Abstract
Pituitary adenomas in children and adolescents are rare tumors that often result from a tumor predisposition syndrome. Several inherited causes for pituitary adenomas have been identified in the last few years, including multiple endocrine neoplasia type 1 and 4, Carney’s complex, Tuberous sclerosis, DICER1 syndrome, neurofibromatosis type 1, McCune Albright syndrome, familial isolated pituitary adenoma, and pituitary adenoma association due to defects in succinate dehydrogenase genes. Recently, our group discovered X-linked acrogigantism (X-LAG), a new pediatric disorder that is caused by an Xq26.3 genomic duplication (involving the GPR101 gene). Genes that predispose to pediatric Cushing disease, including CABLES1 and USP8, were also recently identified. Genetic screening and counseling of affected or at risk individuals is a key component of their comprehensive care. In this review, we provide an up-to-date discussion on the latest pediatric genetic discoveries associated with pituitary adenomas with a focus on familial syndromes.
Keywords: Pituitary adenoma, Cushing disease, Gigantism, GH, Genetics, USP8
Introduction
The adenohypophysis of the pituitary gland arises from epithelial cells of endodermal origins and consists of a heterogeneous population of well-differentiated hormone-secreting cells. These include somatotrophs, lactotrophs, mammosomatotrophs, corticotrophs, thyrotrophs and gonadotrophs [1], and comprise ~3.5–6% of all surgically treated pediatric pituitary tumors [2]. Pediatric pituitary adenomas are typically benign with the most frequently encountered tumors being prolactinomas (most are in adolescents), followed by corticotropinomas and somatotropinomas [3]. Pediatric nonfunctioning pituitary adenomas are encountered in 3–6% of all cases [4, 5].
Over the past three decades, our evolving understanding of molecular and genetic investigations have revealed a number of genetic defects predisposing to pituitary adenomas in childhood (Table 1) [6]. It is now well established that known germline gene abnormalities may cause up to approximately one-fifth of pituitary adenomas in children and adolescents. Multiple familial syndromes were identified, including multiple endocrine neoplasia type 1 and 4 (MEN1 and MEN4), Carney’s complex (CNC), DICER1 syndrome, Tuberous sclerosis complex (TSC), neurofibromatosis type 1 (NF1), McCune Albright syndrome (MAS), familial isolated pituitary adenoma (FIPA), and pituitary adenoma association due to defects in familial succinate dehydrogenase genes (3PAs) [7, 8]. Recently, our group has discovered X-linked acrogigantism (X-LAG), a new pediatric disorder of gigantism that is caused by an Xq26.3 genomic duplication (GPR101) [9]. Additionally, genes that predispose to pediatric CD, including CABLES1 and USP8, have also been uncovered [10, 11]. In this review, we provide an up-to-date discussion on the latest genetic discoveries in pediatric pituitary adenomas with a focus on familial syndromes that predispose to CD and gigantism and provide an algorithm for genetic counseling and testing of these conditions.
Table 1.
Genetic syndromes associated with familial pituitary adenomas.
| Syndrome | Gene | Chromosome | Function | Inheritance | Pituitary tumor type |
|---|---|---|---|---|---|
|
| |||||
| Familial isolated pituitary adenoma (FIPA) | AIP | 11q13.3 | Tumor suppressor | AD | Somatotropinoma |
| Somatomammotropinoma | |||||
| Corticotropinoma | |||||
| Non-functional | |||||
| Prolactinoma | |||||
|
| |||||
| X-linked acrogigantism (X-LAG) | GPR101 | Xq26.3 | Unknown function | Sporadic, X-linked dominant | Somatotropinoma |
| Somatomammotropinoma | |||||
|
| |||||
| Carney complex (CNC) | PRKAR1A | 17q24.2 | Tumor suppressor | AD | Somatotropinoma |
| Somatomammotropinoma | |||||
| Corticotropinoma | |||||
|
| |||||
| Multiple endocrine neoplasia type 1 (MEN-1) | MEN1 | 11q13.1 | Tumor suppressor | AD | Somatotropinoma |
| Somatomammotropinoma | |||||
| Corticotropinoma | |||||
| Non-functional | |||||
| Prolactinoma | |||||
|
| |||||
| Multiple endocrine neoplasia type 2A (MEN-2A) | RET | 10q11.21 | Oncogene | AD | Corticotropinoma |
|
| |||||
| Multiple endocrine neoplasia type 2A (MEN-2B) | RET | 10q11.21 | Oncogene | AD | Corticotropinoma |
|
| |||||
| Multiple endocrine neoplasia type 4 (MEN 4) | CDKN14 | 12p13.1 | Tumor suppressor | AD | Somatotropinoma |
| Corticotropinoma | |||||
| Non-functional | |||||
| Prolactinoma | |||||
|
| |||||
| DICER1 syndrome | DICER1 | 14q32.13 | Tumor suppressor | AD | Corticotropinoma |
|
| |||||
| McCune Albright Syndrome (MAS) | GNAS1 | 20q13.32 | Oncogene | Sporadic (mosaicism) | Somatotropinoma |
| Somatomammotropinoma | |||||
|
| |||||
| Tuberous sclerosis complex (TSC) | TSC1 | 9q34.13 | Tumor suppressor | AD | Corticotropinoma |
| TSC2 | 16p13.3 | ||||
|
| |||||
| Paraganglioma, pheochromocytoma, and pituitary adenoma association (3PA) | SDHA | 5p15.33 | Tumor suppressor | AD, sporadic | Somatotropinoma |
| SDHB | 1p36.13 | ||||
| SDHC | 1q23.3 | ||||
| SDHD | 11q23.1 | ||||
Genetics of Cushing disease
Cushing disease (CD) is a rare condition with an incidence of 1.2–1.7 cases per million per year [12]. CD arises from monoclonal proliferation of corticotrophs leading to endogenous ACTH-dependent hypercortisolemia. Pediatric CD is evenly distributed throughout childhood, and is the commonest cause of Cushing syndrome beyond infancy [3]. Genetic alterations in corticotropinomas rarely occur in the known proto-oncogenes or tumor suppressor genes (Table 1). In contrast with other pituitary tumor types, the genetic causes of corticotropinomas are largely unknown. Recent studies have shown that the most common genetic alteration found in over one third of pediatric corticotropinomas were recurrent activating somatic heterozygous driver mutations located in a hotspot region in exon 14 of the ubiquitin-specific protease 8 gene (USP8; chromosome 15q21.2) [10, 13]. Patients with CD harboring mutations in USP8 were older at diagnosis with a higher likelihood of recurrence when compared with patients without mutations [10]. Moreover, USP8 mutations lead to activation of epidermal growth factor receptor (EGFR) signaling, a potential target for CD treatment. There are no known germline USP8 mutations in humans.
Recently, our group has identified 4 potentially pathogenic missense germline variants in CDK5 and ABL1 enzyme substrate 1 (CABLES1; chromosome 18q11.2), a tumor suppressor gene that regulates cell cycle progression. Genetic alterations in CABLES1 were found in 4 female patients (2 young adults and 2 children) with large corticotropinomas that were difficult to manage [11]. Other somatic events reported in CD with aggressive behavior include genetic alterations in p53 (chromosome 17p13.1) tumor suppressor gene [14].
Several familial syndromes predispose to CD. MEN’s are a diverse group of autosomal dominant (AD) syndromes that predispose to tumor formation in multiple organs. MEN-1 (OMIM #131100) is characterized by tumor formation in over 30 tissues, including pituitary, parathyroid, and pancreas. MEN-1 is caused by germline (and rarely somatic) mutations in MEN1 (chromosome 11q13) [15], with over 1300 germline mutations reported [16]. Pediatric corticotropinomas are rare in MEN-1 but have been reported as its first manifestation [17]. In one study of 74 patients with sporadic CD and 4 patients with syndromic CD, MEN-1 mutations were only identified in 2 syndromic patients with genetically confirmed MEN-1 relatives [18].
MEN-2 is divided into MEN-2A (OMIM # 171400) and MEN-2B (OMIM #162300) and caused by activating mutations of the proto-oncogene RET (chromosome 10q11.21) [19]. Pituitary involvement in MEN-2 is exceedingly rare; our group has recently reported the a case of pediatric CD due to a microcorticotropinoma in MEN-2B [20], providing evidence for the role of this gene in corticotroph tumorigenesis.
MEN-4 (OMIM #610755) is MEN-1 like, and caused by germline inactivating mutations in CDKN1B (chromosome 12p13.1), a putative tumor suppressor gene coding for p27 that regulates cell cycle progression [21]. Pituitary adenomas are the second most common phenotypic feature of MEN-4, affecting ~ 37% of the reported cases with an age of diagnosis of 30–79 years [21]. CD has been reported in only one adult with MEN-4 due to a heterozygous 19-bp duplication (c.59_77dup19) in CDKN1B, leading to a truncated protein [22], and none in pediatric cohorts with CD [18, 23]. In one study, the common CDKN1B rs2066827 polymorphism was shown to play a role in corticotropinoma susceptibility and tumorigenesis likely through epigenetic mechanisms [24].
CNC (OMIM #160980) is an AD syndrome that is primarily caused by mutations in the tumor suppressor gene PRKAR1A (chromosome 17q22-24; CNC1 locus). A specific genetic alteration on chromosome 2p16 (CNC2 locus) has not yet been identified, whereas a single case of CNC has been described in association with PRKACB amplification (CNC3 locus) [25–27]. CNC manifests with skin pigmentation, cardiac myxomas, GH and prolactin-secreting pituitary tumors or hyperplasia, and adrenal Cushing syndrome primarily from primary pigmented nodular adrenocortical disease (PPNAD) [27, 28]. Previous investigations did not reveal somatic or germline PRKAR1A mutations in pediatric CD [18]. Recently, our group reported a pediatric case of CD that was subsequently followed by PPNAD in a patient carrying an inactivating PRKAR1A germline mutation [29], providing evidence for the role of PRKAR1A in corticotroph tumorigenesis.
FIPA (OMIM #605555) is characterized by the occurrence of pituitary adenomas in multiple family members. The tumor suppressor gene aryl hydrocarbon receptor-interacting protein (AIP, chromosome 11q13.3) is identified in approximately 15–20% of familial FIPA [30, 31]. These mutations typically affect young patients, with or without a family history, with a low penetrance of ~ 15–30%.
Other syndromes that predispose to pediatric CD include MAS (OMIM #174800) due to gain-of-function mutations in GNAS1 in the mosaic state [32, 33], TSC (OMIM #191100 and #613254) as a result of germline mutations in 2 tumor suppressor genes (TSC1; chromosome 9q34.13, and TSC2; chromosome 12q15) [34], and DICER1 syndrome as a result of loss-of-function mutations in the DICER1 gene (chromosome 14q32.13) [35]. The recently discovered syndrome, X-LAG (see below) due to Xq26.3 genomic duplication (GPR101) has not been implicated in pediatric CD. Screening a cohort of pediatric CD did not reveal germline or somatic GPR101 mutations [36].
Newer genetic approaches, such as whole exome sequencing and transcriptomic analysis, have helped uncover new genes in the predisposition of sporadic and familial pediatric CD. These include cadherin-related 23 (CDH23; chromosome 10q22.1), cyclin D2 (CCND2; 12p13.32), Zinc-finger 676 protein (ZNF676; chromosome 19p12), death-associated protein kinase 1 (DAPK1; chromosome 9q21.33) and metalloproteinase inhibitor 2 (TIMP2; chromosome 17q25.3) [37, 38]. Their role in the pathogenesis of pediatric CD should be further ascertained in well-designed studies.
Genetics of Gigantism
Disorders of GH-excess can be grossly divided into two major categories; gigantism and acromegaly. The two disorders represent a continuum of clinical manifestations and depend on whether the epiphyseal growth plates are not fused (gigantism), or fused (acromegaly). The most common pituitary pathology is a benign GH-secreting pituitary tumor, called somatotropinoma. The incidence of pituitary gigantism and acromegaly are approximately 8 and 11 cases per million person-years, respectively [39]. The cyclic AMP pathway is frequently dysregulated in sporadic somatotropinomas; somatic activating mutations in GNAS, which encodes for Gsα, are found in the heterozygous state [40], and on the maternal allele [41], representing the first and largest somatic genetic alteration in somatotropinomas [42].
Most cases of pediatric gigantism are familial. X-LAG (OMIM #300942) is the most common cause of early childhood-onset gigantism in ~80% of pre-pubertal gigantism. X-LAG is caused by GH (and prolactin) over secretion due to a pituitary macroadenoma or hyperplasia [9], with a median age of onset of 12 months. Germline microduplications on chromosome Xq26.3 causing X-LAG mainly to arise de novo. The culprit gene in this duplicated region is GPR101, which codes for an orphan G-protein coupled receptor (GPCR) [9, 43]. In sporadic acromegaly, a rare missense variant in GPR101 (p.E308D) was identified in approximately 4% of cases [44].
Gigantism can occur in association with AIP mutations (FIPA) and seen in ~30% of patients with a somatotropinoma [8, 45]. In one study, Daly et al. [46] showed that AIP mutation-positive acromegalics were predominantly young males with the majority presenting during childhood or adolescence. These cases were associated with higher levels of GH and prolactin, were more likely to undergo transphenoidal surgery, and were less responsive to somatostatin analogues. The prevalence of AIP mutations in patients with sporadic pituitary adenomas is ~4% [47]. However, there are no reports to date of somatic mutations of AIP.
In MAS, GH-excess is seen in ~20–30%, with a mean age at diagnosis of 24.4 years [48]. Somatotroph hyperplasia involves the entire pituitary gland, with or without somatotroph adenoma [49]. The incidence of GH-secreting pituitary adenomas in MEN-1 is ~10% by age 40 and rarely occurs in childhood [15]. GH-excess is rare in MEN-4; only one case of gigantism due to a heterozygous mutation in the 5'-UTR region (c.-29_-26delAGAG) of CDKN1B [21, 50]. Mutations in CDNK1B rarely occur in association with sporadic gigantism or acromegaly [51]. In CNC, GH-excess is seen in ~79% of patients and usually due to somatotroph cell hyperplasia [27]. Somatic alterations in PRKAR1A or PRKACB have never been found in sporadic GH-secreting pituitary adenomas.
Recently, our group has identified a new syndrome, that we termed 3PAs, which refers to the co-existence of familial paragangliomas and pheochromocytomas (PPGL) and pituitary adenomas. 3PAs is caused by germline SDHx mutations [52]. The first case was in an acromegalic with paraganglioma due to a pathogenic mutation in SDHD [53].
Other rare genetic defects that have been implicated in the pathogenesis of gigantism include NF1 (OMIM #162200) [54, 55] and pathogenic germline variant (p.N604T) in IGSF1 (OMIM #300137), a membrane of the immunoglobulin superfamily, identified in a family with somatomammotroph lesions [56].
Genetic counseling and testing
The identification of the genes responsible for syndromic CD and gigantism has enabled the genetic diagnosis and early identification of patients and their at-risk family members. Genetic testing in clinical practice for familial syndrome has become routine. When faced with a rare endocrinopathy, such as CD, clinicians are encouraged to obtain a detailed family history and pedigree to deduce dominance and distinguish autosomal from X-linked inheritance.
Most pediatric CD cases are sporadic but can rarely arise from familial syndromes. Conversely, most pediatric gigantism is caused by familial syndromes such as FIPA, X-LAG, CNC, and MEN and rarely occur sporadically. Thus, when a clinician encounters a pediatric patient with gigantism, genetic testing and counseling regardless of family history should be considered as many of these conditions (such as FIPA or MEN-1) have decreased penetrance and first-degree relatives that are carriers may not be affected. In cases of pediatric CD, genetic testing and counseling should be performed particularly if the clinical presentation is in keeping with a familial syndrome (e.g.: spotty pigmentation that may suggest CNC). The clinician may encounter an occasional patient with presumed sporadic gigantism or CD that may harbor an underlying germline genetic alteration that predisposes to any of the familial syndromes in this review. In such cases, a low threshold for exploring genetic testing is important particularly if the clinical phenotype warrants it.
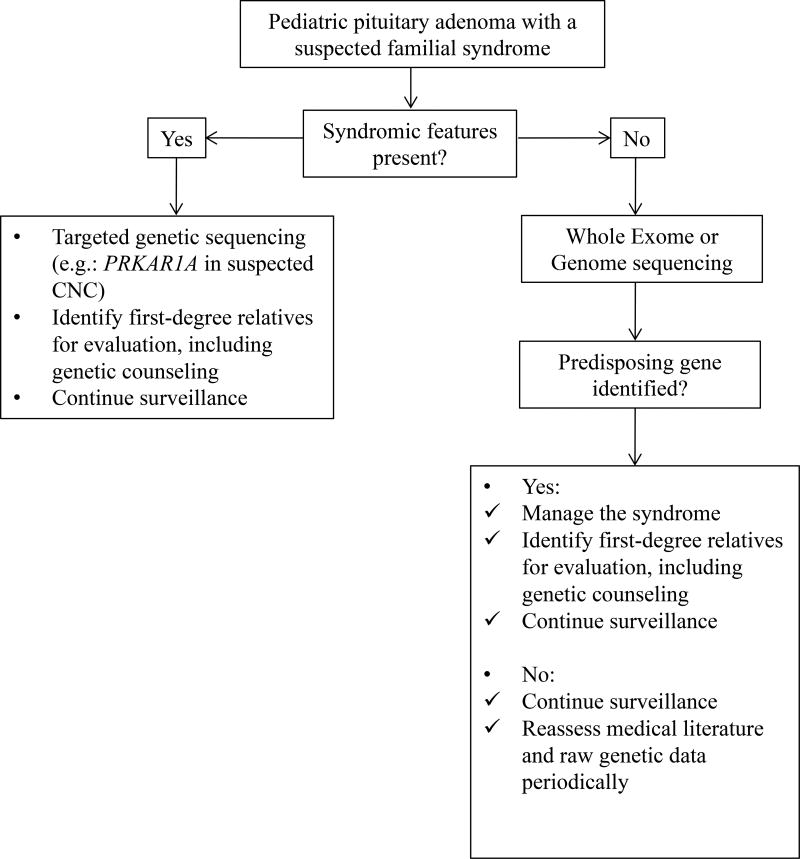
Figure 1 details an approach to screening in familial pituitary adenomas. Index cases or individuals with syndromic features should be offered targeted genetic testing (e.g.: PRKAR1A in CNC) for the syndrome in question. Index cases or individuals with negative targeted sequencing or those without a defined syndrome should be offered whole exome or genome sequencing. Screening should also be offered to a first-degree relative when a germline mutation has been identified. Additionally, the identification of a germline mutation should prompt periodic clinical, biochemical and radiological screening for the syndrome in question. Periodic (e.g.: every 2 years) reassessment of the medical literature and raw genetic data is encouraged to identify new genes or syndromes in individuals with a suspected syndrome and an unidentified genetic mutation.
Figure 1.
An approach to screening in familial pituitary adenomas. Index cases or individuals with syndromic features should be offered targeted genetic testing for the syndrome in question. Index cases or individuals with negative targeted sequencing or those without a defined syndrome should be offered whole exome or genome sequencing. Screening should also be offered to a first-degree relative when a germline mutation has been identified. Additionally, the identification of a germline mutation should prompt periodic clinical, biochemical and radiological screening for the syndrome in question.
Conclusions
Pituitary tumors are rare in children and adolescents but they could be, more often that in adults, associated with a tumor predisposition syndrome. Over the past three decades, advances in molecular genetics uncovered several molecular causes of pituitary adenomas changing the way these patients are approached by the clinicians. It is now imperative that genetic screening and counseling of affected or at risk individuals are offered to children and adolescents with pituitary tumors and an inherited tumor predisposition syndrome.
Highlights.
X-linked acrogigantism (X-LAG) is a recently described pediatric disorder that is caused by an Xq26.3 genomic duplication and characterized by early-onset gigantism.
A novel pituitary tumor-predisposing gene, CABLES1, has been uncovered in pediatric corticotropinomas.
Somatic USP8 gene mutations are a common cause of pediatric Cushing disease and associated with a higher likelihood of tumor recurrence.
Acknowledgments
This work was supported by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health (NIH).
Abbreviations
- 3PAs
pituitary adenoma association
- cAMP
cyclic adenosine monophosphate
- CNC
Carney’s complex
- FIPA
familial isolated pituitary adenoma
- GPCRs
G protein-coupled receptors
- MAS
McCune Albright syndrome
- MEN
Multiple endocrine neoplasia
- NF 1
neurofibromatosis 1
- PPNAD
primary pigmented micronodular adrenal disease
- PRKAR1A
protein kinase A regulatory subunit type 1
- X-LAG
X-linked acrogigantism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors declare that the research was conducted in absence of any potential conflict of interest.
Author and Contributors
All authors contributed equally to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND drafting the work or revising it critically for important intellectual content; AND final approval of the version to be published; AND agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Other (non-NIH) Financial support and sponsorship
None.
References
- 1.Watkins-Chow DE, Camper SA. How many homeobox genes does it take to make a pituitary gland? Trends Genet. 1998;14:284–290. doi: 10.1016/s0168-9525(98)01476-0. [DOI] [PubMed] [Google Scholar]
- 2.Kane LA, Leinung MC, Scheithauer BW, et al. Pituitary adenomas in childhood and adolescence. J Clin Endocrinol Metab. 1994;79:1135–1140. doi: 10.1210/jcem.79.4.7525627. [DOI] [PubMed] [Google Scholar]
- 3.Lafferty AR, Chrousos GP. Pituitary tumors in children and adolescents. J Clin Endocrinol Metab. 1999;84:4317–4323. doi: 10.1210/jcem.84.12.6215. [DOI] [PubMed] [Google Scholar]
- 4.Partington MD, Davis DH, Laws ER, Jr, Scheithauer BW. Pituitary adenomas in childhood and adolescence. Results of transsphenoidal surgery. J Neurosurg. 1994;80:209–216. doi: 10.3171/jns.1994.80.2.0209. [DOI] [PubMed] [Google Scholar]
- 5.Mindermann T, Wilson CB. Pediatric pituitary adenomas. Neurosurgery. 1995;36:259–268. doi: 10.1227/00006123-199502000-00004. discussion 269. [DOI] [PubMed] [Google Scholar]
- 6.Xekouki P, Azevedo M, Stratakis CA. Anterior pituitary adenomas: inherited syndromes, novel genes and molecular pathways. Expert review of endocrinology & metabolism. 2010;5:697–709. doi: 10.1586/eem.10.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly AF, Vanbellinghen JF, Khoo SK, et al. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab. 2007;92:1891–1896. doi: 10.1210/jc.2006-2513. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Ramirez LC, Gabrovska P, Denes J, et al. Landscape of Familial Isolated and Young-Onset Pituitary Adenomas: Prospective Diagnosis in AIP Mutation Carriers. J Clin Endocrinol Metab. 2015;100:E1242–1254. doi: 10.1210/jc.2015-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Trivellin G, Daly AF, Faucz FR, et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. 2014;371:2363–2374. doi: 10.1056/NEJMoa1408028. This paper describes a new pediatric disorder, X-linked acrogigantism (X-LAG) that is caused by an Xq26.3 genomic duplication and is characterized by early-onset gigantism resulting from an excess of growth hormone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Faucz FR, Tirosh A, Tatsi C, et al. Somatic USP8 Gene Mutations Are a Common Cause of Pediatric Cushing Disease. J Clin Endocrinol Metab. 2017;102:2836–2843. doi: 10.1210/jc.2017-00161. This paper establishes somatic USP8 gene mutations as a common cause of pediatric Cushing disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Hernandez-Ramirez LC, Gam R, Valdes N, et al. Loss-of-function mutations in the CABLES1 gene are a novel cause of Cushing's disease. Endocr Relat Cancer. 2017;24:379–392. doi: 10.1530/ERC-17-0131. This paper describes the role of CABLES1 as a novel pituitary tumor-predisposing gene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindholm J, Juul S, Jorgensen JO, et al. Incidence and late prognosis of cushing's syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86:117–123. doi: 10.1210/jcem.86.1.7093. [DOI] [PubMed] [Google Scholar]
- 13**.Reincke M, Sbiera S, Hayakawa A, et al. Mutations in the deubiquitinase gene USP8 cause Cushing's disease. Nat Genet. 2015;47:31–38. doi: 10.1038/ng.3166. This study was the first to identify somatic mutations in the USP8 deubiquitinase gene in 4 of 10 corticotropinomas. [DOI] [PubMed] [Google Scholar]
- 14.Kawashima ST, Usui T, Sano T, et al. P53 gene mutation in an atypical corticotroph adenoma with Cushing's disease. Clin Endocrinol (Oxf) 2009;70:656–657. doi: 10.1111/j.1365-2265.2008.03404.x. [DOI] [PubMed] [Google Scholar]
- 15.Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 16.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Human mutation. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 17.Rix M, Hertel NT, Nielsen FC, et al. Cushing's disease in childhood as the first manifestation of multiple endocrine neoplasia syndrome type 1. Eur J Endocrinol. 2004;151:709–715. doi: 10.1530/eje.0.1510709. [DOI] [PubMed] [Google Scholar]
- 18.Stratakis CA, Tichomirowa MA, Boikos S, et al. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet. 2010;78:457–463. doi: 10.1111/j.1399-0004.2010.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marquard J, Eng C. Multiple Endocrine Neoplasia Type 2. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- 20.Kasturi K, Fernandes L, Quezado M, et al. Cushing Disease in a patient with Multiple Endocrine Neoplasia type 2B. J Clin Transl Endocrinol Case Rep. 2017;4:1–4. doi: 10.1016/j.jecr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alrezk R, Hannah-Shmouni F, Stratakis CA. MEN4 and CDKN1B mutations: the latest of the MEN syndromes. Endocr Relat Cancer. 2017;24:T195–T208. doi: 10.1530/ERC-17-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgitsi M, Raitila A, Karhu A, et al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. 2007;92:3321–3325. doi: 10.1210/jc.2006-2843. [DOI] [PubMed] [Google Scholar]
- 23.Igreja S, Chahal HS, Akker SA, et al. Assessment of p27 (cyclin-dependent kinase inhibitor 1B) and aryl hydrocarbon receptor-interacting protein (AIP) genes in multiple endocrine neoplasia (MEN1) syndrome patients without any detectable MEN1 gene mutations. Clin Endocrinol (Oxf) 2009;70:259–264. doi: 10.1111/j.1365-2265.2008.03379.x. [DOI] [PubMed] [Google Scholar]
- 24.Sekiya T, Bronstein MD, Benfini K, et al. p27 variant and corticotropinoma susceptibility: a genetic and in vitro study. Endocr Relat Cancer. 2014;21:395–404. doi: 10.1530/ERC-13-0486. [DOI] [PubMed] [Google Scholar]
- 25.Forlino A, Vetro A, Garavelli L, et al. PRKACB and Carney complex. N Engl J Med. 2014;370:1065–1067. doi: 10.1056/NEJMc1309730. [DOI] [PubMed] [Google Scholar]
- 26.Correa R, Salpea P, Stratakis CA. Carney complex: an update. Eur J Endocrinol. 2015;173:M85–97. doi: 10.1530/EJE-15-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 28.Carney JA, Gordon H, Carpenter PC, et al. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine. 1985;64:270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Hernández-Ramírez CT Laura C, Lodish Maya B, Faucz Fabio R, Pankratz Nathan, Chittiboina Prashant, Lane John, Kay Denise M, Valdés Nuria, Dimopoulos Aggeliki, Mills James L, Stratakis Constantine A. Corticotropinoma as a Component of Carney Complex. Journal of the Endocrine Society. 1:918–925. doi: 10.1210/js.2017-00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leontiou CA, Gueorguiev M, van der Spuy J, et al. The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. J Clin Endocrinol Metab. 2008;93:2390–2401. doi: 10.1210/jc.2007-2611. [DOI] [PubMed] [Google Scholar]
- 31.Vierimaa O, Georgitsi M, Lehtonen R, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312:1228–1230. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein LS, Shenker A, Gejman PV, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 33.Riminucci M, Collins MT, Lala R, et al. An R201H activating mutation of the GNAS1 (Gsalpha) gene in a corticotroph pituitary adenoma. Mol Pathol. 2002;55:58–60. doi: 10.1136/mp.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandagopal R, Vortmeyer A, Oldfield EH, et al. Cushing's syndrome due to a pituitary corticotropinoma in a child with tuberous sclerosis: an association or a coincidence? Clin Endocrinol (Oxf) 2007;67:639–641. doi: 10.1111/j.1365-2265.2007.02941.x. [DOI] [PubMed] [Google Scholar]
- 35.Sahakitrungruang T, Srichomthong C, Pornkunwilai S, et al. Germline and somatic DICER1 mutations in a pituitary blastoma causing infantile-onset Cushing's disease. J Clin Endocrinol Metab. 2014;99:E1487–1492. doi: 10.1210/jc.2014-1016. [DOI] [PubMed] [Google Scholar]
- 36.Trivellin G, Correa RR, Batsis M, et al. Screening for GPR101 defects in pediatric pituitary corticotropinomas. Endocr Relat Cancer. 2016 doi: 10.1530/ERC-16-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Peng C, Song J, et al. Germline Mutations in CDH23, Encoding Cadherin-Related 23, Are Associated with Both Familial and Sporadic Pituitary Adenomas. Am J Hum Genet. 2017;100:817–823. doi: 10.1016/j.ajhg.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Araujo LJ, Lerario AM, de Castro M, et al. Transcriptome Analysis Showed a Differential Signature between Invasive and Non-invasive Corticotrophinomas. Front Endocrinol (Lausanne) 2017;8:55. doi: 10.3389/fendo.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burton T, Le Nestour E, Neary M, Ludlam WH. Incidence and prevalence of acromegaly in a large US health plan database. Pituitary. 2016;19:262–267. doi: 10.1007/s11102-015-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasufuku-Takano J, Takano K, Takei T, et al. Heterozygous gsp mutation renders ion channels of human somatotroph adenoma cells unresponsive to growth hormone-releasing hormone. Endocrinology. 1999;140:2018–2026. doi: 10.1210/endo.140.5.6731. [DOI] [PubMed] [Google Scholar]
- 41.Hayward BE, Barlier A, Korbonits M, et al. Imprinting of the G(s)alpha gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest. 2001;107:R31–36. doi: 10.1172/JCI11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landis CA, Masters SB, Spada A, et al. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 43.Beckers A, Lodish MB, Trivellin G, et al. X-linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocr Relat Cancer. 2015;22:353–367. doi: 10.1530/ERC-15-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lecoq AL, Bouligand J, Hage M, et al. Very low frequency of germline GPR101 genetic variation and no biallelic defects with AIP in a large cohort of patients with sporadic pituitary adenomas. Eur J Endocrinol. 2016;174:523–530. doi: 10.1530/EJE-15-1044. [DOI] [PubMed] [Google Scholar]
- 45.Rostomyan L, Daly AF, Petrossians P, et al. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocrine-related cancer. 2015;22:745–757. doi: 10.1530/ERC-15-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daly AF, Tichomirowa Ma, Petrossians P, et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. The Journal of clinical endocrinology and metabolism. 2010;95:E373–383. doi: 10.1210/jc.2009-2556. [DOI] [PubMed] [Google Scholar]
- 47.Lecoq AL, Kamenicky P, Guiochon-Mantel A, Chanson P. Genetic mutations in sporadic pituitary adenomas--what to screen for? Nature reviews. Endocrinology. 2015;11:43–54. doi: 10.1038/nrendo.2014.181. [DOI] [PubMed] [Google Scholar]
- 48.Salenave S, Boyce AM, Collins MT, Chanson P. Acromegaly and McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99:1955–1969. doi: 10.1210/jc.2013-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vortmeyer AO, Glasker S, Mehta GU, et al. Somatic GNAS mutation causes widespread and diffuse pituitary disease in acromegalic patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2012;97:2404–2413. doi: 10.1210/jc.2012-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambugaro S, Di Ruvo M, Ambrosio MR, et al. Early onset acromegaly associated with a novel deletion in CDKN1B 5'UTR region. Endocrine. 2015;49:58–64. doi: 10.1007/s12020-015-0540-y. [DOI] [PubMed] [Google Scholar]
- 51.Schernthaner-Reiter MH, Trivellin G, Stratakis CA. MEN1, MEN4, and Carney Complex: Pathology and Molecular Genetics. Neuroendocrinology. 2016;103:18–31. doi: 10.1159/000371819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Xekouki P, Szarek E, Bullova P, et al. Pituitary adenoma with paraganglioma/pheochromocytoma (3PAs) and succinate dehydrogenase defects in humans and mice. J Clin Endocrinol Metab. 2015;100:E710–719. doi: 10.1210/jc.2014-4297. This study describes a new association between germline SDHx mutations and pituitary adenomas/paragangliomas, called 3PAs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xekouki P, Pacak K, Almeida M, et al. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: a new association for SDH? J Clin Endocrinol Metab. 2012;97:E357–366. doi: 10.1210/jc.2011-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferner RE, Gutmann DH. Neurofibromatosis type 1 (NF1): diagnosis and management. Handbook of clinical neurology. 2013;115:939–955. doi: 10.1016/B978-0-444-52902-2.00053-9. [DOI] [PubMed] [Google Scholar]
- 55.Wimmer K, Yao S, Claes K, et al. Spectrum of single- and multiexon NF1 copy number changes in a cohort of 1,100 unselected NF1 patients. Genes, chromosomes & cancer. 2006;45:265–276. doi: 10.1002/gcc.20289. [DOI] [PubMed] [Google Scholar]
- 56.Faucz FR, Horvath AD, Azevedo MF, et al. Is IGSF1 involved in human pituitary tumor formation? Endocrine-related cancer. 2015;22:47–54. doi: 10.1530/ERC-14-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]