Abstract
Ezrin is a critical structural protein that organizes receptor complexes and orchestrates their signal transduction. In this study, we review the ezrin-meditated regulation of critical receptor complexes, including the epidermal growth factor receptor (EGFR), CD44, vascular cell adhesion molecule (VCAM), and the deleted in colorectal cancer (DCC) receptor. We also analyze the ezrin-meditated regulation of critical pathways associated with asthma, such as the RhoA, Rho-associated protein kinase (ROCK), and protein kinase A (cAMP/PKA) pathways. Mounting evidence suggests that ezrin plays a role in controlling airway cell function and potentially contributes to respiratory diseases. Ezrin can participate in asthma pathogenesis by affecting bronchial epithelium repair, T lymphocyte regulation, and the contraction of the airway smooth muscle cells. These studies provide new insights for the design of novel therapeutic strategies for asthma treatment.
Keywords: Actin-binding proteins, Airway cells, Asthma, Ezrin
1. Introduction
Asthma is a clinical challenge in modern medicine that affects over 300 million people and causes over 250,000 deaths annually worldwide (D’Amato et al. 2016; Lambrecht and Hammad 2015). Structural airway cells, such as smooth muscle and epithelial cells, are critical factors that contribute to asthma. Multiple studies suggest that alterations in the actin cytoskeleton cause a pathological contraction of the structural airway cells, which contributes to asthma (Fletcher and Mullins 2010; Noble et al. 2014). In clinical studies, biopsied tissue from asthmatic patients showed reduced β-actin mRNA levels (Glare et al. 2002). Altogether, these results encouraged investigators to study the actin-binding proteins in the airway cells and their clinical implications in asthma (Tang 2015).
This review analyzes recent studies on ezrin and its potential implications in asthma. Ezrin is a principal member of the ERM (ezrin–radixin–moesin) protein family, which includes actin-binding proteins of the band 4.1 superfamily because their N-termini are similar to those of the erythrocyte cytoskeletal protein band 4.1 (Sagara et al. 1995; Vaheri et al. 1997; Gould et al. 1989; Ng et al. 2001). The ERM proteins are known as structural organizers that link membrane proteins to the underlying actin cytoskeleton. In addition to their structural role, the ERMs also regulate the interaction between receptor complexes and intracellular proteins, thereby modulating signal transduction pathways, such as the RhoA, Rho-associated protein kinase (ROCK), and cyclic AMP/protein kinase A (cAMP/PKA) pathways (Iontcheva et al. 2004; Ponta et al. 2003; Celik et al. 2015). The ERMs are expressed in a developmental and tissue-specific pattern. Ezrin is mainly expressed in lymphocytes and epithelial cells, moesin is expressed in endothelial cells, and radixin is expressed in hepatocytes. Ezrin has recently attracted the attention of multiple investigators due to its role in key biological processes such as the immunological synapsis in T lymphocytes and the epidermal growth factor (EGF)-induced stimulation of human carcinoma tumor differentiation and metastasis (Bretscher et al. 1997; Yoshida et al. 2016). The interaction between ezrin and various receptor complexes and intracellular targets is mainly regulated by phosphorylation (Neisch and Fehon 2011). In this study, we review recent results of ezrin regulation and its physiological and clinical implications.
2. Molecular Features of Ezrin
2.1. Discovery of Ezrin
Ezrin was discovered in multiple cellular processes and was thought to be different proteins because of its different electrophoretic mobility on the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Gould et al. 1989; Chambers and Bretscher 2005). Ezrin was first identified in 1981 as a polypeptide with an apparent molecular weight of 81-kDa on SDS-PAGE that was quickly phosphorylated after the stimulation of human A431 carcinoma cells with EGF (Hunter and Cooper 1981). In 1983, ezrin was purified as a polypeptide with an apparent molecular weight of 80-kDa on SDS-PAGE from the microvillus cytoskeleton in chicken intestinal epithelial cells (Bretscher 1983). In 1986, both polypeptides were compared and identified as ezrin (true molecular weight of 69-kDa), which was confirmed by immunoblotting, immunoprecipitation, two-dimensional gel electrophoresis, and protein sequencing (Gould et al. 1986). Ezrin was also purified as an 82-kDa tumor antigen from the cytosol of a methylcholanthrene-induced sarcoma (Ullrich et al. 1986; Fazioli et al. 1993). In 1988, ezrin was isolated from the microvillar membranes of human choriocarcinoma cells as a 75-kDa protein named cytovillin (Pakkanen et al. 1987; Turunen et al. 1989). Ezrin was also purified as a 78-kDa cyclic AMP-dependent kinase anchoring protein (originally named AKAP78) enriched in murine gastric parietal cells (Dransfield et al. 1997). These studies showed that ezrin is involved in multiple cellular processes ranging from the EGF stimulation of human carcinoma cells to tumor antigens. These results also indicated that the original confusion was due to the different electrophoretic mobility of ezrin on SDS-PAGE (Gould et al. 1989). These differences were mostly due to its phosphorylation, indicating that ezrin is regulated by phosphorylation, which induces structural changes that affect its electrophoretic mobility.
2.2. Ezrin Phosphorylation
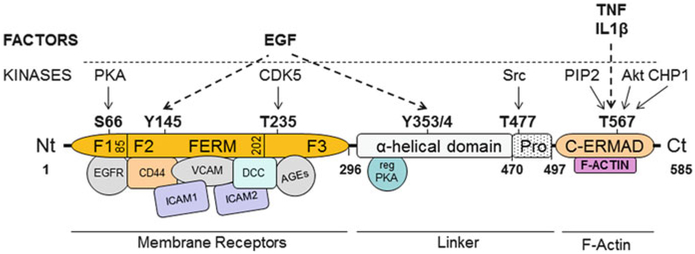
Ezrin contains an N-terminal FERM (four-point one, ezrin, radixin, moesin) domain (~300 residues), a central linker region (~200 residues), and a C-terminal ERM-associated domain (C-ERMAD, ~80 residues; Fig. 1) (Jayasundar et al. 2012). The N-terminal FERM domain consists of the following three subdomains: F1–F3. These subdomains have structural (but not sequence) homology to known folded proteins. F1 is similar to ubiquitin, F2 is similar to acyl-CoA-binding protein, and F3 is similar to the PTB (phosphotyrosine binding)-domain. All ERMs have a central helical linker region composed of the predicted α-helical domain. Ezrin and radixin, but not moesin, have a proline-rich linker domain (~470–497 residues) preceding the C-ERMAD. Ezrin is normally a dormant inactive protein due to the intramolecular interaction between the N- and C-terminal domains. Ezrin is activated by phosphorylation, which dissociates the intramolecular interaction between the N- and C-terminal domains and allows the N-terminal domain to interact with membrane receptor complexes and the C-terminal domain to interact with F-actin (Bretscher et al. 1997). Thus, the interaction between ezrin and other proteins is regulated by its phosphorylation in multiple domains by various kinases (McRobert et al. 2003; Fehon et al. 2010). A detailed list of the ezrin phosphorylation sites, kinases, and biological activity is provided in Table 1.
Fig. 1.
Structural characteristics of ezrin. Ezrin contains an N-terminal FERM domain (~300 residues), a central linker region (~200 residues), and a C-terminal ERM-associated (C-ERMAD) domain. The N-terminal domain binds to membrane receptor complexes. The linker is an α-helix with a proline-rich domain. The C-terminal C-ERMAD domain binds to F-actin. Ezrin is regulated by phosphorylation at serine-66, tyrosine-145, threonine-235, tyrosine-353, threonine-477, and threonine-567. These phosphorylations are regulated by multiple extracellular factors (EGF, TNF, and IL1β) and intracellular kinases (PKA, CDK5, Src, and Akt). FERM four-point one, ezrin, radixin, moesin, EGF epidermal growth factor, TNF tumor necrosis factor, IL1β interleukin-1β, PKA protein kinase A, CDK5 cyclin-dependent kinase 5, SRC proto-oncogene tyrosine-protein kinase Src, PIP2 phosphatidylinositol 4,5-bisphosphate, CHP1 calcineurin homologous protein-1
Table 1.
Phosphorylation sites for the ezrin activation
| Phosphorylation site |
Kinases/ phosphorylation factors |
Cell line | Biological activity | References |
|---|---|---|---|---|
| Threonine-567 | Phosphatidylinositol 4,5-bisphosphate (PIP2) | LLC-PK1 epithelial cell line | Epithelial cell morphogenesis | Fievet et al. (2004) |
| Threonine-567 | Calyculin A | LLC-PK1 epithelial cell line | Cytoskeleton arrangement and development of multicellular epithelial structures | Gautreau et al. (2000) |
| Threonine-567 | Calcineurin homologous protein-1 | Opossum kidney cells | Na+ transport | Di Sole et al. (2009) |
| Threonine-567 | TNF-α and IL-1β | Human synoviocytes | Migration and invasion of fibroblast-like synoviocytes | Xiao et al. (2014) |
| Threonine-567 | Akt | BeWo trophoblastic cells from human choriocarcinoma | Microvilli-mediated mechanoresponsive cellular functions, such as epithelial absorption, signal perception, and mechanotransduction | Miura et al. (2015) |
| Tyrosine-353 | Akt (but not ERK1/2, ROCK1) pathway | Tongue squamous cell carcinomas (TSCC) cell | Metastasis of TSCC cells | Wang et al. (2014) |
| Threonine-567/Tyrosine-353 | N/A | Pancreas tissue | Associated with positive lymph node metastasis, less differentiation, pAkt overexpression, and shorter survival times | Cui et al. (2010) |
| Threonine-567/Tyrosine-353 | N/A | Tissue of intraductal papillary mucinous neoplasms (IPMNs) and pancreatic intraepithelial neoplasia (PanINs) | Associated with tumor invasion and related to early development of PanINs | Oda et al. (2013) |
| Tyrosine-145/Tyrosine-353 | EGFR (epidermal growth factor receptor) | Human epidermoid carcinoma A431 cell | Unclear | Krieg and Hunter (1992) |
| Threonine-235 | CDK5 (cyclin-dependent kinase 5) | Human osteosarcoma cell line SAOS-2 | pRb activity and cytoskeletal regulation | Yang and Hinds (2003) |
| Threonine-235 | CDK5 | Human osteosarcoma cell line SAOS-2, senescent human diploid fibroblasts | Prevent senescence-associated flat cell formation | Yang and Hinds (2006) |
| Serine-66 | PKA | Gastric parietal cells | Remodeling of the apical membrane cytoskeleton associated with acid secretion | Zhou et al. (2003) |
| Threonine-477 | Src | Human embryonic kidney 293 cells | Unclear | Heiska and Carpen (2005) |
The most common ezrin phosphorylation site is threonine-567 in the C-terminal domain (Zhu et al. 2007). Ezrin threonine-567 is phosphorylated by calcineurin homologous protein-1 (CHP1), and the inhibition of CHP1 abrogates the interaction between ezrin and the Na+/H+ exchanger 3 (Di Sole et al. 2009). The phosphorylation of threonine-567 via phosphatidylinositol 4,5-bisphosphate (PIP2) is necessary for ezrin activation during kidney epithelial LLC-PK1 cell morphogenesis (Fievet et al. 2004). Ezrin threonine-567 is also phosphorylated by Akt, which induces microvilli in human BeWo trophoblastic cells (Miura et al. 2015). The phosphorylation of threonine-567 enhances the binding of ezrin to the actin cytoskeleton and is crucial for establishing epithelial polarity in epithelial LLC-PK1 cells (Gautreau et al. 2000). The phosphorylation of ezrin threonine-567 contributes to the migration and invasion of fibroblast-like synoviocytes in rheumatoid arthritis (Xiao et al. 2014). The ezrin C-terminal domain is also regulated by the phosphorylation of threonine-477. The inhibition of Src by the PP2 chemical inhibitor prevented the phosphorylation of ezrin threonine-477 that is induced by pervanadate in human embryonic kidney 293 cells (Heiska and Carpen 2005). The phosphorylation of threonine-477 by Src enhances the binding of ezrin to the Kelch-repeat and BTB/POZ domain containing 2 (KBTBD2) complex, which suggests that ezrin regulates cellular morphology and adhesion (Heiska and Carpen 2005).
The phosphorylation of the N-terminal domain regulates the binding of ezrin to a variety of proteins and membrane complexes. PKA phosphorylates ezrin serine-66 during the remodeling of the apical membrane during the acid secretion of gastric parietal cells (Zhou et al. 2003). CDK5 (cyclin-dependent kinase 5) induces the phosphorylation of ezrin threonine-235, which regulates the cellular shape and cytoskeletal activity in human osteosarcoma SAOS-2 cells (Yang and Hinds 2003). The phosphorylation of threonine-235 by CDK5 causes ezrin to dissociate from the Rho GDP-inhibitor (Rho-GDI) and prevents senescence-associated flat cell formation in SAOS-2 cells and human diploid fibroblasts (Yang and Hinds 2006).
Ezrin is also regulated by tyrosine phosphorylation in both the N-terminal domain (Y145) and the helical linker (Y353/4) (Fehon et al. 2010). No tyrosine phosphorylation has been reported in the C-terminal domain, suggesting that tyrosine phosphorylation does not regulate the binding of the ezrin C-terminal domain to F-actin (Fehon et al. 2010). As mentioned above, ezrin was originally identified as a protein that was quickly phosphorylated at tyrosine-353 after the stimulation of human epidermoid carcinoma A431 cells with EGF (Hunter and Cooper 1981; Krieg and Hunter 1992). Ezrin is phosphorylated at both tyrosine-145 and 353 during carcinoma differentiation and invasion (Saygideger-Kont et al. 2016; Bretscher 1989). The phosphorylation of ezrin tyrosine-353 is related to tumor differentiation associated with positive lymph node metastasis and shorter survival times in human invasive pancreatic carcinomas (Wang et al. 2014; Fehon et al. 2010; Cui et al. 2010). The phosphorylation of ezrin tyrosine-353 by Akt (but not ERK1/2 or ROCK1) has been associated with the metastasis of tongue squamous cell carcinomas (Wang et al. 2014). This phosphorylation induced invasive ductal carcinoma in human pancreatic intraepithelial neoplasia (Oda et al. 2013). These results indicate that ezrin is regulated by multiple kinases (PKA, Akt, and Src) during critical cellular processes including carcinoma tumor differentiation, survival, and metastasis.
3. Ezrin Counterparts: From Receptors to Scaffold Proteins
Ezrin is considered a key regulator of airway cells that modulates the membrane receptor complexes and their signal transduction pathways (Neisch and Fehon 2011; Miura et al. 2015; Perez-Cornejo et al. 2012; Fievet et al. 2007). Ezrin is expressed in airway cells, including both epithelial and smooth muscle cells, and interacts with receptor complexes via its N-terminal domain and the F-actin cytoskeleton via its C-terminal domain (Miura et al. 2015). A schematic of the interaction between ezrin and the membrane receptor complexes is shown in Fig. 2. These interactions are critical for modulating receptor localization, complex organization, and signal transduction pathways. Ezrin regulates critical protein including epidermal growth factor receptor (EGFR), CD44, vascular cell adhesion molecule (VCAM), and deleted in colorectal cancer (DCC) receptor.
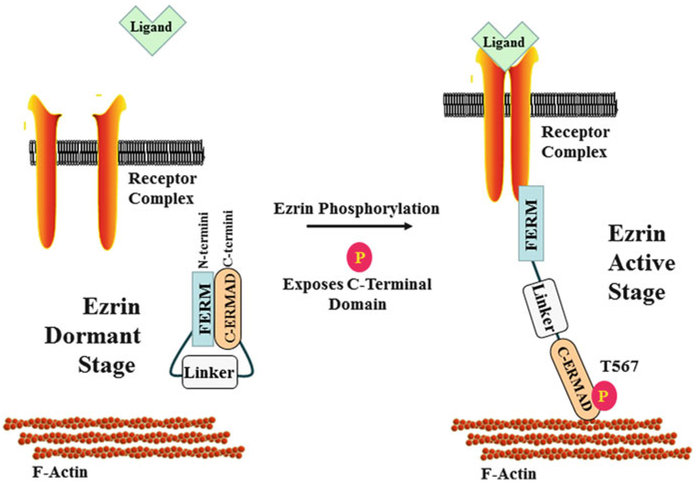
Fig. 2.
Structural regulation of ezrin. Ezrin is normally in a dormant inactive stage with its N-terminal domain interacting and blocking the C-terminal domain. Ezrin is activated by phosphorylation. Ezrin threonine-567 is one of the most characteristic phosphorylation sites, and phosphorylation at threonine-567 causes the dissociation of the intramolecular interaction between the N- and C-terminal domains. This dissociation allows the N-terminal domain to interact with multiple receptor complexes and the C-terminal domain to interact with F-actin. P phosphorylation
3.1. EGFR
Ezrin is phosphorylated at tyrosine-145 in the N-terminus and tyrosine-353 in the central helical domain after the stimulation of human A431 epidermoid carcinoma cells with EGF (Hunter and Cooper 1981). These phosphorylations are associated with cellular differentiation and invasion (Saygideger-Kont et al. 2016; Bretscher 1989). Recent studies have shown that ezrin colocalized with EGFR, Na+/H+ exchanger 1 (NHE1), and β1-integrin during invadopodia formation in tumor invasion and metastasis (Antelmi et al. 2013). The airway epithelial cells express high levels of EGFR during immune responses and cell remodeling in asthma and smoking. These results are consistent with multiple studies showing the potential regulation of EGFR signaling by ezrin (Ammar and Alice 2016; Jing et al. 2015; Burgel and Nadel 2008; Koff et al. 2008; Homma et al. 2015). The EGFR is activated by endothelin-1 in asthmatic airway smooth muscle cells and is involved in airway remodeling in asthma (Ammar and Alice 2016). EGFR also mediates the smoking-induced airway epithelium remodeling (Jing et al. 2015). These results suggest that ezrin contributes to the EGFR-induced modulation of airway cell remodeling and, thereby, respiratory disorders, such as asthma.
3.2. CD44 Receptor
Ezrin can also contribute to asthma by inducing CD44 de-polymerization (Lackie et al. 1997; Klagas et al. 2009; Casalino-Matsuda et al. 2009). CD44 is a transmembrane glycoprotein that is highly expressed on the surface of both immune and epithelial airway cells, and its expression is increased in the bronchial epithelium of asthmatic patients (Isacke and Yarwood 2002; Kumar et al. 2016; Lackie et al. 1997). However, CD44 expression is decreased in the airway smooth muscle cells of asthma and chronic obstructive pulmonary disease (COPD) patients, as shown by RT-PCR and Western blot analyses (Klagas et al. 2009). The de-polymerization of CD44 in the airway epithelial cells during inflammation also contributes to the hyper-secretion of mucus in asthma (Casalino-Matsuda et al. 2009). CD44 is also up-regulated in bronchial epithelial cells upon cellular damage in the airway, and the blockade of CD44 by neutralizing antibodies prevents cell migration (Leir et al. 2003). CD44 also interacts with other ERM (ezrin/radixin/moesin)-proteins (Yonemura et al. 1998). The GST-CD44 cytoplasmic domain binds to ERM-proteins with a high affinity, particularly to moesin, which has a KD of 9.3 ± 1.6 × 10−9 M (a smaller equilibrium dissociation constant (KD) indicates a higher affinity) (Hirao et al. 1996). Radixin binds to CD44 cytoplasmic peptides (292–363 residues) via the FERM domain, as demonstrated by structural studies (Mori et al. 2008). Because CD44 plays an important role in allergies, its interaction with ezrin has clinical implications as a potential pharmacological target (Katoh et al. 2011).
Similar to CD44, CD43 is a trans-membrane activation marker that interacts with ERM proteins and can contribute to asthma. CD43 regulates critical cellular functions, including T cell trafficking (Cannon et al. 2011). The activation of the T cell receptor (TCR) enhances the binding of ezrin to CD43, which induces the formation of a scaffold between the membrane and the cytoskeleton at the contact zone between the T cells and the antigen-presenting cells (APC) (Roumier et al. (2001). However, ezrin also colocalizes with CD43 in the opposite region, which is distal to the TCR engagement, suggesting that ezrin may contribute to the removal of inhibitory proteins from the immunological synapse during T cell activation (Allenspach et al. 2001).
3.3. VCAM
VCAM is expressed in tracheal smooth muscle and lung epithelial cells and modulates airway inflammation in asthma (Lin et al. 2015; da Silva et al. 2015). Immunoprecipitation assays have shown that VCAM-1 directly interacts with ezrin in the endothelial actin-rich docking structure, which mediates the leukocyte adhesion to the endothelium during inflammation in asthma (Barreiro et al. 2002). VCAM-1 signaling can be mediated by the advanced glycation end products (AGEs) receptor in pulmonary endothelial cells (Timothy et al. 2016). The N-terminal domain of ezrin binds to immobilized AGEs with a KD value of 5.3 ± 2.1 × 10−7 M. These results suggest that this interaction is specific and likely mediated by the exposed ezrin N-terminal domain because neither the full-length nor the C-terminal domain binds to AGEs. The binding to AGEs inhibits ezrin phosphorylation and the subsequent formation of tubules in kidney LLC-PK1 cells (McRobert et al. 2003).
In addition to VCAM, ezrin interacts with other critical adhesion molecules in airway smooth muscle cells, such as intercellular cell adhesion molecule (ICAM) (Arij et al. 2015). Ezrin interacts with both ICAM-1 and ICAM-2 (but not with ICAM-3), and the ezrin–ICAM-2 interaction has a KD of 3.3 × 10−7 M (Heiska et al. 1998). PIP2 induces the interaction between ezrin and ICAM-1 and ICAM-2 (Heiska et al. 1998). ICAM-2 induces the phosphorylation of ezrin after the activation of Akt, which inhibits apoptosis in naive CD4+ cells (Perez et al. 2002). The crystal structures show that the ICAM-2 cytoplasmic domain binds to the groove of the phosphotyrosine binding (PTB)-like F3 subdomain of the N-terminal domain of radixin (Hamada et al. 2003).
3.4. DCC
The DCC is a part of the receptor complex of netrin-1 in the nervous system (Manhire-Heath et al. 2013). Netrin-1 regulates bleomycin-induced pulmonary fibrosis and fibrocyte accumulation in the lungs. These results suggest that the DCC is implicated in respiratory diseases, such as asthma (Sun et al. 2016). The interaction between the ezrin N-terminal domain and the DCC cytoplasmic domain was originally shown in pull-down assays and later confirmed by co-immunoprecipitation in living DCC-transfected COS-1 cells (Martin et al. 2006). Netrin-1 induces the association between the DCC and ezrin and its subsequent phosphorylation (Antoine-Bertrand et al. 2011). Co-immunoprecipitation assays have shown that a DCC antibody pulls down ezrin after the stimulation of netrin-1 in IMR-32 cells (Antoine-Bertrand et al. 2011). Similar studies indicated that the transfection of DCC in murine neuroblastoma NG108–15 cells induces the binding of ezrin to PKA (Deming et al. 2015). This interaction has major cellular implications because the inhibition of ezrin protein expression abrogates the DCC-induced PKA activation and cellular growth (Deming et al. 2015).
3.5. ERM-Binding Phosphoprotein 50 (EBP50)
EBP50 (also known as the Na+/H+ exchanger regulatory factor 1 (NHERF1)) is not an integral membrane protein, although it is recruited to the membrane and regulates membrane protein complexes. The binding of ERMs to certain membrane protein complexes is mediated by EBP50 (Fouassier and Fiorotto 2015). EBP50 is mainly expressed in airway epithelial cells and regulates vascular smooth muscle cell migration and cytokinesis in asthma (Fouassier et al. 2009; Baeyens et al. 2011). Ezrin binds EBP50 to complex with PAG (phosphoprotein associated with glycosphingolipid-enriched membrane microdomains) (Stokka et al. 2010). Ezrin appears to be an essential link that colocalizes PKA and Src with EBP50, which inhibits immune responses upon the activation of T cell (Cornez and Taskén 2010). EBP50 was first identified as an ezrin binding protein using affinity chromatography, and the C-terminal domain of EBP50 was sufficient for this association (Reczek and Bretscher 1998). A BIAcore analysis further confirmed the high affinity between ezrin and EBP50 with a KD value of 58.0 ± 7.0 × 10−9 M (Stokka et al. 2010). This interaction is hypothesized to be regulated by ezrin phosphorylation because EBP50 did not bind to full-length ezrin due to its intramolecular association blocking the N- and C-terminal domains (Reczek and Bretscher 1998).
4. Ezrin Modulates Signal Transduction Pathways
Ezrin modulates signal pathways mainly by connecting membrane receptor complexes to signaling pathways (Youn et al. 2009; Pore et al. 2015; Clucas and Valderrama 2014).
4.1. RhoA and the Rho-Associated Protein Kinase (ROCK) Pathway
The RhoA/ROCK pathway is a critical signaling pathway that regulates both ezrin and airway smooth muscle contractions in asthma (Kosako et al. 2000). RhoA interacts with various effectors to regulate the downstream signaling pathways and the actin cytoskeleton (Schmieder et al. 2004; Anastasiadis et al. 2000). RhoA activates ezrin, which promotes the assembly of stress fibers and the polymerization of cortical actin in multiple cells, including fibroblasts, A431 and NIH3T3 cells (Mackay et al. 1997; Yonemura et al. 2002; Song et al. 2000). The activation of RhoA increases the intracellular levels of PIP2, which induces the phosphorylation of ezrin threonine-567 and therefore releases the ezrin C-terminal domain, allowing it to interact with F-actin (Castellani et al. 2012). The EGF-induced ezrin activation is mediated by RhoA because the inhibition of RhoA by fasudil (a potent Rho-kinase chemical inhibitor and vasodilator) prevents the activation of ezrin (Ma et al. 2013). Pretreatment with fasudil before the EGF stimulation decreased the phosphorylation of RhoA and the expression of ezrin in MDA-MB-231 cells. These results show that RhoA acts as an upstream factor that regulates ezrin (Ma et al. 2009). The inhibition of RhoA by C3 transferase (an exoenzyme that inhibits the addition of ADP-ribose moieties to Rho-like proteins) in cortical neurons abrogates the potential of netrin-1 to induce ERM phosphorylation via the DCC receptor (Lawrence et al. 2016).
Ezrin also interacts with Rho-GDI and enhances Rho activation (Takahashi et al. 1997; Nethe and Hordijk 2010). The binding of ezrin to Rho-GDI contributes to the activation of RhoA in MDCK cells after stimulation with podocalyxin (Schmieder et al. 2004). Once activated, RhoA maintains the ezrin activation and allows the connection of podocalyxin to actin at the apical cell membrane (Schmieder et al. 2004). Clinical evidence supports the relationship between RhoA and ezrin because more than 60% of ezrin-positive osteosarcomas show RhoA overexpression (Chiappetta et al. 2014). RhoA colocalizes with ezrin at the membrane ruffles of human endothelial cells that contribute to cellular migration during development (Menager et al. 1999). The phosphorylation of podocalyxin prevents its binding to ezrin and its subsequent dissociation from Rho-GDI. Thus, RhoA prevents the phosphorylation of ezrin by blocking its C-terminal actin-binding domain and inducing the dissociation of the podocalyxin/ezrin complexes from the actin cytoskeleton (Fukasawa et al. 2011). Treatment with podoplanin activates RhoA, which induces an epithelial mesenchymal transition in MDCK cells, and the activation of RhoA during the epithelial mesenchymal transition requires the binding of ezrin to the cytoplasmic tail of podoplanin (RKMSGRYSP) (Martin-Villar et al. 2006). The blockade of ezrin via RNA interference reduced the migration of ectopic endometrial cells and decreased the expression levels of RhoA and ROCK. These results suggest that the Ezrin/RhoA/ROCK pathway is a potential therapeutic target for the treatment of endometriosis (Jiang et al. 2012).
ROCK regulates the cytoskeleton by phosphorylating the myosin regulatory light chain at serine-19 during cytokinesis (Totsukawa et al. 2000). Recent studies have indicated that ROCK regulates the phosphorylation of ezrin, but some of these results are controversial. The ROCK inhibitor Y27632 prevents the phosphorylation of ezrin threonine-567 and its binding to the cytoskeleton after the transfection of RhoGEFs Net and Dbl in NIH3T3 fibroblasts (Tran Quang et al. 2000). However, other studies have indicated that the ROCK inhibitors Y27632 and HA1077 enhanced the total phosphorylation of ezrin in human glioblastoma U251 cells during cytokinesis (Kosako et al. 2000). These results are consistent with previous study showing that cisplatin induces the phosphorylation of ezrin by ROCK (Rebillard et al. 2010).
4.2. Protein Kinase A (PKA) Pathway
The cAMP/PKA pathway regulates ezrin phosphorylation, airway smooth muscle cell contraction, and cytokine production in asthma (Horvat et al. 2012). Ezrin is highly expressed in human airway smooth muscle cells and modulates β2-adrenergic receptor signaling and muscle contraction in asthma (Horvat et al. 2012). Ezrin promotes the interaction between PKA and other proteins, such as the chloride channel cystic fibrosis trans-membrane regulator (CFTR), the transporter Na+/H+ exchanger type 3 (NHE3), and the scaffold protein EBP50 (Vallée 2000; Soares et al. 2016). Ezrin is associated with cystic fibrosis, and several investigators have reported its binding to F508del CFTR, which is the most common mutation associated with this disease. In primary cystic fibrosis airway cells, the phosphorylation of ezrin threonine-567 enhances its binding to F508del CFTR and the actin cytoskeleton, activates the cAMP/PKA pathway, and rescues the F508del CFTR-dependent chloride secretion (Abbattiscianni et al. 2016). In human airway epithelial cells, the formation of the CFTR-ezrin complex increases the cAMP-mediated activation of CFTR (Ribas et al. 2007). In human airway smooth muscle cells, a disruption of the ezrin-PKA complex does not affect the β-agonist-induced accumulation of cAMP, but it increases the duration of plasma membrane-delineated cAMP (Horvat et al. 2012). Similarly, ezrin enhances PKA signaling during the Na+/H+ exchanger activation. In the mammalian kidney, EBP50 enhances the phosphorylation of NHE3 by tethering NHE3 with the PKA-ezrin complex (Weinman et al. 2000). EBP50 also forms a complex with ezrin and the type-2 Na-Pi co-transporter in opossum kidney cells. Ezrin anchors PKA and triggers the phosphorylation of EBP50, which releases Npt2a and thereby inhibits the transport of phosphate (Wang et al. 2012). PKA promotes the phosphorylation of ezrin threonine-567 and its localization at the actin membrane ruffles, which activates the cAMP-induced exchange protein in HEK293T-EPAC1 cells (Parnell et al. 2015). Ezrin, CD99, soluble adenylyl cyclase, and PKA form a signaling complex at the endothelial junctions that regulates the movement of the recycling compartments to the site of the transendothelial migration (Watson et al. 2015). The PKA-ezrin-Cx43 complex regulates the cAMP-induced gap junction connections (Pidoux and Tasken 2015). Ezrin binds to the adenosine A2b receptor and stabilizes the receptor complex after an adenosine stimulation in intestinal epithelial cells (Sitaraman et al. 2002). In contrast, ezrin, PKA, CFTR, and E3KARP (NHE3 kinase A regulatory protein) form a regulatory complex at the apical membranes of human airway epithelial cells to enhance the cAMP-induced activation of CFTR (Sun et al. 2000). Because the cAMP/PKA pathway contributes to asthma, these results suggest that the modulation of this pathway by ezrin can contribute to asthma.
5. Clinical Implications of Ezrin in Asthma
Ezrin is considered a key regulator of airway cells by modulating membrane-cortex interactions. The clinical implications of ezrin in asthma are growing and recent results suggest that ezrin is a potential therapeutic target in asthma. First, ezrin may participate in the repair of the bronchial epithelium during the early stages of asthma (Fig. 3). EGF is a key factor in bronchial epithelial repair and CD44 enhances the repair efficiency (Holgate 2000). The epithelial 3v isoform of CD44 is overexpressed in damaged tracheal epitheliums. Thus, EGF can induce the phosphorylation of ezrin, which links CD44 to the cortical actin cytoskeleton (Holgate 2000). Ezrin has also been reported to induce the secretion of mucin 5 AC (a typical feature of airway remodeling in asthma) after a neutrophil elastase attack in human airway epithelial cells.
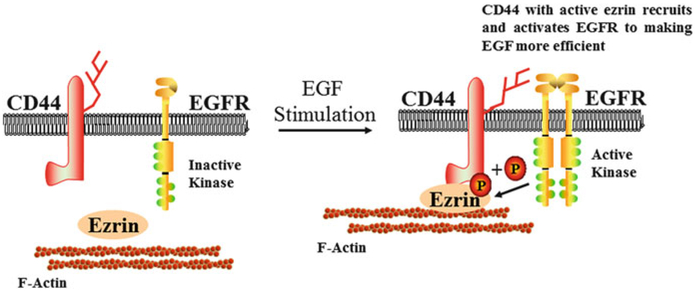
Fig. 3.
Proposed model of the participation of ezrin in the repair of the bronchial epithelium in asthma. EGF stimulation phosphorylates ezrin, which links CD44 to the cortical actin cytoskeleton. The interaction between CD44 and the EGFR enhances the repair efficiency of the airway epithelium. P phosphorylation, EGF epidermal growth factor, EGFR epidermal growth factor receptor
Second, ezrin appears to be required for the activation, morphological change, and apoptosis of T lymphocytes in asthma (Burkhardt et al. 2008). Ezrin is recruited to the immunological synapse between the T lymphocytes and APC (Roumier et al. 2001). TCR activates Rac1 to dephosphorylate ezrin, and the phosphorylation of ezrin can be restored by PIP2 binding (Burkhardt et al. 2008). This regulation of ezrin collapses the microvilli, detaches the membrane from the actin cytoskeleton, and modulates the morphology of T lymphocytes (Fig. 4, upper panel) (Brown et al. 2003). Ezrin also plays an important role in the distal pole complex (DPC) at the opposite site of the immunological synapse (Allenspach et al. 2001; Shaffer et al. 2009). Ezrin binding is crucial for the phosphorylation of CD43 during T lymphocyte activation (Cannon et al. 2011). Similarly, the activation of Rac1 induces ezrin dephosphorylation and regulates its binding to membrane receptors at the DPC of T lymphocytes (Fig. 4, lower panel) (Burkhardt et al. 2008; Nijhara et al. 2004). Chemokine stimulation induces the dephosphorylation of ezrin, which triggers a rapid microvillar collapse in T lymphocytes, leading to the arrest of circulating T lymphocytes (Brown et al. 2003). Meanwhile, ezrin antisense oligonucleotides protect T lymphocytes from CD95-mediated apoptosis (Parlato et al. 2000). These results suggest that ezrin plays a role in T lymphocyte apoptosis and has clinical implications in asthma.
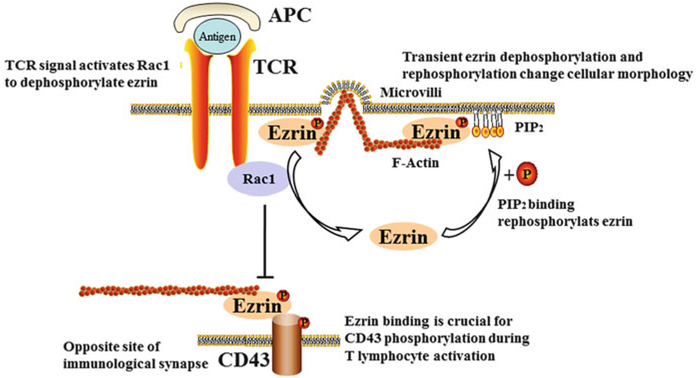
Fig. 4.
Involvement of ezrin in the activation of T lymphocytes in asthma. At the immunological synapse, a TCR signal activates Rac1, which dephosphorylates ezrin, while binding to PIP2 re-phosphorylates ezrin. This transient dephosphorylation and re-phosphorylation of ezrin can change the cellular morphology of T lymphocytes. Ezrin interacts with CD43 during T lymphocyte activation at the DPC. P phosphorylation, TCR T cell receptor, PIP2 phosphatidylinositol 4,5-bisphosphate, APC antigen-presenting cells, Rac1 ras-related C3 botulinum toxin substrate 1, DPC distal pole complex
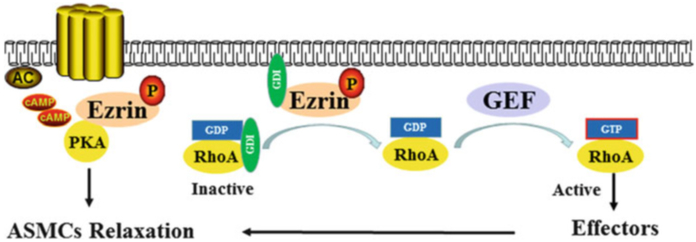
Third, ezrin can control the contraction of airway smooth muscle cells. Phosphorylated ezrin colocalizes with internalized β2-adrenergic receptors and increases the re-sensitization of these receptors to cAMP stimulation, thus relaxing the airway smooth muscle cells (Horvat et al. 2012; Cant and Pitcher 2005). Furthermore, phosphorylated ezrin binds to Rho-GDI, enhances RhoA activation, and induces airway smooth muscle cells relaxation (Fig. 5) (Takahashi et al. 1997; Fukata et al. 2001).
Fig. 5.
Possible mechanism of the ezrin-mediated regulation of the contraction and relaxation of airway smooth muscle cells. The PKA-Ezrin complex is the effector of β2-adrenergic receptor signaling in the regulation of the contraction and relaxation of ASMCs. Phosphorylated ezrin binds to Rho-GDI, which enhances the activation of RhoA and induces the relaxation of ASMCs. P phosphorylation, AC adenylate cyclase, cAMP cyclic adenosine monophosphate, PKA protein kinase A, GDP guanosine diphosphate, GDI GDP dissociation inhibitor, GEF guanine nucleotide exchange factor, GTP guanosine-5′-triphosphate
Altogether, these results suggest that ezrin can contribute to asthma by affecting bronchial epithelium repair, T lymphocyte regulation, and airway smooth muscle cell relaxation.
6. Conclusion and Perspectives
Ezrin is a critical protein that orchestrates membrane receptor complexes and intracellular transduction pathways. Ezrin is also a complex protein in terms of its structural features and functional regulation. Ezrin has multiple phosphorylation sites, but the biological and clinical implications of some of these sites remain unknown. Ezrin interacts with key receptors and adaptors, which modulates their signaling pathways, including the RhoA/ROCK and the cAMP/PKA pathways, in critical cellular processes associated with asthma. The clinical implications of ezrin in asthma are growing, and recent results support that ezrin is a potential therapeutic target for the treatment of asthma. These results warrant future studies to elucidate the interactions between ezrin and membrane receptor complexes and the regulation of airway cells in physiological and clinical conditions, such as asthma.
Acknowledgments
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81473760, 81574058); the Shanghai Talent Development Fund (No. 201610); the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (JZ2016010).
Footnotes
Competing Interests
The authors declare that they have no conflicts of interest.
Contributor Information
Lei-Miao Yin, Laboratory of Molecular Biology, Shanghai Research Institute of Acupuncture and Meridian, Yue Yang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200030, China.
Ting-Ting Duan, Laboratory of Molecular Biology, Shanghai Research Institute of Acupuncture and Meridian, Yue Yang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200030, China.
Luis Ulloa, Laboratory of Molecular Biology, Shanghai Research Institute of Acupuncture and Meridian, Yue Yang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200030, China; Department of Surgery, Center of Immunology and Inflammation, Rutgers-New Jersey Medical School, Rutgers University, Newark, NJ 07101, USA.
Yong-Qing Yang, Laboratory of Molecular Biology, Shanghai Research Institute of Acupuncture and Meridian, Yue Yang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200030, China.
References
- Abbattiscianni AC, Favia M, Mancini MT, Cardone RA, Guerra L, Monterisi S, Castellani S, Laselva O, Di Sole F, Conese M, Zaccolo M, Casavola V (2016) Correctors of mutant CFTR enhance subcortical cAMP-PKA signaling through modulating ezrin phosphorylation and cytoskeleton organization. J Cell Sci 129:1128–1140 [DOI] [PubMed] [Google Scholar]
- Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, Sperling AI (2001) ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15:739–750 [DOI] [PubMed] [Google Scholar]
- Ammar B, Alice G (2016) ET-1 enhances EGFR phosphorylation via Src activation in asthmatic airway smooth muscle. In: A33. Airway smooth muscle. American Thoracic Society international conference abstracts, American Thoracic Society, p A1347 [Google Scholar]
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB (2000) Inhibition of RhoA by p120 catenin. Nat Cell Biol 2:637–644 [DOI] [PubMed] [Google Scholar]
- Antelmi E, Cardone RA, Greco MR, Rubino R, Di Sole F, Martino NA, Casavola V, Carcangiu M, Moro L, Reshkin SJ (2013) ss1 integrin binding phosphorylates ezrin at T567 to activate a lipid raft signalsome driving invadopodia activity and invasion. PLoS One 8:e75113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine-Bertrand J, Ghogha A, Luangrath V, Bedford FK, Lamarche-Vane N (2011) The activation of ezrin-radixin-moesin proteins is regulated by netrin-1 through Src kinase and RhoA/ Rho kinase activities and mediates netrin-1-induced axon outgrowth. Mol Biol Cell 22:3734–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arij F, Britt RD Jr, Elizabeth RV, Michael AT, Christina MP, Richard JM, Prakash YS (2015) Fluticasone propionate attenuates poly (I:C)-induced ICAM-1 and VCAM-1 in the developing human airway smooth muscle. In: C67. Functional mapping of smooth muscle contractome and relaxome. American Thoracic Society international conference abstracts, American Thoracic Society, p A5000 [Google Scholar]
- Baeyens N, Meester CD, Yerna X, Morel N (2011) EBP50 is involved in the regulation of vascular smooth muscle cell migration and cytokinesis. J Cell Biochem 112:2574–2584 [DOI] [PubMed] [Google Scholar]
- Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F (2002) Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 157:1233–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A (1983) Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol 97:425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A (1989) Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol 108:921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Reczek D, Berryman M (1997) Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci 110:3011–3018 [DOI] [PubMed] [Google Scholar]
- Brown MJ, Nijhara R, Hallam JA, Gignac M, Yamada KM, Erlandsen SL, Delon J, Kruhlak M, Shaw S (2003) Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood 102:3890–3899 [DOI] [PubMed] [Google Scholar]
- Burgel PR, Nadel JA (2008) Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur Respir J 32:1068–1081 [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Carrizosa E, Shaffer MH (2008) The actin cytoskeleton in T cell activation. Annu Rev Immunol 26:233–259 [DOI] [PubMed] [Google Scholar]
- Cannon JL, Mody PD, Blaine KM, Chen EJ, Nelson AD, Sayles LJ, Moore TV, Clay BS, Dulin NO, Shilling RA, Burkhardt JK, Sperling AI (2011) CD43 interaction with ezrin-radixin-moesin (ERM) proteins regulates T-cell trafficking and CD43 phosphorylation. Mol Biol Cell 22:954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant SH, Pitcher JA (2005) G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol Biol Cell 16:3088–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino-Matsuda SM, Monzon ME, Day AJ, Forteza RM (2009) Hyaluronan fragments/CD44 mediate oxidative stress-induced MUC5B up-regulation in airway epithelium. Am J Respir Cell Mol Biol 40:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani S, Guerra L, Favia M, Di Gioia S, Casavola V, Conese M (2012) NHERF1 and CFTR restore tight junction organisation and function in cystic fibrosis airway epithelial cells: role of ezrin and the RhoA/ROCK pathway. Lab Investig 92:1527–1540 [DOI] [PubMed] [Google Scholar]
- Celik H, Sajwan KP, Selvanathan SP, Marsh BJ, Pai AV, Kont YS, Han J, Minas TZ, Rahim S, Erkizan HV, Toretsky JA, Uren A (2015) Ezrin binds to DEAD-box RNA helicase DDX3 and regulates its function and protein level. Mol Cell Biol 35:3145–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers DN, Bretscher A (2005) Ezrin mutants affecting dimerization and activation. Biochemistry 44:3926–3932 [DOI] [PubMed] [Google Scholar]
- Chiappetta C, Leopizzi M, Censi F, Puggioni C, Petrozza V, Rocca CD, Di Cristofano C (2014) Correlation of the Rac1/RhoA pathway with ezrin expression in osteosarcoma. Appl Immunohistochem Mol Morphol 22:162–170 [DOI] [PubMed] [Google Scholar]
- Clucas J, Valderrama F (2014) ERM proteins in cancer progression. J Cell Sci 127:267–275 [DOI] [PubMed] [Google Scholar]
- Cornez I, Taskén K (2010) Spatiotemporal control of cyclic AMP immunomodulation through the PKA-Csk inhibitory pathway is achieved by anchoring to an ezrin-EBP50-PAG scaffold in effector T cells. FEBS Lett 584:2681–2688 [DOI] [PubMed] [Google Scholar]
- Cui Y, Li T, Zhang D, Han J (2010) Expression of ezrin and phosphorylated ezrin (pezrin) in pancreatic ductal adenocarcinoma. Cancer Investig 28:242–247 [DOI] [PubMed] [Google Scholar]
- D’Amato G, Vitale C, Molino A, Stanziola A, Sanduzzi A, Vatrella A, Mormile M, Lanza M, Calabrese G, Antonicelli L, D’Amato M (2016) Asthma-related deaths. Multidiscip Respir Med 11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva AR, Madge L, Soroosh P, Tocker J, Croft M (2015) The TNF family molecules LIGHT and lymphotoxin alphabeta induce a distinct steroid-resistant inflammatory phenotype in human lung epithelial cells. J Immunol 195:2429–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming PB, Campbell SL, Stone JB, Rivard RL, Mercier AL, Howe AK (2015) Anchoring of protein kinase A by ERM (ezrin-radixin-moesin) proteins is required for proper netrin signaling through DCC (deleted in colorectal cancer). J Biol Chem 290:5783–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sole F, Babich V, Moe OW (2009) The calcineurin homologous protein-1 increases Na(+)/H(+)-exchanger 3 trafficking via ezrin phosphorylation. J Am Soc Nephrol 20:1776–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR (1997) Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J 16:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazioli F, Wong WT, Ullrich SJ, Sakaguchi K, Appella E, Di Fiore PP (1993) The ezrin-like family of tyrosine kinase substrates: receptor-specific pattern of tyrosine phosphorylation and relationship to malignant transformation. Oncogene 8:1335–1345 [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A (2010) Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11:276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M (2004) Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol 164:653–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievet B, Louvard D, Arpin M (2007) ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta 1773:653–660 [DOI] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD (2010) Cell mechanics and the cytoskeleton. Nature 463:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouassier L, Fiorotto R (2015) Ezrin finds its groove in cholangiocytes. Hepatology 61:1467–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouassier L, Rosenberg P, Mergey M, Saubamea B, Claperon A, Kinnman N, Chignard N, Jacobsson-Ekman G, Strandvik B, Rey C, Barbu V, Hultcrantz R, Housset C (2009) Ezrin-radixin-moesin-binding phosphoprotein (EBP50), an estrogen-inducible scaffold protein, contributes to biliary epithelial cell proliferation. Am J Pathol 174:869–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa H, Obayashi H, Schmieder S, Lee J, Ghosh P, Farquhar MG (2011) Phosphorylation of podocalyxin (Ser415) prevents RhoA and ezrin activation and disrupts its interaction with the actin cytoskeleton. Am J Pathol 179:2254–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Amano M, Kaibuchi K (2001) Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 22:32–39 [DOI] [PubMed] [Google Scholar]
- Gautreau A, Louvard D, Arpin M (2000) Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol 150:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glare EM, Divjak M, Bailey MJ, Walters EH (2002) Beta-actin and GAPDH housekeeping gene expression in asthmatic airways is variable and not suitable for normalising mRNA levels. Thorax 57:765–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Cooper JA, Bretscher A, Hunter T (1986) The protein-tyrosine kinase substrate, p81, is homologous to a chicken microvillar core protein. J Cell Biol 102:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Bretscher A, Esch FS, Hunter T (1989) cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J 8:4133–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K, Shimizu T, Yonemura S, Tsukita S, Tsukita S, Hakoshima T (2003) Structural basis of adhesion-molecule recognition by ERM proteins revealed by the crystal structure of the radixin-ICAM-2 complex. EMBO J 22:502–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiska L, Carpen O (2005) Src phosphorylates ezrin at tyrosine 477 and induces a phosphospecific association between ezrin and a kelch-repeat protein family member. J Biol Chem 280:10244–10252 [DOI] [PubMed] [Google Scholar]
- Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O (1998) Association of ezrin with intercellular adhesion molecule-1 and −2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. J Biol Chem 273:21893–21900 [DOI] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S, Tsukita S (1996) Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol 135:37–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate ST (2000) Epithelial damage and response. Clin Exp Allergy 30(Suppl 1):37–41 [DOI] [PubMed] [Google Scholar]
- Homma T, Kato A, Sakashita M, Norton JE, Suh LA, Carter RG, Schleimer RP (2015) Involvement of Toll-like receptor 2 and epidermal growth factor receptor signaling in epithelial expression of airway remodeling factors. Am J Respir Cell Mol Biol 52:471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat SJ, Deshpande DA, Yan H, Panettieri RA, Codina J, DuBose TD Jr, Xin W, Rich TC, Penn RB (2012) A-kinase anchoring proteins regulate compartmentalized cAMP signaling in airway smooth muscle. FASEB J 26:3670–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Cooper JA (1981) Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell 24:741–752 [DOI] [PubMed] [Google Scholar]
- Iontcheva I, Amar S, Zawawi KH, Kantarci A, Van Dyke TE (2004) Role for moesin in lipopolysaccharide-stimulated signal transduction. Infect Immun 72:2312–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacke CM, Yarwood H (2002) The hyaluronan receptor, CD44. Int J Biochem Cell Biol 34:718–721 [DOI] [PubMed] [Google Scholar]
- Jayasundar JJ, Ju JH, He L, Liu D, Meilleur F, Zhao J, Callaway DJ, Bu Z (2012) Open conformation of ezrin bound to phosphatidylinositol 4,5-bisphosphate and to F-actin revealed by neutron scattering. J Biol Chem 287:37119–37133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang QY, Xia JM, Ding HG, Fei XW, Lin J, Wu RJ (2012) RNAi-mediated blocking of ezrin reduces migration of ectopic endometrial cells in endometriosis. Mol Hum Reprod 18:435–441 [DOI] [PubMed] [Google Scholar]
- Jing Y, Renat S, Wu-Lin Z, Ion WC, Michelle RS, Jacqueline S, Robert JK, Yael S-B, Ronald GC (2015) Distal-to-proximal remodeling of small airway epithelium in smokers mediated by EGF signaling in small airway basal progenitor cells. In: A19. Pathologic programming of airway epithelium. American Thoracic Society international conference abstracts, American Thoracic Society, p A1050 [Google Scholar]
- Katoh S, Kaminuma O, Hiroi T, Mori A, Ohtomo T, Maeda S, Shimizu H, Obase Y, Oka M (2011) CD44 is critical for airway accumulation of antigen-specific Th2, but not Th1, cells induced by antigen challenge in mice. Eur J Immunol 41:3198–3207 [DOI] [PubMed] [Google Scholar]
- Klagas I, Goulet S, Karakiulakis G, Zhong J, Baraket M, Black JL, Papakonstantinou E, Roth M (2009) Decreased hyaluronan in airway smooth muscle cells from patients with asthma and COPD. Eur Respir J 34:616–628 [DOI] [PubMed] [Google Scholar]
- Koff JL, Shao MX, Ueki IF, Nadel JA (2008) Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am J Physiol Lung Cell Mol Physiol 294:L1068–L1075 [DOI] [PubMed] [Google Scholar]
- Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M (2000) Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene 19:6059–6064 [DOI] [PubMed] [Google Scholar]
- Krieg J, Hunter T (1992) Identification of the two major epidermal growth factor-induced tyrosine phosphorylation sites in the microvillar core protein ezrin. J Biol Chem 267:19258–19265 [PubMed] [Google Scholar]
- Kumar S, Lanckacker E, Dentener M, Bracke K, Provoost S, De Grove K, Brusselle G, Wouters E, Maes T, Joos G (2016) Aggravation of allergic airway inflammation by cigarette smoke in mice is CD44-dependent. PLoS One 11:e0151113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackie PM, Baker JE, Gunthert U, Holgate ST (1997) Expression of CD44 isoforms is increased in the airway epithelium of asthmatic subjects. Am J Respir Cell Mol Biol 16:14–22 [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H (2015) The immunology of asthma. Nat Immunol 16:45–56 [DOI] [PubMed] [Google Scholar]
- Lawrence EJ, Boucher E, Mandato CA (2016) Mitochondria-cytoskeleton associations in mammalian cytokinesis. Cell Div 11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leir SH, Holgate ST, Lackie PM (2003) Inflammatory cytokines can enhance CD44-mediated airway epithelial cell adhesion independently of CD44 expression. Am J Physiol Lung Cell Mol Physiol 285:L1305–L1311 [DOI] [PubMed] [Google Scholar]
- Lin CC, Lin WN, Hou WC, Hsiao LD, Yang CM (2015) Endothelin-1 induces VCAM-1 expression-mediated inflammation via receptor tyrosine kinases and Elk/p300 in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 309:L211–L225 [DOI] [PubMed] [Google Scholar]
- Ma L, Liu YP, Zhang XH, Xing LX, Wang JL, Geng CZ (2009) Effect of RhoA signaling transduction on expression of ezrin in breast cancer cell lines. Ai Zheng 28:108–111 [PubMed] [Google Scholar]
- Ma L, Liu YP, Zhang XH, Geng CZ, Li ZH (2013) Relationship of RhoA signaling activity with ezrin expression and its significance in the prognosis for breast cancer patients. Chin Med J 126:242–247 [PubMed] [Google Scholar]
- Mackay DJ, Esch F, Furthmayr H, Hall A (1997) Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol 138:927–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhire-Heath R, Golenkina S, Saint R, Murray MJ (2013) Netrin-dependent downregulation of Frazzled/DCC is required for the dissociation of the peripodial epithelium in Drosophila. Nat Commun 4:2790. [DOI] [PubMed] [Google Scholar]
- Martin M, Simon-Assmann P, Kedinger M, Martin M, Mangeat P, Real FX, Fabre M (2006) DCC regulates cell adhesion in human colon cancer derived HT-29 cells and associates with ezrin. Eur J Cell Biol 85:769–783 [DOI] [PubMed] [Google Scholar]
- Martin-Villar E, Megias D, Castel S, Yurrita MM, Vilaro S, Quintanilla M (2006) Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci 119:4541–4553 [DOI] [PubMed] [Google Scholar]
- McRobert EA, Gallicchio M, Jerums G, Cooper ME, Bach LA (2003) The amino-terminal domains of the ezrin, radixin, and moesin (ERM) proteins bind advanced glycation end products, an interaction that may play a role in the development of diabetic complications. J Biol Chem 278:25783–25789 [DOI] [PubMed] [Google Scholar]
- Menager C, Vassy J, Doliger C, Legrand Y, Karniguian A (1999) Subcellular localization of RhoA and ezrin at membrane ruffles of human endothelial cells: differential role of collagen and fibronectin. Exp Cell Res 249:221–230 [DOI] [PubMed] [Google Scholar]
- Miura S, Sato K, Kato-Negishi M, Teshima T, Takeuchi S (2015) Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6. Nat Commun 6:8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kitano K, Terawaki S, Maesaki R, Fukami Y, Hakoshima T (2008) Structural basis for CD44 recognition by ERM proteins. J Biol Chem 283:29602–29612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisch AL, Fehon RG (2011) Ezrin, radixin and moesin: key regulators of membrane-cortex interactions and signaling. Curr Opin Cell Biol 23:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethe M, Hordijk PL (2010) The role of ubiquitylation and degradation in RhoGTPase signalling. J Cell Sci 123:4011–4018 [DOI] [PubMed] [Google Scholar]
- Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A, Arpin M, Gschmeissner S, Verveer PJ, Bastiaens PI, Parker PJ (2001) Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J 20:2723–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhara R, van Hennik PB, Gignac ML, Kruhlak MJ, Hordijk PL, Delon J, Shaw S (2004) Rac1 mediates collapse of microvilli on chemokine-activated T lymphocytes. J Immunol 173:4985–4993 [DOI] [PubMed] [Google Scholar]
- Noble PB, Pascoe CD, Lan B, Ito S, Kistemaker LE, Tatler AL, Pera T, Brook BS, Gosens R, West AR (2014) Airway smooth muscle in asthma: linking contraction and mechanotransduction to disease pathogenesis and remodelling. Pulm Pharmacol Ther 29:96–107 [DOI] [PubMed] [Google Scholar]
- Oda Y, Aishima S, Morimatsu K, Hayashi A, Shindo K, Fujino M, Mizuuchi Y, Hattori M, Tanaka M, Oda Y (2013) Differential ezrin and phosphorylated ezrin expression profiles between pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasm, and invasive ductal carcinoma of the pancreas. Hum Pathol 44:1487–1498 [DOI] [PubMed] [Google Scholar]
- Pakkanen R, Hedman K, Turunen O, Wahlstrom T, Vaheri A (1987) Microvillus-specific Mr 75,000 plasma membrane protein of human choriocarcinoma cells. J Histochem Cytochem 35:809–816 [DOI] [PubMed] [Google Scholar]
- Parlato S, Giammarioli AM, Logozzi M, Lozupone F, Matarrese P, Luciani F, Falchi M, Malorni W, Fais S (2000) CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J 19:5123–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell E, Koschinski A, Zaccolo M, Cameron RT, Baillie GS, Baillie GL, Porter A, McElroy SP, Yarwood SJ (2015) Phosphorylation of ezrin on Thr567 is required for the synergistic activation of cell spreading by EPAC1 and protein kinase A in HEK293T cells. Biochim Biophys Acta 1853:1749–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez OD, Kinoshita S, Hitoshi Y, Payan DG, Kitamura T, Nolan GP, Lorens JB (2002) Activation of the PKB/AKT pathway by ICAM-2. Immunity 16:51–65 [DOI] [PubMed] [Google Scholar]
- Perez-Cornejo P, Gokhale A, Duran C, Cui Y, Xiao Q, Hartzell HC, Faundez V (2012) Anoctamin 1 (Tmem16A) Ca2+-activated chloride channel stoichiometrically interacts with an ezrin-radixin-moesin network. Proc Natl Acad Sci U S A 109:10376–10381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux G, Tasken K (2015) Anchored PKA as a gatekeeper for gap junctions. Commun Integr Biol 8:e1057361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich PA (2003) CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4:33–45 [DOI] [PubMed] [Google Scholar]
- Pore D, Bodo J, Danda A, Yan D, Phillips JG, Lindner D, Hill BT, Smith MR, Hsi ED, Gupta N (2015) Identification of ezrin-radixin-moesin proteins as novel regulators of pathogenic B-cell receptor signaling and tumor growth in diffuse large B-cell lymphoma. Leukemia 29:1857–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebillard A, Jouan-Lanhouet S, Jouan E, Legembre P, Pizon M, Sergent O, Gilot D, Tekpli X, Lagadic-Gossmann D, Dimanche-Boitrel MT (2010) Cisplatin-induced apoptosis involves a Fas-ROCK-ezrin-dependent actin remodelling in human colon cancer cells. Eur J Cancer 46:1445–1455 [DOI] [PubMed] [Google Scholar]
- Reczek D, Bretscher A (1998) The carboxyl-terminal region of EBP50 binds to a site in the amino-terminal domain of ezrin that is masked in the dormant molecule. J Biol Chem 273:18452–18458 [DOI] [PubMed] [Google Scholar]
- Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F Jr (2007) The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta 1768:913–922 [DOI] [PubMed] [Google Scholar]
- Roumier A, Olivo-Marin JC, Arpin M, Michel F, Martin M, Mangeat P, Acuto O, Dautry-Varsat A, Alcover A (2001) The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity 15:715–728 [DOI] [PubMed] [Google Scholar]
- Sagara J, Tsukita S, Yonemura S, Tsukita S, Kawai A (1995) Cellular actin-binding ezrin-radixin-moesin (ERM) family proteins are incorporated into the rabies virion and closely associated with viral envelope proteins in the cell. Virology 206:485–494 [DOI] [PubMed] [Google Scholar]
- Saygideger-Kont Y, Minas TZ, Jones H, Hour S, Celik H, Temel I, Han J, Atabey N, Erkizan HV, Toretsky JA, Uren A (2016) Ezrin enhances EGFR signaling and modulates erlotinib sensitivity in non-small cell lung cancer cells. Neoplasia 18:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder S, Nagai M, Orlando RA, Takeda T, Farquhar MG (2004) Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and ezrin in MDCK cells. J Am Soc Nephrol 15:2289–2298 [DOI] [PubMed] [Google Scholar]
- Shaffer MH, Dupree RS, Zhu P, Saotome I, Schmidt RF, McClatchey AI, Freedman BD, Burkhardt JK (2009) Ezrin and moesin function together to promote T cell activation. J Immunol 182:1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman SV, Wang L, Wong M, Bruewer M, Hobert M, Yun CH, Merlin D, Madara JL (2002) The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and ezrin upon agonist stimulation. J Biol Chem 277:33188–33195 [DOI] [PubMed] [Google Scholar]
- Soares MA, Cohen OD, Low YC, Sartor RA, Ellison T, Anil U, Anzai L, Chang JB, Saadeh PB, Rabbani PS, Ceradini DJ (2016) Restoration of Nrf2 signaling normalizes the regenerative niche. Diabetes 65:633–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Wong C, Chang DD (2000) Overexpression of wild-type RhoA produces growth arrest by disrupting actin cytoskeleton and microtubules. J Cell Biochem 80:229–240 [DOI] [PubMed] [Google Scholar]
- Stokka AJ, Mosenden R, Ruppelt A, Lygren B, Tasken K (2010) The adaptor protein EBP50 is important for localization of the protein kinase A-ezrin complex in T-cells and the immunomodulating effect of cAMP. Biochem J 425:381–388 [DOI] [PubMed] [Google Scholar]
- Sun F, Hug MJ, Lewarchik CM, Yun CH, Bradbury NA, Frizzell RA (2000) E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J Biol Chem 275:29539–29546 [DOI] [PubMed] [Google Scholar]
- Sun H, Zhu Y, Pan H, Chen X, Balestrini JL, Lam TT, Kanyo JE, Eichmann A, Gulati M, Fares WH, Bai H, Feghali-Bostwick CA, Gan Y, Peng X, Moore MW, White ES, Sava P, Gonzalez AL, Cheng Y, Niklason LE, Herzog EL (2016) Netrin-1 regulates fibrocyte accumulation in the decellularized fibrotic sclerodermatous lung microenvironment and in bleomycin-induced pulmonary fibrosis. Arthritis Rheumatol 68:1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Takai Y (1997) Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem 272:23371–23375 [DOI] [PubMed] [Google Scholar]
- Tang DD (2015) Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling. Respir Res 16:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timothy NP, Elizabeth AO, Gina ED, Timothy DO (2016) The receptor for advanced glycation end-products mediates VCAM-1 expression in pulmonary endothelial cells: implications for asthma and allergic airway disease. In: D31. Novel mechanisms of allergy and airway inflammation. American Thoracic Society international conference abstracts, American Thoracic Society, p A6693 [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F (2000) Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol 150:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Quang C, Gautreau A, Arpin M, Treisman R (2000) Ezrin function is required for ROCK-mediated fibroblast transformation by the Net and Dbl oncogenes. EMBO J 19:4565–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen O, Winqvist R, Pakkanen R, Grzeschik KH, Wahlstrom T, Vaheri A (1989) Cytovillin, a microvillar Mr 75,000 protein. cDNA sequence, prokaryotic expression, and chromosomal localization. J Biol Chem 264:16727–16732 [PubMed] [Google Scholar]
- Ullrich SJ, Robinson EA, Appella E (1986) Characterization of a chemically homogeneous tumor antigen from a methylcholanthrene-induced sarcoma, Meth A. Mol Immunol 23:545–555 [DOI] [PubMed] [Google Scholar]
- Vaheri A, Carpen O, Heiska L, Helander TS, Jaaskelainen J, Majander-Nordenswan P, Sainio M, Timonen T, Turunen O (1997) The ezrin protein family: membrane-cytoskeleton interactions and disease associations. Curr Opin Cell Biol 9:659–666 [DOI] [PubMed] [Google Scholar]
- Vallée L (2000) ERM proteins: from cellular architecture to cell signaling. Biol Cell 92:305–316 [DOI] [PubMed] [Google Scholar]
- Wang B, Means CK, Yang Y, Mamonova T, Bisello A, Altschuler DL, Scott JD, Friedman PA (2012) Ezrin-anchored protein kinase A coordinates phosphorylation-dependent disassembly of a NHERF1 ternary complex to regulate hormone-sensitive phosphate transport. J Biol Chem 287:24148–24163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lin Z, Sun L, Fan S, Huang Z, Zhang D, Yang Z, Li J, Chen W (2014) Akt/ezrin Tyr353/NF-kappaB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br J Cancer 110:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RL, Buck J, Levin LR, Winger RC, Wang J, Arase H, Muller WA (2015) Endothelial CD99 signals through soluble adenylyl cyclase and PKA to regulate leukocyte transendothelial migration. J Exp Med 212:1021–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman EJ, Minkoff C, Shenolikar S (2000) Signal complex regulation of renal transport proteins: NHERF and regulation of NHE3 by PKA. Am J Physiol Renal Physiol 279:F393–F399 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Sun M, Zhan Z, Ye Y, Huang M, Zou Y, Liang L, Yang X, Xu H (2014) Increased phosphorylation of ezrin is associated with the migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Rheumatology 53:1291–1300 [DOI] [PubMed] [Google Scholar]
- Yang HS, Hinds PW (2003) Increased ezrin expression and activation by CDK5 coincident with acquisition of the senescent phenotype. Mol Cell 11:1163–1176 [DOI] [PubMed] [Google Scholar]
- Yang HS, Hinds PW (2006) Phosphorylation of ezrin by cyclin-dependent kinase 5 induces the release of Rho GDP dissociation inhibitor to inhibit Rac1 activity in senescent cells. Cancer Res 66:2708–2715 [DOI] [PubMed] [Google Scholar]
- Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S, Tsukita S (1998) Ezrin/radixin/ moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol 140:885–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Matsui T, Tsukita S, Tsukita S (2002) Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J Cell Sci 115:2569–2580 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Fukutomi T, Kimura T, Sakurai H, Hatano R, Yamamoto H, Mukaisho K, Hattori T, Sugihara H, Asano S (2016) Comprehensive proteome analysis of brush border membrane fraction of ileum of ezrin knockdown mice. Biomed Res 37:127–139 [DOI] [PubMed] [Google Scholar]
- Youn JY, Wang T, Cai H (2009) An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production. Circ Res 104:50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Cao X, Watson C, Miao Y, Guo Z, Forte JG, Yao X (2003) Characterization of protein kinase A-mediated phosphorylation of ezrin in gastric parietal cell activation. J Biol Chem 278:35651–35659 [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhou R, Mettler S, Wu T, Abbas A, Delaney J, Forte JG (2007) High turnover of ezrin T567 phosphorylation: conformation, activity, and cellular function. Am J Physiol Cell Physiol 293: C874–C884 [DOI] [PubMed] [Google Scholar]