Abstract
Context:
Pain may be a potentially modifiable risk factor for expensive and burdensome Emergency Department (ED) visits near the end-of-life for older adults with dementia.
Objectives:
To assess the effect of pain and unmet need for pain management on ED visits in the last month of life in older adults with dementia.
Methods:
Mortality follow-back study of older adults with dementia in the National Health and Aging Trends Study (NHATS) who died between 2012–2014, linked to Medicare claims.
Results:
281 NHATS decedents with dementia met criteria (mean age 86, 61% female, 81% white). Fifty-seven percent had at least one ED visit in the last month of life, and 46.5% had an ED visit that resulted in a hospital admission. Almost 3/4 (73%) of decedents experienced pain in the last month of life, and 10% had an unmet need for pain management. After adjustment for age, gender, race, educational attainment, income, comorbidities, and impairment in activities of daily living, pain was not associated with increased ED use in the last month of life (Adjusted Incident Rate Ratio (aIRR 0.87, 95% Confidence Interval (CI) 0.64–1.17). However, decedents with unmet need for pain management had an almost 50% higher rate of ED visits in the last month of life than those without unmet needs (aIRR 1.46, 95% CI 1.07–1.99).
Conclusions:
Among older adults with dementia, unmet need for pain management was associated with more frequent ED visits in the last month of life.
Keywords: Dementia, End-of-Life, Emergency Department, Pain
Introduction
Due to population aging and a lack of curative treatments, the number of older adults with dementia in the United States (U.S.) is growing rapidly. Currently, more than 5 million adults over the age of 65 in the U.S have dementia. This number will expand to 13.8 million by 2050 in the absence of effective treatment.1 Given estimates that 1/3 of all decedents over the age of 65 die either with or from dementia, improving the quality of care at the end-of-life for persons with dementia is a national priority.2,3
Emergency Department (ED) use by older adults increases dramatically near the end-of life.4,5 Some of these visits may be potentially avoidable with improved access to both primary and palliative care.6 Major health policy intiatives now include mandates for the reduction of potentially avoidable ED visits and hospital admissions by older adults with dementia as part of efforts to improve care quality and value.3,7 Achieving these reductions requires targeting and tailoring interventions to older adults with dementia at highest risk for increased utilization. However, knowledge of patterns of ED use and risk factors associated with higher ED utilization in older adults with dementia at the end-of-life is limited.
Pain may be one potentially modifiable risk factor for increased ED visits for older adults with dementia at end-of-life. The clinical course of dementia is notable for increasing pain near the end-of- life.8 Pain in older adults with dementia commonly manifests as behavioral symptoms, such as agitation, that caregivers often find very challenging to manage.9 Overwhelmed caregivers may panic in response to their loved one’s distress and see the ED as the only means of obtaining treatment, especially outside of regular office hours.10
However, little is currently known specifically about the relationship between pain and ED use at the end-of-life for older adults with dementia. Thus, the objectives of this study were to: 1) assess prevalence of pain and unmet need for pain management in the last month of life in older adults dying with dementia; 2) describe ED use in the last month of life among older adults with dementia; and 3) examine whether pain was associated with increased ED use in the last month of life in older adults with dementia.
Methods
Population and Setting
The National Health and Aging Trends Study (NHATS) is a prospective panel study designed to increase knowledge on trends in late-life functioning of older adults in the U.S. Begun in 2011, NHATS draws from a nationally-representative sampling frame of adults aged 65 years and older in the U.S. and includes oversamples of non-Hispanic black persons and older adults (≥90).11,12 NHATS assessments are performed annually in-person in the participant’s place of residence and include verbal questionnaires, performance-based metrics of cognitive and physical functioning, and facility assessments if applicable. Proxy-respondents are interviewed if participants are unable to respond for themselves. In addition, NHATS includes a mortality-follow back survey (last month of life interview) completed by proxy respondents, which includes questions about type and quality of care NHATS participants received in the last month of life.
We selected all NHATS participants with a complete interview in 2011 that died between 2012 and 2014 and had a last month of life interview completed by a proxy familiar with the decedent. We linked data for NHATS decedents with the Center for Medicare and Medicaid Files, including the Medicare Beneficiary Summary File (MBSF) and Medicare Part A and B claims files. Participants who did not have continuous Medicare fee-for-service Part A and B enrollment in the last 2 months of life were excluded because it was not possible to assess individual level healthcare use for these individuals.
Dementia Ascertainment
Decedent’s dementia status was determined using a previously defined and validated algorithm.13 Briefly, this algorithm is based on (1) self or proxy report of a physician’s diagnosis of dementia; (2) scoring on cognitive testing in 3 domains (orientation, memory, and executive function); and (3) AD8 score for those with a proxy-respondent (8-item measure designed for administration to informants for the purpose of identifying the presence of dementia).14,15 NHATS participants are classified as no dementia, possible dementia, and probable dementia. Sensitivity and specificity of this approach for identifying probable dementia are 65.7% and 87.2%, respectively.13 We assigned dementia status based on results in the survey immediately prior to death (i.e. 2012 dementia status if participant died in 2013). The average number of days between the final survey completed by participants and their date of death was 186 days (standard deviation 122 days). We defined our dementia cohort as including only participants with probable dementia. Participants whose dementia classification was missing in the survey prior to death were excluded.
Pain Assessment
Pain was assessed by asking proxy respondents who completed the NHATS last month of life interview questions regarding whether the decedent experienced pain in the last month of life, and if so, if they received the right amount of help in managing their pain. These questions were only asked about decedents who proxies were alert as reported by proxies (i.e. awake and able to communicate at least some of the time) in the last month of life. We excluded decedents who had missing or don’t know/refused response to any questions regarding pain. We created two dichotomous variables from these questions: 1) decedent experienced pain in last month of life (yes/no) and 2) decedent had unmet need for pain management in last month of life (yes/no) if they answered yes to experiencing pain and did not receive the right amount of help with pain management. This definition of unmet need for pain management is based on previous classifications in the literature.16
ED Visits
ED visit information was obtained from Medicare Part A and Part B claims. We assessed the percentage with and mean number of 3 types of ED visits: 1) any ED visit; 2) ED visit outpatient only and; 3) ED visit with a hospital admission. For each category of ED visit examined, we assessed whether decedents had a visit in the last month of life as well as the average number of visits. Using International Classification of Disease, Ninth Revision (ICD-9) primary diagnosis codes, we assessed the top 10 most frequent diagnoses and rates per 100 persons in the last month of life for the any ED visit category. Online Supplement 2 Table 2 includes the ICD-9 codes used to define diagnostic categories. Where there was crossover, we used the Charlson comorbidity index to guide category definition.17 Otherwise we based categories on our clinical judgment.
Other variables
Age at death was determined from the MBSF file. Gender (male/female), race (white/black/Hispanic or other), and median income were extracted from the initial 2011 NHATS interview. Marital status (married or living with partner/widowed, divorced, separated, other) was assessed in the NHATS survey immediately preceding death. Residential care in last month of life was determined from the NHATS last month of life interview. We defined residential care as including nursing homes, assisted living, board and cares, and other facilities that provide services to older adults. Activities of Daily Living (ADLs) were assessed in the NHATS survey immediately preceding death and included bathing, dressing, toileting, eating, transferring, going inside, and going outside. We defined ADL impairment (0/1–2/3+ impairments) as requiring assistance for the activity from another person. Comorbidities were assessed in the survey immediately preceding death and included a count of 10 conditions, including history of heart attack, heart disease, high blood pressure, arthritis, osteoporosis, diabetes, lung disease, stroke, cancer, and broken hip. We assessed whether decedents received hospice services in the last month of life (yes/no) and, if so, hospice length of stay using Medicare claims files. A full description of variables included in our analysis is available in Online Supplement 2 Table 1.
Statistical Analyses
Descriptive statistics were used to describe the sample characteristics and healthcare use. Using a Poisson regression, we examined the association between: 1) Pain and ED visit count in the last month of life; and 2) Unmet need for pain management and any ED visit count (both outpatient only and with inpatient admission) in the last month of life. For each set of models, we present an unadjusted model, a model adjusted for age, gender, race, and a model adjusted for age, gender, race, educational attainment, income, comorbidities, and ADL impairment. Incident rate ratio (IRR) and 95% confidence intervals (CI) were estimated for all models. All reported analyses, with the exception of ED visit diagnosis, were weighted for the differential probability of selection and took into account the complex survey design of NHATS. Weights from the survey immediately prior to death were used. Taylor linearization was used for variance estimation. Statistical analyses were conducted using Stata software, version 15 (Stata Corp., College Station, TX) and SAS software, version 9.4 (SAS Institute, Inc., Cary, NC). This study was approved by the University of California Institutional Review Board, the NHATS Data Confidentiality Committee, and Centers for Medicare & Medicaid Services Privacy Board.
Results
Between 2012–2014, 1,081 NHATS participants died and had a complete last month of life interview, of whom 736 (68.1%) had fee-for-service Medicare Parts A and B in the last 2 months of life. We excluded 17 (2.3%) decedents who had dementia status missing in the interview prior to death, and 72 (9.8%) decedents who had missing data on pain in the last month of life, either because they were not alert or proxy respondents answered don’t know or refused. Of the remaining 647 decedents, 291 were categorized as having dementia (38.8%). We excluded an additional 10 decedents because proxies were not familiar with their daily routines for a final sample of 281 decedents with dementia (Online Supplement 1 Figure).
Characteristics of the dementia decedent cohort are reported in Table 1. The average age at death was 86.3 (±7.8). The majority were female (61.3%), white (80.5%), high school graduates (50.5%), and just over 1/3 were married (36.3%). Median income was $21,000. Just over 1/3 (36.6%) were living in residential care in the last month of life. Seventy percent of decedents had more than 3 ADL impairments in the survey prior to death and just over a half had 4 or more comorbidities (52.3%). Almost 3/4 of respondents reported that the decedent had pain in the last month of life (73.1%) and approximately 10% had an unmet need for pain management. More than half (56.6%) of decedents with dementia visited the ED in the last month of life. The mean number of ED visits was 0.75. Only a small proportion (14.4%) of decedents had outpatient-only ED visits that did not result in a hospital admission. About half (50.7%) received hospice during the last month of life, with a mean length of stay in hospice of 14.7 days.
Table 1.
Characteristics of NHATS Decedents with Dementia 2011–2014 (n=281)
| Demographic | n (%)a |
|---|---|
| Age at death in years, mean (SD) | 86.3 (7.8) |
| Female gender | 174 (61.3) |
| Race | |
| White | 186 (80.5) |
| Black | 74 (10.2) |
| Hispanic/other | 21 (9.3) |
| Education | |
| Less than high school diploma | 125 (36.4) |
| High school graduate | 119 (50.5) |
| Bachelor’s or higher | 37 (13.1) |
| Median income $ (IQR) | 21,000 (23,000) |
| Marital status | |
| Married or living with partner | 85 (36.3) |
| Widowed/divorced/ separated/never married | 196 (63.7) |
| Living in residential care in last month of life | 86 (36.6) |
| Functional (ADL) Impairments | |
| 0 | 33 (11.9) |
| 1–2 | 54 (18.4) |
| 3+ | 194 (69.7) |
| Comorbidities | |
| 0–1 | 35 (12.8) |
| 2–3 | 98 (34.9) |
| 4+ | 148 (52.3) |
| Pain | |
| Pain in last month of life | 203 (73.1) |
| Unmet need for pain management in last | 28 (10.3) |
| ED Visits | |
| Any ED visit | 151 (56.6) |
| Any ED visit, mean (SD) | 0.75 (0.76) |
| Outpatient only ED visit | 40 (14.4) |
| Outpatient only ED visit, mean (SD) | 0.17 (0.45) |
| ED visit with hospital admission | 126 (46.5) |
| ED visit with hospital admission, mean (SD) | 0.57 (0.68) |
| Hospice Use | |
| Any hospice use | 151 (50.7) |
| Hospice length of stay in days, mean (SD) | 14.7 (11.9) |
Unless otherwise noted
Abbreviations: ADL=Activities of Daily Living; ED=Emergency Department; IQR=Interquartile range; NHATS=National Health and Aging Trends Study; SD=standard deviation
There was no association between pain in the last month of life and ED visits (Table 2) (Adjusted IRR (aIRR) 0.87, 95% CI 0.64–1.17). However, unmet need for pain management increased ED visit rate by almost 50% (aIRR 1.46, 95% CI 1.07–1.99 after adjustment for age, gender, race, educational attainment, income, comorbidities, and ADL impairment. Figure 1 shows the percentage of participants who had 0, 1, or 2+ ED visits by whether they had unmet need for pain management or not. A higher percentage of participants with unmet need for pain management had one (46% versus 39%) or multiple ED visits (26% versus 16%) compared to those without unmet need for pain management. The top 10 most frequent diagnoses for any ED visit were: 1) septicemia; 2) cardiac arrest; 3) pneumonia and other respiratory disease; 4) malignancy; 5) congestive heart failure; 6) cerebrovascular disease; 7) cystitis and other urological infections; 8) hip and other bone fractures; 9) stomach/intestinal disorders; 10) fluid and electrolyte disturbances.
Table 2.
Unadjusted and Adjusted Incident Rate Ratio of Emergency Department (ED) Visits in Last Month of Life in NHATS Decedents with Dementia by Pain Status (n=281)
| Mean number of ED visits in last month of life (SD) |
Unadjusted IRR (95% CI) |
Adjusted IRRb (95% CI) |
Adjusted IRRc (95% CI) |
|
|---|---|---|---|---|
| Pain in last month of life | ||||
| no | 0.80 (0.77) | Reference | Reference | Reference |
| yes | 0.72 (0.75) | 0.90 (0.64–1.25) | 0.87 (0.64–1.17) | 0.86 (0.63–1.17) |
|
Unmet need for pain management in last month of life |
||||
| no | 0.71 (0.75) | Reference | Reference | Reference |
| yes | 1.02 (0.78) | 1.42 (0.99–2.05) | 1.48 (1.10–2.03)a | 1.46 (1.07–1.99)a |
p<0.05
adjusted for age, gender, race
adjusted for age, gender, race, educational attainment, income, comorbidities, and impairment in activities of daily living
Abbreviations: CI=Confidence Interval; IRR=Incident Rate Ratio; NHATS=National Health and Aging Trends Study; SD=standard deviation
Figure 1. Percentage of NHATS Decedents with Dementia (n=281) with 0, 1, or 2+ Emergency Department Visits in the Last Month by Pain Management Status.
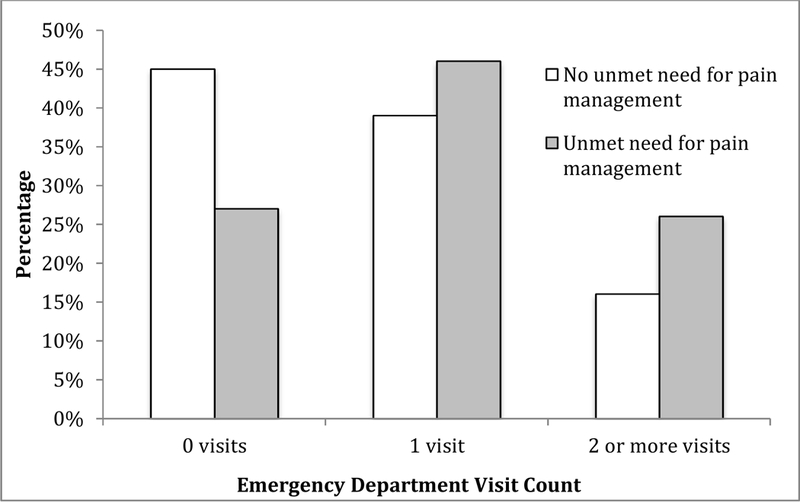
NHATS=National Health and Aging Trends Study
Discussion
We found that almost 3/4 of decedents with dementia experienced pain in the last month of life, the majority visited the ED at least once, and most of these ED visits resulted in a hospital admission. Although prevalence of unmet need for pain management was low (10%), it was associated with an almost 50% higher rate of ED visits in the last month of life than those without unmet need. While many of the most frequent ED visit diagnoses were acutely painful, pain itself was not a top reason for ED visits. These findings suggests that persons with dementia in pain may be at increased risk for ED visits for other conditions, some of which may layer acute on chronic pain (e.g. fracture on osteoarthritis pain). Overall, our results point to a complex interplay between pain and declining health at the end-of-life, and highlight the importance of the quality of pain management on ED use and subsequent hospitalizations at the end-of-life for older adults with dementia.
Our results suggest that interventions aimed at improving pain management for older adults with dementia at the end-of-life may aid in reducing ED visit rates and hospitalizations. Expanding access to hospice care for older adults with dementia, with its focus on pain and symptom management, may be one tactic for improving pain management and reducing ED use and hospitalizations at the end-of-life. Numerous studies that have found that enrollment in and longer length of stay in hospice is associated with lower ED use and hospitalizations.4,18–20 However, although hospice use by persons with dementia has increased dramatically in the past 20 years21, access is still limited. Only half of decedents with dementia in our study received hospice during the last month of life and for most this was only during the last two weeks of life.
Access to hospice for older adults with dementia is currently hindered by strict eligibility requirements for hospice based on prognosis, which is more difficult to determine in dementia compared to other terminal diseases.22 Loosening hospice eligibility criteria and changing payment mechanisms to reflect needs rather than prognosis is one potential strategy to addressing this issue.23 Integrating consultative palliative care services into primary care, nursing home settings, and other models of care— such as home-based primary care—may also improve pain management and reduce ED use at the end-of- life for older adults with dementia.24–26 For example, early palliative care consults with nursing home residents with dementia was associated with an almost 12% reduction in ED visits in the last 30 days of life.24 However, palliative care for people with dementia is in early stages of development, and more work is needed to develop palliative care models and programs tailored to the unique needs of people with dementia near the end-of-life.
ED-based interventions to reduce hospital admissions for older adults with dementia near the end- of-life may be another way to reduce the burdens on patients, families, and the healthcare system. The fact that we found that most ED visits resulted in a hospital admission underscores the high potential for possible reductions in hospital admissions coming from the ED. Novel strategies, such as palliative care consults in the ED and ED rooms reserved for actively dying patients are promising interventions that have been found to reduce hospital admissions and lengths of stay, ameliorate symptom burden, and increase care quality ratings by patients and families.27,28 However, avoiding ED visits in the first place through investment in community-based palliative care, hospice, and supportive models of care that can address pain, symptoms, and other palliative care needs still provides the most leverage for reducing hospital admissions in the last month of life.
Several limitations of this study are noted. The main limitation was the small sample size of individuals with unmet need for pain management. Although our findings did reach statistical significance for the effect of unmet need for pain management on ED use in the adjusted model, this result should be interpreted with caution given the small sample size and the fact that Poisson regression tends to underestimate variance. However, these data provide important preliminary evidence and estimates of effect size that can inform future studies with larger sample sizes, which will become available with longer follow-up times for the NHATS cohort. Second, our study only included individuals with fee-for- service Medicare. Characteristics and healthcare patterns of older adults enrolled in Managed Medicare Organizations—which now comprise about 1/3 of all Medicare beneficiaries and is increasing---may differ.29 Third, we cannot determine the direction of causality based on our data. Unmet need for pain management may prompt patients to seek relief for pain in the ED, but ED visits and hospitalizations could involve painful procedures or prolonged bedrest that may contribute to pain. Furthermore, because our study did not include a control group, we cannot compare our findings to populations without dementia. We opted to focus on a dementia only cohort due to challenges in creating an accurately matched control group and because the assessment and treatment of pain in dementia varies substantially from those without dementia. Finally, pain assessment in the last month of life relied on retrospective report by proxy respondents. Although studies have demonstrated that proxies are able to reliably report pain on behalf of older adults with dementia30, there remains the possibility of proxy-response and recall bias.
In conclusion, addressing the needs of older adults with dementia at the end-of-life is a critical issue facing society today. Our findings show that ED use by older adults with dementia is very high in the last month of life, and most ED visits result in a hospital admission. While the presence of pain was not associated with increased ED use in decedents with dementia, preliminary results are strongly suggestive that unmet need for pain management increases ED use. Despite limitations of sample size, our results indicate that improving pain management in older adults with dementia may result in reduced ED visits and hospital admissions in the last month of life. Enhancing access to hospice and palliative care for older adults with dementia is one potential strategy for achieving these patient-centered outcomes.
Supplementary Material
Table 3.
Top 10 Primary Diagnoses and Rates for Any Emergency Department Visit in the Last Month of Life for NHATS decedents with dementia 2011–2014 (n=281)
| Rank | ICD-9 Primary Diagnosis | Rate per 100 persons |
|---|---|---|
| 1 | Septicemia | 6.4 |
| 2 | Cardiac arrest | 5.0 |
| 3 | Pneumonia and other respiratory disease | 4.3 |
| 4 | Malignancy | 4.0 |
| 5 | Congestive heart failure | 4.0 |
| 6 | Cerebrovascular disease | 3.6 |
| 7 | Cystitis and other urological infections | 2.9 |
| 8 | Hip and other bone fractures | 2.9 |
| 9 | Stomach/intestinal disorders | 2.5 |
| 10 | Fluid and electrolyte disturbances | 2.1 |
Abbreviations: ICD=International Classification of Disease; NHATS=National Health and Aging Trends Study; Not adjusted for survey weights
Acknowledgements
Funding:
This work was supported by the National Institute of Health [NINR F31 NR015380; NIA K76 AG054862] and the VA Quality Scholars Program. The funding source had no role in the study design, data collection, analysis and interpretation of data, writing of the report, or the decision to submit the article for publication
We would like to acknowledge Dr. Joan Teno for her input on data analysis.
Footnotes
Conflicts of Interest
None of the authors report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19): 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015; 11(3):332–384. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. National Plan to Address Alzheimer’s Disease: 2017 Update. Available from: https://aspe.hhs.gov/report/national-plan-address-alzheimers-disease-2017-update. Accessed May 17, 2018.
- 4.Smith AK, McCarthy E, Weber E, et al. Half of older Americans seen in emergency department in last month of life; most admitted to hospital, and many die there. Health Aff (Millwood). 2012;31(6): 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Z, Coots LA, Kaganova Y, et al. Hospital and ED use among medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff (Millwood). 2014;33(4):683–690. [DOI] [PubMed] [Google Scholar]
- 6.Ouslander JG, Berenson RA. Reducing unnecessary hospitalizations of nursing home residents. N Engl J Med. 2011;365(13):1165–1167. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington, D.C. Available from: https://aspe.hhs.gov/report/national-plan-address-alzheimers-disease-2017-update. Accessed May 17, 2018.
- 8.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16): 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husebo BS, Ballard C, Sandvik R, et al. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillet M, Chassagne A, Aubry R. Dying in hospital: Qualitative study among caregivers of terminally ill patients who are transferred to the emergency department. Presse Med. 2018. [DOI] [PubMed] [Google Scholar]
- 11.Montaquila J, Freedman VA, Edwards B, et al. National Health and Aging Trends Study Round 1 Sample Design and Selection 2012. NHATS Technical Paper #1. Baltimore: Johns Hopkins University School of Public Health; Available at http://www.NHATS.org. Accessed May 17, 2018 [Google Scholar]
- 12.Kasper J, Freedman VA. 2017. National Health and Aging Trends Study User Guide: Rounds 1–6 Final Release. Baltimore, MD: Johns Hopkins University School of Public Health; Available at http://www.NHATS.org. Accessed May 17, 2018. [Google Scholar]
- 13.Kasper JD, Freedman VA, Spillman B. Classification of Persons by Dementia Status in the National Health and Aging Trends Study 2013. Technical Paper #5. Baltimore: Johns Hopkins University School of Public Health; Available at http//:www.NHATS.org Accessed May 17, 2018 [Google Scholar]
- 14.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. [DOI] [PubMed] [Google Scholar]
- 15.Galvin JE, Roe CM, Xiong C, et al. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942–1948. [DOI] [PubMed] [Google Scholar]
- 16.Teno JM, Freedman VA, Kasper JD, et al. Is Care for the Dying Improving in the United States? J Palliat Med. 2015;18(8):662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9- CM and ICD-10 administrative data. Med Care. 2005;43(11): 1130–1139. [DOI] [PubMed] [Google Scholar]
- 18.Zuckerman RB, Stearns SC, Sheingold SH. Hospice Use, Hospitalization, and Medicare Spending at the End of Life. J Gerontol B Psychol Sci Soc Sci. 2016;71(3):569–580. [DOI] [PubMed] [Google Scholar]
- 19.Obermeyer Z, Clarke AC, Makar M, et al. Emergency Care Use and the Medicare Hospice Benefit for Individuals with Cancer with a Poor Prognosis. J Am Geriatr Soc. 2016;64(2):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley AS, Deb P, Du Q, et al. Hospice enrollment saves money for Medicare and improves care quality across a number of different lengths-of-stay. Health Aff (Millwood). 2013;32(3):552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller SC, Lima JC, Mitchell SL. Hospice care for persons with dementia: The growth of access in US nursing homes. Am J Alzheimers Dis Other Demen. 2010;25(8):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell SL, Kiely DK, Hamel MB, et al. Estimating prognosis for nursing home residents with advanced dementia. JAMA. 2004;291(22):2734–2740. [DOI] [PubMed] [Google Scholar]
- 23.Wallace CL. Hospice Eligibility and Election:Does Policy Prepare Us to Meet the Need? J Aging Soc Policy. 2015;27(4):364–380. [DOI] [PubMed] [Google Scholar]
- 24.Miller SC, Lima JC, Intrator O, et al. Specialty Palliative Care Consultations for Nursing Home Residents With Dementia. J Pain Symptom Manage. 2017;54(1):9–16.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchie C, Andersen R, Eng J, et al. Implementation of an Interdisciplinary, Team-Based Complex Care Support Health Care Model at an Academic Medical Center: Impact on Health Care Utilization and Quality of Life. PLoS One. 2016;11(2):e0148096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens CE, Hunt LJ, Bui N, et al. Palliative Care Eligibility, Symptom Burden, and Quality-of- Life Ratings in Nursing Home Residents. JAMA Intern Med. 2018; 178(1): 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang DH. Beyond Code Status: Palliative Care Begins in the Emergency Department. Ann Emerg Med. 2017;69(4):437–443. [DOI] [PubMed] [Google Scholar]
- 28.Rojas E, Schultz R, Linsalata HH, et al. Implementation of a Life-Sustaining Management and Alternative Protocol for Actively Dying Patients in the Emergency Department. J Emerg Nurs. 2016;42(3):201–206. [DOI] [PubMed] [Google Scholar]
- 29.Friedman B, Jiang HJ, Steiner CA, et al. Likelihood of hospital readmission after first discharge: Medicare Advantage vs. fee-for-service patients. Inquiry. 2012;49(3):202–213. [DOI] [PubMed] [Google Scholar]
- 30.Neumann PJ, Araki SS, Gutterman EM. The use of proxy respondents in studies of older adults: lessons, challenges, and opportunities. J Am Geriatr Soc. 2000;48(12):1646–1654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


