Abstract
Background
Retroperitoneal sarcomas (RPS) are rare tumors for which complete surgical resection remains the mainstay of treatment. We sought to determine the impact of hospital case volume on RPS outcomes.
Methods
We identified 6950 patients with primary RPS who underwent surgical resection from the National Cancer Data Base (1998–2011). Treating hospitals were classified by annual case volume; low volume hospitals (LVHs) and high volume hospitals (HVHs) were defined as ≤10 and >10 cases/year, respectively. Overall survival (OS) was compared using Kaplan-Meier curves. Cox proportional hazard models were created to compare risks.
Results
Of 1131 reporting hospitals, most (n=1127, 99.6%) were LVHs treating the majority of patients (n=6270; 90.2%). Patients treated at LVHs were more likely to have lower grade and smaller tumors, receive radiation therapy, and undergo incomplete gross (R2) resection. Patients treated at HVHs had lower 30-day readmission rates (1.8% vs 3.4%, p<0.001), 30-day (1.9% vs 3.1%, p=0.004) and 90-day mortality (3.2% vs 5.7%, p=0.007); longer median OS (76.2 vs 64.2 months, p<0.001) and higher 5-year OS (58% vs 52%, p<0.001). After controlling for age, gender, insurance status, tumor size, tumor grade, resection margin status, and radiation administration, treatment at a HVH was independently associated with a reduced risk of death (HR 0.77, 95% CI 0.65–0.91, p=0.003).
Conclusion
Primary RPS are rare tumors for which few surgeons and institutions have significant experience and expertise in their multidisciplinary management and surgical resection. Although additional studies are needed, patient outcomes may be impacted by treating facility case volume and experience.
Keywords: Soft Tissue Sarcoma, Retroperitoneal Sarcoma, Hospital Volume, NCDB, National Cancer Data Base, Registry, Sarcoma Surgery, Soft Tissue Neoplasm, Sarcoma Treatment
Introduction
Soft tissue sarcomas (STS) are rare and heterogeneous tumors for which the mainstay of treatment for primary localized disease is complete surgical resection. Of the 12,390 new cases of STS in the United States in 2017,1 ~15% present in the retroperitoneum.2 Retroperitoneal sarcomas (RPS) pose significant technical challenges for the surgeon, given their typically large size and abutment or involvement of adjacent critical structures. Resection of these tumors commonly necessitates multi-organ resection and vascular reconstruction and achieving a macroscopically complete surgical (R0/R1) resection is often difficult.
European and North American expert sarcoma surgeons in conjunction with the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group have published consensus statements to describe a reproducible and standardized approach to these tumors3 in which they and others recommend that patients with RPS be managed by experienced multidisciplinary teams in specialized sarcoma referral centers.3–5 Whether treatment at specialized referral centers and high volume hospitals (HVHs) compared to low volume hospitals (LVHs) is associated with better cancer outcomes has been the subject of many studies across disease sites6–11 and in patients undergoing complex oncologic resections including head/neck surgery,12 radical cystectomy,13 lung resection,14 esophagectomy,15,16 colorectal surgery,17 and hepato-pancreatico-biliary (HPB) surgery.18–20
Within the sarcoma literature, a small number of retrospective studies have reported an association between sarcoma treatment at either specialty referral centers or HVHs and better patient outcomes. However, these studies are limited as they included 1) STS of multiple disease sites (not limited to the retroperitoneum),21 2) patients who did not undergo RPS resection,22 and 3) often defined hospitals performing as few as 5 RPS resections/year as HVHs.21,22 The aim of this study was to determine the impact of hospital case volume on outcomes among patients who underwent surgical resection of primary RPS using a large national cancer database.
Materials and Methods
Data source
The National Cancer Database (NCDB) is a hospital-based cancer registry sponsored by the American College of Surgeons and the American Cancer Society that prospectively captures approximately 70% of all new cancer cases in the United States. The data includes clinicopathologic, treatment, and outcome variables and are de-identified. This study was thus considered exempt by The University of Texas MD Anderson Cancer Center Institutional Review Board.
Analytic cohort
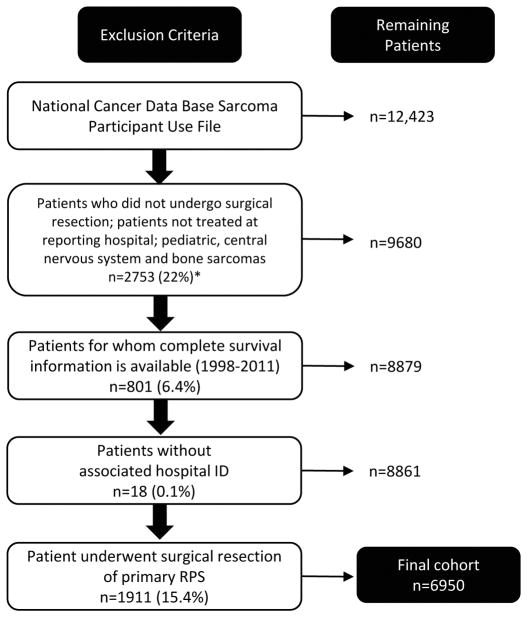
Patients diagnosed with primary RPS (1998–2011) were identified from the NCDB Participant Use File (n=12,423) using the International Classification of Diseases for Oncology third edition (ICD-O-3) code C480 (Figure 1). All histologic subtypes included in the data were individually vetted to exclude non-sarcomatous or mixed histologies (Supplemental Table 1).23 Additional subgroups excluded from the analytical cohort were patients that did not undergo surgical resection, pediatric patients, central nervous system and bone sarcomas, patients not treated at the reporting hospital, and patients with incomplete information. The final cohort (n=6950) was grouped by hospital volume into LVHs (≤10 cases/year) or HVHs (>10 cases/year) (Table 1). We chose not to use the median number of RPS resections performed per year by reporting hospitals as a cutoff to define LVHs versus HVHs because the median number of cases/year was only 1.1. Furthermore, hospitals performing 2 cases/year were among the 90th percentile and 5.4 cases/year among the 99th percentile. Thus, we defined 10 RPS resections/year as a cutoff for LVHs versus HVHs as we felt this volume would endow a hospital with sufficient experience and expertise in the treatment of these rare tumors. Using receiver operating characteristic (ROC) curves, we were unable to identify a better cutoff to define LVHs versus HVHs (data not shown).
Figure 1.
Flow chart demonstrating how the final cohort of 6950 patients who underwent surgical resection of primary retroperitoneal sarcoma was obtained.
*All percentages calculated from the total Participant Use File
Table 1.
Demographics and treatment information of patients with primary retroperitoneal sarcoma who underwent surgical resection, stratified by treating hospital volume.
| All (n=6950) | ≤10/year (n=6270, 90.2%) | >10/year (n=680, 9.8%) | p value | ||||
|---|---|---|---|---|---|---|---|
| N | Col% | N | Col % | N | Col % | ||
| Age (median, range) | 62 (18–90) | 62 (18–90) | 60 (19–90) | <0.001 | |||
| Sex | <0.001 | ||||||
| Male | 3215 | 46.3 | 2853 | 45.5 | 362 | 53.2 | |
| Female | 3735 | 53.7 | 3417 | 54.5 | 318 | 46.8 | |
| Race | <0.001 | ||||||
| Caucasian | 5479 | 78.8 | 4901 | 78.2 | 578 | 85.0 | |
| African American | 667 | 9.6 | 631 | 10.1 | 36 | 5.3 | |
| Asian/Pacific Islander | 304 | 4.4 | 280 | 4.5 | 24 | 3.5 | |
| Hispanic | 445 | 6.4 | 410 | 6.5 | 35 | 5.2 | |
| Other | 55 | 0.8 | 48 | 0.8 | 7 | 1.0 | |
| Median household income in zip code of residence | <0.001 | ||||||
| ≥$63,000 | 2259 | 32.5 | 1978 | 31.6 | 281 | 41.3 | |
| $48,000–$62,999 | 1844 | 26.5 | 1671 | 26.7 | 173 | 25.4 | |
| $38,000–$47,999 | 1553 | 22.4 | 1415 | 22.6 | 138 | 20.3 | |
| <$38,000 | 1095 | 15.8 | 1033 | 16.5 | 62 | 9.1 | |
| Unknown | 199 | 2.9 | 173 | 2.8 | 26 | 3.8 | |
| Educational attainment in zip code of residence, % graduated high school | <0.001 | ||||||
| ≥93% | 1765 | 25.4 | 1535 | 24.5 | 230 | 33.8 | |
| 87.0–92.9% | 2228 | 32.1 | 2018 | 32.2 | 210 | 30.9 | |
| 79–86.9% | 1659 | 23.9 | 1532 | 24.4 | 127 | 18.7 | |
| <79% | 1102 | 15.9 | 1015 | 16.2 | 87 | 12.8 | |
| Unknown | 196 | 2.8 | 170 | 2.7 | 26 | 3.8 | |
| Insurance | <0.001 | ||||||
| Private Insurance | 3344 | 48.1 | 3072 | 49.0 | 272 | 40.0 | |
| Medicare | 2649 | 38.1 | 2478 | 39.5 | 171 | 25.2 | |
| Medicaid | 328 | 4.7 | 309 | 4.9 | 19 | 2.8 | |
| Other Government | 72 | 1.0 | 69 | 1.1 | 3 | 0.4 | |
| Not Insured | 208 | 3.0 | 200 | 3.2 | 8 | 1.2 | |
| Unknown | 349 | 5.0 | 142 | 2.3 | 207 | 30.4 | |
| Charlson-Deyo score | 0.020 | ||||||
| 0 | 3764 | 54.2 | 3361 | 53.6 | 403 | 59.26 | |
| 1 | 801 | 11.5 | 728 | 11.61 | 73 | 10.74 | |
| 2 | 211 | 3.0 | 200 | 3.19 | 11 | 1.62 | |
| Unknown | 2174 | 31.3 | 1981 | 31.59 | 193 | 28.38 | |
| Facility type | <0.001 | ||||||
| Academic/Research Program | 3806 | 54.8 | 3126 | 49.9 | 680 | 100 | |
| Comprehensive Community Cancer Program | 2702 | 38.9 | 2702 | 43.1 | . | . | |
| Community Cancer Program | 434 | 6.2 | 434 | 6.9 | . | . | |
| Other specified types of cancer programs | 8 | 0.1 | 8 | 0.1 | . | . | |
| Grade | <0.001 | ||||||
| Low | 3076 | 44.3 | 2815 | 44.9 | 261 | 38.4 | |
| Intermediate/High | 3874 | 55.7 | 3455 | 55.1 | 419 | 62.6 | |
| Tumor size | 0.048 | ||||||
| ≤5 cm | 594 | 8.6 | 550 | 8.8 | 44 | 6.5 | |
| 5–10 cm | 1239 | 17.8 | 1119 | 17.9 | 120 | 17.7 | |
| >10 cm | 4512 | 64.9 | 4044 | 64.5 | 468 | 68.8 | |
| Unknown | 605 | 8.7 | 557 | 8.9 | 48 | 7.1 | |
| Margin | <0.001 | ||||||
| R0/R1 | 4492 | 64.6 | 4087 | 65.2 | 405 | 59.6 | |
| R2 | 291 | 4.2 | 280 | 4.5 | 11 | 1.6 | |
| Unknown | 2167 | 31.2 | 1903 | 30.4 | 264 | 38.8 | |
| Received chemotherapy | <0.001 | ||||||
| No | 5763 | 82.9 | 5192 | 82.8 | 571 | 84.0 | |
| Yes | 959 | 13.8 | 854 | 13.6 | 105 | 15.4 | |
| Unknown | 228 | 3.3 | 224 | 3.6 | 4 | 0.6 | |
| Radiation therapy | <0.001 | ||||||
| No | 4961 | 71.4 | 4398 | 70.1 | 563 | 82.8 | |
| Yes | 1868 | 26.9 | 1751 | 27.9 | 117 | 17.2 | |
| Unknown | 121 | 1.7 | 121 | 1.9 | . | . | |
| Readmission within 30 days of surgical discharge | <0.001 | ||||||
| No readmission | 4387 | 63.1 | 3913 | 62.4 | 474 | 69.7 | |
| Readmission | 227 | 3.3 | 215 | 3.4 | 12 | 1.8 | |
| Unknown | 2336 | 33.6 | 2142 | 34.2 | 194 | 28.5 | |
| 30 day mortality | 0.004 | ||||||
| Alive or death >30 days of surgery | 6387 | 91.9 | 5818 | 92.8 | 569 | 83.7 | |
| Death ≤30 days of surgery | 359 | 2.9 | 191 | 3.1 | 13 | 1.9 | |
| Alive with <30 days follow-up or date of last contact unknown | 359 | 5.2 | 261 | 4.2 | 98 | 14.4 | |
| 90 day mortality | 0.007 | ||||||
| Alive or death >90 days of surgery | 6355 | 91.4 | 5728 | 91.4 | 627 | 92.2 | |
| Death ≤90 days of surgery | 382 | 5.5 | 360 | 5.7 | 22 | 3.2 | |
| Alive with <90 days follow-up or date of last contact unknown | 213 | 3.1 | 182 | 2.9 | 31 | 4.6 | |
R0/R1: complete gross resection
R2: incomplete gross resection
Study variables
Study variables included patient age, gender, race, income, education, insurance status, Charlson-Deyo comorbidity index, treating facility type (academic, comprehensive, or community), hospital volume (# cases/year reporting to the NCDB), tumor grade, tumor size, and therapies received including radiation and/or chemotherapy. In the NCDB, income is estimated by the median household income in the zip code of each patient’s area of residence based on the 2012 American community Survey data. Educational attainment is estimated by the number of adults in the patient’s zip code who graduated from high school based on the 2012 American Community Survey data. Outcomes of interest included rates of complete (R0/R1) RPS resection, 30-day readmission, 30-day mortality, 90-day mortality, and overall survival (OS).
Statistical analysis
Survival data was available for the years 1998–2011. The Kaplan-Meier method and log-rank test were used to calculate and compare the unadjusted OS curves by hospital volume. Univariate and multivariate Cox regression models were used to identify covariates associated with OS. Age, gender, race, insurance, tumor size, surgical margin, tumor grade, radiation treatment, and hospital volume were considered in the univariate Cox regression models; a stepwise method was used to select the final multivariate models. All variables (except chemotherapy administration) included in the model met the proportional hazards function assumption with exclusion of unknown cases from the analysis except for chemotherapy administration, which consistently violated the proportional hazards assumption. Therefore, two separate models for OS were performed: (1) All patients, stratified by chemotherapy status and (2) patients that did not receive chemotherapy. The adjusted survival curves were estimated from the final Cox regression models after adjusting for significant covariates. We performed a logistic regression analysis to determine factors predictive of treatment at a LVH (Supplemental Table 2). We next performed sensitivity analyses to evaluate the reliability of our model. Propensity score (PS) methods (PS stratification, normalized weighting, and covariate adjustment) were applied to the Cox regression model (Supplemental Table 3) with hospital volume as the dependent variable and age at diagnosis, sex, race, insurance, and education as independent variables. The resulting HRs were similar to that of the original model and thus this was retained as our final model. P<0.05 was considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC) was used to conduct all analyses.
Results
The majority of primary RPS are treated at LVHs
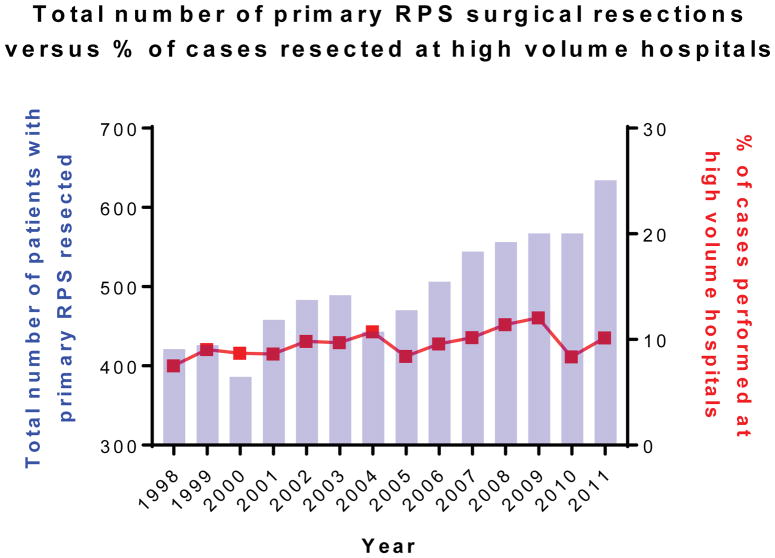
Between 1998–2011, 1131 reporting hospitals performed surgical resection for primary RPS (Table 1). The vast majority of reporting hospitals (n=1127, 99.6%) were LVHs that performed ≤10 primary RPS resections/year. Of the total 6950 patients with primary RPS who underwent surgical resection during the study period, the majority were treated at LVHs (n=6270, 90.2%). A minority of patients (n=680, 9.8%) were treated at the 4 HVHs, defined as facilities which averaged >10 cases/year. Although the number of surgical cases performed for RPS steadily increased from 1998 to 2011, the percentage of resections performed at HVHs remained relatively constant (range 7.6–12.2%) over the study period (Figure 2, Supplemental Table 4).
Figure 2.
Total number of primary retroperitoneal sarcoma surgical resections versus percent of cases resected at high volume hospitals by year.
Patients treated at LVHs versus HVHs differ in their baseline characteristics and treatment modalities
Patient demographics and clinical characteristics and by treating hospital volume are detailed in Table 1. Comparison of patients by treating hospital volume revealed differences between subgroups treated at LVHs versus HVHs.
There were notable socioeconomic differences between RPS patients treated at LVHs versus HVHs. Patients treated at LVHs were more likely to be ethnic minorities rather than white Caucasian and of lower educational background and income (all p<0.001). Additionally, LVHs treated a greater proportion of uninsured (3.2% versus 1.2%) or Medicaid/Medicare (45.4% versus 28%) patients (p<0.001).
HVHs were also distinct from LVHs with respect to patient clinical and tumor characteristics and the multidisciplinary treatment modalities used. HVHs treated patients with RPS that were larger (p=0.048) and of high tumor grade (p<0.001), features associated with poorer clinical outcomes.4,24,25 Those treated at HVHs waited longer between diagnosis and start of treatment (median 28 versus 5 days, p<0.001) and between diagnosis and definitive surgical resection (median 36 versus 10 days, p<0.001). HVHs were less likely to use radiation therapy (17.2% vs 27.9%, p<0.001), although use of systemic therapy was similar between LVHs and HVHs.
Primary RPS treatment at HVHs is associated with improved outcomes
We next compared outcomes of patients who underwent resection of primary RPS at LVHs versus HVHs. One of the most important prognostic factors for patients with RPS is the ability to resect all macroscopic disease (R0/R1 resection). Surgery at HVHs was associated with fewer incomplete (R2) gross resections (1.6% versus 4.5%, p<0.001).
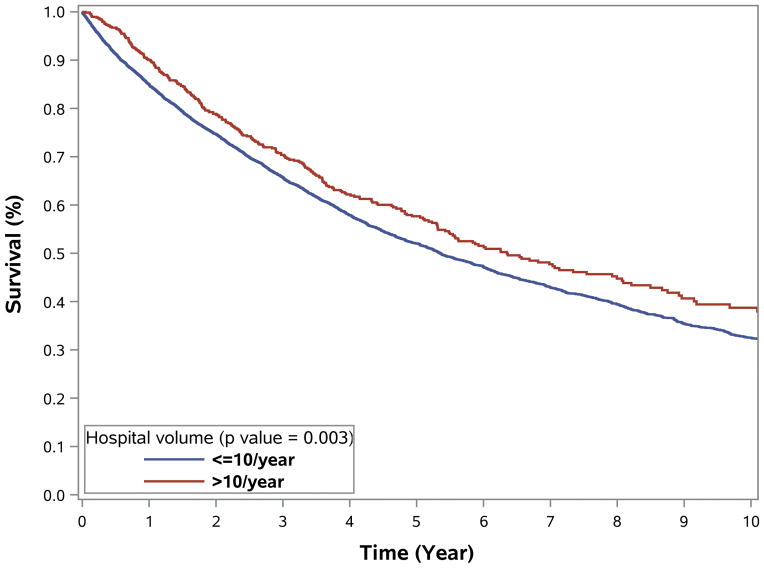
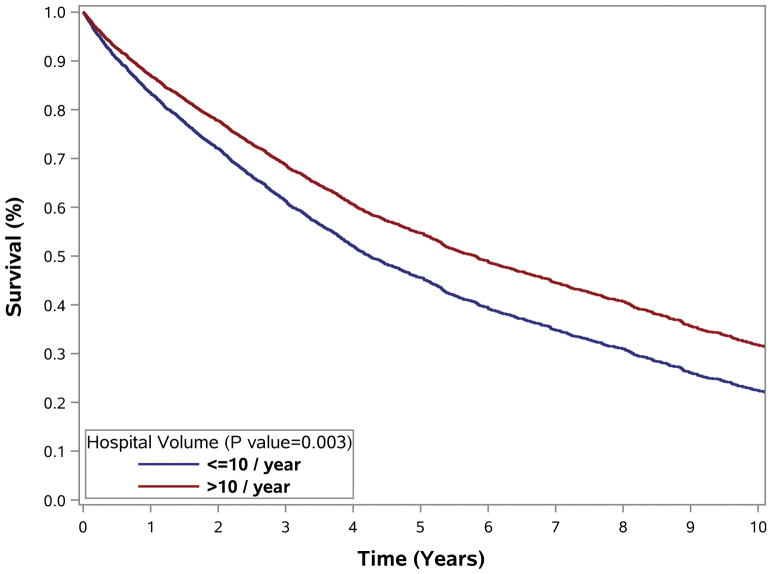
Compared to patients treated at LVHs, those treated at HVHs had lower 30-day readmission rates (1.8% versus 3.4%, p<0.001), 30-day mortality (1.9% versus 3.1%, p=0.004), and 90-day mortality (3.2% versus 5.7%, p=0.007) (Table 1). RPS treatment at HVHs was also associated with longer median OS (76.2 versus 64.2 months) and 5-year OS (57.7% versus 52.0%) (Figure 3). Additional analyses suggest a dose-effect associated with increasing hospital case volume and better patient outcomes and found progressive improvement in patient outcome with increasing hospital case volume (0–5 per year, 6–10 cases/year, and >10 cases per year; Supplemental Figure 1).
Figure 3.
Overall survival of patients with primary retroperitoneal sarcoma undergoing surgical resection by hospital volume (1998–2011). Survival curves for overall survival among (A) all patients, unadjusted; (B) excluding patients who received chemotherapy, unadjusted; (C–D) adjusted for older age, gender, insurance, larger tumor size, radiation therapy and (C) low & (D) intermediate/high grade RPS.
To examine whether hospital volume was independently associated with OS, univariate and multivariate Cox regression models were used to identify covariates associated with OS. Chemotherapy as a covariate violated the proportional hazards assumption in the model. Patients who received chemotherapy (n=959, 13.8%) differed significantly with respect to multiple factors compared to those that did not receive chemotherapy (n=5763, 90.1%) as part of their multidisciplinary treatment plan (Supplemental Table 5). Thus, analysis was limited to patients who did not receive chemotherapy as part of their RPS treatment (Supplementary Table 6).
On multivariate analysis, treatment at a HVH was associated with a reduced risk of death compared to treatment at a LVH (HR 0.77, 95% CI 0.65–0.91, p=0.003; Table 2). Older age, male gender, larger tumor size, higher tumor grade, and incomplete gross (R2) resection were also associated with worse OS. We found similar results for univariate and multivariate analyses to identify factors predictive of OS among all patients, stratified by chemotherapy status (Supplemental Table 7). We also found similar results when separate analyses were performed limited to patients with Charlson-Deyo score available in the NCDB (2003–2011, n=3524) (Supplemental Table 8).
Table 2.
Cox model for overall survival among patients with primary retroperitoneal sarcoma surgically resected, excluding patients who received chemotherapy (n=4969).
| Description | Univariate model
|
Multivariate model
|
||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||
| Age (ref. ≤60) | <0.001 | <0.001 | ||||||
| >60 | 1.96 | 1.80 | 2.14 | 1.64 | 1.47 | 1.84 | ||
| Gender (ref. Male) | <0.001 | <0.001 | ||||||
| Female | 0.81 | 0.75 | 0.88 | 0.84 | 0.77 | 0.91 | ||
| Race (ref. White) | 0.003 | |||||||
| African American | 0.91 | 0.79 | 1.05 | |||||
| Asian/Pacific Islander | 0.71 | 0.56 | 0.88 | |||||
| Hispanic | 0.82 | 0.68 | 0.99 | |||||
| Insurance (ref. Private) | <0.001 | <0.001 | ||||||
| Medicare | 1.85 | 1.70 | 2.01 | 1.31 | 1.17 | 1.46 | ||
| Medicaid | 1.27 | 1.03 | 1.57 | 1.33 | 1.08 | 1.64 | ||
| Other Government | 1.07 | 0.67 | 1.70 | 1.11 | 0.70 | 1.77 | ||
| Not Insured | 1.40 | 1.09 | 1.81 | 1.37 | 1.06 | 1.77 | ||
| Tumor size (ref. ≤5 cm) | <0.001 | <0.001 | ||||||
| 5–10 cm | 1.07 | 0.90 | 1.26 | 1.03 | 0.87 | 1.22 | ||
| >10 cm | 1.29 | 1.11 | 1.49 | 1.28 | 1.11 | 1.48 | ||
| Margin (ref. R0/R1) | <0.001 | <0.001 | ||||||
| R2 | 2.34 | 1.94 | 2.83 | 2.45 | 2.02 | 2.96 | ||
| Unknown | 1.41 | 1.29 | 1.54 | 1.46 | 1.34 | 1.60 | ||
| Hospital volume (ref. >10/year) | <0.001 | 0.003 | ||||||
| ≤10/year | 1.27 | 1.07 | 1.51 | 1.30 | 1.10 | 1.55 | ||
| Received radiation therapy (ref. No) | 0.006 | <0.001 | ||||||
| Yes | 0.89 | 0.81 | 0.97 | 0.80 | 0.73 | 0.88 | ||
| Grade (ref. Low) | <0.001 | <0.001 | ||||||
| Intermediate/High | 2.05 | 1.88 | 2.23 | 2.24 | 2.05 | 2.45 | ||
R0/R1: complete gross resection
R2: incomplete gross resection
Discussion
In this study, we examined the effect of hospital case volume on perioperative and survival outcomes in patients undergoing surgical resection for primary RPS using the NCDB. The results of this study demonstrate that treatment of primary RPS at HVHs is associated with reduced perioperative death and improved OS. Unfortunately, however, the vast majority of primary RPS resections during the study period were performed at LVHs that have limited experience in the multidisciplinary care of these rare tumors. It is important to note that although 10 RPS resections/year (<1 case per month) was chosen as the cutoff for LVHs versus HVHs in this study, expert sarcoma referral centers likely have higher volumes than this cutoff.
To our knowledge, this is the first study to investigate the impact of hospital volume on outcomes among patients undergoing surgical resection for RPS. Two prior studies examined hospital volume and outcomes in sarcomas patients, however these were limited for several reasons.21,22 Gutierrez et al reported outcomes among 4205 patients with STS undergoing surgical resection, finding lower 30- and 90-day mortality in HVHs.21 However, the majority of patients included had STS of the extremity, trunk, and head/neck locations and <42% of patients included had RPS. Also using the NCDB, Maurice et al reported outcomes among 3141 RPS patients, however, 30% of patients in this study did not undergo surgical resection and HVH was defined as a hospital performing at least 5 resections/year.22 RPS treatment at HVHs in this study was associated with higher rates of surgical resection and R0/R1 resection, however the authors did not find a survival difference between patients treated at HVHs and LVHs.
Although the association between hospital volume and patient outcomes has only recently begun to be examined for sarcomas, it has been extensively examined across other patient populations.6–8,11,20,26 Compared to treatment at LVHs, treatment at HVHs has been associated with improved patient outcomes following various complex oncologic resections including head/neck surgery,12 esophagectomy,9,15,16 colorectal surgery,9,17,27 HPB,9,19,20,28 cystectomy,13 nephrectomy,10 and major lung resection.14
The relationship between hospital volume and patient outcomes may reflect differences in multiple aspects of the multidisciplinary care of the oncology patient undergoing complex surgical resection, such as the patient with primary resectable RPS. These may include not only differences in surgeon volume and experience at HVHs which may result in differences in surgical approach to RPS resection, completeness of surgical resection, and quality of surgery but also extend to differences in how multimodality therapy is used, how patients are surveyed, and how disease recurrence is managed.
The impact of surgeon volume on patient outcomes has been demonstrated in other patient populations such as following adrenalectomy,29 thyroidectomy,30 and HPB surgery.18,31 In this study, despite limitations of data available in the NCDB regarding perioperative complications, there are immediate differences in quality of surgery with higher rates of incomplete (R2) RPS resection occurring at LVHs compared to HVHs. Additionally, there is significant differences in utilization of radiation therapy for RPS between HVHs and LVHs. Finally, surgical resection at HVHs compared to LVHs is associated with significantly lower 30-day readmission.
There are also data across disease sites to suggest that HVHs may be better able to “rescue” patients following postoperative complications. Failure to rescue (FTR) is defined as operative mortality after a major perioperative complication and has been demonstrated to differ between HVHs and LVHs in other patient populations, including patients undergoing high-risk cancer operations such as gastrectomy, esophagectomy, pancreatectomy, and liver resection.18,32–34 In this study, although FTR cannot be determined using data available in the NCDB, it might be extrapolated that LVHs may have had higher FTR rates based on the findings of higher 30- and 90-day mortality rates.
Lastly, studies have suggested that there may be differences in rates of adherence to sarcoma treatment guidelines between HVHs and LVHs. Incomplete adherence to sarcoma treatment guidelines has consistently been shown to be associated with worse outcomes in studies of patient populations in the United States35 and Europe.36,37 Furthermore, these studies report that treatment within specialized sarcoma centers, HVHs, and within a cancer network is more likely to adhere to treatment guidelines.38–40
Despite a significant body of literature demonstrating the association between care at HVHs and improved outcomes, a significant proportion of patients seek care at LVHs. Although some patients may prefer to undergo a complex surgical procedure at a local LVH,41 there is concern that socioeconomic barriers may prevent many patients from accessing care at HVHs and specialized referral centers.20,42,43 In this study, demographic data available in the NCDB suggests that patients who underwent surgery at LVHs may be of lower socioeconomic status, raising concerns that socio-economic circumstances may be barriers to patients’ ability to access care at HVHs for RPS.
There are several limitations of this study, including those inherent to retrospective studies using a large national database. Data available through the NCDB are submitted by participating hospitals across the US and thus not only reflect potential differences in RPS management across institutions but may also reflect inconsistencies in pathologic diagnosis of RPS histologies and data entry by research personnel. Furthermore, for each variable examined, a minority of cases within the analytic cohort had missing or insufficient data for analysis. Additionally, the NCDB does not include data pertaining to disease recurrence/progression, details of systemic chemotherapy administered or radiation therapy regimens. Despite these limitations, however, the NCDB remains a valuable resource to study patient- and hospital-related factors that may impact patient care and oncologic outcomes particularly in rare malignancies such as primary RPS.
Conclusion
Multidisciplinary care of the patient with RPS is complex. Although additional studies are needed, the results of the current study suggest that outcomes among patients with primary RPS undergoing surgical resection may be impacted by treating facility case volume and experience. Furthermore, patients who underwent surgery at LVHs in this study were of lower socioeconomic status, raising concerns that financial and social circumstances may be clinically relevant barriers to patients’ ability to seek and access care at HVHs for RPS.
Supplementary Material
Supplemental Table 1. International Classification of Diseases for Oncology (ICD-O-3) topographical and morphological codes included.
Supplemental Table 2. Factors associated with treatment at a low volume hospital (≤ 10 cases/year)
Supplemental Table 3. Sensitivity analysis of hospital volume - results of various propensity score methods
Supplemental Table 4. Total number of primary retroperitoneal sarcoma surgical resections versus percent of cases resected at high volume hospitals by year.
Supplemental Table 5. Demographics and treatment information of patients with primary retroperitoneal sarcoma who underwent surgical resection, stratified by chemotherapy status.
Supplemental Table 6. Demographics and treatment information of patients with primary retroperitoneal sarcoma who underwent surgical resection (without chemotherapy), stratified by treating hospital volume.
Supplemental Table 7. Cox model for overall survival among patients with primary retroperitoneal sarcoma surgically resected, stratified by chemotherapy status.
Supplemental Table 8. Cox model for overall survival among patients with primary retroperitoneal sarcoma surgically resected with Charlson-Deyo score available (2003–2011 only), excluding patients who received chemotherapy (n=3524).
Supplemental Figure 1. Overall survival of patients with primary retroperitoneal sarcoma undergoing surgical resection by hospital volume (1998–2011). Survival curves for overall survival among all patients adjusted for older age, gender, insurance, larger tumor size, radiation therapy and (A) low and (B) intermediate/high grade RPS.
Synopsis.
Few institutions have significant experience in the multidisciplinary management of retroperitoneal sarcomas (RPS), rare tumors that often require complex multi-visceral resection. Treatment of primary RPS at high volume hospitals is associated with improved patient outcomes.
Acknowledgments
Funding: This works was supported by grants from the NIH (EZK is supported by T32CA0095999, CLR is supported by K12 Paul Calabresi Career Development Award for Clinical Oncology).
Footnotes
Conflict of Interest: The authors have no financial or personal relationships to disclose pertinent to the submitted study.
Author contributions statement:
Emily Z. Keung: Conceptualization, methodology, data analysis, investigation, visualization, writing and approval of manuscript
Yi-Ju Chiang: Conceptualization, methodology, data analysis, visualization, writing and approval of manuscript
Janice N. Cormier: Conceptualization, methodology, investigation, writing and approval of manuscript
Keila E. Torres: Conceptualization, writing and approval of manuscript
Kelly K., Hunt: Conceptualization, writing and approval of manuscript
Barry W. Feig: Conceptualization, writing and approval of manuscript
Christina L. Roland: Conceptualization, data analysis, methodology, investigation, visualization, writing and approval of manuscript.
All authors gave final approval
References
- 1. [Accessed January 1, 2018];Adult soft tissue sarcoma treatment. https://www.cancer.gov/types/soft-tissue-sarcoma/hp/adult-soft-tissue-treatment-pdq#cit/section_1.1.
- 2.Fairweather M, Wang J, Jo VY, Baldini EH, Bertagnolli MM, Raut CP. Surgical Management of Primary Retroperitoneal Sarcomas: Rationale for Selective Organ Resection. Ann Surg Oncol. 2018;25(1):98–106. doi: 10.1245/s10434-017-6136-4. [DOI] [PubMed] [Google Scholar]
- 3.Bonvalot S, Raut CP, Pollock RE, et al. Technical considerations in surgery for retroperitoneal sarcomas: position paper from E-Surge, a master class in sarcoma surgery, and EORTC-STBSG. Ann Surg Oncol. 2012;19(9):2981–2991. doi: 10.1245/s10434-012-2342-2. [DOI] [PubMed] [Google Scholar]
- 4.ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2014;25(Suppl 3):iii102–12. doi: 10.1093/annonc/mdu254. [DOI] [PubMed] [Google Scholar]
- 5.Trans-Atlantic RPS Working Group. Management of Recurrent Retroperitoneal Sarcoma (RPS) in the Adult: A Consensus Approach from the Trans-Atlantic RPS Working Group. Ann Surg Oncol. 2016;23(11):3531–3540. doi: 10.1245/s10434-016-5336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Siewers AE, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 7.Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260(2):244–251. doi: 10.1097/SLA.0000000000000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245(5):777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. IMpact of hospital volume on operative mortality for major cancer surgery. Jama. 1998;280(20):1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 10.Abouassaly R, Finelli A, Tomlinson GA, Urbach DR, Alibhai SMH. Volume-outcome relationships in the treatment of renal tumors. J Urol. 2012;187(6):1984–1988. doi: 10.1016/j.juro.2012.01.076. [DOI] [PubMed] [Google Scholar]
- 11.Dudley Ra, Johansen KL, Brand R, Rennie DJ, Milstein A. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. Jama. 2000;283:1159–1166. doi: 10.1001/jama.283.9.1159. [DOI] [PubMed] [Google Scholar]
- 12.Nieman CL, Stewart CM, Eisele DW, Pronovost PJ, Gourin CG. Frailty, hospital volume, and failure to rescue after head and neck cancer surgery. Laryngoscope. 2017:1–6. doi: 10.1002/lary.26952. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Higher surgeon and hospital volume improves long-term survival after radical cystectomy. Cancer. 2013;119(19):3546–3554. doi: 10.1002/cncr.28235. [DOI] [PubMed] [Google Scholar]
- 14.Falcoz PE, Puyraveau M, Rivera C, et al. The impact of hospital and surgeon volume on the 30-day mortality of lung cancer surgery: A nation-based reappraisal. J Thorac Cardiovasc Surg. 2014;148(3):841–848. doi: 10.1016/j.jtcvs.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Fischer C, Lingsma H, Klazinga N, et al. Volume-outcome revisited: The effect of hospital and surgeon volumes on multiple outcome measures in oesophago-gastric cancer surgery. PLoS One. 2017;12(10):1–11. doi: 10.1371/journal.pone.0183955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munasinghe A, Markar SR, Mamidanna R, et al. Is it time to centralize high-risk cancer care in the United States? comparison of outcomes of esophagectomy between England and the United States. Ann Surg. 2015;262(1):79–85. doi: 10.1097/SLA.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 17.Liu CJ, Chou YJ, Teng CJ, et al. Association of surgeon volume and hospital volume with the outcome of patients receiving definitive surgery for colorectal cancer: A nationwide population-based study. Cancer. 2015;121(16):2782–2790. doi: 10.1002/cncr.29356. [DOI] [PubMed] [Google Scholar]
- 18.Buettner S, Gani F, Amini N, et al. The relative effect of hospital and surgeon volume on failure to rescue among patients undergoing liver resection for cancer. Surgery. 2016;159(4):1004–1012. doi: 10.1016/j.surg.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Schneider EB, Ejaz A, Spolverato G, et al. Hospital Volume and Patient Outcomes in Hepato-Pancreatico-Biliary Surgery: Is Assessing Differences in Mortality Enough? J Gastrointest Surg. 2014;18(12):2105–2115. doi: 10.1007/s11605-014-2619-9. [DOI] [PubMed] [Google Scholar]
- 20.Lidsky ME, Sun Z, Nussbaum DP, Adam MA, Speicher PJ, Blazer DG. Going the Extra Mile. Ann Surg. 2017;266(2):333–338. doi: 10.1097/SLA.0000000000001924. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez JC, Perez EA, Moffat FL, Livingstone AS, Franceschi D, Koniaris LG. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg. 2007;245(6):952–958. doi: 10.1097/01.sla.0000250438.04393.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurice MJ, Yih JM, Ammori JB, Abouassaly R. Predictors of surgical quality for retroperitoneal sarcoma: Volume matters. J Surg Oncol. 2017;116(6):766–774. doi: 10.1002/jso.24710. [DOI] [PubMed] [Google Scholar]
- 23.Keung EZ, Chiang Y-J, Voss RK, et al. Defining the incidence and clinical significance of lymph node metastasis in soft tissue sarcoma. Eur J Surg Oncol. 2018;44(1):170–177. doi: 10.1016/j.ejso.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gronchi A, Strauss DC, Miceli R, et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS) Ann Surg. 2016;263(5):1002–1009. doi: 10.1097/SLA.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 25.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228(3):355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel VI, Mukhopadhyay S, Ergul E, et al. Impact of hospital volume and type on outcomes of open and endovascular repair of descending thoracic aneurysms in the United States Medicare population. J Vasc Surg. 2013;58(2):346–354. doi: 10.1016/j.jvs.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 27.Xu Z, Becerra AZ, Justiniano CF, et al. Is the Distance Worth It? Patients With Rectal Cancer Traveling to High-Volume Centers Experience Improved Outcomes. Dis Colon Rectum. 2017;60(12):1250–1259. doi: 10.1097/DCR.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 28.Ravaioli M, Pinna AD, Francioni G, et al. A partnership model between high- And low-volume hospitals to improve results in hepatobiliary pancreatic surgery. Ann Surg. 2014;260(5):871–877. doi: 10.1097/SLA.0000000000000975. [DOI] [PubMed] [Google Scholar]
- 29.Anderson KL, Thomas SM, Adam MA, et al. Each procedure matters: threshold for surgeon volume to minimize complications and decrease cost associated with adrenalectomy. Surgery. 2018;163(1):157–164. doi: 10.1016/j.surg.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Adam MA, Thomas S, Youngwirth L, et al. Is There a Minimum Number of Thyroidectomies a Surgeon Should Perform to Optimize Patient Outcomes? Ann Surg. 2017;265(2):402–407. doi: 10.1097/SLA.0000000000001688. [DOI] [PubMed] [Google Scholar]
- 31.Gani F, Kim Y, Weiss MJ, et al. Effect of surgeon and anesthesiologist volume on surgical outcomes. J Surg Res. 2016;200(2):427–434. doi: 10.1016/j.jss.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Krautz C, Nimptsch U, Weber GF, Mansky T, Grützmann R. Effect of Hospital Volume on In-hospital Morbidity and Mortality Following Pancreatic Surgery in Germany. Ann Surg. 2017 Apr; doi: 10.1097/SLA.0000000000002248. [DOI] [PubMed] [Google Scholar]
- 33.Amini N, Spolverato G, Kim Y, Pawlik TM. Trends in Hospital Volume and Failure to Rescue for Pancreatic Surgery. J Gastrointest Surg. 2015;19(9):1581–1592. doi: 10.1007/s11605-015-2800-9. [DOI] [PubMed] [Google Scholar]
- 34.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49(12):1076–1081. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 35.Voss RK, Chiang Y-J, Torres KE, et al. Adherence to National Comprehensive Cancer Network Guidelines is Associated with Improved Survival for Patients with Stage 2A and Stages 2B and 3 Extremity and Superficial Trunk Soft Tissue Sarcoma. Ann Surg Oncol. 2017;24(11):3271–3278. doi: 10.1245/s10434-017-6015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi CR, Vecchiato A, Mastrangelo G, et al. Adherence to treatment guidelines for primary sarcomas affects patient survival: A side study of the european CONnective TIssue CAncer NETwork (CONTICANET) Ann Oncol. 2013;24(6):1685–1691. doi: 10.1093/annonc/mdt031. [DOI] [PubMed] [Google Scholar]
- 37.Derbel O, Heudel PE, Cropet C, et al. Survival impact of centralization and clinical guidelines for soft tissue sarcoma (A prospective and exhaustive populationbased cohort) PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0158406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray-Coquard I, Thiesse P, Ranchère-Vince D, et al. Conformity to clinical practice guidelines, multidisciplinary management and outcome of treatment for soft tissue sarcomas. Ann Oncol. 2004;15(2):307–315. doi: 10.1093/annonc/mdh058. [DOI] [PubMed] [Google Scholar]
- 39.Nijhuis PHA, Schaapveld M, Otter R, Hoekstra HJ. Soft tissue sarcoma - Compliance with guidelines. Cancer. 2001;91(11):2186–2195. doi: 10.1002/1097-0142(20010601)91:11<2186::aid-cncr1248>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 40.Hoekstra HJ, Haas RLM, Verhoef C, et al. Adherence to Guidelines for Adult (Non-GIST) Soft Tissue Sarcoma in the Netherlands: A Plea for Dedicated Sarcoma Centers. Ann Surg Oncol. 2017;24(11):3279–3288. doi: 10.1245/s10434-017-6003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37(2):204–209. doi: 10.1097/00005650-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Pezzi TA, Schwartz DL, Mohamed ASR, et al. Barriers to Combined-Modality Therapy for Limited-Stage Small-Cell Lung Cancer. JAMA Oncol. 2018;77030:1–5. doi: 10.1001/jamaoncol.2017.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin CC, Bruinooge SS, Kirkwood MK, et al. Association between geographic access to cancer care, insurance, and receipt of chemotherapy: Geographic distribution of oncologists and travel distance. J Clin Oncol. 2015;33(28):3177–3185. doi: 10.1200/JCO.2015.61.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. International Classification of Diseases for Oncology (ICD-O-3) topographical and morphological codes included.
Supplemental Table 2. Factors associated with treatment at a low volume hospital (≤ 10 cases/year)
Supplemental Table 3. Sensitivity analysis of hospital volume - results of various propensity score methods
Supplemental Table 4. Total number of primary retroperitoneal sarcoma surgical resections versus percent of cases resected at high volume hospitals by year.
Supplemental Table 5. Demographics and treatment information of patients with primary retroperitoneal sarcoma who underwent surgical resection, stratified by chemotherapy status.
Supplemental Table 6. Demographics and treatment information of patients with primary retroperitoneal sarcoma who underwent surgical resection (without chemotherapy), stratified by treating hospital volume.
Supplemental Table 7. Cox model for overall survival among patients with primary retroperitoneal sarcoma surgically resected, stratified by chemotherapy status.
Supplemental Table 8. Cox model for overall survival among patients with primary retroperitoneal sarcoma surgically resected with Charlson-Deyo score available (2003–2011 only), excluding patients who received chemotherapy (n=3524).
Supplemental Figure 1. Overall survival of patients with primary retroperitoneal sarcoma undergoing surgical resection by hospital volume (1998–2011). Survival curves for overall survival among all patients adjusted for older age, gender, insurance, larger tumor size, radiation therapy and (A) low and (B) intermediate/high grade RPS.