Abstract
Growing evidence suggests that chronic low back pain (CLBP) is associated with pain sensitization, and that there are sex and race disparities in CLBP. Given the sex and race differences in pain sensitization, this has been hypothesized as a mechanism contributing to the sex and race disparities in CLBP. This study examined sex and race differences in pain sensitization among patients with CLBP as well as the role of catastrophizing as a potential mediator of those differences. We found that compared to males, females required less pressure to produce deep muscle pain and rated mechanical punctate pain as more painful. Compared to non-Hispanic White (NHW) patients, Black patients demonstrated greater pain sensitivity for measures of deep muscle hyperalgesia and mechanical punctate pain. Furthermore, catastrophizing partially mediated the race differences in deep muscle pain such that Black participants endorsed greater pain catastrophizing, which partially accounted for their increased sensitivity to, and temporal summation of, deep muscle pain. Taken together, these results support the need to further examine the role of catastrophizing and pain sensitization in the context of sex and race disparities in the experience of CLBP.
Perspective:
This study identifies sex and race differences in pain sensitization among patients with CLBP. Further, it recognizes the role of catastrophizing as a contributor to such race differences. More research is needed to further dissect these complex relationships.
Keywords: chronic low back pain, sex, race, pain sensitization, QST
INTRODUCTION
Chronic low back pain (CLBP), one of the most common complaints seen by physicians, is a primary cause of disability in the United States and is associated with between $100 and $200 billion in annual costs1, 8, 43, 63, 72. Although some patients with CLBP may have identifiable etiologies, for many there are no discernable mechanisms to account for their pain and dysfunction9. There is growing evidence that like other chronic pain conditions, idiopathic CLBP involves changes in the central nervous system that result in pain augmentation2, 66, 70. Indeed, compared to healthy, pain-free individuals, patients with CLBP show increased sensitivity to a variety of mechanical, thermal, and chemical noxious stimuli32, 46, 53, 58. Providing further support for the relationship between central pain augmentation and changes in the central nervous system, Giesecke and colleagues found that patients with persistent back pain demonstrated hyperalgesia at a CLBP-unaffected anatomic site compared to healthy controls, and this increased pain was associated with increased neuronal activation in multiple cortical areas involved in pain processing33.
Despite consistently high rates of chronic low back pain across the population, there are sex and race disparities in the prevalence and experience of low back pain. Compared to males, there is a greater prevalence of CLBP among females1, 52. Females with back pain also report greater pain intensity than males26. Although the prevalence of CLBP is higher among Whites compared to Blacks1, 52, Black individuals with low back pain report greater pain intensity and worse functional disability7, 68. In addition, there are well-documented sex and race differences in pain sensitization among healthy pain-free individuals; quantitative sensory testing (QST) studies have shown that women (relative to men) and Black participants (relative to non-Hispanic white participants) demonstrate lower pain tolerance and threshold for experimental noxious stimuli3, 6, 11, 14, 27–29, 34, 49, 51, 62, 64, 65, 76. Sex and race differences in the experience of CLBP may be related to sex and race differences in pain sensitivity, however, no studies have yet examined these inter-relationships among patients with CLBP.
Psychosocial factors influence pain experiences both inside and outside the laboratory, and cognitive-emotional processes such as pain-related catastrophizing appear to play an influential role in shaping individual differences in the trajectory of chronic pain symptoms. Patients with chronic low back pain catastrophize more in response to pain than healthy, pain-free individuals57, and CLBP patients who are high in catastrophizing are at greater risk for worsening pain and disability81. Like the sex and race differences in the experience of back pain, there are sex and race differences in pain catastrophizing29, 49–51. Indeed, females and Black individuals catastrophize more in response to pain than do males and Whites, respectively. In addition, catastrophizing has been shown to mediate sex differences in pain sensitivity in both healthy individuals and those with chronic pain as well as race differences in pain sensitivity among healthy, pain-free individuals20, 44, 51, 75, 76. That is, greater catastrophizing is associated with increased pain sensitivity among females and Black individuals compared to males and Whites, respectively. However, these relationships have not been studied in patients with CLBP. The current study aimed to examine sex and race differences in pain sensitization among patients with CLBP and to examine the role of catastrophizing as a potential mediator of those putative group differences. We hypothesized that (1) females and Black individuals would demonstrate greater pain sensitivity and endorse greater levels of pain catastrophizing than males and Whites, respectively; (2) these differences would be independent of one another, not interactive; and (3) catastrophizing would mediate the assumed sex and race differences in pain sensitivity.
METHODS
Participants and Design
Participants were 324 Black and non-Hispanic White (NHW) adults with idiopathic chronic low back pain. They were recruited by email advertising, web and bulletin board announcements in Boston, MA, as well as electronic medical records-based databases. Study inclusion criteria included: the presence of low back pain for at least six months (confirmed by both patient selfreport and electronic medical record review), average pain intensity ratings ≥ 3/10, and low back pain as the primary (i.e., most intense) pain complaint. Potential participants were excluded for pregnancy, severe cognitive impairment, Raynauds Disease, sickle cell anemia, documented neuropathy, a myocardial infarction within the past year, and cancer.
Upon arriving at the laboratory, participants provided informed consent. They then completed a series of questionnaires that assessed demographics, pain ratings, depression, and catastrophizing. After completing questionnaires, participants underwent quantitative sensory testing (QST). Participants were then debriefed and compensated. All procedures were approved by the Brigham and Women’s Hospital institutional review board.
Measures
The Beck Depression Inventory-II (BDI-II) is a 21-item self-report measure of depression. Participants rate the severity with which they have experienced each symptom in the past two weeks from 0 (not present) to 3 (severe). The BDI-II has been validated in patients with chronic pain38.
The Pain Catastrophizing Scale (PCS) is a 13-item self-report measure of cognitive and emotional responses to pain comprised of rumination, magnification, and helplessness54. Participants rated how frequently they endorsed various thoughts and emotions related to pain from 0 (not at all) to 4 (all the time). The PCS has strong criterion-related, concurrent, and discriminant validity15, 54, 55.
Participants used a 0 (“no pain”) to 100 (“most intense pain imaginable”) numeric rating scale to rate their low back pain at the beginning of the laboratory session24, 40. They also provided an average low back pain rating using an 11-point numeric rating scale (NRS) ranging from 0 (no pain) to 10 (worst pain imaginable) for the past week. The NRS is validated for use in chronic pain research25, 39.
Quantitative Sensory Testing
Mechanical pain was assessed using weighted pinprick stimulators17, 21, 23. The lowest-force stimulator that produced a sensation of pain (128 or 256mN for most subjects) was used to apply a train of 10 stimuli to the skin on the dorsum of the hand at the rate of 1 per second. Participants rated the painfulness of the first, fifth, and tenth stimulus as well as painful after-sensations 15 seconds following the final stimulus using a 0–100 numeric rating scale (“no pain” to “most intense pain imaginable”)18, 22. Temporal summation was calculated by subtracting the pain rating of the first stimulus from that of the tenth. The first, fifth, and tenth pain ratings were also averaged to determine the mean pain intensity rating for the probes.
Response to deep pressure pain was ascertained via cuff pressure algometry (CPA)19, 41, 47, 59. Tonic, deep-tissue, mechanical stimulation was applied using a Hokanson rapid cuff inflator. Using a standard blood pressure cuff wrapped comfortably around the gastrocnemius muscle, pressure was increased at approximately 5 mmHg/s. Participants provided pain ratings every 5 seconds until an intensity level of 40/100 pain was reached. At this point the cuff was deflated. After a recovery period, the cuff was inflated to the previously identified pressure needed to produce 40/100 pain and was maintained at this level for 2 minutes. Participants provided pain intensity ratings (0–100) upon inflation and every 30 seconds for the entire 2 minutes. The participants then provided ratings for any painful after sensations 15 seconds following cuff deflation. Temporal summation was calculated by subtracting the initial pain rating from the pain rating at the 120 second time point. The mean pain intensity rating for the pressure cuff was calculated by averaging the pain ratings from the 30, 60, 90, and 120 second marks.
Data Analysis
We examined sex and race differences among demographic variables (e.g., education, employment) using a series of independent samples t-tests and Chi Square analyses. Further, because the potential impact of socioeconomic status on QST outcomes, we conducted a series of analyses of variance (ANOVAs) examining education and employment status differences among all QST variables. Associations between QST responses, clinical pain, catastrophizing, depression, and age were assessed using Pearson correlations. To examine sex and race differences in pain catastrophizing and QST responses, we conducted a series of Analyses of covariance (ANCOVAs).We then used Preacher and Hayes’ bootstrapping procedure and the SPSS Process Macro60, 61 to conduct a series of bias-corrected bootstrapped mediation analyses using 10,000 bootstrapped resamples. This bootstrapping procedure is nonparametric and does not assume the indirect effects are normally distributed. To examine the role of catastrophizing as a potential mediator of the race differences in QST responses, we created mediation models for each QST outcome variable for which there were race differences and an association with catastrophizing Mediation models were considered significant if zero was not contained within the 95% confidence intervals60, 61. All participant characteristic variables that were significantly correlated with QST outcomes at the p<.05 level were included in ANCOVA and mediation models. Given the number of analyses conducted, to reduce the rate of Type I error, we used a Bonferroni correction for all ANCOVA models (.05/8 = .006).
RESULTS
Participant Characteristics
The sample consisted of 324 participants (47% female, 27% Black; see Table 1) approximately 46 years of age with an average pain at the time of the visit at 46/100. Compared to females, males were older (t316 = −2.79, p<.01), more likely to use opioid pain medications [X2(1) = 36.43, p<.01), and less likely to have a college education [X2(1) = 7.19, p<.05; see Table 2]. There were no significant sex differences in current pain ratings, average low back pain severity, depressive symptoms, catastrophizing, education, or employment. The distribution of sex did not differ significantly between races [X2(1) = 3.42, n.s.]. Compared to NHW participants, Black participants were older (t323 = −2.49, p<.05), endorsed greater depressive symptoms (t323 = −4.07, p<.01) and catastrophizing (t323 = −8.74, p<.01), and were more likely to use opioid pain medications [X2 (1) = 10.01, p<.01], to have less education [X2(1) = 20.90, p<.01], and to be unemployed [X2(1) = 7.38, p<.01].
Table 1.
Participant Characteristics
| Characteristics | n (%) |
|---|---|
| Age, mean (SD) | 45.8 (11.8) |
| Pain at Visit, mean (SD) | 45.5 (22.2) |
| Low Back Pain in Last Week, mean (SD) | 5.5 (1.9) |
| Depression, mean (SD) | 10.9 (9.1) |
| Catastrophizing, mean (SD) | 18.0 (11.9) |
| Opioid Use | |
| Using Opioids | 109 (34) |
| Not Using Opioids | 213 (66) |
| Sex | |
| Male | 170 (53) |
| Female | 149 (47) |
| Race | |
| White | 234 (73) |
| Black | 85 (27) |
| Education | |
| Less than College Graduate | 143 (44) |
| College Graduate or Above | 181 (56) |
| Employment | |
| Employed | 112 (35) |
| Unemployed | 212 (65) |
Table 2.
Race and Gender Differences in Participant Characteristics
| Male | Female | X2/t | White | Black | X2/t | |
|---|---|---|---|---|---|---|
| Sex, n (%) | 3.43 | |||||
| Male | 102 (69) | 47 (31) | ||||
| Female | 132 (78) | 38 (22) | ||||
| Age, mean (SD) | 48 (10) | 44 (13) | −2.79** | 45 (12) | 49 (11) | −2.49* |
| Pain at Visit, mean (SD) | 45 (22) | 46 (22) | 0.21 | 44 (22) | 49 (23) | −1.48 |
| Low Back Pain in Last Week, mean (SD) | 5.5 (1.8) | 5.5 (2.1) | −0.21 | 5.4 (1.9) | 5.7 (1.9) | −0.75 |
| Depression, mean (SD). | 11 (9) | 10 (9) | −1.27 | 10 (9) | 14 (10) | −4.07** |
| Catastrophizing, mean (SD) | 18 (11) | 18 (12) | −0.25 | 15 (11) | 27 (11) | −8.74** |
| Opioid Use, n (%) | 36.43** | 10.01** | ||||
| Using Opioids | 73 (50) | 30 (18) | 68 (29) | 41 (48) | ||
| Not Using Opioids | 74 (50) | 140(82) | 166 (71) | 44 (52) | ||
| Education, n (%) | 7.19** | 20.90** | ||||
| Less than College Graduate | 77 (52) | 63 (37) | 87 (37) | 56 (65) | ||
| College Graduate or Above | 71 (48) | 107(63) | 151 (63) | 30 (35) | ||
| Employment, n (%) | 0.42 | 7.38** | ||||
| Employed | 100(68) | 109(64) | 166(70) | 46(54) | ||
| Unemployed | 48 (32) | 61 (36) | 72 (30) | 40 (46) | ||
p<.05
p<.01
Associations Between Participant Characteristics, Catastrophizing, and QST Responses
Results of Pearson correlations indicated that older age was associated with greater temporal summation of deep muscle pressure pain (r=.135; p<.05). Opioid use was associated with requiring greater pressure to produce moderate deep muscle pressure pain (r=.129; p<.05) as well as lower pain intensity ratings for mechanical punctate probes (r=−.126; p<.05). Catastrophizing was associated with requiring less cuff inflation to produce moderate pain (r=−.262) as well as higher ratings for pressure pain aftersensations (r=.233), higher pain intensity ratings for mechanical punctate probes (r=.183), and greater temporal summation for both deep muscle pain (r=.209) and mechanical punctate pain (r=.166; ps<.01; see Table 3). Pain ratings at the time of the study visit were associated with greater temporal summation for mechanical punctate pain (r=.161; p<.01), while average low back pain severity over the last week was associated with greater deep muscle pain intensity ratings (r =.159) and pressure pain painful after sensations (r=.147; ps<.05). Pain and depression were not otherwise associated with any QST outcomes. Results of a series of ANOVAs examining education and employment status differences in QST outcomes indicated that unemployed participants required less pressure to produce deep muscle pressure pain (t322 = −2.27, p<.05) and provided greater pain ratings for deep muscle pain aftersensations (t154 = 2.22, p<.05) and mechanical punctate probes (t168 = 2.29, p<.05).
Table 3.
Pearson Correlations Between Clinical Outcomes and QST Measurements
| Cuff MMHG |
Mean Cuff Intensity Ratings |
Cuff Temporal Summation |
Cuff Aftersensations |
Mean Probe Intensity Ratings |
Probe Temporal Summation |
Probe Aftersensations |
Catastrophizing | |
|---|---|---|---|---|---|---|---|---|
| Age | −.035 | −.020 | .135* | −.037 | −.041 | .005 | −.089 | .126* |
| Opioid Use | .129* | −.070 | −.015 | −.039 | −.126* | −.081 | .028 | .223** |
| Pain At Visit |
−.104 | .011 | −.062 | .055 |
.088 |
.161** | .099 | .313** |
| Mean Low Back Pain in Last Week |
−.084 | .159* | .050 | .147* | .133 | .096 | .111 | .374** |
| Depression | −.053 | −.056 | .006 | .131 | .077 | .086 | −.003 | .601** |
| Catastrophizing | −.262** | .126 | .209** | .233** | .183** | .166** | .118 | 1.00 |
p<.05
p<.01
Race and Sex Differences in QST Responses
Results of a series of 2 (race) by 2 (sex) analyses of covariance (ANCOVAs) are provided below. Results indicated that there were main effects of sex for mechanical punctate painful aftersensations ( no covariates included) and inflation pressure needed to produce moderate pain (controlling for opioid use and employment status). Compared to males, female participants rated aftersensations from the mechanical punctate stimulus as more painful (F1,314 = 24.48, η2=.07) and required less cuff inflation pressure to produce moderate levels of pain (F1, 310 = 25.24, η2=.08; see Table 4). Furthermore, there were significant effects of race for all QST variables except painful aftersensations from the pressure cuff. That is, compared to NHW participants, Black participants provided higher pain intensity ratings for mechanical punctate probes (F1,310 = 7.02; η2 =.11; controlling for opioid use), deep muscle pressure pain (F1,315 = 7.94, η2=.03; no covariates), and aftersensations for mechanical punctate (F1,314 = 25.68,η2=.08; no covariates) pain. Black participants demonstrated greater temporal summation for both mechanical punctate pain (F1,313 = 11.03, η2=.03; controlling for pain at the time of visit) and deep muscle pressure pain (F1,314 = 11.53, η2=.04; controlling for age) compared to NHW participants. Compared to NHW participants, Black participants required less cuff inflation pressure to produce moderate levels of pain (F1,310 = 8.22, η2=.03; controlling for opioid use and employment status). Black participants also endorsed greater catastrophizing than NHW participants (F1,309 = 52.01, η2=.14; controlling for opioid use, age, pain at the time of visit, and depression). There were no sex by race interactions (ps>.05).
Table 4.
Significant Sex and Race Differences in QST Outcomes
| QST Measure | Mean (SD) | |
|---|---|---|
| Cuff mmHg | Male | 174 (60) |
| Female | 135 (55) | |
| White | 158 (57) | |
| Black | 141 (68) | |
| Mean Cuff Pain Intensity | White | 40 (14) |
| Black | 45 (15) | |
| Cuff Temporal Summation | White | 7 (18) |
| Black | 16 (21) | |
| Mean Probe Pain Intensity | White | 22 (15) |
| Black | 34 (21) | |
| Probe Temporal Summation | White | 14 (15) |
| Black | 22 (24) | |
| Probe Aftersensations | Male | 4 (6) |
| Female | 8 (14) | |
| White | 4 (8) | |
| Black | 11 (17) | |
| Catastrophizing | White | 15(11) |
| Black | 26(11) | |
Mediation
Mediation analyses for sex differences in QST variables were not conducted because there were no sex differences in catastrophizing. The role of catastrophizing as a potential mediator of the race differences in QST variables was assessed. Results of the analyses indicated that controlling for sex, opioid use, and employment status, catastrophizing accounted for 14% of the variance in the amount of pressure required to produce moderate deep muscle pain and significantly mediated the relationship between race and cuff inflation pressure such that Blacks endorsed greater catastrophizing which was associated with requiring less pressure to produce moderate deep muscle pain (see Figure 1). Controlling for age, catastrophizing accounted for 5% of the variance in deep muscle pain temporal summation and partially mediated the relationship between race and deep muscle pain temporal summation such that Black participants engaged in more catastrophizing which was associated with greater temporal summation of deep muscle pain (see Figure 2). Catastrophizing was not a significant mediator of the relationships between race and painful aftersensation ratings, mean pain ratings, or temporal summation of mechanical punctate pain (all CIs include 0).
Figure 1.
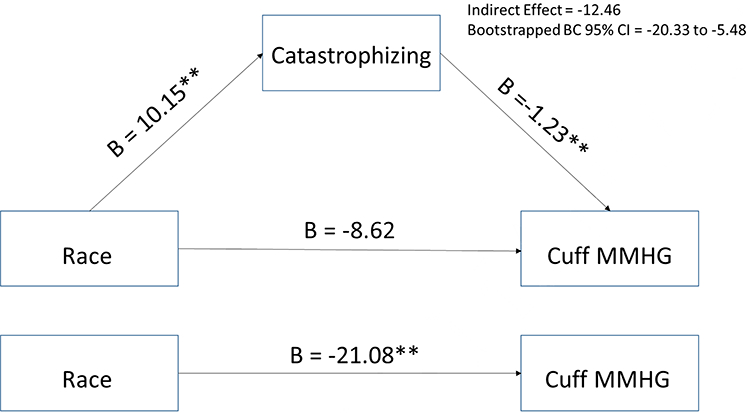
The mediating effect of catastrophizing in the relationship between race and mmHg needed to produce moderate deep muscle pain controlling for sex, opioid use, and employment status.
**p<.01
Figure 2.
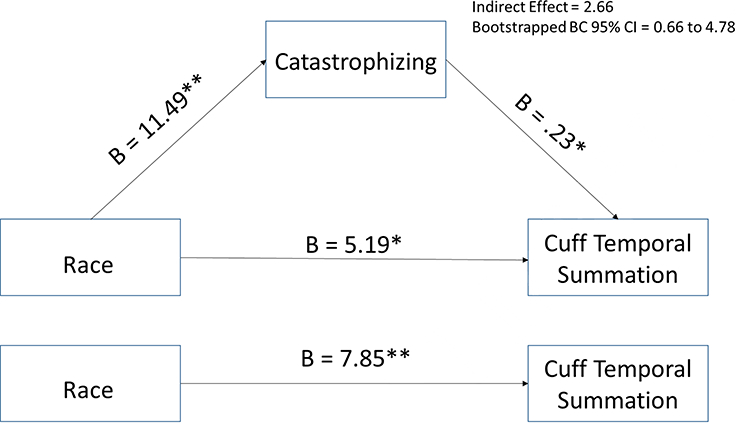
The mediating effect of catastrophizing in the relationship between race and cuff temporal summation controlling for age.
*p<.05; **p<.01
DISCUSSION
Growing evidence suggests that CLBP is associated with pain sensitization2, 66, 70, and that there are sex and race disparities in CLBP1, 7, 26, 52, 68. Given the sex and race differences in pain sensitization, this has been hypothesized as a mechanism contributing to the sex and race disparities in CLBP. This study examined sex and race differences in pain sensitization among patients with CLBP as well as the role of catastrophizing as a potential mediator of those differences. As expected, we found that compared to NHWs, Blacks demonstrated greater pain sensitivity for several measures of deep muscle hyperalgesia as well as mechanical punctate pain. However, there were only sex differences in two measures of pain sensitivity; compared to males, females required less pressure to produce deep muscle pain and rated aftersensations of mechanical punctate pain as more painful. Collectively, the present study suggests that, among patients with chronic pain, race differences in suprathreshold mechanical pain sensitivity are at least as robust as sex differences, which is consistent with prior reports56.
The identified race differences across various deep muscle pain and mechanical punctate pain measures were consistent with our hypotheses as well as the current literature. According to a recent meta-analysis, Black individuals demonstrate a lower pain tolerance and provide higher pain intensity ratings across experimental stimuli compared to their NHW counterparts45. Specifically, Black participants report higher pain ratings for mechanical punctate pain compared to non-Hispanic NHWs. However, the evidence for race differences in pressure pain sensitivity has been mixed, with some studies suggesting no race differences while others indicate that Black individuals demonstrated a lower threshold for pressure pain45. It is important to note, however, that previous studies have used a standard pressure algometer to induce pressure pain. Our study is novel in that it used cuff pressure algometry to identify such differences.
In addition to race differences across QST outcomes, we also found race differences in opioid use. There are mixed findings in the literature with regard to race differences in opioid use. While there are well-documented disparities in the prescription of opioids37, results from 1999–2012 NHANES data suggest there are no differences in opioid use between Black and non-Hispanic White individuals30. In our sample, Black participants were more likely to be using prescription opioid medications. Perhaps the race differences in opioid prescribing seen in other parts of the country can be explained, in part, by the link between race and socioeconomic status. Indeed, patients from poorer areas are less likely to receive opioids42. However, Boston is a relatively affluent city with minorities having a median income above the poverty line79. Thus, opioid prescribing patterns may differ as a result.
While we anticipated sex differences across experimental measures, females in this study differed from males on only two measures of pain sensitization: pressure required to produce moderate deep muscle pain and pain intensity ratings for mechanical punctate stimuli. The sex difference in pressure to produce deep muscle pain is aligned with previous findings indicating that males consistently demonstrate a higher pressure pain threshold and tolerance compared to females36, 65. Likewise, our findings replicate research from a pain-free sample demonstrating that females provide higher pain intensity ratings for mechanical punctate pain67. However, we did not find sex differences in temporal summation or aftersensation ratings for mechanical punctate pain, which is inconsistent with previous literature examining healthy pain-free individuals67. It is possible that the general pain sensitization experienced across patients with CLBP eliminated any sex differences that are present within the general, pain-free population.
We also sought to examine the role of catastrophizing as a potential mediator of sex and race differences in pain sensitization. Previous studies have suggested that catastrophizing mediates observed sex and race differences in experimental pain responses among healthy individuals49, 51, 75, 77 as well as the sex differences in clinical arthritis pain44. In the current study, there were no sex differences in catastrophizing. However, there were notable race differences in catastrophizing with Black participants endorsing greater levels of catastrophizing than NHWs. Further, higher levels of pain catastrophizing were associated with less pressure required to produce moderate deep muscle pain, greater temporal summation and aftersensations of deep muscle pain, and greater pain ratings and temporal summation for mechanical pain ratings across all participants. This is consistent with Taub and colleagues’ findings suggesting that catastrophizing is associated with central sensitization of pain among patients with CLBP78. We also found that catastrophizing accounted for the race differences in deep muscle pain. That is, Black participants endorsed greater pain catastrophizing which partially accounted for their requiring less pressure to produce deep muscle pain as well as greater pain ratings and temporal summation for deep muscle pain.
Catastrophizing, a passive coping strategy involving magnification, rumination, and helplessness cognitions, is thought to augment pain perception through increased attention to painful stimuli as well as a greater emotional response to pain12, 13, 31. Indeed, neuroimaging research has demonstrated that catastrophizing is associated with altered anticipatory brain responses48 and with amplified pain-related activity in the dorsolateral prefrontal, insula, rostral anterior cingulate, premotor, and parietal cortices during mild pain, areas associated with the attentional and affective aspects of pain35, 69. Taken together with our findings, this may help explain the race differences observed in the experience of CLBP. It is possible that Black individuals’ increased frequency of catastrophizing results in enhanced pain sensitization through attentional and emotional response mechanisms. This, in turn, may be associated with the greater pain intensity and worse functional disability experienced by Black patients with CLBP7, 68.
Our results have important clinical implications. The association between catastrophizing and poor pain outcomes is well documented in the literature80. However, according to the communal coping model of catastrophizing, individuals may engage in catastrophizing to secure social or interpersonal resources, to alter the expectations of others, reduce performance demands, or manage interpersonal conflict73, 74. As different cultural groups exhibit varying degrees of collectivist orientation10, catastrophizing may be more subject to social reinforcement in particular groups, which could contribute to racial/ethnic differences in catastrophizing. Despite the goal of increasing social support, there is evidence that catastrophizing is not only associated with greater pain and worse physical functioning16, 71, but is also associated with perceived punishing responses from significant others4, 5. Thus, it may be important to not only reduce catastrophic thinking to improve pain outcomes but to also provide social skills such that patients can secure social support and assistance from others. Future studies of group differences in catastrophizing may benefit from also assessing social support.
Several limitations should be considered when interpreting these findings. First, we examined only two (mechanical) modalities of quantitative sensory testing: cuff pressure algometry and punctate pain. Although these testing modalities were sufficient to detect race and sex differences in pain sensitization, they are not comprehensive. Thus, future studies should attempt to replicate these findings using other experimental modalities (e.g., heat, pain, cold pain, conditioned pain modulation). Additionally, this study was cross-sectional and thus we cannot identify causal relationships between the variables of interest. Future studies should consider a longitudinal approach to examine baseline race differences in catastrophizing and future development of pain sensitization. Furthermore, we were not able to investigate other aspects of catastrophizing such as its bi-directional association with the social environment such as social support. Finally, we did not have data regarding potential selection biases in our sample, or regarding patients’ pain impact, workers’ compensation status, comorbidity, previous treatment history, or sleep limiting the generalizability of our findings to other samples. Future studies should consider these important patient characteristics in their analyses of sex and race differences in CLBP.
Despite these limitations, our study provides new insights into the race and sex differences in the experience of CLBP. We found both race and sex differences in deep muscle hyperalgesia as well as mechanical punctate pain such that Black participants and females experience greater pain sensitization compared to NHW participants and males, respectively. Further, we found that Black participants’ greater degree of pain-related catastrophizing partially accounts for their increased sensitivity for deep muscle hyperalgesia. The present findings support the need to further examine the role of catastrophizing and sensitization in the context of race and sex disparities in the experience of CLBP.
Highlights.
We examined race and sex differences in pain sensitivity in patients with CLBP.
There were race and sex differences in deep muscle and mechanical punctate pain.
Catastrophizing contributed to race differences in deep muscle pain.
Further research is needed to better understand these complex relationships.
Acknowledgments
Disclosures: This work was supported by grants from the National Institutes of Health under award numbers R21 DA0441020 and T32 AR055885. The authors have no conflicts of interest.
Footnotes
Author Contributions:
All authors have made substantial contributions to the conception and design of this manuscript, have assisted in drafting and revising the manuscript, and have read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 354:581–585, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci 15:1117–1119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartley EJ, King CD, Sibille KT, Cruz-Almeida Y, Riley JL, Glover TL, Goodin BR, Sotolongo AS, Herbert MS, Bulls HW. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res. (Hoboken). 68:472–480, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boothby J, Thorn B, Overduin L, Ward L. Catastrophizing and perceived partner responses to pain. Pain. 109:500–506, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Buenaver LF, Edwards RR, Haythornthwaite JA. Pain-related catastrophizing and perceived social responses: Interrelationships in the context of chronic pain. Pain. 127:234–242, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell C, Edwards R, Fillingim R. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 113:20–26, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Carey TS, Garrett JM. The relation of race to outcomes and the use of health care services for acute low back pain. Spine (Phila Pa 1976). 28:390–394, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention: Impact of arthritis and other rheumatic conditions on the health-care system--United States, 1997 In: Morbidity and mortality weekly report, 1999, pp. 349–353. [PubMed] [Google Scholar]
- 9.Chou R, Deyo RA, Jarvik JG. Appropriate use of lumbar imaging for evaluation of low back pain. Radiol. Clin. North Am 50:569–585, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Coon H, Kemmelmeier M. Cultural orientations in the United States (re) examining differences among ethnic groups. Journal of Cross-Cultural Psychology. 32:348–364, 2001 [Google Scholar]
- 11.Creamer P, Lethbridge-Cejku M, Hochberg MC. Determinants of pain severity in knee osteoarthritis: effect of demographic and psychosocial variables using 3 pain measures. J. Rheumatol 26:1785–1792, 1999 [PubMed] [Google Scholar]
- 12.Crombez G, Eccleston C, Baeyens F, Eelen P. When somatic information threatens, catastrophic thinking enhances attentional interference. Pain. 75:187–198, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Crombez G, Eccleston C, Van den Broeck A, Van Houdenhove B, Goubert L. The effects of catastrophic thinking about pain on attentional interference by pain: no mediation of negative affectivity in healthy volunteers and in patients with low back pain. Pain Research and Management. 7:31–39, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Almeida Y, Sibille KT, Goodin BR, Petrov ME, Bartley EJ, Riley JL, King CD, Glover TL, Sotolongo A, Herbert MS. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheum. 66:1800–1810, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Eon J, Harris CA, Ellis JA. Testing factorial validity and gender invariance of the pain catastrophizing scale. J. Behav. Med 27:361–372, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Edwards R, Cahalan C, Mensing G, Smith M. Pain, catastrophizing, and depression in the rheumatic diseases. Nat. Rev. Rheumatol 7:216–225, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Edwards R, Dolman A, Michna E, Katz J, Nedeljkovic S, Janfaza D, Isaac Z, Martel M, Jamison R, Wasan A. Changes in pain sensitivity and pain modulation during oral opioid treatment: the impact of negative affect. Pain Med. 17:1882–1891, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards R, Grace E, Peterson S, Klick B, Haythornthwaite J, Smith M. Sleep continuity and architecture: Associations with pain-inhibitory processes in patients with temporomandibular joint disorder. European Journal of Pain. 13:1043–1047, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards RR, Dolman AJ, Martel MO, Finan PH, Lazaridou A, Cornelius M, Wasan AD. Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMCMusculoskelet. Disord 17:284, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: differential effects for daily pain versus laboratory-induced pain. Pain. 111:335–341, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Edwards RR, Mensing G, Cahalan C, Greenbaum S, Narang S, Belfer I, Schreiber KL, Campbell C, Wasan AD, Jamison RN. Alteration in pain modulation in women with persistent pain after lumpectomy: influence of catastrophizing. J. Pain Symptom Manage. 46:30–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards RR, Wasan AD, Bingham CO, Bathon J, Haythornthwaite JA, Smith MT, Page GG. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res. Ther 11:R61, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, Jamison RN. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. The Journal of Pain. 12:953–963, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekblom A, Hansson P. Pain intensity measurements in patients with acute pain receiving afferent stimulation. J. Neurol. Neurosurg. Psychiatry 51:481–486, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 94:149–158, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Fillingim R, Doleys D, Edwards R, Lowery D. Clinical characteristics of chronic back pain as a function of gender and oral opioid use. Spine (Phila Pa 1976). 28:143–150, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Fillingim R, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 75:121–127, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Fillingim RB: Sex, Gender, and Pain. Fillingim RB (Ed.), IASP Press, Seattle, 2000. [Google Scholar]
- 29.Forsythe LP, Thorn B, Day M, Shelby G. Race and Sex Differences in Primary Appraisals, Catastrophizing, and Experimental Pain Outcomes. The Journal of Pain. 12:563–572, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Frenk SM, Porter KS, Paulozzi L: Prescription opioid analgesic use among adults: United States, 1999–2012, US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, 2015. [Google Scholar]
- 31.Geisser M, Robinson M, Keefe F, Weiner M. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain. 59:79–83, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Giesbrecht J, Battie M. A Comparison of Pressure Pain Detection Thresholds in People With Chronic Low Back Pain and Volunteers Without Pain. Physical therapy. 85:1085–1092, 2005 [PubMed] [Google Scholar]
- 33.Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 50:613–623, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Glover T, Goodin B, Horgas A, Kindler L, King C, Sibille K, Peloquin C, Riley J, Staud R, Bradley L. Vitamin D, race, and experimental pain sensitivity in older adults with knee osteoarthritis. Arthritis Rheum. 64:3926–3935, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gracely R, Geisser M, Giesecke T, Grant M. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 127:835–843, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Graven-Nielsen T, Vaegter HB, Finocchietti S, Handberg G, Arendt-Nielsen L. Assessment of musculoskeletal pain sensitivity and temporal summation by cuff pressure algometry: a reliability study. Pain. 156:2193–2202, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kaloukalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Med. 4:277–294, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Harris CA, Joyce L. Psychometric properties of the Beck Depression Inventory-(BDI-II) in individuals with chronic pain. PAIN®. 137:609–622, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J. Pain Symptom Manage. 41:1073–1093, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Jensen M, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986 [DOI] [PubMed] [Google Scholar]
- 41.Jespersen A, Dreyer L, Kendall S, Graven-Nielsen T, Arendt-Nielsen L, Bliddal H, Danneskiold-Samsoe B. Computerized cuff pressure algometry: A new method to assess deep-tissue hypersensitivity in fibromyalgia. Pain. 131:57–62, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Joynt M, Train MK, Robbins BW, Halterman JS, Caiola E, Fortuna RJ. The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the United States. J. Gen. Intern. Med 28:1604–1610, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. JBJS. 88:21–24, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Keefe F, Lefebvre J, Egert J, Affleck G, Sullivan M. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 87:325–334, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Kim HJ, Yang GS, Greenspan JD, Downton KD, Griffith KA, Renn CL, Johantgen M, Dorsey SG. Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. Pain. 158:194–211, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Laursen BS, Bajaj P, Olesen AS, Delmar C, Arendt-Nielsen L. Health related quality of life and quantitative pain measurement in females with chronic non-malignant pain. European Journal of Pain. 9:267–267, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Lemming D, Graven-Nielsen T, Sörensen J, Arendt-Nielsen L, Gerdie B. Widespread pain hypersensitivity and facilitated temporal summation of deep tissue pain in whiplash associated disorder: an explorative study of women. J. Rehabil. Med 44:648–657, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Loggia M, Berna C, Kim J, Cahalan C, Martel M. The lateral prefrontal cortex mediates the hyperalgesic effects of negative cognitions in chronic pain patients. The Journal of Pain. 16:692–699, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meints SM, Hirsh AT. In vivo praying and catastrophizing mediate the race differences in experimental pain sensitivity. The Journal of Pain. 16:491–497, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Meints SM, Miller MM, Hirsh AT. Differences in pain coping between Black and White Americans: A meta-analysis. The Journal of Pain. 17:642–653, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meints SM, Stout M, Abplanalp S, Hirsh AT. Pain-Related Rumination, But Not Magnification or Helplessness, Mediates Race and Sex Differences in Experimental Pain. The Journal of Pain. 18:332–339, 2017 [DOI] [PubMed] [Google Scholar]
- 52.Meucci RD, Fassa AG, Faria NMX. Prevalence of chronic low back pain: systematic review. Rev. Saude Publica. 49:73–73, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. European Journal of Pain. 11:415–420, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J. Behav. Med 23:351–365, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J. Behav. Med 20:589–605, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Ostrom C, Bair E, Maixner W, Dubner R, Fillingim RB, Ohrbach R, Slade GD, Greenspan JD. Demographic predictors of pain sensitivity: results from the OPPERA study. The Journal of Pain. 18:295–307, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Owens MA, Bulls HW, Trost Z, Terry SC, Gossett EW, Wesson-Sides KM, Goodin BR. An examination of pain catastrophizing and endogenous pain modulatory processes in adults with chronic low back pain. Pain Med. 17:1452–1464, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters ML, Schmidt AJ. Differences in pain perception and sensory discrimination between chronic low back pain patients and healthy controls. J. Psychosom. Res 36:47–53, 1992 [DOI] [PubMed] [Google Scholar]
- 59.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Spatial and temporal aspects of deep tissue pain assessed by cuff algometry. Pain. 100:19–26, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput 36:717–731,2004 [DOI] [PubMed] [Google Scholar]
- 61.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 40:879–891, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Rahim-Williams FB, Riley JL, Herrera D, Campbell CM, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. 129:177–184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ricci JA, Stewart WF, Chee E, Leotta C, Foley K, Hochberg MC. Back pain exacerbations and lost productive time costs in United States workers. Spine (Phila Pa 1976). 31:3052–3060, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Riley JL, Cruz-Almeida Y, Glover TL, King CD, Goodin BR, Sibille KT, Bartley EJ, Herbert MS, Sotolongo A, Fessler BJ. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. The Journal of Pain. 15:272282, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riley JL, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 74:181–187, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Structural brain changes in chronic pain reflect probably neither damage nor atrophy. PLoS One. 8:e54475, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. Pain. 109:115–123, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Selim A, Fincke G, Ren X, Deyo R, Lee A, Skinner K, Kazis L. Racial differences in the use of lumbar spine radiographs: Results from the veterans health study. Spine (Phila Pa 1976). 26:1364–1369, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Seminowicz D, Davis K. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 120:297–306, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Seminowicz D, Wideman T, Naso L. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci 31:7540–7550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin. J. Pain 17:165–172, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 290:2443–2454, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Sullivan M, Adams H, Sullivan M. Communicative dimensions of pain catastrophizing: social cueing effects on pain behaviour and coping. Pain. 107:220–226, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Sullivan M, Martel M, Tripp D, Savard A. The relation between catastrophizing and the communication of pain experience. Pain. 122:282–288, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Sullivan M, Tripp D, Rodgers W. Catastrophizing and pain perception in sport participants. J. Appl. Sport Psychol 12:151–167, 2000 [Google Scholar]
- 76.Sullivan M, Tripp D, Santor D. Gender differences in pain and pain behavior: the role of catastrophizing. Cognitive Ther Res. 24:121–134, 2000 [Google Scholar]
- 77.Sullivan MJL, Tripp DA, Santor D. Gender differences in pain and pain behavior: The role of catastrophizing. Cognit. Ther. Res 24:121–134, 2000 [Google Scholar]
- 78.Taub CJ, Sturgeon JA, Johnson KA, Mackey SC, Darnall BD. Effects of a pain catastrophizing induction on sensory testing in women with chronic low back pain: A pilot study. Pain Research and Management. 2017, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.United States Census Bureau: 2016 American Community Survey 1-Year Estimates, United States Census Bureau, 2016. [Google Scholar]
- 80.Wertli MM, Burgstaller JM, Weiser S, Steurer J, Kofmehl R, Held U. Influence of catastrophizing on treatment outcome in patients with nonspecific low back pain: a systematic review. Spine (Phila Pa 1976). 39:263. [DOI] [PubMed] [Google Scholar]
- 81.Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, Weiser S. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. 14:2639–2657, 2014 [DOI] [PubMed] [Google Scholar]


