Abstract
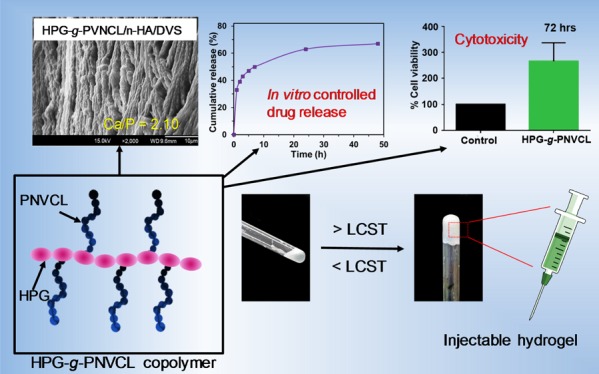
In this study, an injectable thermoresponsive hydroxypropyl guar-graft-poly(N-vinylcaprolactam) (HPG-g-PNVCL) copolymer was synthesized by graft polymerization. The reaction parameters such as temperature, time, monomer, and initiator concentrations were varied. In addition, the HPG-g-PNVCL copolymer was modified with nano-hydroxyapatite (n-HA) by in situ covalent cross-linking using divinyl sulfone (DVS) cross-linker to obtain HPG-g-PNVCL/n-HA/DVS composite material. Grafted copolymer and composite materials were characterized using Fourier transform infrared spectroscopy, thermogravimetric analysis, proton nuclear magnetic resonance spectroscopy (1H NMR), and differential scanning calorimetry. The morphology of the grafted copolymer (HPG-g-PNVCL) and the composite (HPG-g-PNVCL/n-HA/DVS) was examined using scanning electron microscopy (SEM), which showed interconnected porous honeycomb-like structures. Using Ultraviolet−visible spectroscopy, low critical solution temperature for HPG-g-PNVCL was observed at 34 °C, which is close to the rheology gel point at 33.5 °C. The thermoreversibility of HPG-g-PNVCL was proved by rheological analysis. The HPG-g-PNVCL hydrogel was employed for slow release of the drug molecule. Ciprofloxacin, a commonly known antibiotic, was used for sustainable release from the HPG-g-PNVCL hydrogel as a function of time at 37 °C because of viscous nature and thermogelation of the copolymer. In vitro cytotoxicity study reveals that the HPG-g-PNVCL thermogelling polymer works as a biocompatible scaffold for osteoblastic cell growth. Additionally, in vitro biomineralization study of HPG-g-PNVCL/n-HA/DVS was conducted using a simulated body fluid, and apatite-like structure formation was observed by SEM.
1. Introduction
Injectable polymer hydrogels, with their similar water content to tissue, are a class of polymeric materials whose excellent shape flexibility can mold them to defects of any size and shape via a minimally invasive procedure.1,2 As a result, they have been widely explored for potential use in the field of medicine.3−5 In this context, there has been a growing interest among researchers in polymeric injectable hydrogel systems as candidates for developing suitable artificial extracellular matrices (ECMs), for example, in bone tissue regeneration.6,7 In such scenarios, researchers have been focusing on developing a spectrum of biomaterials for tissue engineering,8,9 including naturally occurring materials to synthetically derived materials.10 One more area of interest in the similar direction is developing a method for controlled drug delivery.11−13 Poor bioavailability of conventional drug administration leads to lower patient compliance, which results in severe side effects and even toxicity.14,15 In such cases, controlled drug delivery systems are providing hope over a conventional dosage form. The most interesting feature of this drug delivery system is to deliver the drugs at the desirable rate, time, and specific sites to achieve the therapeutic objectives.16,17 Additionally, hydrogel materials are known to serve as a site for localized drug delivery over a period of time for a wide range of drugs.18
Generally speaking, there are several injectable hydrogel systems that have been developed using an aqueous solution of stimuli-responsive polymers that gel in situ in response to pH, electric field, temperature, and other environmental changes.19−23 In particular, temperature-responsive polymers are widely employed in the preparation of injectable hydrogels for apatite formation and drug delivery.24,25
Among various thermoresponsive polymers, poly(N-isopropylacrylamide) (PNIPAM), a synthetic polymer, has been widely studied as an injectable hydrogel for bone tissue regeneration and drug delivery applications.26 PNIPAM’s lower critical solution temperature (LCST) is low, with sol-to-gel transition occurring at 32 °C.27 However, application of PNIPAM is limited to injectable ECM development and drug delivery because of its neurotoxicity28 and impairment of fibrinogen activity.29 However, an injectable thermoresponsive hydrogel fabricated using minimally toxic biodegradable materials, such as poly(N-vinylcaprolactam) (PNVCL), would be the possible solution to overcome the aforementioned problems. PNVCL has a phase transition temperature of 33 °C, which is comparable to that of PNIPAM.30 PNVCL has excellent biocompatibility31 and low cytotoxicity,29 but it lacks bioactivity and may cause inflammation and an immune response.32 Fortunately, these problems can be solved by combining synthetic and natural polymers using methods such as graft polymerization.33 In this context, guar gum (GG) or its derivatives, hydroxypropyl guar (HPG), which are naturally originated, biodegradable, and low-cost polymers, can be highly useful to design degradable and biocompatible injectable thermoresponsive polymers.34−36 Because GG and HPG contain several hydroxyl groups, such polymers can be grafted chemically with PNVCL for making thermoresponsive injectable hydrogels.37
Previous studies have shown that the thermoresoponsive hydrogel can be a substituent scaffold material to repair and replace bone.13,38,39 Human bone is mainly composed of hydroxyapatite (HA) and collagen, which render both toughness and high strength to bone. HA, which is a major inorganic component of bone tissue, can be introduced with a hybrid hydrogel as a scaffold to improve the mechanical property of bone in tissue engineering.40 For example, Wang et al. synthesized a novel triblock injectable and thermosensitive hydrogel composite constituting nano-HA (n-HA) as one of the components. The hydrogel has an interconnected porous structure and exhibited very good thermosensitivity.38 Thus, n-HA is an excellent substituent material for enhancing the bioactivity and mechanical properties of hydrogel scaffolds, making it more suitable for bone tissue engineering.
As mentioned before, sustainable drug delivery using an injectable hydrogel is an important area of research. Ciprofloxacin is one of most efficient antimicrobial drugs used to treat and prevent the infections caused by bacteria, including skin, eye, nose, and ear infections.41 Usually, restoration of tissues at the wound area susceptible to bacterial infection can be avoided by antimicrobial drugs loaded on injectable hydrogels.42−45 The injectable hydrogel loaded with drugs at the specific injected site in the body slowly releases the loaded drugs through its three-dimensional structure for a long time. Additionally, such a hydrogel can also help to increase the poor solubility of drugs in aqueous solution and minimize the side effect associated with it. From these points of view, injectable hydrogels based on external stimuli are of growing interest in the formulation of drug delivery systems in recent years.11,12
Herein, we report the synthesis of a graft copolymer (HPG-g-PNVCL), which acts as an injectable thermoresponsive hydrogel (Figure 1). The graft polymer was characterized using scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, thermogravimetric analysis (TGA), proton nuclear magnetic resonance spectroscopy (1H NMR), ultraviolet–visible (UV–vis) spectroscopy, and differential scanning calorimetry (DSC). The copolymer (HPG-g-PNVCL) was found to be soluble in water, and the viscous solution had an LCST of 34 °C. The thermoreversibility of the grafted copolymer hydrogel was determined using rheology. The temperature-dependent rheology study showed that the gelling point of the grafted copolymer was 33.5 °C. The graft copolymer showed excellent thermogelling and injectable properties suitable for in vitro osteoblast differentiation and controlled drug delivery of ciprofloxacin. To the best of our knowledge, this is the first example of HPG modified with the PNVCL polymer, which shows thermosensitive and injectable hydrogel properties for various applications. Composite samples of a slightly chemically cross-linked HPG-g-PNVCL and n-HA were also obtained and studied. An in vitro biomineralization study was conducted using a simulated body fluid (SBF), and apatite-like structure formation was observed by SEM.
Figure 1.
Schematic representation of the synthesis of HPG-g-PNVCL using AIBN as the initiator.
2. Results and Discussion
2.1. Graft Copolymerization of HPG and NVCL
To study the effect of polymerization time, temperature, monomer, and initiator concentrations on the grafting yield, reaction conditions were optimized during the synthesis of the HPG-g-PNVCL graft copolymer. The effect of polymerization time on the grafting yield was studied by varying the time between 1 and 5 h. As shown in Figure 2a, the grafting yield steadily increased as the polymerization time reached 3 h. The propagation of the grafting chain increased with time because of the accessibility of more active reaction sites on HPG, leading to a higher grafting yield. However, after 3 h, a gradual decrease in grafting yield was observed. This was attributed to a decrease in the monomer and initiator concentration and mutual annihilation of growing chains.46
Figure 2.
Effect of (a) time, (b) temperature, (c) NVCL concentration, and (d) AIBN concentration on grafting yield.
The effect of temperature, ranging from 55 to 80 °C, on graft copolymerization was observed; the results are shown in Figure 2b. When the temperature increased from 55 to 65 °C, the grafting yield increased steadily, reaching its maximum of 140%. This can be attributed to the increased N-vinylcaprolactam (NVCL) monomer diffusion into the reaction site, accelerated decomposition of the initiator, and improvement in the propagation step. Increasing the temperature above 65 °C resulted in reduced grafting yield, which may be due to the enhanced homopolymerization or the termination process by the combination and disproportionation step and chain-transfer reactions. These results are supported by similar findings obtained previously.46
The grafting yield was also studied by changing the NVCL concentration (0.2–0.6 M) while keeping all other parameters constant. As shown in Figure 2c, the grafting yield increased gradually with the increase in monomer concentration up to 0.4 M, most likely because of the large availability of monomer molecules around the HPG macroradical that leads to the initiation and propagation of the grafting reaction. However, higher concentrations of NVCL caused a decrease in grafting yield because of the nonavailability of monomer molecules to attack the HPG macroradical, as the homopolymerization rate of NVCL increases with increased monomer concentration.46,47
Initiator concentration is another important parameter that affects the grafting yield. As shown in Figure 2d, when the azobisisobutyronitrile (AIBN) concentration increased up to 2.2 × 10–3 M by keeping other parameters constant, the grafting yield rapidly increased. Increased AIBN concentration likely facilitated more HPG macroradicals through the direct abstraction of a hydrogen atom from the hydroxyl propyl group of the HPG backbone. This generated more active sites for NVCL attachment, promoting the grafting process. However, upon further increase in the initiator concentration, the grafting yield decreased continuously, possibly because of the termination step with HPG macroradicals overriding the initiation step. This trend of decrease in grafting yield with increase in initiator concentration has also been observed in previous studies.46,47
2.2. Characterizations
Following reaction optimization, HPG-g-PNVCL was characterized using different spectroscopic techniques. Figure S1 (Supporting Information) shows the FTIR spectra of HPG, PNVCL, HPG-g-PNVCL, and HPG-g-PNVCL/n-HA/DVS. Divinyl sulfone (DVS) was used to chemically cross-link HPG-g-PNVCL. A very strong and broad absorption band observed at 3349 cm–1 (Figure S1a, Supporting Information) for HPG is due to O–H bond stretching frequency, whereas the sharp peak located at around 2880 cm–1 is attributed to C–H stretching frequency. A bending of CH2–O–CH2 units of HPG appears at 1014 cm–1 stretching frequency.48 Figure S1b (Supporting Information) shows two peaks at 1627 and 1483 cm–1 that correspond to carbonyl (C=O) bond stretching and amide (C–N) stretching present in PNVCL, respectively.49 In the HPG-g-PNVCL spectrum (Figure S1c, Supporting Information), the increased intensity of O–H bond stretching at 3617 cm–1 was observed and it proves the grafting of PNVCL on HPG. Furthermore, peaks arising due to C=O stretching vibration at 1627 cm–1 and C–N bending vibration at 1474 cm–1 illustrate the successful grafting of PNVCL on HPG.49 Compared to HPG-g-PNVCL, the FTIR spectrum of HPG-g-PNVCL/n-HA/DVS (Figure S1e, Supporting Information) shows a slight decrease in the intensity of O–H bond stretching frequency. Additionally, the appearance of phosphate (PO43–) asymmetric bond stretching at 1024 cm–1 arising from n-HA (Figure S1d, Supporting Information) and the presence of S=O bond stretching frequency at 1130 cm–1 indicate the successful cross-linking of the hydroxyl groups of HPG by DVS in HPG-g-PNVCL/n-HA/DVS.50
Further confirmation on the structural features of HPG-g-PNVCL was obtained by proton NMR (1H NMR) spectroscopy. Figure S2 (Supporting Information) shows the 1H NMR spectra of HPG and HPG-g-PNVCL. 1H NMR of HPG shows a broad peak in the range of 3.3–4.1 ppm (a, b, c, and d), which was assigned to all protons present in the guar carbohydrate structures.50 Additionally, similar peaks are also visible in HPG-g-PNVCL in the same region, indicating the covalent grafting of HPG with PNVCL. In the HPG-g-PNVCL NMR spectrum, methylene (CH2) groups of the caprolactam ring in the vicinity of N and C=O groups are assigned to 3.4 ppm (a) and 2.5 ppm (b), respectively. Similar peak positions for CH2 groups adjacent to N and C=O groups were observed in the PNVCL homopolymer as reported in the previous literature.51 Therefore, grafting of PNVCL on the HPG polymer backbone is also confirmed by 1H NMR.
TGA is a crucial method to characterize and verify the thermal stability of different materials. TGA results obtained at the scan rate of 10 °C/min in N2 atmosphere for HPG, HPG-g-PNVCL, and HPG-g-PNVCL/n-HA/DVS are presented in Figure S3 (Supporting Information). HPG showed two different temperature zones of weight loss. HPG began losing water at 40 °C and continued up to 102 °C. The maximum weight loss occurred in the second zone, representing the degradation of the HPG backbone, which started at 240 °C and continued up to 358 °C as revealed by the analysis.52 As shown in Figure S3 (Supporting Information), HPG-g-PNVCL showed a slightly different pattern compared to HPG. The initial weight loss profile in the region of 40–110 °C is essentially due to the removal of water. Furthermore, the thermal degradation profile of HPG-g-PNVCL showed the weight loss from 216 to 333 °C, which is indicative of the depolymerization of the HPG polymer chain, and it is slightly shifted compared to pure HPG. In addition to two degradation zones as shown in HPG, a third degradation zone which appeared in the region of 375–541 °C with 4.7 wt % weight loss, resulting from the incorporation of PNVCL chains into the HPG backbone.52 However, the absence of significant weight loss in HPG-g-PNVCL/n-HA/DVS (with 50 wt % residues) above 400 °C compared to HPG and HPG-g-PNVCL is attributed to the increase in thermal stability, additional cross-linking, and good interaction of HPG-g-PNVCL polymers with thermally stable n-HA.53,54
Figure S4 (Supporting Information) indicates DSC results for HPG, HPG-g-PNVCL, and HPG-g-PNVCL/n-HA/DVS scaffolds. DSC was performed in the temperature range of 25–600 °C with a temperature ramp of 10 °C/min under N2 atmosphere. The HPG thermogram showed two major endothermic peaks at 93 and 295 °C, respectively. The first endothermic peak at 93 °C is attributed to the removal of moisture, whereas the second endothermic peak observed at 295 °C is due to the thermal decomposition of main-chain HPG polymers.52 In contrast to HPG, HPG-g-PNVCL exhibited an additional endothermic melting peak at 439 °C, which may be attributed to the grafted PNVCL chain on the HPG backbone.51 In HPG-g-PNVCL/n-HA/DVS, there was no significant change in the thermogram observed as compared to HPG-g-PNVCL.
SEM images were obtained to characterize the microstructural morphologies of the lyophilized materials. The cross-sectional SEM images of lyophilized HPG-g-PNVCL and HPG-g-PNVCL/n-HA/DVS hydrogels are presented in Figure 3. As shown in Figure 3a,b, after lyophilization, the HPG-g-PNVCL hydrogel showed a continuous porous honeycomb-like structure because of freezing of the hydrogel, which creates ice crystals in the inner morphology of the sample and subsequent vacuum removal of ice crystals from the hydrogel structure produces porous morphology.55 SEM imaging of the HPG-g-PNVCL/n-HA/DVS hydrogel (Figure 3c,d), on the other hand, showed a more planar-like morphology with relatively less porous structures because of the cross-linking with DVS and the further addition of n-HA resulted in the thicker and denser pore walls.56 Additionally, a good dispersion of n-HA (shown by red arrows) in the cross-linked HPG-g-PNVCL/n-HA/DVS was observed.
Figure 3.
SEM images of HPG-g-PNVCL at (a) 200× and (b) 500× magnifications and HPG-g-PNVCL/n-HA/DVS at (c) 200× and (d) 500× magnifications.
2.3. LCST Measurement
The thermoresponsivity of aqueous solutions of 1 wt % HPG and HPG-g-PNVCL determined by the LCST measurement is given in Figure 4. Figure 4a shows the transmittance response of the hydrogels measured at 470 nm over the temperature range of 20–50 °C. Initially, HPG did not show any phase transition in the given temperature range (especially near body temperature). However, HPG-g-PNVCL showed an optical transmittance of 44% when the temperature was below 34 °C and had almost zero transmittance above this temperature. Thus, the LCST was found to be 34 °C because of the presence of thermosensitive PNVCL graft chains.51Figure 4b shows excellent flowability of the HPG-g-PNVCL hydrogel at room temperature with no gel formation and a relatively transparent appearance of the hydrogel. When the hydrogel was exposed to a temperature of 37 °C (near body temperature), it became a gel (<15 min), as shown in Figure 4c. Furthermore, an injectability test (3 wt % in distilled water) was conducted, and the gel showed good injectability in the solution at 37 °C (Figure 4d–g).
Figure 4.
(a) Phase transition diagram of HPG and HPG-g-PNVCL hydrogels [1 wt %, phosphate-buffered saline (PBS) buffer pH 7.4]. Gelling behavior of HPG-g-PNVCL in PBS buffer pH 7.4 at (b) 25 and (c) 37 °C. (d–g) Injectability of the HPG-g-PNVCL hydrogel (3 wt %, PBS buffer pH 7.4) at 37 °C.
2.4. Rheology Study and Apatite Formation Ability of Scaffolds
An oscillatory shear rheometer was used to find the rheological properties of HPG and HPG-g-PNVCL at a heating rate of 1 °C/min over a 25–40 °C temperature range. Figure 5 shows the thermosensitive gelation behavior of HPG and HPG-g-PNVCL suspended in distilled water at 1% (w/v). In the case of HPG, when the temperature is increased from 25 to 34 °C, the value of the storage modulus (G′) and loss modulus (G″) increased, and no crossover between them was observed at around 40 °C (Figure 5a), indicating that they maintained a viscous liquid-like behavior until 40 °C. In contrast, in the HPG-g-PNVCL sample, as the temperature increased, a crossover between G′ and G″ was observed at a critical point, the gel point (Tgel), where tan δ (δ = G″/G′) = 1 and the sol–gel transition occurs.53 An elastic solid-like behavior was observed beyond this gel point.56 Thus, the gelation temperature (Tgel) of HPG-g-PNVCL was found to be 33.5 °C (Figure 5b), which corresponds well with the LCST results obtained from the phase transition diagram (Figure 4a). From these results, it can be concluded that HPG-g-PNVCL is thermoresponsive around the body temperature.
Figure 5.
Rheological properties of G′ and G″ for 1 wt % aqueous suspension of (a) HPG and (b) HPG-g-PNVCL.
The thermoreversibility of HPG-g-PNVCL was further evaluated by a second temperature sweep of G′ and G″ after the sample was cooled down to the initial temperature (Figure S5, Supporting Information). Interestingly, the two graphs are almost overlapped, indicating that the sol–gel transition was reversible. A slight reduction in sample diameter was observed, most likely because of water loss after the initial heating cycle.
We have used the thermoresponsive hydrogel for in vitro slow release of a pharmaceutically relevant drug molecule. Figure 6 shows the cumulative percentage of ciprofloxacin released from the HPG-g-PNVCL hydrogel as a function of time at 37 °C. The release studies of ciprofloxacin were examined by UV–vis spectroscopy of the solution taken at predetermined time intervals. Ciprofloxacin release from 3 wt % HPG-g-PNVCL, 4 wt % HPG-g-PNVCL, and 5 wt % HPG-g-PNVCL hydrogels showed a biphasic manner with initial burst release, followed by controlled release pattern of sustained release. In vitro drug release profiles of different concentrations of HPG-g-PNVCL are shown in Figure 6. HPG-g-PNVCL (3 wt %) exhibits higher release than 5 wt % HPG-g-PNVCL, whereas 4 wt % HPG-g-PNVCL exhibits an intermediate release pattern, indicating the dependence of drug release on the concentration of HPG-g-PNVCL. For instance, the release is slower for formulation containing a high concentration of HPG-g-PNVCL. This may be due to the presence of higher grafted polymers, which undergo chain entanglements among themselves, leading to the entrapment of drug molecules tightly and releasing in a controlled manner.
Figure 6.

In vitro drug release profile of ciprofloxacin from the injectable HPG-g-PNVCL hydrogel with various concentrations (3–5 wt %). The study was performed at 37 °C.
To check the utility of HPG-g-PNVCL as a scaffold, we grew mouse osteoblastic MC3T3 cells on it for various time periods to determine precisely the extent to which cells grew on the HPG-g-PNVCL scaffolds. Figure 7 shows the results of cell growths on the HPG-g-PNVCL scaffold obtained for the time period of 24 (a), 48 (b), and 72 h (c), respectively. In these studies, a control and HPG-g-PNVCL scaffolds were tested. After seeding of cells, most cells in the HPG-g-PNVCL hydrogel were found to be viable. As revealed in Figure 7, MC3T3 cells remained drastically viable compared to control in the HPG-g-PNVCL scaffold hydrogel for 24, 48, and 72 h after encapsulation. After 24 h of cell culture, there was a drastic increase in viable cell counts on HPG-g-PNVCL scaffolds (>5 folds) compared to the control. Figure 7c shows that viable cell counts on HPG-g-PNVCL continued to increase and reached to more than 5-fold in 3 days (72 h) compared to the control. These in vitro studies demonstrate that HPG-g-PNVCL would be a good choice as a scaffold material for bone tissue engineering. As discussed previously, highly biocompatible nature of HPG of the HPG-g-PNVCL scaffold is important for better cell growth and proliferation. These data prove the general applicability of the HPG-g-PNVCL hydrogel for culture and transplantation of viable cells in tissue engineering.
Figure 7.
In vitro cytotoxicity studies of the injectable thermoresponsive HPG-g-PNVCL hydrogels at (a) 24, (b) 48, and (c) 72 h. The data were compared against the control surface which is just the cell culture plate.
The in vitro biomineralization (apatite formation ability) of the HPG-g-PNVCL and HPG-g-PNVCL/n-HA/DVS biocomposite scaffolds was evaluated by immersing them in an SBF solution for 14 days. The results are shown in Figure 8. We have found that HPG-g-PNVCL shows a thermogel behavior near body temperature. However, the gel is not strong enough that can be used in an aqueous environment for long time. Therefore, we decided to use DVS, which can form additional chemical cross-linking furnishing a strong gel suitable for prolonged water exposure and utilization. Recently, several studies have shown that carbohydrate polymers which contain hydroxyl (−OH) or amine (−NH2) functionalities could be cross-linked by DVS. DVS reacts with −OH or −NH2 groups of the polymers via Michael addition either intra- or intermolecularly to form cross-linked gels.57 Therefore, HPG, which has plenty of −OH groups, can be easily cross-linked in a slightly alkaline water solution using DVS and such a cross-linking reaction can proceed even at 25 °C.58,59
Figure 8.
In vitro biomineralization of (a) HPG-g-PNVCL without n-HA, (b) HPG-g-PNVCL/n-HA/DVS on day 0, and HPG-g-PNVCL/n-HA/DVS after (c) 7 and (d) 14 days.
SBF solution is ionic in nature mimicking human blood plasma and it was prepared according to the previous experimental procedures.57 Additionally, after soaking in SBF for stipulated time, each sample was washed with distilled water and dried in an oven for further studies. Because of the absence of n-HA, the HPG-g-PNVCL scaffold did not exhibit apatite-like formation (Ca/P ratio = 0; Figure 8a). The initial Ca/P ratio in HPG-g-PNVCL/n-HA/DVS before SBF immersion (day 0) was 0.83 (Figure 8b), so the initial scaffold was a calcium-deficient apatite-like biocomposite, because in stoichiometric HA, the Ca/P ratio is 1.67.60 However, we found the deposition of apatite-like particles (and particle aggregates) on the HPG-g-PNVCL/n-HA/DVS scaffold surface after immersion in SBF for 7 and 14 days. These deposits were caused by the initiation of apatite nuclei on the scaffold surface, which produced crystals by absorbing more calcium (Ca2+) and phosphorous (PO43–) from the SBF, thus displaying bioactivity. After a 7 day period, the Ca/P ratio increased from 0.83 to 2.00—similar to the Ca/P ratio in stoichiometric HA (1.67) and the theoretical Ca/P ratio in human bone (1.75).60 After 14 days, formation of apatite on the surface was slightly greater. These results confirm that calcium-rich apatite was deposited on the scaffold’s surface forming a bone-like structure and composition.
3. Conclusions
In this study, HPG was successfully grafted with NVCL using AIBN as the initiator to synthesize HPG-g-PNVCL. The polymer was characterized using various analytical techniques. HPG-g-PNVCL showed excellent thermal stability, as well as thermoresponsive behavior at ∼34 °C and a reversible soluble-insoluble characteristic. The hydrogel (HPG-g-PNVCL) was found to be highly promising for sustained controlled delivery of macromolecular drugs as an injectable form. Additionally, the HPG-g-PNVCL hydrogel also performed as an excellent scaffold for osteoblast cell differentiation. A chemically cross-linked hydrogel containing the n-HA (HPG-g-PNVCL/n-HA/DVS) scaffold was obtained to enhance the mechanical strength of the hydrogel and employed in in vitro biomineralization study. The scaffold showed apatite-like structure formation on the surface after soaking for 7 days in SBF solution. As the immersion period was increased up to 14 days, the Ca/P ratio also increased. Therefore, this novel n-HA-containing biocompatible and plant-based hydrogel has promising potential applications in bone tissue regeneration because of its calcium-rich apatite-forming ability and good bioactivity.
4. Experimental Methods
4.1. General
HPG with a specific gravity of 1.47 was obtained from Halliburton, USA, as a gift. AIBN, ethanol, n-HA, and acetone were all obtained from VWR International, USA, and used as received without further purification, unless otherwise noted. DVS and ciprofloxacin hydrochloride were purchased from Sigma-Aldrich, USA. NVCL was purchased from Alfa Aesar, USA. Ultrahigh purity nitrogen gas was obtained from NLR Welding Supply, USA. Doubly distilled water was used throughout the experiments. The FTIR spectra of HPG, PNVCL, HPG-g-PNVCL, and HPG-g-PNVCL/n-HA/DVS were collected using a Thermo Scientific Nicolet 6700 FTIR spectrometer, USA. The spectra were recorded in the wavenumber range of 400–4000 cm–1 using a standard KBr pellet method. The 1H NMR spectra of the samples (HPG and HPG-g-PNVCL) were collected at 25 °C with a JEOL ECS 400 MHz spectrometer (USA) that has a 5 mm triple resonance inverse probe. The chemical shifts were represented in parts per million with D2O as a solvent. SEM images were recorded using a scanning electron microscope (JSM 7000F JEOL, USA). The dried samples for the SEM imaging were obtained by freeze-drying the hydrogels using Labconco FreeZone 2.5 L freeze dryers, USA. The UV–vis spectra were recorded using a Varian Cary 5000 UV–vis–NIR spectrophotometer, USA. For rheological measurements, a Discovery Hybrid Rheometer (HR-1, TA Instruments, USA) with a parallel-plate geometry was used. TGA and DSC were performed using a TGA/DSC 3+ analyzer and a DSC 823e analyzer (Mettler-Toledo, UK), respectively. Mouse osteoblastic cells (MC3T3), minimum essential medium, penicillin, and streptomycin were purchased from the American Type Culture Collection, Manassas, VA, USA. Fetal bovine serum, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich, St. Louis, MO, USA.
4.2. Synthesis of the Graft Copolymer of HPG-g-PNVCL
Reactions were performed under a nitrogen atmosphere in a 250 mL three-necked flask that was immersed in a constant temperature bath and equipped with a magnetic stirrer, reflux condenser, and gas inlet system, as described previously.46 In brief, HPG (0.25 g) was dissolved in 12.5 mL of distilled water with constant stirring at room temperature for 2 h. The desired amount of NVCL which was initially dissolved in 6.25 mL of distilled water was added to the above solution, and the mixture was stirred under a slow stream of nitrogen. The mixture was placed in an oil bath set at 65 °C and stirred for 10 min. After that, the desired concentration of the AIBN initiator (dissolved in 2 mL of acetone) was slowly added to the reaction mixture, followed by the addition of distilled water to reach a total volume of 50 mL. As soon as AIBN was added, the free radical graft polymerization of NVCL started. The whole reaction mixture was kept under constant stirring under an inert nitrogen atmosphere. At the end of the polymerization time, copious amount of acetone was added to the reaction mixture to precipitate out the product. The resultant precipitate was collected by filtration and washed with absolute ethanol four times to remove any homopolymer (PNVCL). The obtained solid product (HPG-g-PNVCL graft copolymer) was vacuum-dried at 60 °C for 12 h to a constant weight. A scheme for the synthesis of HPG-g-PNVCL is given in Figure 1. The grafting yield was calculated using eq 1.
| 1 |
where W0 and W1 are the initial and final weights of ungrafted (HPG) and grafted polymer (HPG-g-PNVCL), respectively.
4.3. LCST Determination
The LCST of the 1 wt % HPG and HPG-g-PNVCL aqueous solutions in distilled water was determined by measuring the absorbance at 470 nm from 20 to 50 °C using a UV–vis spectrophotometer. Prior to the measurement, the hydrogels were equilibrated in a thermostatic water bath for at least 10 min to ensure a stable temperature. The temperature corresponding to the sudden drop in transmittance between 30 and 40 °C was defined as the LCST.
4.4. In Vitro Drug Release Study
Drug release studies were conducted using an incubator shaker at 37 °C. Ciprofloxacin was used as a model drug. To determine the release of ciprofloxacin from the injectable HPG-g-PNVCL hydrogel, an experimental system was followed according to previous studies with slight modifications.61 Initially, the graft copolymer was dissolved in PBS buffer (pH 7.4) in each vial with different concentrations of 3, 4, and 5 wt %, respectively. One milliliter of prepared solution with ciprofloxacin (6 mg/mL) was poured into test tubes (diameter = 12 mm). Test tubes were kept at 37 °C for thermal gelation, and then a release medium (PBS, pH 7.4) of 10 mL was added to each test tube. Then, 3 mL sample aliquot was withdrawn at different time intervals to evaluate the released content of the drug, and 3 mL of fresh release media was replenished back to maintain the sink conditions. The amount of ciprofloxacin released was analyzed using UV–vis spectroscopy at 377 nm (λmax). Ciprofloxacin standard curve was obtained using known concentrations of the drug and used to determine the amount of drug released.
4.5. MTT Assay
Cytotoxicity studies were performed using osteoblastic MC3T3 cells by the MTT assay as described previously.62 To study the cytotoxicity of the scaffold material, an MTT assay was performed. In this assay, the mitochondrial succinate dehydrogenase activity was used as an indicator of cell viability, where mitochondria-dependent reduction of MTT to formazan was measured spectrophotometrically. Prior to using the HPG-g-PNVCL copolymer for the cytotoxicity study, this material was sterilized by washing with absolute ethanol several times and then exposing to UV light for 5 h in fume hood. For this study, 6 wt % solution of HPG-g-PNVCL was used. A 24-well cell culture plate was used for the study. Initially, 250 μL of HPG-g-PNVCL was taken in the cell culture plate. The plate was then kept at 37 °C for 2 h. This method ensures thermogelling sol-to-gel transition of the polymer. The provided scaffold was sterilized under UV light for 5 h before use. Approximately 1.5 × 104 cells/mL per well were seeded in 48-well plates directly in the absence of a scaffold to serve as a control and onto the solidified scaffold for stipulated time period. After the cells were grown on the scaffold for a predetermined time period, the HPG-g-PNVCL scaffold with cells attached to it was transferred into fresh multiwell plates and to the well 400 μL of the fresh cell culture medium supplemented with 0.25 mg/mL MTT solution was added. The cells were incubated for an additional 4 h in the dark condition. Thereafter, the medium was discarded, and 400 μL of DMSO was added to the well. The plate was then wrapped with an aluminum foil and placed on a shaker for 10 min to dissolve the purple insoluble MTT formazan complex. A small aliquot of 100 μL of DMSO containing MTT formazan solution was transferred to a new 96-well plate, and the absorbance was then measured at 570 nm using an automated microplate reader (Benchmark Plus, Bio-Rad Laboratories, Hercules, California, USA). The cell viability data were obtained as % of the control values obtained from untreated cells. We consider here control as just regular MC3T3 cells in medium under normal condition. All measurements were performed in triplicate and repeated independently at least three times.
4.6. Synthesis of HPG-g-PNVCL/n-HA/DVS
Our strategy was to modify HPG-g-PNVCL with n-HA to form bone apatite in situ because n-HA, a major component of natural bone, is osteoconductive, biodegradable, and bioactive,36,63 improving its use in biomedical applications such as bone defect treatments.64 Also, for bone replacement applications, we need a stronger hydrogel. In situ covalent cross-linking of the hydrogel system at 37 °C in the presence of a chemical cross-linker such as DVS is a suitable option. DVS is widely used as a cross-linker because of its reactivity, stability, low cost, and solubility in water. DVS has a high chemical affinity to various polysaccharide networks such as dextran, agarose, cellulose, and hyaluronic acid because of the presence of two electrophilic double bonds.65 Combining the thermogelling property of modified guar and the chemical cross-linking of DVS, it is possible to develop an injectable material with the desired mechanical properties for bone tissue engineering. To synthesize a DVS cross-linked gel, at first 30 mg of the HPG-g-PNVCL graft copolymer was mixed with 1 mL of distilled water in a test tube. The mixture was then vortexed until it became a clear solution. Then, 40 mg (2 wt %) of n-HA was added, and the mixture was sonicated for 1 h. Subsequently, 15 μL of DVS was added in order to initiate the cross-linking of the polymer composite, primarily through −OH bonds of HPG in HPG-g-PNVCL. The HPG-g-PNVCL/n-HA/DVS cross-linked hydrogel was formed after incubation of the mixture in an oven maintained at 37 °C for a certain period. After that, the purified cross-linked hydrogel was obtained by washing with distilled water until a neutral pH was achieved for the washing liquid.
Acknowledgments
The authors would like to acknowledge US Army Medical Research and Materiel Command award number W81XWH-15-1-0666 for the financial support of this research. The authors would also like to thank N. Ali and P. Singh for helping with the cell culture studies.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01765.
FTIR spectra of virgin HPG, PNVCL, HPG-g-PNVCL, n-HA, and HPG-g-PNVCL/n-HA/DVS; 1H NMR spectra of HPG and HPG-g-PNVCL; TGA curves of HPG, HPG-g-PNVCL, and HPG-g-PNVCL/n-HA/DVS; DSC curves of HPG, HPG-g-PNVCL, and HPG-g-PNVCL/n-HA/DVS; and thermoreversibility of HPG-g-PNVCL (PDF)
Author Contributions
A.P.-T., C.M.P., F.W., A.B.R., B.P.C., P.K.S., and A.S.B. contributed to the experimental and data interpretation. A.G. conceived the study and experimental design. A.P.-T., C.M.P., and A.G. contributed to the experimental design and wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Sivashanmugam A.; Arun Kumar R.; Vishnu Priya M.; Nair S. V.; Jayakumar R. An Overview of Injectable Polymeric Hydrogels for Tissue Engineering. Eur. Polym. J. 2015, 72, 543–565. 10.1016/j.eurpolymj.2015.05.014. [DOI] [Google Scholar]
- Tan R.; Niu X.; Gan S.; Feng Q. Preparation and Characterization of an Injectable Composite. J. Mater. Sci.: Mater. Med. 2009, 20, 1245–1253. 10.1007/s10856-009-3692-6. [DOI] [PubMed] [Google Scholar]
- Lee K. Y.; Mooney D. J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Temenoff J. S.; Mikos A. G. Injectable Biodegradable Materials for Orthopedic Tissue Engineering. Biomaterials 2000, 21, 2405–2412. 10.1016/s0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- Lee J. W.; Jung M. C.; Park H. D.; Park K. D.; Ryu G. H. Synthesis and Characterization of Thermosensitive Chitosan Copolymer as a Novel Biomaterial. J. Biomater. Sci., Polym. Ed. 2004, 15, 1065–1079. 10.1163/1568562041526496. [DOI] [PubMed] [Google Scholar]
- Dhivya S.; Saravanan S.; Sastry T. P.; Selvamurugan N. Nanohydroxyapatite-Reinforced Chitosan Composite Hydrogel for Bone Tissue Repair in Vitro and in Vivo. J. Nanobiotechnol. 2015, 13, 40. 10.1186/s12951-015-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretlow J. D.; Mikos A. G. Review: Mineralization of Synthetic Polymer Scaffolds for Bone Tissue Engineering. Tissue Eng. 2007, 13, 927–938. 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- Langer R.; Tirrell D. A. Designing Materials for Biology and Medicine. Nature 2004, 428, 487–492. 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- Mahanta A. K.; Senapati S.; Maiti P. A polyurethane-chitosan brush as an injectable hydrogel for controlled drug delivery and tissue engineering. Polym. Chem. 2017, 8, 6233–6249. 10.1039/c7py01218g. [DOI] [Google Scholar]
- Han Y.; Zeng Q.; Li H.; Chang J. The Calcium Silicate/alginate Composite: Preparation and Evaluation of Its Behavior as Bioactive Injectable Hydrogels. Acta Biomater. 2013, 9, 9107–9117. 10.1016/j.actbio.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Yang W. J.; Zhou P.; Liang L.; Cao Y.; Qiao J.; Li X.; Teng Z.; Wang L. Nanogel-Incorporated Injectable Hydrogel for Synergistic Therapy Based on Sequential Local Delivery of Combretastatin-A4 Phosphate (CA4P) and Doxorubicin (DOX). ACS Appl. Mater. Interfaces 2018, 10, 18560–18573. 10.1021/acsami.8b04394. [DOI] [PubMed] [Google Scholar]
- Kang G.; Cheon S.; Song S. Controlled Release of Doxorubicin from Thermosensitive Poly(organophosphazene) Hydrogels. Int. J. Pharm. 2006, 319, 29–36. 10.1016/j.ijpharm.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Yan J.; Miao Y.; Tan H.; Zhou T.; Ling Z.; Chen Y.; Xing X.; Hu X. Injectable Alginate/hydroxyapatite Gel Scaffold Combined with Gelatin Microspheres for Drug Delivery and Bone Tissue Engineering. Mater. Sci. Eng., C 2016, 63, 274–284. 10.1016/j.msec.2016.02.071. [DOI] [PubMed] [Google Scholar]
- Hoare T. R.; Kohane D. S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- Liechty W. B.; Kryscio D. R.; Slaughter B. V.; Peppas N. A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y.; Park K. Environment-Sensitive Hydrogels for Drug Delivery. Adv. Drug Delivery Rev. 2001, 53, 321–339. 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- Gupta P.; Vermani K.; Garg S. Hydrogels: From Controlled Release to pH-Responsive Drug Delivery. Drug Discovery Today 2002, 7, 569–579. 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- Dimatteo R.; Darling N. J.; Segura T. In Situ Forming Injectable Hydrogels for Drug Delivery and Wound Repair. Adv. Drug Delivery Rev. 2018, 127, 167–184. 10.1016/j.addr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denuziere A.; Ferrier D.; Damour O.; Domard A. Chitosan-Chondroitin Sulfate and Chitosan-Hyaluronate Polyelectrolyte Complexes: Biological Properties. Biomaterials 1998, 19, 1275–1285. 10.1016/s0142-9612(98)00036-2. [DOI] [PubMed] [Google Scholar]
- Morikawaand N.; Matsuda T. Thermoresponsive Artificial Extracellular Matrix: N-Isopropylacrylamide-Graft-Copolymerized Gelatin. J. Biomater. Sci., Polym. Ed. 2002, 13, 167–183. 10.1163/156856202317414357. [DOI] [PubMed] [Google Scholar]
- Bryant S. J.; Bender R. J.; Durand K. L.; Anseth K. S. Encapsulating Chondrocytes in Degrading PEG Hydrogels with High Modulus: Engineering Gel Structural Changes to Facilitate Cartilaginous Tissue Production. Biotechnol. Bioeng. 2004, 86, 747–755. 10.1002/bit.20160. [DOI] [PubMed] [Google Scholar]
- Ohya S.; Nakayama Y.; Matsuda T. Thermoresponsive Artificial Extracellular Matrix for Tissue Engineering: Hyaluronic Acid Bioconjugated with Poly(N-isopropylacrylamide) Grafts. Biomacromolecules 2001, 2, 856–863. 10.1021/bm010040a. [DOI] [PubMed] [Google Scholar]
- Choi B. G.; Park M. H.; Cho S.-H.; Joo M. K.; Oh H. J.; Kim E. H.; Park K.; Han D. K.; Jeong B. Thermal Gelling Polyalanine-Poloxamine-Polyalanine Aqueous Solution for Chondrocytes 3D Culture: Initial Concentration Effect. Soft Matter 2011, 7, 456–462. 10.1039/c0sm00611d. [DOI] [Google Scholar]
- Ho E.; Lowman A.; Marcolongo M. In Situ Apatite Forming Injectable Hydrogel. J. Biomed. Mater. Res., Part A 2007, 83, 249–256. 10.1002/jbm.a.31457. [DOI] [PubMed] [Google Scholar]
- Kang Derwent J. J.; Mieler W. F. Thermoresponsive Hydrogels as a New Ocular Drug Delivery Platform to the Posterior Segment of the Eye. Trans. Am. Ophthalmol. Soc. 2008, 106, 206–213. 10.6084/m9.figshare.1422037.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodorescu M.; Andrei M.; Turturicǎ G.; Stǎnescu P. O.; Zaharia A.; Sârbu A. Novel Thermoreversible Injectable Hydrogel Formulations Based on Sodium Alginate and Poly(N-Isopropylacrylamide). Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 763–771. 10.1080/00914037.2015.1030646. [DOI] [Google Scholar]
- Heskins M.; Guillet J. E. Solution Properties of Poly(N-Isopropylacrylamide). J. Macromol. Sci. Part A—Chem. 1968, 2, 1441–1455. 10.1080/10601326808051910. [DOI] [Google Scholar]
- Zhang Y.; Cai J.; Li C.; Wei J.; Liu Z.; Xue W. Effects of Thermosensitive poly(N-Isopropylacrylamide) on Blood Coagulation. J. Mater. Chem. B 2016, 4, 3733–3749. 10.1039/c6tb00823b. [DOI] [PubMed] [Google Scholar]
- Vihola H.; Laukkanen A.; Valtola L.; Tenhu H.; Hirvonen J. Cytotoxicity of Thermosensitive Polymers poly(N-Isopropylacrylamide), poly(N-Vinylcaprolactam) and Amphiphilically Modified poly(N-Vinylcaprolactam). Biomaterials 2005, 26, 3055–3064. 10.1016/j.biomaterials.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Mikheeva L. M.; Grinberg N. V.; Mashkevich A. Y.; Grinberg V. Y.; Thanh L. T. M.; Makhaeva E. E.; Khokhlov A. R. Microcalorimetric Study of Thermal Cooperative Transitions in Poly(N-Vinylcaprolactam) Hydrogels. Macromolecules 1997, 30, 2693–2699. 10.1021/ma9615112. [DOI] [Google Scholar]
- Galaev I.; Mattiasson B. ‘Smart’ polymers and what they could do in biotechnology and medicine. Trends Biotechnol. 1999, 17, 335–340. 10.1016/s0167-7799(99)01345-1. [DOI] [PubMed] [Google Scholar]
- Yao J.; Tao S. L.; Young M. J. Synthetic Polymer Scaffolds for Stem Cell Transplantation in Retinal Tissue Engineering. Polymers 2011, 3, 899–914. 10.3390/polym3020899. [DOI] [Google Scholar]
- Polysaccharide Based Graft Copolymers; Kalia S., Sabaa M. W., Eds.; Springer: Berlin, 2013; pp 1–353. [Google Scholar]
- Chourasia M. K.; Jain S. K. Potential of Guar Gum Microspheres for Target Specific Drug Release to Colon. J. Drug Targeting 2004, 12, 435–442. 10.1080/10611860400006604. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T.; Hay S.; Macfarlane S.; Gibson G. R. Effect of different carbohydrates on growth, polysaccharidase and glycosidase production byBacteroides ovatus, in batch and continuous culture. J. Appl. Bacteriol. 1990, 68, 179–187. 10.1111/j.1365-2672.1990.tb02564.x. [DOI] [PubMed] [Google Scholar]
- Rizzi S. C.; Heath D. J.; Coombes A. G. A.; Bock N.; Textor M.; Downes S. Biodegradable Polymer/hydroxyapatite Composites: Surface Analysis and Initial Attachment of Human Osteoblasts. J. Biomed. Mater. Res. 2001, 55, 475–486. . [DOI] [PubMed] [Google Scholar]
- Srivastava A.; Behari K. Synthesis and characterization of graft copolymer (guar gum-g-N-vinyl-2-pyrrolidone) and investigation of metal ion sorption and swelling behavior. J. Appl. Polym. Sci. 2006, 100, 2480–2489. 10.1002/app.23594. [DOI] [Google Scholar]
- Fu S.; Ni P.; Wang B.; Chu B.; Zheng L.; Luo F.; Luo J.; Qian Z. Injectable and Thermo-Sensitive PEG-PCL-PEG Copolymer/collagen/n-HA Hydrogel Composite for Guided Bone Regeneration. Biomaterials 2012, 33, 4801–4809. 10.1016/j.biomaterials.2012.03.040. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Zhang X.; Wu A.; Xu H. An Injectable Nano-Hydroxyapatite (N-HA)/glycol Chitosan (G-CS)/hyaluronic Acid (HyA) Composite Hydrogel for Bone Tissue Engineering. RSC Adv. 2016, 6, 33529–33536. 10.1039/c5ra26160k. [DOI] [Google Scholar]
- Liu M.; Zeng X.; Ma C.; Yi H.; Ali Z.; Mou X.; Li S.; Deng Y.; He N. Injectable Hydrogels for Cartilage and Bone Tissue Engineering. Bone Res. 2017, 5, 17014. 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talan D. A.; Naber K. G.; Palou J.; Elkharrat D. Extended-Release Ciprofloxacin (Cipro XR) for Treatment of Urinary Tract Infections. Int. J. Antimicrob. Agents 2004, 23, 54–66. 10.1016/j.ijantimicag.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Pal S.; Tak Y. K.; Song J. M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia Coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. 10.1128/aem.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden P. A.; Schimel J. P.; Godwin H. A. Five Reasons to Use Bacteria When Assessing Manufactured Nanomaterial Environmental Hazards and Fates. Curr. Opin. Biotechnol. 2014, 27, 73–78. 10.1016/j.copbio.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Shahverdi A. R.; Fakhimi A.; Shahverdi H. R.; Minaian S. Synthesis and Effect of Silver Nanoparticles on the Antibacterial Activity of Different Antibiotics against Staphylococcus Aureus and Escherichia Coli. Nanomedicine 2007, 3, 168–171. 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Xiu Z.-m.; Zhang Q.-b.; Puppala H. L.; Colvin V. L.; Alvarez P. J. J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. 10.1021/nl301934w. [DOI] [PubMed] [Google Scholar]
- Prasad S. S.; Rao K. M.; Reddy P. R. S.; Reddy N. S.; Rao K. K.; Subha M. C. S. Synthesis and Characterisation of Guar gum-g-Poly (acrylamidoglycolic acid) by Redox Initiator. Indian J. Adv. Chem. Sci. 2012, 1, 28–32. [Google Scholar]
- Rao K. M.; Krishna Rao K. S. V.; Sudhakar P.; Chowdoji Rao K.; Subha M. C. S. Synthesis and Characterization of biodegradable Poly (Vinyl caprolactam) grafted on to sodium alginate and its microgels for controlled release studies of an anticancer drug. J. Appl. Pharm. Sci. 2013, 3, 061–069. 10.7324/JAPS.2013.3609. [DOI] [Google Scholar]
- Thaker M. D.; Trivedi H. C. Ultraviolet-Radiation-Induced Graft Copolymerization of Methyl Acrylate onto the Sodium Salt of Partially Carboxymethylated Guar Gum. J. Appl. Polym. Sci. 2005, 97, 1977–1986. 10.1002/app.20988. [DOI] [Google Scholar]
- Yu Z.; Gu H.; Tang D.; Lv H.; Ren Y.; Gu S. Fabrication of PVCL-Co-PMMA Nanofibers with Tunable Volume Phase Transition Temperatures and Maintainable Shape for Anti-Cancer Drug Release. RSC Adv. 2015, 5, 64944–64950. 10.1039/c5ra10808j. [DOI] [Google Scholar]
- Ellzy M. W.; Jensen J. O.; Kay J. G. Vibrational Frequencies and Structural Determinations of Di-Vinyl Sulfone. Spectrochim. Acta, Part A 2003, 59, 867–881. 10.1016/s1386-1425(02)00239-1. [DOI] [PubMed] [Google Scholar]
- Kozanoǧlu S.; Özdemir T.; Usanmaz A. Polymerization of N-Vinylcaprolactam and Characterization of Poly(N-Vinylcaprolactam). J. Macromol. Sci., Part A: Pure Appl.Chem. 2011, 48, 467–477. 10.1080/10601325.2011.573350. [DOI] [Google Scholar]
- Nayak B. R.; Singh R. P. Synthesis and Characterization of Grafted Hydroxypropyl Guar Gum by Ceric Ion Induced Initiation. Eur. Polym. J. 2001, 37, 1655–1666. 10.1016/s0014-3057(01)00035-0. [DOI] [Google Scholar]
- Zhang J.; Wu Q.; Li M.-C.; Song K.; Sun X.; Lee S.-Y.; Lei T. Thermoresponsive Copolymer Poly(N-Vinylcaprolactam) Grafted Cellulose Nanocrystals: Synthesis, Structure, and Properties. ACS Sustainable Chem. Eng. 2017, 5, 7439–7447. 10.1021/acssuschemeng.7b02033. [DOI] [Google Scholar]
- Kumar A.; Negi Y. S.; Choudhary V.; Bhardwaj N. K. Fabrication of Poly (Vinyl Alcohol)/ovalbumin/cellulose Nanocrystals/nanohydroxyapatite Based Biocomposite Scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 191–201. 10.1080/00914037.2015.1099102. [DOI] [Google Scholar]
- Chen D.; Xia X.; Wong T. W.; Bai H.; Behl M.; Zhao Q.; Lendlein A.; Xie T. Omnidirectional Shape Memory Effect via Lyophilization of PEG Hydrogels. Macromol. Rapid Commun. 2017, 38, 1600746. 10.1002/marc.201600746. [DOI] [PubMed] [Google Scholar]
- Narbat M. K.; Orang F.; Hashtjin M. S.; Goudarzi A. Fabrication of Porous Hydroxyapatite-Gelatin Composite Scaffolds for Bone Tissue Engineering. Iran. Biomed. J. 2006, 10, 215–223. [Google Scholar]
- Zhang J.; Ma X.; Hui J.; Ma P.; Deng J.; Fan D. Crosslinking of Hyaluronic Acid and Human-like Collagen with Divinyl Sulfone. J. Chem. Pharm. Res. 2014, 6, 726–730. [Google Scholar]
- Shimojo A. A. M.; Pires A. M. B.; Lichy R.; Santana M. H. A. The Performance of Crosslinking with Divinyl Sulfone as Controlled by the Interplay between the Chemical Modification and Conformation of Hyaluronic Acid. J. Braz. Chem. Soc. 2015, 26, 506–512. 10.5935/0103-5053.20150003. [DOI] [Google Scholar]
- Kriegel R. M.Divinyl Sulfone Crosslinking Agents and Methods of Use in Subterranean Applications. U.S. Patent 20,060,081,369 A1, Nov 7, 2006.
- Geçer A.; Yıldız N.; Kavak D.; Çalımlı A. Comparison of Chitosan Apatite Composites Synthesized by Different Methods. Polym. Compos. 2009, 30, 288–295. 10.1002/pc.20635. [DOI] [Google Scholar]
- Ha D. I.; Lee S. B.; Chong M. S.; Lee Y. M.; Kim S. Y.; Park Y. H. Preparation of Thermo-Responsive and Injectable Hydrogels Based on Hyaluronic Acid and poly(N-Isopropylacrylamide) and Their Drug Release Behaviors. Macromol. Res. 2006, 14, 87–93. 10.1007/bf03219073. [DOI] [Google Scholar]
- Thankam A.; Al-Anbaky Q.; Al-karakooly Z.; Rangu Magar A. B.; Chhetri B. P.; Ali N.; Ghosh A. Fabrication and Characterization of Hydroxypropyl Guar-Poly (Vinylalcohol)-Nano Hydroxyapatite Composite Hydrogels for Bone Tissue Engineering. J. Biomater. Sci., Polym. Ed. 2018, 1–44. 10.1080/09205063.2018.1494437. [DOI] [PubMed] [Google Scholar]
- Fu S.; Guo G.; Gong C.; Zeng S.; Liang H.; Luo F.; Zhang X.; Zhao X.; Wei Y.; Qian Z. Injectable Biodegradable Thermosensitive Hydrogel Composite for Orthopedic Tissue Engineering. 1. Preparation and Characterization of Nanohydroxyapatite/Poly(ethylene glycol)–Poly(ε-caprolactone)–Poly(ethylene glycol) Hydrogel Nanocomposites. J. Phys. Chem. B 2009, 113, 16518–16525. 10.1021/jp907974d. [DOI] [PubMed] [Google Scholar]
- Wang C.; Ge X. G.; Yang K. K.; Chen S. C.; Wang Y. Z. Preparation and Characterization of Biodegradable Poly(p-dioxanone)/Hydroxyapatite Composites. Soft Mater. 2009, 7, 116–131. 10.1080/15394450903163391. [DOI] [Google Scholar]
- Morales-Sanfrutos J.; Lopez-Jaramillo F.; Elremaily M.; Hernández-Mateo F.; Santoyo-Gonzalez F. Divinyl Sulfone Cross-Linked Cyclodextrin-Based Polymeric Materials: Synthesis and Applications as Sorbents and Encapsulating Agents. Molecules 2015, 20, 3565–3581. 10.3390/molecules20033565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









