Abstract
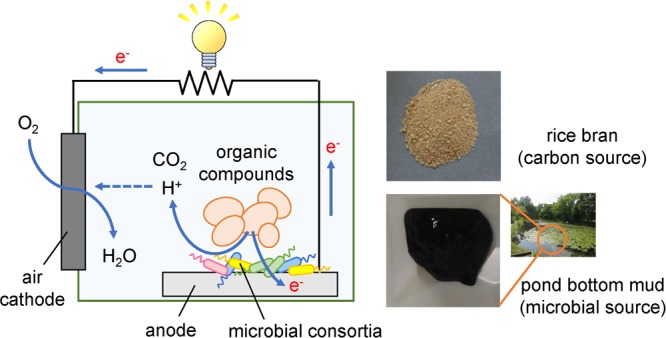
Single-chamber microbial fuel cells (MFCs) were constructed using rice bran (carbon source) and pond bottom mud (microbial source). The total electric charge obtained in the MFC combining rice bran with pond bottom mud was four times higher than that in MFC using only rice bran. Phylogenetic analyses revealed dominant growth of fermentative bacteria such as Bacteroides and Clostridium species, and exoelectrogenic Geobacter species in the anode biofilms, suggesting that mutualism of these bacteria is a key factor for effective electricity generation in the MFC. Furthermore, rice bran, consisting of persistent polysaccharide, was pretreated by the hydrodynamic cavitation system to improve the digestibility and enhance the efficiency in MFC, resulting in 26% increase in the total production of electricity.
1. Introduction
Microbial fuel cells (MFCs) are promising for energy recovery and electricity generation from organic compounds using microbes as electrocatalysts.1,2 Because of the large variation in microbial metabolisms, many different organic compounds can be used as a substrate for electricity generation in MFCs.3−5 Many studies have applied MFCs to organic compounds derived from food wastes, such as a brewery wastewater,6 cheese whey,7 yogurt waste,8 and palm oil mill effluent.9 Food waste is useful for electricity generation in MFCs because it is rich in organic compounds that can be assimilated by microbes. However, most of the above studies used liquid food waste and few studies have focused on the solid food waste as a substrate for MFCs. Solid organic compounds are promising substrates for electricity generation in MFCs because they have higher energy density than soluble organic compounds.3
Rice bran is a major by-product of rice milling.10 About 600 million tons of rice is harvested worldwide annually, and rice bran accounts for 7% of the mass of rice.11 Rice bran has been used for oil production, animal feed, fertilizer, and in industrial applications.12 Recently, rice bran has attracted much attention as a functional food because it contains bioactive ingredients, such as polysaccharides, proteins, minerals, and other micronutrients.10 Rice bran is a potential source material for MFCs because of its rich nutrient and organic compounds.13
However, one problem in the use of solid biomass for MFCs is low digestibility. Rice bran contains cellulose, which has a crystalline structure and is difficult to degrade. Thus, pretreatment of solid biomass is necessary for efficient degradation by microbes in a MFC. Ultrasonication causes cavitation in a solution, triggering a hotspot generation with localized extreme temperature and pressure.14 Ultrasonication has been used to improve the performance of sludge-based MFC for the purpose of pretreatment of the sludge15,16 and removal of the biofilm17 by the action of the localized high energy. A flowing fluid system also gives cavitation, which is called hydrodynamic cavitation. Cavitation is generated in a flow of fluid passing through a venturi tube or an orifice plate with a constriction, resulting in hotspot generation.18 Application of hydrodynamic cavitation has been investigated in various fields including food industry and water treatment.19 We have recently developed a biomass pretreatment technique using hydrodynamic cavitation with low energy input and demonstrated a higher pretreatment efficiency compared to ultrasonication.20
In the present study, electricity was generated from solid rice bran using mud from the bottom of a pond as a bacterial source. A single chamber MFC equipped with an air cathode was constructed. For electric generation without stirring, an anode was attached to the bottom of the MFC reactor, where solid compounds decomposed and electrons collected. The use of hydrodynamic cavitation pretreatment of solid rice bran for electricity generation was examined.
2. Results and Discussion
2.1. Electricity Generation Using Rice Bran in MFCs
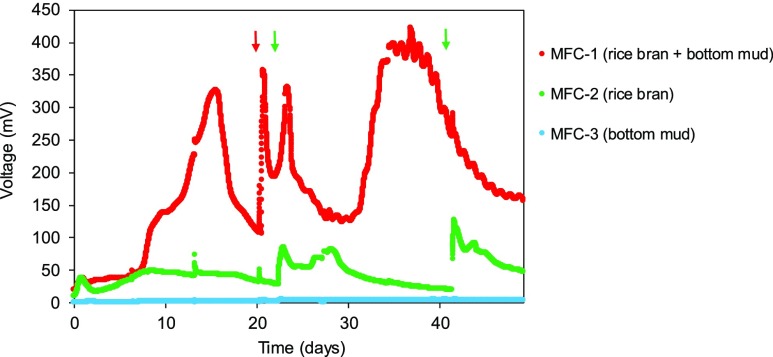
Electricity generation test was conducted using an MFC system shown in Figure 1. The voltage was measured for each MFC (MFC-1, MFC-2, and MFC-3) during operation (Figure 2). Initially, the voltage was low in all cases. The voltage in MFC-1 (rice bran + bottom mud) increased gradually after day 7. Rice bran contains macromolecular biopolymers such as cellulose, which are gradually degraded to low-molecular-weight compounds by microbes around the anode to be assimilated, resulting in a gradual increase in the voltage. The higher voltage (325 mV) was achieved on day 15, and then it began to decrease. After additional rice bran was supplied to MFC-1 on day 20, two peaks showing sharp rise and drastic decline in the voltage were observed. The first peak would be attributed to the voltage increase by the assimilation of low-molecular weight compounds such as sugars and amino acids in additionally supplied biomass. The second peak could be because of the electricity generation from degradation products of oligomers such as oligosaccharides and/or peptides. Subsequently, the increase in the voltage was observed again after 30 days, probably due to the degradation of macromolecular compounds such as cellulose and proteins. In contrast, the voltage for MFC-2 (rice bran) was quite low. In this system, some degradation and redox reactions catalyzed by microbes would occur moderately on the anode and the cathode even though the main microbial source (bottom mud) was not added in MFC-2. The voltage for MFC-3 (bottom mud) was negligible (average voltage 2.45 mV) throughout the experiment, suggesting that microbes could not grow and generate electricity in this MFC because of the lack of organic compounds in the electrolyte. The lowest internal resistance and total electric charge are shown in Table 1. MFC-1 had lower internal resistance (298.7 Ω) than MFC-2 (1786 Ω) and MFC-3 (9710 Ω). Furthermore, the total electric charge with MFC-1 (1.58 × 103 C) was more than four times that of MFC-2 (3.69 × 102 C), indicating that MFC-1 performed the best among the three MFCs. On the basis of these results, both rice bran and bottom mud are important in our MFC system for electricity generation, where they provide organic compounds and effective biocatalysis, respectively. In terms of power density, we obtained maximum power density of 16.5 mW/m2 in MFC-1. Previous works reported power density of 4.2,21 10,22 67,23 37,24 436 mW/m23 using cattle waste, and 360 mW/m2 using rice bran,13 where paddy field soil was used as a microbial source. The actual value of power density would be significantly and susceptibly affected by several factors such as the performance of anode and cathode including the efficiency of metal catalyst, types of substrate (i.e. biomass or organic compounds), and microbial source and community employed in the system. As for the types of substrate, electricity generation would be improved by the treatment of biomass as shown in the latter part.
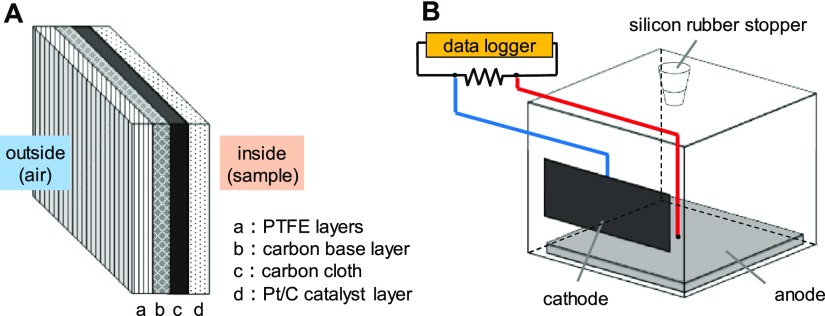
Figure 1.
Schematic diagrams of (A) the air cathode and (B) single chamber MFC.
Figure 2.
Time course of voltage changes in MFC-1, MFC-2, and MFC-3. Additional rice bran was supplemented to each MFC at the point indicated by the arrow in the same color.
Table 1. Internal Resistance and Total Electric Charges MFC-1, MFC-2, and MFC-3.
| lowest internal resistance r (Ω) | total charge amount (∼day 49) Q (C) | |
|---|---|---|
| MFC-1 | 298.7 | 1.58 × 103 |
| MFC-2 | 1876 | 3.69 × 102 |
| MFC-3 | 9710 | 2.04 × 101 |
2.2. Phylogenetic Compositions of Bacteria
Phylogenetic compositions of bacteria in the original rice bran, bottom mud, and anode biofilms of MFC-1 were determined (Figure 3). Chloroplasts made up 88% of the rice bran, and were detected using the amplified 16S rRNA gene. Thus, bacteria in the class Alphaproteobacteria (10.8%) were predominant in the original rice bran. In the original bottom mud, bacteria in the class Betaproteobacteria (18.0%) were dominant as well as the classes Deltaproteobacteria (16.3%) and Alphaproteobacteria (14.7%). In the anode biofilms, bacteria in the classes Bacteroidia (27.5%) and Clostridia (21.4%) were enriched compared with the levels in the original bottom mud. Among the bacteria in the class Bacteroidia, the most predominant genus was Bacteroides (37.1% of the class Bacteroidia). Some bacteria belonging to the genus Bacteroides are anaerobic glycolytic bacteria that can produce organic acids such as acetate and succinate.25 The bacteria in the Clostridia class were mainly in the genus Clostridium (61.7%). Many kinds of Clostridium species are known to be able to decompose cellulosic materials to oligo- and mono-saccharides under anaerobic conditions,3 suggesting that they would play an important role in decomposing high-molecular-weight compounds in rice bran to low-molecular-weight compounds in the MFC systems. Followed by bacteria in the classes Bacteroidia and Clostridia, bacteria in the class Deltaproteobacteria (13.2%) were enriched in the anode biofilms, as was the case with original bottom mud (16.3%). Among the bacteria in the class Deltaproteobacteria, 88.0% belonged to the genus Geobacter. The genus Geobacter are exoelectrogenic bacteria which use organic acids as a substrate and can directly transfer electrons to electrodes without a mediator.26,27 This suggests that bacteria in the genus Geobacter are crucial for voltage generation in MFCs. Furthermore, a recent study reported phenol-degrading MFCs with graphite electrodes, where Geobacter sp. is working as a phenol degrader in the anode biofilm.28 Therefore, Geobacter sp. could possibly degrade polyphenolic compounds in the rice bran to generate electricity in our MFC system. On the basis of these results, we hypothesize the following concerted electric generation system; the genus Clostridium degrades polymeric cellulose in rice bran to glucose, some of which is converted to organic acids by the genus Bacteroides and/or are directly converted to organic acids by anaerobic Clostridium such as Clostridium butylicum. Finally, these organic acids are used for electron transfer to the electrode by the genus Geobacter. The degrading, fermentative, and exoelectrogenic bacteria would cooperatively and sequentially function for effective electricity generation in the MFC system using rice bran and bottom mud.
Figure 3.
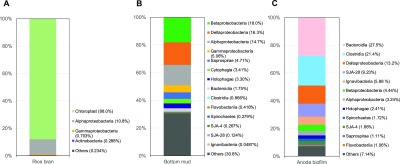
Phylogenetic compositions of bacteria in (A) rice bran, (B) bottom mud, and (C) anode biofilms of MFC-1.
2.3. Effect of Hydrodynamic Cavitation Pretreatment of Rice Bran on the MFC Performance
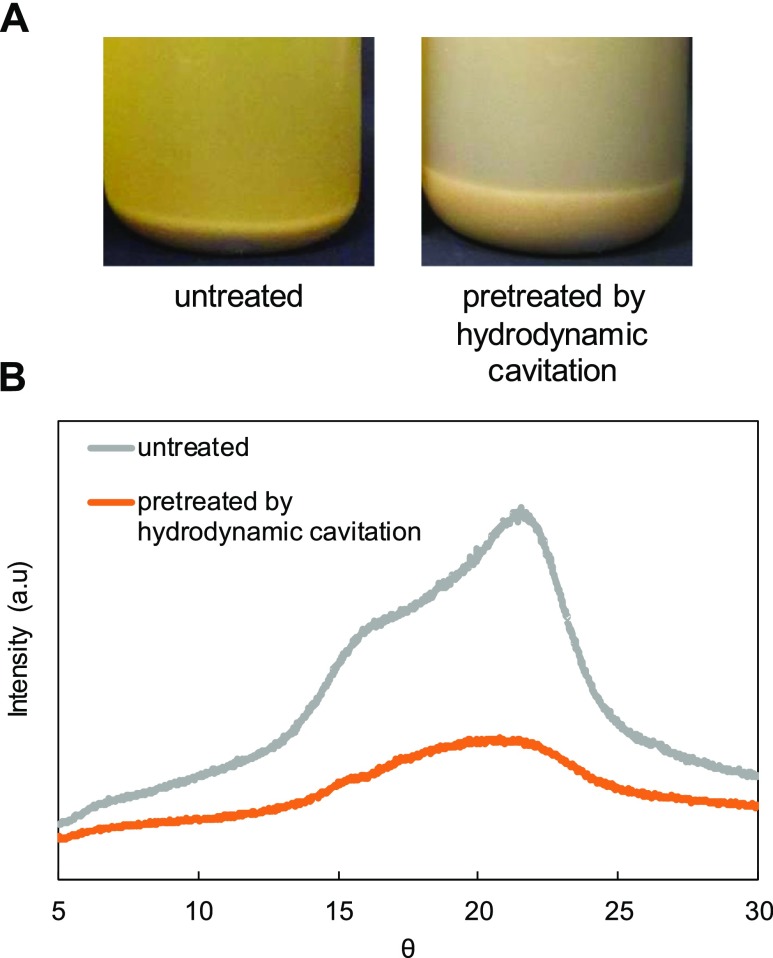
Because rice bran contains a large quantity of biopolymer such as cellulose, these biopolymers should be altered to be easily digested by microbes in the MFC system. To improve the digestibility of biomass, pretreatment is the most powerful tool. We thus examine pretreatment of biomass by hydrodynamic cavitation, which was found to be efficient for the pretreatment of lignocellulosic biomass.20Figure 4A shows the photos of untreated and pretreated rice bran by hydrodynamic cavitation. Swelling of the rice bran was observed after the pretreatment compared to the untreated. The powder X-ray diffraction pattern of the untreated sample (Figure 4B) showed a characteristic peak for crystalline cellulose at around θ = 22°.29 This peak was significantly decreased after hydrodynamic cavitation pretreatment, which indicated that the cellulose crystallinity of the rice bran decreased by the treatment. Hot spots with high temperature and high pressure are generated locally in a hydrodynamic cavitation system.18 This would lead to disruption of hydrogen-bond in cellulose in the biomass, resulting in decrease in crystallinity.
Figure 4.
(A) Photographs and (B) powder X-ray diffraction patterns of rice bran without pretreatment and pretreated by hydrodynamic cavitation (at 30 °C for 1 h).
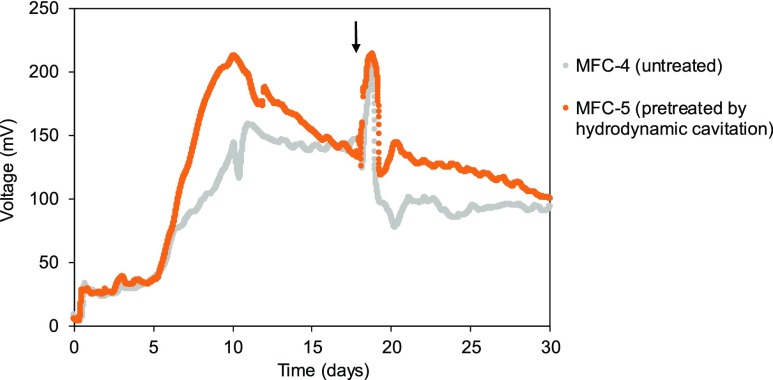
Figure 5 shows the voltage of MFC-4 (untreated) and MFC-5 (pretreated by hydrodynamic cavitation). MFC-5 (213 mV on day 10) gave higher voltage than MFC-4 (158 mV on day 11). The total electric charges obtained with MFC-4 and MFC-5 were 4.91 × 102 and 6.19 × 102 C, respectively (Table 2), indicating hydrodynamic cavitation increased the total electric charge by 26%. Pretreatment of rice bran by hydrodynamic cavitation would increase the digestibility of the biomass by microbes around the anode, which leads to higher efficiency in electric generation in MFC-5. Pretreatment of biomass was demonstrated to be also important in the MFC system.
Figure 5.
Time course of voltage changes in MFC-4 (untreated) and MFC-5 (pretreated by hydrodynamic cavitation). The electrolyte was replaced at the points indicated with arrows.
Table 2. Internal Resistance and Total Electric Charges of MFC-4 and MFC-5.
| lowest internal resistance r (Ω) | total charge amount (∼day 30) Q (C) | |
|---|---|---|
| MFC-4 | 442.1 | 4.91 × 102 |
| MFC-5 | 355.1 | 6.19 × 102 |
3. Conclusions
We constructed the MFC system using rice bran and pond bottom mud. Electricity generation from rice bran was significantly improved by employing pond bottom mud as the microbial source. Cooperative action of Clostridium, Bacteroides, and Geobacter species would be quite important in a sequential biological process that includes degradation of biopolymers, conversion of saccharides to organic acids, and electricity generation on anode. Furthermore, the pretreatment of rice bran by hydrodynamic cavitation apparently increased the efficiency of MFC.
4. Experimental Section
4.1. MFC Construction
A carbon-felt anode (9 cm × 9 cm, 0.5 mm thick; Sogo Carbon, Kanagawa, Japan) was attached to the bottom of a plastic case (10 cm × 10 cm × 8 cm) with silicone sealant. The air cathode contained a carbon cloth (EC-CC1-06; Electrochem, Inc., MA, USA) modified with polytetrafluoroethylene (Sigma-Aldrich Co. LLC., MO, USA) layers and a carbon base layer of carbon powder (Vulcan XC-72, MOUBIC INC., Shizuoka, Japan) plus polytetrafluoroethylene on one side, and a platinum carbon layer (TEC10E40E; Tanaka Kikinzoku Kogyo, Tokyo, Japan) on the opposite side (Figure 1A). A small window (8 cm × 3 cm) was cut out of the side of the plastic case for placement of the air cathode (Figure 1B).
4.2. MFC Operation Using Rice Bran
Rice bran (Mori Sangyo Co., Ltd, Hokkaido, Japan) was stored in a refrigerator at 4 °C until required. Bottom mud was collected from Ono pond (Hokkaido University, Hokkaido, Japan) and used as an inoculum. We examined three MFC systems, MFC-1 (rice bran 4.0 g and bottom mud 12 g in 500 mL of distilled water), MFC-2 (rice bran 4.0 g in 500 mL of distilled water), and MFC-3 (bottom mud 12 g in 500 mL of distilled water). The three MFCs were connected to an external resistance of 510 Ω and left in an incubator at a constant temperature (30 °C) to avoid variation in MFC efficiency caused by temperature change. The voltage (V) was measured at 10 min intervals using a data logger (GL240; Graphtec, Tokyo, Japan). Additional rice bran (1.3 g) was added to MFC-1 and MFC-2 when the voltage decreased. The internal resistance (r) was calculated from the external resistance (R), recorded voltage (V), and current (I = V/R) according to an established method.4 Maximum power density was obtained from power curve (current I vs power P = I·V). The total electric charge (Q) was calculated from the voltage measurement interval (t) and average current for this interval (I′) as Q = ∑(I′ × t).30
4.3. Phylogenetic Analysis of Bacterial Communities
Phylogenetic analysis of bacterial communities was conducted by Repertoire Genesis Inc. (Osaka, Japan). Briefly, genome DNA was extracted from each sample (rice bran, bottom mud, and anode biofilm) and used as a template for PCR amplification of 16S rRNA gene fragments (V1–V2 region). The amplicon was analyzed by MiSeq next-generation sequencer (Illumina, Inc., CA, USA). Phylogenetic analyses were performed using Flora Genesis database.
4.4. Pretreatment of Rice Bran by Hydrodynamic Cavitation
A hydrodynamic cavitation system was constructed according to a previous study.20 A Venturi tube with a throat diameter of 1.4 mm was used in this experiment. Rice bran was screened to obtain particles of size ranging from 75 to 200 μm. Rice bran (4 g) was suspended in 400 mL of 0.1 mol/L NaOH solution in a holding tank. Hydrodynamic cavitation pretreatment was carried out by circulating the suspension with diaphragm pump (Duplex II AC Demand Pump D3635E7011A, FLOJET, 50 W; CA, USA) at a flow rate of 1.8 L/min in a water bath (30 °C, 1 h). The pretreated sample was neutralized with 2 mol/L HCl. For comparison, an untreated sample was prepared by adding rice bran to a neutralized solution with the same salinity. We examined two MFC systems, which we labeled MFC-4 (untreated), and MFC-5 (pretreated by hydrodynamic cavitation), both containing bottom mud (12 g) as a microbial source. The experimental conditions were the same as described above. On day 18, 250 mL of biomass suspension in each MFC was replaced with 250 mL of a fresh suspension of rice bran prepared under the same conditions.
Powder X-ray diffraction was used to observe changes in the crystalline structure after pretreatment. Samples with hydrodynamic cavitation pretreatment and no pretreatment were dried at 90 °C for 24 h and scanned with an X-ray diffractometer (MultiFlex 2 kW; Rigaku, Tokyo, Japan) at 2θ = 5–30°. The scan speed was 1°/min, and the step size was 0.02°.
Acknowledgments
This work is partly supported by JSPS KAKENHI grant number JP15K18273 and financial support by Hokkaido Gas Co., Ltd.
The authors declare no competing financial interest.
References
- Lovley D. R. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. 10.1038/nrmicro1442. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T.; Miyahara M.; Kouzuma A.; Watanabe K. Conversion of activated-sludge reactors to microbial fuel cells for wastewater treatment coupled to electricity generation. J. Biosci. Bioeng. 2014, 118, 533–539. 10.1016/j.jbiosc.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Inoue K.; Ito T.; Kawano Y.; Iguchi A.; Miyahara M.; Suzuki Y.; Watanabe K. Electricity generation from cattle manure slurry by cassette-electrode microbial fuel cells. J. Biosci. Bioeng. 2013, 116, 610–615. 10.1016/j.jbiosc.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Watanabe K. Recent Developments in microbial fuel cell technologies for sustainable bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. 10.1263/jbb.106.528. [DOI] [PubMed] [Google Scholar]
- Logan B. E.; Hamelers B.; Rozendal R.; Schröder U.; Keller J.; Freguia S.; Aelterman P.; Verstraete W.; Rabaey K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- Zhuang L.; Yuan Y.; Wang Y.; Zhou S. Long-term evaluation of a 10-liter serpentine-type microbial fuel cell stack treating brewery wastewater. Bioresour. Technol. 2012, 123, 406–412. 10.1016/j.biortech.2012.07.038. [DOI] [PubMed] [Google Scholar]
- Stamatelatou K.; Antonopoulou G.; Tremouli A.; Lyberatos G. Production of gaseous biofuels and electricity from cheese whey. Ind. Eng. Chem. Res. 2011, 50, 639–644. 10.1021/ie1002262. [DOI] [Google Scholar]
- Cercado-Quezada B.; Delia M.-L.; Bergel A. Testing various food-industry wastes for electricity production in microbial fuel cell. Bioresour. Technol. 2010, 101, 2748–2754. 10.1016/j.biortech.2009.11.076. [DOI] [PubMed] [Google Scholar]
- Leaño E. P.; Anceno A. J.; Babel S. Ultrasonic pretreatment of palm oil mill effluent: Impact on biohydrogen production, bioelectricity generation, and underlying microbial communities. Int. J. Hydrogen Energy 2012, 37, 12241–12249. 10.1016/j.ijhydene.2012.06.007. [DOI] [Google Scholar]
- Park H.-Y.; Lee K.-W.; Choi H.-D. Rice bran constituents: immunomodulatory and therapeutic activities. Food Funct. 2017, 8, 935–943. 10.1039/c6fo01763k. [DOI] [PubMed] [Google Scholar]
- Chen M.-H.; Choi S. H.; Kozukue N.; Kim H.-J.; Friedman M. Growth-inhibitory effects of pigmented rice bran extracts and three red bran fractions against human cancer cells: Relationships with composition and antioxidative activities. J. Agric. Food Chem. 2012, 60, 9151–9161. 10.1021/jf3025453. [DOI] [PubMed] [Google Scholar]
- Esa N. M.; Ling T. B.; Peng L. S. By-products of rice processing: an overview of health benefits and applications. J. Rice Res. 2013, 1, 107. 10.4172/2375-4338.1000107. [DOI] [Google Scholar]
- Takahashi S.; Miyahara M.; Kouzuma A.; Watanabe K. Electricity generation from rice bran in microbial fuel cells. Bioresour. Bioprocess. 2016, 3, 50. 10.1186/s40643-016-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslick K. S. Sonochemistry. Science 1990, 247, 1439–1445. 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- Oh S.-E.; Yoon J. Y.; Gurung A.; Kim D.-J. Evaluation of electricity generation from ultrasonic and heat/alkaline pretreatment of different sludge types using microbial fuel cells. Bioresour. Technol. 2014, 165, 21–26. 10.1016/j.biortech.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Tiwari B. R.; Ghangrekar M. M. Enhancing electrogenesis by pretreatment of mixed anaerobic sludge to be used as inoculum in microbial fuel cells. Energy Fuels 2015, 29, 3518–3524. 10.1021/ef5028197. [DOI] [Google Scholar]
- Islam M. A.; Woon C. W.; Ethiraj B.; Cheng C. K.; Yousuf A.; Khan M. M. R. Ultrasound driven biofilm removal for stable power generation in microbial fuel cell. Energy Fuels 2017, 31, 968–976. 10.1021/acs.energyfuels.6b02294. [DOI] [Google Scholar]
- Gogate P. R.; Pandit A. B. Engineering design methods for cavitation reactors II: Hydrodynamic cavitation. AIChE J. 2000, 46, 1641–1649. 10.1002/aic.690460815. [DOI] [Google Scholar]
- Gogate P. R. Hydrodynamic cavitation for food and water processing. Food Bioprocess Technol. 2011, 4, 996–1011. 10.1007/s11947-010-0418-1. [DOI] [Google Scholar]
- Nakashima K.; Ebi Y.; Shibasaki-Kitakawa N.; Soyama H.; Yonemoto T. Hydrodynamic cavitation reactor for efficient pretreatment of lignocellulosic biomass. Ind. Eng. Chem. Res. 2016, 55, 1866–1871. 10.1021/acs.iecr.5b04375. [DOI] [Google Scholar]
- Scott K.; Murano C. Microbial fuel cells utilising carbohydrates. J. Chem. Technol. Biotechnol. 2007, 82, 92–100. 10.1002/jctb.1641. [DOI] [Google Scholar]
- Scott K.; Murano C. A study of a microbial fuel cell battery using manure sludge waste. J. Chem. Technol. Biotechnol. 2007, 82, 809–817. 10.1002/jctb.1745. [DOI] [Google Scholar]
- Zheng X.; Nirmalakhandan N. Cattle wastes as substrates for bioelectricity production via microbial fuel cells. Biotechnol. Lett. 2010, 32, 1809–1814. 10.1007/s10529-010-0360-3. [DOI] [PubMed] [Google Scholar]
- Lee Y.; Nirmalakhandan N. Electricity production in membrane-less microbial fuel cell fed with livestock organic solid waste. Bioresour. Technol. 2011, 102, 5831–5835. 10.1016/j.biortech.2011.02.090. [DOI] [PubMed] [Google Scholar]
- Madigan M. T.; Martinko J. M.; Stahl D. A.; Clark D. P.. Brock Biology of Microorganism, 13th ed.; Pearson, 2010. [Google Scholar]
- Bond D. R.; Lovley D. R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. 10.1128/aem.69.3.1548-1555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan B. E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. 10.1038/nrmicro2113. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Li Z.; Zhang C.; Zhou X.; Xiao Z.; Awata T.; Katayama A. Phenol-degrading anode biofilm with high coulombic efficiency in graphite electrodes microbial fuel cell. J. Biosci. Bioeng. 2017, 123, 364–369. 10.1016/j.jbiosc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Mittal A.; Katahira R.; Himmel M. E.; Johnson D. K. Effects of alkaline or liquid-ammonia treatment on crystalline cellulose: changes in crystalline structure and effects on enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 41. 10.1186/1754-6834-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Wang X.; Zhao Q.; Wan L.; Li Y.; Zhou Q. Carbon fiber enhanced bioelectricity generation in soil microbial fuel cells. Biosens. Bioelectron. 2016, 85, 135–141. 10.1016/j.bios.2016.05.001. [DOI] [PubMed] [Google Scholar]