Abstract
Background
Male sex is associated with an increased risk of childhood cancer as is high birthweight. Given that sex determination precedes birthweight we conducted a mediation analysis to estimate the direct effect of sex in association with childhood cancer tumor type with birthweight as the mediator.
Methods
Cases (n=12,632) and controls (n=64,439) (ages 0–14 years) were identified from population-based cancer and birth registries in Minnesota, New York, and Washington states (1970–2014). An inverse odds weighting (IOW) mediation analysis was used to estimate odds ratios (OR) and 95% confidence intervals (95% CI) as the measure of association between sex and cancer.
Results
A significant indirect effect was observed for sex and lymphoid leukemia, mediated by birthweight (indirectOR: 1.03; 95% CI: 1.02–1.04). We observed significant direct effects for male sex and lymphoid leukemia (directOR: 1.16; 95% CI: 1.08–1.25), Hodgkin lymphoma (directOR: 1.48; 95% CI: 1.22–1.81), Burkitt lymphoma (directOR: 5.02; 95% CI: 3.40–7.42), other non- Hodgkin lymphoma (directOR: 1.42; 95% CI: 1.18–1.70), intracranial embryonal tumors (directOR: 1.49; 95% CI: 1.26–1.76), hepatoblastoma (directOR: 1.90; 95% CI: 1.40–2.59), and rhabdomyosarcoma (directOR: 1.47; 95% CI: 1.19–1.81). There were also inverse associations for extracranial GCTs (directOR: 0.41; 95% CI: 0.26–0.63) and thyroid carcinoma (directOR: 0.35; 95% CI: 0.25–0.50).
Conclusion
Significant direct effects for sex and numerous childhood cancer types suggests sex- specific factors such as differences in gene expression from the autosomes or the X chromosome, rather than birthweight, may underlie sex differences in tumor risk.
Keywords: childhood cancer, sex-disparity, birthweight, mediation analysis
Introduction
The scientific evidence showing an increased risk of cancer among males relative to females over the life course is well established[1–5]. Differences in risk between the sexes in adulthood are attributable in part to risk factor differences for behaviors such as alcohol and tobacco intake between males and females[6,7]. In studies of the US population, males have a higher incidence rate of childhood cancer in general and by tumor type for acute lymphoblastic leukemia, Non-Hodgkin lymphoma, medulloblastoma, hepatic tumors, osteosarcoma, and germ cell tumors compared with females[5]. However, unlike adult cancers the mechanisms underlying the differences in childhood cancer incidence by sex are largely unknown[8]. Compared to females, male children are often of higher birthweight[9], experience a higher number of childhood infections[10–13], have an accelerated pubertal growth rate[14], and experience a different hormonal milieu over the childhood and adolescent periods[15]; therefore, these differences in risk factors may contribute to the observed sex disparity in childhood cancer incidence.
Although both sex and birthweight have been examined in association with childhood cancer, there has been no formal mediation analysis to quantify the direct effect of sex on childhood cancer risk. Male infants generally have higher birthweights than females[9] of the same gestational age[16–18] by approximately 100–200 grams[9,18]. Increasing birthweight is an established risk factor for childhood malignancy[19] including leukemia[19–22] and central nervous system (CNS) tumors[19,20,23–25], the two most common classes of childhood cancers. High birthweight may increase the risk of childhood cancer by increasing the number of mitotic events and thus the frequency of somatic mutations in larger babies[20], or by alterations in maternal hormones and growth factors that encourage rapid fetal growth [26,27]; factors that may ultimately impact cancer occurrence.
Since sex determination precedes birthweight, we conducted a mediation analysis using an inverse odds weighting (IOW) method to estimate the indirect, direct, and total effects of sex on childhood cancer risk while treating birthweight as the mediator. A benefit of a using mediation analysis is that we can examine the direct effect of sex on the risk of childhood cancer while also determining the degree to which this association operates through birthweight. Quantifying the direct effect of sex on childhood cancer risk may guide future studies to uncover the biologic mechanisms contributing to the observed sex-disparity in the incidence of most childhood cancer types. We carried out the IOW mediation analysis using a pooled study of 12,632 cases and 64,439 controls from population-based cancer registries and birth registries in Minnesota, New York, and Washington states with cancer diagnoses captured from 1970–2014.
Methods
Study population.
The present analysis includes 12,632 cases and 64,439 controls from 3 of the 5 states included in an earlier childhood cancer study [19,28–32], including incident cancer cases diagnosed in children aged 0–14 years identified from population-based cancer registries in Minnesota (MN), California (CA), New York (NY [excluding New York City]), Texas (TX), and Washington (WA) state. CA and TX individually matched cases and controls on sex and were therefore excluded from the present analysis. The current analysis is restricted to data from MN, NY, and WA, which did not match on sex and therefore could be used to estimate its association with cancer. For MN, NY, and WA, cases and controls were frequency matched on birth year (MN:1976–2004; NY: 1970–2001; WA: 1980–2004)[28]. The MN data also arose from a linkage between the Department of Health (MN DOH) Minnesota Cancer Surveillance System and the Minnesota birth registry for all cancers diagnosed from 1989–2014 as described previously[33] where cases and controls were frequency matched on birth year (1989–2010). Approval for the study was obtained from each state’s respective department of health and from Institutional Review Boards at all participating institutions and informed consent was waived.
Cancer type was classified using the International Classification of Childhood Cancer, Third Edition (ICCC-3)[34]. Tumor types with fewer than 20 cases in either males or females in the fully adjusted models or types labeled as “other” or “miscellaneous” were excluded from the cancer type-specific analyses (case counts for included tumor types are presented in Supplemental Table 1). Excluded tumor types were leukemia, not otherwise specified (NOS); myelodysplastic syndrome; lymphoma, NOS; miscellaneous lymphoma; CNS, NOS; other gliomas; other intracranial, chondrosarcoma; soft tissue sarcomas (STS), NOS; other STS; adrenocortical carcinoma and other, unspecified carcinomas. Data was harmonized across the studies for maternal and birth characteristics as previously described[28–30]. Children diagnosed with cancer <28 days of life and those with Down syndrome listed on their birth certificate (57 cases and 24 controls) were excluded.
Variables of interest.
Covariates were selected a priori for inclusion based on established associations with sex and childhood cancer[19,28,31,35] and in consideration of the study design (state and birth year). Final models included birthweight category (grams) (>350–<2,500, 2,500–<3,000, 3,000–<3,500, 3,500–<4,000, ≥4,000), gestational age (weeks) continuous (18–46), maternal race/ethnicity (Non-Hispanic white, Hispanic, other), maternal age (continuous) (13–54 years), maternal education (≤high school graduate, some college, college graduate), state of birth (MN, NY, WA), and birth year category (1970–1984, 1985–1990, 1991–1996, 1997–2010).
Statistical analysis.
We used a semiparametric, inverse odds weighting (IOW) method described by Nguyen et al. (2014)[36] and others[37,38] to test for mediation of the association between sex and childhood cancer by birthweight for tumor types with a significant total effect (IOW code is available in Nguyen et al. (2014)[36]). The advantage of the IOW method is that the generation of the IOW renders the mediator, birthweight, and the exposure, sex, independent. IOW first leg results where the exposure, sex, was regressed onto the mediator, birthweight, while adjusting for gestational age, maternal race/ethnicity, maternal education, and state and year of birth are presented in Supplemental Table 2.
The IOW method was used to estimate the direct effect of sex on cancer risk (Supplemental Figure 1), independent of birthweight, through the use of a weighted logistic model accounting for the IOW generated for each subject from the multivariate logistic regression model for birthweight in association with sex and adjusted for the additional covariates mentioned above. The weight, defined by the inverse of the odds from the aforementioned model was assigned to males. Females, the referent category, were assigned a weight value of 1. To estimate the indirect effect of sex on childhood cancer operating through birthweight, the beta from the logistic model for the direct effect was subtracted from the beta for the total effect, which was estimated from a standard logistic regression model without the IOW specification (βindirect=βtotal-βdirect). For the total, direct, and indirect effects, the resulting odds ratios (OR) and bootstrapped standard errors (1000 replications) were used to estimate the 95% confidence intervals (95% CI). The statistical significance of an indirect effect is interpreted as evidence of mediation by birthweight on the sex- childhood cancer association. We also quantified the degree of mediation by calculating the percent change from the total effect to the direct effect such that a larger percent change indicated a stronger mediation effect. ORs and 95% CIs were estimated using Stata 15.0 (College Station, Texas) for the IOW analysis and SAS version 9.4 (Cary, North Carolina) for multivariable logistic regression models. Cases and controls with missing data for any of the covariates were excluded from the relevant adjusted models. P-values for tests of statistical significance were generated for two-sided hypotheses tests with alpha equal to 0.05.
Results
Compared to controls, cases were more likely to be male (cases 54.8%; controls 51.7%, of higher birthweight (≥3,500 grams: cases 48.8%; controls 45.1%), born at gestational age of ≤40 weeks (cases 75.2%; controls 72.1%), born to non-Hispanic, white mothers (cases 87.7%; controls 84.5%), and were more likely to have mothers with some college education (cases 52.5%; controls 50.6%) (Table 1). Compared to females, male cases were more likely to weigh ≥3,500 grams at birth (males 53.4%; females 43.3%).
Table 1.
Infant and maternal characteristics of children in the 3-state analyses (1970–2014)
| Controls | Cases | ||||||
|---|---|---|---|---|---|---|---|
| Males N = 33,295 |
Females N = 31,144 |
Total N = 64,439 |
Males N = 6,924 |
Females N = 5,708 |
Total N = 12,632 |
||
| Birthweight (grams) | |||||||
| mean (standard deviation) | 3,477 | 3,350 | 3,416 | 3,504 | 3,386 | 3,450 | |
| >350 – <2,500 | 1,663 | 1,877 | 3,540 | 352 | 341 (6.0) | 693 | |
| 2,500 – <3,000 | 3,850 | 4,881 | 8,731 | 770 | 820 | 1,590 | |
| 3,000 – <3,500 | 10,886 | 12,124 | 23,010 | 2,096 | 2,067 | 4,163 | |
| 3,500 – <4,000 | 11,439 | 9,090 | 20,529 | 2,484 | 1,822 | 4,306 | |
| ≥4000 | 5,350 | 3,068 | 8,418 | 1,203 | 640 | 1,843 | |
| Missing | 107 | 104 | 211 | 19 | 18 | 37 | |
| Gestational age (weeks) | |||||||
| mean (standard deviation) | 39.3 | 39.4 | 39.4 | 39.2 | 39.3 | 39.2 | |
| <37 | 2,651 | 2,182 | 4,833 | 574 | 448 (8.3) | 1,022 | |
| 37–38 | 5,816 | 5,078 | 10,894 | 1,265 | 999 | 2,264 | |
| 39–40 | 14,416 | 13,975 | 28,391 | 3,141 | 2,592 | 5,733 | |
| 41 | 5,061 | 4,747 | 9,808 | 946 | 808 | 1,754 | |
| ≥42 | 3,636 | 3,612 | 7,248 | 651 | 579 | 1,230 | |
| Missing | 1,715 | 1,550 | 3,265 | 347 | 282 | 629 | |
| Maternal Race | |||||||
| Non-Hispanic, white | 27,817 | 26,024 | 53,841 | 6,002 | 4,988 | 10,990 | |
| Hispanic | 1,488 | 1,358 | 2,846 | 283 | 204 (3.6) | 487 | |
| Other | 3,618 | 3,395 | 7,013 | 597 | 461 (8.2) | 1,058 | |
| Missing | 372 | 367 | 739 | 42 | 55 | 97 | |
| Maternal Education | |||||||
| ≤High school | 12,097 | 11,175 | 23,272 | 2,614 | 2,222 | 4,836 | |
| Some college | 6,051 | 5,812 | 11,863 | 1,491 | 1,136 | 2,627 | |
| College graduate | 6,181 | 5,800 | 11,981 | 1,506 | 1,223 | 2,729 | |
| Missing | 8,966 | 8,357 | 17,323 | 1,313 | 1,127 | 2,440 | |
| Birth year | |||||||
| 1970–1984 | 7,796 | 7,172 | 14,968 | 1,624 | 1,347 | 2,971 | |
| 1985–1990 | 8,719 | 8,174 | 16,893 | 1,997 | 1,640 | 3,637 | |
| 1991–1996 | 8,806 | 8,235 | 17,041 | 1,659 | 1,428 | 3,087 | |
| 1997–2010 | 7,974 | 7,563 | 15,537 | 1,644 | 1,293 | 2,937 | |
| State | |||||||
| Minnesota | 14,674 | 13,999 | 28,673 | 3,316 | 2,638 | 5,954 | |
| New York | 6,280 | 5,759 | 12,039 | 2,393 | 1,964 | 4,357 | |
| Washington | 12,341 | 11,386 | 23,727 | 1,215 | 1,106 | 2,321 | |
Differences in the risk of childhood cancer by sex are apparent when comparing crude and multivariable adjusted ORs for childhood cancer overall and by cancer type (Figure 1). ORs >1 indicate tumor types associated with male sex, whereas ORs<1 represents tumor types associated with female sex. The ORs from multivariable logistic regression correspond to the total effects estimates presented in Table 2.
Figure 1.
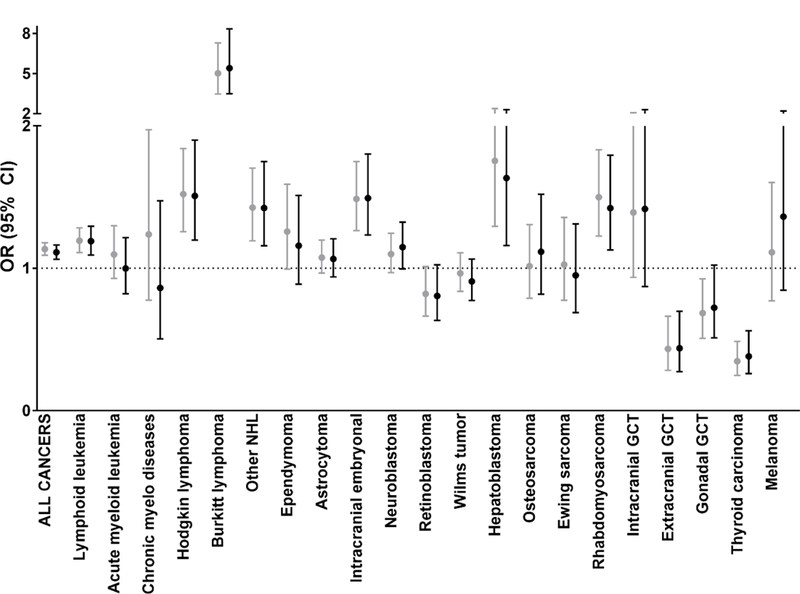
Relationship between crude and adjusteda odds ratios (OR) and 95% confidence intervals (95% CI) for male sex by childhood cancer type, 3-state pooled analysis (1970–2014)
Table 2.
Odds ratios (OR) and 95% confidence intervals (95% CI) from the mediation analysis for the crude association between sex and childhood cancer risk mediated by birthweighta, 3-state pooled analysis (1970–2014)
| Indirect | Direct | Total | % Change from total to direct |
||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p- | OR (95% CI) | p- | OR (95% | p- | ||
| All cancers combined | 1.01 (1.01- | <0.001 | 1.12 (1.08- | <0.001 | 1.13 (1.09- | <0.001 | 11 |
| Lymphoid leukemia | 1.03 (1.02- | <0.001 | 1.16 (1.08- | <0.001 | 1.19 (1.11- | <0.001 | 16 |
| Hodgkin lymphoma | 1.01 (0.98- | 0.4 | 1.48 (1.22- | <0.001 | 1.50 (1.24- | <0.001 | 3 |
| Burkitt lymphoma | 1.00 (0.96- | 0.9 | 5.02 (3.40- | <0.001 | 5.02 (3.42- | <0.001 | 2 |
| Other non-Hodgkin | 1.01 (0.98- | 0.7 | 1.42 (1.18- | <0.001 | 1.43 (1.20- | <0.001 | 0 |
| Intracranial embryonal | 0.99 (0.97- | 0.6 | 1.49 (1.26- | <0.001 | 1.48 (1.26- | <0.001 | −2 |
| Hepatoblastoma | 0.92 (0.88- | <0.001 | 1.90 (1.40- | <0.001 | 1.75 (1.29- | <0.001 | −15 |
| Rhabdomyosarcoma | 1.01 (0.98- | 0.5 | 1.47 (1.19- | <0.001 | 1.49 (1.21- | <0.001 | 3 |
| Extracranial GCT | 1.06 (0.97- | 0.2 | 0.41 (0.26- | <0.001 | 0.43 (0.28- | <0.001 | −7 |
| Thyroid carcinoma | 0.99 (0.94- | 0.8 | 0.35 (0.25- | <0.001 | 0.35 (0.25- | <0.001 | 1 |
Percent change from total to direct effect ((βtotal-βdirect)/ βtotal)*100
Male sex was significantly associated with childhood cancer overall (adjusted OR [adjOR]: 1.11; 95% confidence interval [95% CI]: 1.06–1.16) as well as lymphoid leukemia (adjOR: 1.19; 95% CI: 1.09–1.30), Hodgkin lymphoma (adjOR: 1.51; 95% CI: 1.20–1.90), Burkitt lymphoma (adjOR: 5.39; 95% CI: 3.49–8.34), other non-Hodgkin lymphoma (adjOR: 1.42; 95% CI: 1.16–1.75), intracranial embryonal tumors (adjOR: 1.49; 95% CI: 1.23–1.80), hepatoblastoma (adjOR: 1.63; 95% CI: 1.16–2.30), and rhabdomyosarcoma (adjOR: 1.42; 95% CI: 1.13–1.79) (Figure 1). Conversely, male sex displayed a significant, inverse association with extracranial germ cell tumors (GCTs) (adjOR: 0.44; 95% CI: 0.27–0.70) and thyroid carcinoma (adjOR: 0.38; 95% CI: 0.26–0.56). Adjustment for gestational age, maternal age, maternal education, state and year of birth did not substantially alter results (Figure 1). The largest amount of missing data arose from maternal education (27% controls, 19% cases). Estimates from models excluding maternal education (results not shown) were similar in direction and magnitude to those observed in our fully adjusted model.
IOW analysis first leg results are presented in Supplemental Table 2 where high birthweight (≥4,000g), relative to normal birthweight (2,500-<4,000g), is generally positively associated with male sex for each tumor type. We did not observe a significant total effect for sex and the following tumor types: acute myeloid leukemia, chronic myelodysplastic diseases, ependymoma, astrocytoma, neuroblastoma, retinoblastoma, Wilms tumor, osteosarcoma, Ewing sarcoma, intracranial germ cell tumor (GCT), gonadal GCT, or melanoma. In the IOW mediation analysis among tumor types where a significant total effect was observed (Table 2), birthweight showed a modest, but significant mediation effect on the association between male sex and all cancer types combined (indirectOR: 1.02; 95% CI: 1.01–1.03) and among lymphoid leukemia (indirectOR: 1.03; 95% CI: 1.02–1.05).
When we examined the direct effect of sex on childhood cancer overall and by tumor type (Table 2), the ORs mirror those observed for the total effects, suggesting that birthweight does not have a strong mediating effect on the association between sex and childhood cancer overall and among many tumor types, which is also represented in the small values for the percent change from total to direct effect. We found significant direct effects for male sex and all childhood cancers combined (directOR: 1.12; 95% CI: 1.08– 1.17) along with lymphoid leukemia (directOR: 1.16; 95% CI: 1.08–1.25), Hodgkin lymphoma (directOR: 1.48; 95% CI: 1.22–1.81), Burkitt lymphoma (directOR: 5.02; 95% CI: 3.40–7.42), other non-Hodgkin lymphoma (directOR: 1.42; 95% CI: 1.18–1.70), intracranial embryonal tumors (directOR: 1.49; 95% CI: 1.26–1.76), hepatoblastoma (directOR: 1.90; 95% CI: 1.40–2.59), and rhabdomyosarcoma (directOR: 1.47; 95% CI: 1.19–1.81). We also observed inverse associations between male sex and extracranial GCTs (directOR: 0.41; 95% CI: 0.26–0.63) and thyroid carcinoma (directOR: 0.35; 95% CI: 0.25– 0.50). The estimates from the fully adjusted IOW mediation analysis (Supplemental Table 3) were similar to those from the crude model (Table 2) in both direction and magnitude.
Discussion
In our pooled analysis from population-based case-control data of the association between sex and risk of childhood cancer, we observed evidence of a modest, but significant mediation effect by birthweight for childhood cancer overall and for lymphoid leukemia. However, the direct effect of male sex was significant and much stronger than that of birthweight for several tumor types. Among children, we and others have reported that males are at greater risk of, or were more frequently diagnosed with acute lymphoblastic leukemia (ALL)[39,40], but not acute myeloid leukemia (AML)[2,41], intracranial embryonal tumors (assumed to be predominantly medulloblastomas)[2,42–44], lymphomas[2,39,41], hepatoblastoma[41], and rhabdomyosarcoma[2,39,45]. We observed an increased, marginally significant (p=0.05) risk of neuroblastoma among males as observed elsewhere[41]. We observed a significant inverse association between male sex and extracranial germ cell tumors (GCTs), consistent with SEER data showing an increased risk of GCTs among females aged 0–14 years[46,47], and an increased incidence rate of extragonadal tumors among females aged 0–9 years[48]. We also observed an inverse association between male sex and thyroid carcinoma, consistent with numerous reports based on subjects of all ages[1,4,45,49–51].
The American Cancer Society reports that males aged 0–14 years have an overall cancer incidence rate of 178.0 per 1,000,000 while females have a rate of 160.1 cases per 1,000,000[2], corresponding to a crude incidence rate ratio for childhood cancer of 1.11 for male versus female sex in relation to childhood cancer. Analyses of sex disparities in childhood cancer incidence are limited with few earlier focused studies[39]. Many childhood cancer studies match cases to controls on sex, which precludes analysis of this relationship. However, consistent with one prior study[39], there are several tumor types associated strongly with male sex including hematologic malignancies, embryonal tumors, and sarcomas; therefore, these tumor types may be especially sensitive to the sex-specific biologic differences in childhood. Our results indicate these associations are independent of birthweight, suggesting that other biologic factors may be responsible for increased cancer diagnoses among boys.
The increased risk of childhood cancer among males may have a genetic basis, as the differences in male and female hormones during childhood are minimal compared to the differences observed between adults[10]. Although sex differences in tissue-specific gene expression have been observed during development in animal models[52], there are few studies of sex differences in gene expression during development available for humans. Among adults, genes on the X chromosome have shown more variation in expression between the sexes than is observed for autosomal genes and this may depend on sex-differences in chromatin accessibility[53].
Other possible genetic mechanisms may depend on acquiring somatic mutations on the X chromosome. This may differentially impact males and females as skewing toward one X over the other can occur with aging such that mutations on the X chromosome could be preferentially confined to the inactivated copy due to selective pressures[52], whereas the expression of any X- mutation in males is obligate over the life course and may be more likely to have an impact on gene expression and carcinogenesis[54]. The X chromosome may contain multiple tumor suppressors and genes that promote cell growth[54]; therefore, it is plausible that the X chromosome plays an important role in the observed sex disparity in a childhood cancer[10]. The Y chromosome, which contains less than 100 genes[55], may also play a role in the increased risk of cancer among males. To date the Y chromosome has largely been viewed as responsible for sex determination and germ cell differentiation and is usually not investigated in association with cancer; however, work in adult hepatocellular carcinoma, which is more frequent in males, suggests genes on the Y chromosome, particularly TSPY, may in fact play a role in carcinogenesis[56–58]. The contribution of Y chromosome genes to the observed male excess in childhood cancers should be explored.
Further, there is some evidence that the difference in cancer risk for boys and girls may lie in the immune response to tumor development. Males have lower innate and adaptive immune responses than females as demonstrated by higher rates of infectious diseases in males and greater levels of autoimmune diseases in females[10–13]. The X chromosome houses a large number of immune-related genes and differential expression of such genes [10,59], which may underlie the observed differences in immunity between males and females. Sex differences in immunity could also be due to X mosaicism in females[52], for whom the increased genetic diversity may result in a more comprehensive immune response; conversely, males rely upon the single X chromosome of maternal origin leading to less diversity in their immune response[10,12]. Evaluating tissue-specific germline and somatic differences in the X chromosome for DNA mutations, DNA methylation, and gene expression between boys and girls with childhood cancer will be critical for understanding the role of the X chromosome in carcinogenesis in children and adolescents.
Our study should be interpreted in light of some limitations. Even though we have accounted for known reliably measured risk factors for childhood cancer in our adjusted models such as birthweight, gestational age and maternal age, we may be missing other confounders of the association between sex and cancer risk; however, we do not expect there to be tremendous residual confounding as there are few strong risk factors for childhood cancer in children aged 0–14 years that we have not accounted for herein. We cannot rule out misclassification of disease status among controls, but the misclassification bias is likely to be negligible as Johnson et al. have described in detail[28]. Limitations to the IOW method, as suggested by Nguyen et al. [36], arise from larger variances than what might be observed in other mediation analysis techniques, which may hinder the discovery of smaller indirect effects. Concerning missing data, we do not expect the values of the missing data to differ between cases and controls as the maternal characteristic data were derived from birth registries, which collected the data prospectively.
Conclusions
Our findings suggest that the association between sex and childhood cancer may operate to a small degree through birthweight for lymphoid leukemia. For most tumors, though, there was little evidence of mediation by birthweight on the association between sex and childhood cancer suggesting that factors associated with sex other than birthweight, play a critical biologic role in carcinogenesis among children. Suspected biological mechanisms may be rooted in differences in sex chromosome gene expression or methylation or may be due to differences in immune function between males and females during infancy and childhood. Characterization of genomic differences on the X chromosome between males and females during prenatal development and childhood will be important for discovering the underlying biologic mechanisms responsible for the observed sex differences in childhood cancer risk.
Supplementary Material
Highlights.
Males have a higher childhood cancer incidence than females, but little work has focused on biologic mechanisms explaining this phenomenon
Male sex is associated with an increased risk of a number of childhood cancer types, these associations are not mediated by birthweight, and are independent of other perinatal risk factors
Biologic mechanisms underlying the increased risk of childhood cancer among males may be due to genomic differences between the sexes
Acknowledgments
Funding
This work was supported by the National Institutes of Health (grant numbers T32 CA099936 to LAW (MN;) N01 CN05230 to WA); Children’s Cancer Research Fund, Minneapolis, MN; Fred Hutchinson Cancer Research Center; Centers for Disease Control and Prevention’s National Program of Cancer Registries by cooperative agreement (U58 DP000783-01 to NY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
Cancer types for which the models failed to produce adjusted ORs are excluded.
Models adjusted for gestational age, maternal race/ethnicity, maternal age, maternal education, state of birth, and birth year
References
- [1].Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, Devesa SS, McGlynn KA, Sex disparities in cancer incidence by period and age, Cancer Epidemiol. Biomarkers Prev 18 (2009) 1174–1182. doi:10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ward E, Desantis C, Robbins A, Kohler B, Jemal A, Childhood and Adolescent Cancer Statistics, 2014, Ca Cancer J Clin 64 (2014) 83–103. doi:10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- [3].American Cancer Society, Cancer Facts and Figures 2017, Genes Dev 21 (2017) 2525–2538. doi:10.1101/gad.1593107. [Google Scholar]
- [4].Edgren G, Liang L, Adami HO, Chang ET, Enigmatic sex disparities in cancer incidence, Eur. J. Epidemiol 27 (2012) 187–196. doi:10.1007/s10654-011-9647-5. [DOI] [PubMed] [Google Scholar]
- [5].American Cancer Society, Cancer in Children & Adolescents, Spec. Sect. Cancer Child. Adolesc 1 (2014) 25–42. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/. [Google Scholar]
- [6].Paul LA, Grubaugh AL, Frueh BC, Ellis C, Egede LE, Associations between binge and heavy drinking and health behaviors in a nationally representative sample, Addict. Behav 36 (2011) 1240–1245. doi:10.1016/j.addbeh.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ, Current Cigarette Smoking Among Adults — United States, 2016, MMWR. Morb. Mortal. Wkly. Rep 67 (2018) 53–59. doi:10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tevfik Dorak M, Karpuzoglu E, Gender differences in cancer susceptibility: An inadequately addressed issue, Front. Genet 3 (2012) 1–11. doi:10.3389/fgene.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lunde A, Melve KK, Gjessing HK, Skjærven R, Irgens LM, Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data, Am. J. Epidemiol 165 (2007) 734–741. doi:10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- [10].Libert C, Dejager L, Pinheiro I, The X chromosome in immune functions: When a chromosome makes the difference, Nat. Rev. Immunol 10 (2010) 594–604. doi:10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- [11].Klein SL, Flanagan KL, Sex differences in immune responses, Nat. Rev. Immunol 16 (2016) 626–638. doi:10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- [12].Washburn T, Medearis D, Child B, Sex differences in susceptibilty to infections, Pediatrics 35 (1965) 57–64. [PubMed] [Google Scholar]
- [13].Piccini P, Montagnani C, De Martino M, Gender disparity in pediatrics: A review of the current literature, Ital. J. Pediatr 44 (2018) 4–9. doi:10.1186/s13052-017-0437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Granados A, Gebremariam A, Lee JM, Relationship Between Timing of Peak Height Velocity and Pubertal Staging in Boys and Girls, J. Clin. Res. Pediatr. Endocrinol 7 (2015) 235–237. doi:10.4274/jcrpe.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lenroot RK, Giedd JN, Sex differences in the adolescent brain, Brain Cogn 72 (2010) 1–19. doi:10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Skjærven R, Gjessing HK’, Bakketeig, Birthweight by gestational age in Norway, Acta Obstet. Gynecol. Scand 79 (2000) 440–449. doi:10.1034/j.1600-0412.2000.079006440.x. [PubMed] [Google Scholar]
- [17].Niklasson A, Albertsson-Wikland K, Continuous growth reference from 24th week of gestation to 24 months by gender, BMC Pediatr 8 (2008) 1–14. doi:10.1186/1471-2431-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Arbuckle TE, Sherman GJ, An analysis of birth weight by gestational age in Canada, Anadian Med. Assoc. J 140 (1989) 157. [PMC free article] [PubMed] [Google Scholar]
- [19].O’Neill KA, Murphy MFG, Bunch KJ, Puumala SE, Carozza SE, Chow EJ, Mueller BA, McLaughlin CC, Reynolds P, Vincent TJ, Von Behren J, Spector LG, Infant birthweight and risk of childhood cancer: international population-based case control studies of 40 000 cases, Int. J. Epidemiol 44 (2015) 153–168. doi:10.1093/ije/dyu265. [DOI] [PubMed] [Google Scholar]
- [20].Samuelsen SO, Bakketeig LS, Tretli S, Johannesen TB, Magnus P, Birth weight and childhood cancer, Epidemiology 20 (2009) 484–487. doi:10.1097/EDE.0b013e3181a7786d. [DOI] [PubMed] [Google Scholar]
- [21].Caughey RW, Michels KB, Birth weight and childhood leukemia: a meta-analysis and review of the current evidence, Int. J. Cancer 124 (2009) 2658–2670. doi:10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- [22].Westergaard T, Andersen PK, Pedersen JB, Olsen JH, Frisch M, Sorensen HT, Wohlfahrt J, Melbye M, Birth characteristics, sibling patterns, and acute leukemia risk in childhood: a population-based cohort study, J Natl Cancer Inst 89 (1997) 939–947. doi:10.1093/jnci/89.13.939. [DOI] [PubMed] [Google Scholar]
- [23].Dahlhaus A, Prengel P, Spector L, Pieper D, Birth weight and subsequent risk of childhood primary brain tumors: An updated meta-analysis, Pediatr. Blood Cancer 64 (2017) e26299. doi:10.1002/pbc.26299. [DOI] [PubMed] [Google Scholar]
- [24].Harder T, Plagemann A, Harder A, Birth weight and subsequent risk of childhood primary brain tumors: A meta-analysis, Am. J. Epidemiol 168 (2008) 366–373. doi:10.1093/aje/kwn144. [DOI] [PubMed] [Google Scholar]
- [25].Yan J, Yin M, Dreyer ZE, Scheurer ME, Kamdar K, Wei Q, Okcu MF, A meta-analysis of MTHFR C677T and A1298C polymorphisms and risk of acute lymphoblastic leukemia in children, Pediatr Blood Cancer 58 (2012) 513–8. doi:10.1002/pbc.23137. [DOI] [PubMed] [Google Scholar]
- [26].Lagiou P, Samoli E, Hsieh CC, Lagiou A, Xu B, Yu GP, Onoyama S, Chie L, Adami HO, Vatten LJ, Trichopoulos D, Williams MA, Maternal and cord blood hormones in relation to birth size, Eur. J. Epidemiol 29 (2014) 343–351. doi:10.1007/s10654-014-9914-3. [DOI] [PubMed] [Google Scholar]
- [27].Nagata C, Iwasa S, Shiraki M, Shimizu H, Estrogen and α-fetoprotein levels in maternal and umbilical cord blood samples in relation to birth weight, Cancer Epidemiol. Biomarkers Prev 15 (2006) 1469–1472. doi:10.1158/1055-9965.EPI-06-0158. [DOI] [PubMed] [Google Scholar]
- [28].Johnson KJ, Carozza SE, Chow EJ, Fox EE, Horel S, Mclaughlin CC, Mueller BA, Puumala SE, Reynolds P, Von Behren J, Spector LG, Parental age and risk of childhood cancer: A pooled analysis, Epidemiology 20 (2009) 475–483. doi:10.1097/EDE.0b013e3181a5a332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Von Behren J, Spector LG, Mueller BA, Carozza SE, Chow EJ, Fox EE, Horel S, Johnson KJ, McLaughlin C, Puumala SE, Ross JA, Reynolds P, Birth order and risk of childhood cancer: a pooled analysis from five US States, Int. J. Cancer 128 (2011) 2709–2716. doi:10.1002/ijc.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Puumala SE, Carozza SE, Chow EJ, Fox EE, Horel S, Johnson KJ, McLaughlin C, Mueller BA, Reynolds P, Von Behren J, Spector LG, Childhood cancer among twins and higher order multiples, Cancer Epidemiol. Biomarkers Prev 18 (2009) 162–168. doi:10.1158/1055-9965.EPI-08-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chow EJ, Puumala SE, Mueller BA, Carozza SE, Fox EE, Horel S, Johnson KJ, McLaughlin CC, Reynolds P, Von Behren J, Spector LG, Childhood cancer in relation to parental race and ethnicity: a 5-state pooled analysis, Cancer 116 (2010) 3045–3053. doi:10.1002/cncr.25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Johnson KJ, Carozza SE, Chow EJ, Fox EE, Horel S, McLaughlin CC, Mueller BA, Puumala SE, Reynolds P, Von Behren J, Spector LG, Birth characteristics and childhood carcinomas, Br. J. Cancer 105 (2011) 1396–1401. doi:10.1038/bjc.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kehm RD, Spector LG, Poynter JN, Vock DM, Osypuck TL, Socioeconomic Status and Childhood Cancer Incidence: A Population-Based Multilevel Analysis, Am. J. Epidemiol (2017) 612–626. doi:10.1093/aje/kwx292/4080179/Cigarette-Smoking-and-Risk-of-Early-Natural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P, International classification of childhood cancer, third edition, Cancer 103 (2005) 1457–1467. doi:10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- [35].Bjorge T, Sorensen HT, Grotmol T, Engeland A, Stephansson O, Gissler M, Tretli S, Troisi R, Fetal Growth and Childhood Cancer: A Population-Based Study, Pediatrics 132 (2013) e1265–e1275. doi:10.1542/peds.2013-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen EJT, Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting, Am. J. Epidemiol 181 (2015) 349–356. doi:10.1093/aje/kwu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tchetgen Tchetgen EJ, Inverse odds ratio-weighted estimation for causal mediation analysis, Stat. Med 32 (2013) 4567–4580. doi:10.1002/sim.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kehm RD, Spector LG, Poynter JN, Vock DM, Altekruse SF, Osypuk TL, Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival?, Cancer In press (2018). [DOI] [PMC free article] [PubMed]
- [39].Gurney JG RL, Severson RK, Scott D, Incidence of Cancer in Children in the United States, Cancer 75 (1995) 2186–2195. [DOI] [PubMed] [Google Scholar]
- [40].Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL, Racial and ethnic differences in survival of children with acute lymphoblastic leukemia, Blood 100 (2002) 1957–1964. doi:10.1182/blood-2002-02-0395.Supported. [DOI] [PubMed] [Google Scholar]
- [41].Schüz J, Luta G, Erdmann F, Ferro G, Bautz A, Simony SB, Dalton SO, Lightfoot T, Winther JF, Birth order and risk of childhood cancer in the Danish birth cohort of 1973– 2010, Cancer Causes Control 26 (2015) 1575–1582. doi:10.1007/s10552-015-0651-z. [DOI] [PubMed] [Google Scholar]
- [42].Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG, Descriptive epidemiology of primary brain and CNS tumors: Results from the Central Brain Tumor Registry of the United States, 1990–1994, Neuro. Oncol 1 (1999) 14–25. doi:10.1093/neuonc/1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Heuch JM, Heuch I, Akslen LA, Kvåle G, Risk of primary childhood brain tumors related to birth characteristics: A Norwegian prospective study, Int. J. Cancer 77 (1998) 498–503. doi:10.1002/(SICI)1097-0215(19980812)77:4<498::AID-IJC4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- [44].Crump C, Sundquist J, Sieh W, Winkleby MA, Sundquist K, Perinatal and Familial Risk Factors for Brain Tumors in Childhood through Young Adulthood, Cancer Res 75 (2015) 576–583. doi:10.1158/0008-5472.CAN-14-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Silva IDS, Swerdlow AJ, Sex differences in the risks of hormone-dependent cancers, Am. J. Epidemiol 138 (1993) 10–28. doi:10.1093/oxfordjournals.aje.a116773. [DOI] [PubMed] [Google Scholar]
- [46].Poynter JN, Pediatric Germ Cell Tumors: Epidemiology of Germ Cell Tumors, First, Springer-Verlag, Heidelberg New York Dordrecht London, 2014. [Google Scholar]
- [47].Ries L. a. G., Smith M. a., Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995., NIH Pub. No. 99–4649 (1999) 179 pp.
- [48].Poynter JN, Amatruda JF, Ross JA, Trends in incidence and survival of pediatric and adolescent patients with germ cell tumors in the United States, 1975 to 2006, Cancer 116 (2010) 612–625. doi:10.1002/cncr.25454.Trends. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rahbari R, Zhang L, Kebebew E, Thyroid cancer gender disparity., Future Oncol 6 (2010) 1771–9. doi:10.2217/fon.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Machens A, Hauptmann S, Dralle H, Disparities between male and female patients with thyroid cancers: Sex difference or gender divide?, Clin. Endocrinol. (Oxf) 65 (2006) 500– 505. doi:10.1111/j.1365-2265.2006.02623.x. [DOI] [PubMed] [Google Scholar]
- [51].Hogan AR, Zhuge Y, Perez EA, Koniaris LG,Lew JI, Sola JE, Pediatric Thyroid Carcinoma: Incidence and Outcomes in 1753 Patients, J. Surg. Res 156 (2009) 167–172. doi:10.1016/j.jss.2009.03.098. [DOI] [PubMed] [Google Scholar]
- [52].Sun T, Plutynski A, Ward S, Rubin JB, An integrative view on sex differences in brain tumors, Cell. Mol. Life Sci 72 (2015) 3323–3342. doi:10.1007/s00018-015-1930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kukurba KR, Parsana P, Balliu B, Smith KS, Zappala Z, Knowles DA, Favé MJ, Davis JR, Li X, Zhu X, Potash JB, Weissman MM, Shi J, Kundaje A, Levinson DF, Awadalla P, Mostafavi S, Battle A, Montgomery SB, Impact of the X chromosome and sex on regulatory variation, Genome Res 26 (2016) 768–777. doi:10.1101/gr.197897.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Spatz A, Borg C, Feunteun J, X-chromosome genetics and human cancer, Nat. Rev. Cancer 4 (2004) 617–629. doi:10.1038/nrc1413. [DOI] [PubMed] [Google Scholar]
- [55].Colaco S, Modi D, Genetics of the human Y chromosome and its association with male infertility, Reprod. Biol. Endocrinol 16 (2018) 14. doi:10.1186/s12958-018-0330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Clocchiatti A, Cora E, Zhang Y, Dotto GP, Sexual dimorphism in cancer, Nat. Rev. Cancer 16 (2016) 330–339. doi:10.1038/nrc.2016.30. [DOI] [PubMed] [Google Scholar]
- [57].Yin Y, Li Y, Qiao H, Wang H, Yang X, Zhang H, Pang X, Zhang Y, Chen W, TSPY is a cancer testis antigen expressed in human hepatocellular carcinoma, Br. J. Cancer 94 (2005) 458–463. doi:10.1038/sj.bjc.6602716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kido T, Fai Y, Lau C, Roles of the Y chromosome genes in human cancers, Asian J. Androl 17 (2015) 373–380. doi:10.4103/1008-682X.150842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bianchi I, Lleo A, Gershwin ME, Invernizzi P, The X chromosome and immune associated genes, J. Autoimmun 38 (2012) J187–J192. doi:10.1016/j.jaut.2011.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


