Abstract
ZIP8 is a membrane transporter that facilitates the uptake of divalent metals (e.g., Zn, Mn, Fe, Cd) and the mineral selenite in anionic form. ZIP8 functionality has been recently reported to regulate cell proliferation, migration and cytoskeleton arrangement, exhibiting an essential role for normal physiology. In this study, we report a ZIP8 role in chemotherapy response. We show ZIP8 regulates cell sensitivity to the anti-cancer drug cisplatin. Overexpression of ZIP8 in mouse embryonic fibroblast (MEF) cells induces cisplatin sensitivity, while knockout of ZIP8 in leukemia HAP1 cells leads to cisplatin resistance. In ZIP8 altered cells and transgenic mice, we show cisplatin is not a direct ZIP8 substrate. Further studies demonstrate that ZIP8 regulates anti-apoptotic protein Bcl-2. ZIP8 overexpression decreases Bcl-2 levels in cultured cells, mice lung and liver tissue while loss of ZIP8 elevates Bcl-2 expression in HAP1 cells and liver tissue. We also observe that ZIP8 overexpression modulates cisplatin-induced cell apoptosis, manifested by the increased protein level of cleaved Caspase-3. Since Bcl-2 elevation was previously discovered to induce cisplatin drug resistance, our results suggest ZIP8 may modulate cisplatin drug responses as well as apoptosis through Bcl-2. We therefore conclude ZIP8 is a new molecule to be involved in cisplatin drug responses and is predicted as a genetic factor to be considered in cisplatin therapy.
Keywords: ZIP8, transporter, cisplatin, Bcl-2, apoptosis, drug-resistance
1. Introduction
ZIP8 is a multifunctional membrane transporter that regulates the cellular influx of divalent metals including: Zn2+, Fe2+, Co2+, Mn2+ and toxic Cd 2+(He et al., 2006; Liu et al., 2008; He et al., 2009; Wang et al., 2011; Wang et al., 2012). ZIP8 transports divalent metal ions through coupling with anionic bicarbonate (HCO3−). ZIP8 is also identified as the major transporter for inorganic selenite, which is a monovalent anion (HSeO3−) at physiological pH. The ZIP8 selenite transport requires both Zn2+ and bicarbonate as co-substrates (McDermott et al., 2016).
ZIP8 is an essential gene in mice and is involved in organogenesis (Galvez-Peralta et al., 2012). Aberrant expression of ZIP8 is related to multiple human diseases, such as low HDL-cholesterol, elevated blood pressure, increased body mass index, osteoarthritis, asthma and increased risk of schizophrenia (Speliotes et al., 2010; Waterworth et al., 2010; Kim et al., 2014; Zhang et al., 2016; Costas, 2018; Mak et al., 2018). ZIP8 is also known to be highly responsive to inflammatory stimuli, such as lipid polysaccharide (LPS), TNF-α and IL-1β (Besecker et al., 2008; Liu et al., 2013). Our results showed ZIP8 is involved in regulation of cytoskeleton arrangement, proliferation and migration (Geng et al., 2018). ZIP8 deficiency in liver decreases liver selenium and expression of anti-oxidant selenoproteins (Liu et al., 2018). However, as a transporter for multiple functional essential biometals, ZIP8 may regulate many downstream targets and pathways, which remain to be elucidated.
Platinum-based drugs, such as cis-diamminedichloroplatinum(II) (CCCD, cisplatin), have been used to treat various malignancies, including testicular, ovarian, head and neck, colorectal, bladder, and non-small cell lung cancers (Prestayko et al., 1979; Lebwohl and Canetta, 1998; Galanski, 2006). Cisplatin exerts anticancer effects via multiple mechanisms - the best understood involves he generation of DNA lesions and the induction of DNA damage (Dasari and Tchounwou, 2014a). In addition, cisplatin can also elevate oxidative stress and induce cell mitochondrial apoptosis (Dasari and Tchounwou, 2014b).
Despite a consistent rate of initial responses, cisplatin resistance forms a therapeutic impediment for its clinical efficacy. Numerous mechanisms are currently reported to be involved in tumor cell resistance to cisplatin. The mechanisms include: 1) decreased intracellular Pt accumulation via drug influx and efflux pumps, e.g., down-regulation of copper transporter receptor 1 (CTR1) (Ishida et al., 2002), organic cation transporters (OCT3) (Guttmann et al., 2018), up-regulation of multidrug resistance-associated protein (Kool et al., 1997), CTR2 (Huang et al., 2014) and Copper-transporting ATPases (ATP7A and ATP7B) (Samimi et al., 2004; Yoshizawa et al., 2007), 2) enhanced DNA repair capability (Furuta et al., 2002), 3) elevated detoxification, e.g., increased glutathione (GSH) level, which conjugates to platinum rendering inactivation of cisplatin (Zhang et al., 2001), Glutathione S-transferase (GST) which catalyzes conjugation of GSH to drug(Townsend et al., 2009), overexpressed superoxide dismutase 1 (SOD1) which protects resistant-cells against apoptosis(Kim et al., 2010) and elevated metallothioneins (MTs) that binds to cisplatin contributing to resistance (Hagrman et al., 2003), 4) altered cell signaling pathway, e.g., activation of MEK/ERK and PI3K/AKT pathway (Fu et al., 2014) and 5) altered apoptotic signaling pathway, e.g., up-regulation of anti-apoptotic proteins and down-regulation of pro-apoptotic proteins (Stewart, 2007). In addition to these mechanisms, proteomic studies have identified additional proteins that are significantly correlated with cisplatin drug resistance from clinical specimen, which involve many previously uncharacterized candidates (Severi et al., 2018).
In the present study, we found that ZIP8 expression is negatively associated with cisplatin resistance; overexpression of ZIP8 increases cisplatin sensitivity while downregulation of ZIP8 leads to cisplatin tolerance. We have excluded the possibility that ZIP8 may serve as a direct cisplatin transporter in ZIP8 overexpressed cells and ZIP8 transgenic mice. Further studies have shown that ZIP8 deficiency induces upregulation of anti-apoptotic Bcl-2, while ZIP8 overexpression downregulates Bcl-2 levels in cells and mouse tissue. Bak, the apoptotic signal, is elevated following ZIP8 overexpression. ZIP8 overexpression triggers cisplatin induced apoptosis, shown by elevation of cleaved Caspase-3. Based on these results, we conclude that ZIP8 overexpression modulate Bcl-2 expression and cell apoptosis, results altered cisplatin drug responses.
2. Materials and methods
2.1. Cell culture
Mouse embryonic fibroblast cell line was transfected with either a firefly Luciferase or slc39a8 (ZIP8) gene in a pRevTre vector to derive the two cell lines, LUC-MEF and ZIP8-MEF(He et al., 2006), with different ZIP8 expression. We showed ZIP8-MEF had a 2-fold overexpression of ZIP8 (Geng et al., 2018).
Another cell line, HAP1, was derived from chronic myelogenous leukemia cell line and was largely in haploid form. Human ZIP8 knockout (KO) HAP1 cells were constructed by CRISPR by Horizon (Catalog no. HZGHC002069c001) and maintained in IMDM medium (Gibco). The knockout of ZIP8 was validated by PCR amplification and Sanger Sequencing to confirm 67bp insertion in exon 3, which resulted a frame-shift. Medium was supplemented with 10% FBS (Denville Scientific Inc.) and antibiotics (HyClone). All cells were cultured at 37°C in a humidified atmosphere with 5% CO2.
2.2. Animals
BTZIP8–3 mice were constructed in the lab of Dr. Daniel Nebert, University of Cincinnati (Wang et al., 2007). BTZIP8–3 mice (C57BL/6J background) were generated by gDNA random insertion of three slc39a8 (ZIP8) (129S6/SvEvTac) -containing BAC fragments in tandem. Compared with wild-type (WT) mice carrying two copies (diploid) of the slc39a8 (ZIP8) gene, BTZIP8–3 mice carry a total of five gene copies and exhibit ~2.5-fold increased ZIP8 mRNA and protein expression in every tissue examined (Wang et al., 2007). The BTZIP8–3 homozygotes along with WT were used for our studies, without bias of males and females.
When adenovirus mediated shRNA was performed, the WT C57BL/6J male mice at 8–12 weeks were used. Tail-vein injection was performed at a total volume of 50–100 μL. The recombinant adenoviruses, including Ad-shZIP8 along with control (Ad-Control), were delivered to mice at 1×1011 viral particles (Welgen) per injection. Mice were sacrificed one week later and livers were collected for western-blot.
For intraperitoneal (IP) cisplatin injection, stock cisplatin (10 mg/mL) was diluted in deionized water. The mice were sacrificed by CO2 asphyxiation 30 minutes after the IP injection and tissues for Pt quantification were isolated and standardized according to wet weight.
All experimental studies on the animals were approved by Oakland University Animal Care and Use Committee (IACUC). Mice were housed in a pathogen-free facility with 12-h light/dark cycle and free access to water with a regular chow diet.
2.3. Immunofluorescence staining in cells
To probe expression of target protein Bcl-2 in cells, immunofluorescent staining using Blc-2 antibody was adopted. Cells were seeded into coverslip for 24 h and fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. The coverslip was blocked in 3% BSA/10% FBS for 1 h at room temperature. Cells were then permeabilized with 0.2% Triton-X100/PBS for 10 min. After washing with PBS 3 times, the cells were incubated with primary antibody overnight at 4°C against Bcl-2 (Bioss) and Alexa Fluor 488 or 555 conjugated secondary antibodies (Cell signaling) for 1 h at room temperature. After counterstained with 1 μg/mL DAPI (Acros Organics) for 5 min, cells were mounted on the slide with mounting medium (SouthernBiotech) and imaged by confocal fluorescent microscope (Nikon Eclipse). NIS Element software was used to qualify fluorescence intensity.
2.4. MTT assay
MTT assays were performed to determine the cell proliferation. In brief, 1X105 cells/well were plated into 96-well plate. After 24 h, cells were treated with 10 μg/mL and 20 μg/mL cisplatin (Platinum-Q) for 24 h. Then 20 μL of 5 mg/mL MTT (Sigma) was added, following by incubation for another 4 h. Cells were washed with PBS once and then 100 μL DMSO (Sigma) was added to each well and the plate was gently shaken for 10 min at room temperature. The absorbance of MTT formazan crystals dissolved in 100 μL DMSO was measured at 490 nm by a microplate reader (Bio-Tek). The MTT assay was performed in triplicate and the relative cell viability (%) was expressed as a percentage relative to cells without cisplatin treatment.
2.5. q-RT-PCR
ZIP8 expression in mRNA in MEF and HAP1 cells were evaluated with real-time RT-PCR. Briefly, total RNA was extracted using RNeasy Mini Kit (Qiagen) and quantified using a NanoDrop spectrophotometer (ThermoFisher Scientific). RNA was reverse-transcribed) in Bio-Rad CFX96 under the following conditions: 95°C for 10 min, 40 cycles for 95°C for 15 sec and 60°C for 60 sec. The relative quantification values for ZIP8 (For MEF cells: 5’-GGGACTAGCTTTCGGCATTT-3’ and 5’-GCATGTCGTTCATCTCTGGA-3’; For HAP1 cells: 5’-GCTACGCTGCCACTTCAATG-3’ and 5’-CTGCTCCGAGTCAGAGGTGG-3’) gene expression were calculated from the accurate threshold cycle (Ct), which was acquired from into cDNA using M-MuLV Reverse Transcriptase (NEB) containing random hexamers (IDT). Quantitative real-time PCR was performed using EvaGreen qPCR Mastermix (MIDSCI instrument’s software. The expression levels of slc39a8 were normalized to GAPDH. Fold change was determined by 2-ΔΔCt method. The fold values for triplicate wells were averaged.
2.6. Western-blot analysis
The Bcl-2 proteins were quantified in cells and tissues by western-blot. For cisplatin treatment, cells were treated with cisplatin for 24 h. Whole cells and mouse tissues were obtained by lysis in an appropriate volume of RIPA buffer (150 mM NaCl, 1.0% Triton-100, 1mM Na3VO4, 0.1% SDS, 1mM EDTA and 25 mM Tris-HCl, pH 7.6) containing protease inhibitor (Roche) and phosphatase inhibitor (Roche). Cellular extracts were centrifuged at 13000 × g for 10 min at 4°C and then protein concentration was measured by a BCA protein assay kit (PIERCE). Cell lysates were boiled for 10 min and separated by SDS-PAGE and then transferred to PVDF membrane. Non-binding site of membrane was blocked with 5% nonfat milk and 0.1% (v/v) Tween-20 in PBS for 1 h at room temperature. Membranes were then probed by primary antibody in blocking solution overnight at 4°C against Bcl-2 (Santa Cruz), Bak (Cell Signaling), Bax (Cell Signaling), cleaved PAPR (Cell Signaling) and cleaved Caspase-3 (Cell Signaling), followed by incubation with HRP-conjugated secondary antibody (Santa Cruz for Bcl-2, Proteintech for Bak, Bax, cleaved PARP and cleaved Caspase-3) for 1 h. After washing 3 times in PBST for 5 min, the bands were detected by performing ECL by using Western Bright ECL reagent (Advansta) for 2 min incubation at room temperature and visualized by a ChemiDoc Touch Imaging System (Bio-Rad).
2.7. Transport assay
Transport assay was performed to quantify cisplatin transport in cells. MEF cells were pre-incubated with serum free medium for 20 min. Then cisplatin was added to a final concentration at 3 μg/mL and 6 μg/mL, respectively. After 40 min incubation, cells were washed 3 times with cold PBS to inactivate transport.
2.8. Platinum (Pt) quantification
Platinum (Pt) was quantified in cells and tissues. Following transport assay or IP injection, cells or tissues were digested with 70% nitric acid until dissolved, and then diluted in deionized water for Pt qualification by ICP-MS (Perkin Elmer, Nexion300), as described (McDermott et al., 2010).
2.9. Statistics
The statistical significance of the experiential data was determined using ANOVA as appropriated by SPSS 10.0 for windows (Chicago, IL, USA) after a comparison of the homogeneity of variance (Levene’s test). When P<0.05, Independent-Samples T Test was applied for univariate comparisons between groups. Data were expressed as the mean ± S.E. P<0.05 was considered statistically significant in this study.
3. Results
3.1. ZIP8 expression regulates cell responses to cisplatin.
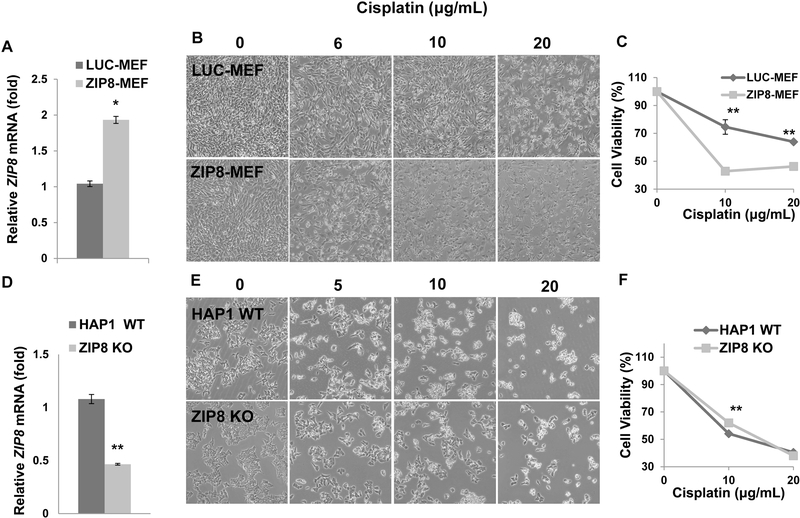
First, ZIP8 mRNA levels were qualified in ZIP8-MEF cells using qRT-PCR. There was 2-fold increase of ZIP8 expression in ZIP8-MEF cells compared with LUC-MEF cells (Fig. 1A). The role of ZIP8 in regulating cisplatin sensitivity was examined in MEF cells with ZIP8 overexpression. As shown in Fig. 1B, overexpression of ZIP8 in MEF cells induced cisplatin sensitivity. MTT assay in MEF cells showed ZIP8-MEF cells have sensitivity to cisplatin and lower proliferation rates at 10 and 20 μg/mL concentrations compared with LUC-MEF cells (Fig. 1C). To further test the ZIP8-dependent drug response, we acquired ZIP8 knockout (KO) HAP1 cells. HAP1 is a near haploid human cell line that has been derived from the male chronic myelogenous leukemia (CML) cell line KBM-7 (Carette et al., 2011). ZIP8 KO HAP1 cell line was constructed by CRISPR/Cas9 system to introduce frame-shift mutations into the coding sequence of genes of interest (New Horizon). The HAP1 knockout cell line was validated by PCR amplification and Sanger Sequencing to confirm the mutation at the genomic level. Although q-RT-PCR showed a 50% decrease in ZIP8 mRNA in HAP KO cells (Fig. 1D), we expect these transcripts cannot be functionally expressed. Treatment of HAP1 WT cells with 5- and 10 μg/mL cisplatin exhibited significant growth inhibition in comparison with ZIP8 KO cells (Fig. 1E), suggesting ZIP8 KO in HAP1 cells have slightly elevated cisplatin resistance. When treated with 10 μg/mL cisplatin, the ZIP8 KO HAP1 cells showed higher viability, whereas at a higher concentration (20 μg/mL) there was no significant difference observed (Fig. 1F). This result can be explained by 1) high levels of cisplatin lead to cell death in both WT and ZIP8 KO cells regardless of ZIP8 expression and 2) HAP1 cells were relatively more sensitive to cisplatin in comparison with MEF cells (Fig. 1B versus Fig. 1E). Taking together, these results demonstrated ZIP8 overexpression leads to elevated cisplatin sensitivity phenotype while downregulation of ZIP8 contributes to visible cisplatin resistance.
Fig. 1. ZIP8 regulates cisplatin response in MEF and HAP1 cells.
(A) Total RNA was used for quantitive RT-PCR to determine ZIP8 expression in MEF cells. (B) LUC-MEF and ZIP8-MEF cells were treated with 6, 10 and 20 μg/mL cisplatin for 24 h and phenotype was recorded with an inverted microscope. (C) MEF cell viability was determined by MTT assay after treatment with 10 and 20 μg/mL cisplatin for 24 h. Data is expressed as means ± SE of three independent experiments (N=3). (D) Total RNA was used for quantitative RT-PCR to determine ZIP8 expression in HAP1 cells. (E) HAP1 WT and ZIP8-KO cells were treated with 5, 10 and 20 μg/mL cisplatin for 24 h and phenotype was recorded with an inverted microscope. (F) HAP1 cell viability was determined by MTT assay. Three replicates were used to determine the statistics (N=3). *P<0.05 and **P<0.01.
3.2. ZIP8 does not facilitate cisplatin uptake.
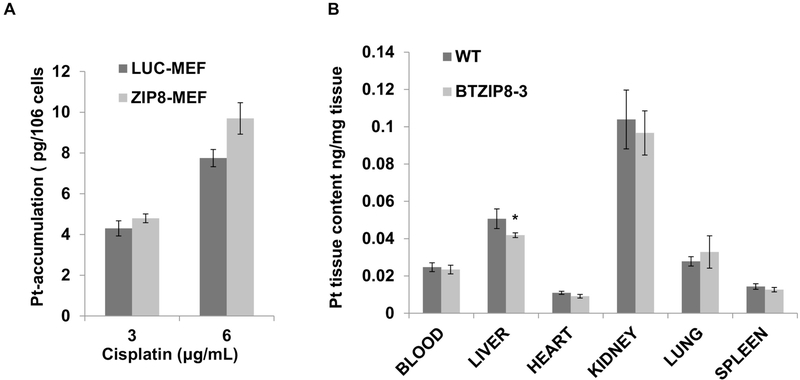
We here tested whether ZIP8 transports cisplatin as a substrate. In LUC-MEF and ZIP8-MEF cells, we compared the Pt cellular uptake using transport assays. Assays were performed at two concentrations and total Pt cellular retention was determined by ICP-MS. As shown in Fig. 2A, Pt cellular accumulation exhibited no significant difference between LUC-MEF and ZIP8-MEF cells following cisplatin treatment. This result supports that ZIP8 does not transport cisplatin.
Fig. 2. ZIP8 does not facilitate cisplatin uptake in MEF cells and transgenic mouse.
(A) Pt uptake was determined in LUC-MEF and ZIP8-MEF cells after exposure to 3 and 6 μg/mL cisplatin for 40 min. Three replicates (N=3) were used to determine the statistics. (B) Intracellular Pt content was measured in WT (N=5) and BTZIP8–3 (N=16) tissues at 30 min after IP cisplatin (10 mg/Kg) injection. Intracellular Pt content was determined by ICP-MS. *P<0.05.
The role of ZIP8 in affecting cisplatin tissue accumulation was studied in ZIP8 transgenic mice BTZIP8–3 and WT controls. BTZIP8–3 contains three extra copies of Slc39a8 (ZIP8), with overexpression in multiple tissue (~2.5 fold higher expression) (Wang et al., 2007). In these mice, cisplatin was delivered by intraperitoneal (IP) injection at a dose of 10 mg/Kg. After 30 minutes, mice were sacrificed and tissues were collected for Pt quantification by ICP-MS. Our results showed that the Pt content in BTZIP8–3 had no significant difference with WT controls in the tissues of blood, liver, heart, kidney, lung and spleen (Fig. 2B). Overall, we conclude ZIP8 does not transport cisplatin directly and ZIP8 regulated cisplatin resistance is mediated by alternative mechanisms.
3.3. ZIP8 regulates anti-apoptotic protein Bcl-2 expression.
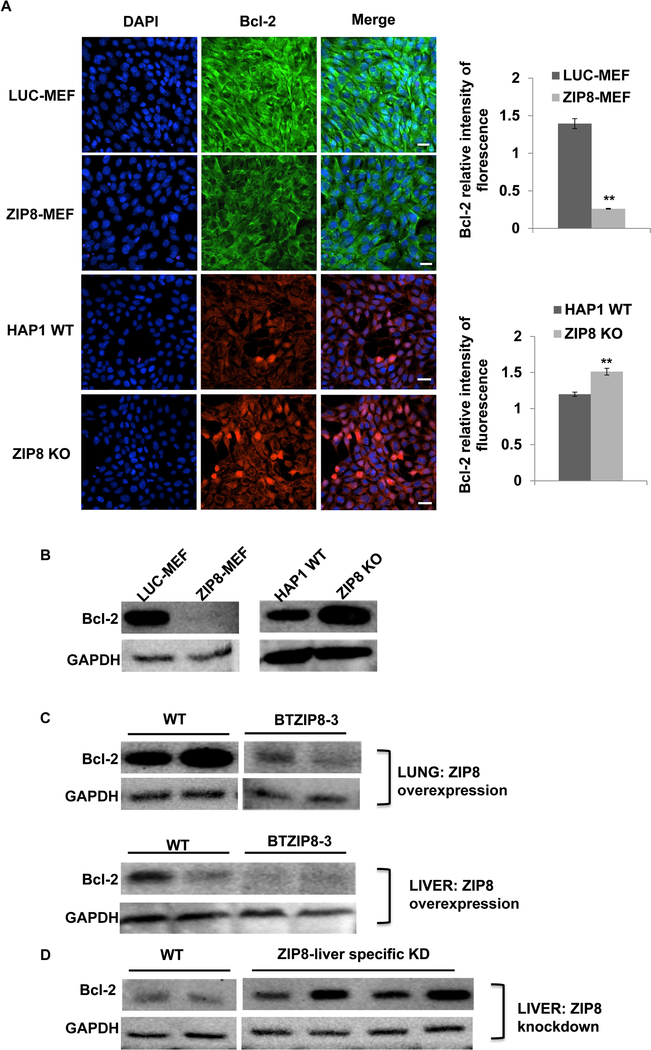
Next, we examined whether ZIP8 regulates downstream targets that are involved in the ZIP8 associated cisplatin sensitivity. The expression of anti-apoptotic Bcl-2 in cell cultures and mouse tissues with altered ZIP8 expression was further assessed. Immunofluorescent staining showed that Bcl-2 was significantly decreased in ZIP8 overexpressed MEF cells (Fig. 3A). In HAP1 cells, knockout of ZIP8 induced an elevation of Bcl-2 (Fig. 3A). The quantification of Bcl-2 expression in ZIP8-altered MEF and HAP1 cells was performed by western-blot. Results support that ZIP8 overexpression induces the decrease of Bcl-2 while ZIP8-deficiency results in elevation of Bcl-2 in vitro (Fig. 3B).
Fig. 3. ZIP8 regulates Bcl-2 expression in ZIP8 gain and loss cells and transgenic mouse.
(A) Bcl-2 expression was visualized by immunofluorescence in MEF and HAP1 cells, with ZIP8 overexpression and knockout, respectively. Scale bar: 20 μM. (B) Bcl-2 expression was quantified by western-blotting in MEF and HAP1 cells, with ZIP8 overexpression and knockout, respectively. GAPDH was used as loading control. (C) Bcl-2 expression was quantified by western-blotting in BTZIP8–3 lung and liver tissues. (D) Bcl-2 expression in liver-specific knockdown of ZIP8 was determined by western-blotting.
The role of ZIP8 on Bcl-2 expression was further investigated in ZIP8 overexpressed liver and lung tissue. Our results showed Bcl-2 expression was significantly lower in both BTZIP8–3 liver and lung, compared with that in WT tissues (Fig. 3C). On the other hand, when ZIP8 was downregulated specifically in liver tissue by adenovirus mediated shRNA knockdown (Liu et al., 2018), Bcl-2 expression was significantly elevated, as shown in Fig. 3D. Therefore, results from gain and loss of ZIP8 mice demonstrated that ZIP8 regulates Bcl-2 expression, which is predicted to be involved in ZIP8-regulated cisplatin responses.
3.4. ZIP8 expression modulates cisplatin-induced apoptotic signaling.
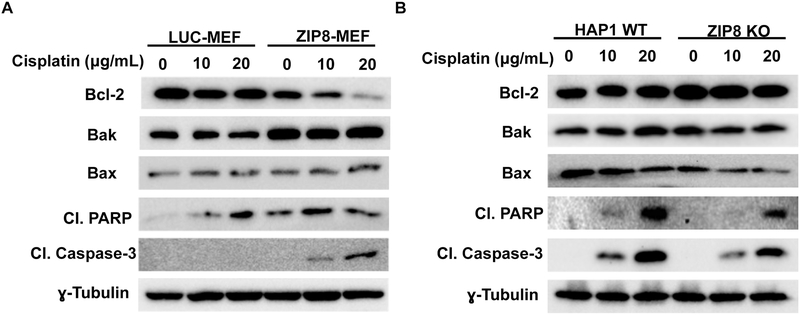
To investigate whether ZIP8-mediated cisplatin resistance is associated with cell apoptosis signaling, we examined expression of apoptosis molecules including: Bcl-2, Bak, Bax, cleaved PARP and cleaved Caspase-3 under treatment by cisplatin in MEF and HAP1 cells. In ZIP8-MEF cells, our results showed cleavage Caspase-3 was induced with cisplatin treatment, indicating cell apoptosis was initiated (Fig. 4A). PARP can be cleaved by Caspase-3 into 2 specific fragments of 89-kD and 24-kD (Lazebnik et al., 1994; Margolin et al., 1997). Caspase-1,Caspase-3 and Caspase-7 all cleave PARP (Germain et al., 1999; Kirsch et al., 1999). We discovered that cleaved PARP was also elevated in ZIP8-MEF cells following cisplatin treatment (Fig. 4A). In addition, higher expression of pro-apoptotic Bak and lower expression of Bcl-2 in ZIP8-MEF cells were observed and showed cisplatin readily induces apoptosis when ZIP8 is overexpressed. (Fig. 4A).
Fig. 4. ZIP8 affects cisplatin induced Caspase-3 cleavage.
MEF (A) and HAP1 (B) cells were treated with cisplatin for 24 h. Anti-apoptotic Bcl-2, proapoptotic Bak, proapoptotic Bax, cleaved PARP and cleaved Caspase-3 expression was analyzed by western-blotting. ɣ-tubulin was used as loading control.
The cisplatin-induced apoptotic signaling was also examined in HAP1 cells with ZIP8 KO. The cleaved Caspase-3 expression was remarkably decreased in HAP1 ZIP8 KO cells compared with WT cells under cisplatin treatment (Fig. 4B). In ZIP8 KO HAP1 cells, elevated Bcl-2, decreased Bax/Bak, cleaved PARP, and cleaved Caspase-3 were also observed (Fig. 4B). These results further support that ZIP8 KO in HAP1 cells benefit cell survival in cisplatin through inhibition of cellular apoptotic process. Overall, results from ZIP8-overexpressed MEF and ZIP8 KO HAP1 cells supports that ZIP8 regulates Bcl-2 expression and modulates cell apoptosis, which further regulates cisplatin toxicity.
4. Discussion
Chemotherapies efficacy is significantly influenced by drug resistance. Elucidation of mechanisms involved in drug resistance could improve treatment outcome and aid the development of chemo-sensitization strategies in clinical settings. Our results showed ZIP8 expression correlates to cisplatin sensitivity: overexpression of ZIP8 induced elevation of cisplatin sensitivity and knockout of ZIP8 led to cisplatin resistance. This is the first report to link ZIP8 function to cisplatin drug responses, which suggests ZIP8 a new molecule to be evaluated involved in cisplatin therapy and drug resistance management.
Why would ZIP8 transporter affects cisplatin sensitivity? A direct rationale is that ZIP8 serves as a cisplatin transporter and facilitates cisplatin uptake. Cisplatin is transported by copper transporter Ctr1 (Lin et al., 2002), while ZIP8 transports many divalent biometals, such as Zn, Fe, and Mn. Although there is no direct evidence to show ZIP8 transports Cu, Cu can inhibit ZIP8 uptake of Zn (Koike et al., 2017). Therefore, we tested whether ZIP8 directly transports cisplatin. Our results clearly excluded this possibility due to no significant difference of cellular Pt content observed in ZIP8 altered cells and mice tissues. It has been shown that kidney and lung tissue have the highest ZIP8 expression (Wang et al., 2007) and cisplatin is mainly metabolized in the kidney (Oh et al., 2016). Our results showed that the kidney has the highest Pt retention, followed by the liver. The BTZIP8–3 mice showed no significant elevation of Pt accumulation in all examined tissues. Based on these results we concluded ZIP8 is not a transporter for cisplatin.
Further studies showed ZIP8 regulates a well-known anti-apoptotic molecule, Bcl-2. ZIP8 knockout in HAP1 cells and ZIP8 knockdown in liver induced elevation of Bcl-2 expression. ZIP8 overexpression in MEF cells and liver and lung tissue was associated with decreased Bcl-2 expression. Therefore, it is reasonable to propose that ZIP8 modulates cisplatin responses through Bcl-2 regulation.
Bcl-2 locates at the outer membrane of mitochondria and the endoplasmic reticulum, where it plays a role in either pro- or anti-apoptotic activity (Youle and Strasser, 2008). Overexpression of anti-apoptotic proteins or inhibition of pro-apoptotic proteins can protect cells against cell death induced by stimuli, such as oxidative stress, chemotherapy, and growth factor deprivation. Bcl-2 overexpression is observed in a number of cancers, including leukemia, prostate, breast, and small cell and non-small cell lung cancers (Yip and Reed, 2008). Overexpression of Bcl-2 protein can inhibit Bax and Bak activation and prevent subsequent cytochrome C release and caspase activation (Cheng et al., 2001), together alters cell apoptosis.
Overexpression of Bcl-2 has been observed to be associated with cisplatin resistance. For example, cancer cells can circumvent cisplatin-induced toxicity by up-regulating Bcl-2 expression (Leisching et al., 2015). Down-regulation of Bcl-2 activity by inhibitor or siRNA sensitizes resistant-cancer cells to cisplatin and promotes apoptosis (Wang et al., 2009) (Cho et al., 2006). These results suggest Bcl-2 overexpression increases cisplatin resistance and affects the cisplatin therapeutic outcome.
Our study showed Bcl-2 was negatively regulated by ZIP8. ZIP8-deficiency-induced Bcl-2 expression can protect HAP1 cells against cisplatin toxicity, whereas overexpression of ZIP-8 decreased Bcl-2 sensitivity of MEF cells to cisplatin treatment. Upon cisplatin treatment, Bcl-2 alternation was accompanied with changes of apoptotic Bak and Bax signaling, which collectively lead to a change of cell apoptosis progress, manifested by the expression of cleaved Caspase-3 and PARP.
Why would the nutrient transporter ZIP8 regulate apoptotic signaling Bcl-2 expression? ZIP8 transports multiple substrates, including Mn, Zn and Se; one or more of which might regulate apoptotic signaling. While Bcl-2 is not metal-dependent molecule, none of the ZIP8 substrate should have direct effects on Bcl-2 function. Instead, Bcl-2 may be indirectly modulated by ZIP8 substrate selenium. Selenium is reported to be associated with cell apoptotic reactions to T-2 toxin through changing Bcl-2/Bax ratio (Chen et al., 2008). Lack of selenium transporter ZIP8 may induce a selenium deficiency which contributes to the Bcl-2/Bax ratio and apoptosis. Moreover, ZIP8 may have a role in regulating GST activity and GSH level through controlling selenium availability (Arthur et al., 1987). Therefore, we propose that ZIP8 may regulate Bcl-2 via its substrate indirectly through uncharacterized pathway(s).
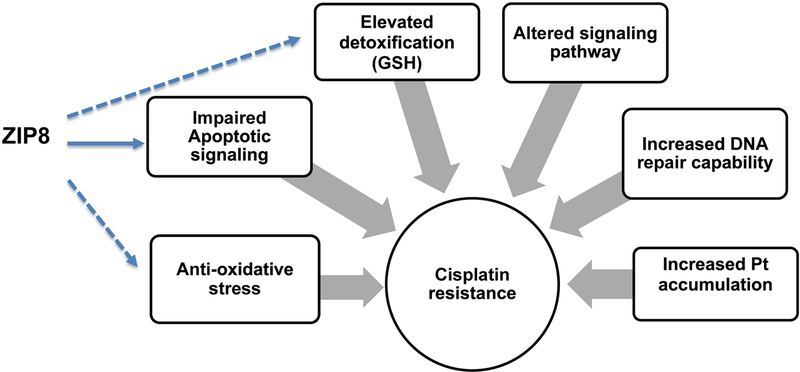
In addition to Bcl-2, other mechanisms might also be involved in ZIP8-associated cisplatin responses. We discovered ZIP8 regulates cell morphology, adhesion and proliferation through NF-κB and Snail2, both involved in the regulation of many downstream targets (Geng et al., 2018). The ZIP8-regulated cisplatin responses may be affected by the molecules involved in cell adhesion and migration. Further studies will be followed to further identify additional ZIP8 downstream targets and their connection with cisplatin chemosensitivity. Proposed mechanisms of ZIP8-regulated cisplatin response were summarized in Fig. 5.
Fig. 5. Proposed mechanisms of ZIP8 cisplatin-resistance.
Pathways involved in cisplatin resistance were summarized. Mechanisms of possible ZIP8-regulated cisplatin responses was proposed. ZIP8 may regulate anti-apoptotic Bcl-2 expression and contribute to altered cisplatin responses.
Overall, we present results to show that ZIP8 regulates cisplatin chemotherapy response in both non-cancer and cancer cells. We also demonstrate that ZIP8 regulates anti-apoptotic Bcl-2 expression, which is predicted to link ZIP8 with cisplatin sensitivity. Future studies need to look into how ZIP8 regulates Bcl-2 and identify the additional ZIP8 regulated pathways. Our results offer a potential candidate involved in cisplatin drug resistance and suggest ZIP8 and mineral homeostasis to be considered in managing chemotherapy resistance.
Highlights:
ZIP8 expression is associated with cisplatin toxicity
Overexpression of ZIP8 leads to elevated cisplatin sensitivity in MEF cells while ZIP8 knockout induces cisplatin resistance
ZIP8 overexpression does not increase cisplatin accumulation in cells and in ZIP8 transgenic mice
ZIP8 expression is reversely associated with Bcl-2 expression and cisplatin induced apoptosis
Acknowledgments
We acknowledge Gerard Madlambayan for his support in materials. We also appreciate Mrs. Constance Arsenault and Mr. Dane Deemer for their careful review and edit of the manuscript.
Financial Support:
This work was supported by Oakland University Research Excellent Fund (REF) and NIH ES022800 to Liu Z.
Footnotes
Disclosures of Potential Conflicts of Interest:
The authors indicate no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Besecker B, Bao SY, Bohacova B, Papp A, Sadee W, Knoell DL, 2008. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol-Lung C 294, L1127–L1136. [DOI] [PubMed] [Google Scholar]
- Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, Dal Cin P, Dye JM, Whelan SP, Chandran K, Brummelkamp TR, 2011. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477, 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Cao JL, Chu YL, Wang ZL, Yang ZT, Wang HL, 2008. T-2 toxin-induced apoptosis involving Fas, p53, Bcl-xL, Bcl-2, Bax and caspase-3 signaling pathways in human chondrocytes. J Zhejiang Univ Sci B 9, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EHYA, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ, 2001. BCL-2, BCL-X-L sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Molecular Cell 8, 705–711. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Kim JK, Kim KD, Yoon HK, Cho MY, Park YP, Jeon JH, Lee ES, Byun SS, Lim HM, Song EY, Lim JS, Yoon DY, Lee HG, Choe YK, 2006. Upregulation of Bcl-2 is associated with cisplatin-resistance via inhibition of Bax translocation in human bladder cancer cells. Cancer Letters 237, 56–66. [DOI] [PubMed] [Google Scholar]
- Costas J, 2018. The highly pleiotropic gene SLC39A8 as an opportunity to gain insight into the molecular pathogenesis of schizophrenia. Am J Med Genet B 177, 274–283. [DOI] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB, 2014a. Cisplatin in cancer therapy: Molecular mechanisms of action. European Journal of Pharmacology 740, 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB, 2014b. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740, 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Feng J, Zeng D, Ding Y, Yu C, Yang B, 2014. PAK4 confers cisplatin resistance in gastric cancer cells via PI3K/Akt- and MEK/ERK-dependent pathways. Biosci Rep 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Ueda T, Aune G, Sarasin A, Kraemer KH, Pommier Y, 2002. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res 62, 4899–4902. [PubMed] [Google Scholar]
- Galanski M, 2006. Recent developments in the field of anticancer platinum complexes. Recent Pat Anti-Canc 1, 285–295. [DOI] [PubMed] [Google Scholar]
- Galvez-Peralta M, He L, Jorge-Nebert LF, Wang B, Miller ML, Eppert BL, Afton S, Nebert DW, 2012. ZIP8 Zinc Transporter: Indispensable Role for Both Multiple-Organ Organogenesis and Hematopoiesis In Utero. Plos One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Liu L, Banes-Berceli A, Yang Z, Kang P, Shen J, Tsai K-J, Liu Z, 2018. Role of ZIP8 in regulating cell morphology and NF-κB/Snail2 signaling. Metallomics. [DOI] [PubMed] [Google Scholar]
- Germain M, Affar EB, D’Amours D, Dixit VM, Salvesen GS, Poirier GG, 1999. Cleavage of automodified poly(ADP-ribose) polymerase during apoptosis - Evidence for involvement of caspase-7. J Biol Chem 274, 28379–28384. [DOI] [PubMed] [Google Scholar]
- Guttmann S, Chandhok G, Groba SR, Niemietz C, Sauer V, Gomes A, Ciarimboli G, Karst U, Zibert A, Schmidt HH, 2018. Organic cation transporter 3 mediates cisplatin and copper cross-resistance in hepatoma cells. Oncotarget 9, 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagrman D, Goodisman J, Dabrowiak JC, Souid AK, 2003. Kinetic study on the reaction of cisplatin with metallothionein. Drug Metab Dispos 31, 916–923. [DOI] [PubMed] [Google Scholar]
- He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW, 2006. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: Characterization of transporter properties. Mol Pharmacol 70, 171–180. [DOI] [PubMed] [Google Scholar]
- He L, Wang B, Hay EB, Nebert DW, 2009. Discovery of ZIP transporters that participate in cadmium damage to testis and kidney. Toxicol Appl Pharm 238, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CP, Fofana M, Chan J, Chang CJ, Howell SB, 2014. Copper transporter 2 regulates intracellular copper and sensitivity to cisplatin. Metallomics 6, 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I, 2002. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A 99, 14298–14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, Lee G, Rhee J, Ryu JH, Chun CH, Chun JS, 2014. Regulation of the Catabolic Cascade in Osteoarthritis by the Zinc-ZIP8-MTF1 Axis. Cell 156, 730–743. [DOI] [PubMed] [Google Scholar]
- Kim JW, Sahm H, You J, Wang M, 2010. Knock-down of Superoxide Dismutase 1 Sensitizes Cisplatin-resistant Human Ovarian Cancer Cells. Anticancer Res 30, 2577–2581. [PubMed] [Google Scholar]
- Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM, 1999. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem 274, 21155–21161. [DOI] [PubMed] [Google Scholar]
- Koike A, Sou J, Ohishi A, Nishida K, Nagasawa K, 2017. Inhibitory effect of divalent metal cations on zinc uptake via mouse Zrt-/Irt-like protein 8 (ZIP8). Life Sci 173, 80–85. [DOI] [PubMed] [Google Scholar]
- Kool M, deHaas M, Scheffer GL, Scheper RJ, vanEijk MJT, Juijn JA, Baas F, Borst P, 1997. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res 57, 3537–3547. [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC, 1994. Cleavage of Poly(Adp-Ribose) Polymerase by a Proteinase with Properties Like Ice. Nature 371, 346–347. [DOI] [PubMed] [Google Scholar]
- Lebwohl D, Canetta R, 1998. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer 34, 1522–1534. [DOI] [PubMed] [Google Scholar]
- Leisching G, Loos B, Botha M, Engelbrecht AM, 2015. Bcl-2 confers survival in cisplatin treated cervical cancer cells: circumventing cisplatin dose-dependent toxicity and resistance. J Transl Med 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XJ, Okuda T, Holzer A, Howell SB, 2002. The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol 62, 1154–1159. [DOI] [PubMed] [Google Scholar]
- Liu L, Geng X, Cai Y, Copple BL, Yoshinaga M, Shen J, Nebert DW, Wang H, Liu Z, 2018. Hepatic ZIP8 deficiency is associated with disrupted selenium homeostasis, liver pathology and tumor formation. American Journal of Physiology-Gastrointestinal and Liver Physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MJ, Bao SY, Galvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li CL, Nebert DW, Wewers MD, Knoell DL, 2013. ZIP8 Regulates Host Defense through Zinc-Mediated Inhibition of NF-kappa B. Cell Reports 3, 386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li H, Soleimani M, Girijashanker K, Reed JM, He L, Dalton TP, Nebert DW, 2008. Cd2+ versus Zn2+ uptake by the ZIP8 HCO3--dependent symporter: Kinetics, electrogenicity and trafficking. Biochem Bioph Res Co 365, 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak ACY, et al. Consortium, N.T.-O.f.P.M., 2018. Whole-Genome Sequencing of Pharmacogenetic Drug Response in Racially Diverse Children with Asthma. Am J Respir Crit Care Med 197, 1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin N, Raybuck SA, Wilson KP, Chen WY, Fox T, Gu Y, Livingston DJ, 1997. Substrate and inhibitor specificity of interleukin-1 beta-converting enzyme and related caspases. J Biol Chem 272, 7223–7228. [DOI] [PubMed] [Google Scholar]
- McDermott JR, Geng X, Jiang L, Galvez-Peralta M, Chen F, Nebert DW, Liu Z, 2016. Zinc- and bicarbonate-dependent ZIP8 transporter mediates selenite uptake. Oncotarget 7, 35327–35340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JR, Jiang X, Beene LC, Rosen BP, Liu ZJ, 2010. Pentavalent methylated arsenicals are substrates of human AQP9. Biometals 23, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh GS, Kim HJ, Shen A, Lee SB, Yang SH, Shim H, Cho EY, Kwon KB, Kwak TH, So HS, 2016. New Therapeutic Concept of NAD Redox Balance for Cisplatin Nephrotoxicity. Biomed Res Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestayko AW, Daoust JC, Issell BF, Crooke ST, 1979. Cisplatin (Cis-Diamminedichloroplatinum-Ii). Cancer Treat Rev 6, 17–39. [DOI] [PubMed] [Google Scholar]
- Samimi G, Safaei R, Katano K, Holzer AK, Rochdi M, Tomioka M, Goodman M, Howell SB, 2004. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin Cancer Res 10, 4661–4669. [DOI] [PubMed] [Google Scholar]
- Severi L, Losi L, Fonda S, Taddia L, Gozzi G, Marverti G, Magni F, Chinello C, Stella M, Sheouli J, Braicu EI, Genovese F, Lauriola A, Marraccini C, Gualandi A, D’Arca D, Ferrari S, Costi MP, 2018. Proteomic and Bioinformatic Studies for the Characterization of Response to Pemetrexed in Platinum Drug Resistant Ovarian Cancer. Front Pharmacol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speliotes EK, et al. , 2010. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42, 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DJ, 2007. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol 63, 12–31. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD, He L, King JB, Hanigan MH, 2009. Role of glutathione S-transferase Pi in cisplatin-induced nephrotoxicity. Biomed Pharmacother 63, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, He L, Dong HB, Dalton TP, Nebert DW, 2011. Generation of a Slc39a8 hypomorph mouse: Markedly decreased ZIP8 Zn2+/(HCO3-)(2) transporter expression. Biochem Bioph Res Co 410, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Schneider SN, Dragin N, Girijashanker K, Dalton TP, He L, Miller ML, Stringer KF, Soleimani M, Richardson DD, Nebert DW, 2007. Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am J Physiol Cell Physiol 292, C1523–1535. [DOI] [PubMed] [Google Scholar]
- Wang CY, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD, 2012. ZIP8 Is an Iron and Zinc Transporter Whose Cell-surface Expression Is Up-regulated by Cellular Iron Loading. J Biol Chem 287, 34032–34043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou JY, Zhang LF, Wu GS, 2009. Involvement of MKP-1 and Bcl-2 in acquired cisplatin resistance in ovarian cancer cells. Cell Cycle 8, 3191–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth DM, et al. , 2010. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol 30, 2264–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip KW, Reed JC, 2008. Bcl-2 family proteins and cancer. Oncogene 27, 6398–6406. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Nozaki S, Kitahara H, Ohara T, Kato K, Kawashiri S, Yamamoto E, 2007. Copper efflux transporter (ATP7B) contributes to the acquisition of cisplatin-resistance in human oral squamous cell lines. Oncol Rep 18, 987–991. [PubMed] [Google Scholar]
- Youle RJ, Strasser A, 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Bio 9, 47–59. [DOI] [PubMed] [Google Scholar]
- Zhang K, Chew M, Yang EB, Wong KP, Mack P, 2001. Modulation of cisplatin cytotoxicity and cisplatin-induced DNA cross-links in HepG2 cells by regulation of glutathione-related mechanisms. Mol Pharmacol 59, 837–843. [DOI] [PubMed] [Google Scholar]
- Zhang R, Witkowska K, Afonso Guerra-Assuncao J, Ren M, Ng FL, Mauro C, Tucker AT, Caulfield MJ, Ye S, 2016. A blood pressure-associated variant of the SLC39A8 gene influences cellular cadmium accumulation and toxicity. Hum Mol Genet 25, 4117–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]