothermic effects can be assigned to a stepwise combustion into steps of the rest of organic polymer. Our conclusion from the thermal analysis is that Boc-Cys-Cur is stable at ambient temperatures while Cys-Cur self-degrades at temperatures as low as 6 °C and thus will self-degrade when deployed as a therapeutic.
Figure 5.
The TG-DTA analysis of Boc-Cys-Cur recorded in air.
Cancer Study
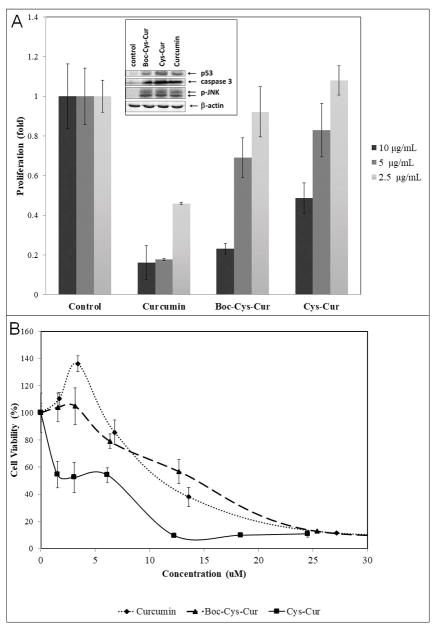
Both Boc-Cys-Cur and Cys-Cur showed growth inhibitory activity against HT29 human colon cancer cells and DU-145 prostate cancer cells. DU-145 cells were treated with 10, 5 and 2.5 μg/mL of the polymers and curcumin. At a dose of 10 μg/mL Boc-Cys-Cur activity is on par with that of curcumin, while the self-degrading polymer Cys-Cur is approximately half as effective at inhibiting cell proliferation. At a dose of 10 μg/mL curcumin showed a decrease to 0.16 fold DU-145 cell proliferation and Boc-Cys-Cur showed a comparable 0.23 fold decrease. Cys-Cur however performed significantly less with a decrease to 0.48 fold. At less concentrated doses the polymers become considerably less effective than curcumin. Examination of the mechanisms of cell death shows that the polymers induced apoptosis similarly to curcumin by activating p53, caspase 3 and JNK. The anti-cancer activity of the polymers is reversed against HT29 human colon cancer cells. The IC50 values (the concentration that caused 50% inhibition of cell proliferation) were approximately: Cys-Cur: (MW: 816) 7 μM; Boc-Cys-Cur: (MW: 788) 14 μM; curcumin: (MW: 368) 11 μM (Figure 3c). Thus when calculated per equivalent of curcumin the self-degrading polymer Cys-Cur was more active than its precursor Boc-Cys-Cur and curcumin. These results showed that incorporation of curcumin into a polymeric form can improve or diminish its effect against cancer cells depending on the cell line targeted.
CONCLUSIONS
Self-degradable polymers of curcumin were demonstrated through the condensation polymerization of curcumin with boc protected cystine. Deprotection of the amine made the polymer self-degrading with a shelf life of less than a day in physiological conditions. Self-degradability of Cys-Cur was confirmed by thermal analysis. Polymers Boc-Cys-Cur and Cys-Cur demonstrated activity against HT29 human colon cancer cells and DU-145 prostate cancer cells with the self-degradable polymer demonstrating higher activity than curcumin against colon cancer cells. This positively charged polymer has the promise of being a source material for developing anti-cancer nanoparticles by conjugation with anionic polymers or materials via layer-by-layer electrodeposition.
Figure 1.
Synthesis of self-degrading polymeric Curcumin.
Figure 6.
Cytoxicity of Boc-Cys-Cur, Cys-Cur and curcumin towards a) DU-145 prostate cancer cells (inset: Western blot analysis of mechanism of cell death) and b) HT29 colon cancer cells.
Acknowledgments
This work was possible due to grants from the Professional Staff Congress CUNY, National Institute of Health (5SC3GM111194).
References
- 1.Gupta SC, Kismali G, Aggarwal BB. BioFactors. 2013;39:2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 2.Dulbecco P, Savarino V. World J Gastroenterol. 2013;19:9256–9270. doi: 10.3748/wjg.v19.i48.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen TA, Friedman AJ. J Drugs Dermatol. 2013;12:1131–1137. [PubMed] [Google Scholar]
- 4.Gunes H, Gulen D, Mutlu R, Gumus A, Tas T, Eren Topkaya A. Toxicol Ind Health. 2013 doi: 10.1177/0748233713498458. [DOI] [PubMed] [Google Scholar]
- 5.Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K. Biomed Res Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravindran J, Prasad S, Aggarwal BB. AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopal PK, Paul M, Paul S. Asian Pac J Cancer Prev. 2014;15:93–100. doi: 10.7314/apjcp.2014.15.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Jee SH, Shen SC, Tseng CR, Chiu HC, Kuo ML. J Invest Dermatol. 1998;111:656–661. doi: 10.1046/j.1523-1747.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- 9.Lu HF, Yang JS, Lai KC, Hsu SC, Hsueh SC, Chen YL, Chiang JH, Lu CC, Lo C, Yang MD, Chung JG. Neurochem Res. 2009;34:1491–1497. doi: 10.1007/s11064-009-9936-5. [DOI] [PubMed] [Google Scholar]
- 10.Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, Min DS, Chang JS, Jeong YJ, Lee YH, Park JW, Kwon TK. Carcinogenesis. 2003;24:1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 11.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 12.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 13.Sahu A, Kasoju N, Bora U. Biomacromolecules. 2008;9:2905–2912. doi: 10.1021/bm800683f. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Braiteh FS, Kurzrock R. Cancer. 2005;104:1322–1331. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 15.Bisht S, Mizuma M, Feldmann G, Ottenhof NA, Hong SM, Pramanik D, Chenna V, Karikari C, Sharma R, Goggins MG, Rudek MA, Ravi R, Maitra A. Mol Cancer Ther. 2010;9:2255–2264. doi: 10.1158/1535-7163.MCT-10-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB. Biochem Pharmacol. 2010;79:330–338. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Dubey SK, Sharma AK, Narain U, Misra K, Pati U. Eur J Med Chem. 2008;43:1837–1846. doi: 10.1016/j.ejmech.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Narain U, Tripathi S, Misra K. Bioconjugate Chem. 2001;12:464–469. doi: 10.1021/bc0000482. [DOI] [PubMed] [Google Scholar]
- 19.Singh RK, Rai D, Yadav D, Bhargava A, Balzarini J, De Clercq E. Eur J Med Chem. 2010;45:1078–1086. doi: 10.1016/j.ejmech.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang H, Murphy CJ, Zhang B, Shen Y, Van Kirk EA, Murdoch WJ, Radosz M. Biomaterials. 2010;31:7139–7149. doi: 10.1016/j.biomaterials.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 21.De Smedt SC, Demeester J, Hennink WE. Pharm Res. 2000;17:113–126. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- 22.Ramos J, Forcada J, Hidalgo-Alvarez R. Chem Rev. 2014;114:367–428. doi: 10.1021/cr3002643. [DOI] [PubMed] [Google Scholar]
- 23.Persaud L, Zhong X, Alvarado G, Do W, Dejoie J, Zybtseva A, Aktas BH, Sauane M. Mol Cancer Res. 2017;15:1117–1124. doi: 10.1158/1541-7786.MCR-16-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeTar DF, Silverstein R. J Am Chem Soc. 1966;88:1013–1019. [Google Scholar]
- 25.Odian G. Principles of Polymerization. John Wiley& Sons Inc; 1993. pp. 6–9. [Google Scholar]
- 26.Rawal VH, Cava MP. Tet Lett. 1985;26:6141–6142. [Google Scholar]