Abstract
Microvessels of the blood-brain barrier (BBB) regulate transport into the brain. The highly specialized brain microvascular endothelial cells, a major component of the BBB, express tight junctions and efflux transporters which regulate paracellular and transcellular permeability. However, most existing models of BBB microvessels fail to exhibit physiological barrier function. Here, using (iPSC)-derived human brain microvascular endothelial cells (dhBMECs) within templated type I collagen channels we mimic the cylindrical geometry, cell-extracellular matrix interactions, and shear flow typical of human brain post-capillary venules. We characterize the structure and barrier function in comparison to non-brain-specific microvessels, and show that dhBMEC microvessels recapitulate physiologically low solute permeability and quiescent endothelial cell behavior. Transcellular permeability is increased two-fold using a clinically relevant dose of a p-glycoprotein inhibitor tariquidar, while paracellular permeability is increased using a bolus dose of hyperosmolar agent mannitol. Lastly, we show that our human BBB microvessels are responsive to inflammatory cytokines via upregulation of surface adhesion molecules and increased leukocyte adhesion, but no changes in permeability. Human iPSC-derived blood-brain barrier microvessels support quantitative analysis of barrier function and endothelial cell dynamics in quiescence and in response to biologically- and clinicallyrelevant perturbations.
Keywords: blood-brain barrier, permeability, microvessels, induced pluripotent stem cells, brain microvascular endothelial cells, tissue engineering
1. Introduction
The blood-brain barrier (BBB) maintains tight control of the brain microenvironment by regulating fluctuations in chemistry, transport of immune cells, and entry of pathogens and toxins.1–3 The brain microvascular endothelial cells (BMECs) that form the lumen of the cerebrovasculature are highly specialized, expressing tight junctions which effectively block paracellular transport, and an array of transporters and efflux pumps which regulate transcellular transport. Additionally, BMECs display low turnover and motility to maintain a quiescent state in the healthy cerebrovasculature. Differences in the cerebrovasculature between humans and rodents mean that animal models do not always recapitulate human disease.4 Therefore, in vitro models can provide an important link between human physiology and animal models, and have the potential to contribute to elucidating disease mechanisms and developing new strategies for drug and gene delivery to the brain. However, for widespread adoption, these in vitro models must achieve physiological barrier function and endothelial cell behavior.
Advances in tissue engineering have led to the development of a new generation of perfusable three-dimensional (3D) models of the BBB.5–8 However, recapitulating physiological tight junction formation and barrier function has been particularly challenging, largely due to the fact that primary and immortalized human and animal brain microvascular endothelial cells exhibit transendothelial electrical resistance (TEER) values well below the range thought to be physiological (1,500 – 8,000 Ω cm2).9–12 To overcome this limitation, many existing BBB models incorporate supporting cell types of the neurovascular unit (i.e. astrocytes and pericytes) which improve barrier function, but still do not achieve physiological TEER or permeability. Human induced pluripotent stem cells (iPSCs) differentiated into BMECs (dhBMECs) display many of the hallmarks of the BBB in two-dimensional (2D) transwell assays including physiological TEER, permeability and efflux behavior.13–16 Interestingly, BBB phenotype is achieved without supporting cell types. In previous work we have reported on the role of matrix composition and stiffness on the adhesion and barrier formation of dhBMECs relevant to tissue engineering of 3D microvessel models.17
Here, we report on characterization of an in vitro human iPSC-derived blood-brain barrier microvessel model, resembling brain post-capillary venules (PCVs). PCVs are characterized by diameters of around 100 μm, a relatively thick basement membrane, a perivascular space with limited supporting cells, and a wall shear stress of 1 – 4 dyne cm-2.18–21 PCVs are the site for immune surveillance and preferential extravasation of leukocytes, tumor cells, parasites and viruses.22–28 We report on the structure (i.e. endothelial cell behavior) and function (i.e. permeability) of human iPSC-derived blood-brain barrier microvessels, in comparison to nonbrain-specific microvessels constructed using human umbilical vein endothelial cells (HUVECs). We show that the microvessels comprised of dhBMECs recapitulate key aspects of the human BBB including physiologically low solute permeability, cell turnover, and cell motility. Compared to self-organizing approaches recently developed for BBB modeling, which mimic angiogenesis and vasculogenesis, our microvessels are functional after only two days of culture and without the complexities of co-culture with astrocytes or pericytes.29, 30 We provide proof-of-principle examples of how a physiological BBB microvessel model can be used to study biologically- and clinically-relevant processes, including patient-specific models of neurodegenerative disease, efflux inhibition, hyperosmolar opening of the BBB, and activation by inflammatory cytokines.
2. Materials and Methods
2.1. Cell culture
Human brain microvascular endothelial cells (dhBMECs) were differentiated from induced pluripotent stem cells (iPSCs) as previously reported.15 Briefly, iPSCs were singularized using StemPro accutase (Thermo Fisher) and seeded at 15,000 cells cm−2 on Matrigel-treated six-well plates (Corning). Cell lines used: BC1 and iPS12,31 KW01, and AD6.32 The BC1 and iPS12 lines were derived from healthy individuals. The AD6 line was derived from skin fibroblasts (Coriell Institute) of a 56-year-old male with Alzheimer’s disease harboring the familial AD PSEN1 mutation A246E.32 The KW01 line was derived from a 73-year-old male with multiple sclerosis. All iPSC lines were used between passage 30 – 70. Cells were cultured in TeSR-E8 media (Stem Cell Technologies) for three days to approximately 40% confluence, with 10 μM ROCK inhibitor Y27632 (ATCC) supplemented for the first 24 hours. The differentiation was then initiated in unconditioned media without bFGF (UM/F-): DMEM/F12 (Life Technologies) supplemented with 20% knockout serum replacement (Life Technologies), 1% non-essential amino acids (Life Technologies), 0.5% GlutaMAX (Life Technologies) and 0.836 μM beta-mercaptoethanol (Life Technologies). After six days of UM/F- culture, cells were cultured for two days in BBB induction media: human endothelial cell serum-free media (Life Technologies) supplemented with 1% human platelet poor derived serum (Sigma), 2 ng mL−1 bFGF (R&D Systems), and 10 μM all-trans retinoic acid (Sigma). Media was changed every 24 hours. After a total of 11 days in culture, cells were detached using accutase and sub-cultured onto plates coated overnight with 50 μg mL−1 human placental collagen IV (Sigma) and 25 μg mL−1 fibronectin from human plasma (Sigma). Sub-cultures were conducted at a 1:1 surface area ratio (one well of differentiated cells sub-cultured onto one well of six-well plate) and in BBB induction media supplemented with 10 μM ROCK inhibitor Y27632. After one hour, cell monolayers were washed three times with phosphate buffered saline (PBS; Thermo Fisher), detached with accutase, and seeded to form microvessels once resuspended in BBB induction media supplemented with 10 μM ROCK inhibitor Y27632 to 50 million cells mL-1.
Human umbilical vein endothelial cells (HUVECs) were used up to passage six. HUVECs were grown in MCDB 131 (Caisson) supplemented with 10% fetal bovine serum (Sigma), 1% pen-strep-glut (Thermo Fisher), 1 μg mL−1 hydrocortisone (Sigma), 10 μg mL−1 heparin (Sigma), 25 μg mL−1 endothelial cell growth supplement (Thermo Fisher), 0.2 mM ascorbic acid 2phosphate (Sigma), and 80 μM dibutyryl cyclic-adenosine monophosphate (db-cAMP; Sigma). TrypLE Express (Life Technologies) was used to detach cells for routine passing and seeding of microvessels. HUVECs were resuspended to 10 million cells mL−1 in media for seeding microvessels.
2.2. Microvessel fabrication, perfusion, and maintenance
To fabricate microvessels, 1 cm (length) x 1.75 mm (width) x 1 mm (height) channels were patterned in a Sylgard 184 polydimethylsiloxane (PDMS; Dow Corning) chip using a custom-made aluminum mold (Fig. 1). Inlet and outlet ports, along with other access ports, were formed using a hole punch (McMaster-Carr). The PDMS chip was then plasma-treated and adhered to 24 × 50 mm glass slides (Dow Corning). A 150 μm diameter super-elastic nitinol wire (Malin Co.) was suspended within the chip using guidance channels patterned within the PDMS as previously reported.33 Devices were then cleaned with ethanol and treated with trimethoxysilane (Sigma) to reduce formation of bubbles at the PDMS-collagen interface. Neutralized 7 mg mL−1 rat tail type I collagen (Corning) was gelled around a template wire for 30 minutes at 37°C. To prevent delamination of the gel, 2% agarose was added to both sides of the collagen gel. Following removal of the template wires, the bare channels were cross-linked with 20 mM genipin (Wako Biosciences) for two hours. Residual genipin was removed by perfusing channels under high flow (~0.5 mL h−1) with PBS for at least 12 hours. Lastly, channels were coated overnight with 50 μg mL−1 collagen IV and 25 μg mL−1 fibronectin in either HUVEC or dhBMEC microvessel media (composition outlined below). dhBMEC microvessels were seeded and perfused using brain microvessel media: endothelial cell serum-free media (Life Technologies) supplemented with 1% human platelet poor derived serum (Sigma), 1% penicillin-streptomycin (Life Technologies), 400 μM db-cAMP, 20 μM phosphodiesterase inhibitor Ro-201724 (Calbiochem), and 3% 70-kDa dextran (Sigma). Both retinoic acid and bFGF (present during the last two days of the differentiation) were removed from the microvessel media. Additionally, 10 μM ROCK inhibitor Y27632 was supplemented during the first 24 hours of microvessel perfusion to increase adhesion.17, 34
Figure 1.
Fabrication, perfusion, and maintenance of human iPSC-derived blood-brain barrier microvessels. (A,B) Schematic illustrations of the side- and end-view of 150 μm diameter microvessel fabrication. Type I collagen and agarose is gelled around suspended wire. Wire removal results in a bare channel, which is sequentially treated with genipin and ECM proteins. 3D microvessels form following cell seeding. (C) Phase contrast images of sequential microvessel fabrication steps. (D) PDMS-based microfluidic chip. (E) Perfusion system comprised of tubing connecting inlet and outlet ports to an upper and lower media reservoir (Δh = 5 cm). (F) Shear stress for BC1 dhBMEC and HUVEC microvessels over six days. (G) Phase contrast images of dhBMEC microvessels constructed from various iPSC lines including BC1, iPS12, KW01, and AD6 on day two after seeding. (H,I) Fluorescence images of blood-brain barrier markers in BC1 dhBMEC microvessels on day 2: zona occluden-1 (ZO1), occludin, claudin-5, glucose transporter-1 (GLUT1), and P-glycoprotein (P-gp). Images shown are a 0.4 μm confocal z-slice of the bottom microvessel pole, or a cross-section of the microvessel. Nuclei visualized with DAPI (blue).
HUVEC microvessels were seeded and perfused using HUVEC microvessel media: HUVEC cell culture media supplemented with 400 μM db-cAMP, 20 μM Ro-20–1724, and 3% 70-kDa dextran. These additions have previously been shown to promote barrier function and long-term stability of in vitro microvessels.35–37 Confluent HUVEC microvessels do not form using brain microvessel media (data not shown).
Microvessel shear stress was estimated by Poiseuille’s equation: τ = μQ / 2πd3 where μ is the fluid dynamic viscosity (~1.4 cP for media supplemented with 3% dextran), Q is the volumetric flow rate, and d is the microvessel diameter. The volumetric flow rate was measured daily as the increase in fluid volume in the lower media reservoir. A physiological shear stress of ~4 dyn cm−2 was maintained with a reservoir height difference (Δh) of 5 cm, corresponding to a volumetric flow rate of ~ 0.5 mL h-1. Changes in Δh due to changes in media levels within the upper and lower reservoirs were minimal (~6% over 24 hours), and hence we can assume that the wall shear stress remains approximately constant during the experiments.
2.3. Live-cell imaging
The strategy for live-cell imaging of dhBMEC and HUVEC microvessels is summarized in Figure 2. Microvessels were imaged using an inverted microscope (Nikon Eclipse Ti-E) maintained at 37° C and 5% CO2. Epifluorescence illumination was provided by an X-Cite 120LEDBoost (Excelitas Technologies). A 10x objective (Nikon) was used for all epifluorescence and phase-contrast images. Imaging of microvessels was conducted on days two, four and six after seeding (Fig. 2A).
Figure 2.
Live-cell imaging of human iPSC-derived blood-brain barrier microvessels. (A) representative phase contrast images of a BC1-derived brain microvessel on days two, four and six under a wall shear stress of about 4 dyne cm-2. (B) Phase contrast and fluorescence images of perfusion with Lucifer yellow, Rhodamine 123, and 10 kDa dextran at the microvessel midplane. (C,D) Phase contrast images at the top and bottom planes, respectively. (E) Representative fluorescence intensity for Lucifer yellow for a region of interest comprising both the microvessel and surrounding matrix: (i) Prior to perfusion of the dye. (ii) luminal filling where ΔI represents the increase in fluorescence intensity. (iii) Penetration of the dye into the surrounding matrix results in a linear increase in fluorescence intensity (dI/dt).
Permeability was simultaneously measured for three different molecular weight solutes introduced into the upper media reservoir at a final concentration of 200 μM Lucifer yellow (CH dilithium salt; LY) (Sigma), 5 μM Rhodamine 123 (R123) (Thermo Fisher), and 2 μM Alexa Flour-647-conjugated 10 kDa dextran (Thermo Fisher). LY is a small (444.3 Da) negatively charged water-soluble dye used in vivo and in vitro to assess BBB integrity. R123 is a small molecule (380.8 Da) that is a substrate for efflux pumps, including p-glycoprotein. 10 kDa dextran conjugated to Alexa Fluor-647 is a large molecular-weight polysaccharide used as a marker of vascular permeability. A NIS Elements (Nikon) imaging protocol was initiated to acquire both phase contrast and fluorescence images every two minutes for two hours (61 total frames). At every time point six images were collected (Fig. 2B-D): (1) a phase contrast image of the top of the microvessel (located and maintained by autofocus), (2–5) phase contrast and fluorescence images of the microvessel midplane, and (6) a phase contrast image of the bottom of the microvessel. To independently excite and collect the emission from each fluorophore, three filter cubes were used: Chroma 39008 for Lucifer yellow (20 ms exposure), Chroma 49003 for Rhodamine 123 (50 ms exposure), and Chroma 41008 for Alexa Fluor-647-conjugated dextran (200 ms exposure). The total image area was 8.18 mm x 0.67 mm, corresponding to ten adjacent frames using a 10x objective.
2.4. Permeability
Large images were cropped to an area of 1 mm x 0.67 mm (corresponding to a single 10x magnification frame); a central region of interest (ROI) was chosen to minimize contributions of interstitial flow of solutes from the inlet and outlet. Intensity profiles were obtained using ImageJ (NIH). The apparent permeability (cm s−1) is calculated as P = (d/4)(1/ΔI)(dI/dt), where d is the vessel diameter, ΔI is the initial increase in fluorescence intensity upon luminal filling, and (dI/dt)0 is the rate of increase in fluorescence intensity as solute exits into the gel (Fig. 2E).38, 39 Over the course of two hour imaging experiments there are four phases: (i) before the solute reaches the microvessel lumen, (ii) while the solute is filling the microvessel lumen (typically 20–30 minutes), (iii) while the solute permeates into the ECM, and (iv) when solute continues to permeate into ECM and where interstitial flow of solutes interferes with quantification. After luminal filling, the intensity increase (dI/dt) was calculated over twenty minutes. For Rhodamine 123, to eliminate the contributions of intracellular accumulation, the permeability was also calculated by considering only the rate of increase in fluorescence intensity in the ECM; unless otherwise noted this value is reported for Rhodamine 123 permeability.33 The detection limit for permeability of Alexa Flour-647-conjugated 10 kDa dextran (≤ 1 × 10−7 cm s−1) was limited by photobleaching of the genipin cross-linked collagen gel. Some dhBMEC microvessels displayed macroscopic breakdown past day four of perfusion (~30% of devices); these microvessels were not considered in time course permeability analysis. Monolayer breakdown is not observed on transwells where the substrate is much stiffer. Thus, further increasing the stiffness of the matrix, or incorporation of additional factors to promote adhesion could further support microvessel longevity.
For studies of efflux inhibition, 2 μM tariquidar (Sigma) was supplemented in brain microvessel media at 36 hours after cell seeding. Day two permeability and cell behavior analysis was conducted as outlined above after 12 hours of tariquidar exposure. To determine the relative contributions of sequestration and transport of solutes we calculated permeability considering an ROI excluding the lumen and intracellular compartment, and an ROI of the entire image frame.
2.5. Cell behavior
To assess endothelial cell behavior in microvessels, we quantified cell area (μm2), proliferation rate (% h−1), the rate of cell loss (% h−1), turnover rate (% h−1), root mean square (RMS) displacement (μm), path length (μm), number of nearest neighbors, and frequency of nearest neighbor change (h−1) of BC1 dhBMEC microvessels. Details of the protocols have been published previously.40–43 Phase contrast image sequences from the top and bottom planes were independently analyzed at each time point using ImageJ. Analysis was performed on an ROI ~100 μm wide centered along the top or bottom of the microvessel and over the entire length (typically 5 – 7 mm). This width was selected to minimize foreshortening of the lumen due to microvessel curvature. Any regions along the microvessel length that were significantly out of focus were excluded from the ROI. The total number of cells was quantified by identifying individual cell nuclei within the ROI. Cell area was calculated by dividing the total area of the region of interest by the number of cell nuclei. From analysis of fluorescence images following staining with DAPI and f-actin, we independently verified that there was less than 10% error between the measurements (see Supplementary Figure S1 for details).
The rates of cell proliferation and cell loss were quantified through identification of cell division and loss events within the ROI. Proliferation events are easily identified in time-lapse phase contrast images from cell compression, alignment of chromosomes, and the formation of daughter cells. These steps occur over ~40 minutes, allowing us to visualize cell division over a minimum of 5 – 20 frames. Cell loss from the monolayer was identified by constriction of the cell body, removal of the cell from the monolayer, and the subsequent movement of the surrounding cells to fill the area of the lost cell. Cell loss is more challenging to identify than cell proliferation as the lysed cell contents create debris in the monolayer. Despite these challenges, identification of cell loss events are clearly seen in an image sequence. Events were monitored over the duration of time-lapse imaging and labeled with their location and time at which they occurred. The rates of cell proliferation and cell loss were determined by summing the number of events in the imaging window and dividing by the total number of cells within the ROI (n = 16753 dHBMECs and n = 14042 HUVEC cells sampled for analysis).
To assess cell motility, we tracked the location of the cell nuclei within the region of interest over two hours of imaging. RMS displacement is a measure of how far the cell moved from its original position, while path length is a measure of the total distance cells traverse. At least ten cells were randomly selected over the length of the microvessel per timepoint for motility analysis (n = 291 dHBMECs and n = 171 HUVEC cells sampled for analysis). Using ImageJ, a point was placed on the centroid of the cell nucleus at each frame, resulting in an array of (x, y) coordinate pairs for each time point within the time-lapse sequence. A reference point outside the microvessel was tracked to correct for x-y shifts in the imaging plane.
Additionally, from phase contrast images we quantified the number of cell neighbors for each cell, as well as the frequency of change in cell neighbors. Cells are considered neighbors when they share a border, and the number of cell neighbors can change due to proliferation, cell loss, cell movement, or due to redistribution of tight junctions. From analysis of fluorescence images stained for f-actin, we verified that there was less than 10% error in the number of cell neighbors between the two methods (see Supplementary Figure S1 for details).
2.6. Immunocytochemistry
On day two after seeding, microvessels were washed with PBS for 5 minutes, fixed with 3.7% paraformaldehyde (Sigma) for 15 minutes, permeabilized with 0.1% Triton X-100 (Sigma) for 15 minutes, and blocked with 1% donkey serum (Sigma) overnight at 4°C. Microvessels were incubated for 6 hours at 4°C with primary antibodies (see Supplementary Table 1 for details) and for 20 minutes at room temperature with Alexa Flour-647 and Alexa Flour-488 secondary antibodies (Life Technologies). To localize nuclei or f-actin, 1:500 DAPI solution (Thermo Scientific) and 1:50 Alexa Fluor-647 phalloidin (Invitrogen) were added, respectively. Confocal z-stacks were obtained on a swept field confocal microscope system (Prairie Technologies) with illumination provided by MLC 400 monolithic laser combiner (Keysight Technologies). To fully reconstruct microvessels, approximately four hundred 0.4 μm slices were acquired using a 40x objective (Nikon).
2.7. Hyperosmolar microvessel opening
An intra-arterial osmotic procedure is used in neuro-oncology for transient opening of the blood-brain barrier to facilitate drug delivery to tumors. To model this procedure, 250 mg mL−1 d-mannitol (Sigma) in brain microvessel media was introduced into the upper media reservoir for five minutes on day two following seeding. Mannitol was then replaced with 200 μM Lucifer yellow and 2 μM Alexa Fluor-647-conjugated 10 kDa dextran in brain vessel media. Live-cell imaging and analysis was conducted as previously outlined. Additionally, structural changes observed after exposure to mannitol (i.e. intracellular vacuoles) were counted in ImageJ using time-lapse phase contrast images of microvessel poles cropped to an area of twenty cells; vacuole count was normalized to the number of cells.
2.8. Leukocyte adhesion
To determine the effect of tumor necrosis factor alpha (TNFα) activation on permeability and leukocyte adhesion, two media conditions were used. In control experiments, microvessels were perfused with endothelial cell serum-free media supplemented with 1% human platelet poor derived serum and 1% penicillin-streptomycin. To model inflammatory conditions, microvessels were perfused with control media supplemented with 10 ng mL−1 TNFα (Thermo Fisher) for 12 hours. On day two, either permeability was tested (as previously summarized) or leukocyte adhesion was tested. For both conditions, microvessels were maintained at ~1 dyn cm−2 shear stress using fluid reservoirs directly connected to the inlet and outlets. Human peripheral blood mononuclear cells (PBMCs, StemCell Technologies), predominately comprised on lymphocytes and monocytes, were used for adhesion studies. PBMCs were freshly thawed and resuspended in RPMI-1640 media supplemented with 10% FBS and 1% penicillin-streptomycin before use. Prior to the microvessel experiments, PBMCs were labeled by incubating with 5 μM Calcein AM (Thermo Fisher) in their resuspension media for 30 minutes. PBMCs were added to the inlet at a concentration of 106 cells mL−1 and allowed to flow through the microvessel for a duration of 30 minutes. After five minutes of washout, fluorescent images of the microvessel poles and midplane were taken to quantify adherent cells, which were normalized to microvessel length. VCAM-1 and ICAM-1 expression was visualized using immunocytochemistry as previously described.
2.9. Statistical Analysis
All statistical analysis was performed using Prism ver. 6 (GraphPad). Experimental metrics (i.e. permeabilities, shear stresses, turnover) are presented as means ± standard error of the mean (SEM). All experimental metrics were collected across at least three biological replicates, representing at least two independent differentiations of dhBMECs. The principle statistical tests used were a student’s unpaired t-test (two-tailed with unequal variance) for comparison of two groups and an analysis of variance (ANOVA) for comparison of three or more groups. Reported p values were multiplicity adjusted using a Tukey test. A one sample t-test was used to determine if sample mean was statistically different from zero. Differences were considered statistically significant for p < 0.05, with the following thresholds: * p < 0.05, ** p < 0.01, *** p < 0.001.
3. Results
3.1. Human iPSC-derived blood-brain barrier microvessels express blood-brain barrier markers and maintain stable perfusion over at least six days
Using a templating method we have engineered a functional model of a human PCV using dhBMECs (Fig. 1). In previous work we screened matrix composition, stiffness, and soluble factors to promote adhesion and spreading of dhBMECs in tissue-engineered microvessels.17 Here, dhBMECs were seeded into a cylindrical 150 μm diameter channel in 7 mg mL−1 type I collagen gels, cross-linked with 20 mM genipin and coated with fibronectin and type IV collagen. Additionally, ROCK inhibitor Y27632 was added to promote cell survival over the first 24 hours of culture. Microvessels were continually perfused using gravity-driven flow reservoirs (Fig 1E), and from analysis of volumetric flow rates the wall shear stress was determined to be around 4 dyne cm−2, typical of PCVs (Fig. 1F). This approach enabled the formation of microvessels with dhBMECs derived from multiple iPSC lines (Fig. 1G). dhBMEC microvessels formed from the BC1 iPSC line displayed proper localization of the tight junction proteins zonula occludens-1 (ZO-1), claudin-5 (CLDN-5) and occludin (OCLN), and blanket expression of glucose transporter 1 (GLUT1) and the p-glycoprotein (P-gp) efflux pump (Fig. 1H,I).
3.2. Blood-brain barrier microvessels display physiological barrier function
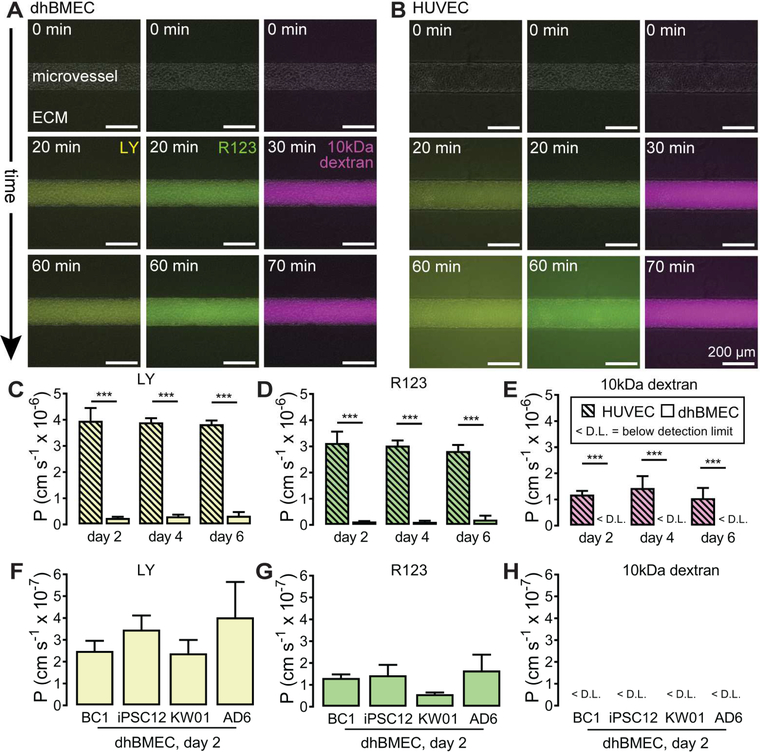
The permeability of dhBMEC and HUVEC microvessels was determined on day two, four and six from simultaneous measurement of the transport of three fluorescent probes: LY, R123, and 10 kDa dextran. Representative phase / fluorescence overlays of microvessels during permeability measurements show perfusion of the solute in the lumen and transport into the ECM (Fig. 3A,B, Supplementary Video 1). BC1 dhBMEC microvessels displayed stable permeability over six days (Fig. 3C-E). The permeability of LY was 2.50 ± 0.46 × 10−7 cm s−1 on day two and did not increase on days four and six (p > 0.05), with an average value of 2.84 ± 0.41 × 10−7 cm s−1 across all time points. The permeability of R123 in dhBMEC microvessels was 6.61 ± 0.26 × 10−7 cm s−1 on day two when considering the entire ROI for analysis; however, if the microvessel lumen and cells are excluded it was 1.32 ± 0.16 × 10−7 cm s-1. This dramatic difference, which was not observed with other solutes (data not shown), was due to significant intracellular accumulation of the dye. R123 permeability remained stable on day four and six (p > 0.05). The permeability of 10 kDa dextran in dhBMEC microvessels was below the detection limit at all timepoints. We observed no plumes of solute entering the ECM associated with focal leaks, indicating tight junction stability (Supplementary Video 1).
Figure 3.
Permeability of human iPSC-derived blood-brain barrier microvessels. Representative phase / fluorescence overlays of Lucifer yellow (LY), Rhodamine 123 (R123), and 10kDa dextran perfusion in (A) BC1 dhBMEC microvessels, and (B) HUVEC microvessels. t = 0 min represents the frame prior to initiation of luminal filling. Luminal filling occurred on average over 20 minutes for LY and R123, and 30 minutes for 10 kDa dextran. The rate of fluorescence change was determined over subsequent 20 minutes after luminal filling. (C-E) Permeability of LY, R123, and 10 kDa dextran in HUVEC and BC1 dhBMEC microvessels. (FH) Permeability of LY, R123, and 10kDa dextran in dhBMEC microvessels derived from multiple iPS cell lines (BC1, iPS12, KW01, AD6) on day two following seeding. N = 7 BC1 dhBMEC microvessels on day 2, N = 4 BC1 dhBMEC microvessels on day 4 and 6, N = 4 HUVEC microvessels across all timepoints, N = 3 iPSC12, KW01 and AD6 microvessels. *** p < 0.001.
To assess whether the barrier function of BC1 dhBMEC microvessels was robust, we formed microvessels with dhBMECs derived from the iPS12, KW01 and AD6 lines. Microvessels generated from the iPS12, KW01, and AD6 lines also display low solute permeability (Fig. 3F-H). There were no statistically significant differences in LY, R123, or 10 kDa dextran permeability between BC1, iPS12, KW01 and AD6 microvessels on day two (p > 0.05 for all comparisons). The barrier function of these four cell lines was also assessed by measuring TEER in a standard transwell assay, as previously reported.15 Day two TEER was 2,260 ± 39 Ω cm2 for BC1s, 2260 ± 73 Ω cm2 for iPS12s, 1330 ± 343 Ω cm2 for KW01s, and 2160 ± 514 Ω cm2 for AD6s. TEER values for the KWO1 cell line were significantly lower than for the BC1 (p = 0.010) and iPSC12 (p = 0.021) lines from healthy individuals.
To provide a comparison for the permeability of BC1 dhBMEC microvessels, we performed the same experiments in HUVEC microvessels. HUVEC microvessels also display stable permeability over six days (Fig. 3C-E). There were no statistically significant differences in permeability between day two, four and six for LY, R123 and 10 kDa dextran (p > 0.05 for all comparisons). From the phase / fluorescence overlays (Fig. 3B), it is evident that the extent of penetration of these solutes into the ECM was significantly larger than in the dhBMEC microvessels. The mean permeability for LY over six days was 3.90 ± 0.16 × 10−6 cm s−1, more than 10-fold higher than in BC1 dhBMEC microvessels. Similarly, the permeability of R123 was 3.00 ± 0.16 × 10−6 cm s−1, about 20-fold higher than in BC1 dhBMEC microvessels. The permeability of 10 kDa dextran over six days was 1.23 ± 0.19 × 10−6 cm s-1. No focal leaks were observed in HUVEC microvessels during solute perfusion. A similar value for the permeability of 10 kDa dextran and the absence of focal leaks have previously been reported in HUVEC microvessels cultured under similar conditions.35
3.3. Blood-brain barrier microvessels display distinct endothelial cell dynamics
The behavior of endothelial cells was assessed from analysis of phase contrast images at the microvessel poles (Fig. 4, Supplementary Video 2). Rates of cell proliferation, loss, and turnover were determined by direct counting of cell events to eliminates possible errors associated with staining markers to quantify proliferation and cell loss (Fig. 4A,B).44
Figure 4.
Endothelial cell turnover of human iPSC-derived blood-brain barrier microvessels. (A) Representative time-lapse images depicting cell division. Identification of cell division begins with alignment of DNA along the equatorial plate (t = 32 mins, white arrow), chromosomes can be identified pulling apart (t = 34 mins, white arrows), directly proceeding the formation of two daughter cells. (B) Representative time-lapse images depicting cell loss. Identification of cell loss begins with contraction of cell boundaries (t = 6 mins), followed by lysing of cell contents into the lumen associated with a breakdown of the cell membrane (t = 12 mins, white arrow). As the remaining portions of the cell envelope are shed into circulation the surrounding cells migrate to fill the space where the cell was removed. (C-E) Rates of proliferation, cell loss, and turnover on days 2, 4, and 6 for BC1 dhBMEC and HUVEC microvessels. (F) Cumulative proliferative and cell loss events during imaging of BC1 dhBMEC microvessels. N = 3 BC1 dhBMEC and HUVEC microvessels. * p < 0.05, ** p < 0.01, *** p < 0.001.
In dhBMEC microvessels the proliferation rate decreased significantly over time, from 1.66 ± 0.281 % h−1 on day two to 0.199 ± 0.064 % h−1 on day six (p < 0.001) (Fig. 4C). The cell loss rate also decreased significantly from 1.015 ± 0.067 % h−1 on day two to 0.334 ± 0.083 % h−1 on day six (p < 0.001) (Fig. 4D). The net turnover calculated by subtracting the rate of cell loss from the rate of proliferation, decreased from 0.648 ± 0.302 % h−1 on day two to −0.134 ± 0.050 % h−1 on day six (p = 0.012); the resulting day six turnover was not statistically different from zero (p = 0.115) (Fig. 4E). Proliferation and cell loss events were equally distributed over the entire two-hour imaging window, as evident by linear increases in cumulative events as a function of time (r > 0.98 for each plot); while on day two proliferation events occurred more frequently than cell loss events, this trend reversed by day six (Fig. 4F).
In HUVEC microvessels the rates of proliferation, cell loss, and turnover remained stable over six days (p > 0.05 for all comparisons). HUVECs displayed lower cell rates of proliferation and loss than dhBMECs on day two and four (p < 0.05 for all comparisons) (Fig. 4C,D). By day six, the rates of proliferation, cell loss, and turnover were not statistically different between HUVECs and dhBMECs (p > 0.05 for all comparisons) (Fig. 4C-E). It is difficult to completely decouple endothelial cell behavior across both cell lines due to differences in media composition; however, high db-cAMP levels used in both microvessel medias have previously been found to reduce cell turnover.35
Phase contrast images, DAPI stains, and f-actin stains confirm that the average dhBMEC area ranges from about 600 to 700 μm2, corresponding to an average cell diameter of ~28 μm (Supplementary Figure S1). This is a useful metric for comparing length scales of cell motion. RMS displacement for dHBMECs did not change over time (p > 0.05 for all comparisons) and was on average 8.72 ± 0.75 μm over two hours of imaging (Fig. 5A). The RMS displacement for HUVECs was not significantly different than dhBMECs (p > 0.05 for all comparisons), similarly remaining below 10 μm. We observe that the typical dhBMEC and HUVEC displacement was less than the diameter of an average cell, and that the cell motility corresponds predominantly to small fluctuations in position within the monolayer (Fig. 5B-D). There was no clear spatial directionality of cell motility, as across all dhBMECs the average displacement vector over two hours of imaging was 1.16 μm, 0.22 μm (x, y) (Fig. 5C). Furthermore, cell path length widely varied between individual dhBMECs, but was on average an order of magnitude higher than RMS displacement (Fig. 5D).
Figure 5.
Endothelial cell motility of human iPSC-derived blood-brain barrier microvessels. (A) Root mean square (RMS) displacement. (B) Representative path for a dhBMEC (blue line) and HUVEC (black line) cell on day 2 following seeding. (C) Scatter plot showing the change in dhBMEC position over two hours normalized to the initial position for all cells tracked, the average cell displacement vector (x, y) = (1.16 μm, 0.22 μm). (D) Scatter plots of dhBMEC cell path and RMS displacement (dotted black line denotes average cell diameter). (E) Number of cell neighbors. (F) Fluorescence image showing representative six cell neighbors of dhBMEC microvessels. (G) The frequency of change in the number of cell neighbors. (H-I) Fluorescence images showing a stain for f-actin and basement membrane protein laminin α4 on the bottom pole of a microvessel on days 2 and 6. (J) Cross section of laminin α4 on days 2 and 6. N = 3 BC1 dhBMEC and HUVEC microvessels.
To assess the spatial organization of endothelial cells within the microvessels, we determined the number of neighbors that share a boundary for each cell. The average number of cell neighbors for both dhBMECs and HUVECs was between six and seven (Fig. 5E,F). The frequency of change in number of cell neighbors for dhBMEC microvessels was 0.406 ± 0.054, suggesting that cells experience a change in number of cell neighbors once every 2.5 hours. This frequency was the same across cell types and time points (p > 0.05 for all comparisons) (Fig. 5G).
To assess cytoskeletal organization, we stained dhBMEC microvessels for f-actin (Fig. 5H). On day two, f-actin was localized to cell-cell junctions with no preferred orientation. After six days, we also observe some intracellular stress fibers in addition to localization at cell-cell junctions, although they do not appear to be aligned with flow. These observations have previously been reported for primary human BMECs in 2D monolayers.42 To assess basement membrane maturation we stained dhBMEC microvessels for laminin α4 (Fig. 5I,J); we did not stain for collagen IV and fibronectin as they were used to coat collagen channels to promote cell adhesion. On day two, expression was punctate, but by day six, laminin expression was uniform around the microvessel.
3.4. Blood-brain barrier microvessels are responsive to P-gp inhibition
Treatment with P-gp inhibitor tariquidar increased R123 (a P-gp substrate) permeability from 1.32 ± 0.16 × 10−7 cm s−1 to 2.66 ± 0.44 × 10−7 cm s−1 (p = 0.015) (Fig. 6A,B). The permeability of LY did not change in the presence of tariquidar (p > 0.05), while the permeability of 10 kDa dextran remained below the detection limit (Fig. 6B). R123 accumulates within endothelial cells and results in increasing fluorescence intensity during permeability experiments. The ratio of permeability calculated from the ECM (by excluding the lumen / intracellular compartment) and permeability calculated by the entire imaging frame (PECM / Ptotal) was ~0.8 for LY in BC1 dhBMEC and HUVEC microvessels, indicating low intracellular accumulation. For R123, PECM / Ptotal was 0.749 ± 0.022 for HUVEC microvessels, 0.203 ± 0.027 for BC1 dhBMEC microvessels, and 0.381 ± 0.031 for tariquidar treated BC1 dhBMEC microvessels (Fig. 6C). These results show that: (1) dhBMEC microvessels display greater intracellular R123 accumulation compared to HUVECs (as indicated by a lower ratio, p < 0.0001) and (2) tariquidar treatment increases the contribution of transcellular transport compared to intracellular accumulation compared to non-treated controls (as indicated by a higher ratio, p = 0.0046). The ratio of LY to R123 permeabilities (PLY / PR123) indicates the relative rates of paracellular and transcellular transport (Fig. 6D). dHBMECs and HUVECs display values of PLY / PR123 of ~1.9 and ~1.3, respectively. With 12 hours of tariquidar perfusion PLY / PR123 was < 1 suggesting an increase in the transcellular transport (no statistical significance, p > 0.05). We note that R123 is an active mitochondrial stain and increased accumulation could result from a higher mitochondrial density in dhBMECs. Indeed, increased punctate localization of R123 is visible in dhBMEC microvessels compared to HUVECs, although tariquidar had no noticeable effect on mitochondrial localization (Fig. 6E). Additionally, 12 hours of tariquidar exposure did not result in apparent cytotoxicity as observed by preserved barrier function and microvessel structure (Fig. 6F).
Figure 6.
Efflux inhibition of human iPSC-derived blood-brain barrier microvessels. (A) Phase / fluorescence overlays showing Rhodamine 123 (R123) permeability in BC1 dhBMEC microvessels treated with 2 μM tariquidar for 12 hours. (B) Comparison of BC1 dhBMEC microvessel permeability with and without tariquidar treatment. (C) The ratio of R123 permeability calculated only considering the ECM to including the ECM, lumen and intracellular compartments (PECM/Ptotal) in HUVEC and BC1 dhBMEC microvessels with and without tariquidar treatment. (D) Ratio of Lucifer yellow (LY) permeability to R123 permeability (PLY / PR123). (E) Cross section and pole confocal z-slice of R123 accumulation within HUVEC and BC1 dhBMEC microvessels with and without tariquidar treatment. (F) Phase contrast image of BC1 dhBMEC microvessels after tariquidar treatment. N = 3 BC1 dhBMEC microvessels exposed to tariquidar, compared to control BC1 dhBMEC microvessels. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.5. Hyperosmolar blood-brain barrier opening
To model a protocol used clinically for transient opening of the BBB, on day two we introduced mannitol to the upper fluid reservoir for five minutes and then immediately perfused dhBMEC microvessels with LY and 10 kDa dextran to study changes in permeability (Fig. 7AC, Supplementary Video 3). Fluorescence images show both global transport of LY and 10 kDa dextran into the ECM, as well as focal leaks indicating disruption of endothelial cell-cell junctions (Fig. 7B,C). Following mannitol injection, the average permeability of LY was 3.67 ± 1.39 × 10−6 cm s−1, approximately 15-fold higher than homeostatic BC1 dhBMEC microvessels on day two (p = 0.003) (Fig. 7D). Similarly, 10 kDa dextran permeability increased to 2.32 ± 0.86 × 10−6 cm s−1 (p = 0.005). Recall that the permeability of 10 kDa dextran was not detectable in dhBMEC microvessels. The average 10 kDa dextran permeability was also approximately two-fold higher than the baseline permeability in HUVEC microvessels (1.23 ± 0.19 × 10−6 cm s−1), although not statistically significant (p > 0.05).
Figure 7.
Hyperosmolar blood-brain barrier opening of human iPSC-derived blood-brain barrier microvessels. (A) Phase contrast images at the pole of a BC1 dhBMEC microvessel following five minute mannitol exposure. t = 0 represents the frame prior to arrival of mannitol into the lumen. A vacuole formed following mannitol exposure is indicated by an arrow. Corresponding phase / fluorescence overlays for (B) Lucifer yellow (LY) and (C) 10 kDa dextran. Plumes of 10kDa dextran entering the ECM are marked with arrows. (D) Day two permeability increases with mannitol exposure. (E) Vacuoles per cell over two hours of imaging. (F) Fluorescence image showing f-actin phalloidin stain at the pole of a microvessel following mannitol exposure. N = 3 BC1 dhBMEC microvessels exposed to mannitol, compared to control BC1 dhBMEC microvessels. ** p < 0.01.
The loss of intracellular water following mannitol exposure is associated with formation of vacuoles,45 which are observed in phase contrast images (Fig. 7A). The number of vacuoles plateaued over approximately 20 minutes following injection of mannitol, reaching a maximum value of approximately six per cell (Fig. 7E). LY and 10 kDa dextran remain excluded from endothelial cells (images not shown), indicating that vacuoles are not formed by invagination of the plasma membrane. Phalloidin staining following mannitol exposure shows increased stress fiber formation indicating actin cytoskeleton disruption (Fig. 7F).
3.6. Blood-brain barrier microvessels are responsive to cytokine activation
TNFα exposure resulted in upregulation of adhesion molecules ICAM-1 and VCAM-1 in dhBMEC microvessels (Fig. 8A). In non-treated microvessels ICAM-1 and VCAM-1 expression was punctate, however, following TNFα exposure expression of these adhesion molecules was uniform across the microvessel lumen. Treatment with TNFα did not alter the permeability of LY, R123 or 10 kDa dextran when compared to control microvessels (p > 0.05 for all comparisons) (Fig. 8B). PBMCs were perfused in the microvessels for 30 minutes, and then washed out for 5 minutes at a shear stress of ~1 dyne cm−2; afterwards, adherent PBMCs were clearly visible attached to the endothelium (Fig. 8C). Microvessels exposed to TNFα showed higher numbers of adherent PBMCs (23.0 cells cm−1) as compared to control microvessels (1.75 cells cm−1) (p < 0.001) (Fig. 8D). While transendothelial migration events were not observed during the experiments, microvessels exposed to TNFα with adherent PBMCs displayed macroscopic breakdown within 24 hours (Fig. 8E).
Figure 8.
Brain post-capillary venular model of leukocyte adhesion during inflammation. (A) Fluorescence images of the cross section of BC1 dhBMEC microvessels showing upregulation of surface adhesion markers (VCAM-1, ICAM-1) following 12-hour cytokine treatment with 10 ng mL−1 TNF-α. (B) Permeability of Lucifer yellow (LY), Rhodamine 123 (R123), and 10 kDa dextran in BC1 dhBMEC microvessels following treatment with TNF-α. (C) Representative fluorescence images showing human peripheral blood mononuclear cells (PBMCs) adhered to the bottom pole of BC1 dhBMEC microvessels with and without cytokine activation. (D) Adhesion of PBMCs increases with TNF-α exposure. (E) Phase contrast image showing macroscopic breakdown of cytokine treated microvessels exposed to PBMCs within 24 hours. N = 3 BC1 dhBMEC microvessels exposed and not exposed to TNF-α. *** p < 0.001.
4. Discussion
4.1. Barrier function
We have characterized the structure and barrier function of 150 μm diameter microvessels formed using human iPSC-derived brain microvascular endothelial cells (dhBMECs). Microvessels mimic the cylindrical geometry, cell-extracellular matrix interactions, and shear flow typical of human brain post-capillary venules (PCVs). Physiological barrier function was established after only two days and maintained for at least four additional days, enabling time course studies of biologically- and clinically-relevant perturbations.
One of the most important functions of the BBB is to restrict access to the brain. Lucifer yellow permeability of BC1 dhBMEC microvessels was 2.84 ± 0.41 × 10−7 cm s−1 over six days, very close to values that we have previously reported for dhBMEC monolayers in a 2D transwell assay,15 and close to estimated values of 1 – 2 × 10−7 cm s−1 in 15 μm pial PCV microvessels in rats.46 In addition, the permeability of 10 kDa dextran remained below the detection limit, showing that dhBMEC microvessels effectively block transport of large molecular weight solutes. Numerous studies have shown that dyes (i.e. Evans Blue), which bind to albumin (66.5 kDa), are excluded from the brain implying extremely low permeability.47 Similarly, four hours after systemic injection of 20 kDa dextran, no dye extravasation was found in the brain parenchyma in a hamster model.48 However, more recent in vivo imaging in rat pial postcapillary venules have reported finite and relatively high permeabilities of about 1 – 2 × 10−7 cm s−1 for 10 – 70 kDa dextrans,49, 50 which is inconsistent with other results showing restricted transport of large molecular weight solutes across the BBB. Reconciling these differences in in vivo permeabilities, which may be due to the substantial regional BBB heterogeneity,51 is important towards validating tissue-engineered models.
Physiological values for TEER are thought to be in the range of 1,500 – 8,000 Ω cm2.9–12 As Lucifer yellow permeability in dhBMEC microvessels was the same as values obtained in transwell assays (where TEER was above 1,500 Ω cm2), this implies that the TEER was also likely similar, as previously shown for dhBMECs cultured on hydrogel materials in 2D.17 Together, these results suggest that we have achieved physiological barrier function within perfusable 3D microvessels. A further implication of these results is that physiological barrier function is dependent on the ability of the hBMECs to form tight junctions and that other cell types, such as astrocytes and pericytes, are not required for establishment of barrier function. Evidence for this hypothesis comes primarily from in vitro transwell experiments where the presence of astrocytes or pericytes (or astrocyte extract) in the basolateral chamber increases TEER.52–58 However, these experiments are usually performed with primary or immortalized cells where the TEER is low and does not reach physiological values (> 1,500 Ω cm2) in the presence of supporting cells. While some studies have shown an increase in TEER for stem cell-derived dhBMECs with supporting cells,14, 59 physiological TEER values can be achieved without them.
Other strategies for engineering microvessels using primary BMECs, regardless of inclusion of supporting cell types, result in permeabilities still significantly higher than values reported here.5–8 For example, in one model incorporating primary human BMECs, astrocytes, and pericytes, the permeability of 3 kDa dextran was reported to be 2 – 4 × 10−6 cm s-1.6 As acknowledged by the authors, this high permeability for a large molecule did not recapitulate BBB barrier function, and was likely associated with their reported low TEER values of the primary BMECs (40 – 50 Ω cm2). Therefore, we hypothesize that rather than being responsible for maintenance of barrier function, it is likely that these supporting cells provide factors that promote recovery of barrier function towards physiological values. This hypothesis is further supported by studies that utilize astrocytes and/or pericytes to improve the barrier function of non-brain specific endothelial cells (HUVEC or iPSC-derived) in microvascular networks.29, 30
In addition to forming functional microvessels from iPSCs from two healthy individuals (BC1 and iPS12), we also created microvessels from iPSCs from an individual with multiple sclerosis (KW01), and an individual with Alzheimer’s disease (AD6). Disruption of the BBB is associated with many neurodegenerative disorders and may manifest through non-cell autonomous toxicity of endothelial cells.60, 61 Recently, iPSC-derived BMECs from individuals with Huntington’s disease were found to display abnormal barrier function, which may contribute to HD pathology.62 Here we show that the barrier function of microvessels formed from dhBMECs from individuals with Alzheimer’s disease and multiple sclerosis show no difference in permeability to Lucifer yellow, Rhodamine 123, and 10 kDa dextran compared to iPSCs from healthy individuals. While these results suggest that mutations associated with neurodegenerative disease in these iPSC lines do not induce changes in paracellular permeability, other transport systems have not yet been studied; for example, impaired amino acid transport has been implicated as a cause of autism spectrum disorder.63 Additionally, although TEER values for the KW01 line from an individual with multiple sclerosis (1330 ± 343 Ω cm2) were lower than for the lines from healthy individuals (BC1 and iPS12), they are close to values thought to be physiological (> 1,500 Ω cm2) and much higher than for primary and immortalized cell lines. Therefore, it is not surprising that there was no difference in small molecule permeability. A recent study using the transwell assay showed that the permeability of fluorescein increased only for TEER values less than 500 Ω cm2.64
4.2. Endothelial cell dynamics
Turnover is an important but often overlooked parameter in benchmarking tissue engineered vascular models. The turnover of BMECs in the human brain has not been reported, and there are only limited data from animal models65–67 and other tissue beds.68, 69 Similarly, there are very few studies on the effect of disease (i.e. brain cancer, neurogenerative disease) on brain endothelial cell turnover. Over six days, the turnover of dhBMECs in BC1 microvessels decreases to reach a net turnover rate close to zero. While the proliferation rate was less than values previously reported in 2D dhBMEC monolayers cultured on glass under similar shear stress (0.35 % h−1), the rate of cell loss was significantly higher than in 2D (0.01% h−1).41 Results from thymidine labeling studies in mice suggest proliferation rates for BMECs of about 0.04 % h−1,65 an order of magnitude or more lower than endothelial cells in other tissues.68, 69 Two-photon intravital microscopy studies in adult mice have shown: (1) no change in capillary segment diameter, capillary segment length, and the position of branch points over about 30 days,66 and (2) no detectable endothelial cell division over ten days based on BrdU labeling of cortical microvessels.67 These results suggest that the rates of cell proliferation and loss need to be further decreased to achieve physiological turnover.
Tracking of dhBMECs in microvessels showed that 96.5% of cells do not move more than an average cell diameter (28 μm) over two hours, as we have shown previously in 2D dhBMEC monolayers.41 The maximum number of neighbors for an isotropic object in 2D is six, corresponding to a hexagonal or close-packed lattice, whereas for a random distribution the number of cell neighbors is expected to be five. In dhBMEC microvessels, the average number of neighbors for each cell was between six and seven. As we have hypothesized previously, a close packed network of cells (hexagons) is a way to minimize the total length of cell-cell junctions per unit length of vessel.70 To verify cell shape we traced junctional f-actin stains for 150 cells fixed on day six. dhBMECs were relatively rounded, with a circularity of about 0.73 and an inverse aspect ratio of about 0.57, similar to values obtained in 2D under static conditions,41 suggesting that the circularity and orientation of dhBMECs was independent of geometry or shear stress. The number of cell neighbors changed once approximately every 2.5 hours for dhBMECs; this rate of change was not due to turnover since the rates of proliferation (0.19 % h−1) and cell loss (0.33 % h−1) mean that the average time for loss / gain of a neighboring cell is about 50 hours. Similarly, this change was not due to migration of cells within the monolayer because most cells do not move more than one cell length. Based on observations from phase contrast videos, we suggest that the changes in neighboring cells are associated with formation and loss of triple junctions that result in dynamic changes in the number of neighbors. Whether these transient changes in the number of cell neighbors play a significant role in determining paracellular permeability remains to be established.
The basement membrane is an important component of the BBB, comprising several proteins including collagen IV, fibronectin, and laminin.71, 72 Time course actin and laminin stains indicate that dhBMECs are actively modify the surrounding ECM and reorganizing their actin cytoskeleton; the links between these changes and cell behavior remain to be established.
4.3. Efflux inhibition
ATP-binding cassette efflux transporters including P-glycoprotein (P-gp), actively transport many lipophilic compounds out of brain microvascular endothelial cells, limiting their penetration into the CNS.73 Important P-gp substrates include anticancer drugs, immunosuppressive agents, antibiotics, corticoids, antivirals, antidepressants, antiepileptic drugs, and many other therapeutics.74 Thus, modulation of efflux transporters represents a key strategy to improve CNS drug delivery. However, P-gp inhibition has had limited clinical success due to issues with drug toxicity and poor pharmacokinetics.75 Existing in vitro and in vivo models of Pgp inhibition have several limitations. In vitro models, such as transwell assays or substrate accumulation assays, enable confirmation of the polarization of efflux transporters to the luminal membrane but do not provide direct visualization of transport phenomena. In contrast, the pharmacokinetics in vivo in animal model do not precisely match that of humans.76–78
Tariquidar is a potent third generation P-gp inhibitor that has undergone multiple preclinical trials to improve drug delivery to the brain.79–81 Tariquidar plasma concentrations between 2 – 3 μM are utilized to effectively limit P-gp efflux. In our model, 12-hour perfusion of 2 μM tariquidar increased permeability of P-gp substrate Rhodamine 123 two-fold, while disruptions in paracellular permeability were not observed. In healthy humans, continuous infusion of tariquidar, which was predicted to achieve near complete inhibition of p-glycoprotein, increased the verapamil distribution volume 2.73-fold, similar to the results reported here.80
4.4. Blood-brain barrier opening
Many techniques have emerged to modulate BBB permeability for delivery of therapeutic compounds, including: focused ultrasound,82 enterotoxin analogs,83 siRNA delivery systems,84 and exosomes85, however, few have been translated to the clinic. Systemic intravenous injection of the hyperosmolar agent mannitol is used clinically to reduce cerebral edema in a wide range of acute conditions.86 However, bolus intra-arterial injection over 1 – 2 minutes at a high concentration (close to the solubility limit of around 1.4 M), results in endothelial cell shrinkage that is sufficient to induce transient opening of the BBB.87 Hyperosmolar BBB opening has been used to improve delivery of chemotherapeutics, stem cells, and viral vectors into the brain.88–90 Despite the more than 100-year history of research into hyperosmotic agents in the brain and the renewed interest in image-guided opening of the BBB,91 the procedure is not reproducible92 highlighting the need for mechanistic studies at the cellular level. Understanding the processes occurring during hyperosmotic therapy is complicated by the complex structure and function of brain vasculature. Several major arteries supply the brain and regulation of blood flow by the Circle of Willis results in variable and irreproducible mixing and distribution of solutes throughout the brain. Animal models are valuable for studying these phenomena; however, there are substantial differences in vascular anatomy and cerebral blood flow between humans and animal models.93, 94 Thus, in vitro models provide the opportunity to study hyperosmotic therapy under controlled conditions.
In response to a five-minute bolus of 1.4 M mannitol, we observed a 15-fold increase in the permeability of Lucifer yellow, confirming blood-brain barrier opening. In addition, the permeability of 10 kDa dextran, which was below the detection limit under baseline conditions, increased to 10-fold higher than Lucifer yellow under baseline conditions. Over two hours of imaging, barrier function remained disrupted, although some leakage of dye was transient. Previous studies have found that mannitol exposure results in transient reductions in TEER both in vivo9 and in in vitro culture of primary brain microvascular endothelial cells.95 From histological studies, brain penetration of herpes simplex virus following hyperosmolar BBB opening suggests a transient defect size up to 120 nm.96 In our model, mannitol results in leakage of 10 kDa dextran, which has a hydrodynamic size of 4.8 nm.97 Future studies will explore the reversibility of BBB opening and the dynamics of pore formation. Additionally, hyperosmotic stress resulted in formation of vacuoles within endothelial cells. Vacuole formation is a mechanism to buffer cells against increases in ion concentration that occurs due to the associated decrease in cell volume. Vacuoles formation plateaus after only 20 minutes and remains steady over the subsequent 1.5 hours (despite an only transient exposure to mannitol). Fluorescent solutes were not found to accumulate within the cells suggesting that vacuoles are not direct mediators of BBB disruption during hyperosmotic therapy, as suggested previously in vivo.45
4.5. Inflammation
Studies of leukocyte interactions in the cerebrovasculature are usually performed in the autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis or in 2D in vitro models.98, 99 We show that it is possible to study leukocyte dynamics within an in vitro 3D model of human brain PCVs, which supports visualization in a well-controlled environment.100 Immune trafficking across the BBB is low under physiological conditions, however, increased infiltration occurs during neurological disease.101 Activation of microglia and astrocytes during neurological disease results in release of inflammatory cytokines (including TNFα) which may underly changes in immune infiltration and barrier function.102, 103 Endothelial ICAM-1 and VCAM-1 expression promotes leukocyte adhesion,104, 105 an early step in extravasation, which occurs preferentially in PCVs in the brain.22–24 In our 3D model of human PCVs, exposure to TNFα resulted in upregulation of VCAM-1 and ICAM-1, and corresponding increased adhesion of human peripheral blood mononuclear cells.
We also found that TNFα activation resulted in no change in barrier function. This observation is contradictory to results from in vitro 2D models,106–109 existing 3D BBB models,6,7 and in vivo animal models,110 all of which report increased permeability of BMECs exposed to TNFα. However, as described previously, in vitro models utilizing BMECs with non-physiological TEER may be more susceptible to disruptive changes in barrier function.111 Therefore, our results suggest that TNFα stimulation alone, without other blood components and conditions associated with an immune response, is insufficient to induce BBB disruption. Interestingly, 24 hours after TNFα activation and PBMC adhesion studies, microvessels displayed macroscopic breakdown suggesting a possible role of cytokine secretion. In our model, no transendothelial migration events were observed, presumably since there was change in barrier function, but also potentially due to the restrictive nature of a dense cross-linked collagen hydrogel. Although they lack the biological complexity of animal models, these proof-of-principle results highlight a potential advantage of in vitro models to deconvolve complex processes occurring during inflammation.
5. Conclusions
We report on barrier function and endothelial cell behavior of human iPSC-derived bloodbrain barrier microvessels resembling in vivo post-capillary venules. Microvessels are generated from multiple iPS cell lines and display localization of tight junction proteins, and expression of key transporters and efflux pumps. Physiological barrier function is achieved within two days, requires no co-cultured cells, and is maintained for at least four days. Over the course of six days, endothelial cells reach quiescence and develop a uniform basement membrane. We demonstrate successful modulation of both transcellular and paracellular permeability using P-gp inhibitor tariquidar and hyperosmolar agent mannitol, respectively. Cytokine activation using TNFα resulted in upregulation of leukocyte adhesion molecules (VCAM-1 and ICAM-1) and increased leukocyte adhesion, but resulted in no change in permeability suggesting that activation alone is insufficient to induce BBB disruption. Human iPSC-derived blood-brain barrier microvessels serve as a robust patient-specific platform to study transport phenomena and endothelial cell behavior during health and disease.
Supplementary Material
Supplementary Figure 1. Verification of cell area and neighbors. (A) Average cell area for three different quantification methods: Phase, the total area of the microvessel plane was divided by the number of cells counted within that region. DAPI, the total area of the microvessel plane was divided by the number of DAPI labeled nuclei within that region. F-actin junction trace, cell-cell junctions were traced freehand in ImageJ and the total area for each cell was quantified. (B) Example images of phase, DAPI, and f-actin images used to quantify cell area. (C) Average cell neighbors for two quantification methods. Cell neighbors were manually counted from phase contrast images on day 6 and compared to counts from f-actin junction stains on day 6. There is less than a 10% error between quantification methods.
Supplementary Table 1. Antibodies used in this study.
Supplementary Video 1. Fluorescence / phase overlay of perfusion with Lucifer yellow (left), Rhodamine 123 (middle) and 10 kDa dextran (right) in a dhBMEC microvessel (top row) and HUVEC microvessel (bottom row) over two hours.
Supplementary Video 2. Phase contrast imaging of dhBMECs over two hours. (left) proliferation event, (middle) cell loss events, (right) cell motility. Cell proliferation is marked with a circle, alignment of chromosomes along the equatorial plane is clearly visible proceeding cell division and integration of daughter cells into the monolayer. Cell loss events are marked with an arrow and display contraction of cell body, lysing of cell contents into the lumen, followed by removal of the cell from the monolayer. Cell motility is visible in each video, one cell is identified with a rectangle showing change in nearest neighbors through adjacent cell division and through change in cell-cell borders.
Supplementary Video 3. Blood-brain barrier opening following five-minute bolus of mannitol. (left) phase contrast images show rapid vacuolation of endothelial cells, (middle) Lucifer yellow and (right) 10 kDa dextran display elevated permeability. t = 0 represents the frame prior to arrival of mannitol into the lumen.
Acknowledgements
This work was supported by DTRA (HDTRA1–15-1–0046). RML acknowledges a National Science Foundation Graduate Research Fellowship under Grant No. DGE1746891, JD acknowledges support from the Nanotechnology for Cancer Research training program, ZSX acknowledges National Institute of Neurological Disorders and Stroke of the National Institutes of Health Graduate Research Fellowship (NIH F31NS097209), PW acknowledges support from NIH/NINDS R01NS09111, and PAC acknowledges support from the Maryland Stem Cell Research Foundation. The authors acknowledge many helpful discussions with Andrew D. Wong and Moriah E. Katt.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Data availability statement
The raw/processed data required to reproduce these findings are available from the corresponding author on reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pardridge WM Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 32, 1959–1972 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong AD et al. The blood-brain barrier: an engineering perspective. Frontiers in Neuroengineering 6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR & Begley DJ Structure and function of the blood-brain barrier. Neurobiol Dis 37, 13–25 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Watase K & Zoghbi HY Modelling brain diseases in mice: the challenges of design and analysis. Nat Rev Genet 4, 296–307 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Cho H et al. Three-Dimensional Blood-Brain Barrier Model for in vitro Studies of Neurovascular Pathology. Sci Rep 5, 15222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herland A et al. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS One 11, e0150360 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partyka PP et al. Mechanical stress regulates transport in a compliant 3D model of the blood-brain barrier. Biomaterials 115, 30–39 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Adriani G, Ma D, Pavesi A, Kamm RD & Goh ELA 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 17, 448–459 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Butt AM, Jones HC & Abbott NJ Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol 429, 47–62 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith QR & Rapoport SI Cerebrovascular permeability coefficients to sodium, potassium, and chloride. J Neurochem 46, 1732–1742 (1986). [DOI] [PubMed] [Google Scholar]
- 11.Butt AM & Jones HC Effect of histamine and antagonists on electrical resistance across the blood-brain barrier in rat brain-surface microvessels. Brain Res 569, 100–105 (1992). [DOI] [PubMed] [Google Scholar]
- 12.Crone C & Olesen SP Electrical resistance of brain microvascular endothelium. Brain Res 241, 49–55 (1982). [DOI] [PubMed] [Google Scholar]
- 13.Lippmann ES et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nature biotechnology 30, 783–791 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP & Shusta EV A retinoic acidenhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep 4, 4160 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katt ME, Xu ZS, Gerecht S & Searson PC Human Brain Microvascular Endothelial Cells Derived from the BC1 iPS Cell Line Exhibit a Blood-Brain Barrier Phenotype. PLoS One 11, e0152105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian T et al. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci Adv 3, e1701679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katt ME, Linville RM, Mayo LN, Xu ZS & Searson PC Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: the role of matrix composition on monolayer formation. Fluids Barriers CNS 15, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turitto VT Blood viscosity, mass transport, and thrombogenesis. Prog Hemost Thromb 6, 139–177 (1982). [PubMed] [Google Scholar]
- 19.Kamiya A, Bukhari R & Togawa T Adaptive regulation of wall shear stress optimizing vascular tree function. Bulletin of Mathematical Biology 46, 127–137 (1984). [DOI] [PubMed] [Google Scholar]
- 20.Koutsiaris AG et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology 44, 375–386 (2007). [PubMed] [Google Scholar]
- 21.Santisakultarm TP et al. In vivo two-photon excited fluorescence microscopy reveals cardiac- and respiration-dependent pulsatile blood flow in cortical blood vessels in mice. Am J Physiol Heart Circ Physiol 302, H1367–1377 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeshita Y & Ransohoff RM Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol Rev 248, 228–239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens T, Bechmann I & Engelhardt B Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol 67, 1113–1121 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Banks WA & Erickson MA The blood-brain barrier and immune function and dysfunction. Neurobiol Dis 37, 26–32 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Kienast Y et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16, 116–122 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Kristensson K, Nygard M, Bertini G & Bentivoglio M African trypanosome infections of the nervous system: Parasite entry and effects on sleep and synaptic functions. Prog Neurobiol 91, 152–171 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Spindler KR & Hsu TH Viral disruption of the blood brain barrier. Trends Microbiol 20, 282–290 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dando SJ et al. Pathogens Penetrating the Central Nervous System: Infection Pathways and the Cellular and Molecular Mechanisms of Invasion. Clin Microbiol Rev 27, 691–726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bang S et al. A Low Permeability Microfluidic Blood-Brain Barrier Platform with Direct Contact between Perfusable Vascular Network and Astrocytes. Sci Rep 7, 8083 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campisi M et al. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180, 117–129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou BK et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res 21, 518–529 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahairaki V et al. Induced pluripotent stem cells from familial Alzheimer’s disease patients differentiate into mature neurons with amyloidogenic properties. Stem Cells Dev 23, 2996–3010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogorad MI & Searson PC Real-time imaging and quantitative analysis of doxorubicin transport in a perfusable microvessel platform. Integr Biol (Camb) 8, 976–984 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson HK, Faubion MG, Hjortness MK, Palecek SP & Shusta EV Cryopreservation of Brain Endothelial Cells Derived from Human Induced Pluripotent Stem Cells Is Enhanced by Rho-Associated Coiled Coil-Containing Kinase Inhibition. Tissue Eng Part C Methods 22, 1085–1094 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong KH, Truslow JG & Tien J The role of cyclic AMP in normalizing the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 31, 4706–4714 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung AD, Wong KH & Tien J Plasma expanders stabilize human microvessels in microfluidic scaffolds. J Biomed Mater Res A 100, 1815–1822 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linville RM, Boland NF, Covarrubias G, Price GM & Tien J Physical and Chemical Signals That Promote Vascularization of Capillary-Scale Channels. Cell Mol Bioeng 9, 73–84 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huxley VH, Curry FE & Adamson RH Quantitative fluorescence microscopy on single capillaries: alpha-lactalbumin transport. Am J Physiol 252, H188–197 (1987). [DOI] [PubMed] [Google Scholar]
- 39.Chrobak KM, Potter DR & Tien J Formation of perfused, functional microvascular tubes in vitro. Microvasc Res 71, 185–196 (2006). [DOI] [PubMed] [Google Scholar]
- 40.DeStefano JG et al. Real-time quantification of endothelial response to shear stress and vascular modulators. Integr Biol (Camb) 9, 362–374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeStefano JG, Xu ZS, Williams AJ, Yimam N & Searson PC Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs). Fluids Barriers CNS 14, 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinitz A, DeStefano J, Ye M, Wong AD & Searson PC Human brain microvascular endothelial cells resist elongation due to shear stress. Microvasc Res 99, 8–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogorad MI, DeStefano J, Wong AD & Searson PC Tissue‐engineered 3D microvessel and capillary network models for the study of vascular phenomena. Microcirculation 24, e12360 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muskhelishvili L, Latendresse JR, Kodell RL & Henderson EB Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67 (MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. Journal of Histochemistry & Cytochemistry 51, 1681–1688 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Cosolo WC, Martinello P, Louis WJ & Christophidis N Blood-brain barrier disruption using mannitol: time course and electron microscopy studies. Am J Physiol 256, R443447 (1989). [DOI] [PubMed] [Google Scholar]
- 46.Easton AS, Sarker MH & Fraser PA Two components of blood-brain barrier disruption in the rat. J Physiol 503 ( Pt 3), 613–623 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saunders NR et al. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front Neurosci-Switz 8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsson Y, Svensjo E, Arfors KE & Hultstrom D Fluorescein labelled dextrans as tracers for vascular permeability studies in the nervous system. Acta Neuropathol 33, 45–50 (1975). [DOI] [PubMed] [Google Scholar]
- 49.Yuan W, Lv Y, Zeng M & Fu BM Non-invasive measurement of solute permeability in cerebral microvessels of the rat. Microvasc Res 77, 166–173 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Shi L, Zeng M, Sun Y & Fu BM Quantification of blood-brain barrier solute permeability and brain transport by multiphoton microscopy. J Biomech Eng 136, 031005 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Noumbissi ME, Galasso B & Stins MF Brain vascular heterogeneity: implications for disease pathogenesis and design of in vitro blood-brain barrier models. Fluids Barriers CNS 15, 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daneman R, Zhou L, Kebede AA & Barres BA Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakagawa S et al. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int 54, 253–263 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Nakagawa S et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol 27, 687–694 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siddharthan V, Kim YV, Liu S & Kim KS Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res 1147, 39–50 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomsen LB, Burkhart A & Moos T A Triple Culture Model of the Blood-Brain Barrier Using Porcine Brain Endothelial cells, Astrocytes and Pericytes. Plos One 10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malina KCK, Cooper I & Teichberg VI Closing the gap between the in-vivo and invitro blood-brain barrier tightness. Brain Research 1284, 12–21 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Hatherell K, Couraud PO, Romero IA, Weksler B & Pilkington GJ Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Meth 199, 223–229 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Wang YI, Abaci HE & Shuler ML Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng 114, 184194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sweeney MD, Sagare AP & Zlokovic BV Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14, 133–150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ilieva H, Polymenidou M & Cleveland DW Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187, 761–772 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim RG et al. Huntington’s Disease iPSC-Derived Brain Microvascular Endothelial Cells Reveal WNT-Mediated Angiogenic and Blood-Brain Barrier Deficits. Cell Rep 19, 13651377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarlungeanu DC et al. Impaired Amino Acid Transport at the Blood Brain Barrier Is a Cause of Autism Spectrum Disorder. Cell 167, 1481–1494 e1418 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mantle JL, Min L & Lee KH Minimum Transendothelial Electrical Resistance Thresholds for the Study of Small and Large Molecule Drug Transport in a Human in Vitro Blood-Brain Barrier Model. Mol Pharm 13, 4191–4198 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Hobson B & Denekamp J Endothelial proliferation in tumours and normal tissues: continuous labelling studies. British journal of cancer 49, 405 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cudmore RH, Dougherty SE & Linden DJ Cerebral vascular structure in the motor cortex of adult mice is stable and is not altered by voluntary exercise (vol 37, pg 3725, 2017). J Cerebr Blood F Met 37, 3824–3824 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harb R, Whiteus C, Freitas C & Grutzendler J In vivo imaging of cerebral microvascular plasticity from birth to death. Journal of Cerebral Blood Flow & Metabolism 33, 146–156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tannock IF & Hayashi S The proliferation of capillary endothelial cells. Cancer research 32, 77–82 (1972). [PubMed] [Google Scholar]
- 69.Spaet T & Lejnieks I Mitotic activity of rabbit blood vessels. Proceedings of the Society for Experimental Biology and Medicine 125, 1197–1201 (1967). [DOI] [PubMed] [Google Scholar]
- 70.Ye M et al. Brain microvascular endothelial cells resist elongation due to curvature and shear stress. Sci Rep 4, 4681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tilling T et al. Expression and adhesive properties of basement membrane proteins in cerebral capillary endothelial cell cultures. Cell Tissue Res 310, 19–29 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Tilling T, Korte D, Hoheisel D & Galla HJ Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J Neurochem 71, 1151–1157 (1998). [DOI] [PubMed] [Google Scholar]
- 73.International Transporter C et al. Membrane transporters in drug development. Nat Rev Drug Discov 9, 215–236 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loscher W & Potschka H Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2, 86–98 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Callaghan R, Luk F & Bebawy M Inhibition of the multidrug resistance P-glycoprotein: time for a change of strategy? Drug Metab Dispos 42, 623–631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Syvanen S et al. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab Dispos 37, 635–643 (2009). [DOI] [PubMed] [Google Scholar]
- 77.Warren MS et al. Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human. Pharmacol Res 59, 404–413 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Bauer M et al. Pgp-mediated interaction between (R)-[11C]verapamil and tariquidar at the human blood-brain barrier: a comparison with rat data. Clin Pharmacol Ther 91, 227–233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bauer M et al. Pharmacokinetics of single ascending doses of the P-glycoprotein inhibitor tariquidar in healthy subjects. Pharmacology 91, 12–19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bauer M et al. Approaching complete inhibition of P-glycoprotein at the human blood-brain barrier: an (R)-[11C]verapamil PET study. J Cereb Blood Flow Metab 35, 743–746 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kreisl WC et al. Increased permeability-glycoprotein inhibition at the human blood-brain barrier can be safely achieved by performing PET during peak plasma concentrations of tariquidar. J Nucl Med 56, 82–87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ting CY et al. Concurrent blood-brain barrier opening and local drug delivery using drugcarrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 33, 704–712 (2012). [DOI] [PubMed] [Google Scholar]
- 83.Neuhaus W et al. Reversible opening of the blood-brain barrier by claudin-5-binding variants of Clostridium perfringens enterotoxin’s claudin-binding domain. Biomaterials 161, 129–143 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Park TE et al. Enhanced BBB permeability of osmotically active poly(mannitol-co-PEI) modified with rabies virus glycoprotein via selective stimulation of caveolar endocytosis for RNAi therapeutics in Alzheimer’s disease. Biomaterials 38, 61–71 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Jia G et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 178, 302–316 (2018). [DOI] [PubMed] [Google Scholar]
- 86.Diringer MN New trends in hyperosmolar therapy? Curr Opin Crit Care 19, 77–82 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rapoport SI Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol 20, 217–230 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gonzales-Portillo GS et al. Mannitol-enhanced delivery of stem cells and their growth factors across the blood-brain barrier. Cell Transplant 23, 531–539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Foley CP et al. Intra-arterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption. J Control Release 196, 71–78 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burkhardt JK et al. Intra-arterial delivery of bevacizumab after blood-brain barrier disruption for the treatment of recurrent glioblastoma: progression-free survival and overall survival. World Neurosurg 77, 130–134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janowski M, Walczak P & Pearl MS Predicting and optimizing the territory of bloodbrain barrier opening by superselective intra-arterial cerebral infusion under dynamic susceptibility contrast MRI guidance. J Cereb Blood Flow Metab 36, 569–575 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zylber-Katz E et al. Pharmacokinetics of methotrexate in cerebrospinal fluid and serum after osmotic blood-brain barrier disruption in patients with brain lymphoma. Clin Pharmacol Ther 67, 631–641 (2000). [DOI] [PubMed] [Google Scholar]
- 93.Joshi S et al. Inconsistent blood brain barrier disruption by intraarterial mannitol in rabbits: implications for chemotherapy. J Neurooncol 104, 11–19 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Janowski M, Walczak P & Pearl MS Predicting and optimizing the territory of blood–brain barrier opening by superselective intra-arterial cerebral infusion under dynamic susceptibility contrast MRI guidance. Journal of Cerebral Blood Flow & Metabolism 36, 569–575 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cucullo L, Marchi N, Hossain M & Janigro D A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab 31, 767–777 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muldoon LL et al. Comparison of intracerebral inoculation and osmotic blood-brain barrier disruption for delivery of adenovirus, herpesvirus, and iron oxide particles to normal rat brain. Am J Pathol 147, 1840–1851 (1995). [PMC free article] [PubMed] [Google Scholar]
- 97.Granath KA Solution properties of branched dextran. Journal of Colloid Science 31, 308328 (1958). [Google Scholar]
- 98.Coisne C, Lyck R & Engelhardt B Live cell imaging techniques to study T cell trafficking across the blood-brain barrier in vitro and in vivo. Fluids Barriers CNS 10, 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahad D et al. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain 129, 212–223 (2006). [DOI] [PubMed] [Google Scholar]
- 100.Irimia D & Wang X Inflammation-on-a-Chip: Probing the Immune System Ex Vivo. Trends Biotechnol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prinz M & Priller J The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci 20, 136–144 (2017). [DOI] [PubMed] [Google Scholar]
- 102.da Fonseca AC et al. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci 8, 362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saili KS et al. Blood-brain barrier development: Systems modeling and predictive toxicology. Birth Defects Res 109, 1680–1710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ley K, Laudanna C, Cybulsky MI & Nourshargh S Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7, 678–689 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Steiner O et al. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J Immunol 185, 4846–4855 (2010). [DOI] [PubMed] [Google Scholar]
- 106.Deli MA et al. Exposure of tumor necrosis factor-alpha to luminal membrane of bovine brain capillary endothelial cells cocultured with astrocytes induces a delayed increase of permeability and cytoplasmic stress fiber formation of actin. J Neurosci Res 41, 717–726 (1995). [DOI] [PubMed] [Google Scholar]
- 107.Capaldo CT & Nusrat A Cytokine regulation of tight junctions. Biochim Biophys Acta 1788, 864–871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mark KS & Miller DW Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-alpha exposure. Life Sci 64, 1941–1953 (1999). [DOI] [PubMed] [Google Scholar]
- 109.Lopez-Ramirez MA et al. Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells. J Immunol 189, 3130–3139 (2012). [DOI] [PubMed] [Google Scholar]
- 110.Mayhan WG Cellular mechanisms by which tumor necrosis factor-alpha produces disruption of the blood-brain barrier. Brain Res 927, 144–152 (2002). [DOI] [PubMed] [Google Scholar]
- 111.Varatharaj A & Galea I The blood-brain barrier in systemic inflammation. Brain Behav Immun 60, 1–12 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Verification of cell area and neighbors. (A) Average cell area for three different quantification methods: Phase, the total area of the microvessel plane was divided by the number of cells counted within that region. DAPI, the total area of the microvessel plane was divided by the number of DAPI labeled nuclei within that region. F-actin junction trace, cell-cell junctions were traced freehand in ImageJ and the total area for each cell was quantified. (B) Example images of phase, DAPI, and f-actin images used to quantify cell area. (C) Average cell neighbors for two quantification methods. Cell neighbors were manually counted from phase contrast images on day 6 and compared to counts from f-actin junction stains on day 6. There is less than a 10% error between quantification methods.
Supplementary Table 1. Antibodies used in this study.
Supplementary Video 1. Fluorescence / phase overlay of perfusion with Lucifer yellow (left), Rhodamine 123 (middle) and 10 kDa dextran (right) in a dhBMEC microvessel (top row) and HUVEC microvessel (bottom row) over two hours.
Supplementary Video 2. Phase contrast imaging of dhBMECs over two hours. (left) proliferation event, (middle) cell loss events, (right) cell motility. Cell proliferation is marked with a circle, alignment of chromosomes along the equatorial plane is clearly visible proceeding cell division and integration of daughter cells into the monolayer. Cell loss events are marked with an arrow and display contraction of cell body, lysing of cell contents into the lumen, followed by removal of the cell from the monolayer. Cell motility is visible in each video, one cell is identified with a rectangle showing change in nearest neighbors through adjacent cell division and through change in cell-cell borders.
Supplementary Video 3. Blood-brain barrier opening following five-minute bolus of mannitol. (left) phase contrast images show rapid vacuolation of endothelial cells, (middle) Lucifer yellow and (right) 10 kDa dextran display elevated permeability. t = 0 represents the frame prior to arrival of mannitol into the lumen.