Abstract
The potential of sustaining release of very small (Mw < 250 g/mol) hydrophilic drugs up to several days from layer-by-layer (LbL) polyelectrolyte coated alginate microgels (Alg-Ms) was investigated. One purpose is to minimize post-surgical adhesions, which develop in 12 h to 3 days after surgery. The LbL polyelectrolyte layer would serve as a diffusion barrier for their release. The LbL polyelectrolyte bilayers were prepared using poly(allylamine) (PAH) and poly(styrene sulfonate) (PSS). Sodium benzoate (NaB, Mw = 144 g/mol) and zosteric acid (ZA, Mw = 244 g/mol), two anti-inflammatory and anti-microbial compounds, were used as model drugs. A higher number of PAH/PSS bilayer lead to a greater sustained release of both drugs, and with 4 bilayers, the release of NaB and ZA was prolonged from 24 h to 72 h and 120 h, respectively. Fitting the data to the Ritger-Peppas’ equation showed that as the bilayer number increased, the release constant and/or exponent decreased, indicating the LbL PAH/PSS bilayer effectively reduced the permeability of these two very small hydrophilic drugs. The ability to prolong the release of such small hydrophilic molecules, which has rarely been investigated previously, would find broad applications in fields such as anti-adhesion treatment and antifouling coatings.
Keywords: LbL polyelectrolyte layer, alginate microgel, hydrophilic drugs, zosteric acid, sodium benzoate
Graphical abstract

Introduction
One major aim for developing drug delivery systems is to achieve greater drug effectiveness with sustained release and reduced toxicity [1–3]. Such system is critical when the drug is designated for local delivery on the surface of an organ. An example is the application of anti-adhesion drugs for preventing post-operative adhesions, which normally develop in 12 h to 3 days after a surgery [4–6]. In an effort to prevent surgical adhesions, numerous pharmacological and barrier-based approaches have been reported [7–10]. When pharmacological agents are applied, the rapid clearance of drugs from the peritoneum leads to limited effectiveness of the intraperitoneally applied drugs [4, 6]. Instead of burst release, a sustained release would be more suitable for delivering certain therapeutics to local target sites.
Some of the anti-adhesion drugs of interests are low molecular weight (Mw < 500 g/mol) hydrophilic (solubility in water > 33 mg/mL) compounds [11–13]. One group of these hydrophilic drugs is defined as nonsteroidal anti-inflammatory drugs, which are commonly used for treating inflammation, pain, and fever [14, 15]. They can also regulate cell growth and reduce inflammatory activities when applied to a wound [4, 5]. One of the challenges for delivering such small hydrophilic drug is their high initial burst release on the target sites and their quick depletion [16–21]. Various drug carriers including micelles, liposomes, and nano/micro-particles have been designed to achieve sustained release [22–24]. However, these systems still suffer from some drawbacks such as poor drug release control, weak chemical stability, or low drug loading.
In recent years, the particulates made of hydrogels (i.e., nano-or microgels) with a size ranging from 50 nm to 500 μm have been widely recognized as promising drug carriers for controlled drug delivery. The main advantages of hydrogel based drug carriers include easy synthesis with precise size control, long-term stability, and biocompatibility [25]. However, the ease and high permeability of small molecules in hydrogels are problematic for them to serve as sustained release drug carriers. A potential approach to reduce drug permeability is to create a barrier over the hydrogels that carry hydrophilic drugs [11, 26, 27]. In this regard, the layer-by-layer (LbL) deposition of alternating cationic and anionic polyelectrolytes to form a barrier coating for sustaining drug delivery has been actively investigated [28, 29] primarily due to the process being simple, cost-effective and safe [29, 30].
The LbL coatings have been utilized for sustaining the delivery of various biomolecules including DNA, proteins and peptides, particles, and small hydrophobic drugs [31–35]. Several recent studies have also examined the release of small (Mw: 300 – 500 g/mol) hydrophilic compounds (e.g., fluorescein, ciprofloxacin hydrochloride, rhodamine B) utilizing LbL polyelectrolyte bilayers as the barrier. The total release of these drugs, however, still occurs within minutes to a few hours [29, 30, 36, 37], and a faster depletion is expected when the molecule is smaller and/or more soluble in water [35]. However, the release, especially sustaining the release, of very small (Mw < 250 g/mol) hydrophilic drugs from LbL polyelectrolyte coated hydrogel based carriers has rarely been investigated [33]. In order to expand the applications of this class of drugs (e.g., for anti-adhesion treatment), investigations into designing suitable carriers that prolong (e.g., up to 72 h) their release are needed.
In our study, we created LbL deposited polyelectrolyte bilayers on alginate microgels (LbL-Alg-Ms) as drug carriers. Alginate, a linear anionic polysaccharide, has been widely investigated as a carrier material for drug delivery [38–40]. The LbL deposited polyelectrolyte bilayers were hypothesized to serve as a diffusion barrier that would significantly retard the release of very small hydrophilic drugs (Mw < 250 g/mol, solubility in water > 33 mg/mL) by increasing the diffusion path and potentially introducing molecular interactions, such as electrostatic interactions and hydrogen-bonding, between drug molecules and the bilayers. The primary goal of this study was to evaluate the effectiveness of the LbL Poly (allylamine)/Poly (sodium 4-styrenesulfonate) (PAH/PSS) bilayers in retarding the release of small hydrophilic drugs from alginate microgels, and desirably to prolong their release up to three days, the critical period for the development of post-surgical adhesions. PAH/PSS is the most commonly used and well-studied polyelectrolyte pair for LbL deposition in drug delivery applications [29, 30, 36, 41– 44]. The two drugs to be evaluated were sodium benzoate (NaB, Mw = 144 g/mol, solubility in water = 630 mg/mL) and zosteric acid (ZA, Mw = 244 g/mol, solubility in water = 300 mg/mL). They are potential anti-microbial and anti-inflammatory compounds [45–47] that would reduce inflammatory activities and be beneficial in post-surgical adhesions’ management. The release was monitored via a topical Franz diffusion cell to mimic the conditions where the drugs would be mixed in a gel to be applied on the surgical sites. Our results showed that the LbL PAH/PSS bilayers were effective barriers in slowing down the release of these two very small hydrophilic drugs and sustained the release of NaB and ZA up to 3 days and 5 days, respectively, sufficiently long enough for treating potential adhesions post-surgery.
Experimental section
Materials
Most of the starting materials were purchased commercially and used as received. All reagents and solvents were of ACS or HPLC grade. Sodium alginate, sodium benzoate (NaB), phosphate buffered saline (PBS) and sodium chloride (NaCl) were obtained from Sigma-Aldrich. Calcium chloride (CaCl2) anhydrous was purchased from EMD. Zosteric acid (ZA), with a purity > 95 %, was synthesized in house by using p-coumaric acid (98% pure) and chlorosulphonic acid (99% pure) [48] – both purchased from Sigma-Aldrich – as the reactants. Crocein orange G (CG) was obtained from TCI. Poly (allylamine) (PAH) (average Mw ~ 15,000, 15 wt. % solution in water) was purchased from Polysciences, Inc. Poly (sodium 4-styrenesulfonate) (PSS) (average Mw ~ 70,000) and acridine orange (AO) were purchased from Sigma-Aldrich. Deionized water (DI) was purified in house and had a conductivity of ~1 μS/cm or less. Some main materials used in this study and their properties are summarized in Table 1.
Table 1.
Materials used in this study and their structures and some basic properties
| Materials | Chemical structure | Molecular weight (g/mol) |
Solubility in water (mg/mL) |
Polar surface area (Å2) |
|---|---|---|---|---|
| sodium benzoate (NaB) |

|
144 | 630a | 40a |
| zosteric acid (ZA) |

|
244 | 300b | 109a |
| crocein orange G (CG) |
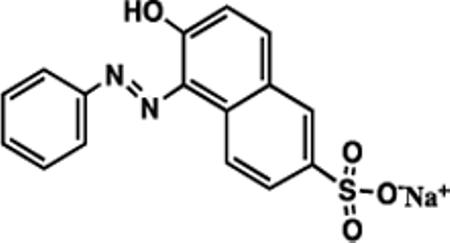
|
350 | 26b | 107a |
| poly (styrene sulfonate) (PSS) |

|
~ 70,000 | soluble | -- |
| poly (allylamine) PAH |

|
~ 15,000 | soluble | -- |
The value was obtained from pubchem.ncbi.nlm.nih.gov;
The value was determined experimentally.
Preparation of alginate microgels (Alg-Ms)
Alg-Ms were produced by a special air droplet generator. The drug, either NaB or ZA, was added into both alginate solution and gelation solution for microgels preparation. Briefly, calcium chloride (1.5 % w/v) and the drug (3 % w/v) were dissolved in DI water (200 mL) as the gelling medium. Alginate (2 % w/v) and the drug (4 % w/v) were dissolved in DI water (8 mL) and co-axial extruded as droplets through a 25-gauge needle using a 10-mL syringe barrel. The droplets formed on the needle tip were sheared off by the air flow at a rate of 5 LPM. The gelation process took place in the gelling medium for 30 min under gentle stirring using a magnetic stirring bar. Eventually, the Alg-Ms were collected by filtering the Alg-Ms containing gelling solution through a stainless-steel mash with a grid size of ~ 100 μm.
Deposition of polyelectrolytes bilayer(s) on Alg-Ms
The PAH solution was prepared at a concentration of 2 mg/mL in DI water containing 0.1 M NaCl, and the PSS solution was also prepared at a concentration of 2 mg/mL in DI water and contained either 0.1 M NaCl or 1.5 – 2 wt.% of the drug (NaB or ZA). For deposition of the first layer, 1 mL of PAH solution was added to a 2 mL micro-centrifuge tube containing 270 mg of negatively charged alginate microgels. Adsorption was allowed to proceed for 2 min. After the microgels settled, the solution was removed, and the microgels were washed, twice, by adding DI water to the centrifuge tube followed with gentle shaking and discarding of the liquid to remove free polyelectrolytes on the surface. A 1 mL aliquot of PSS solution was then added to the PAH-coated alginate microgels and allowed to interact for 2 min, followed by the removal of the solution and washing with DI water. The process was repeated to deposit polyelectrolyte multilayers onto Alg-Ms with a desired number of PAH/PSS bilayers.
Determination of drug loading
For drug loading in the carriers (Alg-Ms and LbL-Alg-Ms), ~ 200 mg of each carrier-drug pair was used. The polyelectrolyte bilayers were dissembled and the alginate cores were de-gelled by using 40 mL 1M NaOH solution at 80 °C under stirring for 2 h. After vortexing and centrifuging the solution, the supernatant was collected and diluted 20× using 1× PBA to measure the sodium benzoate (NaB) or zosteric acid (ZA) concentration by using UV-vis spectroscopy (Model UV-1601, Shimadzu Corporation, Columbia, MD) at 225 nm or 273 nm, respectively. The exact concentration of each drug was determining by using a pre-constructed calibration curve of absorbance intensity vs. concentrations (0–20 mg/mL) of the drug. All measurements were performed in triplicate. The amount of drug loaded is estimated using:
Characterizations of microgels: morphology and size
A drop of Alg-Ms or LbL-Alg-Ms suspended in DI water was placed on a glass slide and observed with an inverted IX-70 microscope (Olympus IX71, B&B Microscope, Pittsburg, PA) equipped with a differential interference contrast (DIC) slider (U-DICT, Olympus). Bright field images were taken using either 4× (Olympus, PLAN4 /0.1 NA) or 10X (Olympus, UPLFLN10×PH /0.3 NA, W.D. 10) objective lens with the DIC slider in. For fluorescence images, the LbL-Alg-Ms spread on glass slide were illuminated with a Xenon light passing through a blue/green fluorescent filter. The images were viewed by an eye-piece digital camera (HDCB-90D) connected to the microscope, and captured by the YAWCAM software (version 0.5.0, Magnus Lundvall). The size, in term of radius, of the microgels, and the fluorescence intensity of LbL-Alg-Ms were measured using ImageJ (Version 1.43t, National Institute of Health) from the captured images. To obtain reliable values, images of at least 30 microgels were measured for each sample.
In vitro release of NaB or ZA from Alg-Ms and LbL-Alg-Ms
In vitro drug release from LbL-Alg-Ms was followed using a Franz-cell diffusion setup to simulate the release conditions of drugs applied topically on a surgical site. The Franz diffusion cell had two compartments, the donor compartment, which was filled with drug carriers, and the receptor compartment, which was filled with ~ 60 mL of the release medium (i.e., PBS). A thin cotton pad (thickness ~ 1 mm) was used as the membrane in between the two compartments. The volume of the receptor was maintained by replacing the sampled volume with fresh PBS solution. ~ 200 mg of LbL-Alg-Ms containing a certain drug was used for each release study. At predetermined time points (sampling every 10 min during the first hour, and then every hour up to 16 h, and finally every 4 or 8 h after 24 h), 0.6 mL aliquot was withdrawn from the release medium, and the withdrawn aliquot volume was replaced with fresh PBS. Each sample was diluted to 3 mL using 1× PBS prior to measuring the NaB or ZA concentration via UV-vis. The amount of drug released from each carrier was determined and the cumulative percentage release was calculated and plotted vs. time. All experiments were carried out in triplicate.
The release data was also fitted with the Ritger-Peppas equation [49]. Based on the constrains of the equation and the sampling uncertainty of the first few samples, the cumulative release % from 10% to 60% for each individual data set was fitted using MATLAB to obtain a pair of n (order) and k (coefficient) values. For each carrier-drug combination, the average and standard error of n value and k value were calculated from at least three independent sets of n and k values and reported.
Statistical analysis
The statistical analysis on drug loading of Alg-Ms and LbL-Alg-Ms was performed using the analysis of variance (ANOVA) and the student t-test. The differences were considered statistically significant at the level of p < 0.05.
Results and discussion
Formation and structure of LbL-Alg-Ms
The schematic of LbL deposition of polyelectrolyte layers of PAH and PSS on the surface of alginate microgels (Alg-Ms) is illustrated in Figure 1. Alg-Ms, prepared by the co-axial air-flow technique using a G25 needle and an air flow rate of 5 L/min, had a mean radius of 333.3 ± 21.1 µm (Table 2). They were negatively charges, due to carboxylic acid groups on the polyguluronate units of alginate, and served as templates for LbL deposition of polyelectrolytes [50, 51]. The most widely used and well-studied polyelectrolyte pair for LbL deposition in drug delivery applications is PAH and PSS [29, 30, 41–43], which was chosen for this study. The positively charged PAH, having one amine group per monomer unit, was chosen as the first layer to deposit on the negatively charged Alg-Ms, and electrostatic interactions promoted the formation of this first PAH layer and additional PSS-PAH alternating layers. Evidence of LbL PAH/PSS bilayers on Alg-Ms was observed (Figure 2B & 2D). Without LbL PAH/PSS bilayer(s), the edge of Alg-Ms appeared transparent. 4 bilayers of PAH/PSS formed a homogenous and smooth shell on the surface of Alg-Ms, caused the edges of microgels to appear thicker and darker as compared to Alg-Ms alone. Wrinkling features could also be seen on the surface of LbL-Alg-Ms (Figure 2B). The observed LbL layer could be the result of the inner polyelectrolyte chains diffused to the surface and formed a complex with the counter polymer [52]. The size of LbL-Alg-Ms was basically the same as that of Alg-Ms (Table 2). The indifference in the size of Alg-Ms and LbL-Alg-Ms was due to the relatively thin polyelectrolyte bilayer [43, 53] (10–50 nm) as compared to the size of microgels (~ 330 μm).
Fig. 1.
Schematic representation of an alginate microgel deposited with poly (allylamine) (PAH) and poly (styrene sulfonate) (PSS) bilayers by the layer-by-layer (LbL) technique. Steps 1–4 show the deposition of one bilayer of PAH/PSS (LbL-1-Alg-Ms) on an alginate microgel loaded with drug. Step 5 shows additional drug uptake and step 6 illustrates the release.
Table 2.
The average mass, drug loading and size of the microgels
|
Samples
(carrier-drug) |
Average microgels mass (mg) |
Drug loading
(wt %) |
radius
(before release) (μm) |
|
|---|---|---|---|---|
| Before release | After release | |||
| Alg-Ms-NaB | 203.0 ± 4.4 | 285.0 ± 37.1 | 2.78± 0.15 | 333.3 ± 21.1 |
| LbL-1 -Alg-Ms-NaB | 200.2 ± 17.5 | 280.0 ± 23.4 | 2.68 ± 0.13 | 319.7 ± 16.5 |
| LbL-2-Alg-Ms-NaB | 202.5 ± 3.9 | 247.7 ± 44.5 | 2.53 ± 0.12 | 327.0 ± 37.2 |
| LbL-4-Alg-Ms-NaB | 199.6 ± 4.0 | 256.0 ± 29.0 | 2.22 ± 0.10 | 339.5 ± 26.3 |
| LbL-6-Alg-Ms-NaB | 225.0 ± 14.8 | 228.7 ± 4.0 | 2.43 ± 0.29 | 330.9 ± 12.5 |
| dry Alg-Ms-NaB | 21.0 ± 2.5 | 220.0 ± 24.2 | 30.55 ± 9.94 | 173.9 ± 17.5 |
| dry LbL-4-Alg-Ms | 20.1 ± 2.9 | 229.3 ± 18.3 | 45.48 ± 5.03 | 327.5 ± 33.2 |
| Alg-Ms-ZA | 200.7 ± 17.8 | 262.3 ± 22.0 | 1.79 ± 0.24 | 342.3± 14.4 |
| LbL-4-Alg-Ms-ZA | 205.3 ± 7.4 | 245.33 ± 28.4 | 1.78 ± 0.19 | 334.7± 34.4 |
The mean values and standard deviations were estimated from three sets of measurements.
Fig. 2.
Optical microscopic bright field images of Alg-Ms [(A), (C)] and LbL-6-Alg-Ms [(B), (D)]. A and B are wet microgels in water, and C and D are one-day air-dried microgels. The inset in each image is a zoom-in of a microgel. The increase in edge roughness of LbL-6-Alg-Ms is noticed. The scale bar in the A & B main images and insets are 500 µm and 200 µ m, respectively. The scale bar for C & D main images and insets are 200 µm and 33 µ m, respectively.
The presence of PAH/PSS bilayer in LbL-Alg-Ms was better assessed by the difference between dried Alg-Ms and dried LbL-Alg-Ms. We subjected wet microgels to one-day air-drying. The optical microscopic images of dried Alg-Ms and dried LbL-4-Alg-Ms were taken and analyzed (Figure 2C & 2D). The Alg-Ms lost their spherical shape after drying. As compared to wet LbL-4-Alg-Ms, dried Alg-Ms showed smoother and clearer edges, but appeared to have a fragile surface morphology, and the size reduced dramatically after drying (to 173.9 ± 17.5 µm, Table 2). For dried LbL-4-Alg-Ms, the size only decreased slightly (to 327.5 µm) from its wet state (Table 2), but their surface contained many folds with rough edges (Figure 2D). The folds and creases are the characteristics of collapsed membrane of polyelectrolytes. Similar observations have been reported earlier for hollow polymer shells comprising PAH and PSS [41, 44, 54–55]. The formation of these membranes demonstrated that LbL of PAH/PSS was successfully assembled on alginate microgels.
Since most commonly used characterization techniques, such as atomic force microscopy (AFM), for quantifying polyelectrolyte layers deposited on planar surfaces or on nano/microparticles [32, 43] could not be easily applied to our large microgels (~ 330 μm in radius) with rough surfaces, the exact thickness of the PAH/PSS layer on our microgels was not measured. The difficulty stemmed from the presence of alginate core that, even with a complete de-gelation, could not diffuse out of the PAH/PSS membrane. Upon drying, the topography of alginate core inside the PAH/PSS shell became quite heterogeneous, making it challenging to obtain the polyelectrolyte membrane thickness using AFM or confocal as done in other studies where the core had been completely dissolved away [35, 36, 44]. As a result, a fluorescence based method was used as an alternative for tracking the deposition of PAH/PSS bilayer. For this purpose, acridine orange (AO), which can electrically bind to the PSS layer to form PSSAO [56], was used as a tracking agent. The green fluorescent observed on Alg-Ms (Figure 3A-D) suggested the deposition of PSS, and the fluorescence intensity increased with the increase of bilayer number. The mean fluorescence intensity for LbL-Alg-Ms was found to positively correlate to the number of bilayers deposited (Figure 3E), which agreed with what was reported previously [37, 52].
Fig. 3.
Fluorescence microscopic images of alginate microgels deposited with (A) 1 bilayer, (B) 2 bilayers, (C) 4 bilayers, and (D) 6 bilayers of PAH/PSS (with a scale bar of 200 µ m). The fluorescence intensities of the images, taken of 30 microgels, are analyzed and summarized in (E). The error bars are the standard derivations from the measurements.
Drug loading and release of loaded drug from LbL-Alg-Ms
Figure 4 shows drug loading of NaB and ZA in Alg-Ms or LbL-Alg-Ms under different preparation conditions. For NaB (Figure 4A), the drug loading in Alg-Ms was ~ 2.8 wt %. Using the PSS solution without NaB, the loading decreased significantly to ~ 2.3 wt.%, ~ 1.5 wt.%, ~ 0.6 wt.%, and ~ 0.3 wt.% after depositing 1, 2, 4 and 6 bilayers of PAH/PSS, which was caused by the fast diffusion of NaB out from Alg-Ms-NaB into the polyelectrolyte solutions during LbL deposition. By adding 1.5 wt.% of NaB in the PSS solution during LbL deposition, this diffusion issue was alleviated, leading to a drug loading of 2.2 wt % after depositing 6 bilayers. Also, by increasing NaB concentration in the PSS solution to 2 wt.%, the final drug loading increased slightly, while not statistically different, to 2.4 wt.%. Similar results were observed for ZA (Figure 4B). The drug loading in Alg-Ms-ZA was 1.8 wt.%, the value decreased, significantly, to 1 wt.% after depositing 6 PAH/PSS bilayers when no ZA was added to the PSS deposition solution. By adding 1.5 wt.% of ZA to the PSS solution during bilayer deposition, the drug loading significantly increased (p < 0.05) to ~ 1.8 wt. % after 6 bilayers, which appeared to further increase slightly (to 1.9 wt.%) by using 2 wt.% of ZA in the PSS solution. Therefore, by adding NaB or ZA into the polyelectrolyte solutions used for LbL deposition, drug loading of these small hydrophilic drugs in the LbL-Alg-Ms greatly increased.
Fig. 4.
Drug loading of (A) sodium benzoate (NaB) and (B) Zosteric acid (ZA) in LbL-Alg-Ms containing different number of bilayer and prepared using a PSS solution containing a drug concentration of 0 wt.% (dotted column), 1.5 wt.% (grey column), and 2 wt.% (lined column). The error bars are the standard derivations from the measurements. Symbols above the bars are used to indicate that there is a statistical difference in NaB or ZA loading for carriers prepared under two different conditions (*: LbL-Alg-Ms prepared using PSS solution containing no drug vs. Alg-Ms, &: LbL-Alg-Ms prepared using PSS solution containing 1.5 wt.% of drug vs. those prepared using PSS solution containing no drug; #: LbL-Alg-Ms prepared using PSS solution containing 2 wt.% of drug vs. those prepared using PSS solution containing no drug; and @: LbL-Alg-Ms prepared using the PSS solution containing 2 wt.% of drug vs. those prepared using PSS solution containing 1.5 wt.% of drug).
The releases study of NaB from Alg-Ms-NaB and LbL-Alg-Ms-NaB (Figure 5) into a phosphate-buffered saline (PBS) medium at 37°C was carried out by using a Franz diffusion cell, which we believe, as compared to a dialysis bag, is better at mimicking the quantities of water involved on the surface of a surgical sites. The NaB release profile from 4 LbL PAH/PSS bilayers coated Alg-Ms, i.e., LbL-4-Alg-Ms-NaB, is presented in Figure 5A and5B. As compared to Alg-Ms-NaB (Figure 5A), the initial burst release was greatly reduced. In fact, ~ 10% of NaB released within 20 min from Alg-Ms-NaB, and it prolonged to ~ 1.8 h from LbL-4-Alg-NaB. For Alg-Ms-NaB, ~ 90 % NaB was released in ~ 13.5 h, but it took ~ 47 h for ~ 90% of NaB to release from LbL-4-Alg-Ms-NaB (Table 3).
Fig. 5.
The release profiles of sodium benzoate (NaB) from (A) Alg-Ms-NaB and LbL-4-Alg-Ms-NaB; and (B) LbL-Alg-Ms-NaB with 1, 2, 4, and 6 bilayers of PAH/PSS. The release medium was PBS solution at 37 °C. (C) and (D) are the release profiles of zosteric acid (ZA) and crocein orange G (CG) from LbL-4-Alg-Ms, respectively.
Table 3.
Time taken to reach 10 %, 60 % and 90 % (Mt/M∞) of NaB released
|
Samples
(carrier-drug) |
NaB release time (h) at Mt/M∞ of |
||
|---|---|---|---|
| 10% | 60% | 90% | |
| Alg-Ms-NaB | 0.4 ± 0.1 | 3.0 ± 0.8 | 13.5 ± 2.1 |
| LbL-1 -Alg-Ms-NaB | 1.7 ± 0.6 | 10.6 ± 1.9 | 22.0 ± 2.0 |
| LbL-2-Alg-Ms-NaB | 1.7 ± 0.4 | 16.7 ± 2.4 | 41.6 ± 3.0 |
| LbL-4-Alg-Ms-NaB | 1.8 ± 0.6 | 22.2 ± 2.3 | 47.3 ± 5.2 |
| LbL-6-Alg-Ms-NaB | 2.5 ± 0.5 | 27.3 ± 3.4 | 60.8 ± 4.2 |
To further illustrate that NaB release was sustained by coating Alg-Ms-NaB with PAH/PSS bilayer(s), NaB release from Alg-Ms-NaB coated with different numbers of bilayers was also carried out (Figure 5B). As expected, as the number of PAH/PSS bilayer increased, the release of NaB decreased. The time taken for a cumulative release of 10% (relating to the initial burst release), 60% (relating to the end of data fitting period of the Ritger-Peppas’ equation) and 90% (towards the end of release) of NaB from these carriers was extracted and summarized in Table 3. The data clearly showed that the initial burst release was prolonged from 0.3 h to ~ 2 h by depositing PAH/PSS bilayer(s) on Alg-Ms. The time needed for 60% cumulative release extended from 3 h to 11 h, 17 h, 22 h, and 27 h with 1, 2, 4 and 6 bilayers of PAH/PSS deposited on Alg-Ms, respectively. Similar results were observed for 90% cumulative release. By embedding crystals/particles of hydrophilic substances inside capsules made of polyelectrolyte layers, slower release of these substances, due to reduced diffusion coefficient with the presence of polyelectrolyte layers, has been reported [30, 35, 36]. Such diffusion coefficient reduction was also expected with PAH/PSS bilayers on Alg-Ms. In addition, two other factors would further retard drug diffusion in our case: (1) an alginate core was utilized for distributing drug molecules in our study, which would lead to an increased diffusion path, and (2) potential interactions between the PAH/PSS bilayers and the alginate core would result in a less permeable shell. As a result, as compared to Alg-Ms alone, the release of NaB from LbL-Alg-Ms prolonged (4 – 9)×. Therefore, the PAH/PSS bilayer served as an effective transport barrier in slowing down the transport of NaB, a very small hydrophilic compound, from an alginate core to an aqueous medium; also a higher number of PAH/PSS bilayer lead to a greater barrier.
A second model drug – zosteric acid (ZA) – was loaded in Alg-Ms and LbL-4-Alg-Ms to further demonstrate that LbL-Alg-Ms could sustain the release of small hydrophilic drugs. The release results of ZA are shown in Figure 5C. 90% of ZA released from LbL-4-Alg-Ms-ZA in ~ 87 hr as compared to ~ 17 h for 90% of ZA to release from Alg-Ms. ZA (Mw = 244 g/mol, polar surface area = 109 Å2) is larger and more polar than NaB (Mw = 144 g/mol, polar surface area = 40.1 Å2), it could be more difficult for ZA to permeate/diffuse, especially through the PAH/PSS bilayers, into the release medium. To further verify that the molecular size has an effect on drug release from LbL-Alg-Ms, we entrapped a hydrophilic dye [57], crocein orange G (CG, Mw = 350 g/mol, polar surface area = 107 Å2, similar to that of ZA), in LbL-4-Alg-Ms and followed its release. As shown in Figure 5D, after 168 h (7 days) and 336 h (14 days), respectively, only ~ 80% of CG from Alg-Ms-CG and ~ 70% of CG from LbL-4-Alg-Ms-CG, had been released, much slower than those for ZA and NaB.
Release mechanism and kinetics
In order to understand the release behaviors of hydrophilic drugs from LbL-Alg-Ms, the experimental release data was fitted with drug release models. While the release from these microgels were likely complicated by potential variations of polymer properties, drug solubility, and hydrogel swelling, a simplified model developed by Ritger and Peppas based on the assumptions of no bulk flow, no reaction, and constant diffusivity was utilized to provide some understanding. The model leads to a power law relationship, or the Ritger-Peppas equation [49]:, where Mt is the amount of drug released out to medium at specific time point, M∞ is the total amount of drug released at equilibrium, k is a mass transfer coefficient relating to diffusivity, and n is the diffusional exponent, which is relative to release mechanism and drug carrier geometry [49]. For slightly swellable (i.e., volume increase ≤ 25%) spherical carriers such as the case studied here, when the carriers are uniform in size, an n value of 0.43 would suggest that the release is governed by Fickian diffusion, an n value between 0.43 and 0.85 suggests a combination of diffusion and swelling controlled release, an n value of 0.85 indicates Case-II transport or transport controlled by polymer relaxation/swelling, and an n value > 0.85 means super Case-II transport where carrier erosion plays a dominate role in the release [57]. When the drug carriers are heterogeneous in size, the n value would deviate from those having a homogenous size [57].
The release data were fitted to the Ritger-Peppas equation and the obtained release parameters are summarized in Table 4. For all the microgel carriers of NaB, n value ranged from 0.82 to 0.60, suggesting that the release of NaB from these carriers was controlled by both diffusion and carrier swelling. The swelling extent (Table 2) of Alg-Ms-NaB and LbL-1-Alg-Ms-NaB, however, was greater (~ 40%), as compared to those carriers with a higher number of bilayer deposited, and as the number of PAH/PSS bilayer increased, the swelling extent decreased (e.g., ~ 1% for LbL-6-Alg-Ms-NaB). The k values for those carriers with PAH/PSS bilayer(s) were significantly smaller than that of Alg-Ms-NaB, meaning the release rate decreased when Alg-Ms-NaB were coated by PAH/PSS bilayer(s). The k value maintained at ~ 11 for 1, 2 and 4 bilayers of PAH/PSS, while the n value showed a slight decreasing trend. The decrease in n value could simply be the result of the microgels being more heterogeneous in size [57] (8–11% variation from the average value, Table 2) for 2 and 4 bilayer deposited Alg-Ms. The k value dropped to ~ 6 with 6 PAH/PSS bilayers coated Alg-Ms-NaB. In this case, as compared to LbL-1-Alg-Ms-NaB, the n value was also smaller. Even though we could not conclude that the release mechanism had been altered by depositing PAH/PSS bilayer(s) to Alg-Ms-NaB, the decrease in n value and/or k value resulted, leading to a slower release of NaB from PAH/PSS deposited Alg-Ms-NaB.
Table 4.
Parameters obtained by fitting the release data to Ritger-Peppas equation
|
Samples
(carrier-drug) |
k | n |
|---|---|---|
| Alg-Ms-NaB | 34.9 ± 7.6 | 0.74 ± 0.03 |
| LbL-1-Alg-Ms-NaB | 10.6 ± 2.4 | 0.82 ± 0.07 |
| LbL-2-Alg-Ms-NaB | 11.6 ± 2.4 | 0.64 ± 0.06 |
| LbL-4-Alg-Ms-NaB | 11.0 ± 3.4 | 0.60 ± 0.10 |
| LbL-6-Alg-Ms-NaB | 6.3 ± 1.3 | 0.71 ± 0.04 |
| Alg-Ms-ZA | 19.2 ± 3.0 | 1.04 ± 0.12 |
| LbL-4-Alg-Ms-ZA | 10.8 ± 0.8 | 0.51 ± 0.05 |
For ZA, both n and k values decreased, from ~ 1.0 to ~ 0.5 and from ~ 19 to ~ 11, respectively, as Alg-Ms-ZA were coated with 4 PAH/PSS bilayers. The slower release of ZA from these carriers, as compared to NaB, could be due to drug properties, especially their polarity, influencing the release. Drug molecules with a higher charge (e.g., ZA) likely had more interactions with the polyelectrolyte bilayer as they migrated through the barrier, hence slowed down their release. Also, the greater interactions could potentially alter the release mechanism from a combination of diffusion/swelling/erosion (e.g., Alg-Ms-ZA, n = 1.04) to a mainly diffusion controlled release (e.g., LbL-4-Alg-Ms-ZA, n = 0.51).
Conclusions
We have demonstrated that the layer-by-layer (LbL) deposited PAH/PSS on alginate microgels served as an effective barrier for slowing the release of very small hydrophilic drugs (Mw < 250 g/mol) from such carriers. Negative charged alginate microgels (Alg-Ms) were used as templates for LbL deposition of PAH and PSS. The PAH/PSS bilayers could be sequentially adsorbed onto the surface of Alg-Ms, and formed a surface membrane. In vitro drug release of sodium benzoate (NaB) from LbL-PAH/PSS coated Alg-Ms-NaB was found to be sustained, and the extent of slowed release was proportional to the number of PAH/PSS bilayer. Increasing the number of PAH/PSS bilayer up to 4 rendered the release of NaB from LbL-4-Alg-Ms-NaB up to 72 h (3 days). While the swelling of microgels was suppressed by depositing the PAH/PSS bilayers to Alg-Ms, the release of NaB from these carriers was found to still be governed by a combination of diffusion and swelling as that of un-coated Alg-Ms. Nevertheless, the decrease of n value and/or k value was observed as the number of PAH/PSS bilayer increased. For zosteric acid (ZA), having a higher polarity and molecular weight than those of NaB, the transport of its molecules through PAH/PSS layer became harder, and the release of ZA was prolonged further and the release mechanism switched from the combined diffusion and swelling to mainly diffusion controlled. This study illustrates the potential of polyelectrolyte layers to be used as effective barriers for hydrogel drug carriers in sustaining the release of small hydrophilic drugs, especially those with a molecular weight of < 250 g/mol of which controlled release is generally difficult to achieve. This type of carriers, which are designed for entrapping and releasing very small hydrophilic anti-inflammatory anti-microbial drugs, could be incorporated with other materials (e.g., thermos-reversible gels for spreading on a surface) as implantable devices for anti-adhesion treatments or as transdermal patches for anti-inflammatory treatments. The ability to prolong the release of very small hydrophilic compounds would also expand the applications of this type of carriers in other fields including antifouling coatings and catalysis.
Highlights.
Layer-by-layer (LbL) deposition of poly (allylamine) (PAH) and poly (styrene sulfonate) (PSS) on alginate microgels (Alg-Ms) was achieved
The sustained release of two small hydrophilic anti-inflammatory drugs: sodium benzoate (NaB) and zosteric acid (ZA), from the LbL bilayer coated Alg-Ms was evaluated.
The PAH/PSS bilayers effectively retarded the release of NaB and ZA
The release was prolonged from hours to at least 3 days, the critical period for the development of post-surgical adhesions.
The PAH/PSS bilayers altered the release mechanism from a combined diffusion/swelling to solely diffusion as bilayer number increased.
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 1R15GM09762601A1.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sokolsky-Papkov M, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007;59:187–206. doi: 10.1016/j.addr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Malmsten M, Bysell H, Hansson P. Biomacromolecules in microgels — Opportunities and challenges for drug delivery. Curr. Opin. Colloid Interface Sci. 2010;15:435–444. [Google Scholar]
- 3.Ariga K, Lvov YM, Kawakami K, Ji Q, Hill JP. Layer-by-layer self-assembled shells for drug delivery. Adv. Drug Deliv. Rev. 2011;63:762–771. doi: 10.1016/j.addr.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Yeo Y, Kohane DS. Polymers in the prevention of peritoneal adhesions. Eur. J. Pharm. Biopharm. 2008;68:57–66. doi: 10.1016/j.ejpb.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maciver AH, McCall M, James Shapiro AM. Intra-abdominal adhesions: cellular mechanisms and strategies for prevention. Int. J. Surg. 2011;9:589–594. doi: 10.1016/j.ijsu.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J. Surg. Res. 2011;165:91–111. doi: 10.1016/j.jss.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, Yeo Y, Highley CB, Bellas E, Benitez CA, Kohane DS. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials. 2007;28:975–983. doi: 10.1016/j.biomaterials.2006.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falabella CA, Melendez MM, Weng L, Chen W. Novel macromolecular crosslinking hydrogel to reduce intra-abdominal adhesions. J. Surg. Res. 2010;159:772–778. doi: 10.1016/j.jss.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Ni J, Chen L, Yu L, Xu J, Ding J. Biodegradable and thermoreversible PCLA– PEG–PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials. 2011;32:4725–4736. doi: 10.1016/j.biomaterials.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Wei C-Z, Hou C-L, Gu Q-S, Jiang L-X, Zhu B, Sheng A-L. A thermosensitive chitosan-based hydrogel barrier for post-operative adhesions' prevention. Biomaterials. 2009;30:5534–5540. doi: 10.1016/j.biomaterials.2009.05.084. [DOI] [PubMed] [Google Scholar]
- 11.Arpicco S, Battaglia L, Brusa P, Cavalli R, Chirio D, Dosio F, Gallarate M, Milla P, Peira E, Rocco F, Sapino S, Stella B, Ugazio E, Ceruti M. Recent studies on the delivery of hydrophilic drugs in nanoparticulate systems. J. Drug Deliv. Sci. Technol. 2016;32:298–312. [Google Scholar]
- 12.Vrignaud S, Benoit J-P, Saulnier P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials. 2011;32:8593–8604. doi: 10.1016/j.biomaterials.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 13.Eloy JO, Claro de Souza M, Petrilli R, Barcellos JPA, Lee RJ, Marchetti JM. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf. B: Biointerfaces. 2014;123:345–363. doi: 10.1016/j.colsurfb.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AG, Quinn DI, Day RO. Non-steroidal anti-inflammatory drugs. Med. J. Aust. 1995;163:155–158. doi: 10.5694/j.1326-5377.1995.tb127972.x. [DOI] [PubMed] [Google Scholar]
- 15.Whelton A, Sturmer T, Porter GA. Non-steroidal anti-inflammatory drugs. In: de Broe ME, Porter GA, Bennett WM, Verpooten GA, editors. Clinical Nephrotoxins: Renal Injury from Drugs and Chemicals. Springer; Netherlands: 2003. pp. 279–306. [Google Scholar]
- 16.Natarajan JV, Nugraha C, Ng XW, Venkatraman S. Sustained-release from nanocarriers: a review. J. Control. Release. 2014;193:122–138. doi: 10.1016/j.jconrel.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Cook RO, Pannu RK, Kellaway IW. Novel sustained release microspheres for pulmonary drug delivery. J. Control. Release. 2005;104:79–90. doi: 10.1016/j.jconrel.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Mao Z, Ma L, Gao C, Shen J. Preformed microcapsules for loading and sustained release of ciprofloxacin hydrochloride. J. Control. Release. 2005;104:193–202. doi: 10.1016/j.jconrel.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Coughlan DC, Corrigan OI. Release kinetics of benzoic acid and its sodium salt from a series of poly(N-isopropylacrylamide) matrices with various percentage crosslinking. J. Pharm. Sci. 2008;97:318–330. doi: 10.1002/jps.21095. [DOI] [PubMed] [Google Scholar]
- 20.Byrne RS, Deasy PB. Use of porous aluminosilicate pellets for drug delivery. J. Microencapsul. 2005;22:423–437. doi: 10.1080/02652040500100196. [DOI] [PubMed] [Google Scholar]
- 21.Lewis G, Coughlan DC, Lane ME, Corrigan OI. Preparation and release of model drugs from thermally sensitive poly(N-isopropylacrylamide) based macrospheres. J. Microencapsul. 2006;23:677–685. doi: 10.1080/02652040600789237. [DOI] [PubMed] [Google Scholar]
- 22.Sirousazar M, Forough M, Farhadi K, Shaabani Y, Molaei R. Applications in Tissue Engineering, Drug, Delivery and Wound Care, Advanced Healthcare Materials. John Wiley & Sons; 2014. Hydrogels: Properties, Preparation, Characterization and Biomedical; pp. 295–357. [Google Scholar]
- 23.Shim TS, Kim S-H, Yang S-M. Elaborate design strategies toward novel microcarriers for controlled encapsulation and release. Part. Part. Syst. Charact. 2013;30:9–45. [Google Scholar]
- 24.Chen C-K, Wang Q, Jones CH, Yu Y, Zhang H, Law W-C, Lai CK, Zeng Q, Prasad PN, Pfeifer BA, Cheng C. Synthesis of pH-responsive chitosan nanocapsules for the controlled delivery of doxorubicin. Langmuir. 2014;30:4111–4119. doi: 10.1021/la4040485. [DOI] [PubMed] [Google Scholar]
- 25.Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008;33:448–477. [Google Scholar]
- 26.Chin SF, Jimmy FB, Pang SC. Size controlled fabrication of cellulose nanoparticles for drug delivery applications. J. Drug Deliv. Sci. Technol. 2018;43:262–266. [Google Scholar]
- 27.Cassano R, Nicoletta FP, Mellace S, Grande F, Picci N, Trombino S. Liquid crystalline microspheres for 5-fluorouracil specific release. J. Drug Deliv. Sci. Technol. 2017;41:482–487. [Google Scholar]
- 28.Borges J, Mano JF. Molecular interactions driving thelayer-by-layer assembly of multilayers. Chem. Rev. 2014;114:8883–8942. doi: 10.1021/cr400531v. [DOI] [PubMed] [Google Scholar]
- 29.Cuomo F, Ceglie A, Piludu M, Miguel MG, Lindman B, Lopez F. Loading and protection of hydrophilic molecules into liposome-templated polyelectrolyte nanocapsules. Langmuir. 2014;30:7993–7999. doi: 10.1021/la501978u. [DOI] [PubMed] [Google Scholar]
- 30.Anandhakumar S, Debapriya M, Nagaraja V, Raichur AM. Polyelectrolyte microcapsules for sustained delivery of water-soluble drugs. Mater. Sci. Eng. C. 2011;31:342–349. [Google Scholar]
- 31.Keeney M, Jiang XY, Yamane M, Lee M, Goodman S, Yang F. Nanocoating for biomolecule delivery using layer-by-layer self-assembly. J. Mater. Chem. B. 2015;3:8757–8770. doi: 10.1039/c5tb00450k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balabushevich NG, Lebedeva OV, Vinogradova OI, Larionova NI. Polyelectrolyte assembling for protein microencapsulation. J. Drug Deliv. Sci. Technol. 2006;16:315–319. [Google Scholar]
- 33.Mandapalli PK, Labala S, Vanamala D, Koranglekar MP, Sakimalla LA, Venuganti VVK. Influence of charge on encapsulation and release behavior of small molecules in self-assembled layer-by-layer microcapsules. Drug Deliv. 2014;21:605–614. doi: 10.3109/10717544.2013.867381. [DOI] [PubMed] [Google Scholar]
- 34.Ariga K, McShane M, Lvov YM, Ji Q, Hill JP. Layer-by-layer assembly for drug delivery and related applications. Expert Opin. Drug Deliv. 2011;8:633–644. doi: 10.1517/17425247.2011.566268. [DOI] [PubMed] [Google Scholar]
- 35.Qiu X, Leporatti S, Donath E, Möhwald H. Studies on the drug release properties of polysaccharide multilayers encapsulated ibuprofen microparticles. Langmuir. 2001;17:5375–5380. [Google Scholar]
- 36.Antipov AA, Sukhorukov GB, Donath E, Möhwald H. Sustained release properties of polyelectrolyte multilayer capsules. J. Mater. Chem. B. 2001;105:2281–2284. [Google Scholar]
- 37.Yuan W, Lu Z, Li CM. Charged drug delivery by ultrafast exponentially grown weak polyelectrolyte multilayers: amphoteric properties, ultrahigh loading capacity and pH-responsiveness. J. Mater. Chem. 2012;22:9351–9357. [Google Scholar]
- 38.Alvarez-Lorenzo C, Blanco-Fernandez B, Puga AM, Concheiro A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013;65:1148–1171. doi: 10.1016/j.addr.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Santalices I, Gonella A, Torres D, Alonso MJ. Advances on the formulation of proteins using nanotechnologies. J. Drug Deliv. Sci. Technol. 2017;42:155–180. [Google Scholar]
- 40.Fuchs S, Coester C. Protein-based nanoparticles as a drug delivery system: chances, risks, perspectives. J. Drug Deliv. Sci. Technol. 2010;20:331–342. [Google Scholar]
- 41.De Geest B, Déjugnat C, Verhoeven E, Sukhorukov G, Jonas AM, Plain J, Demeester J, De Smedt S. Layer-by-layer coating of degradable microgels for pulsed drug delivery. J. Control. Release. 2006;116:159–169. doi: 10.1016/j.jconrel.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Zhou G, Lu Y, Zhang H, Chen Y, Yu Y, Gao J, Sun D, Zhang G, Zou H, Zhong Y. A novel pulsed drug-delivery system: polyelectrolyte layer-by-layer coating of chitosan–alginate microgels. Int. J. Nanomedicine. 2013;8:877–887. doi: 10.2147/IJN.S38144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habibi N, Pastorino L, Babolmorad G, Ruggiero C, Guda T, Ong JL. Polyelectrolyte multilayers and capsules: S-layer functionalization for improving stability and biocompatibility. J. Drug Deliv. Sci. Technol. 2017;38:1–8. [Google Scholar]
- 44.Leporatti S, Voigt A, Mitlöhner R, Sukhorukov G, Donath E, Möhwald H. Scanning force microscopy investigation of polyelectrolyte nano- and microcapsule wall texture. Langmuir. 2000;16:4059–4063. [Google Scholar]
- 45.Alam MA, Subhan N, Hossain H, Hossain M, Reza HM, Rahman MM, Ullah MO. Hydroxycinnamic acid derivatives: a potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016;13:27. doi: 10.1186/s12986-016-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brahmachari S, Pahan K. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J. Immunol. 2007;179:275–283. doi: 10.4049/jimmunol.179.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kilani-Jaziri S, Mokdad-Bzeouich I, Krifa M, Nasr N, Ghedira K, Chekir-Ghedira L. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: a structure–activity relationship study. Drug Chem. Toxicol. 2017;40:416–424. doi: 10.1080/01480545.2016.1252919. [DOI] [PubMed] [Google Scholar]
- 48.Barrios CA, Xu QW, Cutright TJ, Zhang Newby B.-m. Incorporating zosteric acid into silicone coatings to achieve its slow release while reducing fresh water bacterial attachment. Colloids Surf. B: Biointerfaces. 2005;41:83–93. doi: 10.1016/j.colsurfb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release. 1987;5:23–36. [PubMed] [Google Scholar]
- 50.Tan JPK, Wang Q, Tam KC. Control of burst release from nanogels via layer by layer assembly. J. Control. Release. 2008;128:248–254. doi: 10.1016/j.jconrel.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Zhu H, Srivastava R, McShane MJ. Spontaneous loading of positively charged macromolecules into alginate-templated polyelectrolyte multilayer microcapsules. Biomacromolecules. 2005;6:2221–2228. doi: 10.1021/bm0501656. [DOI] [PubMed] [Google Scholar]
- 52.Picart C, Mutterer J, Richert L, Luo Y, Prestwich GD, Schaaf P, Voegel J-C, Lavalle P. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. 2002;99:12531–12535. doi: 10.1073/pnas.202486099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Villiers MM, Otto DP, Strydom SJ, Lvov YM. Introduction to nanocoatings produced by layer-by-layer (LbL) self-assembly. Adv. Drug Deliv. Rev. 2011;63:701–715. doi: 10.1016/j.addr.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Caruso F, Schüler C, Kurth DG. Core−Shell Particles and Hollow Shells Containing Metallo-Supramolecular Components. Chem. Mater. 1999;11:3394–3399. [Google Scholar]
- 55.Caruso RA, Susha A, Caruso F. Multilayered Titania, Silica, and Laponite Nanoparticle Coatings on Polystyrene Colloidal Templates and Resulting Inorganic Hollow Spheres. Chem. Mater. 2001;13:400–409. [Google Scholar]
- 56.Wang Y, An Q, Zhou Y, Niu Y, Akram R, Zhang Y, Shi F. Post-infiltration and subsequent photo-crosslinking strategy for layer-by-layer fabrication of stable dendrimers enabling repeated loading and release of hydrophobic molecules. J. Mater. Chem. B. 2015;3:562–569. doi: 10.1039/c4tb01688b. [DOI] [PubMed] [Google Scholar]
- 57.Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release. 1987;5:37–42. [PubMed] [Google Scholar]







