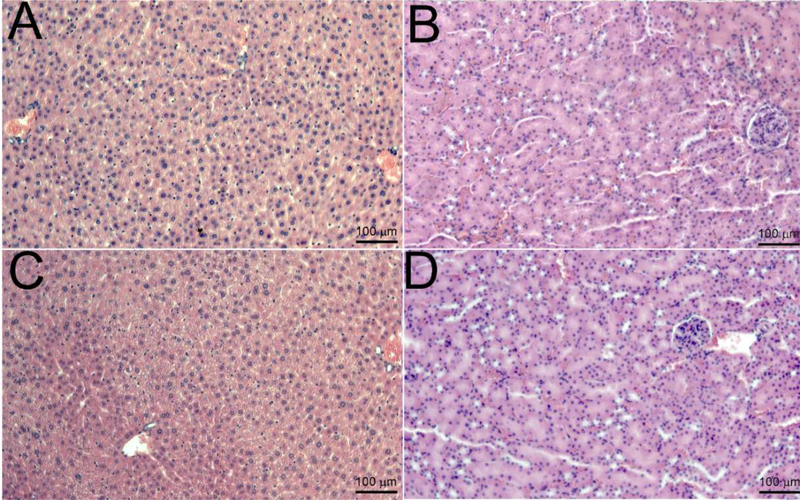
Figure 7: Histopathological examination to identify in vivo toxicity of the V7-RUBY.
Histological evaluation of the liver and kidney samples were analyzed (24hrs) after intravenous administration of the V7-RUBY. The tissue samples were fixed with paraformaldehyde and paraffin embedded followed by staining of sections from the paraffin block with hematoxylin & eosin. The tissue sections were evaluated at X 200. The scale bars represent 100 μm for all images. The pictures are representative of at least 4 independent sections. Notably, after the V7-RUBY treatment, our results indicate that hepatocytes appeared viable and the liver had no apparent changes in inflammation (Figure 7). Similarly, treatment with V7-RUBY did not cause detectable abnormalities in the kidney samples compared to untreated kidney controls (Figure 7).