Abstract
During mammalian development, the challenge for the embryo is to override intrinsic cellular plasticity to drive cells to distinct fates. Here, we unveil novel roles for the HIPPO signaling pathway in controlling cell positioning and expression of Sox2, the first marker of pluripotency in the mouse early embryo. We show that maternal and zygotic YAP1 and WWTR1 repress Sox2 while promoting expression of the trophectoderm gene Cdx2 in parallel. Yet, Sox2 is more sensitive than Cdx2 to Yap1/Wwtr1 dosage, leading cells to a state of conflicted cell fate when YAP1/WWTR1 activity is moderate. Remarkably, HIPPO signaling activity resolves conflicted cell fate by repositioning cells to the interior of the embryo, independent of its role in regulating Sox2 expression. Rather, HIPPO antagonizes apical localization of Par complex components PARD6B and aPKC. Thus, negative feedback between HIPPO and Par complex components ensure robust lineage segregation.
Research organism: Mouse
eLife digest
As an embryo develops, its cells divide, grow and migrate in specific patterns to build an organized collection of cells that go on to form our tissues and organs. One of the first steps – well before the embryo has implanted into the womb – is to allocate cells to make part of the placenta.
Once this process is complete, the remaining cells continue building the organism. These cells are pluripotent, meaning they can develop into any part of the body. Scientists think that the embryo manages to sort ‘placenta cells’ from pluripotent ones with the help of certain proteins, which the mother has packaged into her eggs.
To investigate this further, Frum et al. used genetic tools to track a specific gene called Sox2 that identifies pluripotent cells as soon as they are formed in mouse embryos. The experiments revealed that the mother places two closely related proteins known as YAP1 and WWTR1 within each egg, which help to make placenta cells different from pluripotent cells. Moreover, both proteins enable the embryo to segregate these two cell types to two different locations: placenta cells are moved to the outer layer of the embryo, while pluripotent cells are moved to the inside.
Current technologies allow researchers to create pluripotent cells in the laboratory. But these approaches often result in error, failing to replicate the embryo’s natural ability. By studying how embryos form and arrange pluripotent cells, scientists hope to advance stem cell technology (which emerge from pluripotent cells). This may help to find new ways to heal damaged tissues and organs, or to treat or even prevent many diseases.
Introduction
During embryogenesis cells gradually differentiate, adopting distinct gene expression profiles and fates. In mammals, the first cellular differentiation is the segregation of trophectoderm and inner cell mass. The trophectoderm, which comprises the polarized outer surface of the blastocyst, will mainly produce cells of the placenta, while the inner cell mass will produce pluripotent cells, which are progenitors of both fetus and embryonic stem cells. Understanding how pluripotent inner cell mass cells are segregated from non-pluripotent cells therefore reveals how pluripotency is induced in a naturally occurring setting.
Progenitors of inner cell mass are first morphologically apparent at the 16 cell stage as unpolarized cells residing inside the morula (reviewed in Frum and Ralston, 2017). However, at this stage, pluripotency genes such as Pou5f1 (Oct4) and Nanog, do not specifically label inside cells (Dietrich and Hiiragi, 2007; Niwa et al., 2005; Palmieri et al., 1994; Strumpf et al., 2005). Thus, the first cell fate decision has been studied mainly from the perspective of trophectoderm specification because the transcription factor CDX2, which is essential for trophectoderm development (Strumpf et al., 2005), is expressed specifically in outer cells of the 16 cell embryo (Ralston and Rossant, 2008), and has provided a way to distinguish future trophectoderm cells from non-trophectoderm cells. Knowledge of CDX2 as a marker of trophectoderm cell fate enabled the discovery of mechanisms that sense cellular differences in polarity and position in the embryo, and then respond by regulating expression of Cdx2 (Nishioka et al., 2009). However, the exclusive study of Cdx2 regulation does not provide direct knowledge of how pluripotency is established because the absence of Cdx2 expression does not necessarily indicate acquisition of pluripotency. As such, our understanding of the first cell fate decision in the early mouse embryo is incomplete.
In contrast to other markers of pluripotency, Sox2 is expressed specifically in inside cells at the 16 cell stage, and is therefore the first marker of pluripotency in the embryo (Guo et al., 2010; Wicklow et al., 2014). The discovery of how Sox2 expression is regulated in the embryo therefore provides unique insight into how pluripotency is first established in vivo. Genes promoting expression of Sox2 in the embryo have been described (Cui et al., 2016; Wallingford et al., 2017). However, it is currently unclear how expression of Sox2 becomes restricted to inside cells. We previously showed that Sox2 is restricted to inside cells by a Cdx2-independent mechanism (Wicklow et al., 2014), which differs from Oct4 and Nanog, which are restricted to the inner cell mass by CDX2 (Niwa et al., 2005; Strumpf et al., 2005). Thus, Sox2 and Cdx2 are regulated in parallel, leading to complementary inside/outside expression patterns. However, it is not known whether Sox2 is regulated by the same pathway that regulates Cdx2 or whether a distinct pathway could be in use.
The expression of Cdx2 is regulated by members of the HIPPO signaling pathway. In particular, the HIPPO pathway kinases LATS1/2 become active in unpolarized cells located deep inside the embryo, where they antagonize activity of the YAP1/WWTR1/TEAD4 transcriptional complex that is thought to promote expression of Cdx2 (Anani et al., 2014; Cockburn et al., 2013; Hirate et al., 2013; Kono et al., 2014; Korotkevich et al., 2017; Leung and Zernicka-Goetz, 2013; Lorthongpanich et al., 2013; Mihajlović and Bruce, 2016; Nishioka et al., 2009; Nishioka et al., 2008; Posfai et al., 2017; Rayon et al., 2014; Watanabe et al., 2017; Yagi et al., 2007; Zhu et al., 2017). In this way, the initially ubiquitous expression of Cdx2 becomes restricted to outer trophectoderm cells. However, the specific requirements for Yap1 and Wwtr1 in the regulation of Cdx2 has been inferred from overexpression of wild type and dominant-negative variants, neither of which provide the standard of gene expression analysis that null alleles can provide. Nonetheless, the roles of Yap1 and Wwtr1 in regulating expression of Sox2 have not been investigated. Here, we evaluate the roles of maternal and zygotic YAP1/WWTR1 in regulating expression of Sox2 and cell fate during blastocyst formation.
Results
Patterning of Sox2 is ROCK-dependent
To identify the mechanisms regulating Sox2 expression during blastocyst formation, we focused on how Sox2 expression is normally repressed in the trophectoderm to achieve inside cell-specific expression. We previously showed that SOX2 is specific to inside cells in the absence of the trophectoderm factor CDX2 (Wicklow et al., 2014), suggesting that mechanisms that repress Sox2 in the trophectoderm act upstream of Cdx2. Rho-associated, coiled-coil containing protein kinases (ROCK1 and 2) are thought to act upstream of Cdx2 because embryos developing in the presence of a ROCK-inhibitor (Y-27632, ROCKi) exhibit reduced Cdx2 expression (Kono et al., 2014). Additionally, quantitative RT-PCR showed that Sox2 mRNA levels are elevated in ROCKi-treated embryos (Kono et al., 2014), suggesting that ROCK1/2 activity leads to transcriptional repression of Sox2. However, the role of ROCK1/2 in regulating the spatial expression of Sox2 has not been investigated.
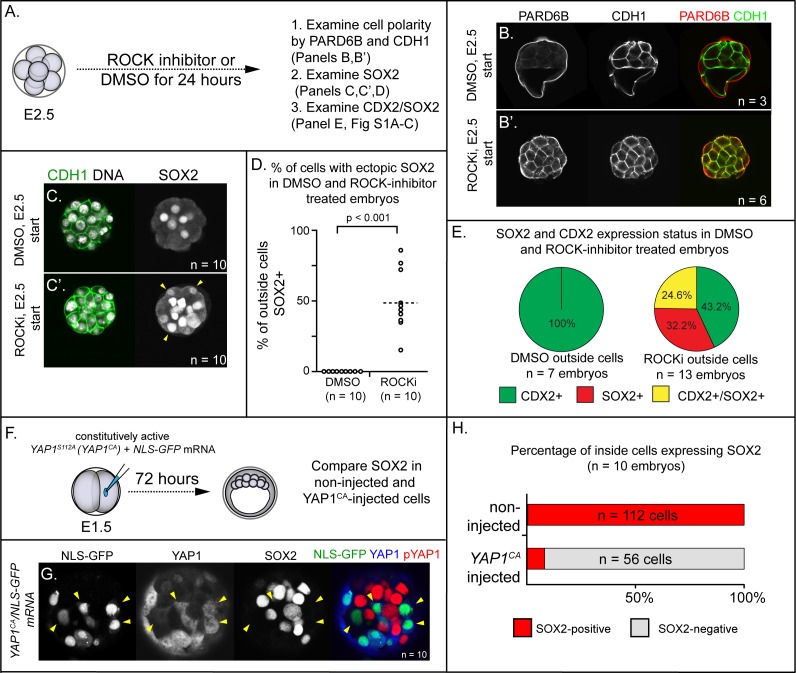
To evaluate the roles of ROCK1/2 in patterning Sox2 expression, we collected 8-cell stage embryos prior to embryo compaction (E2.5), and then cultured these either in control medium or in the presence of ROCKi for 24 hr (Figure 1A). Embryos cultured in control medium exhibited normal cell polarity, evidenced by the apical localization of PARD6B and basolateral localization of E-cadherin (CDH1) in outside cells (Figure 1B,C) as expected (Vestweber et al., 1987; Vinot et al., 2005). Additionally, SOX2 was detected only in inside cells in control embryos (Figure 1C,D). By contrast, embryos cultured in ROCKi exhibited defects in cell polarity (Figure 1B’, C’), consistent with prior studies (Kono et al., 2014). Interestingly, in ROCKi-treated embryos, we observed ectopic SOX2 expression in cells located on the outer surface of the embryo (Figure 1C’, D), indicating that ROCK1/2 participates in repressing expression of Sox2 in the trophectoderm.
Figure 1. ROCK1/2 and nuclear YAP1 repress expression of SOX2.
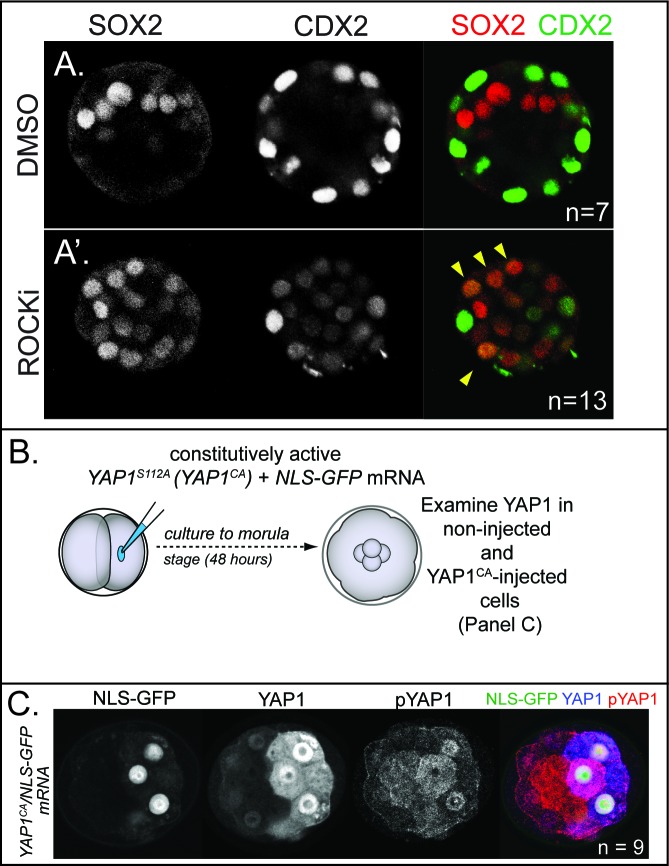
(A) Experimental design: embryos were collected at E2.5 and treated with ROCK inhibitor Y-27632 (ROCKi) or DMSO (control) for 24 hr. (B–B’) Confocal images of apical (PARD6B) and basolateral (CDH1) membrane components in control and ROCKi-treated embryos. As expected, PARD6B and CDH1 are mislocalized to the entire cell membrane of all cells in ROCKi-treated embryos, demonstrating effective ROCK inhibition (n = number of embryos examined). (C–C’) In control embryos, SOX2 is detected only in inside cells, while in ROCKi-treated embryos, SOX2 is detected in inside and outside cells (arrowheads, outside cells; n = embryos). (D) Quantification of ectopic SOX2 detected in outside cells of control and ROCKi-treated embryos (p, student’s t-test, n = embryos). (E) SOX2 and CDX2 staining in outside cells of control and ROCKi-treated embryos. ROCK-inhibitor treatment leads to outside cells with mixed lineage marker expression (CDX2+/SOX2+). (F) Experimental design: embryos were collected at E1.5 and one of two blastomeres injected with mRNAs encoding YAP1CA and GFP. Embryos were cultured for 72 hr, fixed, and then analyzed by immunofluorescence and confocal microscopy. (G) SOX2 is detected non-injected inside cells. SOX2 is not detected in YAP1CA-overexpressing inside cells (arrowheads), n = embryos. (H) Across multiple embryos, all non-injected inside cells express SOX2, whereas the vast majority of YAP1CA-injected inside cells fail to express SOX2.
Figure 1—figure supplement 1. Effect of ROCK1/2 inhibition on Cdx2 expression and effect Yap1CA overexpression on YAP1 localization and phosphorylation.
To scrutinize the identity of outside-positioned SOX2-positive cells in ROCKi-treated embryos, we co-stained an additional cohort of control and ROCKi-treated embryos with CDX2 and SOX2 and compared the overlap of lineage marker expression. In control embryos, CDX2 was detected only in outside cells (Figure 1—figure supplement 1A) as expected at this stage (Ralston and Rossant, 2008; Strumpf et al., 2005). In ROCKi-treated embryos, CDX2 expression levels were reduced (Figure 1—figure supplement 1A’) as was the proportion of outside cells in which CDX2 was detected (Figure 1E), as previously reported (Kono et al., 2014). However, among outside cells, a substantial proportion coexpressed CDX2 and SOX2 in ROCKi-treated embryos compared with controls (Figure 1E and Figure 1—figure supplement 1A), suggesting that ROCK inhibition leads to an increase in outside cells of mixed lineage. Since SOX2 expression does not regulate expression of CDX2 (Wicklow et al., 2014), these observations suggest that ROCK1/2 activity regulates these genes through parallel mechanisms. We next sought to identify mediators that act downstream of ROCK1/2 to repress expression of Sox2 in the trophectoderm.
YAP1 is sufficient to repress expression of SOX2 in the inner cell mass
Several direct and indirect targets of ROCK1/2 kinases in the early embryo have been described (Alarcon and Marikawa, 2018; Shi et al., 2017). Among these is YAP1, a transcriptional partner of TEAD4 (Nishioka et al., 2009), since ROCK activity is required for the nuclear localization of YAP1 (Kono et al., 2014). Notably, Tead4 is required to repress expression of Sox2 in the trophectoderm (Wicklow et al., 2014), consistent with the possibility that YAP1 partners with TEAD4 to repress Sox2 expression in the trophectoderm. To test this hypothesis, we overexpressed a constitutively active variant of YAP1 (YAP1CA). Substitution of alanine at serine 112 leads YAP1 to be constitutively nuclear and constitutively active (YAP1CA hereafter) (Dong et al., 2007; Nishioka et al., 2009; Zhao et al., 2007). We injected mRNAs encoding YAP1CA and GFP into one of two blastomeres at the 2-cell stage, and then cultured these to the blastocyst stage (Figure 1F). This mosaic approach to overexpression permitted comparison of Yap1CA-overexpressing with non-injected cells, which served as internal negative controls. We first examined localization of YAP1 in these embryos at the morula stage, with the expectation that YAP1 would be detected in nuclei of both inside and outside cells in YAP1CA-overexpressing cells (Nishioka et al., 2009). As expected, YAP1 was observed in nuclei of all Yap1CA-overexpressing cells (Figure 1—figure supplement 1B,C). We next evaluated the consequences of ectopic nuclear YAP1 on expression of SOX2 in inside cells. We observed a conspicuous decrease in the proportion of Yap1CA-overexpressing inside cells expressing detectable SOX2 (Figure 1G,H). Therefore, nuclear YAP1 is sufficient to repress Sox2 expression in the inner cell mass, indicative of a likely role for YAP1 in repressing expression of Sox2 in the trophectoderm downstream of ROCK1/2.
LATS kinase is sufficient to induce inside cell positioning
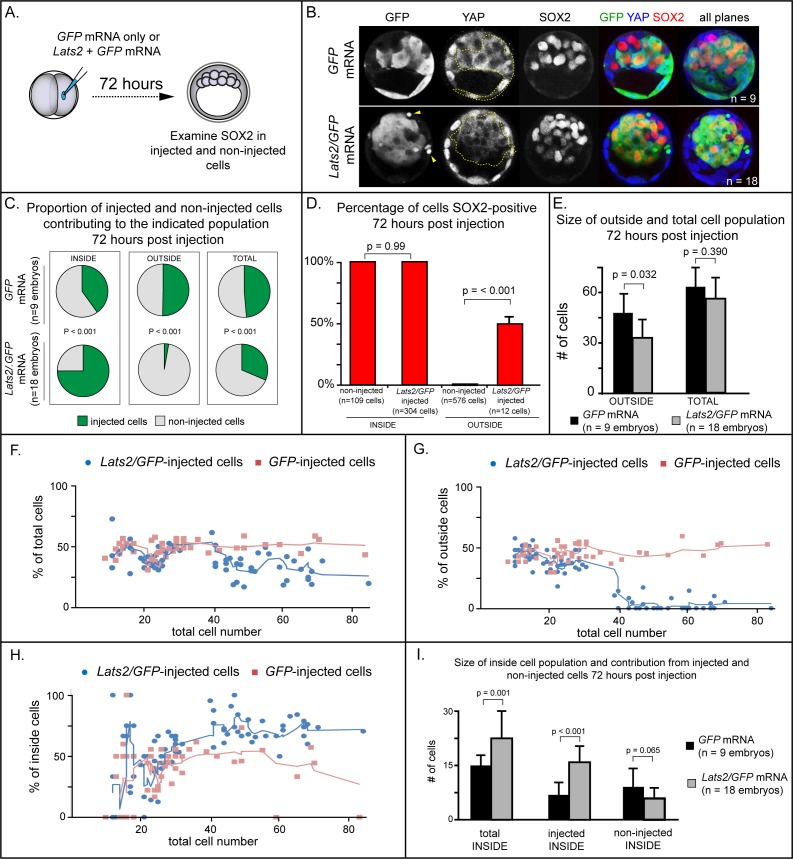
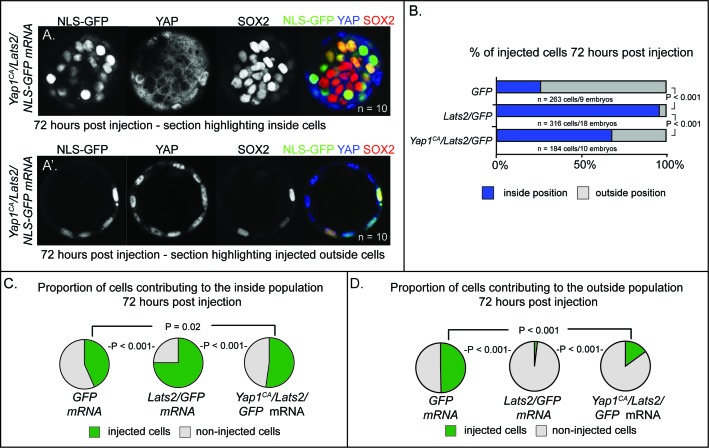
To functionally test of the role of YAP1 in repressing expression of Sox2, we injected one of two blastomeres with mRNA encoding LATS2 kinase, which inactivates YAP1 and, presumably, the related protein WWTR1 by phosphorylation, causing their cytoplasmic retention (Nishioka et al., 2008). We then examined expression of SOX2 after culturing embryos to the blastocyst stage (Figure 2A), predicting that LATS2 kinase would induce the ectopic expression of Sox2 in outside cells. Surprisingly, we observed that almost all Lats2-overexpressing cells ended up within the inner cell mass by the blastocyst stage (Figure 2B,C), in contrast to cells injected with GFP mRNA only, which contributed to both inner cell mass and trophectoderm. Notably, SOX2 was detected in all Lats2-overexpressing cells observed within the inner cell mass (Figure 2D), suggesting that Lats2-overexpressing cells were not only localized to the inner cell mass but also exhibited position-appropriate regulation of Sox2.
Figure 2. LATS2 kinase is sufficient to direct cells to inner cell mass fate.
(A) Embryos were collected at E1.5 and one of two blastomeres was injected with mRNAs encoding LATS2 and GFP or GFP alone. Embryos were cultured for 72 hr, fixed, and then analyzed by immunofluorescence and confocal microscopy. (B) Cells injected with GFP (dotted line) contributed to trophectoderm and inner cell mass, while cells injected with Lats2 and GFP (dotted line) contributed almost exclusively to the inner cell mass, leaving only cellular fragments in the trophectoderm (arrows), suggestive of cell death (n = embryos). (C) Proportion of inside, outside, and total cell populations across multiple embryos, which were comprised of non-injected cells, or cells injected with either GFP or GFP/Lats2 mRNAs. Cells injected with GFP/Lats2 were overrepresented within the inside cell population and underrepresented in the outside and total cell populations, relative to cells injected with GFP alone (P, chi-squared test). (D) Percentage of SOX2-positive cells within non-injected and GFP-injected or Lats2/GFP-injected populations observed inside and outside of the embryo. SOX2 was detected in all of the Lats2/GFP-injected inside cells, and in half of the rare, Lats2/GFP-injected outside cells (same number of embryos as in panel C) (p, student’s t-test). (E) Average number of outside and total cells per embryo. The average number of outside cells is reduced in embryos injected with Lats2/GFP, relative to GFP-injected (p, student’s t-test). (F) Proportion of GFP and Lats2/GFP-injected cells, relative to total cell number, over the course of development to the ~80-cell blastocyst (solid lines = average of indicated data point and four previous data points). (G) Data as shown in panel H, shown relative to outside cell number. (H) Data as shown in panel H, shown relative to inside cell number. (I) Contribution of injected and non-injected cells to the inside cell population, following injection with GFP or Lats2/GFP. Injection with Lats2/GFP increases the overall number of inside cells compared to injection with GFP only through increasing the number of injected cells contributing to the inside cell population, without affecting the number of non-injected cells contributing to the inside cell population (p, student’s t-test).
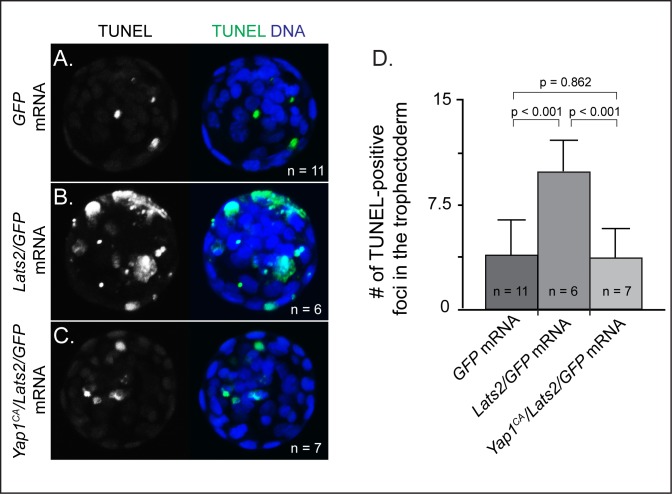
Figure 2—figure supplement 1. Lats2-overexpressing cells die on the surface of the embryo (A) Merge of all confocal sections from TUNEL assay performed on an embryo injected with GFP mRNA into one blastomere at the two-cell stage and then cultured until the blastocyst stage.
Figure 2—figure supplement 2. LATS2 drives cells to an inside position by inhibiting YAP1 activity (A–A’) Cooverexpression of Yap1CA and Lats2 partially rescues the ability of Lats2-overexpressing cells to contribute to trophectoderm and to repress Sox2.
The strikingly increased prevalence of Lats2-overexpressing cells in the inner cell mass was also associated with a stark decrease in the number of Lats2-overexpressing cells detected within the trophectoderm and a decrease in the number of outside cells compared to embryos injected with GFP mRNA alone (Figure 2C,E), suggesting that Lats2-overexpressing outside cells either internalize or undergo cell death. Furthermore, we observed cellular fragments within the trophectoderm of Lats2-overexpressing embryos (Figure 2B, yellow arrowheads), as well as increased TUNEL staining in Lats2-overexpressing embryos compared to embryos injected with GFP mRNA only (Figure 2—figure supplement 1A–B,D), consistent with increased death of Lats2-overexpressing cells.
In addition to detecting SOX2 in all Lats2-overexpressing cells located inside the embryo, SOX2 was also detected in rare Lats2-overexpressing cells that remained on the embryo surface (Figure 2D). Therefore, LATS2 is sufficient to induce expression of SOX2 in cells regardless of their position within the embryo. We predicted that, if Lats2 overexpression drove cells to adopt inner cell mass fate by influencing YAP1 and WWTR1 activity, then co-overexpression of Yap1CA would enable Lats2-overexpressing cells to contribute to trophectoderm. Consistent with this prediction, cooverexpression of Lats2 and Yap1CA led to a significant decrease in the proportion of Lats2-overexpressing cells contributing to the inside cell position, and a concomitant increase in the proportion of Lats2-overexpressing cells remaining in the outside position (Figure Figure 2—figure supplement 2A–D). Moreover, cooverexpression of Lats2 and Yap1CA reduced the number of TUNEL positive nuclei, consistent with Yap1CA rescuing survival of outside-positioned Lats2-overexpressing cells (Figure 2—figure supplement 1C–D). Collectively, these observations strongly suggest that LATS2 promotes inside cell positioning by regulating the activities of YAP1 and, likely, the related protein WWTR1.
To pinpoint when Lats2-overexpressing cells come to occupy the inside of the embryo, we performed a time course, examining the position of injected and non-injected cells from the 16-cell to the blastocyst stage (~80 cells). Surprisingly, between the 16 and 32-cell stages, the proportion of injected and non-injected cells in the total, outside, and inside cell populations were comparable whether embryos had been injected with Lats2 and GFP or GFP mRNA alone (Figure 2F–H). In embryos injected with GFP mRNA alone, the proportion of injected and non-injected cells making up the total, outside, and inside cell populations remained constant throughout the time course. In contrast, starting around the 32-cell stage, the average proportion of Lats2-overexpressing cells making up the inside population began to increase dramatically. This increase was associated with a decrease in the proportion Lats2-overexpressing cells making up the outside population, consistent with internalization of Lats2-overexpressing cells after the 32-cell stage (Figure 2G). After the 32-cell stage, Lats2-injected cells became underrepresented as a proportion of the total cell population (Figure 2H), lending further support to the idea that Lats2-overexpressing cells that fail to internalize undergo cell death. Interestingly, the inside-skewed contribution of Lats2-overexpressing cells did not influence the ability of non-injected cells to contribute to the ICM (Figure 2I), arguing that Lats2-overexpression drives inside positioning cell-autonomously. We therefore conclude that Lats2 overexpression acts on cell position and survival around the time of blastocyst formation.
LATS2 induces positional changes independent of Sox2
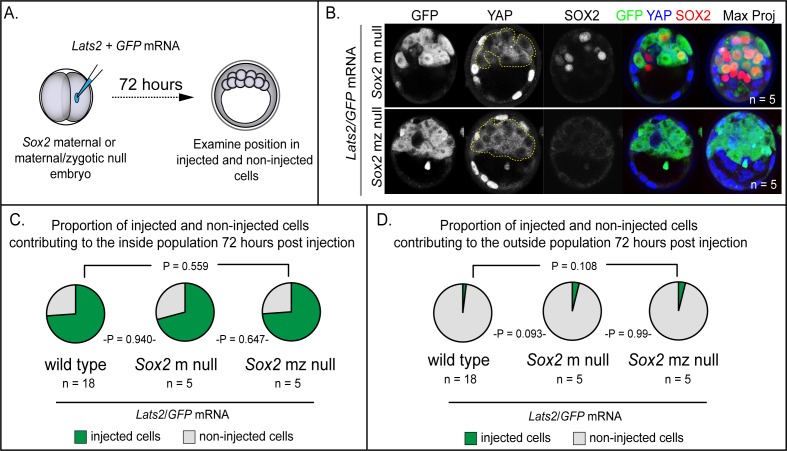
Our observation that Lats2-overexpression induces both the expression of SOX2 and cell repositioning to inner cell mass prompted us to investigate whether SOX2 itself drives cell repositioning downstream of Lats2. In support of this hypothesis, SOX2 activity has been proposed to bias inner cell mass fate (Goolam et al., 2016; White et al., 2016). We therefore investigated whether Sox2 is required for the inner cell mass-inducing activity of LATS2 by overexpressing Lats2 in embryos lacking maternal and zygotic Sox2 (Figure 3A), as previously described (Wicklow et al., 2014). However, we observed that Lats2-overexpressing cells were equally likely to occupy inside position in the presence and absence of Sox2 (Figure 3B,C). Furthermore, Lats2-overexpressing cells were equally unlikely to occupy outside position in the presence and absence of Sox2 (Figure 3D). Therefore, although Lats2 overexpression is sufficient to induce expression of Sox2, LATS2 acts on cell positioning/survival independently of Sox2.
Figure 3. LATS2 directs inner cell mass fate independently of Sox2 (A) Lats2 and GFP or GFP alone were overexpressed in embryos lacking maternal or maternal and zygotic Sox2.
(B) Lats2/GFP-overexpressing cells (dotted line) contribute almost exclusively to the inner cell mass in the presence or absence of Sox2 (n = embryos). (C) Proportion of non-injected cells and cells injected with Lats2/GFP mRNAs contributing to inner cell mass in the indicated genetic backgrounds. No significant differences were observed based on embryo genotype, indicating that Sox2 is dispensable for inside positioning by Lats2-overexpression (P, chi-squared test; n = embryos). (D) Proportion of non-injected cells and cells injected with the indicated mRNAs contributing to trophectoderm in the indicated genetic backgrounds. No significant differences were observed based on embryo genotype (P, chi-squared test; n = embryos).
LATS2 antagonizes formation of the apical domain
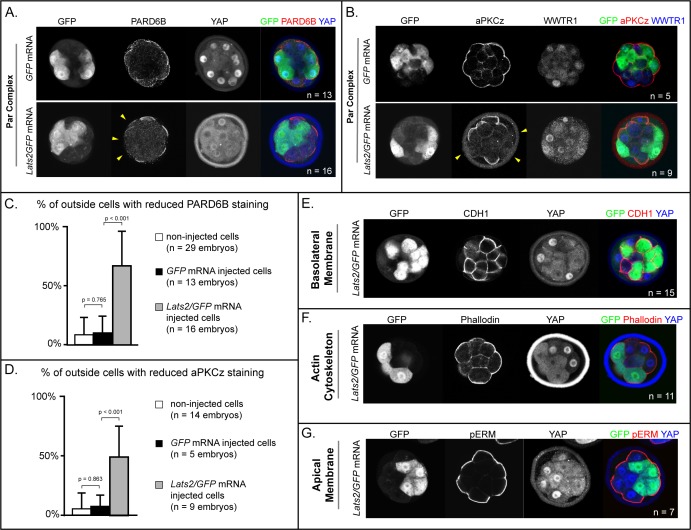
Trophectoderm cell fate has been proposed to be determined by apically localized membrane components that maintain the position of future trophectoderm cells on the embryo surface (Anani et al., 2014; Korotkevich et al., 2017; Maître et al., 2016; Maître et al., 2015; Samarage et al., 2015; Zenker et al., 2018). For example, the apical membrane components aPKC and PARD6B are required for maintaining outside cell position and trophectoderm fate (Alarcon, 2010; Dard et al., 2009; Hirate et al., 2015; Plusa et al., 2005). Because Lats2 overexpression led cells to adopt an inside position, this raised the testable possibility that LATS2 antagonizes localization of aPKC and PARD6B.
Since Lats2 overexpression leads to cell positioning starting around the 32-cell stage, we examined the localization of aPKCz and PARD6B in embryos just prior to the 32-cell stage. At this stage, apical membrane components PARD6B and aPKCz were detected at the apical membrane of non-injected outside cells and outside cells injected with GFP only (Figure 4A–D). By contrast, most Lats2-overexpressing outside cells lacked detectable aPKCz and PARD6B (Figure 4A–D). Therefore, LATS2 is sufficient to antagonize localization of key apical domain proteins in outside cells, providing a compelling mechanism for the observed repositioning of Lats2-overexpressing outside cells.
Figure 4. LATS2 antagonizes formation of the apical domain (A) In embryos at 16–32 cell stages, PARD6B is detectable in GFP-overexpressing and in non-injected cells, but not in Lats2-overexpressing cells (arrowheads, n = embryos).
(B) At 16–32 cell stages, aPKCz is detectable in GFP-overexpressing and in non-injected cells, but not in Lats2-overexpressing cells (arrowheads, n = embryos). (C) Quantification of embryos shown in panel A (p, student’s t-test). (D) Quantification of embryos shown in panel B (p, student’s t-test). (E) At 16–32 cell stages, CDH1 is localized to the basolateral membrane in both Lats2-overexpressing and non-injected cells (n = embryos). (F) At 16–32 cell stages, Phalloidin staining demonstrates that filamentous Actin is apically enriched in Lats2-overexpressing and non-injected cells (n = embryos). (G) At 16–32 cell stages, pERM is localized to the apical membrane in both Lats2-overexpressing and non-injected cells (n = embryos).
We also examined other markers of apicobasal polarization in Lats2-overexpressing outside cells prior to the 32-cell stage. Curiously, other markers of apicobasal polarization were properly localized in all cells examined. For example, CDH1 was restricted to the basolateral membrane (Figure 4E), while filamentous Actin and phospho-ERM were restricted to the apical domain in outside cells of both Lats2-overexpressing and non-injected outside cells (Figure 4F,G). Thus, we propose that Lats2-overexpressing outside cells initially possess hallmarks of apicobasal polarization, but aPKC and PARD6B fail to properly localize, leading to the eventual depolarization and internalization of outside cells.
YAP1 and WWTR1 restrict Sox2 expression to the inner cell mass
Our overexpression data suggested that the activities of YAP1 and WWTR1 are important for regulating cell fate and gene expression. Next, we aimed to test the requirements for Yap1 and Wwtr1 in embryogenesis. Yap1 null embryos survive until E9.0 (Morin-Kensicki et al., 2006), suggesting that oocyte-expressed (maternal) Yap1 (Yu et al., 2016), or the Yap1 paralogue Wwtr1 (Varelas et al., 2010) are important for preimplantation development. However, embryos lacking maternal and zygotic Wwtr1 and Yap1 have not been scrutinized.
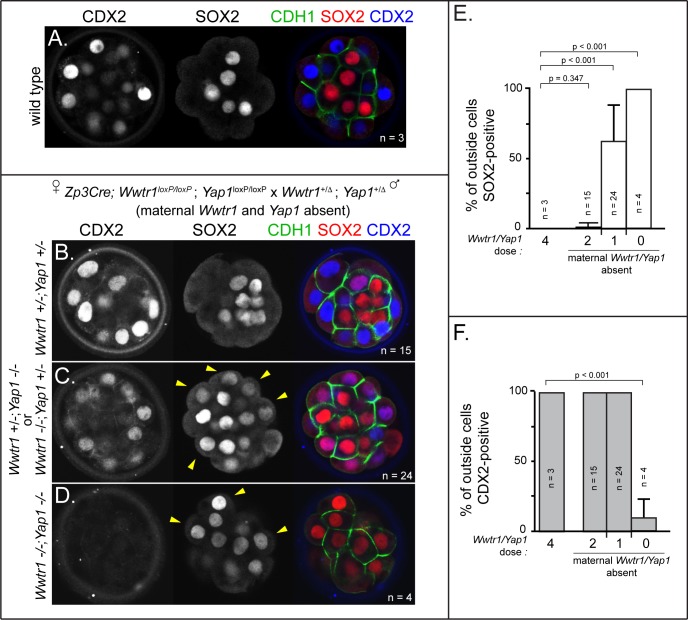
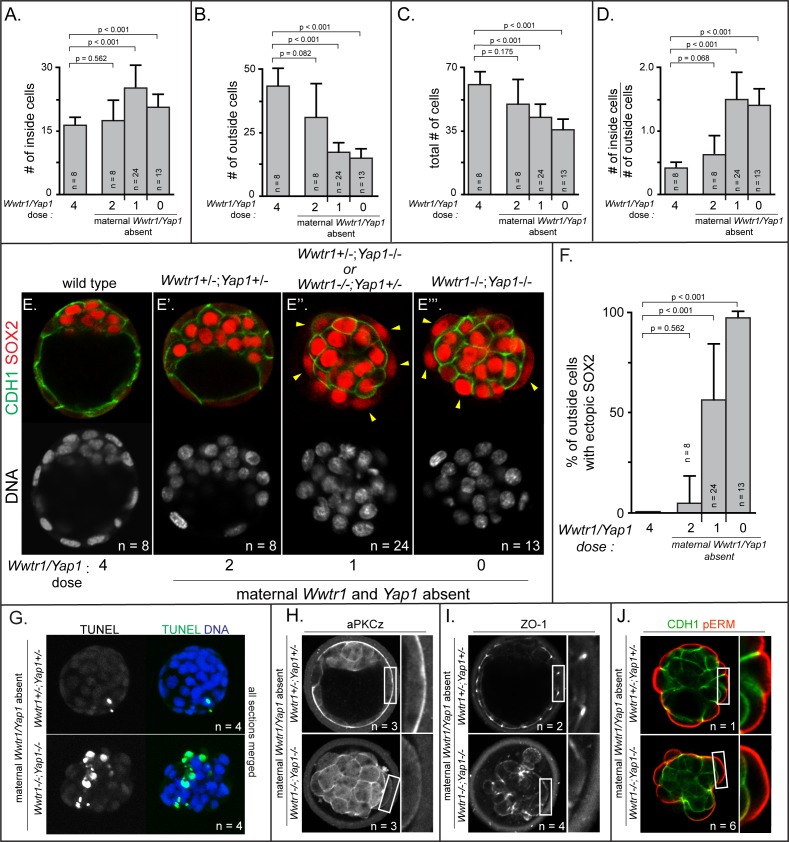
To generate embryos lacking maternal and zygotic Wwtr1 and Yap1, we deleted Wwtr1 and Yap1 from the female germ line using mice carrying conditional alleles of Wwtr1 and Yap1 (Xin et al., 2013; Xin et al., 2011) and the female germ line-specific Zp3Cre (de Vries et al., 2000). We then crossed these females to males heterozygous for deleted alleles of Wwtr1 and Yap1 (see Materials and methods). From these crosses, we obtained embryos lacking maternally provided Wwtr1 and Yap1 and either heterozygous or null for Wwtr1 and/or Yap1 (Supplementary file 1). At E3.25 (≤32 cells), SOX2 and CDX2 are normally mutually exclusive (Figure 5A). However, with decreasing number of wild type zygotic alleles of Wwtr1 and Yap1, we observed worsening phenotypes (Figure 5B–F). In the complete absence of Wwtr1 and Yap1, we observed a severe loss of CDX2 and expansion of SOX2 in outside cells (Figure 5D–F), phenocopying Lats2 overexpression. However, in embryos of intermediate genotypes, we observed expanded SOX2 and persistent, yet lower, expression levels of CDX2 (Figure 5C,E–F). Thus, regulation of Sox2 expression is more sensitive to Wwtr1 and Yap1 dosage than is Cdx2. Moreover, these observations indicate that intermediate doses of Wwtr1 and Yap1 produce outside cells expressing markers of mixed cell lineage at E3.25.
Figure 5. Wwtr1 and Yap1 are required to repress SOX2 expression in outside cells.
(A) CDX2 and SOX2 in wild type embryos at E3.25 (16–32 cell stages). CDX2 staining is more intense in outside cells than inside cells and SOX2 staining is specific to inside cells (n = embryos). (B) Embryos lacking maternal Wwtr1 and Yap1 with and heterozygous for Wwtr1 and Yap1 (which we consider to have 2 doses of WWTR1/YAP1) exhibit normal CDX2 and SOX2 expression (n = embryos). (C) Embryos lacking maternal Wwtr1 and Yap1 and heterozygous for either Wwtr1 or Yap1 (1 dose of WWTR1/YAP1) exhibit a high degree of ectopic SOX2 in outside cells (arrowheads), but continue to express CDX2, although the levels appear reduced (n = embryos). (D) Embryos lacking maternal and zygotic Wwtr1 and Yap1 (0 doses of WWTR1/YAP1) have the most severe phenotype, with a high degree of ectopic SOX2 in outside cells (arrowheads) and little or no detectable CDX2 (n = embryos). (E) Quantification of the percentage of outside cells in which ectopic SOX2 is detected in the presence of decreasing dose of Wwtr1 and Yap1 (t = student’s t-test, n = embryos). (F) Quantification of the percentage of outside cells in which CDX2 is detected in the presence of decreasing dose of Wwtr1 and Yap1 (t = student’s t-test, n = embryos).
YAP1 and WWWTR1 maintain outside cell positioning
Based on our observations of Lats2-overexpressing embryos, we anticipated that defects in cell positioning in embryos lacking maternal and zygotic Wwtr1 and Yap1 could arise after the 32-cell stage. We therefore examined embryos lacking Wwtr1 and Yap1 at E3.75, when embryos possess more than 32 cells. Indeed, we observed skewed lineage contributions, correlating with the dosage of Wwtr1 and Yap1 (Figure 6A–D). Embryos with one or fewer wild type alleles of Wwtr1 or Yap1 exhibited an increase in the number of inside cells and a reduction in the number of outside cells (Figure 6A–B), consistent with altered cell positioning.
Figure 6. Positioning and epithelialization defects in embryos with Wwtr1 and Yap1 null alleles (A) Quantification of the average number of inside cells per embryo with decreasing dose of Wwtr1 and Yap1.
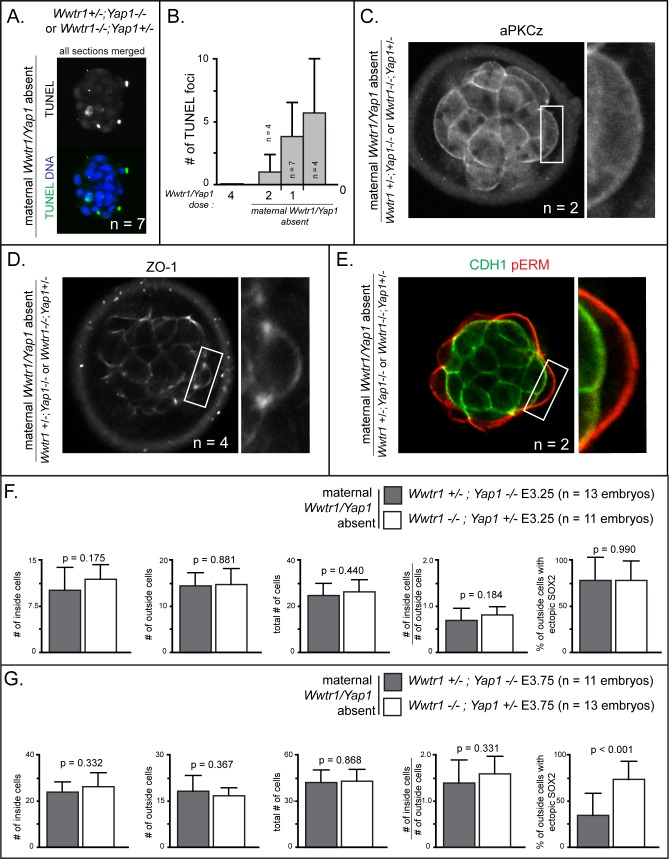
The number of inside cells increases as the dose of wild type Wwtr1 and Yap1 alleles is reduced (p, student’s t-test, n = embryos). (B) Quantification of the average number of outside cells per embryo with decreasing dose of Wwtr1 and Yap1. The number of outside cells decreases as the dose of wild type Wwtr1 and Yap1 alleles is reduced (p, student’s t-test, n = embryos). (C) Quantification of the average number of total cells per embryos with decreasing dose of wild type zygotic Wwtr1 and Yap1. The number of total cells decreases as the dose of wild type Wwtr1 and Yap1 is reduced (p, student’s t-test, n = embryos). (D) Quantification of the average ratio of inside to outside cells per embryo with decreasing dose of Wwtr1 and Yap1. The ratio of inside to outside cells increases as the dose of wild type Wwtr1 and Yap1 is reduced (p, student’s t-test, n = embryos). (E) Wild type embryos at E3.75 exhibit inner cell mass-specific expression of SOX2 (n = embryos). (E’) E3.75 embryos lacking maternal Wwtr1 and Yap1 and heterozygous for zygotic Wwtr1 and Yap1 cavitate and repress Sox2 in outside cells, leading to inner cell mass-specific expression of SOX2 similar to wild type embryos (n = embryos). (E’’) Embryos lacking maternal Wwtr1 and Yap1 but with only one wild type allele of Wwtr1 or Yap1 fail to cavitate and repress Sox2 in outside cells, leading to ectopic SOX2 in outside cells (arrowheads, n = embryos). (E’’’) Embryos lacking maternal and zygotic Wwtr1 and Yap1 fail to cavitate and repress Sox2 in outside cells, leading to ectopic SOX2 in outside cells (arrowheads, n = embryos). (F) Quantification of ectopic SOX2 detected in embryos such as those shown in panels E-E’’’. The percentage of outside cells with ectopic SOX2 increases as the dose of wild type Wwtr1 and Yap1 alleles is reduced (p, student’s t-test, n = embryos). (G) TUNEL analysis of embryos lacking maternal Wwtr1 and Yap1 heterozygous for zygotic Wwtr1 and Yap1 or lacking maternal and zygotic Wwtr1 and Yap1. Extensive TUNEL staining is observed in embryos lacking maternal and zygotic Wwtr1 and Yap1 indicative of cell death. Max projections of all confocal sections from a single embryo are shown (n = embryos). (H) aPKCz staining in embryos lacking maternal Wwtr1 and Yap1, either heterozygous for zygotic Wwtr1 and Yap1 or with no zygotic Wwtr1 and Yap1. aPKC is not localized to the apical membrane of embryos with no zygotic Wwtr1 and Yap1 (n = embryos). (I) ZO-1 staining in embryos lacking maternal Wwtr1 and Yap1, either heterozygous for zygotic Wwtr1 and Yap1 or with no zygotic Wwtr1 and Yap1. ZO-1 is disorganized in embryos with no zygotic Wwtr1 and Yap1, suggesting that formation of a mature epithelium depends on Wwtr1 and Yap1 (n = embryos). (J) pERM and CDH1 staining in embryos lacking maternal Wwtr1 and Yap1, either heterozygous for zygotic Wwtr1 and Yap1 or with no zygotic Wwtr1 and Yap1. pERM is localized to apical membranes and CDH1 to basolateral membranes regardless of the dose of wild type Wwtr1 and Yap1 alleles (n = embryos).
Figure 6—figure supplement 1. Increased cell death and epithelialization defects in embryos lacking maternal Wwtr1 and Yap1 with a single wild type allele of Wwtr1 or Yap1.
Although the average total number of cells was also reduced in these embryos (Figure 6C), the reduction in total cell number did not alone account for the loss of cells on the outside of the embryo (Supplementary file 2). This observation suggested that, similar to Lats2-overexpressing cells, cells with reduced Wwtr1 and Yap1 exhibit an increased frequency of outside cell death, in addition to increased outside cell internalization. Consistent with this, embryos with one or fewer wild type alleles of Wwtr1 or Yap1 exhibited an increase in the ratio of inside to outside cells (Figure 6D) and an increase in cells undergoing apoptosis by TUNEL assay (Figure 6G and Figure 6—figure supplement 1A,B).
Critically, the fewer outside cells in embryos lacking Wwtr1 and Yap1, which appeared stretched over the mass of inside cells, exhibited ectopic expression of SOX2 (Figure 6E–F). Therefore, WWTR1/YAP1 repress inner cell mass fate, downstream of LATS kinases. Intriguingly, our data also indicate that WWTR1 is a more potent repressor of Sox2 at E3.75 than YAP1 since embryos with a single wild type allele of Wwtr1 had significantly fewer cells expressing ectopic SOX2 then embryos with a single wild type allele of Yap1 (Figure 6—figure supplement 1F,G).
Since loss of Wwtr1 and Yap1 phenocopied Lats2 overexpression in terms of Sox2 expression, cell death, and cell repositioning, we next evaluated the apical domain and cell polarization in outside cells of embryos lacking Wwtr1 and Yap1 at E3.75. As expected, observed greatly reduced aPKC at the apical membrane of outside cells in embryos with one or fewer doses of Wwtr1 or Yap1 (Figure 6H and Figure 6—figure supplement 1C). In addition, we evaluated the localization of the tight junction protein ZO-1, which suggested failure in tight junction formation in embryos with one or fewer doses of Wwtr1 and Yap1 (Figure 6I and Figure 6—figure supplement 1D). Notably, however, other markers of apicobasal polarity, such as CDH1 and pERM were correctly localized in outside cells of mutant embryos at this stage (Figure 6J and Figure 6—figure supplement 1E), consistent with some normal cell polarization. Our observations indicate that WWTR1 and YAP1 play a crucial role in the formation of the apical domain and maintaining the positioning and survival of outside cells while repressing expression of Sox2.
Discussion
During preimplantation development, lineage-specific transcription factors are commonly expressed in ‘noisy’ domains before refining to a lineage-appropriate pattern (Simon et al., 2018). For example, Oct4 and Nanog are expressed in both inner cell mass and trophectoderm until after blastocyst formation (Dietrich and Hiiragi, 2007; Strumpf et al., 2005). Similarly, CDX2 is detected in inner cell mass, as well as trophectoderm, until blastocyst stages (McDole and Zheng, 2012; Ralston and Rossant, 2008; Strumpf et al., 2005). In striking contrast to these genes, SOX2 is never detected in outside cells (Wicklow et al., 2014), indicating that robust mechanisms must exist to minimize noise and prevent its aberrant expression in trophectoderm. Here, we identify YAP1/WWTR1 as key components that repress Sox2 expression in outside cells of the embryo. Notably, manipulations known to antagonize YAP1/WWTR1 activity, including chemical inhibition of ROCK and overexpression of LATS2, lead to ectopic expression of SOX2 in outside cells, reinforcing the notion that YAP1/WWTR1 activity are crucial for repression of Sox2 in outside cells.
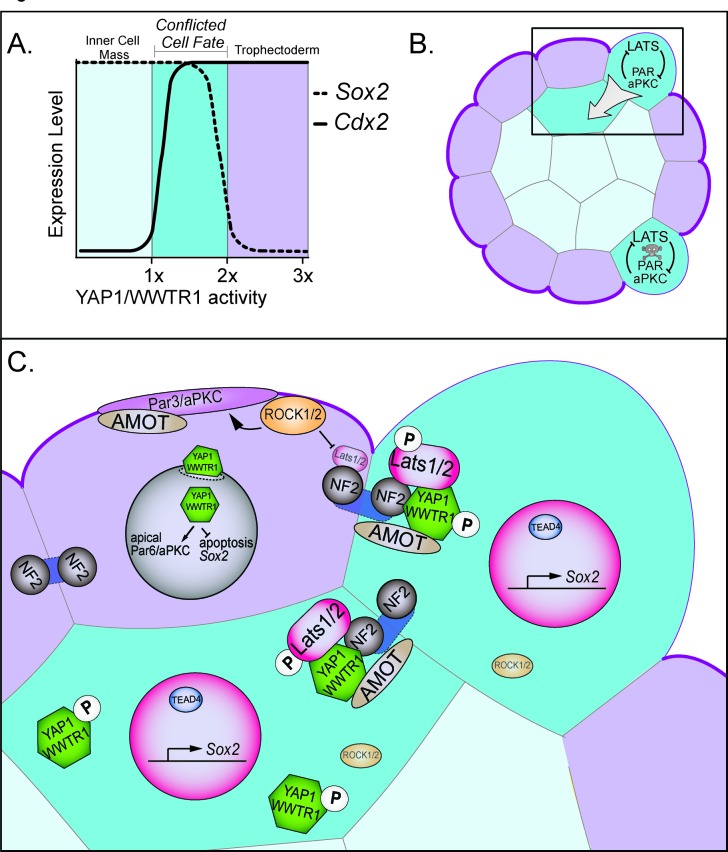
Additionally, we find that Sox2 expression is more sensitive than is Cdx2 to YAP1/WWTR1 activity, since intermediate doses of active YAP1/WWTR1 yield cells that coexpress both SOX2 and CDX2 (Figure 7A). This observation is consistent with the fact that CDX2 is initially detected in inside cells of the embryo during blastocyst formation (Dietrich and Hiiragi, 2007; McDole and Zheng, 2012; Ralston and Rossant, 2008), where SOX2 is also expressed (Wicklow et al., 2014). Thus, inside cells could initially possess intermediate doses of active YAP1/WWTR1 at this early stage. By contrast, outside cells have greatly reduced YAP1/WWTR1 activity, owing to elevated LATS activity. In this way, the HIPPO pathway ensures robust developmental transitions, by rapidly nudging SOX2-expressing cells into their correct and final positions inside the embryo (Figure 7B).
Figure 7. Resolution of cell fate conflicts in the preimplantation mouse embryo.
(A) The expression of Sox2 and Cdx2 is differentially sensitive to YAP1/WWTR1 activity, leading to co-expression of both lineage markers in cells when YAP1/WWTR1 activity levels are intermediate. (B) During division from the 16 to the 32-cell stage, cells that inherit the apical membrane repress HIPPO signaling and maintain an outside position. However, cells that inherit a smaller portion of the apical membrane would initially elevate their HIPPO signaling. We propose that elevated HIPPO then feeds back onto polarity by further antagonizing PAR-aPKC complex formation, leading to a snowball effect on repression of Sox2 expression, and thus ensuring that SOX2 is never detected in outside cells because these cells are rapidly internalized or apoptosed. (C) A closeup of the boxed region in panel B. In most outside cells, low LATS2 activity enables high levels of YAP1/WWTR1 activity, which repress Sox2 and apoptosis and promote Cdx2 expression and apical localization of aPKC and PARD6B, which in turn repress the HIPPO pathway. In rare outside cells, LATS2 activity becomes elevated, leading to lower activity of YAP1/WWTR1, which then leads these cells to become internalized or to undergo apoptosis.
Consistent with our proposed model, the timing of HIPPO-induced cell internalization coincides with loss of cell fate plasticity around the 32-cell stage (Posfai et al., 2017). This timing also coincides with the formation of mature tight junctions among outside cells (Sheth et al., 1997), which reinforce and intensify differences in HIPPO signaling activity between inside and outside compartments of the embryo (Hirate and Sasaki, 2014; Leung and Zernicka-Goetz, 2013). Our observations indicate that HIPPO signaling can, in turn, interfere with trophectoderm epithelialization. Therefore, we propose that HIPPO engages in a negative feedback loop with cell polarity components (Figure 7B).
We propose two mechanisms by which HIPPO signaling eliminates cells from the trophectoderm, both of which are downstream of YAP1/WWTR1 (Figure 7C). First, a small proportion of conflicted cells undergo cell death. This is in line with the observed increase in the level of apoptosis detected after the 32-cell stage (Copp, 1978). We showed that cell lethality due to elevated HIPPO can be rescued by increasing levels of nuclear YAP1, suggesting that YAP1 activity normally provides a pro-survival signal to trophectoderm cells, consistent with the proposed role of YAP1 in promoting proliferation in non-eutherian mammals (Frankenberg, 2018). Moreover, deletion of Sox2 did not rescue survival of outside cells in which HIPPO signaling was artificially elevated, arguing that HIPPO resolves cell fate conflicts independently of lineage-specific genes.
The second way that conflicted cells are eliminated from the trophectoderm is that cells with elevated HIPPO signaling drive their own internalization. This is consistent with the observation that cells in which Tead4 has been knocked down become internalized (Mihajlović et al., 2015). However, in contrast to Tead4 loss of function, which preserves the apical domain in outside cells (Mihajlović et al., 2015; Nishioka et al., 2008), we observed that Yap1/Wwtr1 loss of function leads loss of apical PARD6D/aPKC. These observations suggest that YAP1/WWTR1 could partner with proteins other than TEAD4 to regulate apical domain formation. Consistent with this proposal, TEAD1 has been proposed to play an essential role in the early embryo (Sasaki, 2017). Nevertheless, since PARD6B/aPKC are essential for outside cell positioning (Dard et al., 2009; Hirate et al., 2015; Plusa et al., 2005), the loss of the apical domain could affect cell positioning in several ways. For instance, loss of PARD6B/aPKC would eventually lead to cell depolarization (Alarcon, 2010), which could influence any of the processes normally governing the allocation of inside cells, such as oriented cleavage, cell contractility, or apical constriction (Korotkevich et al., 2017; Maître et al., 2016; Samarage et al., 2015). Identifying the downstream mechanisms by which HIPPO drives cells to inner cell mass will be a stimulating topic of future study.
Our studies also revealed that SOX2 does not play a role in cell positioning. This observation sheds light on a recent study, which showed that SOX2 dwells longer in select nuclei of four-cell stage embryos that are destined to contribute to the inner cell mass (White et al., 2016). We propose that SOX2 is associated with future pluripotent state but does not alone contribute to all aspects of pluripotency, such as inside positioning. It is therefore still unclear why it is important to establish the inside cell-specific SOX2 expression during embryogenesis. Identification pathways that function downstream of YAP1/WWTR1 and in parallel to SOX2 to promote formation of pluripotent cells will provide meaningful insights into the natural origins of mammalian pluripotent stem cell progenitors.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference |
Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) |
CD-1 | Charles River Laboratories |
RRID:IMSR_CRL:22 | |
| Strain, strain background (M. musculus) |
Sox2tm1.1Lan |
Smith et al. (2009) PMID:19666824 |
RRID:IMSR_JAX:013093 | mixed background, Sox2 null refers to recombined allele |
| Strain, strain background (M. musculus) |
Wwtr1
conditional allele; Wwtr1tm1.1Eno; Wwtr1loxp |
Xin et al., 2013
Xin et al., 2013 PMID:23918388 |
MGI:5544289 | mixed background, ‘Wwtr1-’ or ‘Wwtr1’ refers to recombined allele |
| Strain, strain background (M. musculus) |
Yap1
conditional allele; Yap1tm1.1Eno; Yap1loxp |
Xin et al., 2011
Xin et al., 2011 PMID:22028467 |
MGI:5446483 | mixed background, , ‘Yap1-’ or ‘Yap1’ refers to recombined allele |
| Strain, strain background (M. musculus) |
Tg(Zp3-cre)
93Knw; Zp3Cre |
de Vries et al., 2000
de Vries et al., 2000 de Vries et al., 2000 PMID:10686600 |
RRID:MGI:3835503 | mixed background |
| Strain, strain background (M. musculus) |
129-Alpl tm(cre)Nagy |
Lomelí et al., 2000
Lomelí et al., 2000 Lomelí et al., 2000 PMID:10686602 |
RRID:IMSR_
JAX:008569 |
mixed background |
| Antibody | mouse anti-CDX2 |
Biogenex | BioGenex Cat# AM392; RRID:AB_2650531 |
(1:200) |
| Antibody | goat anti -SOX2 |
Neuromics | Neuromics Cat# GT15098; RRID:AB_2195800 |
(1:200) |
| Antibody | rabbit anti-PARD6B |
Novus Biologicals |
Novus Cat# NBP1-87337; RRID:AB_11034389 |
(1:100) |
| Antibody | rabbit anti-PARD6B |
Santa Cruz Biotechnology |
Santa Cruz Biotechnology Cat# sc-67393; RRID:AB_2267889 |
(1:100) |
| Antibody | mouse anti-PKCζ |
Santa Cruz Biotechnology |
Santa Cruz Biotechnology Cat# sc-17781; RRID:AB_628148 |
(1:100) |
| Antibody | mouse anti-YAP1 |
Santa Cruz Biotechnology |
Santa Cruz Biotechnology Cat# sc-101199; RRID:AB_1131430 |
(1:200) |
| Antibody | mouse anti-pYAP1 |
Cell Signaling Technology |
Cell Signaling Technology Cat# 4911; RRID:AB_2218913 |
(1:800) |
| Antibody | chicken anti-GFP |
Aves Labs | Aves Labs Cat# GFP-1020; RRID:AB_10000240 |
(1:2000) |
| Antibody | rat anti- CDH1 |
Sigma- Aldrich |
Sigma-Aldrich Cat# U3254; RRID:AB_477600 |
(1:500) |
| Antibody | mouse anti-ZO1 |
Thermo Fisher Scienctific |
Thermo Fisher Scientific Cat# 33–9100; RRID:AB_2533147 |
(1:1000) |
| Recombinant DNA reagent |
Lats2
mRNA; LATS2 |
Nishioka et al., 2009
Nishioka et al., 2009 Nishioka et al., 2009 PMID:19289085 |
pcDNA3.1 -pA83-Lats2; RIKEN: RDB12200 |
In Vitro Transcription template for Lats2 mRNA |
| Recombinant DNA reagent |
Yap1CA
mRNA; YAP1CA |
Nishioka et al., 2009
Nishioka et al., 2009 Nishioka et al., 2009 PMID:19289085 |
pcDNA3.1-pA83 -HA-Yap-S112A; RIKEN: RDB12194 |
In Vitro Transcription template for Yap1CA mRNA |
| Recombinant DNA reagent |
nls-GFP mRNA; nls-GFP |
Ariotti et al., 2015
Ariotti et al., 2015 Ariotti et al., 2015 PMID:26585296 |
Addgene: Plasmid #67652 |
In Vitro Transcription template for nls- GFP mRNA |
| Recombinant DNA reagent |
pCS2-EGFP; EGFP mRNA; GFP mRNA; GFP |
Chazaud et al., 2006
PMID: 16678776 |
In Vitro Transcription template for GFP mRNA |
|
| Commercial assay or kit |
mMessage mMachine Sp6 Transcription Kit |
Thermo Fisher Scienctific |
Thermo Fisher Scientific Cat# AM1340 |
|
| Commercial assay or kit |
mMessage mMachine T7 Transcription Kit |
Thermo Fisher Scienctific |
Thermo Fisher Scientific Cat# AM1344 |
|
| Commercial assay or kit |
MEGAClear Transcription Clean-Up Kit |
Thermo Fisher Scienctific |
Thermo Fisher Scientific Cat# AM1908 |
|
| Commercial assay or kit |
In-Situ Cell Death Detection Kit, Fluorescein; TUNEL assay |
Sigma-Aldrich | Sigma-Aldrich Cat# 11684795910 |
|
| Commercial assay or kit |
Extract-N -Amp Kit |
Sigma- Aldrich |
Sigma- Aldrich Cat # XNAT2 |
|
| Chemical compound, drug |
Y-27632; ROCK-inhibitor |
Millipore | Millipore Cat# SCM075 |
|
| Software, algorithm |
Adobe Photoshop |
Adobe | RRID:SCR_014199 | |
| Software, algorithm |
Fiji | http://fiji.sc | RRID:SCR_002285 |
Mouse strains and genotyping
All animal research was conducted in accordance with the guidelines of the Michigan State University Institutional Animal Care and Use Committee. Wild type embryos were derived from CD-1 mice (Charles River). The following alleles or transgenes were used in this study, and maintained in a CD-1 background: Sox2tm1.1Lan (Smith et al., 2009), Yaptm1.1Eno (Xin et al., 2011), Wwtr1tm1.1Eno (Xin et al., 2013), Tg(Zp3-cre)93Knw (de Vries et al., 2000). Null alleles were generated by breeding mice carrying floxed alleles and mice carrying ubiquitously expressed Cre, 129-Alpltm(cre)Nagy (Lomelí et al., 2000).
Embryo collection and culture
Mice were maintained on a 12 hr light/dark cycle. Embryos were collected by flushing the oviduct or uterus with M2 medium (Millipore). For embryo culture, KSOM medium (Millipore) was equilibrated overnight prior to embryo collection. Y-27632 (Millipore) was included in embryo culture medium at a concentration of 80 µM with 0.4% DMSO, or 0.4% DMSO as control, where indicated. Embryos were cultured at 37°C in a 5% CO2 incubator under light mineral oil.
Embryo microinjection
Lats2 and Yap1S112A (Yap1CA) mRNA was synthesized from cDNAs cloned into the pcDNA3.1-poly(A)83 plasmid (Yamagata et al., 2005) using the mMESSAGE mMACHINE T7 transcription kit (Invitrogen). EGFP or nls-GFP mRNA were synthesized from EGFP cloned into the pCS2 plasmid or the nls-GFP plasmid (Ariotti et al., 2015) using the mMESSAGE mMACHINE SP6 transcription kit (Invitrogen). mRNAs were cleaned and concentrated prior to injection using the MEGAclear Transcription Clean-Up Kit (Invitrogen). Lats2 and YAP1CA mRNAs were injected into one blastomere of two-cell stage embryos at a concentration of 500 ng/µl, mixed with 350 ng/µl EGFP or nls-GFP mRNA diluted in 10 mM Tris-HCl (pH 7.4), 0.1 mM EDTA.
Immunofluorescence and confocal microscopy
Embryos were fixed with 4% formaldehyde (Polysciences) for 10 min, permeabilized with 0.5% Triton X-100 (Sigma Aldrich) for 30 min, and then blocked with blocking solution (10% Fetal Bovine Serum (Hyclone), 0.1% Triton X-100) for 1 hr at room temperature, or overnight at 4°C. Primary Antibodies used were: mouse anti-CDX2 (Biogenex, CDX2-88), goat anti-SOX2 (Neuromics, GT15098), rabbit anti-PARD6B (Santa Cruz, sc-67393), rabbit anti-PARD6B (Novus Biologicals, NBP1-87337), mouse anti-PKCζ (Santa Cruz Biotechnology, sc-17781), rat anti-CDH1 (Sigma Aldrich, U3254), mouse anti-ZO1 (Thermo Fisher Scientific, 33–9100), mouse anti-YAP (Santa Cruz Biotechnology, sc101199), rabbit anti phospho-YAP (Cell Signaling Technologies, 4911), chicken anti-GFP (Aves, GFP-1020). Stains used were: Phallodin-633 (Invitrogen), DRAQ5 (Cell Signaling Technologies) and DAPI (Sigma Aldrich). Secondary antibodies conjugated to DyLight 488, Cy3 or Alexa Flour 647 fluorophores were obtained from Jackson ImmunoResearch. Embryos were imaged using an Olympus FluoView FV1000 Confocal Laser Scanning Microscope system with 20x UPlanFLN objective (0.5 NA) and 5x digital zoom. For each embryo, z-stacks were collected, with 5 µm intervals between optical sections. All embryos were imaged prior to knowledge of their genotypes.
Embryo analysis
For each embryo, z-stacks were analyzed using Photoshop or Fiji, which enabled the virtual labeling, based on DNA stain, of all individual cell nuclei. Using this label to identify individual cells, each cell in each embryo was then assigned to relevant phenotypic categories, without knowledge of embryo genotype. Phenotypic categories included marker expression (e.g., SOX2 or CDX2 positive or negative), protein localization (e.g., aPKC or CDH1 apical, basal, absent, or unlocalized), and cell position, where cells making contact with the external environment were considered ‘outside’ and cells surrounded by other cells were considered ‘inside’ cells.
TUNEL assay
Embryos were fixed, permeabilized, and blocked as described for immunofluorescence. Zonae pellucida were removed using Tyrode’s Acid treatment prior to performing the TUNEL assay (In Situ Cell Death Detection Kit, Fluorescein, Millipore-Sigma). Embryos were incubated in 200 µl of a 1:10 dilution of enzyme in label solution for 2 hr at 37°C. Embryos were then washed twice with blocking solution for 10 min each, and then mounted in a 1 to 400 dilution of DRAQ5 in blocking solution to stain DNA.
Embryo genotyping
To determine embryo genotypes, embryos were collected after imaging and genomic DNA extracted using the Extract-N-Amp kit (Sigma) in a final volume of 10 µl. Genomic extracts (1–2 µl) were then subjected to PCR using allele-specific primers (Supplementary file 3).
Acknowledgements
We are grateful to Dr. Hiroshi Sasaki for providing expression constructs, to Dr. Randy L Johnson for providing mice carrying conditional alleles of Yap1 and Wwtr1, and to Dr. Jason Knott for embryo microinjection training. We also thank Dr. Ripla Arora, Dr. Julia Ganz, and members of the Ralston Lab for comments. This work was supported by NIH R01 GM104009 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number T32HD087166. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank anonymous reviewers for insightful questions and suggestions.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Amy Ralston, Email: aralston@msu.edu.
Elizabeth Robertson, University of Oxford, United Kingdom.
Marianne E Bronner, California Institute of Technology, United States.
Funding Information
This paper was supported by the following grants:
National Institute of General Medical Sciences R01 GM104009 to Amy Ralston.
National Institute of Child Health and Human Development T32HD087166 to Tayler M Murphy.
Additional information
Competing interests
No competing interests declared.
Author contributions
Data curation, Formal analysis, Investigation.
Data curation, Formal analysis.
Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration.
Additional files
Data availability
All data appear within the manuscript and associated files.
References
- Alarcon VB. Cell polarity regulator PARD6B is essential for trophectoderm formation in the preimplantation mouse embryo. Biology of Reproduction. 2010;83:347–358. doi: 10.1095/biolreprod.110.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon VB, Marikawa Y. ROCK and RHO Playlist for Preimplantation Development: Streaming to HIPPO Pathway and Apicobasal Polarity in the First Cell Differentiation. Advances in Anatomy, Embryology, and Cell Biology. 2018;229:47–68. doi: 10.1007/978-3-319-63187-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anani S, Bhat S, Honma-Yamanaka N, Krawchuk D, Yamanaka Y. Initiation of Hippo signaling is linked to polarity rather than to cell position in the pre-implantation mouse embryo. Development. 2014;141:2813–2824. doi: 10.1242/dev.107276. [DOI] [PubMed] [Google Scholar]
- Ariotti N, Hall TE, Rae J, Ferguson C, McMahon KA, Martel N, Webb RE, Webb RI, Teasdale RD, Parton RG. Modular detection of gfp-labeled proteins for rapid screening by electron microscopy in cells and organisms. Developmental Cell. 2015;35:513–525. doi: 10.1016/j.devcel.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Developmental Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Cockburn K, Biechele S, Garner J, Rossant J. The Hippo pathway member Nf2 is required for inner cell mass specification. Current Biology. 2013;23:1195–1201. doi: 10.1016/j.cub.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Copp AJ. Interaction between inner cell mass and trophectoderm of the mouse blastocyst. I. A study of cellular proliferation. Journal of Embryology and Experimental Morphology. 1978;48:109–125. [PubMed] [Google Scholar]
- Cui W, Dai X, Marcho C, Han Z, Zhang K, Tremblay KD, Mager J. Towards functional annotation of the preimplantation transcriptome: an rnai screen in mammalian embryos. Scientific Reports. 2016;6:37396. doi: 10.1038/srep37396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dard N, Le T, Maro B, Louvet-Vallée S. Inactivation of aPKClambda reveals a context dependent allocation of cell lineages in preimplantation mouse embryos. PLoS One. 2009;4:e7117. doi: 10.1371/journal.pone.0007117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries WN, Binns LT, Fancher KS, Dean J, Moore R, Kemler R, Knowles BB. Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis. 2000;26:110–112. doi: 10.1002/(SICI)1526-968X(200002)26:2<110::AID-GENE2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberg S. Pre-gastrula development of non-eutherian mammals. Current Topics in Developmental Biology. 2018;128:237–266. doi: 10.1016/bs.ctdb.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Frum T, Ralston A. Pluripotency—what does cell polarity have to do with it? In: Houston D, editor. Cell Polarity in Development and Disease. First edition. Academic Press; 2017. pp. 31–57. [Google Scholar]
- Goolam M, Scialdone A, Graham SJL, Macaulay IC, Jedrusik A, Hupalowska A, Voet T, Marioni JC, Zernicka-Goetz M. Heterogeneity in oct4 and sox2 targets biases cell fate in 4-cell mouse embryos. Cell. 2016;165:61–74. doi: 10.1016/j.cell.2016.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Huss M, Tong GQ, Wang C, Li Sun L, Clarke ND, Robson P. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Developmental Cell. 2010;18:675–685. doi: 10.1016/j.devcel.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Hirate Y, Hirahara S, Inoue K, Suzuki A, Alarcon VB, Akimoto K, Hirai T, Hara T, Adachi M, Chida K, Ohno S, Marikawa Y, Nakao K, Shimono A, Sasaki H. Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Current Biology. 2013;23:1181–1194. doi: 10.1016/j.cub.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirate Y, Sasaki H. The role of angiomotin phosphorylation in the Hippo pathway during preimplantation mouse development. Tissue Barriers. 2014;2:e28127. doi: 10.4161/tisb.28127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirate Y, Hirahara S, Inoue K, Kiyonari H, Niwa H, Sasaki H. Par-aPKC-dependent and -independent mechanisms cooperatively control cell polarity, Hippo signaling, and cell positioning in 16-cell stage mouse embryos. Development, Growth & Differentiation. 2015;57:544–556. doi: 10.1111/dgd.12235. [DOI] [PubMed] [Google Scholar]
- Kono K, Tamashiro DA, Alarcon VB. Inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst. Developmental Biology. 2014;394:142–155. doi: 10.1016/j.ydbio.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkevich E, Niwayama R, Courtois A, Friese S, Berger N, Buchholz F, Hiiragi T. The apical domain is required and sufficient for the first lineage segregation in the mouse embryo. Developmental Cell. 2017;40:235–247. doi: 10.1016/j.devcel.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CY, Zernicka-Goetz M. Angiomotin prevents pluripotent lineage differentiation in mouse embryos via Hippo pathway-dependent and -independent mechanisms. Nature Communications. 2013;4:2251. doi: 10.1038/ncomms3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomelí H, Ramos-Mejía V, Gertsenstein M, Lobe CG, Nagy A. Targeted insertion of Cre recombinase into the TNAP gene: excision in primordial germ cells. Genesis. 2000;26:116–117. doi: 10.1002/(SICI)1526-968X(200002)26:2<116::AID-GENE4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lorthongpanich C, Messerschmidt DM, Chan SW, Hong W, Knowles BB, Solter D. Temporal reduction of LATS kinases in the early preimplantation embryo prevents ICM lineage differentiation. Genes & Development. 2013;27:1441–1446. doi: 10.1101/gad.219618.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maître JL, Niwayama R, Turlier H, Nédélec F, Hiiragi T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nature Cell Biology. 2015;17:849–855. doi: 10.1038/ncb3185. [DOI] [PubMed] [Google Scholar]
- Maître JL, Turlier H, Illukkumbura R, Eismann B, Niwayama R, Nédélec F, Hiiragi T. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature. 2016;536:344–348. doi: 10.1038/nature18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDole K, Zheng Y. Generation and live imaging of an endogenous Cdx2 reporter mouse line. Genesis. 2012;50:775–782. doi: 10.1002/dvg.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihajlović AI, Thamodaran V, Bruce AW. The first two cell-fate decisions of preimplantation mouse embryo development are not functionally independent. Scientific Reports. 2015;5:15034. doi: 10.1038/srep15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihajlović AI, Bruce AW. Rho-associated protein kinase regulates subcellular localisation of Angiomotin and Hippo-signalling during preimplantation mouse embryo development. Reproductive BioMedicine Online. 2016;33:381–390. doi: 10.1016/j.rbmo.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O'Neal W, Milgram SL. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Molecular and Cellular Biology. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N, Yamamoto S, Kiyonari H, Sato H, Sawada A, Ota M, Nakao K, Sasaki H. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mechanisms of Development. 2008;125:270–283. doi: 10.1016/j.mod.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, Makita R, Kurihara H, Morin-Kensicki EM, Nojima H, Rossant J, Nakao K, Niwa H, Sasaki H. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Developmental Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Palmieri SL, Peter W, Hess H, Schöler HR. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Developmental Biology. 1994;166:259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- Plusa B, Frankenberg S, Chalmers A, Hadjantonakis AK, Moore CA, Papalopulu N, Papaioannou VE, Glover DM, Zernicka-Goetz M. Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. Journal of Cell Science. 2005;118:505–515. doi: 10.1242/jcs.01666. [DOI] [PubMed] [Google Scholar]
- Posfai E, Petropoulos S, de Barros FRO, Schell JP, Jurisica I, Sandberg R, Lanner F, Rossant J. Position- and Hippo signaling-dependent plasticity during lineage segregation in the early mouse embryo. eLife. 2017;6:e22906. doi: 10.7554/eLife.22906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston A, Rossant J. Cdx2 acts downstream of cell polarization to cell-autonomously promote trophectoderm fate in the early mouse embryo. Developmental Biology. 2008;313:614–629. doi: 10.1016/j.ydbio.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Rayon T, Menchero S, Nieto A, Xenopoulos P, Crespo M, Cockburn K, Cañon S, Sasaki H, Hadjantonakis AK, de la Pompa JL, Rossant J, Manzanares M. Notch and hippo converge on Cdx2 to specify the trophectoderm lineage in the mouse blastocyst. Developmental Cell. 2014;30:410–422. doi: 10.1016/j.devcel.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarage CR, White MD, Álvarez YD, Fierro-González JC, Henon Y, Jesudason EC, Bissiere S, Fouras A, Plachta N. Cortical tension allocates the first inner cells of the mammalian embryo. Developmental Cell. 2015;34:435–447. doi: 10.1016/j.devcel.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Sasaki H. Roles and regulations of Hippo signaling during preimplantation mouse development. Development, Growth & Differentiation. 2017;59:12–20. doi: 10.1111/dgd.12335. [DOI] [PubMed] [Google Scholar]
- Sheth B, Fesenko I, Collins JE, Moran B, Wild AE, Anderson JM, Fleming TP. Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha+ isoform. Development. 1997;124:2027–2037. doi: 10.1242/dev.124.10.2027. [DOI] [PubMed] [Google Scholar]
- Shi X, Yin Z, Ling B, Wang L, Liu C, Ruan X, Zhang W, Chen L. Rho differentially regulates the Hippo pathway by modulating the interaction between Amot and Nf2 in the blastocyst. Development. 2017;144:3957–3967. doi: 10.1242/dev.157917. [DOI] [PubMed] [Google Scholar]
- Simon CS, Hadjantonakis AK, Schröter C. Making lineage decisions with biological noise: Lessons from the early mouse embryo. Wiley Interdisciplinary Reviews: Developmental Biology. 2018;7:e319. doi: 10.1002/wdev.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Radice G, Ashery-Padan R, Lang RA. Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development. 2009;136:2977–2985. doi: 10.1242/dev.037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Developmental Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Gossler A, Boller K, Kemler R. Expression and distribution of cell adhesion molecule uvomorulin in mouse preimplantation embryos. Developmental Biology. 1987;124:451–456. doi: 10.1016/0012-1606(87)90498-2. [DOI] [PubMed] [Google Scholar]
- Vinot S, Le T, Ohno S, Pawson T, Maro B, Louvet-Vallée S. Asymmetric distribution of PAR proteins in the mouse embryo begins at the 8-cell stage during compaction. Developmental Biology. 2005;282:307–319. doi: 10.1016/j.ydbio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wallingford MC, Hiller J, Zhang K, Mager J. YY1 Is Required for Posttranscriptional Stability of SOX2 and OCT4 Proteins. Cellular Reprogramming. 2017;19:263–269. doi: 10.1089/cell.2017.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Miyasaka KY, Kubo A, Kida YS, Nakagawa O, Hirate Y, Sasaki H, Ogura T. Notch and Hippo signaling converge on Strawberry Notch 1 (Sbno1) to synergistically activate Cdx2 during specification of the trophectoderm. Scientific Reports. 2017;7:46135. doi: 10.1038/srep46135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MD, Angiolini JF, Alvarez YD, Kaur G, Zhao ZW, Mocskos E, Bruno L, Bissiere S, Levi V, Plachta N. Long-lived binding of sox2 to dna predicts cell fate in the four-cell mouse embryo. Cell. 2016;165:75–87. doi: 10.1016/j.cell.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Wicklow E, Blij S, Frum T, Hirate Y, Lang RA, Sasaki H, Ralston A. HIPPO pathway members restrict SOX2 to the inner cell mass where it promotes ICM fates in the mouse blastocyst. PLoS Genetics. 2014;10:e1004618. doi: 10.1371/journal.pgen.1004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin like growth factor signaling by yap governs cardiomyocyte proliferation and embryonic heart size. Science Signaling. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. PNAS. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134:3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Yamazaki T, Yamashita M, Hara Y, Ogonuki N, Ogura A. Noninvasive visualization of molecular events in the mammalian zygote. Genesis. 2005;43:71–79. doi: 10.1002/gene.20158. [DOI] [PubMed] [Google Scholar]
- Yu C, Ji SY, Dang YJ, Sha QQ, Yuan YF, Zhou JJ, Yan LY, Qiao J, Tang F, Fan HY. Oocyte-expressed yes-associated protein is a key activator of the early zygotic genome in mouse. Cell Research. 2016;26:275–287. doi: 10.1038/cr.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker J, White MD, Gasnier M, Alvarez YD, Lim HYG, Bissiere S, Biro M, Plachta N. Expanding actin rings zipper the mouse embryo for blastocyst formation. Cell. 2018;173:776–791. doi: 10.1016/j.cell.2018.02.035. [DOI] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & Development. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Leung CY, Shahbazi MN, Zernicka-Goetz M. Actomyosin polarisation through PLC-PKC triggers symmetry breaking of the mouse embryo. Nature Communications. 2017;8:921. doi: 10.1038/s41467-017-00977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]