Abstract
Background:
Direct acting oral anticoagulants (DOAC) are associated with less bleeding than traditional venous thromboembolism (VTE) treatments in the general population but are little studied in cancer-associated VTE (CA-VTE).
Objective:
To determine whether different anticoagulation strategies for CA-VTE have different hospitalized bleeding rates.
Patients/Methods:
We conducted a retrospective study of patients with CA-VTE diagnosed between 2011–2015 in a large administrative database. Using validated algorithms, we identified 26,894 CA-VTE patients treated with anticoagulants and followed them for hospitalized severe bleeding. Cox models were used to assess bleeding risk adjusted for age, sex, high dimensional propensity score, and frailty.
Results:
Over 27,281 person-years of follow-up (median 0.6 years), 1,204 bleeding events occurred for a bleeding rate of 4.4% per patient-year. Bleeding rates varied by cancer type with the highest rate for upper gastrointestinal cancers (8.6%) and the lowest for breast cancer (2.9%). In Cox models (HR; 95% CI), compared with warfarin, DOACS and LMWH had similar hazards of bleeding (HR 0.88; 0.69–1.11 and HR 0.98; 0.85–1.13). Compared to LMWH, there was no difference in hazard of bleeding with DOACs (0.86; 0.66–1.12). There was heterogeneity in bleeding risk with DOACs by cancer type, with a higher risk of bleeding in upper gastrointestinal cancers and lower bleeding risk in prostate cancer and hematologic cancers.
Conclusions:
In this practice-based sample of CA-VTE patients, DOACs were associated with similar bleeding risks to warfarin and LMWH. These findings suggest a complex association of bleeding risk with anticoagulant choice in cancer patients.
Keywords: Anticoagulants, Drug Utilization, Hemorrhage, Neoplasms, Venous Thrombosis
One in five of the 900,000 annual venous thromboembolism (VTE) in the United States are associated with cancer[1, 2]. While in the general population treatment of VTE with anticoagulation is highly effective and safe, patients with cancer-associated VTE (CA-VTE) experience a greater burden of anticoagulation failure and bleeding than other patients with traditional treatments for VTE[3, 4]. Thrombosis, including venous thromboembolism (VTE), is the leading cause of death among cancer patients after the cancer itself[5].
Before the introduction of the direct acting oral anticoagulants (DOAC) to treat VTE, options for outpatient anticoagulation treatment were limited to oral vitamin K antagonists (mainly warfarin in the United States) and sub-cutaneous heparin preparations (including low molecular weight heparins (LMWH))[4]. Vitamin K antagonists have many limitations in patients with cancer including drug interactions and difficulty scaling the anticoagulant effect with rapidly changing bleeding risk factors. As such, heparin preparations, which lack these limitations are the preferred treatment for CA-VTE. However, they are inconvenient as they require sub-cutaneous injections[6].
Since 2012, a series of DOACs (apixaban[7], dabigatran[8], edoxaban[9], and rivaroxaban[10]) have been approved to treat VTE[4]. The DOACs have reliable dosing in most individuals and are as effective as warfarin for treating VTE with similar to reduced bleeding rates, making DOACs an attractive potential treatment option for cancer-associated VTE (CA-VTE)[11]. Unfortunately, given the relative novelty of DOACs and the small numbers of active cancer patients in clinical trials of DOACs to treat VTE, the best way to incorporate DOACs into the care of patients with CA-VTE is not established[12, 13].
To address this knowledge gap, we used MarketScan, a commercial claims database, to assess the impact of anticoagulation choice on hospitalized bleeding risk during the treatment of CA-VTE. We hypothesized that warfarin would have the highest bleeding risk, with DOACs and LMWH having lower bleeding risks.
Methods
Study Population / MarketScan Database
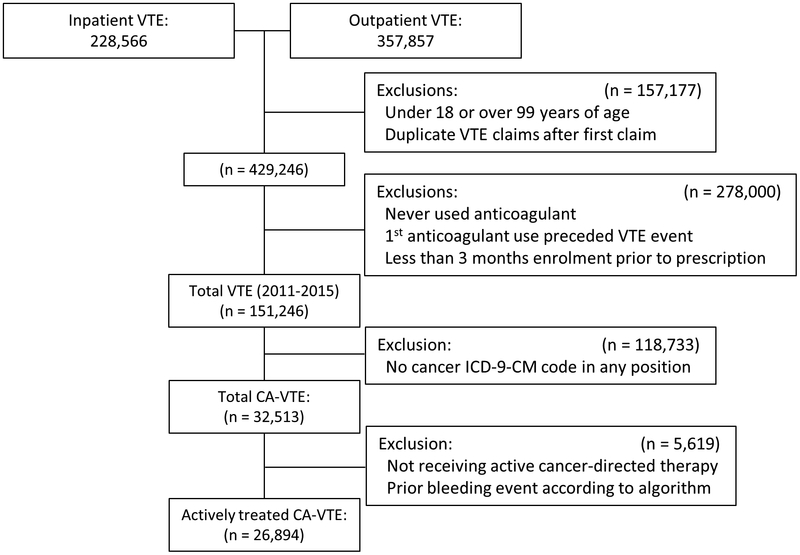
The Truven Health MarketScan Commercial Claims and Encounters Database and the Medicare Supplemental and Coordination of Benefits Database (Truven Health Analytics Inc., Ann Arbor, MI) provide a representative sample of ~43.6 million Americans each year. We conducted a retrospective cohort study from January 1st, 2011 through September 30th, 2015 with information collected via inpatient and outpatient claims including medication and procedure claims. The initial sample included 429,246 patients ages 18–99 with at least one inpatient or 2 outpatient claims for VTE 7 to 365 days apart and one outpatient anticoagulation prescription within 4 weeks of the VTE (International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes 415.1×, 451.1×, 453.2, 453.4×, 453.5×, 453.8×, or 453.9 in any position). ICD-9-CM codes for identifying VTE have a positive predictive value (PPV) of approximately 85% which increases to 91% when requiring treatment of VTE[14–20]. After restricting our sample to those with >90 days of continuous enrolment prior to their anticoagulant prescription, and including only the first enrolment period in the event of a temporary dis-enrolment, there 151,246 patients with VTE.
Among patients with VTE, we identified 32,513 patients with a cancer ICD-9-CM diagnosis claim in any position prior to their VTE diagnosis (eTable 1). Patients were considered to have active cancer-directed therapy if they had at least 1 claim for any of the following before the diagnosis of VTE: 1) an inpatient ICD-9-CM chemotherapy encounter or J-code, 2) a chemotherapy “therapeutic class” or “therapeutic group” drug code, 3) a radiation therapy code, 4) an inpatient chemotherapy or surgery MS-DRG code, or 5) an outpatient cancer surgery procedure code (eTable 2)[21]. The final analytic sample included 26,894 patients being actively treated for cancer concurrent with or before anticoagulant initiation (Figure 1).
Figure 1.
Flowchart for Sample Selection of Cancer-Associated (CA)-Venous Thromboembolism (VTE) Patients Receiving Active Cancer-Directed Therapy
Anticoagulant Use
Patient exposure was categorized based on the first anticoagulant prescribed concurrent with or within 4 weeks after a VTE diagnosis (to mirror an intention to treat analysis), as a new warfarin user, a new LMWH user (enoxaparin, dalteparin, fondaparinux, or heparin), or a new DOAC user (apixaban, dabigatran, or rivaroxaban). To account for anticoagulation overlap while becoming therapeutic on warfarin, individuals whose first anticoagulant was LMWH but received a warfarin prescription within 16 days were classified as warfarin users. Individuals on an oral anticoagulant prior to their first VTE were excluded from further analysis. Though not LMWH, heparin and fondaparinux were included in this category due to the similar clinical profiles. The validity of warfarin claims in administrative databases has a sensitivity of 94% and PPV of 99%; the validity for LMWH and DOACs claims is likely to be similar in the context of this study[22]. Comparisons of anticoagulants for this analysis included warfarin vs. DOACs, warfarin vs. LMWH, and LMWH vs. DOACs. Any comparisons with DOACs were restricted to after November 2nd, 2012, the FDA approval date for rivaroxaban.
Outcome Ascertainment
The main outcome of the study was hospitalized bleeding events defined as intracranial bleeding, gastrointestinal (GI) bleeding, or other bleeding events identified from inpatient claims using validated algorithms[23] in those without a history of major bleeding (eTable 3). This algorithm has a positive predictive value of 86% for identifying serious bleeding events and is comparable to other peer-reviewed algorithms [24, 25].
Assessment of covariates
Covariates including frailty were defined based on inpatient, outpatient and pharmacy claims that occurred within 3 months prior to the index date using validated published algorithms (eTables 4 and 5)[23, 26–28].
Statistical analysis
High dimensional propensity scores (HDPS) were calculated and included predefined variables of age, sex, and calendar year[29, 30]. Empirical covariates were defined using 5 domains: inpatient diagnostic codes, outpatient diagnostic codes, inpatient procedure codes, outpatient procedure codes, and pharmacy claims excluding anticoagulants. Within each of the 5 domains, the top 200 most prevalent conditions were selected resulting in 1000 candidate covariates. The variables were then ranked based on the ratio of prevalence of the candidate covariates in the exposed versus the unexposed. The top 500 candidate covariates were then selected along with the pre-defined covariates to calculate propensity scores. Separate HDPS were calculated for each of the anticoagulant-outcome pairs (1 outcome × 3 comparison groups = 3 total HDPS).
Cox proportional hazards models were used to estimate the association between anticoagulant choice and the time to severe bleeding event for the following comparisons: 1) new warfarin users to new DOAC users; 2) new warfarin users to new LMWH users and 3) new LMWH users to new DOAC users. Follow-up began at the date of anticoagulant initiation and continued until hospitalized bleeding, health plan disenrollment, or the end of study follow-up, whichever occurred first. Four models were conducted for each comparison for all active cancer patients: 1) Crude association; 2) Adjusted for age, sex, and calendar year; 3) Adjusted for age, sex, calendar year and HDPS and 4) Adjusted for age, sex, calendar year, HDPS and frailty. Stratified analyses were conducted for specific cancer types including lung, breast, prostate, colorectal, upper gastrointestinal (GI), and hematologic (leukemia, lymphoma, and myeloma) cancers. Effect modification by sex, age (<75, >75), and kidney disease were explored using stratified analyses. Sensitivity analyses were done excluding individuals not receiving chemotherapy as part of their cancer treatment. All statistical analyses were performed with SAS v 9.4 (SAS Inc., Cary, NC).
We conformed with all regulations from the Health Insurance Portability and Accountability Act and the Declaration of Helsinki. The protocol was reviewed by the University of Minnesota Institutional Review Board and determined exempt from review.
Results
There were 26,894 CA-VTE cases occurring between 2011–2015, of which 14.6% patients had lung cancer, 14.5% had breast cancer, 13.2% had hematologic cancers, 9.6% had colon cancer, 9.5% had prostate cancer, and 3.5% had upper gastrointestinal cancer (Table 1). Of the CA-VTE, 14,833 were treated with warfarin (55.2%), 8,803 with LMWH (32.7%), and 3,258 with a DOAC (12.1%) of whom 2,922 were treated with rivaroxaban (89.7% of DOAC usage). Table 1 presents the characteristics of the analytic sample by anticoagulant choice. In general, the population characteristics were similar for oral anticoagulants (DOACs and warfarin) though individuals treated with LMWH were younger and had a lower burden of chronic diseases. A lower percentage of breast and prostate cancer VTE cases were treated with LMWH.
Table 1.
Characteristics of Venous Thromboembolism Patients with Active Cancer by Anticoagulant Use
| All (n=26,826) |
Warfarin (n=14,833) |
LMWH (n=8,803) |
DOAC (n=3,258) |
Rivaroxaban (n=2,922) |
Apixaban (n=252) |
Dabigatran (n=84) |
|
|---|---|---|---|---|---|---|---|
| Person-years follow-up | 27,281 | 17,725 | 7,184 | 2,372 | 2,196 | 86 | 90 |
| Number of Bleeding Events | 1,204 | 716 | 373 | 115 | 107 | 4 | 4 |
| Age (years, Standard Deviation) | 63.2 (13.2) | 64.8 (13.2) | 60.0 (12.6) | 64.7 (13.3) | 64.2 (13.2) | 69.0 (13.7) | 68.8 (12.7) |
| Age ≥75 years (n, %) | 5,689 (21.2) | 3,767 (25.4) | 1,103 (12.5) | 819 (25.1) | 694 (23.8) | 96 (38.1) | 29 (34.5) |
| Female (n, %) | 13,848 (51.5) | 7,403 (49.9) | 4,768 (54.2) | 1,677 (51.5) | 1,509 (51.6) | 130 (51.6) | 38 (45.2) |
| Baseline Comorbid Conditions | |||||||
| Hypertension (n, %) | 17,121 (63.7) | 9,827 (66.3) | 5,053 (57.4) | 2,241 (68.8) | 1,987 (68.0) | 196 (77.8) | 58 (69.1) |
| Diabetes (n, %) | 7,038 (26.2) | 4,003 (27.0) | 2,083 (23.7) | 952 (29.2) | 833 (28.5) | 96 (38.1) | 23 (27.4) |
| Myocardial Infarction (n, %) | 1,876 (7.0) | 1,166 (7.9) | 470 (5.3) | 240 (7.4) | 202 (6.9) | 33 (13.1) | 5 (6.0) |
| Heart Failure (n, %) | 3,982 (14.8) | 2,421 (16.3) | 990 (11.3) | 571 (17.5) | 483 (16.5) | 66 (26.2) | 22 (26.2) |
| Ischemic Stroke (n, %) | 4,431 (16.5) | 2,538 (17.1) | 1,241 (14.1) | 652 (20.0) | 549 (18.8) | 78 (31.0) | 25 (29.8) |
| Hemorrhagic Stroke (n, %) | 457 (1.7) | 225 (1.5) | 199 (2.3) | 33 (1.0) | 26 (0.9) | 6 (2.4) | 1 (1.2) |
| Kidney Disease (n, %) | 3,351 (12.5) | 2,213 (14.9) | 705 (8.0) | 433 (13.3) | 357 (12.2) | 63 (25.0) | 13 (15.5) |
| Chronic Pulmonary Disease (n, %) | 8,881 (33.0) | 5,058 (34.1) | 2,613 (29.7) | 1,210 (37.1) | 1,071 (36.6) | 106 (42.1) | 33 (39.3) |
| Liver Disease (n, %) | 5,500 (20.5) | 2,428 (16.8) | 2,379 (27.0) | 693 (21.3) | 620 (21.2) | 57 (22.6) | 16 (19.1) |
| Depression (n, %) | 4,618 (17.2) | 2,430 (16.4) | 1,565 (17.8) | 623 (19.1) | 559 (19.1) | 50 (19.8) | 14 (16.7) |
| Alcohol Abuse (n, %) | 156 (0.6) | 85 (0.6) | 56 (0.6) | 15 (0.5) | 13 (0.4) | 2 (0.8) | 0 (0.0) |
| Gastrointestinal Bleeding (n, %) | 3,109 (11.6) | 1,615 (10.9) | 1,029 (11.7) | 465 (14.3) | 415 (14.2) | 41 (16.3) | 9 (10.7) |
| Other Bleeding (n, %) | 2,250 (8.4) | 1,121 (7.6) | 833 (9.5) | 296 (9.1) | 259 (8.9) | 27 (10.7) | 10 (11.9) |
| Frail (n, %) | 12,572 (46.8) | 6,859 (46.2) | 3,975 (45.2) | 1,738 (53.4) | 1,525 (52.2) | 165 (65.5) | 48 (57.1) |
| Medication Use | |||||||
| Anti-platelet medication (n, %) | 333 (1.2) | 198 (1.3) | 85 (1.0) | 50 (1.5) | 42 (1.4) | 4 (1.6) | 4 (4.8) |
| Statins (n, %) | 10,051 (37.4) | 5,833 (39.3) | 2,815 (32.0) | 1,403 (43.1) | 1,234 (42.2) | 134 (53.2) | 35 (41.7) |
| Hormone-Modifying agents (n, %) | 3,949 (14.7) | 2,255 (15.2) | 1,184 (13.5) | 510 (15.7) | 464 (15.9) | 31 (12.3) | 15 (17.9) |
| Selective Serotonin Reuptake Inhibitors (n, %) | 8,446 (31.4) | 4,579 (30.9) | 2,782 (31.6) | 1,085 (33.3) | 964 (33.0) | 100 (39.7) | 21 (25.0) |
| Dexamethasone (n, %) | 6,679 (24.8) | 2,907 (19.6) | 3,167 (36.0) | 605 (18.6) | 552 (18.9) | 33 (13.1) | 20 (23.8) |
| Cancer Type | |||||||
| Lung (n, %) | 3,929 (14.6) | 2,015 (13.6) | 1,494 (17.0) | 420 (12.9) | 381 (13.0) | 25 (9.9) | 14 (16.7) |
| Breast (n, %) | 3,909 (14.5) | 2,330 (15.7) | 1,004 (11.4) | 575 (17.7) | 520 (17.8) | 40 (15.9) | 15 (17.9) |
| Colon (n, %) | 2,580 (9.6) | 1,460 (9.8) | 812 (9.2) | 308 (9.5) | 282 (9.7) | 23 (9.1) | 3 (3.6) |
| Upper Gastrointestinal (n, %) | 930 (3.5) | 429 (2.9) | 415 (4.7) | 86 (2.6) | 84 (2.9) | 2 (0.1) | 0 (0.0) |
| Prostate (n, %) | 2,550 (9.48) | 1,794 (12.1) | 362 (4.1) | 394 (12.1) | 335 (11.5) | 45 (17.9) | 14 (16.7) |
| Hematologic (n, %) | 3,549 (13.2) | 1,889 (12.7) | 1,273 (14.5) | 387 (11.9) | 342 (11.7) | 31 (12.3) | 14 (16.7) |
| Other (n, %) | 3,929 (14.6) | 4,916 (33.1) | 3,443 (39.1) | 1,088 (33.4) | 978 (33.5) | 86 (34.1) | 924 (28.6) |
| Cancer Treatments | |||||||
| Surgery Alone (n, %) | 2,079 (7.7) | 1,375 (9.3) | 322 (3.7) | 382 (11.7) | 331 (11.3) | 41 (16.3) | 10 (11.9) |
| Radiation Therapy Alone (n, %) | 1,150 (4.3) | 577 (3.9) | 470 (5.3) | 103 (3.2) | 196 (3.3) | 4 (1.6) | 3 (3.6) |
| Chemotherapy with or without other treatments(n, %) | 14,567 (54.2) | 6,750 (45.5) | 6,420 (72.9) | 1,397 (42.9) | 1,284 (43.9) | 79 (31.4) | 34 (40.5) |
| Other, unclassified treatments and combinations | 9,098 (33.8) | 6,131 (41.3) | 1,591 (18.1) | 1,376 (42.2) | 1,111 (38.0) | 128 (50.8) | 37 (44.0) |
A total of 1,204 bleeding events occurred over 27,281 person-years of follow-up (mean follow-up 1.00 years, SD: 1.00 years; maximum 4.5 years). Table 2 presents the unadjusted number of bleeding events and bleeding rates by anticoagulant choice and cancer type. The overall bleeding rate was 4.4% per patient-year. Bleeding rates differed by cancer type; the unadjusted rate was highest in patients with upper gastrointestinal cancers (8.6% per patient-year), with lower rates in lung cancer (6.0% per patient-year), colorectal cancer (4.5% per patient-year), prostate cancer (4.0% per patient-year), hematologic cancer (3.5% per patient-year), and breast cancer (2.9% per patient-year).
Table 2.
Number of Hospitalized Bleeding Events and Bleeding Rates (per Patient-Year) by Site of Bleeding and Cancer Type
| All | Warfarin | LMWH | DOACS | |||||
|---|---|---|---|---|---|---|---|---|
| Count | Rate | Count | Rate | Count | Rate | Count | Rate | |
| All bleeding | 1,204 | 4.41 (4.17–4.67) | 716 | 4.04 (3.75–4.34) | 373 | 5.19 (4.69 −5.74) | 115 | 4.85 (4.02–5.80) |
| Cancer Type | ||||||||
| Lung | 167 | 6.19 (5.30–7.18) | 98 | 6.01 (4.91–7.30) | 58 | 6.72 (5.15–8.63) | 11 | 5.31 (2.79–9.24) |
| Breast | 137 | 2.87 (2.42–3.38) | 91 | 2.78 (2.25–3.40) | 26 | 2.48 (1.66–3.58) | 20 | 4.44 (2.79–6.74) |
| Colorectal | 126 | 4.56 (3.81–5.41) | 78 | 4.33 (3.45–538) | 31 | 4.20 (2.90–5.88) | 17 | 7.59 (4.57–11.90) |
| Upper Gastrointestinal | 56 | 8.64 (6.49–11.14) | 24 | 6.59 (4.32–9.66) | 22 | 9.32 (5.99–13.88) | 10 | 20.83 (10.58–37.14) |
| Prostate | 130 | 3.98 (3.34–4.71) | 99 | 3.86 (3.16–4.68) | 22 | 6.53 (4.20–9.72) | 9 | 2.43 (1.18–4.45) |
| Hematologic | 145 | 3.48 (2.94 −4.08) | 81 | 3.23 (2.58–3.99) | 51 | 3.75 (2.82–4.89) | 13 | 4.32 (2.40–7.20) |
| Other | 462 | 4.94 (4.50–5.40) | 256 | 4.41 (3.89–4.97) | 171 | 6.23 (5.35–7.22) | 35 | 4.34 (3.07–5.97) |
| Bleeding Site | ||||||||
| Intracranial | 136 | 0.51 (0.43–0.60) | 68 | 0.39 (0.31–0.50) | 58 | 0.82 (0.63–1.05) | 10 | 0.43 (0.22–0.76) |
| Gastrointestinal | 740 | 2.74 (2.54–2.94) | 459 | 2.61 (2.38–2.86) | 214 | 3.01 (2.62–3.43) | 67 | 2.84 (2.22–3.59) |
| Other | 328 | 1.23 (1.10–1.36) | 189 | 1.09 (0.94–1.25) | 101 | 1.42 (1.17–1.73) | 38 | 1.62 (1.16–2.20) |
We next assessed the association of initial anticoagulant choice to treat CA-VTE with bleeding both overall and by cancer type using sequentially adjusted Cox proportional hazard models (Table 3). In multivariable-adjusted Cox models which included all cancer types, users of DOACs had a similar hazard of bleeding compared to users of warfarin, with little indication of confounding after adjusting for age, sex, year, the HDPS, or frailty. In the unadjusted model (Model 1), the HR (95% CI) of DOACs versus warfarin for bleeding was 0.91 (0.73–1.13), and 0.88 (0.69–1.11) when adjusting for age, sex, the HDPS, and frailty (Model 4). There was a similar magnitude bleeding risk for rivaroxaban alone versus warfarin (HR 0.88; 95% CI 0.70–1.12, Model 4). In the fully adjusted models, there was no significant difference in bleeding for LMWH versus warfarin (HR 0.98; 95% CI 0.85–1.13; Model 4) nor the association of DOACS vs. LMWH in bleeding risk (HR 0.86; 95% CI 0.66–1.12, Model 4).
Table 3.
Association of Anticoagulant Use to Treat Cancer-Associated (CA)-Venous Thromboembolism (VTE) with Hospitalized Bleeding
| Comparison* | N | Events | Person-Years | Model 1† | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|---|---|
| All Cancer | |||||||
| Warfarin (reference) | 6,951 | 309 | 6,556.3 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 3,183 | 111 | 2,278.9 | 0.91 (0.73–1.13) | 0.86 (0.69–1.08) | 0.87 (0.69–1.10) | 0.88 (0.69–1.11) |
| Rivaroxaban | 2,895 | 106 | 2,166.7 | 0.92 (0.74–1.15) | 0.88 (0.70–1.10) | 0.88 (0.70, 1.12) | 0.88 (0.70–1.12) |
| Warfarin (reference) | 14,833 | 716 | 17,724.7 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| LMWH | 8,803 | 373 | 7,184.4 | 1.10 (0.97–1.24) | 1.13 (0.99–1.29) | 0.97 (0.84–1.12) | 0.98 (0.85–1.13) |
| LMWH (reference) | 5,205 | 209 | 3401.6 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 3,183 | 111 | 2,278.9 | 0.82 (0.65–1.03) | 0.78 (0.63–1.01) | 0.86 (0.66–1.13) | 0.86 (0.66–1.12) |
| Rivaroxaban | 2,895 | 106 | 2,166.7 | 0.84 (0.67–1.06) | 0.82 (0.64–1.04) | 0.86 (0.65–1.13) | 0.86 (0.65–1.13) |
| Chemotherapy Treated Cancer | |||||||
| Warfarin (reference) | 2,912 | 144 | 2,389.9 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 1,373 | 56 | 848.6 | 0.97 (0.71–1.33) | 0.98 (0.71–1.35) | 0.95 (0.68–1.33) | 0.95 (0.68–1.34) |
| Rivaroxaban | 1,281 | 54 | 819.9 | 0.98 (0.72–1.34) | 0.98 (0.71–1.36) | 0.94 (0.67–1.32) | 0.94 (0.67 −1.32) |
| Warfarin (reference) | 6,750 | 368 | 6,861.1 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| LMWH | 6,420 | 290 | 5,118.7 | 0.95 (0.81–1.11) | 0.99 (0.84–1.15) | 0.92 (0.78–1.09) | 0.93 (0.78–1.10) |
| LMWH (reference) | 3,778 | 157 | 2453.4 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 1,373 | 56 | 848.6 | 0.99 (0.73–1.35) | 1.01 (0.74–1.38) | 1.05 (0.75–1.47) | 1.05 (0.75–1.47) |
| Rivaroxaban | 1,281 | 54 | 819.9 | 1.01 (0.74–1.37) | 1.03 (0.75–1.41) | 1.05 (0.74 −1.47) | 1.04 (0.74–1.47) |
| Lung Cancer | |||||||
| Warfarin (reference) | 871 | 35 | 651.1 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 409 | 9 | 200.5 | 0.68 (0.32–1.41) | 0.76 (0.31–1.46) | 0.73 (0.32–1.67) | 0.73 (0.32–1.67) |
| Rivaroxaban | 379 | 9 | 190.4 | 0.72 (0.34–1.49) | 0.71 (0.33–1.53) | 0.76 (0.33–1.74) | 0.76 (0.33–1.74) |
| Warfarin (reference) | 2,015 | 98 | 1,630.3 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| LMWH | 1,494 | 58 | 862.6 | 0.99 (0.71–1.37) | 0.98 (0.70–1.37) | 0.89 (0.62–1.28) | 0.89 (0.62–1.28) |
| LMWH (reference) | 880 | 35 | 493.9 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 409 | 9 | 200.5 | 0.57 (0.27–1.18) | 0.56 (0.26–1.19) | 0.85 (0.38–1.90) | 0.86 (0.39–1.92) |
| Rivaroxaban | 379 | 9 | 190.4 | 0.61 (0.29–1.26) | 0.59 (0.28–1.26) | 0.83 (0.37–1.85) | 0.83 (0.37–1.86) |
| Breast Cancer | |||||||
| Warfarin (reference) | 1,113 | 37 | 1,203.9 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 568 | 20 | 445.1 | 1.30 (0.75–2.25) | 1.24 (0.70–2.20) | 1.29 (0.72–2.31) | 1.35 (0.76–2.42) |
| Rivaroxaban | 518 | 19 | 421.0 | 1.32 (0.76–2.30) | 1.27 (0.71–2.26) | 1.32 (0.73–2.39) | 1.38 (0.77–2.49) |
| Warfarin (reference) | 2,330 | 91 | 3,272.9 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| LMWH | 1,004 | 26 | 1,047.6 | 0.83 (0.53–1.28) | 0.88 (0.56–1.37) | 0.70 (0.43–1.15) | 0.71 (0.43–1.16) |
| LMWH (reference) | 602 | 13 | 467.2 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 568 | 20 | 445.1 | 1.57 (0.78–3.16) | 1.37 (0.67–2.80) | 1.61 (0.71–3.65) | 1.67 (0.73–3.83) |
| Rivaroxaban | 518 | 19 | 421.0 | 1.61 (0.80–3.26) | 1.41 (0.69–2.91) | 1.55 (0.68–3.52) | 1.60 (0.70–3.68) |
| Colon Cancer | |||||||
| Warfarin (reference) | 686 | 40 | 631.9 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 304 | 16 | 217.1 | 1.05 (0.59–1.88) | 1.09 (0.60–1.99) | 0.99 (0.52–1.88) | 1.00 (0.53–1.90) |
| Rivaroxaban | 280 | 15 | 209.7 | 1.03 (0.57–1.87) | 1.07 (0.58–1.98) | 0.95 (0.50–1.83) | 0.97 (0.50–1.85) |
| Warfarin (reference) | 1,460 | 78 | 1,800.5 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| LMWH | 812 | 31 | 738.8 | 0.88 (0.58–1.33) | 0.88 (0.58–1.35) | 0.86 (0.55–1.34) | 0.86 (0.55–1.35) |
| LMWH (reference) | 496 | 15 | 372.3 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 304 | 16 | 217.1 | 1.81 (0.89–3.66) | 1.72 (0.83–3.57) | 1.46 (0.67–3.17) | 1.49 (0.69–3.23) |
| Rivaroxaban | 280 | 15 | 209.7 | 1.77 (0.87–3.62) | 1.72 (0.82–3.58) | 1.50 (0.69–3.28) | 1.52 (0.70–3.34) |
| Upper Gastrointestinal | |||||||
| Warfarin (reference) | 200 | 10 | 152.4 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 81 | 10 | 47.4 | 2.88 (1.20–6.93) | 3.09 (1.23–7.73) | 2.68 (0.95–7.55) | 2.69 (0.95–7.62) |
| Rivaroxaban | 79 | 10 | 47.0 | 2.91 (1.21–7.01) | 3.11 (1.24–7.78) | 2.63 (0.94–7.36) | 3.64 (0.94–7.41) |
| Warfarin (reference) | 408 | 24 | 364.5 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| LMWH | 386 | 22 | 236.3 | 1.20 (0.67–2.14) | 1.16 (0.64–2.10) | 1.09 (0.58–2.06) | 1.11 (0.59–2.10) |
| LMWH (reference) | 210 | 12 | 110.6 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 81 | 10 | 47.4 | 2.10 (0.91–4.87) | 2.28 (0.97–5.39) | 2.42 (0.95–6.20) | 2.36 (0.92–6.07) |
| Rivaroxaban | 79 | 10 | 47.0 | 2.14 (0.92–4.96) | 2.30 (0.98–5.43) | 2.27 (0.89–5.82) | 2.21 (0.86–5.70) |
| Prostate Cancer | |||||||
| Warfarin (reference) | 889 | 52 | 947.2 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 382 | 9 | 344.7 | 0.42 (0.21–0.86) | 0.38 (0.19–0.79) | 0.41 (0.19–0.86) | 0.40 (0.19–0.84) |
| Rivaroxaban | 332 | 7 | 326.5 | 0.36 (0.16–0.79) | 0.33 (0.15–0.73) | 0.36 (0.16–0.81) | 0.35 (0.15–0.79) |
| Warfarin (reference) | 1,794 | 99 | 2,562.3 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| LMWH | 362 | 22 | 336.9 | 1.44 (0.91–2.29) | 1.40 (0.87–2.24) | 1.00 (0.59–1.69) | 0.98 (0.58–1.66) |
| LMWH (reference) | 233 | 16 | 162.8 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 382 | 9 | 344.7 | 0.29 (0.13–0.65) | 0.30 (0.13–0.68) | 0.37 (0.15–0.93) | 0.37 (0.15–0.93) |
| Rivaroxaban | 332 | 7 | 326.5 | 0.24 (0.10–0.60) | 0.25 (0.10–0.62) | 0.31 (0.12–0.83) | 0.31 (0.12–0.83) |
| Hematologic Cancer | |||||||
| Warfarin (reference) | 822 | 39 | 821.0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 367 | 13 | 275.2 | 0.85 (0.45–1.60) | 0.68 (0.36–1.31) | 0.71 (0.36–1.39) | 0.71 (0.36–1.39) |
| Rivaroxaban | 334 | 13 | 264.6 | 0.91 (0.48–1.70) | 0.74 (0.38–1.41) | 0.80 (0.41–1.56) | 0.80 (0.41–1.57) |
| Warfarin (reference) | 1,851 | 81 | 2,509.9 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| LMWH | 1,230 | 51 | 1,360.9 | 1.05 (0.74–1.50) | 1.01 (0.70–1.46) | 1.04 (0.70–1.56) | 1.05 (0.70–1.57) |
| LMWH (reference) | 740 | 27 | 626.1 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| DOAC | 367 | 13 | 275.2 | 0.99 (0.51–1.92) | 0.96 (0.49–1.89) | 0.88 (0.41–1.90) | 0.88 (0.41–1.90) |
| Rivaroxaban | 334 | 13 | 264.6 | 1.07 (0.55 −2.07) | 1.04 (0.53–2.04) | 1.00 (0.46–2.16) | 1.01 (0.47–2.17) |
Comparisons between any agent and DOACs restricted to after 11/2/2012 (initial approval of first DOAC to treat VTE)
Models:Model 1: No adjustment, Model 2: Adjusted for age, sex, year, Model 3: Adjusted for Model 2 + high dimension propensity score, Model 4: Adjusted for Model 3 + frailty
When stratified by cancer type, there was no association of anticoagulant choice with bleeding risk for DOACs versus warfarin, LMWH versus warfarin, and DOACs vs. LMWH for lung cancer, breast cancer, or colon cancer. For prostate cancer, there was a reduced hazard of bleeding for DOACs versus warfarin (HR 0.40; 95% CI 0.19–0.84, Model 4) and for DOACs versus LMWH (HR 0.37; 95% CI 0.15–0.93, Model 4) and a similar risk for LMWH versus warfarin (HR 0.98; 95% CI 0.58–1.66, Model 4). The association of bleeding with anticoagulants to treat CA-VTE associated with hematologic cancers showed a HR of 0.71; 95% CI 0.36–1.39; Model 4) for DOACs versus warfarin, and a HR of 0.88 (95% CI 0.41–1.90, Model 4) for DOACs versus LMWH. There was no evidence of any association of LMWH versus warfarin for bleeding risk (HR 1.05; 95% CI 0.70–1.57; Model 4). For upper GI malignancies, there was a borderline increased bleeding risk for DOACs versus warfarin (HR 2.69; 95% CI 0.95–7.62) and for increased bleeding for DOACs versus LMWH (HR 2.36; 95% CI 0.92–6.07). The numbers however for this analysis were limited with wide confidence intervals.
While there were borderline interactions between anticoagulant choice and age (stratified as less than 75 or greater than or equal to 75 years) and sex (but not kidney disease), the point estimates either crossed 1 or could be explained by different cancer type prevalences in men versus women (i.e. prostate and breast cancer, eTable 6). When the population was restricted to those receiving chemotherapy as part of their cancer treatment (Table 3), the association of DOACs with bleeding versus warfarin was closer to 1 (HR 0.95; 95% CI 0.68 −1.34) with a similar finding for DOACs versus LMWH (HR1.05; 95% CI 0.75–1.47).
Discussion
In this analysis of nearly 27,000 patients with anticoagulant treated CA-VTE there were 1,204 hospitalized bleeding events, resulting in an anticoagulation-associated bleeding rate of 4.4% per patient-year. DOACs were associated with a similar hazard of bleeding compared to warfarin (−12% 95% CI: −11%–31%) and a similar hazard of bleeding compared to LMWH (−14%; 95% CI: −12% - 33%). There were lower point estimates for the risks of bleeding for DOACs versus warfarin in prostate and hematologic cancers, and a higher point estimate for bleeding for DOACs versus warfarin for upper gastrointestinal cancers. Our findings are consistent with the current knowledge about warfarin versus LMWH for the treatment of CA-VTE and extend knowledge about bleeding rates with the use of DOACs in CA-VTE, specifically demonstrating that DOACs are associated with similar bleeding risks to warfarin and LMWH with potentially different bleeding risks by cancer site.
LMWH versus warfarin
We observed no difference in bleeding risk with LMWH versus warfarin for treatment of CA-VTE. Recommendations to use LMWH over vitamin K antagonists to treat CA-VTE[3, 31] are driven by reduced VTE recurrence, not a reduction in bleeding[32, 33]. The annual incidence rate of bleeding in the current study’s LMWH treated group (5.2%, 373 bleeds / 8,803 patients) was similar to the annual incidence rate of major bleeding in the LMWH group (4.0%; 21 bleeds / 524 patients) reported in a recently published randomized trial of LMWH and edoxaban versus LMWH alone to treat CA-VTE[34]. Notably, the rates or incidences of bleeding in patients treated with CA-VTE are variable in the literature. Trials have often reported higher incidences or rates[35] than observed in the present study, though with different definitions of bleeding including outpatient bleeding events.
DOACs versus warfarin and LMWH
Based on trials of DOACs versus warfarin, DOACs are thought to have a similar or lower risk of bleeding as warfarin[11]. However, these trials were of general VTE patients, and included very few VTE patients with cancer, let alone cancer being actively treated. Until recently the only data on bleeding risk among CA-VTE patients using DOACs has come from smaller observational studies[36] or pooled data from randomized controlled trials of VTE in more general populations stratifying by cancer patients[13, 35, 37, 38]. The number of bleeding events among DOAC-treated CA-VTE in these analyses was limited, with 41 major bleeds and 74 clinically relevant bleeding events among 600 patients[33, 35]. In our study 115 hospitalized major bleeding events occurred among 3,258 CA-VTE patients using DOACs. We demonstrated a marginally lower risk of bleeding with DOACs versus warfarin (HR 0.88; 95% CI 0.69–1.11) consistent with two different meta-analyses[33, 39], with poorer precision than the present analysis, which demonstrated the incidence of bleeding was similar between DOACS and warfarin for CA-VTE (Study 1: HR 0.94; 95% CI 0.70, 1.28: Study 2: relative risk 1.08 (95% CI 0.70–1.66).
Far less is known about bleeding risks associated with DOACs versus LMWH in the context of CA-VTE. In an indirect comparison of DOACs versus LMWH (done by comparing bleeding rates in trials of LMWH versus warfarin and trials of DOACs versus warfarin) the relative risk of bleeding for DOACs versus LMWH was 0.67 (95% CI 0.31–1.46)[39], similar to our findings (HR 0.86; 95% CI 0.66–1.12). Confusing the issue, in a randomized controlled trial of LMWH bridge followed by edoxaban versus LMWH alone to treat CA-VTE, the risk of major bleeding was higher in the edoxaban plus LMWH group versus LMWH group (HR 1.77; 95% CI 1.03–3.04). However, precision was poor for that analysis as there were only 36 bleeding events in the edoxaban arm and 21 bleeding events in the LMWH arm among 1,046 patients. The higher rate of major bleeding seen with edoxaban in this study could be due to drug-specific effects of edoxaban versus rivaroxaban (the most commonly used DOAC in our analyses), unappreciated differences in the patient populations, or chance. In a pilot study of 406 patients with CA-VTE randomized to rivaroxaban versus LMWH to treat CA-VTE, rivaroxaban did not have a higher risk of major bleeding than LMWH, but did have an increased risk of clinically relevant non-major bleeding[40].
Thus, the current study demonstrated a similar bleeding risk for DOACs versus LMWH, but a slightly reduced rate of major bleeding associated with DOACs versus warfarin. These findings are consistent with smaller pooled analyses of CA-VTE who participated in randomized clinical trials of DOACs versus warfarin, which demonstrated an equivalent rate of major bleeding for DOACs versus warfarin. However, our findings differ from one randomized controlled trial specifically in CA-VTE patients that demonstrated an increased risk of bleeding for edoxaban versus LMWH.
DOACs and bleeding risk by cancer type
In the present analysis there was some evidence that DOACs were particularly beneficial in the context of VTE associated with prostate and to a lesser extent hematologic cancers, though precision was limited for the cancer-specific analyses. There is a strong theoretic rational to support differences in bleeding risk with an interaction by cancer type and by anticoagulant choice. Patients with cancers which have a minimal impact on organ function or with cancers that are typically treated with agents which have less impact on DOAC metabolism should resemble non-cancer patients and have a lower bleeding risk with DOACs, consistent to trials in the general VTE population[11]. Patients whose cancers impact their organ function more or who are treated more often with agents which reduce the metabolism of DOACs may be exposed to a greater anticoagulant effect of DOACs and suffer increased bleeding risk as a result. As the anticoagulant effect of DOACs is not readily measureable, unlike for warfarin or LMWH, these differences in anticoagulant exposure likely go unrealized[41]. In the data presented here, patients with prostate cancer had a strong trend for a lower risk of bleeding with DOACs, versus warfarin or LMWH. Patients with prostate cancer are often treated with local therapies alone (radiation or surgery) or androgen deprivation therapy which are not known to affect DOAC metabolism[42, 43]. Other cancer types, such as breast cancer or upper gastrointestinal cancers, are treated with agents which may interfere with DOAC metabolism and increase exposure to the anticoagulant effects of DOACs[43, 44]. Different impacts on bleeding risk by cancer type could explain why the current results differ from the two randomized controlled trials demonstrating increased bleeding risk with edoxaban and rivaroxaban versus LMWH[34, 40]. These hypotheses are preliminary but highlight the need to assess risks and benefits of anticoagulants in diverse cancer patient populations.
Strengths and limitations
The use of administrative data represents both the greatest strength and the greatest limitation of our analyses. We have large numbers of people with CA-VTE in our analyses, but lose the data granularity of prospectively recruited patient populations as risk factors such as obesity are impossible to assess. Further we could detect only the most severe bleeding events resulting in hospitalization and missed clinically relevant bleeding not resulting in hospitalization. Ideally, we would conduct a large randomized controlled trial comparing multiple agents, however this is not feasible since the resources and time needed to prospectively enrol and follow 30,000 patients with CA-VTE would be immense. In order to minimize the weaknesses associated with administrative data such as misclassification and confounding biases, ‘best practice’ pharmacoepidemiology approaches were utilized when analysing the data. First, we used well-validated definitions for administrative data for VTE and for bleeding. Though some misclassification was certainly present, both characteristics of our patient population and bleeding rates in the present analysis were consistent with prior studies, supporting the validity of our methods. In terms of defining OACs using administrative data, the validity of warfarin claims is excellent, and that of DOACs is likely to be similar[22]. However, patients often switch anticoagulants during the treatment of CA-VTE and those who switch may be different than those who do not, thus leading to bias[45]. To mitigate this, our analyses were based on OAC initially prescribed, following the intent-to-treat principle, which has been shown to be advantageous in making observational data more closely resemble clinical trial data[46]. Secondly, adjustment for high dimensional propensity scores and frailty was used to minimize confounding. We do, however, acknowledge that providers who do not follow guidelines for treatment of CA-VTE and used DOACs or warfarin may not follow other standard practices and outcomes for these patients may differ. A final issue, despite having nearly 30,000 CA-VTE patients who experienced over 1,200 bleeding events in our analysis, we had poor precision for some analyses such as comparisons of various DOACs with each other and stratified analyses by cancer type, age, sex, and organ function.
Conclusions
DOACs (mostly rivaroxaban) were associated with a similar incidence of bleeding versus warfarin and LMWH in this population of CA-VTE patients. There was heterogeneity of the association by cancer type, especially for individuals with prostate cancer and upper GI malignancies. These data in addition to other observational and randomized trial data suggest that DOACs may be appropriate for treating some CA-VTE and support clinical equipoise for further randomized clinical trials in appropriate populations. Future studies must address effects of individual DOACs on bleeding risk as well as the impact of cancer type on bleeding risk.
Supplementary Material
Essentials:
Bleeding risk by anticoagulant choice for cancer-associated venous thrombosis [CA-VTE] is unknown.
26 826 people with CA-VTE were followed for bleeding in a claims database in the United States.
Hospitalized bleeding risk was similar with direct acting oral anticoagulants vs. warfarin
Relative hospitalized bleeding risk varied by cancer type and anticoagulant choice
Acknowledgements
This study was funded by grants R01-HL122200 (PI Alonso) and R01HL131579 (P. L. Lutsey) from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016; 41: 3–14. 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017; 135: e146–e603. 10.1161/cir.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrier M, Lazo-Langner A, Shivakumar S, Tagalakis V, Gross PL, Blais N, Butts CA, Crowther M. Clinical challenges in patients with cancer-associated thrombosis: Canadian expert consensus recommendations. Curr Oncol. 2015; 22: 49–59. 10.3747/co.22.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016; 149: 315–52. 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007; 5: 632–4. 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 6.Robert F The potential benefits of low-molecular-weight heparins in cancer patients. J Hematol Oncol. 2010; 3: 3 10.1186/1756-8722-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE, Weitz JI. Oral Apixaban for the Treatment of Acute Venous Thromboembolism. New England Journal of Medicine. 2013; 369: 799–808. 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 8.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ. Dabigatran versus Warfarin in the Treatment of Acute Venous Thromboembolism. New England Journal of Medicine. 2009; 361: 2342–52. 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 9.Hokusai VTEI, Buller HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob GE, Schellong SM, Schwocho L, Segers A, Shi M, Verhamme P, Wells P. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013; 369: 1406–15. 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 10.Prins MH, Lensing AW, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, Cohen AT, Davidson BL, Decousus H, Raskob GE, Berkowitz SD, Wells PS, Investigators E. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013; 11: 21 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Outes A, Terleira-Fernandez AI, Lecumberri R, Suarez-Gea ML, Vargas-Castrillon E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014; 134: 774–82. 10.1016/j.thromres.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 12.KA A, N S, L AYY, S G, M G, OC C, C M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. Journal of Thrombosis and Haemostasis. 10.1111/jth.14219. [DOI] [PubMed] [Google Scholar]
- 13.McBride A, Katragadda C, Abraham I. Safety and efficacy of direct oral anticoagulants (DOAC) in cancer patients: Meta-analysis of randomized controlled trials (RCT). Journal of Clinical Oncology. 2017; 35: e18264–e. 10.1200/JCO.2017.35.15_suppl.e18264. [Google Scholar]
- 14.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Medical care. 2005; 43: 480–5. [DOI] [PubMed] [Google Scholar]
- 15.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004; 117: 19–25. 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Fang MC, Fan D, Sung SH, Witt DM, Schmelzer JR, Steinhubl SR, Yale SH, Go AS. Validity of Using Inpatient and Outpatient Administrative Codes to Identify Acute Venous Thromboembolism: The CVRN VTE Study. Medical care. 2017; 55: e137–e43. 10.1097/MLR.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH, Curb JD. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol. 2004; 160: 1152–8. 10.1093/aje/. [DOI] [PubMed] [Google Scholar]
- 18.Kniffin WD Jr., Baron JA, Barrett J, Birkmeyer JD, Anderson FA Jr. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med. 1994; 154: 861–6. [PubMed] [Google Scholar]
- 19.White RH, Zhou H, Romano PS. Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Ann Intern Med. 1998; 128: 737–40. [DOI] [PubMed] [Google Scholar]
- 20.Sanfilippo KM, Wang TF, Gage BF, Liu W, Carson KR. Improving accuracy of International Classification of Diseases codes for venous thromboembolism in administrative data. Thromb Res. 2015; 135: 616–20. 10.1016/j.thromres.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitch K, Pelizzari PM, Pyenson B. Cost Drivers of Cancer Care: A Retrospective Analysis of Medicare and Commercially Insured Poulation Claim Data 2004–2014. Milliman; (http://us.milliman.com/uploadedFiles/insight/2016/trends-in-cancer-care.pdf): Commissioned by the Community Oncology Alliance, 2016. [Google Scholar]
- 22.Garg RK, Glazer NL, Wiggins KL, Newton KM, Thacker EL, Smith NL, Siscovick DS, Psaty BM, Heckbert SR. Ascertainment of warfarin and aspirin use by medical record review compared with automated pharmacy data. Pharmacoepidemiology and drug safety. 2011; 20: 313–6. 10.1002/pds.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiology and drug safety. 2011; 20: 560–6. 10.1002/pds.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiology and drug safety. 2012; 21 Suppl 1: 100–28. 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahl PM, Rodgers K, Schneeweiss S, Gage BF, Butler J, Wilmer C, Nash M, Esper G, Gitlin N, Osborn N, Short LJ, Bohn RL. Validation of claims-based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially-insured population. Pharmacoepidemiology and drug safety. 2010; 19: 596–603. 10.1002/pds.1924. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005; 43: 1130–9. [DOI] [PubMed] [Google Scholar]
- 27.Kim DH, Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiology and drug safety. 2014; 23: 891–901. 10.1002/pds.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faurot KR, Jonsson Funk M, Pate V, Brookhart MA, Patrick A, Hanson LC, Castillo WC, Sturmer T. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiology and drug safety. 2015; 24: 59–66. 10.1002/pds.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology (Cambridge, Mass). 2009; 20: 512–22. 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rassen JA, Glynn RJ, Rothman KJ, Setoguchi S, Schneeweiss S. Applying propensity scores estimated in a full cohort to adjust for confounding in subgroup analyses. Pharmacoepidemiology and drug safety. 2012; 21: 697–709. 10.1002/pds.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elewa H, Elrefai R, Barnes GD. Cancer-Associated Venous Thromboembolism. Current Treatment Options in Cardiovascular Medicine. 2016; 18: 23 10.1007/s11936-016-0445-y. [DOI] [PubMed] [Google Scholar]
- 32.Akl EA, Kahale LA, Barba M, Neumann I, Labedi N, Terrenato I, Sperati F, Muti P, Schünemann H. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database of Systematic Reviews. 2014. 10.1002/14651858.CD006650.pub4. [DOI] [PubMed] [Google Scholar]
- 33.Carrier M, Cameron C, Delluc A, Castellucci L, Khorana AA, Lee AY. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer-associated thrombosis: a systematic review and meta-analysis. Thromb Res. 2014; 134: 1214–9. 10.1016/j.thromres.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 34.Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang TF, Yeo E, Zhang G, Zwicker JI, Weitz JI, Buller HR, Hokusai VTECI. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2017; 0: null. 10.1056/NEJMoa1711948. [Google Scholar]
- 35.van der Hulle T, den Exter PL, Kooiman J, van der Hoeven JJ, Huisman MV, Klok FA. Meta-analysis of the efficacy and safety of new oral anticoagulants in patients with cancer-associated acute venous thromboembolism. J Thromb Haemost. 2014; 12: 1116–20. 10.1111/jth.12605. [DOI] [PubMed] [Google Scholar]
- 36.Mantha S, Laube E, Miao Y, Sarasohn DM, Parameswaran R, Stefanik S, Brar G, Samedy P, Wills J, Harnicar S, Soff GA. Safe and effective use of rivaroxaban for treatment of cancer-associated venous thromboembolic disease: a prospective cohort study. Journal of Thrombosis and Thrombolysis. 2017; 43: 166–71. 10.1007/s11239-016-1429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khorana AA, Carrier M, Garcia DA, Lee AY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis. 2016; 41: 81–91. 10.1007/s11239-015-1313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prins MH, Lensing AW, Brighton TA, Lyons RM, Rehm J, Trajanovic M, Davidson BL, Beyer-Westendorf J, Pap AF, Berkowitz SD, Cohen AT, Kovacs MJ, Wells PS, Prandoni P. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014; 1: e37–46. 10.1016/S2352-3026(14)70018-3. [DOI] [PubMed] [Google Scholar]
- 39.Posch F, Konigsbrugge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015; 136: 582–9. 10.1016/j.thromres.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, Hale D, Dunn JA, Lyman GH, Hutchinson C, MacCallum P, Kakkar A, Hobbs FDR, Petrou S, Dale J, Poole CJ, Maraveyas A, Levine M. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol. 2018; 0: JCO2018788034. 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 41.Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014; 64: 1128–39. 10.1016/j.jacc.2014.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litwin MS, Tan HJ. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA. 2017; 317: 2532–42. 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 43.Short NJ, Connors JM. New Oral Anticoagulants and the Cancer Patient. The Oncologist. 2014; 19: 82–93. 10.1634/theoncologist.2013-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo B, Turner NC, Jones A. An update on the medical management of breast cancer. BMJ. 2014; 348: g3608 10.1136/bmj.g3608. [DOI] [PubMed] [Google Scholar]
- 45.Khorana AA, Yannicelli D, McCrae KR, Milentijevic D, Crivera C, Nelson WW, Schein JR. Evaluation of US prescription patterns: Are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb Res. 2016; 145: 51–3. 10.1016/j.thromres.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Hernan MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, Manson JE, Robins JM. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology (Cambridge, Mass). 2008; 19: 766–79. 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.