Abstract
Objective:
To evaluate the relationship between radiographic fundal fluid cap in the lateral internal auditory canal, pre-operative clinical characteristics, and post-operative outcomes in patients with vestibular schwannoma (VS) who underwent microsurgical excision.
Study Design:
Retrospective chart review
Setting:
Academic tertiary referral center
Patients:
36 consecutive patients (mean age 49.4 years (range 29–74)) who underwent microsurgical VS excision.
Interventions:
Microsurgical excision
Main outcome measures:
Linear fundal fluid size and tumor size calculated using volumetric analysis were measured on pre-operative MR imaging, and correlated to hearing status and post-operative facial nerve function.
Results:
Mean fundal fluid size was 2.18 mm (range 0 – 7.32). Mean tumor volume was 5.58 cm3 (range, 0.210 – 40.3 cm3). Short- and long-term post-operative House Brackmann scores were 2.4 and 1.4, respectively. Fundal fluid size was associated with tumor volume (rs = 0.488, p = 0.003) but not pre-operative hearing status (p = 0.333). The presence of fundal fluid and larger tumor volumes were statistically associated with poorer short-term and long-term post-operative facial nerve function (p < 0.05).
Conclusions:
Radiographic fundal fluid size is correlated to tumor volume.
Introduction
The first surgical resection of a vestibular schwannoma (VS) was performed by Ballance in 1895.1 Since that time, the morbidity and mortality has decreased as microsurgical and imaging techniques have been developed. Many predictive factors have been studied in an effort to prognosticate post-operative outcome, including tumor size, pre-operative hearing status, etc.2,3 Cerebrospinal fluid in the lateral internal auditory canal (IAC) or a “fundal fluid cap” on pre-operative magnetic resonance imaging (MRI) has emerged as a variable in multiple studies as important in predicting clinical outcomes, especially the likelihood of successful hearing preservation in small intracanalicular VS.4,5 However, conflicting evidence has emerged from recent studies on the importance of a radiographic fundal fluid cap.5–7 In particular, a recent study suggested that fundal fluid size was not only unrelated to the likelihood of achieving hearing preservation during microsurgery, but also its presence was associated with linear tumor size.6 In addition, in our experience and in concordance with reports by others, a fundal fluid cap demonstrated on pre-operative MRI is seldom seen in the lateral IAC intraoperatively.8 These observations are also consistent with the finding that nearly all VSs originate in the lateral internal auditory canal (IAC) associated with Scarpa’s ganglia.9,10 These observations call into question the prognostic value of a fundal fluid cap.
Previous studies of the clinical significance of the fundal fluid cap were limited to patients who underwent middle cranial fossa (MCF) approach for smaller intracanalicular tumors or retrosigmoid approach where the lateral IAC could not be fully exposed.5,11 While this cohort allowed for assessment of hearing preservation, a translabyrinthine (TL) approach offers the optimal exposure of the lateral IAC for detailed intraoperative examination, and is used on a wider range of tumor sizes. Therefore, we sought to extend our previous findings on fundal fluid size to patients with a wider spectrum of tumor sizes, including those who underwent TL excision. In this study, we examine the relationship between tumor volume, linear fundal fluid distance, and clinical outcomes, including facial nerve outcomes.
Materials and Methods
Subjects
Institutional review board approval was obtained for this study. A retrospective case series analysis was conducted at a tertiary academic referral center. Inclusion criteria were microsurgical excision via either MCF or TL approach, histopathologic confirmation of schwannoma, and volumetric determination of preoperative tumor volume by a neuroradiologist. Exclusion criteria included NF 2, recurrent tumor, and schwannoma arising from a nerve other than VIII. Although it is not typical in our institution to routinely measure preoperative tumor volume, between 2009 and 2014 36 patients met these inclusion and exclusion criteria and their clinical, audiometric, and radiographic data were compiled in a database. Selection of surgical approach was based on patient factors, tumor size, and pre-operative hearing status in the affected ear. In accordance with our institutional practice patients 65 years of age or younger who presented with serviceable hearing and tumor not contacting the brainstem were offered microsurgical excision via the MCF approach with intent for hearing preservation.12,13 Patients electing for microsurgical resection whose tumors contacted the brainstem or were older than 65 years in age with evidence of tumor growth underwent TL resection. Facial nerve function was assessed using the House-Brackmann (HB) scale by Otolaryngologists on the study team at both short-term (mean 9.1 days) and long-term (mean 36.6 months) follow-up.14
Volumetric calculations and tumor characteristics
Volumetric analysis of tumor size was calculated from postcontrast axial T1 sequences on preoperative MRI scans using Vitrea software (version 6.6.2, Vital Images, Toshiba Medical Systems, Minnetonka, MN). This protocol has been previously described and used by Bathla et al.15 Briefly, a 3-D volumetric model was constructed by completing manual segmentation of axial contrast enhanced images (slice thickness 3 mm, interslice gap 0.3 mm) on all sections followed by computerized summation. Post-volumetric data was reanalyzed to ensure accuracy of measurements. When present, fundal fluid size was determined by measuring the length of the CSF space from the lateral edge of tumor to the IAC fundus on axial, highly T2-weighted, thin cut images, such as Fast Imaging Employing Steady-State Acquisition (FIESTA) or Constructive Interface in Steady State (CISS) sequences. When fundal fluid was captured on multiple slices, the slice with the largest fundal fluid cap was used. Picture Archiving and Communication Systems (PACS) was used to calculate linear distances, and less than 1.5 mm was considered no fundal fluid.
Audiometry
Pure tone audiometry (PTA) thresholds and word recognition score (WRS) were extracted from pre-operative audiologic evaluations. PTA thresholds were averaged from air conduction thresholds at 0.5, 1, 2, and 3 kHz. When 3kHz was not available, the mean between thresholds at 2 and 4kHz were used.16
Statistical Analysis
Statistical analysis was performed using SPSS 24, Inc., (Chicago, IL). Non-parametric analyses were completed with Spearman correlation and Wilcoxon-Mann-Whitney. Fisher’s exact test was used for analysis of small categorical data. All tests were considered statistically significant with p < 0.05.
Results
Demographic and clinical characteristics are shown in Table 1. The study population included 23 (63.9%) females and 13 (36.1%) males, with an average age of 49.4 years (range 29–74). Presenting signs and symptoms were hearing loss (78%), tinnitus (42%), disequilibrium (42%), headaches (6%), and facial weakness (3%). The tumor originated from the inferior vestibular nerve 33% of the time, from the superior vestibular nerve 16.7% of the time, and the origin was indeterminate 50% of the time. Mean tumor volume was 5.58 cm3 (range, 0.210–40.3 cm3). Mean tumor volume was 2.52 cm3 (range 0.210 – 2.82 cm3) for patients who underwent an MCF approach compared to 8.03 cm3 (range 0.241 – 40.3 cm3) for patients who underwent a TL approach. Mean fundal fluid size was 2.18 mm (range, 0–7.32 mm). MCF and TL approaches were performed in 44% and 56% of patients, respectively.
Table 1:
Summary of clinical, demographic, and imaging characteristics
| n = 36 | |
|---|---|
| Mean age (range), years | 49.4 (29–74) |
| Gender, n (%) | |
| Female | 23 (63.9%) |
| Male | 13 (36.1%) |
| Clinical | |
| Hearing loss | 78% |
| Tinnitus | 42% |
| Disequilibrium/Imbalance | 42% |
| Headaches | 6% |
| Facial weakness (pre-op HB weakness) | 3% |
| Tumor: | |
| Right (%) | 19 (52.8%) |
| Left (%) | 17 (47.2%) |
| Nerve of tumor origin: IVN/SVN/unknown | 12/6/18 |
| Mean tumor size (range), cm3 | 5.58 (0.210–40.34) |
| Mean fundal fluid size (range), mm | 2.18 (0–7.32) |
| Surgical approach: | |
| Translabyrinthine (%) | 20 (56%) |
| Middle cranial fossa (%) | 16 (44%) |
| Post-operative House-Brackmann score | |
| Short-term (SD) | 2.4 (1.7) |
| Long-term (SD) | 1.4 (0.8) |
Abbreviations: IVN, inferior vestibular nerve; SVN, superior vestibular nerve; HB, House-Brackmann
Association of clinical, demographic, and radiographic findings with the presence of fundal fluid are shown in Table 2. Figure 1 illustrates a tumor with a large (6.33 mm) fundal fluid cap. Comparing cohorts, larger tumor volume and male gender were associated with the presence of fundal fluid (p = 0.004 and p = 0.005, respectively, Table 2). As illustrated in a scatterplot of the data fundal fluid size was positively correlated with tumor volume on pre-operative MRI rs = 0.488 (p = 0.003) (Fig. 2). The scatterplot also highlights that even with this fairly robust correlation between tumor size and fundal fluid cap, there is significant heterogeneity in the relationship of these variables. Some small tumors had larger fluid caps while some large tumors had negligible fluid caps. The presence of fundal fluid was not associated with age, tumor laterality, nerve of tumor origin, pre-operative PTA, pre-operative SDS, or surgical approach (p > 0.05). Larger tumor volume was associated with a higher pre-operative PTA (p = 0.036) but not pre-operative SDS, age, gender, or tumor laterality (p > 0.05). (Table 3).
Table 2.
– Univariate association of clinical and demographic factors as a function of the presence of fundal fluid
| Characteristic | No fluid (n=17) | Any fluid (n=18) | pa |
|---|---|---|---|
| Age in year, mean(SD) | 49.24(13.71) | 49.56(13.76) | 0.96 |
| Gender, n(%) | |||
| Male | 2(11.76) | 11(61.11) | |
| Female | 15(88.24) | 7(38.89) | 0.005 |
| Laterality, n(%) | |||
| Right | 10(58.82) | 9(50) | |
| Left | 7(41.18) | 9(50) | 0.74 |
| Preoperative PTA, mean(SD) | 51.84(23.55) | 47.42(22.86) | 0.63 |
| Preoperative SDS, mean(SD) | 49.88(41.74) | 65.78(37.85) | 0.18 |
| Tumor volume (cm3), mean(SD) |
4.51(11.41) | 6.74(8.56) | 0.004 |
| Tumor origin, n(%) | |||
| IVN | 7(41.18) | 5(27.78) | |
| SVN | 5(29.41) | 1(5.56) | |
| Unknown | 5(29.41) | 12(66.67) | 0.06 |
| Surgical approach, n(%) | |||
| TL | 9(52.94) | 10(55.56) | |
| MCF | 8(47.06) | 8(44.44) | >0.99 |
Abbreviations: std, standard deviation; PTA, pure tone average on the affected side; SDS, speech discrimination score on the affected side; IVN, inferior vestibular nerve; SVN, superior vestibular nerve; TL, translabyrinthine; MCF, middle cranial fossa
Wilcoxon–Mann–Whitney test for continuous variables and Fisher’s exact test for categorical variables.
Figure 1.

A) Pre-operative representative Constructive Interface Steady-State (CISS) 3D axial image demonstrating a ‘fundal fluid cap’ (white arrow) from the lateral aspect of the right cerebellopontine angle mass extending to the fundus of the IAC B) Pre-operative post-contrast coronal image demonstrating a right enhancing cerebellopontine angle mass (white arrow) used to calculate total tumor volume.
Figure 2.

Scatter plot demonstrating a positive correlation of tumor volume on pre-operative MRI with a radiographic fundal fluid cap. Spearman correlation (rs)=0.488, p<0.003
Table 3.
– Correlation of clinical and demographic findings with radiographic tumor volume
| Rho1 | p | |
|---|---|---|
| Age | −0.109 | 0.528 |
| Female | −0.320 | 0.057 |
| Laterality – left | 0.083 | 0.630 |
| Pre-operative PTA | 0.351 | 0.036 |
| Pre-operative SDS | −0.274 | 0.106 |
PTA, pure tone audiogram; SDS, speech discrimination score
Spearman correlation
For patients who underwent microsurgical excision via an MCF approach, fundal fluid distance was not correlated with post-operative PTA rs = 0.078 (p = 0.792) and SDS rs = −0.056 (p = 0.850). Similarly, tumor volume was not correlated with post-operative PTA rs = 0.369 (p = 0.195) and SDS rs = −0.038 (p = 0.896).
Mean House-Brackmann score immediately post-operatively was 2.4 (SD 1.7) and at the most recent follow-up was 1.4 (SD 0.8). Six patients had a post-operative HB score of VI immediately post-operatively, which subsequently improved to a II-IV long-term post-operatively. Univariate analysis demonstrated that the presence of fundal fluid and larger total tumor volume were associated with poorer facial nerve outcome (HB III-VI) in the short post-operative period (mean 9.1 days) (Table 4). The presence of fundal fluid was associated with a poorer facial nerve outcome (HB III-IV) in the long-term (mean 36.6 months). When the cohort was analyzed by surgical approaches independently, the presence of fundal fluid was associated with a poorer short-term post-operative facial nerve outcome (p = 0.011) in the TL cohort, but there was no association (p > 0.05) in the MCF cohort between the presence of fundal fluid and post-operative facial nerve outcome, either short-term or long-term. The MCF subpopulation is smaller and insufficiently powered to specifically study this relationship.
Table 4:
Descriptive statistics of the study population in comparison with post-operative facial nerve outcomes
| Early post-operative House- Brackmann score |
pa | Most recent post-operative House-Brackmann score |
pa | |||
|---|---|---|---|---|---|---|
| I - II (n=23) | III - VI (n=13) | I - II (n=32) | III - IV (n=4) | |||
| Age in year, mean(SD) | 51.78(14.02) | 45.31(11.4) | 0.13 | 49.94(13.5) | 45.5(13.03) | 0.67 |
| Gender, n(%) | ||||||
| Male | 7(30.43) | 6(46.15) | 10(31.25) | 3(75) | ||
| Female | 16(69.57) | 7(53.85) | 0.47 | 22(68.75) | 1(25) | 0.12 |
| Laterality, n(%) | ||||||
| Right | 13(56.52) | 6(46.15) | 16(50) | 3(75) | ||
| Left | 10(43.48) | 7(53.85) | 0.73 | 16(50) | 1(25) | 0.61 |
| Preoperative PTA, mean(SD) | 48.54(20.32) | 53.13(27.65) | 0.72 | 48.74(21.4) | 61.88(34.77) | 0.51 |
| Preoperative SDS, mean(SD) | 62.96(37.66) | 45.85(43.65) | 0.27 | 60.75(37.87) | 25(50) | 0.22 |
| Tumor volume (cm3), mean(SD) | 3.06(6.28) | 10.05(13.25) | 0.016 | 4.68(9.55) | 12.81(10.33) | 0.2 |
| Fundus distance (mm), mean(SD) | 1.54(2.17) | 3.41(2.51) | 0.034 | 1.89(2.38) | 4.4(1.65) | 0.047 |
| Tumor origin, n(%) | ||||||
| IVN | 9(39.13) | 3(23.08) | 10(31.25) | 2(50) | ||
| SVN | 5(21.74) | 1(7.69) | 6(18.75) | 0(0) | ||
| Unknown | 9(39.13) | 9(69.23) | 0.32 | 16(50) | 2(50) | 0.81 |
| Surgical approach, n(%) | ||||||
| TL | 14(60.87) | 6(46.15) | 17(53.13) | 3(75) | ||
| MCF | 9(39.13) | 7(53.85) | 0.49 | 15(46.88) | 1(25) | 0.61 |
Abbreviations: std, standard deviation; PTA, pure tone average on the affected side; SDS, speech discrimination score on the affected side; IVN, inferior vestibular nerve; SVN, superior vestibular nerve; TL, translabyrinthine; MCF, middle cranial fossa.
Wilcoxon–Mann–Whitney test for continuous variables and Fisher’s exact test for categorical variables.
Translabyrinthine resection of VS offers an ideal opportunity to examine the anatomic relationships in the lateral IAC by allowing its full decompression and exposure. Interestingly, even in tumors with a sizeable fund fluid cap on preoperative MRI, intraoperative examination revealed tumor extending to the IAC fundus without any visible fundal fluid cap in the tumors in this series (Fig. 3).
Figure 3.
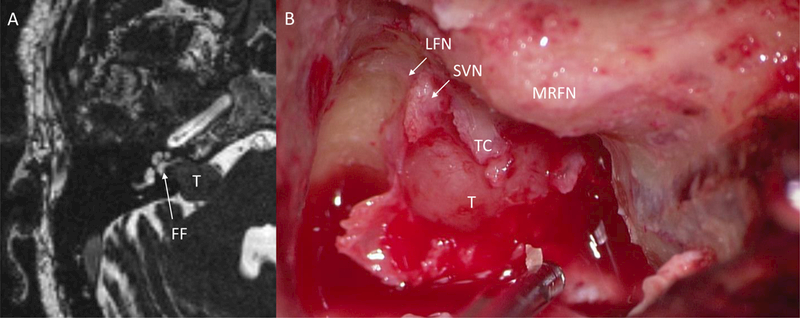
Comparison of pre-operative MRI with intraoperative findings. A) Pre-operative CISS MRI demonstrating a fundal fluid cap (white arrow) in the lateral IAC from the lateral aspect of the tumor to the cochlea aperture. B) Intraoperative photograph during a translabyrinthine approach showing tumor filling the fundus with tumor spanning the transverse crest. Fundal fluid was absent on intraoperative examination. T, tumor; FF, fundal fluid; LFN, labyrinthine facial nerve; SVN, superior vestibular nerve; MRFN, mastoid ridge facial nerve; TC, transverse crest
Discussion
In the current era of advanced magnetic resonance sequences that offer high-resolution anatomical detail, a cerebrospinal ‘fundal fluid cap’ in the lateral IAC has become an area of interest in patients with VS. While prior studies have suggested the presence of a fundal fluid cap to be a favorable prognostic indicator for hearing preservation,11,17–19 recent studies using a variety of hearing outcome classifications have called this into question.6,20 In a retrospective analysis by Goddard et al, with respect to tumors of similar size, the presence of fundal fluid was associated with preservation of measurable (change in PTA ≤ 90 dB and any SDS) post-operative hearing but not with preservation (change in PTA ≤ 15 dB and SDS ≤ 15%) of post-operative hearing status, serviceable (change in PTA ≤ 50 dB and SDS ≥ 50%) hearing, or preservation of American Academy of Otolaryngology – Head and Neck Surgery hearing classification.5 Given this finding, tumors of similar size with a fundal fluid cap may portend to have better post-operative maintenance of hearing, but our institutional experience has not shown the presence of a fundal fluid cap to be an independent predictor of post-operative hearing preservation irrespective of tumor size.
Due to the focus on hearing outcome in previous studies, fundal fluid size has only been studied in smaller tumors. Emerging evidence however, has suggested that its presence in fact may be associated with tumor size.6 In a group of patients with a wider range of tumor sizes, we validate that a larger radiographic fundal fluid cap is in fact associated with larger tumor size using volumetric analysis of tumors on pre-operative MRIs. One possible explanation for the correlation between tumor volume and fluid cap size is that imaging is obtained with the patient in the supine position. In this position gravity may distract some larger tumor masses medially. In these cases, the fundal fluid cap may be position dependent. Further studies are needed to explore this possibility.
Previous studies focusing on the role of fundal fluid size and post-operative hearing preservation necessarily relied on MCF and retrosigmoid approaches for IAC decompression.5,7 An apparent discordance between radiographic and intraoperative fundal fluid size has been noted previously in MCF-based studies.6,8 The current study corroborates those findings by verifying tumor extension to the IAC fundus after full decompression. This clinical finding concords with current understanding of VS tumorigenesis that occurs laterally at Scarpa’s ganglia.9,10 Taken together with recent studies by Goddard et al and Sun et al indicating lack of association between fundal fluid size and likelihood of successful hearing preservation, these findings bring into question the clinical relevance of a radiographic fundal fluid cap.5,6
Fundal fluid cap has been hypothesized to be a favorable prognostic indicator as the more medial location of tumor not only provides a ready dissection plane laterally, which can be important in retrosigmoid approaches where the lateral IAC cannot be visualized, but also portends more favorable pre-operative hearing status as the tumor does not theoretically impact into the cochlear aperture. However, a prior study demonstrated that radiographic fundal fluid caps are in fact not associated with pre-operative hearing.5,6 In addition, even tumors that appear to reside medially in the IAC based on a large radiographic fundal fluid cap, intraoperative examination has revealed that the fluid space in the lateral IAC may in fact be illusory. Taken together, these findings again call into question the extent to which radiographic fundal fluid caps can be reliably used in pre-operative patient counselling.
A trend existed for the association of larger fundal fluid size and poorer short-term and long-term facial nerve outcome. One possibility is that the fundal fluid size reflects underlying differences in tumor biology that may lead to differential degrees of tumor growth and adherence to adjacent nerves. A second possibility is that fundal fluid cap’s association on facial nerve outcome is due to the interdependence of fundal fluid cap with tumor volume. In this study, fundal fluid caps positively correlated with larger tumor volumes. Tumor size is well-known to be a predictor of post-operative facial nerve outcome. 1,21 Thus, facial nerve outcome as it relates to a fundal fluid cap is likely due, at least in part, to the positive correlation of increasing tumor size with increasing fundal fluid. The etiology of this relationship has not been determined and needs to be validated in a larger study population able to construct multivariate models.
This study is limited by its retrospective nature and small sample size. Due to prevailing referral patterns, most pre-operative MR imaging was obtained outside the study institution and consequently only a small subset of scans met the requisite technical and compatibility specifications for volumetric analysis. Despite the small sample size, a significant correlation between fundal fluid and tumor size was found. These exploratory findings require further corroboration in a larger study population where multivariate models may better elucidate the degree to which fundal fluid size is confounded by its tumor size in their respective effects on surgical outcomes. The small and unbalanced sample size also increased the likelihood of spurious correlations, such as the observed association between male gender and larger fundal fluid size. Prior studies have also assessed fundal fluid in patients who have undergone retrosigmoid approaches for tumor resection, which was not addressed in the current study.18,19 Multivariate analysis was not completed because the small sample size did not provide adequate power for analysis.
Conclusion
In this review of patients who underwent microsurgical excision of VS and for which volumetric tumor volumes were available, larger fundal fluid size was positively associated with tumor volume on pre-operative MRI. Larger fundal fluid caps trended toward worse post-operative facial nerve outcomes in the short and long-term, and radiographic fundal fluid caps were not reliably observed during tumor dissection.
Acknowledgments
Support: NCATS UL1TR002537
References:
- 1.Brackmann DE, Cullen RD, Fisher LM. Facial nerve function after translabyrinthine vestibular schwannoma surgery. Otolaryngol Head Neck Surg 2007;136(5):773–777. doi:10.1016/j.otohns.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Glasscock ME, Kveton JF, Jackson CG, Levine SC, McKennan KX. A systematic approach to the surgical management of acoustic neuroma. Laryngoscope 1986;96(10):1088–1094. http://www.ncbi.nlm.nih.gov/pubmed/3531748. [DOI] [PubMed] [Google Scholar]
- 3.Slavit DH, Harner SG, Harper CM, Beatty CW. Auditory monitoring during acoustic neuroma removal. Arch Otolaryngol Head Neck Surg 1991;117(10):1153–1157. http://www.ncbi.nlm.nih.gov/pubmed/1910703. [DOI] [PubMed] [Google Scholar]
- 4.Kocaoglu M, Bulakbasi N, Ucoz T, et al. Comparison of contrast-enhanced T1-weighted and 3D constructive interference in steady state images for predicting outcome after hearing-preservation surgery for vestibular schwannoma. Neuroradiology 2003;45(7):476–481. doi:10.1007/s00234-003-1006-0. [DOI] [PubMed] [Google Scholar]
- 5.Goddard JC, Schwartz MS, Friedman R a. Fundal fluid as a predictor of hearing preservation in the middle cranial fossa approach for vestibular schwannoma. Otol Neurotol 2010;31(7):1128–1134. doi:10.1097/MAO.0b013e3181e8fc3f. [DOI] [PubMed] [Google Scholar]
- 6.Sun DQ, Kung RW, Hansen MR, Gantz BJ. Does a “Fundal Fluid Cap” Predict Successful Hearing Preservation in Vestibular Schwannoma Resections Via the Middle Cranial Fossa Approach? Otol Neurotol 2018;39(6):772–777. doi:10.1097/MAO.0000000000001811. [DOI] [PubMed] [Google Scholar]
- 7.Chen BS, Roberts DS, Lekovic GP. Endoscopic-Assisted Middle Fossa Craniotomy for Resection of Vestibular Schwannoma. J Neurol Surg reports 2016;77(1):e001–7. doi:10.1055/s-0035-1564604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selesnick SH, Rebol J, Heier LA, Wise JB, Gutin PH, Lavyne MH. Internal auditory canal involvement of acoustic neuromas: surgical correlates to magnetic resonance imaging findings. Otol Neurotol 2001;22(6):912–916. http://www.ncbi.nlm.nih.gov/pubmed/11698818. [DOI] [PubMed] [Google Scholar]
- 9.Tryggvason G, Barnett A, Kim J, Soken H, Maley J, Hansen MR. Radiographic association of schwannomas with sensory ganglia. Otol Neurotol 2012;33(7):1276–1282. doi:10.1097/MAO.0b013e318263d315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roosli C, Linthicum FH, Cureoglu S, Merchant SN. What is the site of origin of cochleovestibular schwannomas? Audiol Neurootol 2012;17(2):121–125. doi:10.1159/000331394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubrulle F, Ernst O, Vincent C, Vaneecloo FM, Lejeune JP, Lemaitre L. Cochlear fossa enhancement at MR evaluation of vestibular Schwannoma: correlation with success at hearing-preservation surgery. Radiology 2000;215(2):458–462. doi:10.1148/radiology.215.2.r00ma20458. [DOI] [PubMed] [Google Scholar]
- 12.Woodson EA, Dempewolf RD, Gubbels SP, et al. Long-term hearing preservation after microsurgical excision of vestibular schwannoma. Otol Neurotol 2010;31(7):1144–1152. doi:10.1097/MAO.0b013e3181edb8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer TA, Canty PA, Wilkinson EP, Hansen MR, Rubinstein JT, Gantz BJ. Small acoustic neuromas: surgical outcomes versus observation or radiation. Otol Neurotol 2006;27(3):380–392. http://www.ncbi.nlm.nih.gov/pubmed/16639278. [DOI] [PubMed] [Google Scholar]
- 14.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg 1985;93(2):146–147. doi:10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 15.Bathla G, Case BM, Berbaum K, Hansen MR, Policeni B. Vestibular Schwannomas: Do Linear and Volumetric Parameters on MRI Correlate With Hearing Loss? Otol Neurotol 2016;37(8):1168–1173. doi:10.1097/MAO.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 16.Gurgel RK, Popelka GR, Oghalai JS, Blevins NH, Chang KW, Jackler RK. Is it valid to calculate the 3-kilohertz threshold by averaging 2 and 4 kilohertz? Otolaryngol Head Neck Surg 2012;147(1):102–104. doi:10.1177/0194599812437156. [DOI] [PubMed] [Google Scholar]
- 17.Somers T, Casselman J, de Ceulaer G, Govaerts P, Offeciers E. Prognostic value of magnetic resonance imaging findings in hearing preservation surgery for vestibular schwannoma. Otol Neurotol 2001;22(1):87–94. http://www.ncbi.nlm.nih.gov/pubmed/11314723. [DOI] [PubMed] [Google Scholar]
- 18.Mohr G, Sade B, Dufour J-J, Rappaport JM. Preservation of hearing in patients undergoing microsurgery for vestibular schwannoma: degree of meatal filling. J Neurosurg 2005;102(1):1–5. doi:10.3171/jns.2005.102.1.0001. [DOI] [PubMed] [Google Scholar]
- 19.Ferber-Viart C, Laoust L, Boulud B, Duclaux R, Dubreuil C. Acuteness of preoperative factors to predict hearing preservation in acoustic neuroma surgery. Laryngoscope 2000;110(1):145–150. doi:10.1097/00005537-200001000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Gjuric M, Mitrecic MZ, Greess H, Berg M. Vestibular schwannoma volume as a predictor of hearing outcome after surgery. Otol Neurotol 2007;28(6):822–827. doi:10.1097/MAO.0b013e318068b2b0. [DOI] [PubMed] [Google Scholar]
- 21.Rinaldi V, Casale M, Bressi F, et al. Facial nerve outcome after vestibular schwannoma surgery: our experience. J Neurol Surg B Skull Base 2012;73(1):21–27. doi:10.1055/s-0032-1304559. [DOI] [PMC free article] [PubMed] [Google Scholar]


