Abstract
Neuronal stimulation improves physiological responses to infection and trauma, but the clinical potential of this strategy is unknown. We hypothesized that transdermal neural stimulation through low-frequency electroacupuncture might control the immune responses to surgical trauma and expedite the postoperative recovery. However, the efficiency of electroacupuncture is questioned due to the placebo effect. Here, electroacupuncture was performed on anesthetized patients to avoid any placebo. This is a prospective double-blinded pilot trial to determine whether intraoperative electroacupuncture on anesthetized patients improves postoperative recovery. Patients with electroacupuncture required 60% less postoperative analgesic, even they had pain scores similar to those in the control patients. Electroacupuncture prevented postoperative hyperglycemia and attenuated serum adrenocorticotropic hormone in the older and heavier group of patients. From an immunological perspective, electroacupuncture did not affect the protective immune responses to surgical trauma, including the induction of interleukin-6 and interleukin-10. The most significant immunological effect of electroacupuncture was enhancing transforming growth factor-β1 production during surgery in the older and lighter group of patients. These results suggest that intraoperative electroacupuncture on anesthetized patients can reduce postoperative use of analgesics and improve immune and stress responses to surgery.
Keywords: cytokines, electroacupuncture, inflammation, pain, physiological stress, surgery
1. Introduction
The Centers for Disease Control and Prevention estimates that there are over 51 million surgical procedures performed annually in the USA. Over 85% of surgical patients report significant postoperative pain, with a higher incidence in female patients [1]. Postoperative pain is treated with opioids that have multiple adverse side effects including respiratory depression and decreased intestinal motility [2]. These side effects increase the risk of surgical complications and delay postoperative recovery [3,4]. Furthermore, surgical trauma induces hyperglycemia, physiological stress, and inflammation that can cause cardiovascular, renal, and neurological complications contributing to postoperative mortality [5–7]. Postoperative hyperglycemia is an insulin resistance process that exacerbates inflammation, delays wound healing, and infections. Therefore, there is a clinical need of novel strategies to reduce hyperglycemia and improve postoperative recovery.
Neuromodulation represents efficient systems selected by evolution to control physiological homeostasis [8–10]. Thus, neural stimulation can be a promising strategy to attenuate surgical trauma. We reported that electrical stimulation of the vagus nerve improves physiological responses to infection and trauma [9,10]. These results were confirmed by other investigators reporting that vagal stimulation improved physiological responses to experimental ischemia and reperfusion, hemorrhage, resuscitation, pancreatitis, colitis, endotoxemia, septic shock, and severe sepsis [9–12]. In humans, surgical implantation of vagus nerve stimulators was first approved by the Food and Drug Administration in 1997 for the treatment of refractory epilepsy [13]. However, these studies have limited clinical implications because they were performed through a surgical stimulation of the vagus nerve. Recently, we reported that transdermal neuronal stimulation with electroacupuncture (EA) also regulates physiological responses to infection and trauma [9]. Thus, transdermal neuronal stimulation with EA can represent a promising clinical approach to alleviate surgical trauma and improve postoperative recovery.
EA is currently endorsed by the National Institutes of Health and the World Health Organization. Previous studies analyzed the potential of EA to alleviate postoperative pain and nausea [14–16]. However, the results from these studies were contradictory [16]. Many investigators question these results because the patients were conscious and therefore susceptible to placebo [14,15,17–19]. Indeed, many clinical studies on EA were not conclusive as the results were statistically similar to the placebo group [18,20]. We recently reported that EA regulated physiological responses to infection and trauma in anesthetized mice [9], which are not susceptible to the placebo effect. Similar studies also indicated that ST36 stimulation induced anti-nociceptive effects via adenosine A1 receptors [17]. The use of EA on anesthetized patients has been previously avoided assuming that general anesthesia may conceal the analgesic effects of EA. We hypothesized that low-frequency EA may prevent physiological stress and improve postoperative recovery. Low frequency EA acts on the arcuate nucleus of the hypothalamus, and converges in the periaqueductal grey matter to induce endomorphin/beta-endorphin/encephalin. The effects of endomorphin/beta-endorphin/encephalin in low-frequency EA are mediated by the mu/delta opioid receptors [21]. Thus, low-frequency EA can induce analgesic effects that depend on the activation of the opioidergic system. Here we performed a prospective double-blinded randomized pilot study to determine whether intraoperative EA (using acupoints LI-4, LI-11, and ST-36) on anesthetized patients undergoing thyroid or parathyroid surgery could reduce the use of analgesic, pain score, physiological stress, or immune cytokine responses. Given that our previous studies indicated that EA inhibited the production of inflammatory cytokines [9], and surgical trauma induces inflammatory cytokines, we also analyzed whether intraoperative EA also regulated the immune cytokine responses.
2. Materials and methods
2.1. Clinical trial
A prospective pilot study approved by the Institutional Review Board (Pro2012002417) of the New Jersey Medical School, Rutgers University, Newark, NJ, USA and registered at clinicaltrials.gov (code NCT01937520). This was a prospective double-blinded study with 20 patients undergoing thyroid and parathyroid surgery randomized in two groups: EA (n = 11) group or sham (control, n = 9) group. Participation was voluntary without economical compensation, and each participant signed a written consent. Exclusion criteria include pre-existing diabetes, cardiovascular conditions, or elevated levels of blood glucose, insulin, or tumor necrosis factor (TNF). Patient #11 was excluded because of preexisting levels of TNF > 1 ug/mL prior to surgery. All patients underwent the induction of anesthesia with midazolam 1—2.5 mg, propofol 1.0—2.0 mg/kg, fentanyl 1—3 μg/kg, and lidocaine 1 mg/kg. Then, anesthesia was maintained with sevoflurane at a minimal alveolar concentration of 1.0—3.0 mixed with 50% oxygen and 50% air.
2.1.1. Electroacupuncture (EA)
Low-frequency EA consisted of two 30-minute treatments stimulating simultaneously the He Gu (LI-4), Qu Chi (LI-11), and Zu San Li (ST-36) acupoints simultaneously during the maintenance phase of general anesthesia (Fig. 1A). LI-4 and LI-11 acupoints lie on the large intestine meridian (pathway). LI-4 point is located in the radial side of the hand at the middle of the second metacarpal bone; LI-11 point is located at the lateral end of the transverse cubital crease toward the elbow. ST-36 point is located on the outside of the anterior crest of the tibia and just below the knee. EA was performed by a licensed anesthesiologist and acupuncturist delivered by a stimulator (Digital Electronic Acupunctoscope 4-C, Model AWQ-104L Hong Kong, distributed by Lhasa Medical, Weymouth, MA, USA) at a 10-Hz frequency with continuous electrical current of wave through 30-gauge EA needles. A symmetrical biphasic wave was delivered to the electrodes so that the electrode would be alternately positive and negative and the bilateral LI-4, LI-11, and ST-36 acupoints would be stimulated alternately. Mild muscle twitching was observed. This setting showed significant antihyperalgesic effects in a rat inflammation model [22,23] and also inhibited the upregulation of interleukin (IL)-1β and its mRNA compared with the sham control in a rat model of bone cancer pain [24].
Figure 1.
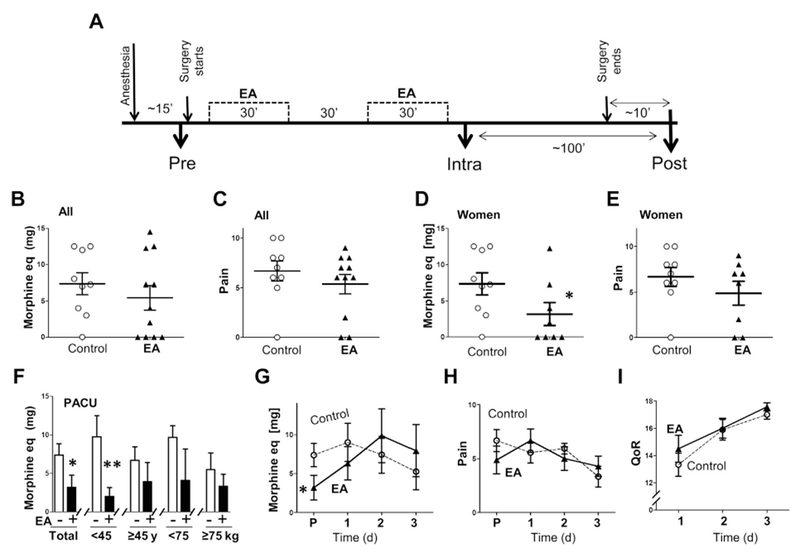
Electroacupuncture attenuated postoperative use of analgesia. (A) Control or two electroacupuncture (EA) treatments of 30 minutes started ~ 15 minutes after the induction of general anesthesia. Blood samples include: pre (before EA and surgery), intra (after EA and during surgery), and post (during anesthesia but right after surgery). (B—E) Use of analgesics and pain score in the control or EA group at the postanalgesia care unit (P) analyzing all the patients or female patients. (F) Use of analgesics at the PACU in the age and body weight subgroups. (G—I) Use of analgesics, pain score, and Quality of Recovery (QoR) mean values of the women with and without EA at the P and the 3 days after surgery. Graphs depict mean ± standard error. * p < 0.1, ** p < 0.05. eq = equivalents.
2.2. Clinical end-points
All analgesic treatments were converted to morphine equivalents of milligrams. Pain score (0—10) was determined using a visual analog scale. The Quality of Recovery scores, a nine-item validated tool (with a maximum best possible score of 18), was used to assess patient oriented outcomes postoperatively.
2.3. Serum analyses
All blood samples were collected during general anesthesia from the radial arterial catheter. Blood samples were coagulated for 120 minutes and centrifuge d at 800 g. Serum was aliquoted, and stored at −80°C. Hormones and immune cytokines were analyzed using enzyme-linked immunosorbent assay as previously described [12], using human adrenocorticotropic hormone (ACTH; CalBiotech, Spring Valley, CA, USA; catalogue number AC018T) and cortisol (CalBiotech; catalogue number CO103S). Glucose was analyzed using the One Touch Ultra test (LifeScan Inc., Milpitas, CA, USA) [22]. TNF was analyzed using recombinant TNF (eBioscience catalogue number 88-7346) as a standard curve and the capture (RRID:AB315249) and detection (Ab RRID:AB315255) antibodies (catalogue number 430201; Biolegend, San Diego, CA, USA). Human cytokines were analyzed using the Biolegend: IL2 (catalogue number 431801), IL6 (catalogue number 430501), IL4 (catalogue number 430301), IL-10 (catalogue numbers 571009, 501401, and 501501; Biolegend), and transforming growth factor-β1 (TGFβ1) with capture (21C11) and detection (19D8) antibodies (catalogue numbers 580709, 525301, and 521705; Biolegend). Plates were read at 450 nm with the VersaMax plate reader and values were interpolated with the Open SoftMax Pro 3.5 point-to-point regression software (Molecular Devices Corp, Sunnyvale, CA, USA).
2.4. Statistical analyses
We hypothesized that intraoperative EA may prevent hyperglycemia, physiological stress, or inflammation. The principal outcome was analyzing serum levels of glucose, ACTH, cortisol, and immune (TNF, IL-2, IL-4, IL-6, IL-10, and TGFβ) cytokines. The second outcome was analyzing the request of analgesics and the pain score. Sample size was determined using standard deviation values and power analyses of our previous studies on EA in surgical-induced trauma and infection [9]. Statistical analyses were performed using the Graphpad Prism 5.0 (La Jolla, CA, USA). Continuous variables were expressed as mean ± standard error. Normality and homogeneity of variance were confirmed with Graphpad Prism 5.0 (GraphPad Software Inc, La Jolla, CA, USA) using the D’Agostino—Pearson omnibus K2 test and the F-ratio of variances, respectively. Results with non-normal distributions were analyzed with the nonparametric Mann—Whitney U test. Mean values with normal distribution of two experimental groups were analyzed using the parametric unpaired homoscedastic Student t test; the Welch’s correction was used for samples with different variances. Statistical analyses of more than two groups were performed with analysis of variance with Bonferroni’s adjustment for multiple hypothesis testing. Two-way analysis of variance was used to analyze the two factors of EA and time. Linear regressions were performed with Graphpad including the calculation of p values and squared correlation coefficients.
3. Results
This prospective pilot study enrolled patients undergoing thyroid and parathyroid surgery to analyze whether EA improves postoperative recovery. The demographics of the patients (age, body weight, and surgical procedure) were similar in the control and EA group (Tables 1 and 2). All patients were under general anesthesia during the EA and the blood collection, and thus they were blinded to the treatment to avoid any placebo effect (Fig. 1A). The patients with EA had a similar use of analgesics and pain score when the heterogeneous group (with both men and women) of EA was compared with the control group (with women only; Figs. 1B and C). Since sex affected the threshold for analgesics and pain and both groups had the same number of women, we analyzed the effects of EA in women only. EA-treated women required 60% less analgesics than control women at the Postanalgesia Care Unit (PACU), but both groups had similar pain scores (Figs. 1D and E). We also compared the use of analgesics and pain scores in the group of patients under or above the average age (45 years) or body weight (75 kg). The request for analgesics and pain scores were similar in the subgroups of control patients with age (< 45 years vs. > 45 years) or body weight (< 75 kg vs. > 75kg). However, EA significantly reduced the request for analgesics in the younger (< 45 years) but not in the older patient group and induces a similar effect in the heavier and lighter patient groups (Fig. 1F). After the hospital discharge, patients with or without EA had the same use of analgesics, pain scores, and quality of recovery during the 3 days after the surgery (Figs. 1G—I).
Table 1.
Demo graphics of the patients. Individual demographics of the patients.
| Patients | Treat | Sex | Age (y) | Weight (kg) | Surgery |
|---|---|---|---|---|---|
| 1 | C | F | 33 | 80 | T |
| 2 | EA | F | 35 | 116 | T |
| 3 | C | F | 51 | 104 | T |
| 4 | C | F | 45 | 67 | T |
| 5 | C | F | 52 | 115 | T |
| 6 | EA | F | 50 | 72.7 | P |
| 7 | EA | F | 61 | 63.6 | P |
| 8 | C | F | 72 | 107 | P |
| 9 | EA | F | 34 | 78 | T |
| 10 | EA | M | 43 | 63.6 | P |
| 11 | EA | F | 43 | 56.8 | P |
| 12 | EA | M | 44 | 99 | T |
| 13 | EA | F | 32 | 76.3 | T |
| 14 | C | F | 64 | 80 | P |
| 15 | EA | F | 55 | – | P |
| 16 | EA | F | 48 | 61 | T |
| 17 | C | F | 62 | 73 | T |
| 18 | C | F | 52 | 74 | P |
| 19 | EA | F | 47 | 90 | T |
| 20 | C | F | 40 | 65 | P |
C = control; EA = electroacupuncture; F = female; M = male; P = parathyroid; T = thyroid.
Table 2.
Distribution of the patients including sample size (n), average age, average body weight, number of patients with thyroid (T) or parathyroid (P) surgery, and number of female (F) patients.
| n | Age (y) | Weight (kg) | T | P | F | |
|---|---|---|---|---|---|---|
| All | 20 | 48.15 ± 2.45 | 81.16 ± 4.27 | 11 | 9 | 18 |
| Control | 9 | 52.33 ± 4.08 | 85.00 ± 6.22 | 5 | 4 | 9 |
| EA | 11 | 44.73 ± 2.68 | 77.70 ± 5.97 | 6 | 5 | 9 |
EA = electroacupuncture.
The molecular mechanisms of EA were studied by analyzing the serum collected at three time points during anesthesia: preoperative before the surgery and EA; intraoperative after the EA; postoperative during anesthesia but right after surgery (Fig. 1A). Surgery increased the serum levels of both ACTH and cortisol in control patients by 10- and three-fold, respectively (Figs. 2A and C). Mean ACTH serum levels were higher in the heavier (> 75kg) patient group but similar between the two age subgroups (Fig. 2B). Cortisol serum levels were statistically similar among all the patient subgroups (Fig. 2D). EA reduced mean serum ACTH levels at the PACU by over 70% without affecting cortisol (Figs. 2A and C). EA reduced serum ACTH levels by over 80% in the older and heavier patient groups without affecting the younger or lighter patient subgroups (Fig. 2B). One of the most significant effects of EA was preventing hyperglycemia. Surgery gradually increased hyperglycemia in the control patients without affecting serum insulin levels (Figs. 2E and G), inducing a similar effect in all the patient subgroups. However, EA prevented hyperglycemia (Fig. 2E) being more significant in the older and heavier patient groups (Fig. 2F).
Figure 2.
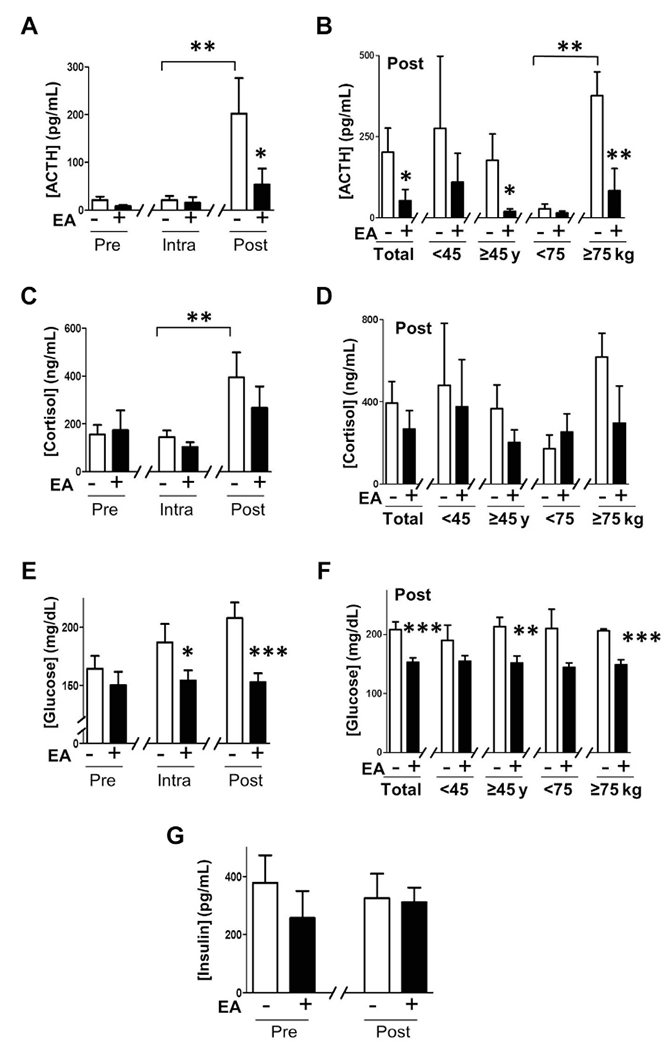
Regulation of physiological stress and glycemia. (A,B) Blood from female patients with or without electroacupuncture (EA) were collected before (Pre), during (Intra), and after (Post) surgery to analyze serum levels of adrenocorticotropic hormone (ACTH). (C,D) Cortisol. (E,F) Glucose. (G) insulin. (B,D,F) Postoperative serum levels of ACTH, cortisol, and glucose in the age and body weight subgroups. Graphs depict mean ± standard error. * p < 0.1, ** p < 0.05, *** p < 0.005.
EA also modulates the immune responses. TNF and IL-6 are critical pyrogen and inflammatory cytokines produced during the surgical trauma. IL-2 and IL-4 are critical cytokines to induce cellular versus humoral immunity. Neither surgery nor EA affected the serum levels of TNF, IL-2, or IL4 (Figs. 3A—C). However, surgery increased serum IL-6 levels by seven-fold postsurgery in the control and EA groups regardless of age and body weight (Figs. 3D and E). We also analyzed the critical anti-inflammatory cytokines IL-10 and TGFβ1. Surgery increased serum IL-10 levels by two-fold postsurgery in the control and EA groups regardless of age and body weight (Figs. 3F and G). The most significant immunological effect of EA was to increase serum TGFβ1, a pivotal factor regulating the inflammation and wound healing. Surgery increased serum TGFâ1 levels by four-fold postsurgery in the control patients (Fig. 3H). Serum TGFâ1 levels were similar among the two control age subgroups but 65% higher in the older than in the younger control group (Fig. 3I). EA induced three-fold higher serum TGFâ1 levels postsurgery (Fig. 3H). EA enhanced TGFâ1 serum levels in the older and lighter patient groups but had no significant effect in the younger and heavier patient groups (Fig. 3I).
Figure 3.
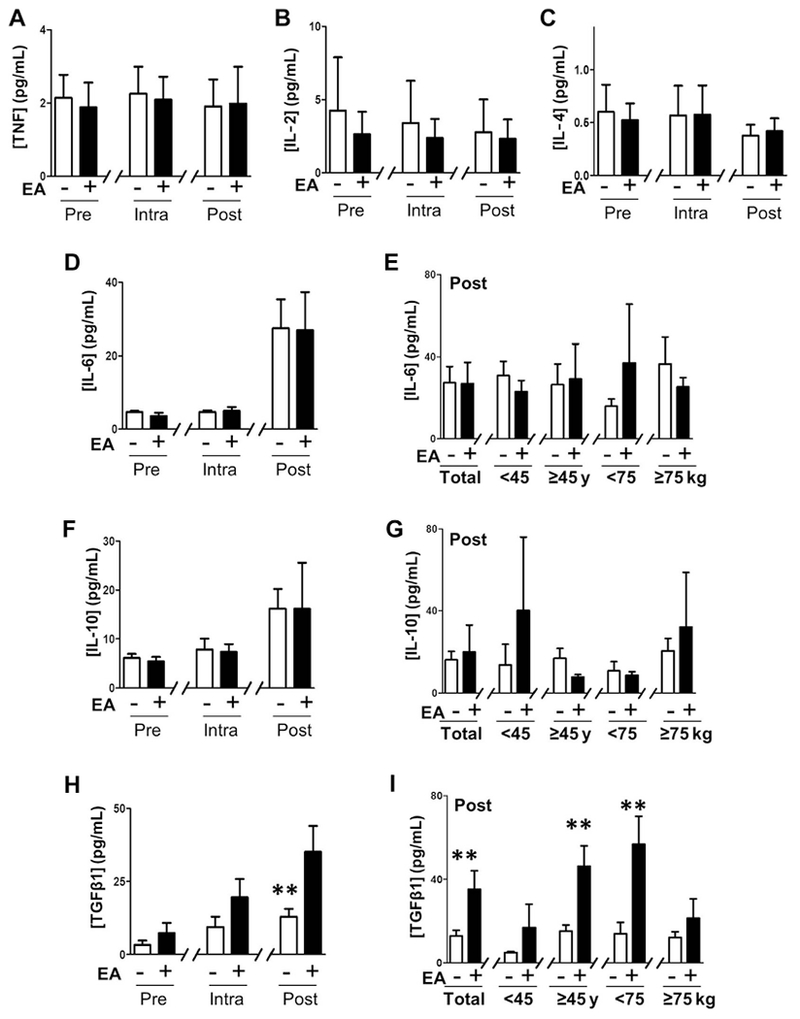
Regulation of the Immune responses to surgical trauma. (A) Serum levels of tumor necrosis factor (TNF). (B) Interleukin (IL)-2. (C) IL-4. (D) IL-6. (E) Glucose. (F,G) IL-10. (H,I) Transforming growth factor (TGF)β1 in control and electroacupuncture (EA) groups at the indicated time points. (E,G,I) Postoperative serum levels of IL-6, IL-10, and TGFβ1 in the postoperative (post) samples. Graphs depict mean ± standard error. * p < 0.1, ** p < 0.05.
4. Discussion
Previous studies of EA were performed on conscious patients before or after anesthesia and therefore the patients were susceptible to a placebo effect [14–16,19]. To our knowledge, our study describes the first clinical trial of EA performed on anesthetized patients with all blood samples collected under general anesthesia. Low-frequency EA on anesthetized patients during surgery attenuated hyperglycemia, physiological stress, immune responses, and postoperative request for analgesics. Despite their similar demographics and pain scores, women with EA required 60% less analgesics at the PACU. These results are important for four reasons: (1) all patients were under general anesthesia, and therefore blinded to the treatment to avoid any placebo effect. Despite the general anesthesia, EA induced an anti-nociceptive effect; (2) the lower amount of analgesics in the EA group did not cause a higher pain score. It is logical to expect a lower pain score in the EA group, if both groups would have received the same amount of analgesics; (3) female patients have a lower threshold for pain and acupuncture than male patients. Thus, our results in women are likely to be replicated in male patients; and (4) after the discharge, the groups had similar analgesic requirements and pain score, and quality of recovery during the 3 days after the discharge. Thus, the lower request for analgesics in the EA group was a transient effect, and not due to a demographic difference in age, sex, or body weight among the groups. In all, these results support the analgesic potential of EA, even when performed under general anesthesia.
The most significant effects of EA were preventing postoperative stress and hyperglycemia. To our knowledge, our study is the first evidence that EA in anesthetized patients prevents postoperative hyperglycemia. Previous studies on EA and glycemia were performed in conscious patients susceptible to placebo. Most studies were performed in diabetic patients with EA at the CV12 acupoint to induce insulin production. However, postoperative hyperglycemia is an insulin resistance process that exacerbates inflammation, delays wound healing, and infections. Given that EA reduced ACTH serum levels and that ACTH can induce insulin-resistant hyperglycemia [23], EA may prevent hyperglycemia by inhibiting ACTH. In agreement with this hypothesis, EA attenuated both ACTH and hyperglycemia with the same pattern in the older and heavier patient groups. EA may inhibit ACTH production through a downstream neuronal network. LI4 and LI11 acupoints lie on the large intestine meridian that attenuates sympathetic signals in the dorsal periaqueductal gray and the rostral ventrolateral medulla that innervates the paraventricular nucleus of hypothalamus. Meanwhile, ST36 activates the afferent sciatic nerve [17], which regulates the rostral ventrolateral nucleus via innervations from the paratrigeminal. Thus, LI4, LI11, and ST36 can converge at the paraventricular nucleus of hypothalamus to inhibit corticotropin-releasing hormone and thereby ACTH production from the pituitary gland. In turn, ACTH inhibition can then prevent surgical-induced hyperglycemia. Our results concur with previous studies indicating that EA affect neither intraoperative ACTH nor cortisol levels during surgery [24]. Our EA did not affect intraoperative serum levels of ACTH or cortisol. However, EA inhibited postoperative ACTH levels after the surgery. The specific regulation of ACTH without affecting cortisol has not been previously reported. By contrast, a recent study showed that EA can regulate both ACTH and cortisol in conscious patients [25]. This correlation likely involves the hypothalamic—pituitary—adrenal axis and the potential of ACTH to activate cortisol production in the adrenal cortex. The inhibition of ACTH without affecting cortisol reveals the other factors regulating cortisol production.
An important result was the potential of EA to induce TGFâ1 serum levels especially in the older and lighter patient groups. This pattern differs from the analgesic effects of EA in the younger patient groups, and the stress regulation of ACTH and hyperglycemia in the older and heavier patient groups. Although these results are limited by the small sample size, the effects of age and body weight by EA are not well established. These different patterns may suggest different mechanisms induced by the stimulation of several acupoints to control TGFâ1, pain, and physiological stress. Indeed, simultaneous stimulation of several acupoints at the same time has stronger effects than the stimulation of single acupoints [26]. For example, concurrent stimulation of LI11, CV12, and ST40 significantly attenuated atherosclerosis in hyperlipemic rats, but single acupoint stimulation did not [26]. Simultaneous stimulations trigger different mechanisms, which makes it harder to determine the discrete mechanisms modulating a specific symptom. In addition to the neuronal networks described above for LI11, LI4, and ST36, these stimulations can trigger other mechanisms to control pain and the immune system. ST36 is the most common acupoint used to control pain and inflammation by inducing endorphins or via adenosine A1 receptor [17]. We reported that ST36 inhibits inflammatory cytokines TNF and IL-6 in septic mice [9]. Likewise, LI4 stimulation in rats prior to endotoxemia prevents TNF, IL-6, and IL-1, without affecting IL-10 or glucocorticoid levels [27]. Here, EA did not affect serum levels of TNF, IL-2, or IL-4 in humans. One possible explanation is that the human and rodent responses to EA may be different. Another possible explanation is that there was no major surgical induction of TNF, IL-2, or IL-4. However, surgery significantly induced the production of IL-6 and IL-10 but they were not affected by EA. These results suggest that EA does not cause immunosuppression preventing the immune responses to trauma and wound healing. Indeed, EA enhanced TGFβ1 production. TGFâ1 is a critical factor that limits inflammation to trauma and triggers wound healing. Our results warrant future studies to determine the potential of EA to control wound healing and its mechanisms to control physiological stress and expedite postoperative recovery.
Acknowledgments
We thank Catherine Schoenberg for her technical assistance and Dr Rafael Torres for his suggestions. L.U. is supported by the faculty program of the Department of Surgery of the New Jersey Medical School, the New Jersey Health Foundation, and the US National Institute of Health (R01GM114180).
Footnotes
Disclosure statement
The authors declare that they have no conflicts of interest and no financial interests related to the material of this manuscript.
References
- [1].Lund I, Lundeberg T. Is it all about sex? Acupuncture for the treatment of pain from a biological and gender perspective. Acupunct Med 2008;26:33–45. [DOI] [PubMed] [Google Scholar]
- [2].Imani F Postoperative pain management. Anesth Pain Med 2011;1:6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol 2008;164:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev 2010;31:98–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ulate KP, Raj S, Rotta AT. Critical illness hyperglycemia in pediatric cardiac surgery. J Diabetes Sci Technol 2012;6:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33–38. [DOI] [PubMed] [Google Scholar]
- [7].Yates AR, Dyke PC 2nd, Taeed R, Hoffman TM, Hayes J, Feltes TF, et al. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med 2006;7:351–355. [DOI] [PubMed] [Google Scholar]
- [8].Steinman L Elaborate interactions between the immune and nervous systems. Nat Immunol 2004;5:575–581. [DOI] [PubMed] [Google Scholar]
- [9].Torres-Rosas R, Yehia G, Pena G, Mishra P, Del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 2014;20:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cai B, Chen F, Lin X, Miller E, Szabo C, Deithch EA, et al. Anti-inflammatory adjuvant in resuscitation fluids improves survival in hemorrhage. Crit Care Med 2009;37:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, et al. Splenectomy inactivates the cholinergic anti-inflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 2006;203:1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 2004;10:1216–1221. [DOI] [PubMed] [Google Scholar]
- [13].Ulloa L The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov 2005;4:673–684. [DOI] [PubMed] [Google Scholar]
- [14].Li G, Li S, An L, Wang B. Electroacupuncture alleviates intraoperative immunosuppression in patients undergoing supratentorial craniotomy. Acupunct Med 2013;31:51–56. [DOI] [PubMed] [Google Scholar]
- [15].Ng SS, Leung WW, Mak TW, Hon SS, Li JC, Wong CY, et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. 2013;144, 307–313.e1. [DOI] [PubMed] [Google Scholar]
- [16].Liodden I, Norheim AJ. Acupuncture and related techniques in ambulatory anesthesia. Curr Opin Anaesthesiol 2013;26: 661–668. [DOI] [PubMed] [Google Scholar]
- [17].Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci 2010;13:883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ernst E, Lee MS, Choi TY. Acupuncture: does it alleviate pain and are there serious risks? A review of reviews. Pain. 2011; 152:755–764. [DOI] [PubMed] [Google Scholar]
- [19].Sahmeddini MA, Farbood A, Ghafaripuor S. Electro-acupuncture for pain relief after nasal septoplasty: a randomized controlled study. J Altern Complement Med 2010;16:53–57. [DOI] [PubMed] [Google Scholar]
- [20].White P, Bishop FL, Prescott P, Scott C, Little P, Lewith G. Practice, practitioner, or placebo? A multifactorial, mixed-methods randomized controlled trial of acupuncture. Pain. 2012;153:455–462. [DOI] [PubMed] [Google Scholar]
- [21].Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci 2003;26:17–22. [DOI] [PubMed] [Google Scholar]
- [22].Halldorsdottir S, Warchal-Windham ME, Wallace JF, Pardo S, Parkes JL, Simmons DA. Accuracy evaluation of five blood glucose monitoring systems: the North American comparator trial. J Diabetes Sci Technol 2013;7:1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Iwen KA, Senyaman O, Schwartz A, Drenckhan M, Meier B, Hadaschik D, et al. Melanocortin crosstalk with adipose functions: ACTH directly induces insulin resistance, promotes a pro-inflammatory adipokine profile and stimulates UCP-1 in adipocytes. J Endocrinol 2008;196:465–472. [DOI] [PubMed] [Google Scholar]
- [24].Kvorning N, Akeson J. Plasma adrenaline increases in anesthetized patients given electro-acupuncture before surgery. Pain Med 2010;11:1126–1131. [DOI] [PubMed] [Google Scholar]
- [25].Jeon JH, Yoon J, Cho CK, Jung IC, Kim S, Lee SH, et al. Effect of acupuncture for radioactive-iodine-induced anorexia in thyroid cancer patients: a randomized, double-blinded, sham-controlled pilot study. Integr Cancer Ther 2015;14:221–230. [DOI] [PubMed] [Google Scholar]
- [26].Zhang GX, Miao JL, Zhang ZY, Wang HJ, Ji LX. Regulation effects of electroacupuncture with different acupoint combinations on blood lipid in rats with hyperlipemia. Zhongguo Zhen Yiu. 2014;34:894–897 [In Chinese]. [PubMed] [Google Scholar]
- [27].Song JG, Li HH, Cao YF, Lv X, Zhang P, Li YS, et al. Electroacupuncture improves survival in rats with lethal endotoxemia via the autonomic nervous system. Anesthesiology. 2012;116: 406–414. [DOI] [PubMed] [Google Scholar]


