Abstract

Glycosylation significantly alters the biological and physicochemical properties of small molecules. β-Lactam alcohols comprise eligible substrates for such a transformation based on their distinct relevance in the chemical and medicinal community. In this framework, the unprecedented enzymatic glycosylation of the rigid and highly strained four-membered β-lactam azaheterocycle was studied. For this purpose, cis-3-hydroxy-β-lactams were efficiently prepared in three steps by means of a classical organic synthesis approach, while a biocatalytic step was implemented for the selective formation of the corresponding 3-O-α- and -β-glucosides, hence overcoming the complexities typically encountered in synthetic glycochemistry and contributing to the increasing demand for sustainable processes in the framework of green chemistry. Two carbohydrate-active enzymes were selected based on their broad acceptor specificity and subsequently applied for the α- or β-selective formation of β-lactam-sugar adducts, using sucrose as a glucosyl donor.
Introduction
The very first β-lactam synthesis by Staudinger back in 1907,1 followed by the accidental discovery of the antibacterial properties of penicillin 1 (penicillin G, R = Bn) by Fleming 20 years later,2 marked the beginning of a revolution in the science of medicine (Figure 1). Up to now, penicillins and more recently discovered classes of β-lactam antibiotics are considered to be the major means of defense against pathogenic bacteria and represent the world’s major biotechnology products.3 Over the years, besides their antibacterial properties, other pharmacological features of β-lactams were also unveiled (e.g., cholesterol absorption inhibition,4 inhibition of human tryptase,5 thrombin6 and chymase,7 vasopressin V1a antagonist activity,8 antiviral,9 antitumor,10 antidiabetic,11 anti-inflammatory,12 antiparkinsonian13 and antitubercular activity,14 etc.), which, in combination with their considerable synthetic importance as four-membered, highly strained cyclic amides,15,72 promoted β-lactams to become one of the most studied classes of azaheterocycles to date.
Figure 1.
Bioactive (β-lactam-containing) compounds.
In general, monocyclic β-lactams unfairly comprise an underexplored subclass, though they are particularly interesting with respect to the treatment of bacterial infections (e.g., commercial 3-aminomonobactam aztreonam 2, Figure 1).16 3-Hydroxy-β-lactams 3, such as glutamine synthetase-inhibiting tabtoxinine β-lactam 4, can also possess antimicrobial activity,17,18 whereas others have been proven to exhibit significant antitumor effects. For example, several 1,4-diarylazetidin-2-ones (such as compound 5), which are conformationally restricted analogues of (Z)-combretastatin A-4 6, a natural product that inhibits tubulin polymerization, showed strong potencies against human neuroblastoma cell growth.10 Furthermore, the renowned “β-lactam synthon method” has been applied repeatedly to convert versatile 3-hydroxy-β-lactam precursors into a variety of highly functionalized scaffolds of biological and medicinal interest,15 such as the side chain of the anticancer drug taxol,19 antibiotics,20 alkaloids,21 amino acids, peptides,22 etc.
In the framework of the biological and synthetic relevance of the class of 3-hydroxy-β-lactams (3, Figure 1), we proposed to subject them to a glucosylation reaction, i.e. glycosylation with glucose.23 Glycosylation can be defined as the attachment of a carbohydrate onto a chemical scaffold, known as the aglycone, via an O-, N-, S-, or C-atom. Hence, in this study, the β-lactam 3-hydroxy-entity serves as the anchor point. Glycosylation is a very common phenomenon in nature and probably concerns one of the most frequent and important modifications of biomolecules.24 It is, for instance, believed to play a key role in cellular communication in plants through accumulation, storage, and transport of hydrophobic substances.25 Moreover, glycosylation has numerous practical applications in synthesis, where it might act as a chiral source or lead to bioactive precursors,26−28 or in industry, where it can be applied to detergents and cosmetics (e.g., long alkyl chain aglycones),29,30 antifungal and antimicrobial compounds (e.g., unsaturated alkyl chain aglycones),31 antitumor formulations and cardiac-related drugs (e.g., peptide and steroid aglycones)31,32 and “controlled release” compounds (e.g., flavor and fragrance aglycones).33
This endless series of examples arises from the influence glycosylation can exert on various physicochemical and biological properties. The most obvious advantage concerns the solubility and bioavailability increase of hydrophobic aglycones, augmenting their potential in pharmacological applications,23 as is for example the case for glycopeptides and curcumin (20 million-fold better aqueous solubility of the latter).34,35 Besides the effect on solubility, glycosylation can be used to alter the bioactivity of the compound. Although so far not a widespread mechanism of antibiotic resistance, glycosylation does play a role in self-protection of antibiotic-producing organisms,36 while bringing about a spectacular increase in activity, as is well illustrated by vancomycin, a glycopeptide effective against methicillin-resistant Staphylococcus aureus. By means of glycorandomization, which is a way to acquire a library of analogues by changing the carbohydrate moiety, several derivatives have been found to be up to 500 times more effective than vancomycin itself.37 Related to this, and even more triggering for the purpose of this study, is the finding that phenolic O-glycosylated ezetimibe, a β-lactam with cholesterol absorption inhibitory activity, was found to be 4 times as potent as its corresponding aglycone 7 (Figure 2).38 Moreover, glycosylation enables tuning of the oxidative and thermal stability, as is the case for vitamin C,39 or the toxicity of the aglycone, illustrated by a reduced cardiotoxicity of anthracycline antibiotics.40
Figure 2.
Ezetimibe 7.
Undoubtedly, glycosides are of high commercial interest,25 though their synthesis is still far from trivial. Chemical glycosylation reactions using these complex substrates typically suffer from the lack of regioselectivity (primary, secondary, and hemiacetalic hydroxyl groups) and stereoselectivity with respect to the anomeric carbon center. Protection and deprotection steps have to be taken into account, involving additional synthetic manipulations, reagents, catalysts, solvents and hence toxic chemical waste streams, besides low overall yields. The development of economically and ecologically feasible procedures in the field of glycochemistry thus remains challenging.23,24,41 Biocatalytic routes have therefore received increasing attention as efficient and green alternatives, by circumventing the disadvantages associated with chemical glycosylation protocols.42,43 Enzymes are capable of regio-, stereo-, and enantioselectively glycosylating various acceptors under mild conditions using renewable starting materials, rendering additional synthetic steps and the concomitant requirements redundant. However, despite considerable efforts to improve enzymatic glycosylation reactions, no universal enzymatic glycosylation platform exists to date. Furthermore, the methodology still suffers from the lack of long-term enzymatic stability, acceptor solubility issues in the aqueous systems used and mostly expensive donors, hence hampering its broad use.23,44,45
Yet, convinced by the assets of biocatalysis and its implementation in organic synthesis, as was also demonstrated by our preliminary studies on the chemoenzymatic syntheses of biologically relevant azaheterocyclic compounds,46,47 the enzymatic glucosylation of chemically prepared cis-3-hydroxy-β-lactams 8, without affecting the sensitive four-membered ring system, is envisioned in the current work. In fact, enzymatic glycosylation of 3-hydroxy-β-lactams as challenging substrates based on their rigidity and steric bulk, has never been described before. As the configuration of the anomeric center is known to alter the biological properties of the molecule,48,49 the selective formation of both α- and β-glucosides 9 are contemplated, as presented in Scheme 1.
Scheme 1. α- and β-Glucosylation of cis-3-Hydroxy-β-lactams 8 by a Mutant Glycoside Phosphorylase from Thermoanaerobacterium thermosaccharolyticum (TtSPP) or by a Glycosyltransferase from Stevia rebaudiana, Respectively.
Results and Discussion
In a first phase, the selective synthesis of the starting cis-3-hydroxy-β-lactams 8 was performed based on preceding research performed by our group, by means of three consecutive steps.50 Though reported more than 100 years ago, the Staudinger synthesis remains the most general and most frequently applied methodology for the preparation of β-lactams and derived compounds based on its simplicity in reaction procedures and predictability of the stereochemical outcome. The mechanism concerns a [2 + 2]-cyclocondensation between functionalized aldimines, obtained through imination of the corresponding aldehydes, and in situ generated ketenes.1 To that end, primary amines 11 were condensed with aldehydes 10 in the presence of magnesium sulfate under various conditions, depending on the nature of the substrates (Scheme 2 and Table 1). The (E)-N-(alkyl/arylmethylidene)amines 12, thus obtained were reacted with acetoxyacetyl chloride 13, transformed in situ to the corresponding ketene through treatment with triethylamine, affording 3-acetoxyazetidin-2-ones 14. The appropriate alkylic/arylic starting materials were selected (R1 = iPr, Pr, Ph; R2 = Bn, iPr), thus offering a variety of β-lactams with respect to steric bulk and polarity. Almost complete diastereoselectivity toward the cis-β-lactams 14 was observed, as deduced from the 1H NMR spectra (CDCl3). The vicinal coupling constants JH3–H4 between the C3- and C4-protons of the azetidin-2-one ring systems varied between 4.6 and 5.0 Hz, values typical for cis-isomers,51 while for derivative 14a, a minor amount (9%) of trans-β-lactam was formed as well (JH3–H4 = 1.7 Hz, CDCl3). Pure cis-β-lactams 14 were obtained after column chromatography-mediated isolation on silica gel (except for alkyl-derivative 14b, posing difficulties during purification and hence further used in a crude form) and subjected to alkaline conditions in a mixture of methanol and water (1:1–2:1) for deacetylation of the C3-group, thus releasing the premised free alcohols 8 for subsequent glycosylation without affecting the relative cis-stereochemistry.
Scheme 2. Synthesis of 3-Hydroxyazetidin-2-ones 8.
Table 1. Synthesis of 3-Hydroxyazetidin-2-ones 8.
| Cpd | R1 | R2 | solvent, T | 12 (%) | eq 13, solvent, t | 14 (%) (cis/trans)a | MeOH/H2O | 8 (%)d (cis/trans) |
|---|---|---|---|---|---|---|---|---|
| 10a | iPr | Bn | THF, Δ | 99 | 1.3 eq 13, CH2Cl2, 16 h | 47b (91:9) | 2:1 | 47 (100:0) |
| 10b | Pr | iPr | CH2Cl2, rt | 90 | 1.1 eq 13, CH2Cl2, 16 h | 84c (100:0) | 1:1 | 9e (100:0) |
| 10c | Ph | Bn | THF, Δ | 99 | 1.5 eq 13, THF, 72 h | 69b (100:0) | 1:1 | 62 (100:0) |
Determined via 1H NMR analysis of the crude reaction mixture.
After column chromatography (SiO2).
Crude yield.
After recrystallization from EtOAc/hexane (1:1).
Overall yield from imine 12b.
In the past century, organic chemists have developed various methods to glycosylate different acceptors, including hydroxylated β-lactams. Banik and co-workers led this research field when it comes to 3-O-glycosylazetidin-2-ones, more specifically the stereospecific glycosylation with glycals, cyclic enol ether derivatives of sugars. They reported an iodine-catalyzed α-glycosylation reaction of racemic 3-hydroxy-β-lactams with various 3-acetoxyglycals via the Ferrier rearrangement, providing access to an optically pure intermediate ((3R,4S)-3-acetoxy-1-(p-anisyl)-4-phenylazetidin-2-one) for the preparation of the Taxol and Taxotere side chain by means of the optical resolution principle.52 Toward the same objective, some research groups employed the Staudinger synthesis starting from protected glycosylated precursors of ketenes, equally affording 3-O-β-lactam-sugar adducts containing several chiral centers, which served as a source of chirality in asymmetric synthesis.53−55
A variety of other chemical procedures are available enabling coupling of a sugar entity with a β-lactam at the C3-, C4-, or even the N1-position (or further away from the β-lactam nucleus) through carbon–carbon or carbon–heteroatom bond formation.56 However, as mentioned before, these reactions typically suffer from low yields, low atom efficiencies, and excessive burden on the environment, making them barely viable from an economic and ecological point of view. Nonetheless, to be able to compare a chemical and enzymatic approach for the glucosylation of our specific substrates, the classical Koenigs–Knorr glycosylation was tested in this study for the attempted synthesis of 3-O-β-glucosyl-β-lactams 9.57 The mechanism comprises nucleophilic substitution of a glycosyl halide by an alcohol and still constitutes a preferred synthetic method in glycochemistry. Unfortunately, we encountered various obstacles when testing several glucosyl donors, acceptors, and reaction conditions, giving rise only to a minor amount of the expected glycoside and hence confirming the general conclusions reported in the literature with respect to chemical glycosylations.
On the other hand, many of the drawbacks of chemical glycosylation may be overcome by using biocatalysts. Nakayama and co-workers developed the first detailed synthesis of vancomycin and some derivatives through two enzymatic glycosylation steps,44 while the enzymatic glucuronidation of ezetimibe 7 has been reported by Zaks and Dodds, in up to 92 and 88% isolated yield, respectively.58 Although the synthesis of the donor substrates is somewhat demanding, careful comparison with their chemical syntheses, requiring tedious protection–deprotection steps of the glycosyl donors resulting in low overall yields, pointed to the enzymatic approach to be the most efficient one.38,59,60
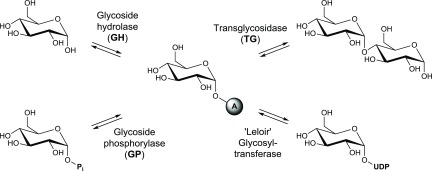
Four types of enzymes that are capable of catalyzing a glycosyl transfer can be distinguished (Scheme 3).61 The vast majority of glycosylation reactions in nature is performed by glycosyltransferases. Although these biocatalysts are highly efficient and provide excellent regio- and stereocontrol, their practical use is hampered due to the excessive cost of the nucleotide-activated carbohydrate donors they require. Transglycosidases are an attractive alternative in this regard, using cheap substrates such as sucrose and amylose, but the variety of different transglycosidase specificities that have been reported to date is rather limited. Finally, glycoside hydrolases and phosphorylases both perform a catabolic role in vivo, cleaving glycosidic bonds using water or phosphate, respectively, but they can be exploited for synthetic purposes under specific process conditions as well.
Scheme 3. Carbohydrate-Active Enzymes Used in Glycosylation Reactions (A = Acceptor; Pi = Inorganic Phosphate; UDP = Uridine Diphosphate)23.
A critical property to consider when choosing a suitable candidate for catalyzing the envisioned glycosylation reactions is the substrate promiscuity. Indeed, acceptor sites that are strictly specific toward a narrow group of molecules will be less likely to bind the rather challenging cis-3-hydroxy-β-lactam targets. Therefore, two biocatalysts that are known to have a broad acceptor scope were selected. The sucrose 6′-phosphate phosphorylase from T. thermosaccharolyticum (TtSPP) is an appealing enzyme for the synthesis of α-glucosides considering the use of sucrose as a cheap and renewable donor substrate, and shows activity on a diverse range of small molecules with retention of the anomeric configuration.62 Moreover, a mutant of TtSPP (R134A) was rationally designed previously in our laboratory to widen the entrance to the active site, triggering affinity for bulky polyphenols such as resveratrol.63 The selected option for the synthesis of β-bonded adducts concerns the stevioside glucosyltransferase from S. rebaudiana (UGT-76G1Sr), which has been employed successfully on structures such as branched alcohols, flavonoids, terpenoids, and more.64 Although its need for the expensive donor substrate uridine diphosphate glucose (UDP-glucose) is a major drawback, this problem is circumvented in this work by coupling the reaction to a sucrose synthase (SuSy).65 Hence, UDP-glucose is regenerated from sucrose in situ while conveniently recycling the UDP byproduct of the transferase reaction (Scheme 4).
Scheme 4. Enzymatic Glucosylation of 3-Hydroxy-β-lactams 8 (TtSPP_R134A, Mutant R134A of the Sucrose 6′-Phosphate Phosphorylase from T. thermosaccharolyticum; UGT-76G1Sr, UDP-Glucosyltransferase from S. rebaudiana; SuSy, Sucrose Synthase from Acidithiobacillus caldus).
The solubility of the β-lactam acceptors 8 is quite poor in the aqueous solutions that these enzymes prefer. Therefore, the performance of various cosolvents (10% v/v) was assessed in small-scale glucosylation reactions. 2-Methylbutan-2-ol and acetonitrile appeared to be toxic to the enzymes, while 2-phenylethanol and isobutanol were unsuitable as they were found to act as acceptor substrates as well, resulting in side products. The desired glucoside was present in highest quantity when diethyl ether, acetone, methanol, dimethyl sulfoxide (DMSO), or methyl t-butyl ether were added. Acetone was finally selected as the most appropriate cosolvent due to its low toxicity, high volatility, and the absence of potentially competing hydroxyl groups. Additionally, the optimal acceptor concentration was determined for both the α- and β-glucosylation reactions. The highest conversions were observed at a concentration of 15 mM in all cases, except for the reaction of 8a with TtSPP_R134A (21 mM).
The α- and β-glucosylation of all three β-lactam acceptors were carried out at a preparative scale (0.375–2.1 mmol acceptor 8) under these optimized conditions, at several degrees below the enzymes’ optimal temperature to slow down irreversible denaturation. All glucosides were successfully synthesized, although the reactions proceeded very slowly and took several days to reach a practical degree of conversion (Table 2). As a comparison, almost complete conversion of resveratrol could be achieved within 24 h with these enzymes.62,63 This difference may be explained by the rigid and bulky structure of the current compounds, which could cause steric clashes in the active site. Finally, after inactivation and removal of the enzymes, the target compounds 9 could be recovered and purified by means of (a sequence of) chromatographic techniques, which resulted in rather low yields. However, it should be noted that the main focus of this research was to obtain and isolate the glucosides in an analytically pure form to allow their spectroscopic analysis, hence the elaborate purification procedures to remove all impurities.
Table 2. Enzymatic Glucosylation of 3-Hydroxyazetidin-2-ones 8.
| Cpd | R1 | R2 | reaction conditions | conversion (%)d | 9 (%) |
|---|---|---|---|---|---|
| α-glucosylation | |||||
| 8a | iPr | Bn | 21 mM 8a, 0.06 mg/mL TtSPP-R134A, 8 d | 23 | 12a |
| 8b | Pr | iPr | 15 mM 8b, 0.04 mg/mL TtSPP-R134A, 22 d | 50 | 17b |
| 8c | Ph | Bn | 15 mM 8c, 0.04 mg/mL TtSPP-R134A, 20 d | 12 | 3b |
| β-glucosylation | |||||
| 8a | iPr | Bn | 15 mM 8a, 0.13 mg/mL UGT-76G1Sr, 0.36 mg/mL SuSy, 6 weeks | 9 | 0.2c |
| 8b | Pr | iPr | 15 mM 8b, 0.19 mg/mL UGT-76G1Sr, 0.31 mg/mL SuSy, 2 weeks | 14 | 1.4c |
| 8c | Ph | Bn | 15 mM 8c, 0.11 mg/mL UGT-76G1Sr, 0.31 mg/mL SuSy, 2 weeks | 4 | 1c |
After column chromatography (SiO2).
After column chromatography (SiO2) and prep. thin-layer chromatography (TLC).
After two-step column chromatography (SiO2 & C18).
Determined via liquid chromatography–mass spectrometry (LC–MS)-analysis of the crude reaction mixture.
The successful synthesis of cis-3-O-d-glucopyranosyl-3-hydroxyazetidin-2-ones 9-αG and 9-βG was confirmed on the basis of the characteristic spectral data and their accordance with reported literature values (Scheme 4, Tables 2 and 3).66 The typical anomeric proton signals are located as a doublet around δ 4.97–5.21 and 4.10–4.70 ppm for the α- and β-glucosides, respectively, and can easily be distinguished from the nearby doublet signals of the protons located on the β-lactam ring (J = ±5 Hz) (1H NMR, D2O). A clear difference is to be noticed between the respective values of the vicinal coupling constants JH1–H2 between the anomeric H1 and its neighboring H2 proton. The values of 3.6–4.0 Hz correspond to α-glucosides, whereas β-glucosides show larger coupling constants of 7.8–8.4 Hz, thus, confirming the expected stereoselective formation of α- and β-isomers by TtSPP_R134A and UGT-76G1Sr, respectively. Finally, the glycosidic anomeric carbon signals are observable as a singlet at δ 98.2–101.6 and 101.1–102.6 ppm, respectively (13C NMR, D2O).
Table 3. Chemical Shift of Glycosidic Anomeric Carbon, Proton, and Vicinal Coupling Constant JH1–H2 (1H and 13C NMR, D2O).
Determined via 1H NMR analysis of purified glycosides.
Upon coupling of racemic cis-3-hydroxy-β-lactams 8 with optically pure glucose, two cis-diastereomers (R,R)-9-G and (S,S)-9-G might be expected as enzymes are chiral catalysts and might therefore discriminate between either of the two enantiomeric acceptor substrates. Indeed, from the analysis of the NMR data presented in Table 3, it becomes clear that one single product is obtained after α-glucosylation with TtSPP_R134A. On the other hand, a very low enantioselectivity was observed in the formation of two isomeric β-glycosides 9-βG using the UGT-76G1Sr/SuSy system (dr = 36:64–44:56, as determined via 1H NMR). It should be noted, however, that these values are determined after the isolation of glycosides, implying the possibility that the recovery of the two diastereomers during purification might not be equal. On the basis of the low concentration of product, though, compared to the amount of non-reacted starting material, sucrose, fructose, UDP, and UDP-glucose in the crude mixture, assessment of the diastereomeric ratios based on the crude NMR data is not reliable and practically infeasible.
In the literature, some examples of glycosyltransferases catalyzing glycosylation reactions with high enantioselectivity are reported, though the reaction conditions such as temperature, (co-)solvent, enzyme formulation, additives, etc. clearly affect the isomeric ratios obtained.67 Similarly, the β-glycosylation of compound 8a at 32 °C with acetone as a cosolvent was found to furnish a more or less equimolar mixture of isomers, whereas a higher selectivity was observed upon incubation at 40 °C in combination with DMSO (dr = 44/56 and 71/29, respectively). A more detailed study on the influence of reaction conditions on the enantioselectivity of transferase-catalyzed transformations has not been published yet and could thus constitute a suitable topic for prospective research. Anyhow, it can be concluded that α-glucosylation of cis-3-hydroxy-β-lactams 8 with TtSPP_R134A takes place with a high enantioselectivity, and thus forms an efficient route toward optically pure α-glucosides 9-αG, while opening up the opportunity to synthesize the corresponding chiral alcohols 8.
Conclusions
Glycosylation can have a profound impact on a compound’s solubility, activity, and bioavailability. Preparing glycosylated molecules is, however, far from trivial. In this work, three cis-3-hydroxy-β-lactams were chemically synthesized via three straightforward steps, after which the potential of two carbohydrate-active enzymes for the production of their corresponding α- or β-glucosides was evaluated. These enzymes, which were selected based on their somewhat promiscuous nature, successfully catalyzed the intended reactions, albeit with low activity in comparison to the reported activities on other, less demanding substrates. However, the limited reaction rate is not surprising considering the bulky and rigid structure of these β-lactams, and was nonetheless sufficient to enable the production of six novel glucosides at a preparative scale and their subsequent isolation in analytically pure form. These findings highlight the potential of the T. thermosaccharolyticum mutant phosphorylase and the S. rebaudiana glycosyltransferase as promising catalysts for the glucosylation of challenging molecules in general, and of cis-3-hydroxy-β-lactams in particular. Furthermore, the glucosyl moiety is transferred from the cheap and renewable sucrose with superior regio- and stereoselectivity (and even enantioselectivity for α-glucosylation) without the need for any protection or deprotection steps. Overall, the complimentary use of classical organic synthesis and biocatalysis for glycosylation reactions was once again shown to provide major advantages within the framework of green chemistry.
Experimental Part
General Methods
Commercially available solvents and reagents were purchased from common chemical suppliers and used without further purification. 1H NMR and 13C NMR spectra were recorded at 400 and 100 MHz (Bruker Avance III-400), respectively, in deuterated solvents with tetramethylsilane as the internal standard. IR spectra were obtained from samples in neat form with an attenuated total reflectance (ATR) accessory with a PerkinElmer Spectrum BX FT-IR or a Shimadzu IRAffinity-1S WL FT-IR spectrophotometer. Melting points were measured using a Kofler bench, type WME Heizbank of Wagner & Munz. High-performance liquid chromatography (HPLC) and HPLC-MS analyses were performed on an Agilent 1200 series HPLC system fitted with an Ascentis Express C18 column (particle size 2.7 μm, length 30 mm, internal diameter 4.6 mm) and connected to a UV–vis detector and an Agilent 1100 series LC/MSD-type SL mass spectrometer (ESI, 70 eV) using a mass-selective single-quadrupole detector. A mixture of acetonitrile/water (5 mM NH4OAc) was used as the eluent. High-resolution electron spray (ES-TOF) mass spectra were obtained with Agilent Technologies 6210 Series Time of Flight. Automated column chromatography was performed on a Büchi Reveleris X2 flash chromatography system (normal phase) or Grace Reveleris X1 flash chromatography system (reversed phase), using Reveleris C18 or Reveleris silica cartridges. Centrifugation for harvesting cultures or performing buffer exchange was carried out in a Sorvall RC-6 Plus rotator. Cell extracts were sonicated in a Branson 250 sonifier (output control 3, 50% duty cycle).
Synthesis of 3-Acetoxyazetidin-2-ones 14
The synthesis of cis-3-acetoxy-1-benzyl-4-phenylazetidin-2-one 14c serves as a general example for the synthesis of 3-acetoxyazetidin-2-ones 14a, 14b (for details, see Table 1 of section “Results and Discussion”).
In a flask of 500 mL, 9.75 g (50 mmol) of (E)-N-(benzylidene)benzylamine 12c, synthesized according to the literature,50 was dissolved in 120 mL dry tetrahydrofuran (THF), followed by the addition of 20.9 mL (150 mmol; 3 equiv) triethylamine. After cooling the reaction mixture to 0 °C using an ice bath, a solution of 8.0 mL (75 mmol; 1.5 equiv) of acetoxyacetyl chloride 13 in 30 mL of dry THF was added dropwise. This mixture was then stirred for 16 h at room temperature, whereupon the solvent was evaporated under reduced pressure. The residue was dissolved in dichloromethane (300 mL), washed twice with 360 mL water, which on its turn was extracted with 150 mL dichloromethane. Next, the combined organic phases were dried over magnesium sulfate. Filtration of the drying agent, evaporation of the solvent, and flash chromatography with petroleum ether/ethyl acetate (2:1) eventually yielded 10.18 g (69%) cis-3-acetoxy-1-benzyl-4-phenylazetidin-2-one 14c.
cis-3-Acetoxy-1-benzyl-4-isopropylazetidin-2-one 14a
The spectral data are derived from the mixture of cis- and trans-isomers (dr = 91:9). Only the major isomer (cis) is described.
1H NMR (400 MHz, CDCl3): δ 0.83 and 0.97 (2 × 3H, 2 × d, J = 6.8 Hz, (CH3)2CH); 1.95 (1H, ∼octet, J = 6.8 Hz, (CH3)2CH); 2.14 (3H, s, CH3C=O); 3.44 (1H, d × d, J = 6.8, 5.0 Hz, OCHCH); 4.11 and 4.86 (2 × 1H, 2 × d, J = 15.1 Hz, N(HCH)); 5.89 (1H, d, J = 5.0 Hz, CHO); 7.21–7.23 and 7.29–7.38 (2H and 3H, 2 × m, CHarom). 13C NMR (100 MHz, CDCl3): δ 19.2 and 19.4 ((CH3)2CH); 20.7 (CH3C=O); 28.4 ((CH3)2CH); 46.0 (NCH2); 62.9 (OCHCH); 74.5 (CHO); 128.0, 128.2, and 128.9 (5 × HCarom); 135.1 (Carom,quat); 166.1 (NC=O); 169.6 (CH3C=O). MS (70 eV): m/z (%) 262 (M+ + 1, 24); 284 (M+ + Na, 27). High resolution electrospray ionization mass spectrometry (HRMS (ESI)) calculated for C15H20NO3+: 262.1438 [M + H]+, found: 262.1440. Yellow oil. Rf = 0.16 (PE/EtOAc 4:1). Yield after column chromatography (SiO2): 47%.
cis-3-Acetoxy-1-isopropyl-4-propylazetidin-2-one 14b
β-Lactam 14b was immediately used in the next reaction step, as purification proved to be impossible at this stage.
cis-3-Acetoxy-1-benzyl-4-phenylazetidin-2-one 14c
The spectral data are in accordance with those reported in the literature.68
Synthesis of 3-Hydroxyazetidin-2-ones 8
The synthesis of cis-1-benzyl-3-hydroxy-4-phenylazetidin-2-one 8c serves as an example for the synthesis of 3-hydroxyazetidin-2-ones 8a and 8b.
In a flask of 50 mL, 1.62 g (5.5 mmol) of cis-3-acetoxy-1-benzyl-4-phenylazetidin-2-one 14c was dissolved in 30 mL of a 1:1-solution of methanol and water (2:1 for 8a). Potassium carbonate (1.52 g, 11 mmol; 2 equiv) was added and the reaction mixture was stirred for 1 h at reflux temperature. Hereafter, methanol was evaporated and an extraction was performed with 50 mL diethyl ether. The organic phase was washed twice with 50 mL water, whereupon the aqueous phase was extracted twice with 50 mL diethyl ether. After drying of the combined organic phases using magnesium sulfate, filtration of the drying agent, evaporation of the solvent and recrystallization from a mixture of ethyl acetate and hexane (1:1), 0.86 g (62%) cis-1-benzyl-3-hydroxy-4-phenylazetidin-2-one 8c was obtained.
cis-1-Benzyl-3-hydroxy-4-isopropylazetidin-2-one 8a
1H NMR (400 MHz, CDCl3): δ 0.94 and 1.01 (2 × 3H, 2 × d, J = 6.9 Hz, (CH3)2CH); 2.06 (1H, ∼octet, J = 6.9 Hz, (CH3)2CH); 3.28 (1H, d × d, J = 6.9, 5.2 Hz, OCHCH); 4.09 (1H, d, J = 5.9 Hz, OH); 4.11 and 4.81 (2 × 1H, 2 × d, J = 15.1 Hz, N(HCH)); 4.88 (1H, d × d, J = 5.9, 5.2 Hz, CHO); 7.20–7.22 and 7.27–7.37 (2H and 3H, 2 × m, CHarom). 13C NMR (100 MHz, CDCl3): δ 19.4 and 19.9 ((CH3)2CH); 28.5 ((CH3)2CH); 45.8 (NCH2); 64.6 (OCHCH); 75.9 (CHO); 127.8, 128.2, and 128.8 (5 × HCarom); 135.4 (Carom,quat); 170.8 (C=O). MS (70 eV): m/z (%) 220 (M+ + 1, 100). HRMS (ESI) calculated for C13H18NO2+: 220.1332 [M + H]+, found: 220.1334. White crystals. Yield after recrystallization from EtOAc/hexane (1:1): 47%. Tm = 76 °C.
cis-3-Hydroxy-1-isopropyl-4-propylazetidin-2-one 8b
The spectral data are in accordance with those reported in the literature.1
cis-1-Benzyl-3-hydroxy-4-phenylazetidin-2-one 8c
The spectral data are in accordance with those reported in the literature.69
Enzyme Production and Purification
The genes for mutant R134A of the T. thermosaccharolyticum sucrose 6′-phosphate phosphorylase (TtSPP_R134A; UniProt ID D9TT09), the A. caldus sucrose synthase (SuSy; UniProt ID A0A059ZV61) and the S. rebaudiana UDP-glucosyltransferase (UGT-76G1Sr; UniProt ID Q6VAB4) were ligated into expression vectors, transformed in Escherichia coli and expressed recombinantly as described in earlier work.63,64,70
Cells containing the expressed proteins were harvested by centrifugation (20 000g, 5 min) and frozen overnight at −20 °C. Intracellular proteins were extracted by dissolving 5 g of frozen pellet in 25 mL of lysis buffer consisting of 50 mM 3-(N-morpholino)propanesulfonic acid (MOPS), 100 μM phenylmethylsulfonyl fluoride, 1 mg/mL lysozyme, pH 7.0. In the case of glucosyltransferase, MOPS was replaced by 50 mM sodium phosphate and 500 mM NaCl, pH 7.4. After incubation on ice for 30 min, the extract was exposed three times for 3 min of sonication and centrifuged to remove the insoluble fraction (20 000g, 30 min, 4 °C). Extracts containing sucrose synthase were used as such, while those containing sucrose 6′-phosphate phosphorylase were subjected to heat treatment (60 °C, 1 h) and centrifuged again. Extracts containing the glucosyltransferase were further purified by means of nickel–nitrilotriacetic acid affinity chromatography in gravity-flow columns as described by the supplier (MCLab). Subsequently, the buffer was exchanged to 50 mM MOPS, pH 7.0, in 30 kDa Amicon Ultra centrifugal filters (Merck).
The protein concentration was determined in triplicate using the BCA Protein Assay kit (Pierce) with bovine serum albumin as a standard.
Enzyme Activity Determination
The activity of TtSPP_R134A and SuSy was confirmed by performing reactions where sucrose is broken down (100 mM sucrose and 100 mM phosphate for TtSPP_R134A; 200 mM sucrose and 5 mM UDP for SuSy; 55 °C) and following the release of reducing sugars using the BCA method. The color reagent was prepared by combining 23 parts of a solution containing 1.5 g/L 4,4′-dicarboxy-2,2′-biquinoline dipotassium salt and 62.3 g/L anhydrous Na2CO3, one part of a solution composed of 23 g/L aspartic acid, 33 g/L anhydrous Na2CO3, and 7.3 g/L CuSO4 and six parts ethanol. The reaction samples (25 μL) were incubated with 150 μL color reagent for 30 min at 70 °C. Absorbance was measured at 560 nm.
The activity of UGT-76G1Sr was confirmed through a colorimetric assay, where UDP released from UDP-glucose is converted to UTP by pyruvate kinase (PK) using phosphoenolpyruvate (PEP). This reaction generates pyruvate as well, which is in turn reduced to lactate while oxidizing NADH to NAD+ by lactate dehydrogenase (LDH).71 The assay mixture consisted of 100 times diluted PK/LDH enzymes from rabbit muscle (900–1400 U/mL LDH, 600–100 U/mL PK), 5 mM MgCl2, 1.6 mg/mL bovine serum albumin, 0.3 mM NADH, and 0.3 mM PEP. A solution containing UGT-76G1Sr and substrates (100 μL; 10 mM UDP-glucose, 20 mM stevioside) was added to the assay mixture (100 μL) and absorbance at 340 nm was measured continuously.
Small-Scale Reactions and Optimization of Reaction Conditions
Small-scale reactions were carried out at 200 μL scale in Eppendorf tubes. The α-glucosylation reactions contained 1 M sucrose, 15 mM acceptor, 10% (v/v) organic solvent, 0.1 mg/mL TtSPP_R134A, and 50 mM MOPS (pH 7.0), and were incubated at 55 °C. The β-glucosylation reactions contained 200 mM sucrose, 15 mM acceptor, 10% (v/v) organic solvent, 0.2 mg/mL UGT-76G1Sr, 1 mg/mL SuSy, and 50 mM MOPS (pH 7.0), and were incubated at 32 °C. A 1 μL sample was taken after 24 h, spotted on silica gel 60 F254 precoated plates (Merck) and analyzed by thin layer chromatography using a mixture of EtOAc/MeOH/H2O (30:5:4) as the eluent. Spots were visualized by charring with 10% (v/v) H2SO4.
Reactions were optimized by assessing various solvents, i.e., 2-phenylethanol, methanol, isobutanol, 2-methylbutan-2-ol, acetone, methyl t-butyl ether, diethyl ether, and acetonitrile. Additionally, the acceptor concentration was varied (5–30 mM).
Synthesis of 3-O-α-d-Glucopyranosyl-3-hydroxyazetidin-2-ones 9-αG and Downstream Processing
The production of α-glucosides 9a-αG, 9b-αG, and 9c-αG was carried out using mutant TtSPP_R134A at a scale of 100, 25, or 50 mL, respectively. Each reaction contained 1 M sucrose, 10 v/v% acetone, 0.04–0.06 mg/mL TtSPP_R134A, 50 mM MOPS buffer (pH 7.0), and 21 mM of acceptor 8a or 15 mM of acceptor 8b or 8c. These reaction samples were incubated for 8–22 days at 55 °C with a reflux condenser, and stirred magnetically (IKA RCt Classic). The reaction samples (50 μL) were inactivated by adding 50 μL acetonitrile and 50 μL ultrapure water, followed by centrifugation. These samples were analyzed by LC-MS (eluent: 10–100% CH3CN in water (5 mM NH4OAc)).
The α-glucosides were first purified by double extraction with isopropyl alcohol, to which NaCl was added (300 mg/mL) to obtain a biphasic system. The organic phase was washed with water to remove any remaining sugars, followed by evaporation under reduced pressure. The remaining product was dissolved in methanol, filtered for the removal of leftover salts and evaporated again. Subsequently, automatic column chromatography was performed (gradual increase in polarity of the eluent: petroleum ether/EtOAc (100:0) to petroleum ether/EtOAc (0:100) to EtOAc/MeOH/H2O (30:5:4)). Minor impurities were finally removed via preparative TLC.
cis-1-Benzyl-3-O-α-d-glucopyranosyl-3-hydroxy-4-isopropylazetidin-2-one 9a-αG
1H NMR (400 MHz, D2O): δ 0.88 and 0.93 (2 × 3H, 2 × d, J = 6.8 Hz, (CH3)2CH); 2.07 (1H, octet, J = 6.8 Hz, (CH3)2CH); 3.39 (1H, d × d, J = 9.9, 9.1 Hz, CHCHCH2OH); 3.56 (1H, d × d, J = 9.9, 3.6 Hz, CHCHα); 3.56–3.72 (4H, m, CHiPr and CHCHCH(HCH)OH); 3.78 (1H, d × d, J = 12.3, 2.2 Hz, O(HCH)); 4.27 and 4.68 (2 × 1H, 2 × d, J = 15.6 Hz, N(HCH)); 4.97 (1H, d, J = 5.0 Hz, CHCHiPr); 5.21 (1H, d, J = 3.6 Hz, CHα); 7.26–7.28 and 7.31–7.41 (2H and 3H, 2 × m, CHarom). 13C NMR (100 MHz, D2O): δ 18.3 and 19.2 ((CH3)2CH); 27.7 ((CH3)2CH); 45.7 (NCH2); 60.4 (OCH2); 64.1 (CHiPr); 69.4 (CHCHCH2OH); 71.1 (CHCHα); 72.7 and 72.8 (CHCHCHCH2OH); 77.7 (CHCHiPr); 98.5 (CHα); 128.0, 128.1 and 129.0 (5 × HCarom); 135.0 (Carom,quat); 170.9 (C=O). IR (ATR, cm–1): νOH = 3348; νC=O = 1730; νmax = 2916, 2849, 2322, 1406, 1078, 1032, 924, 702. MS (70 eV): m/z (%) 404 (M+ + Na, 39); 382 (M+ + 1, 100). HRMS (ESI): calculated for C19H28NO7+: 382.1860 [M + H]+, found: 382.1862. Yellow oil. Rf = 0.5 (EtOAc/MeOH/H2O 30:5:4). Yield after normal phase column chromatography (SiO2): 12%.
cis-3-O-α-d-Glucopyranosyl-3-hydroxy-1-isopropyl-4-propylazetidin-2-one 9b-αG
1H NMR (400 MHz, D2O): δ 0.91 (3H, t, J = 7.4 Hz, CH2CH3); 1.19 and 1.21 (2 × 3H, 2 × d, J = 6.8 Hz, CH(CH3)2); 1.35 (2H, sextet, J = 7.4 Hz, CH2CH3); 1.71–1.77 (2H, m, CH2CH2CH3); 3.39 (1H, ∼t, J = 9.6 Hz, CHCHCH2OH); 3.54 (1H, d × d, J = 9.6, 3.6 Hz, CHCHα); 3.57–3.77 (4H, m, CH(CH3)2 and CHCHCH(HCH)OH); 3.80 (1H, d × d, J = 12.3, 2.1 Hz, O(HCH)); 3.99 (1H, ∼q, J = 5.4 Hz, CHCHPr); 4.88 (1H, d, J = 5.4 Hz, CHCHPr); 5.18 (1H, d, J = 3.6 Hz, CHα). 13C NMR (100 MHz, D2O): δ 13.5 (CH2CH3); 18.8 (CH2CH3); 18.9 and 20.7 ((CH3)2CH); 30.7 (CH2CH2CH3); 44.9 ((CH3)2CH); 57.6 (CHCHPr); 60.4 (OCH2); 69.3 (CHCHCH2OH); 71.2 (CHCHα); 72.67 and 72.70 (CHCHCHCH2OH); 76.7 (CHCHPr); 98.2 (CHα); 169.3 (C=O). IR (ATR, cm–1): νOH = 3339; νC=O = 1726; νmax = 2922, 2851, 2108, 1452, 1155, 1103, 1024, 627. MS (70 eV): m/z (%) 356 (M+ + Na, 100); 334 (M+ + 1.59). Rf = 0.3 (EtOAc/MeOH/H2O 30:5:4). Yield after normal phase column chromatography and preparative TLC (SiO2): 17%.
cis-1-Benzyl-3-O-α-d-glucopyranosyl-3-hydroxy-4-phenylazetidin-2-one 9c-αG
1H NMR (400 MHz, D2O): δ 1.84–1.88 and 3.20–3.22 (1H and 2H, 2 × m, CHCHCHCH2OH); 3.16 (1H, d × d, J = 12.9, 1.9 Hz, (HCH)O); 3.28 (1H, d × d, J = 12.9, 3.1 Hz, (HCH)O); 3.40 (1H, d × d, J = 9.1, 4.0 Hz, CHCHα); 4.14 (1H, d, J = 15.0 Hz, N(HCH)); 4.68 (1H, d, J = 15.0 Hz, N(HCH)); 4.91 (1H, d, J = 4.5 Hz, CHPh); 4.97 (1H, d, J = 4.0 Hz, CHα); 5.16 (1H, d, J = 4.5 Hz, CHCHPh); 7.18–7.22 (2H, m, CHarom); 7.31–7.38 (6H, m, CHarom); 7.40–7.44 (2H, m, CHarom). 13C NMR (100 MHz, D2O): δ 44.6 (NCH2); 59.5 (OCH2); 63.0 (CHPh); 68.1, 72.2, and 72.4 (CHCHCHCH2OH); 71.2 (CHCHα); 83.6 (CHCHPh); 101.6 (CHα); 128.2, 128.7, 128.8, 128.9, 129.0, and 129.2 (10 × HCarom); 132.9 and 134.1 (2 × Carom,quat); 169.7 (C=O). IR (ATR, cm–1): νOH = 3352; νC=O = 1743; νmax = 2926, 1452, 1412, 1350, 1080, 1022, 772, 700. MS (70 eV): m/z (%) 416 (M+ + 1, 100). HRMS (ESI): calculated for C22H26NO7+: 416.1704 [M + H]+, found: 416.1705. Rf = 0.4 (EtOAc/MeOH/H2O 30:5:4). White crystals. Yield after normal phase column chromatography and preparative TLC (SiO2): 3%.
Synthesis of 3-O-β-d-Glucopyranosyl-3-hydroxyazetidin-2-ones 9-βG and Downstream Processing
The β-glucosides 9a-βG, 9b-βG, and 9c-βG were synthesized at 100 mL scale using 200 mM sucrose, 2 mM UDP, 15 mM 8a, 8b, or 8c, 10% (v/v) acetone, 0.11–0.19 mg/mL UGT-76G1Sr, 0.31–0.36 mg/mL SuSy, and 50 mM MOPS buffer (pH 7.0). The reaction samples were incubated at 32 °C in falcon tubes with continuous shaking at 200 rpm for 2 (8b, 8c) or 6 (8a) weeks. The reaction samples (50 μL) were inactivated by adding 50 μL acetonitrile and 50 μL ultrapure water, followed by centrifugation. These samples were analyzed by LC-MS (eluent: 10–100% CH3CN in water (5 mM NH4OAc)).
The products were isolated by extraction and column chromatography as described for the α-glucosides. Finally, any remaining impurities were removed by reverse phase column chromatography (C18) using a gradient of water and acetonitrile as the eluent: CH3CN/H2O (0:100) to CH3CN/H2O (100:0).
cis-1-Benzyl-3-O-β-d-glucopyranosyl-3-hydroxy-4-isopropylazetidin-2-one 9a-βG
The spectral data are derived from the mixture of diastereomers (dr = 44:56).
1H NMR (400 MHz, D2O): δ 0.92–0.99 (2 × 6H, m, 2 × (CH3)2CH); 2.10 (2 × 1H, octet, J = 6.5 Hz, 2 × (CH3)2CH); 3.33–3.41 (2 × 1H, m, 2 × CHCHβ); 3.42–3.55 (2 × 3H, m, 2 × CHCHCHCHCHβ); 3.65–3.72 (2 × 1H, m, CHiPr); 3.73–3.78 (2 × 1H, m, 2 × O(HCH)); 3.89–3.95 (2 × 1H, m, 2 × O(HCH)); 4.34 (1H, d, JAB = 15.8 Hz, N(HCH)); 4.36 (1H, d, JAB = 15.4 Hz, N(HCH)); 4.52 (1H, d, J = 7.9 Hz, CHβ); 4.70 (1H, d, J = 7.8 Hz, CHβ); 4.73 (1H, d, JAB = 15.4 Hz, N(HCH)); 4.75 (1H, d, JAB = 15.8 Hz, N(HCH)); 5.08 (1H, d, J = 4.8 Hz, CHCHiPr); 5.14 (1H, d, J = 4.9 Hz, CHCHiPr); 7.33–7.47 (2 × 5H, m, CHarom). 13C NMR (100 MHz, D2O): δ 18.0, 18.3, 18.8, and 19.1 (2 × (CH3)2CH); 27.5 ((CH3)2CH); 27.6 ((CH3)2CH); 45.8 (NCH2); 45.9 (NCH2); 60.7 (OCH2); 60.8 (OCH2); 64.7 (CHiPr); 64.9 (CHiPr); 69.6, 69.7, 75.69, 75.71, 76.0, and 76.2 (2 × CHCHCHCHCHβ); 73.0 (CHCHβ); 73.2 (CHCHβ); 79.7 (CHCHiPr); 80.7 (CHCHiPr); 102.3 (CHβ); 102.6 (CHβ); 128.0, 128.1, 128.2, and 129.0 (2 × (5 × HCarom)); 135.09 (Carom,quat); 135.15 (Carom,quat); 170.68 (C=O); 170.72 (C=O). IR (ATR, cm–1): νOH = 3356; νC=O = 1724; νmax = 2927, 1680, 1415, 1227, 1078, 1043, 993, 419. MS (70 eV): m/z (%) 382 (M+ + 1, 100). HRMS (ESI): calculated for C19H28NO7+: 382.1860 [M + H]+, found: 382.1863. Colorless oil. Rf = 0.4 (EtOAc/MeOH/H2O 30:5:4). Yield after column chromatography (SiO2 and C18): 0.2%.
cis-3-O-β-d-Glucopyranosyl-3-hydroxy-1-isopropyl-4-propylazetidin-2-one 9b-βG
The spectral data are derived from the mixture of diastereomers (dr = 36:64).
1H NMR (400 MHz, D2O): δ 0.95 (2 × 3H, t, J = 7.3 Hz, 2 × CH2CH3); 1.24–1.31 (2 × 6H, m, 2 × (CH3)2CH); 1.33–1.50 (2 × 2H, m, 2 × CH2CH3); 1.68–1.82 (2 × 2H, m, 2 × CH2CH2CH3); 3.35 (2 × 1H, d × d, J = 8.4, 8.1 Hz, 2 × CHCHβ); 3.38–3.55 (2 × 3H, m, 2 × CHCHCHCHCHβ); 3.72–3.84 (2 × (2 × 1H), m, 2 × CHiPr and 2 × O(HCH)); 3.93 (2 × 1H, d, J = 12.2 Hz, O(HCH)); 4.00–4.05 (2 × 1H, m, 2 × CHCHPr); 4.50 (1H, d, J = 8.1 Hz, CHβ); 4.66 (1H, d, J = 8.1 Hz, CHβ); 5.01 (1H, d, J = 4.8 Hz, CHCHPr); 5.03 (1H, d, J = 4.8 Hz, CHCHPr). 13C NMR (100 MHz, D2O): δ 13.4 (CH2CH3); 13.5 (CH2CH3); 18.5 (CH2CH3); 18.7 (CH2CH3); 18.9 and 20.7 ((CH3)2CH); 19.0 and 20.6 ((CH3)2CH); 30.6 (CH2CH2CH3); 30.9 (CH2CH2CH3); 45.0 ((CH3)2CH); 45.1 ((CH3)2CH); 58.5 (CHPr); 58.7 (CHPr); 60.7 (2 × OCH2); 69.58, 69.66, 75.66, 75.69, 75.9 and 76.2 (2 × CHCHCHCHCHβ); 73.0 (CHCHβ); 73.2 (CHCHβ); 79.4 (CHCHPr); 79.6 (CHCHPr); 102.2 (CHβ); 102.4 (CHβ); 168.8 (C = 0); 169.0 (C = 0). IR (ATR, cm–1): νOH = 3356; νC=O = 1722; νmax = 2932, 1680, 1416, 1225, 1175, 1078, 1043, 419. MS (70 eV): m/z (%) 334 (M+ + 1, 100). Colorless oil. Rf = 0.38 (EtOAc/MeOH/H2O 30:5:4). Yield after column chromatography (SiO2 and C18): 1.4%.
cis-1-Benzyl-3-O-β-d-glucopyranosyl-3-hydroxy-4-phenylazetidin-2-one 9c-βG
The spectral data are derived from the mixture of diastereomers (dr = 41:59).
1H NMR (400 MHz, D2O): δ 3.01 (1H, d × d, J = 8.8, 8.4 Hz, CHCHβ); 3.07 (1H, d × d, J = 8.5, 8.4 Hz, CHCHβ); 3.13–3.41 (2 × 3H, m, 2 × CHCHCHCHCHβ); 3.53 (1H, d × d, J = 11.8, 5.5 Hz, O(HCH)); 3.70 (1H, d × d, J = 11.8, 5.7 Hz, O(HCH)); 3.73 (1H, d, J = 12.0 Hz, O(HCH)); 3.89 (1H, d, J = 12.0 Hz, O(HCH)); 4.10 (1H, d, J = 8.4 Hz, CHβ); 4.13 (1H, d, JAB = 14.8 Hz, N(HCH)); 4.17 (1H, d, JAB = 15.1 Hz, N(HCH)); 4.52 (1H, d, J = 8.4 Hz, CHβ); 4.72 (1H, d, JAB = 14.8 Hz, N(HCH)); 4.73 (1H, d, JAB = 15.1 Hz, N(HCH)); 4.91 (1H, d, J = 4.6 Hz, CHPh); 4.93 (1H, d, J = 4.3 Hz, CHPh); 5.31 (1H, d, J = 4.6 Hz, CHCHPh); 5.37 (1H, d, J = 4.3 Hz, CHCHPh); 7.20–7.21, 7.32–7.35, and 7.41–7.43 (2 × 10H, 3 × m, 2 × (10 × HCarom)). 13C NMR (100 MHz, D2O): δ 44.6 (NCH2); 44.7 (NCH2); 60.66 (OCH2); 60.72 (OCH2); 61.9 (CHPh); 62.6 (CHPh); 69.2, 69.5, 75.1, 75.5, and 75.9 (2 × CHCHCHCHCHβ); 72.5 (CHCHβ); 72.8 (CHCHβ); 80.6 (CHCHPh); 82.3 (CHCHPh); 101.1 (CHβ); 102.6 (CHβ); 128.2, 128.4, 128.66, 128.68, 128.73, 128.75, 128.79, 128.9, and 128.98 (2 × (10 × HCarom)); 132.7 (Carom,quat); 133.3 (Carom,quat); 134.2 (2 × Carom,quat); 169.1 (C=O); 169.3 (C=O). IR (ATR, cm–1): νOH = 3341; νC=O = 1738; νmax = 2924, 1672, 1452, 1416, 1233, 1076, 1045, 700. MS (70 eV): m/z (%) 416 (M+ + 1, 100). Colorless oil. Rf = 0.36 (EtOAc/MeOH/H2O 30:5:4). Yield after column chromatography (SiO2 and C18): 1%.
Acknowledgments
The authors are indebted to the Research Foundation—Flanders (FWO) for financial support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01969.
1H NMR and 13C NMR spectra of compounds 8a, 9a-c-αG, 9a-c-βG, 14a (PDF)
Author Contributions
§ L.D. and J.F. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Staudinger H. Zur Kenntniss der Ketene. Diphenylketen. Justus Liebigs Ann. Chem. 1907, 356, 51–123. 10.1002/jlac.19073560106. [DOI] [Google Scholar]
- Fleming A. On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to their Use in the Isolation of B. influenzæ. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar]
- Elander R. P. Industrial production of beta-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. 10.1007/s00253-003-1274-y. [DOI] [PubMed] [Google Scholar]
- Werder M.; Hauser H.; Carreira E. M. Synthesis and in Vitro Evaluation of Inhibitors of Intestinal Cholesterol Absorption. J. Med. Chem. 2005, 48, 6035–6053. 10.1021/jm050422p. [DOI] [PubMed] [Google Scholar]
- Bisacchi G. S.; Slusarchyk W. A.; Bolton S. A.; Hartl K. S.; Jacobs G.; Mathur A.; Meng W.; Ogletree M. L.; Pi Z.; Sutton J. C.; Treuner U.; Zahler R.; Zhao G.; Seiler S. M. Synthesis of potent and highly selective nonguanidine azetidinone inhibitors of human tryptase. Bioorg. Med. Chem. Lett. 2004, 14, 2227–2231. 10.1016/j.bmcl.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Han W. T.; Trehan A. K.; Wright J. J. K.; Federici M. E.; Seiler S. M.; Meanwell N. A. Azetidin-2-one derivatives as inhibitors of thrombin. Bioorg. Med. Chem. 1995, 3, 1123–1143. 10.1016/0968-0896(95)00101-L. [DOI] [PubMed] [Google Scholar]
- Aoyama Y.; Uenaka M.; Kii M.; Tanaka M.; Konoike T.; Hayasaki-kajiwara Y.; Nakajima M.; et al. Design, synthesis and pharmacological evaluation of 3-benzylazetidine-2-one-based human chymase inhibitors. Bioorg. Med. Chem. 2001, 9, 3065–3075. 10.1016/S0968-0896(01)00209-7. [DOI] [PubMed] [Google Scholar]
- Guillon C. D.; Koppel G. A.; Brownstein M. J.; Chaney M. O.; Ferris C. F.; Lu S. F.; Fabio K. M.; Miller M. J.; Heindel N. D.; Hunden D. C.; Cooper R. D. G.; Kaldor S. W.; Skelton J. J.; Dressman B. A.; Clay M. P.; Steinberg M. I.; Bruns R. F.; Simon N. G. Azetidinones as vasopressin V1a antagonists. Bioorg. Med. Chem. 2007, 15, 2054–2080. 10.1016/j.bmc.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau P. R.; Hasani F.; Plouffe C.; Malenfant E.; LaPlante S. R.; Guse I.; Ogilvie W. W.; Plante R.; Davidson W. C.; Hopkins J. L.; Morelock M. M.; Cordingley M. G.; Déziel R. Inhibition of Human Cytomegalovirus Protease by Monocyclic β-Lactam Derivatives: Kinetic Characterization Using a Fluorescent Probe. J. Am. Chem. Soc. 1999, 121, 2965–2973. 10.1021/ja983905+. [DOI] [Google Scholar]
- Sun L.; Vasilevich N. I.; Fuselier J. A.; Hocart S. J.; Coy D. H. Examination of the 1,4-disubstituted azetidinone ring system as a template for combretastatin A-4 conformationally restricted analogue design. Bioorg. Med. Chem. Lett. 2004, 14, 2041–2046. 10.1016/j.bmcl.2004.02.050. [DOI] [PubMed] [Google Scholar]
- Wang W.; Devasthale P.; Farrelly D.; Gu L.; Harrity T.; Cap M.; Chu C.; Kunselman L.; Morgan N.; Ponticiello R.; Zebo R.; Zhang L.; Locke K.; Lippy J.; O’Malley K.; Hosagrahara V.; Zhang L.; Kadiyala P.; Chang C.; Muckelbauer J.; Doweyko A. M.; Zahler R.; Ryono D.; Hariharan N.; Cheng P. T. W. Discovery of azetidinone acids as conformationally-constrained dual PPARalpha/gamma agonists. Bioorg. Med. Chem. Lett. 2008, 18, 1939–1944. 10.1016/j.bmcl.2008.01.126. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Rajput C. S.; Bhati S. K. Synthesis of 3-[4′-(p-chlorophenyl)-thiazol-2′-yl]-2-[(substituted azetidinone/thiazolidinone)-aminomethyl]-6-bromoquinazolin-4-ones as anti-inflammatory agent. Bioorg. Med. Chem. 2007, 15, 3089–3096. 10.1016/j.bmc.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Kaur H.; Kumar A. Synthesis of new azetidinonyl/thiazolidinonyl quinazolinone derivatives as antiparkinsonian agents. Arabian J. Chem. 2012, 5, 475–484. 10.1016/j.arabjc.2010.09.014. [DOI] [Google Scholar]
- Ilango K.; Arunkumar S. Synthesis, Antimicrobial and Antitubercular Activities of Some Novel Trihydroxy Benzamido Azetidin-2-one Derivatives. Trop. J. Pharm. Res. 2011, 10, 219–229. 10.4314/tjpr.v10i2.66567. [DOI] [Google Scholar]
- Ojima I. Recent Advances in the β-Lactam Synthon Method. Acc. Chem. Res. 1995, 28, 383–389. 10.1021/ar00057a004. [DOI] [Google Scholar]
- Alcaide B.; Almendros P.; Aragoncillo C. β-Lactams: Versatile Building Blocks for the Stereoselective Synthesis of Non-β-Lactam Products. Chem. Rev. 2007, 107, 4437–4492. 10.1021/cr0307300. [DOI] [PubMed] [Google Scholar]
- Decuyper L.; Jukič M.; Sosič I.; Žula A.; D’hooghe M.; Gobec S. Med. Res. Rev. 2018, 38, 426–503. 10.1002/med.21443. [DOI] [PubMed] [Google Scholar]
- Thomas M. D.; Langston-Unkefer P. J.; Uchytil T. F.; Durbin R. D. Inhibition of Glutamine Synthetase from Pea by Tabtoxinine-β-lactam. Plant Physiol. 1983, 71, 912–915. 10.1104/pp.71.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart K. M.; Reck M.; Bowman G. R.; Wencewicz T. A. Tabtoxinine-β-lactam is a “stealth” β-lactam antibiotic that evades β-lactamase-mediated antibiotic resistance. Med. Chem. Commun. 2016, 7, 118–127. 10.1039/C5MD00325C. [DOI] [Google Scholar]
- Ojima I.; Habus I.; Zhao M.; Georg G. I.; Jayasinghe L. R. Efficient and practical asymmetric synthesis of the taxol C-13 side chain, N-benzoyl-(2R,3S)-3-phenylisoserine, and its analogs via chiral 3-hydroxy-4-aryl-beta-lactams through chiral ester enolate-imine cyclocondensation. J. Org. Chem. 1991, 56, 1681–1683. 10.1021/jo00005a003. [DOI] [Google Scholar]
- Mishra R. K.; Coates C. M.; Revell K. D.; Turos E. Synthesis of 2-Oxazolidinones from β-Lactams: Stereospecific Total Synthesis of (−)-Cytoxazone and All of Its Stereoisomers. Org. Lett. 2007, 9, 575–578. 10.1021/ol062752p. [DOI] [PubMed] [Google Scholar]
- Alcaide B.; Almendros P.; Alonso J. M.; Aly M. F.; Torres M. R. Dual Behavior of 2-Azetidinone-Tethered Arylimines as Azadienophiles or Azadienes. Application to the Asymmetric Synthesis of Indolizidine-Type Systems. Synlett 2001, 2001, 1531–1534. 10.1055/s-2001-17453. [DOI] [Google Scholar]
- Kamath A.; Ojima I. Advances in the chemistry of β-lactam and its medicinal applications. Tetrahedron 2012, 68, 10640–10664. 10.1016/j.tet.2012.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet T.; Soetaert W.; Bojarová P.; Křen V.; Dijkhuizen L.; Eastwick-Field V.; Schiller A. Enzymatic Glycosylation of Small Molecules: Challenging Substrates Require Tailored Catalysts. Chem. - Eur. J. 2012, 18, 10786–10801. 10.1002/chem.201103069. [DOI] [PubMed] [Google Scholar]
- De Bruyn F.; Maertens J.; Beauprez J.; Soetaert W.; De Mey M. Biotechnological advances in UDP-sugar based glycosylation of small molecules. Biotechnol. Adv. 2015, 33, 288–302. 10.1016/j.biotechadv.2015.02.005. [DOI] [PubMed] [Google Scholar]
- De Roode B. M.; Franssen M. C. R.; Van Der Padt A.; Boom R. M. Perspectives for the industrial enzymatic production of glycosides. Biotechnol. Prog. 2003, 19, 1391–1402. 10.1021/bp030038q. [DOI] [PubMed] [Google Scholar]
- Postema M. H. D.; Calimente D.; Liu L.; Behrmann T. L. An Olefin Metathesis Route for the Preparation of (1→6)-Linked C-Disaccharide Glycals. A Convergent and Flexible Approach to C-Saccharide Synthesis. J. Org. Chem. 2000, 65, 6061–6068. 10.1021/jo0005159. [DOI] [PubMed] [Google Scholar]
- Palomo C.; Oiarbide M.; Esnal A.; Landa A.; Miranda J. I.; Linden A. Practical Synthesis of α-Amino Acid N-Carboxy Anhydrides of Polyhydroxylated α-Amino Acids from β-Lactam Frameworks. Model Studies toward the Synthesis of Directly Linked Peptidyl Nucleoside Antibiotics. J. Org. Chem. 1998, 63, 5838–5846. 10.1021/jo980354x. [DOI] [PubMed] [Google Scholar]
- Bose A. K.; Mathur C.; Wagle D. R.; Naqvi R.; et al. Chiral β-Lactams as Synthons. Stereospecific Synthesis of a 6-epi-Lincosamine Derivative. Heterocycles 1994, 39, 491–496. 10.3987/COM-94-S(B)74. [DOI] [Google Scholar]
- Goedl C.; Sawangwan T.; Mueller M.; Schwarz A.; Nidetzky B. A high-yielding biocatalytic process for the production of 2-O-(alpha-D-glucopyranosyl)-sn-glycerol, a natural osmolyte and useful moisturizing ingredient. Angew. Chem., Int. Ed. 2008, 47, 10086–10089. 10.1002/anie.200803562. [DOI] [PubMed] [Google Scholar]
- Rather M. Y.; Mishra S.; Chand S. β-Glucosidase catalyzed synthesis of octyl-β-D-glucopyranoside using whole cells of Pichia etchellsii in micro aqueous media. J. Biotechnol. 2010, 150, 490–496. 10.1016/j.jbiotec.2010.09.933. [DOI] [PubMed] [Google Scholar]
- Rivas F.; Parra A.; Martinez A.; Garcia-Granados A. Enzymatic glycosylation of terpenoids. Phytochem. Rev. 2013, 12, 327–339. 10.1007/s11101-013-9301-9. [DOI] [Google Scholar]
- Chen N. D.; Zhang J.; Liu J. H.; Yu B. Y. Microbial conversion of ruscogenin by Gliocladium deliquescens NRRL1086: glycosylation at C-1. Appl. Microbiol. Biotechnol. 2010, 86, 491–497. 10.1007/s00253-009-2315-y. [DOI] [PubMed] [Google Scholar]
- De Roode B. M.; Oliehoek L.; Van der Padt A.; Franssen M. C. R.; Boom R. M. Downstream processing of enzymatically produced geranyl glucoside. Biotechnol. Prog. 2001, 17, 881–886. 10.1021/bp010081i. [DOI] [PubMed] [Google Scholar]
- Jovetic S.; Zhu Y.; Marcone G. L.; Marinelli F.; Tramper J. β-Lactam and glycopeptide antibiotics: first and last line of defense?. Trends Biotechnol. 2010, 28, 596–604. 10.1016/j.tibtech.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Kaminaga Y.; Nagatsu A.; Akiyama T.; Sugimoto N.; Yamazaki T.; Maitani T.; Mizukami H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003, 555, 311–316. 10.1016/S0014-5793(03)01265-1. [DOI] [PubMed] [Google Scholar]
- Wright G. D. Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv. Drug Delivery Rev. 2005, 57, 1451–1470. 10.1016/j.addr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Fu X.; Albermann C.; Jiang J.; Liao J.; Zhang C.; Thorson J. S. Antibiotic optimization via in vitro glycorandomization. Nat. Biotechnol. 2003, 21, 1467–1469. 10.1038/nbt909. [DOI] [PubMed] [Google Scholar]
- Vaccaro W. D.; Davis H. R. Sugar-substituted 2-azetidinone cholesterol absorption inhibitors: Enhanced potency by modification of the sugar. Bioorg. Med. Chem. Lett. 1998, 8, 313–318. 10.1016/S0960-894X(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto I.; Muto N.; Nagata E.; Nakamura T.; Suzuki Y. Formation of a stable L-ascorbic acid alpha-glucoside by mammalian alpha-glucosidase-catalyzed transglucosylation. Biochim. Biophys. Acta 1990, 1035, 44–50. 10.1016/0304-4165(90)90171-R. [DOI] [PubMed] [Google Scholar]
- Kren V.Glycoside vs. Aglycon: the Role of Glycosidic Residue in Biological Activity. In Glycoscience; Fraser-Reid B. O., Tatsuta K., Thiem J., Eds.; Springer: Berlin Heidelberg, 2008; pp 2589–2644. [Google Scholar]
- Das R.; Mukhopadhyay B. Chemical O-Glycosylations: An Overview. ChemistryOpen 2016, 5, 401–433. 10.1002/open.201600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A.; Dordick J. S.; Hauer B.; Kiener A.; Wubbolts M.; Witholt B. Industrial biocatalysis today and tomorrow. Nature 2001, 409, 258–268. 10.1038/35051736. [DOI] [PubMed] [Google Scholar]
- Lim E. K. Plant Glycosyltransferases: Their Potential as Novel Biocatalysts. Chem. - Eur. J. 2005, 11, 5486–5494. 10.1002/chem.200500115. [DOI] [PubMed] [Google Scholar]
- Nakayama A.; Okano A.; Feng Y.; Collins J. C.; Collins K. C.; Walsh C. T.; Boger D. L. Enzymatic Glycosylation of Vancomycin Aglycon: Completion of a Total Synthesis of Vancomycin and N- and C-Terminus Substituent Effects of the Aglycon Substrate. Org. Lett. 2014, 16, 3572–3575. 10.1021/ol501568t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdobbel A.; De Winter K.; Desmet T.; Soetaert W. Sucrose phosphorylase as cross-linked enzyme aggregate: Improved thermal stability for industrial applications. Biotechnol. J. 2010, 5, 1192–1197. 10.1002/biot.201000202. [DOI] [PubMed] [Google Scholar]
- Mollet K.; Decuyper L.; Vander Meeren S.; Piens N.; De Winter K.; Desmet T.; D’hooghe M. Synthesis of 2-aryl-3-(2-cyanoethyl)aziridines and their chemical and enzymatic hydrolysis towards γ-lactams and γ-lactones. Org. Biomol. Chem. 2015, 13, 2716–2725. 10.1039/C4OB02615B. [DOI] [PubMed] [Google Scholar]
- Decuyper L.; Piens N.; Mincke J.; Bomon J.; De Schrijver B.; Mollet K.; De Winter K.; Desmet T.; D’hooghe M. A nitrilase-mediated entry to 4-carboxymethyl-β-lactams from chemically prepared 4-(cyanomethyl)azetidin-2-ones. RSC Adv. 2016, 6, 54573–54579. 10.1039/C6RA08213K. [DOI] [Google Scholar]
- Sassaki G. L.; Rattmann Y. D.; Santana-Filho A. P.; Riter D. S.; Iagher F.; Trindade E. S.; Da Silva M. D.; Santos A. R. S.; De Souza L. M.; Iacomini M.; Gorin P. A. J. Galactofuranosyl glycosides: immunomodulatory effects on macrophages and in vivo enhancement of lethality on sepsis. Chem.-Biol. Interact. 2013, 205, 29–37. 10.1016/j.cbi.2013.05.014. [DOI] [PubMed] [Google Scholar]
- De Winter K.; Dewitte G.; Dirks-Hofmeister M. E.; De Laet S.; Pelantova H.; Kren V.; Desmet T. Enzymatic Glycosylation of Phenolic Antioxidants: Phosphorylase-Mediated Synthesis and Characterization. J. Agric. Food Chem. 2015, 63, 10131–10139. 10.1021/acs.jafc.5b04380. [DOI] [PubMed] [Google Scholar]
- Piens N.; Goossens H.; Hertsen D.; Deketelaere S.; Crul L.; Demeurisse L.; De Moor J.; Van den Broeck E.; Mollet K.; Van Hecke K.; Van Speybroeck V.; D’hooghe M. Reactivity of 3-Oxo-β-lactams with Respect to Primary Amines—An Experimental and Computational Approach. Chem. - Eur. J. 2017, 23, 18002–18009. 10.1002/chem.201703852. [DOI] [PubMed] [Google Scholar]
- Jiao L.; Liang Y.; Xu J. Origin of the Relative Stereoselectivity of the β-Lactam Formation in the Staudinger Reaction. J. Am. Chem. Soc. 2006, 128, 6060–6069. 10.1021/ja056711k. [DOI] [PubMed] [Google Scholar]
- Banik B. K.; Manhas M. S. Stereospecific novel glycosylation of hydroxy β-lactams via iodine-catalyzed reaction: a new method for optical resolution. Tetrahedron 2012, 68, 10769–10779. 10.1016/j.tet.2012.01.078. [DOI] [Google Scholar]
- Banik B. K.; Banik I.; Becker F. F. Asymmetric synthesis of anticancer beta-lactams via Staudinger reaction: utilization of chiral ketene from carbohydrate. Eur. J. Med. Chem. 2010, 45, 846–848. 10.1016/j.ejmech.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Borer B. C.; Balogh D. W. An asymmetric synthesis of a 3-hydroxy-β-lactam by ketene-imine cycloaddition: utilization of chiral ketenes from carbohydrates. Tetrahedron Lett. 1991, 32, 1039–1040. 10.1016/S0040-4039(00)74481-9. [DOI] [Google Scholar]
- Shaikh A. L.; Kale A. S.; Shaikh M. A.; Puranik V. G.; Deshmukh A. R. A. S. Asymmetric synthesis of β-lactams by [2+2] cycloaddition using 1,4:3,6-dianhydro-d-glucitol (isosorbide) derived chiral pools. Tetrahedron 2007, 63, 3380–3388. 10.1016/j.tet.2007.02.022. [DOI] [Google Scholar]
- A small selection of references:; a Adinolfi M.; Giacomini D.; Iadonisi A.; Quintavalla A.; Valerio S. Synthesis of the Mannopeptimycin Disaccharide and Its Conjugation with 4-Alkylidene-β-lactams. Eur. J. Org. Chem. 2008, 2008, 2895–2899. 10.1002/ejoc.200701159. [DOI] [Google Scholar]; b Izquierdo I.; Plaza M. T.; Robles R.; Mota A. J. Synthesis of 4-octuloses. Part 7: Highly stereoselective synthesis of 2,3-anhydrosugar derivatives as key intermediates in the preparation of sugar β-lactams. Tetrahedron: Asymmetry 2000, 11, 4509–4519. 10.1016/S0957-4166(00)00421-3. [DOI] [Google Scholar]; c Sánchez I. P.; Turos E. Glycosylated vinyl ethers by the Julia–Lythgoe–Kocienski olefination: application to the synthesis of 2′,5′-dideoxydisaccharides and carbohydrated β-lactams. Tetrahedron: Asymmetry 2009, 20, 1646–1660. 10.1016/j.tetasy.2009.05.039. [DOI] [Google Scholar]; d Łysek R.; Furman B.; Kałuża Z.; Frelek J.; Suwińska K.; Urbańczyk-Lipkowska Z.; Chmielewski M. [2 + 2] Cycloaddition of chlorosulfonyl isocyanate to allenyl-sugar ethers. Tetrahedron: Asymmetry 2000, 11, 3131–3150. 10.1016/S0957-4166(00)00260-3. [DOI] [Google Scholar]; e Vaccaro W. D.; Sher R.; Davis H. R. Sugar-substituted 2-azetidinones as cholesterol absorption inhibitors. Bioorg. Med. Chem. Lett. 1998, 8, 35–40. 10.1016/S0960-894X(97)10185-8. [DOI] [PubMed] [Google Scholar]; f Zhu X.; Schmidt R. R. New principles for glycoside-bond formation. Angew. Chem., Int. Ed. 2009, 48, 1900–1934. 10.1002/anie.200802036. [DOI] [PubMed] [Google Scholar]; g Arun M.; Joshi S. N.; Puranik V. G.; Bhawal B. M.; Deshmukh A. R. A. S. Asymmetric synthesis of azetidin-2-ones by [2+2] cycloaddition using chiral imines derived from D-(+)-glucose. Tetrahedron 2003, 59, 2309–2316. 10.1016/S0040-4020(03)00239-4. [DOI] [Google Scholar]; h Nagarajan S.; Arjun P.; Raaman N.; Shah A.; Sobhia M. E.; Das T. M. Stereoselective synthesis of sugar-based β-lactam derivatives: docking studies and its biological evaluation. Tetrahedron 2012, 68, 3037–3045. 10.1016/j.tet.2012.02.017. [DOI] [Google Scholar]; i Palomo C.; Aizpurua J. M.; Balentova E.; Azcune I.; Santos J. I.; Jiménez-Barbero J.; Cañada J.; Miranda J. I. “Click” Saccharide/β-Lactam Hybrids for Lectin Inhibition. Org. Lett. 2008, 10, 2227–2230. 10.1021/ol8006259. [DOI] [PubMed] [Google Scholar]
- Capozzi G.; Menichetti S.; Nativi C.. Selective Glycosidation Reactions and Their Use in Medicinal Chemistry. In New Trends in Synthetic Medicinal Chemistry; Gualtieri F., Ed.; Wiley-VCH Verlag GmbH: Weinheim, 2008; pp 221–259. [Google Scholar]
- Zaks A.; Dodds D. R. Enzymatic glucuronidation of a novel cholesterol absorption inhibitor, Sch 58235. Appl. Biochem. Biotechnol. 1998, 73, 205–214. 10.1007/BF02785656. [DOI] [PubMed] [Google Scholar]
- Leimkuhler C.; Chen Z.; Kruger R. G.; Oberthür M.; Lu W.; Walsh C. T.; Kahne D. Glycosylation of glycopeptides: a comparison of chemoenzymatic and chemical methods. Tetrahedron: Asymmetry 2005, 16, 599–603. 10.1016/j.tetasy.2004.12.006. [DOI] [Google Scholar]
- Ritter T. K.; Mong K. K. T.; Liu H.; Nakatani T.; Wong C. H. A Programmable One-Pot Oligosaccharide Synthesis for Diversifying the Sugar Domains of Natural Products: A Case Study of Vancomycin. Angew. Chem., Int. Ed. 2003, 42, 4657–4660. 10.1002/anie.200351534. [DOI] [PubMed] [Google Scholar]
- Desmet T.; Soetaert W. Enzymatic glycosyl transfer: mechanisms and applications. Biocatal. Biotransform. 2011, 29, 1–18. 10.3109/10242422.2010.548557. [DOI] [Google Scholar]
- Verhaeghe T.; Aerts D.; Diricks M.; Soetaert W.; Desmet T. The quest for a thermostable sucrose phosphorylase reveals sucrose 6′-phosphate phosphorylase as a novel specificity. Appl. Microbiol. Biotechnol. 2014, 98, 7027–7037. 10.1007/s00253-014-5621-y. [DOI] [PubMed] [Google Scholar]
- Dirks-Hofmeister M. E.; Verhaeghe T.; De Winter K.; Desmet T. Creating Space for Large Acceptors: Rational Biocatalyst Design for Resveratrol Glycosylation in an Aqueous System. Angew. Chem., Int. Ed. 2015, 127, 9421–9424. 10.1002/ange.201503605. [DOI] [PubMed] [Google Scholar]
- Dewitte G.; Walmagh M.; Diricks M.; Lepak A.; Gutmann A.; Nidetzky B.; Desmet T. Screening of recombinant glycosyltransferases reveals the broad acceptor specificity of stevia UGT-76G1. J. Biotechnol. 2016, 233, 49–55. 10.1016/j.jbiotec.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Masada S.; Kawase Y.; Nagatoshi M.; Oguchi Y.; Terasaka K.; Mizukami H. An efficient chemoenzymatic production of small molecule glucosides with in situ UDP-glucose recycling. FEBS Lett. 2007, 581, 2562–2566. 10.1016/j.febslet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Roslund M. U.; Tähtinen P.; Niemitz M.; Sjöholm R. Complete assignments of the (1)H and (13)C chemical shifts and J(H,H) coupling constants in NMR spectra of D-glucopyranose and all D-glucopyranosyl-D-glucopyranosides. Carbohydr. Res. 2008, 343, 101–112. 10.1016/j.carres.2007.10.008. [DOI] [PubMed] [Google Scholar]
- a Shimoda K.; Kubota N.; Hamada H. Enantioselective glucosylation of (±)-secondary alcohols with plant glucosyltransferases. Tetrahedron: Asymmetry 2004, 15, 2319–2321. 10.1016/j.tetasy.2004.06.022. [DOI] [Google Scholar]; b Lim E. K.; Doucet C. J.; Hou B.; Jackson R. G.; Abrams S. R.; Bowles D. J. Resolution of (+)-abscisic acid using an Arabidopsis glycosyltransferase. Tetrahedron: Asymmetry 2005, 16, 143–147. 10.1016/j.tetasy.2004.11.062. [DOI] [Google Scholar]; c Yu L.; Cabrera R.; Ramirez J.; Malinovskii V. A.; Brew K.; Wang P. G. Chemical and enzymatic synthesis of glycoconjugates 1. Enzymatic galactosylation of conduritol B. Tetrahedron Lett. 1995, 36, 2897–2900. 10.1016/0040-4039(95)00430-K. [DOI] [Google Scholar]; d Nishida Y.; Tamakoshi H.; Kobayashi K.; Thiem J. The First Bovine β1,4-Galactosyltransferase Reaction with an Acyclic Acceptor Substrate, 3-Acetamido-1,2-propanediol, To Yield a 3-O-β-D-Galactopyranosyl-sn-glycerol Skeleton. Org. Lett. 2001, 3, 1–3. 10.1021/ol006590n. [DOI] [PubMed] [Google Scholar]; e Ge H.-X.; Zhang J.; Dong Y.; Cui K.; Yu B.-Y. Unique biocatalytic resolution of racemic tetrahydroberberrubine via kinetic glycosylation and enantio-selective sulfation. Chem. Commun. 2012, 48, 6127–6129. 10.1039/c2cc32175k. [DOI] [PubMed] [Google Scholar]
- Song C. E.; Lee S. W.; Roh E. J.; Lee S.; Lee W.-K. A new synthetic route to (3R,4S)-3-hydroxy-4-phenylazetidin-2-one as a taxol side chain precursor. Tetrahedron: Asymmetry 1998, 9, 983–992. 10.1016/S0957-4166(98)00049-4. [DOI] [Google Scholar]
- Mihovilovic M. D.; Feicht A.; Kayser M. M. An Efficient and Simple Procedure for the Preparation of α-Keto-β-lactams. J. Prakt. Chem. 2000, 342, 585–590. . [DOI] [Google Scholar]
- Diricks M.; De Bruyn F.; Van Daele P.; Walmagh M.; Desmet T. Identification of sucrose synthase in nonphotosynthetic bacteria and characterization of the recombinant enzymes. Appl. Microbiol. Biotechnol. 2015, 99, 8465–8474. 10.1007/s00253-015-6548-7. [DOI] [PubMed] [Google Scholar]
- Gosselin S.; Alhussaini M.; Streiff M. B.; Takabayashi K.; Palcic M. M. A continuous spectrophotometric assay for glycosyltransferases. Anal. Biochem. 1994, 220, 92–97. 10.1006/abio.1994.1303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.