Abstract
The initiation of reverse transcription in human immunodeficiency virus-1 (HIV-1) is a key early step in the virus replication cycle. During this process, the viral enzyme reverse transcriptase (RT) copies the single-stranded viral RNA (vRNA) genome into double-stranded DNA using human tRNALys3 as a primer for initiation. The tRNA primer and vRNA genome contain several complementary sequences that are important for regulating reverse transcription initiation kinetics. Using single-molecule Förster resonance energy transfer (smFRET) spectroscopy, we demonstrate that the vRNA-tRNA initiation complex is conformationally heterogeneous and dynamic in the absence of RT. As shown previously, nucleic acid-RT interaction is characterized by rapid dissociation constants. We show that extension of the vRNA-tRNA primer binding site (PBS) helix from 18 base pairs to 22 base pairs stabilizes RT binding to the complex and that the tRNA 5’ end has a role in modulating RT binding. RT occupancy on the complex stabilizes helix 1 (H1) formation and reduces global structural heterogeneity. The stabilization of H1 upon RT binding may serve to destabilize helix 2 (H2), the first pause site for RT during initiation, during later steps of reverse transcription initiation.
Introduction
RNA structure plays a key role in regulating the initiation of reverse transcription in human immunodeficiency virus-1 (HIV-1). HIV-1 is a retrovirus with a single-stranded, positive-sense RNA genome and uses the process of reverse transcription to convert this viral RNA (vRNA) genome into double-stranded DNA (dsDNA) for integration into the host cell genome1; 2; 3. Defects in reverse transcription are detrimental to virus viability, and drugs targeting reverse transcription are effective antiviral therapies4; 5; 6; 7; 8. Reverse transcription initiates within the highly structured 5′-untranslated region (5’-UTR) of the HIV genome from an annealed host-cell tRNALys3 that was previously packaged during virus assembly (Figure 1A,B)1; 2; 3; 9. The 3’ end of the primer tRNA forms an 18-base pair (bp) helix with a vRNA sequence called the primer binding site (PBS). This 18 bp PBS helix serves as the binding site for HIV-1 reverse transcriptase (RT) and contains the 3′ hydroxyl of the tRNA from which reverse transcription initiates.
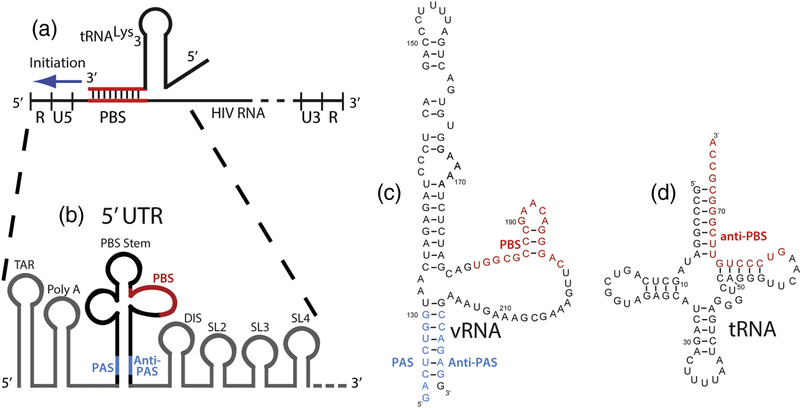
Figure 1.
Overview of reverse transcription initiation in the 5’-UTR. (a) Reverse transcription initiates from the 5’-UTR of the vRNA genome. (b) The vRNA PAS and anti-PAS sequences flank the stem loop containing the PBS where reverse transcription initiates. (c) Sequence and predicted secondary structure for the vRNA used in single-molecule experiments; numbering is for the NL4.3 isolate. (d) Secondary structure of the tRNALys3 primer.
The initiation complex formed between tRNALys3 and HIV-1 vRNA likely extends beyond 18 base pairs formed between the tRNA and vRNA PBS sequences10; 11. Extensive biochemical and in vivo studies have identified sequence elements in both the vRNA and tRNA that are required for efficient initiation12; 13; 14; 15; 16; 17; 18. vRNA elements required for efficient initiation can form secondary structures aside from the PBS and are potential sites of vRNA-tRNA RNA pairing.
These elements include the primer activation signal (PAS) (nucleotides 123–130 in HIV1 NL4.3 RNA) and an A-rich sequence (nucleotides 168–171) that have been proposed to interact with complementary sequences within tRNALys319; 20; 21; 22; 23. These additional interactions between sequences external to the PBS have also been proposed to be strain-dependent14. All of these sequences are contained within an approximately 100 nucleotide region for which a low resolution cryo-EM structure containing the vRNA stem-loop, primer tRNA, and RT was recently solved (Figure 2A)24.
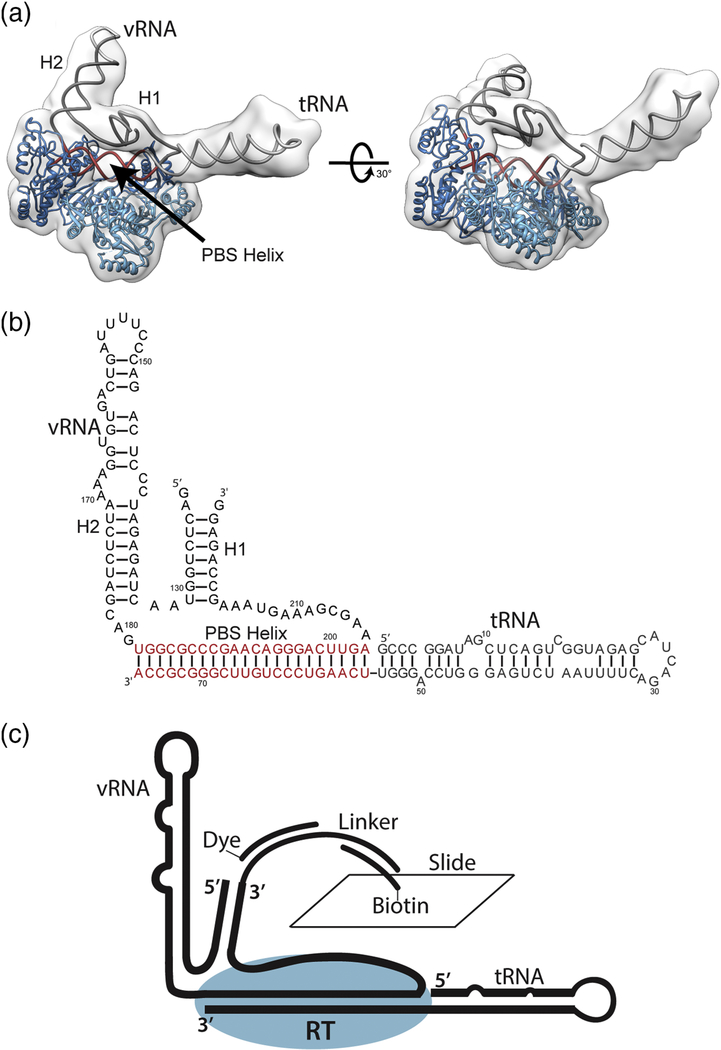
Figure 2.
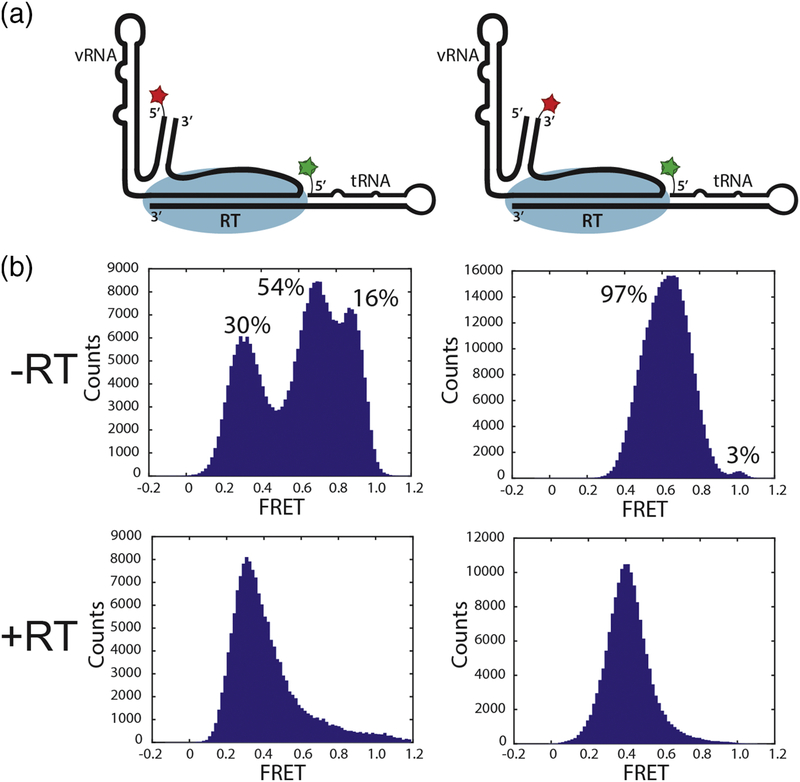
Global architecture of the +1 HIV-1 reverse transcriptase initiation complex. (a) The vRNA and tRNA form an extended PBS helix which occupies the RT binding cleft. H1 and the elongated tRNA protrude from the polymerization active site and RNase H domain respectively. H1 rests on top of the occupied RT binding cleft. (b) Secondary structure of the vRNA and tRNA as fit to cryo-EM electron density maps. (c) Schematic of constructs used for surface immobilization for smFRET experiments.
The process of reverse transcription initiation, during which RT works as an RNAdependent polymerase to incorporate the first ~15 dNTP nucleotides, is 100 to 500-fold slower than the subsequent elongation phase, during which the remainder of the approximately 9.2 kb RNA genome is reverse transcribed, leading eventually to double-stranded DNA as RT works as a DNA-dependent DNA polymerase 25; 26; 27; 28; 29; 30. The 5’-UTR of HIV genomic RNA is highly structured and presents kinetic barriers to initiation, since RT must unfold RNA during genomic replication28; 29. RT is particularly sensitive to RNA structure during initiation, as mutation of structured sequences around the PBS changes the kinetics of initiation, decreases virus viability, and results in reversion to the wild-type sequence13; 16; 19; 20; 31.
To initiate reverse transcription, RT first must bind to the vRNA-tRNA complex within the viral capsid to position the 3’ end of the tRNA primer in the RT active site. RT, a heterodimer of p51 and p66 subunits, has been extensively characterized by structural methods32; 33; 34. The p66 subunit contains a classic palm, fingers, and thumb polymerase active site with a 60Å-long binding cleft that can accommodate nucleic acid double helices 22–24 bp in length. Distal to the polymerase site along this cleft is an RNase H domain that cleaves the RNA strand of RNA-DNA hybrids during replication. During initiation, the 18 bp PBS likely sits within the RT nucleic acid binding cleft. RT-nucleic acid affinity has been measured with dissociation equilibrium constants (KD) of 1–300nM for DNA-DNA, DNA-RNA or RNA-RNA duplexes of various lengths25; 28; 30; 35; 36; 37. Low RT affinities arise from rapid kinetic dissociation constants (koff) of RT from the nucleic acid helical structures30; 36. Additionally, single-molecule fluorescence experiments have highlighted the dynamic positioning of RT on nucleic acid substrates with the 3’ end of the primer positioned either in the RT active site or RNase H domain depending on the orientation in which RT binds38; 39. This kinetic lability has hindered the investigation of RT-nucleic acid complexes, and covalent cross-linking has often been used to stabilize complexes for structural studies24; 33.
Recently, we applied such a cross-linking approach coupled with cryo-electron microscopy (cryo-EM) to determine the three-dimensional architecture of an HIV-1 RTIC containing vRNA, tRNA, and RT (Figure 2A) extended by one nucleotide (+1 initiation complex)24. This structure, solved at a global resolution of 8.0 Å and local resolution around RT of 4.5 Å, showed that the primary RNA secondary structural interaction in this region is the vRNA-tRNA PBS helix. A PAS/anti-PAS interaction between the vRNA and tRNA, previously proposed by several studies was not observed in this +1 initiation complex structure, supportive of work that suggest this interaction may be strain-dependent13; 14; 20; 21; 40; 41; 42. Instead, the tRNALys3 refolds such that the anticodon loop and D-stem loop collapse to form a continuous helix. In this RT-vRNA-tRNA complex structure, the PBS is bound within the RT cleft as proposed and is extended by four additional vRNA-tRNA base pairs allowing additional potential interactions with the RNase H domain of RT; the refolded tRNA helix coaxially stacks on this extended (22 bp) PBS helix (Figure 2B). This refolding of the tRNA was not observed in the free vRNA-tRNA complex in the absence of RT, suggesting that reverse transcriptase induces significant RNA structural rearrangements within the initiation complex upon binding41; 43. The vRNA PAS sequence (nts 125–131) was found to be paired with the vRNA anti-PAS sequence (nucleotides 217–223) to form the vRNA Helix 1 (H1) structure (Figure 2B) that docks near the binding cleft of the RT p66 subunit. The sequestering of the vRNA PAS sequence within H1 is consistent with previous data suggesting that a vRNA PAS / tRNA anti-PAS interaction occurs primarily in HIV-1 MAL isolates and not within the HIV-1 NL4.3 isolate used for these studies14. Helix 2 (H2) within the vRNA extends outward from the RT active site. While poorly resolved in the EM density, H1 and its connecting loops appear to tether H2 and the region of the extended PBS helix located within the RNase H domain.
Although this cryo-EM structure provided global insights into the architecture of the RTIC, it does not provide temporal information on conformational dynamics. For example, the dynamics of H1 may be important for regulating the first initiation pause site at the base of H2, or the extended PBS helix may be important for stabilizing RT on the vRNA-tRNA complex22. Singlemolecule Förster resonance energy transfer (smFRET) spectroscopy is capable of monitoring structurally and compositionally dynamic systems such as the RTIC in real time to probe how dynamic features may regulate reverse transcription initiation36; 38; 39; 40. Here we explore the conformational dynamics of the initiation complex by applying smFRET both in the presence and absence of RT. We use multiple dye-labeling schemes and RNA constructs to understand the detailed dynamic properties of the vRNA-tRNA complex. Using smFRET between dye-labeled RT and vRNA-tRNA complex, we also directly monitor the protein-RNA composition of the initiation complex. From our results, we determined the role of the extended PBS helix and tRNA 5’ end in stabilizing RT on the initiation complex and the effects of RT binding on the global fold of the vRNA-tRNA complex. Our results demonstrate the dynamic nature of the RT initiation complex and the interplay of RNA structure and RT-RNA recognition in modulating the conformation and stability of the RTIC.
Results
Reagent Preparation and Characterization
To employ single-molecule methods, we designed a vRNA construct 136 nucleotides in length for smFRET experiments. This construct spans nucleotides 121–224 in the HIV NL4.3 construct and includes the vRNA PAS, complementary vRNA anti-PAS sequences that form H1, and an extended 3’ end for annealing dye-labeled oligonucleotides (Figure 2C). A similar HIV vRNA construct, spanning 123–223 of NL4.3, but without a 3’ extension was used for our prior cryo-EM studies; the extended vRNA-tRNA initiation complex shows similar RT elongation kinetics (not shown) as the cryoEM complex, suggesting that this extension does not affect initiation complex conformation and function24. Dyes for smFRET experiments were chemically attached at the 5’ end of this RNA via NHS chemistry using a 5’-amino-G-monophosphate incorporated on the 5’ end through in vitro transcription. This method of 5’ labeling yielded approximately 70% labeling efficiency according to UV-vis data. A synthetic oligonucleotide containing a chemically-attached dye at the 3’ end was used to label the extended 3’ end of the vRNA. vRNA-tRNA complexes were formed through heat-annealing in the presence of excess tRNA or truncated tRNA oligonucleotides and purified by size-exclusion chromatography (SEC). SEC chromatograms and subsequent native PAGE gels showed a single band corresponding to one compositionally homogeneous complex similar to our previously employed vRNA-tRNA purification methods41 (Supplementary Figure 1).
Dye-labeled RT activity was confirmed by analyzing the products of reverse transcription on a sequencing gel isolated at different timepoints following initiation (Supplementary Figure 2). The vRNA template for this activity assay was the vRNA used in smFRET experiments, and the primer was full-length synthetic tRNALys3 with a 5’-Cy3 dye (TriLink). Shorter transcripts corresponding to RT pause sites showed similar banding patterns to previously reported data22.
RT Binding Stabilized by an Extended PBS Helix
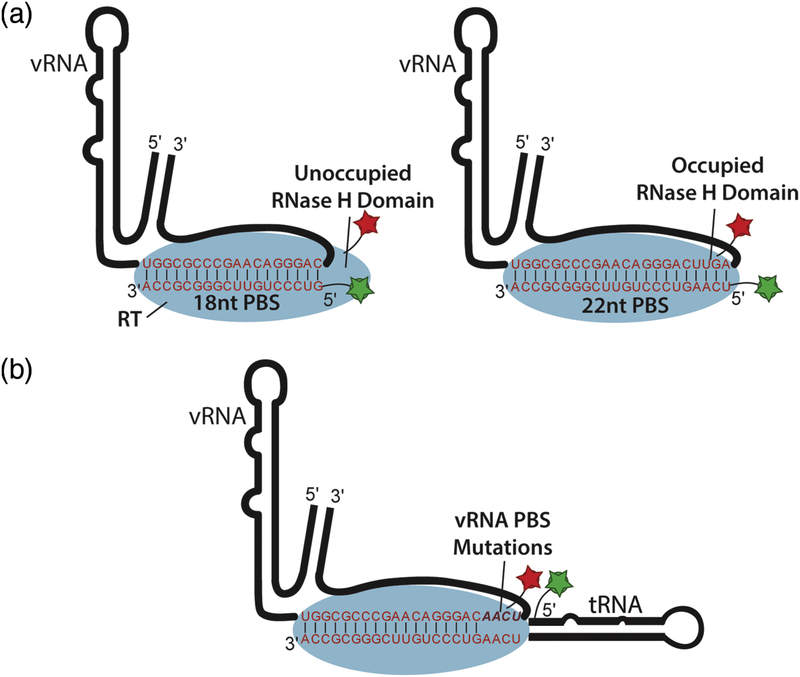
Past biochemical and structural work showed that RT binds to the 18 bp PBS helix formed between the vRNA and tRNA primer to initiate reverse transcription. Our cryo-EM data suggested that four additional base pairs form between complementary sequences in the vRNA and tRNA primer to form an extended 22 bp PBS helix. This helix coaxially stacks with the remainder of the tRNA and can form additional contacts with the RNase H domain of RT. To test whether formation of a 22 bp helix stabilizes RT-RNA interaction, we designed two truncated tRNA constructs (Figure 3A). The first construct contains 18 nucleotides of the 3’ end of the tRNA, and the second contains 22 nucleotides from the 3’ end of the tRNA to simulate the formation of an 18 bp and an extended 22 bp PBS helix, respectively.
Figure 3.
Single-molecule experimental design for measuring RT binding to the vRNA-tRNA complex. (a) Two different RNA oligonucleotides are annealed to the vRNA to promote formation of either an 18 bp PBS helix or 22 bp PBS helix which extends into the RNase H domain of RT.
(b) Design of mutant vRNAs with variable PBS helix lengths in complex with full-length tRNA.
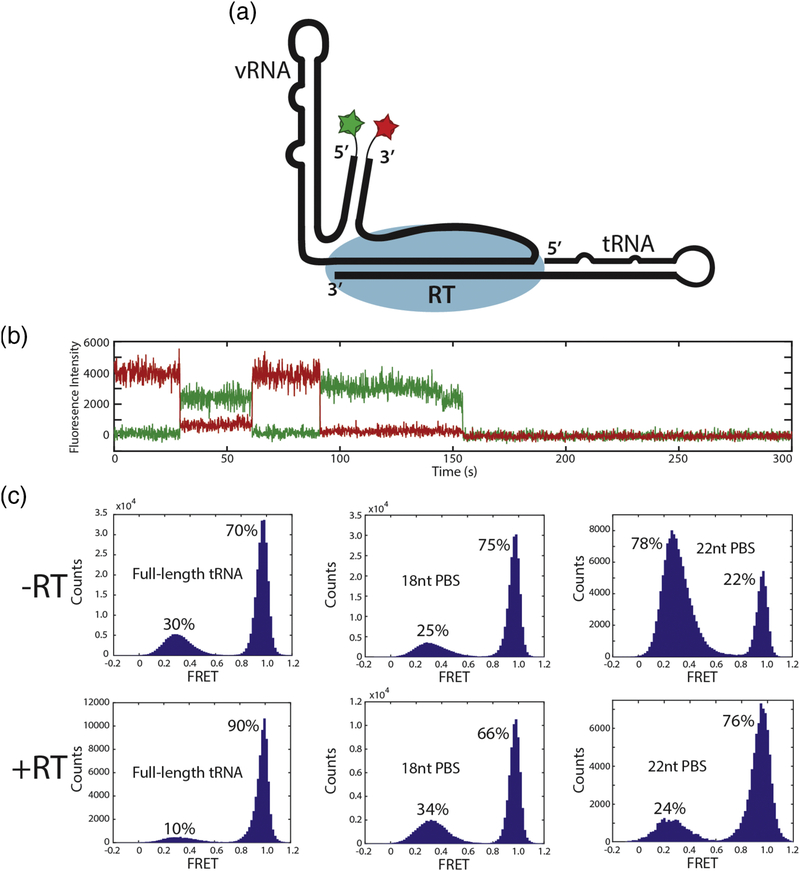
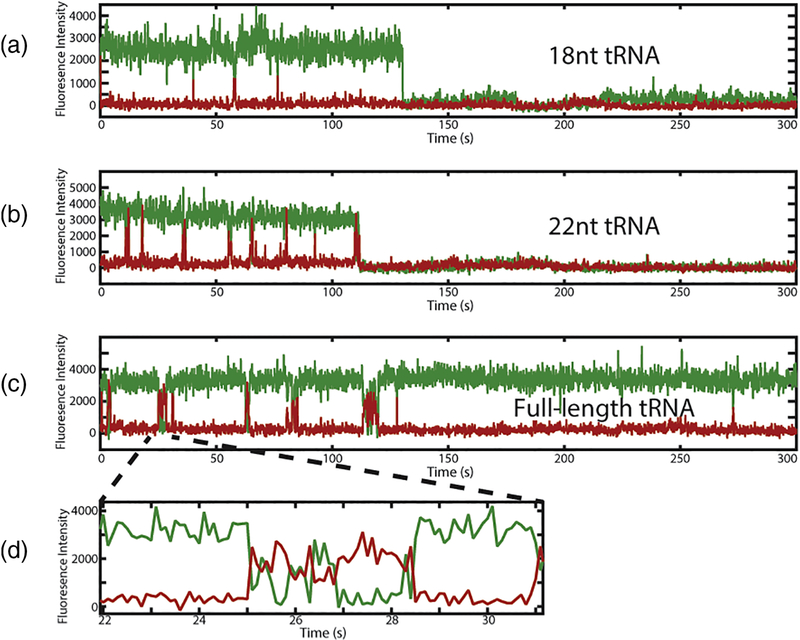
To observe directly the binding of RT to the vRNA-tRNA complex in the presence of the 18nucleotide, 22-nucleotide, and full-length tRNA primers, we engineered a cysteine into the Cterminal end of the RT p66 subunit as previously described38; 39. We modified the RT at this cysteine with a Cy5 acceptor dye to monitor FRET between the RT and the 5’ ends of tRNA constructs labeled with Cy3 donor dye (Figure 3A). Similar dye labeling schemes have been used previously to monitor RT binding and dissociation through RNA / RT FRET38; 39. We measured Cy3 / Cy5 FRET events corresponding to RT binding in the presence of all three tRNA constructs to determine the kinetics of RT association to these RNAs. Longer binding events showed dynamic positioning of RT on the complex as indicated by FRET fluctuations observed within a single RT binding event (Figure 3D). This dynamic behavior is consistent with previously reported single-molecule fluorescence data using a similar labeling scheme for nucleic acid and RT that reported such FRET fluctuations during RT binding events38.
From analysis of the smFRET data, we derived kinetic parameters for RT-RNA complex formation. kon values for dye-labeled RT (10 nM) binding to 18 nucleotide, 22 nucleotide, and fulllength tRNA complexes (Table 1). koff dissociation rates for RT varied according to tRNA construct size. koff for the 18 nucleotide, 22 nucleotide, complexes were fit by single exponentials with rates of 2.59 ± 0.34 s−1 and 1.63 ± 0.18 s−1 respectively. koff for the full-length tRNA complex was fit by a double exponential model with values of 5.21 ± 0.91 s−1 and 0.42 ± 0.028 s−1 with 53% and 47% contributions, respectively, which indicate both productive and nonproductive binding modes for RT (Table 1, Supplementary Figure 3). These data demonstrate that an extended 22 bp PBS helix is more effective at stabilizing RT on the complex than the previously proposed 18 bp helix. RT binding may be further stabilized by additional interactions that may occur with the remaining segments of the full-length tRNA or vRNA found outside of the binding cleft.
Table 1.
Rate constants and 95% confidence interval of exponential fits for RT binding to the vRNA-tRNA complex in the presence of different vRNA and tRNA constructs.
| Construct | kon (s−1μM−1) | koff1 (s−1) | koff2 (s−1) | n kon | n koff |
|---|---|---|---|---|---|
| 18 nt tRNA/WT vRNA | 6.95 ± 0.090 | 2.59 ± 0.34 | n/a | 396 | 400 |
| 22 nt tRNA/WT vRNA | 8.36 ± 0.25 | 1.63 ± 0.18 | n/a | 226 | 228 |
| Full-length tRNA/18 bp PBS vRNA | 3.51 ± 0.097 | 19.2 ± 2.67 (70%) | 2.93 ± 0.52 (30%) | 326 | 326 |
| Full-length tRNA/19 bp PBS vRNA | 3.25 ± 0.054 | 7.90 ± 0.36 (90%) | 0.47 ± 0.36 (10%) | 316 | 317 |
| Full-length tRNA/20 bp PBS vRNA | 2.72 ± 0.048 | 11.6 ± 1.48 (61%) | 1.47 ± 0.18 (39%) | 285 | 285 |
| Full-length tRNA/21 bp PBS vRNA | 2.84 ± 0.020 | 8.78 ± 0.55 (78%) | 0.75 ± 0.089 (22%) | 633 | 633 |
| Full-length tRNA/WT vRNA | 5.48 ± 0.040 | 5.21 ± 0.91 (53%) | 0.42 ± 0.028 (47%) | 433 | 588 |
tRNA Modulation of RT Binding
To test the role of the extended base pairing in the context of the initiation complex with a fulllength tRNA, we designed four vRNA mutants which vary the length of the PBS helix (Figure 3B). These four constructs, named 18 bp PBS vRNA, 19 bp PBS vRNA, 20 bp PBS vRNA, and 21 bp PBS vRNA contained the vRNA mutations U200A-U201A-G202C-A203U, U201AG202CA203U, G202C-A203U, and A203U respectively, allow for RT binding to be studied in the presence of full-length tRNA as a function of a shortened PBS helix. Association rates for constructs with shorter PBS helices were slower than the WT construct, and all four constructs showed dissociation rates with double exponential behavior (Table 1, Supplementary Figure 3 and 4). These data suggest that efficient RT binding is modulated by structures formed within the 5′ end of the tRNA. The contribution from the slower RT dissociation process increases with the number of potential base pairs in the PBS to a maximum in the 22 bp wild-type complex. Additionally, the slower association rates for RT to the mutant PBS complexes suggests that coaxial stacking of the extended tRNA helix onto a fully formed 22 bp PBS helix may be required for binding or that unpaired bases within the RT binding cleft inhibit RT association.
Helix 1 Stability
H1, which resides near the binding cleft of RT, bridges H2 and the end of the PBS helix at the RNase H domain. Our cryo-EM structural data suggested that H1 might be dynamic. To probe H1 stability using FRET, we placed donor and acceptor dyes at the 5′ and 3′ ends of the vRNA similarly to a previously described method (Figure 5A)24. We hypothesized that this labeling scheme would yield high FRET if H1 forms, and lower FRET upon melting of H1. This labeling scheme is similar to Beerens et. al 2013; however, the 3′ dye is placed via an annealed, dyelabeled DNA oligonucleotide, and the biotin moiety is located on a sequence external to the vRNA rather than directly on the vRNA sequence40. Placing the biotin distal to the vRNA-tRNA complex helps prevent interactions of the RNA with the quartz surface during immobilization, which may interfere with RNA conformational dynamics or dye photophysical properties.
Figure 5.
Single-molecule experimental design for measuring the stability of H1 in the presence and absence of RT (1 μM) using the three different tRNA constructs. (a) Schematic of dyelabeling scheme for measuring H1 stability. (b) Representative trace from these experiments which shows two FRET states corresponding to H1 formation (high FRET) and H1 melting (low FRET). (c) Histograms of population densities for H1 stability experiments using all three tRNA constructs in the presence and absence of RT (1 μM).
Dynamic H1 stability was probed using the 18-nucleotide, 22-nucleotide, and full-length tRNA constructs in both absence and presence of RT. The FRET signal from this vRNA dye configuration in complex with full-length tRNA showed two distinct states (high FRET, 1.0 FRET efficiency, and low FRET, 0.3 FRET efficiency) that interconvert (Figure 5B). In the presence of full-length tRNA, the complex exists in the high FRET (H1 formed) state 70% of the time and in a low FRET (H1 melted) state 30% of the time (Figure 5C). These states interconvert slowly on a timescale of 10s of seconds before dye photobleaching is observed.
The stability of H1 was modulated by the length of the potential PBS helix. In the presence of the 18-nucleotide tRNA construct, H1 formation was slightly favored (75%) compared to the full-length tRNA (70%). The H1 equilibrium was shifted to a destabilized conformation in the presence of the 22-nucleotide tRNA construct, with only 22% H1 formation (Figure 5C). This suggests that, in the absence of RT, formation of the additional 4 base pairs between the vRNA and tRNA to extend the PBS destabilizes Helix 1, possibly through the flexible linkers that connect Helix 1 to the PBS and Helix 2.
To determine the effect of RT binding on H1 stability, we repeated the H1 FRET experiment with all three tRNA constructs in the presence of 1 μM RT. A shift in the folded population of H1 from 70% to 90% was observed in the full-length tRNA complex upon addition of RT. These data are consistent with FRET experiments previously performed on the crosslinked cryo-EM RTIC, which observed a single high FRET population (95%) indicative of H1 formation. The population of H1 decreased slightly from 75% to 66% in the presence of RT with the 18 nucleotide tRNA construct, whereas the H1 population increased from 22% to 76% with the 22 nucleotide construct (Figure 5C).
vRNA-tRNA Complex Dynamics and Stabilization by RT
Two additional smFRET experiments were designed to probe the overall conformational dynamics of the initiation complex (Figure 6A). First, the 5′ ends of the tRNA and vRNA were labeled with donor and acceptor dyes Cy3 and Cy5, respectively. Three distinct FRET states were observed between the vRNA 5’-end and tRNA 5’-end, which interconverted on a timescale >10s (Figure 6B, Supplementary Figure 5). When the vRNA acceptor dye was moved from the 5’ end to the 3’ end of the vRNA, two primary FRET states between the vRNA and tRNA were observed (Figure 6B, Supplementary Figure 5). These FRET states also interconvert on a timescale of 10s of seconds. A very small population (<3%) of high FRET was observed for the second labeling scheme, and lifetimes of this state were on a timescale of a few seconds. We repeated the two vRNA-tRNA FRET experiments in the presence of 1 μM RT to measure the effect of RT binding on dynamics between the vRNA 5’ and 3’ ends and the tRNA 5’ end. The FRET distribution for both labeling schemes shifted strongly towards lower FRET states, and the distinct nature of the states observed in the absence of RT was lost (Figure 6B). Multiple lower FRET states with an approximate FRET efficiency of 0.4 were observed in both experiments and were not well defined for kinetic analyses. The observed convergence of multiple distinct FRET states into only low FRET states upon the addition of RT to the vRNA-tRNA complex suggests that RT binding stabilizes the global conformation of the initiation complex.
Figure 6.
Single-molecule experimental design for monitoring the global fold of the initiation complex in the presence and absence of RT (1 μM). (a) Schematics of dye-labeling schemes used to monitor global RNA conformation. (b) Histograms of population densities for both FRET labeling schemes in the presence and absence of RT (1 μM).
These lower FRET states with FRET efficiencies of approximately 0.4 initially did not agree with distance measurements made on the cryoEM structure that predicts that the vRNA 5’ and tRNA 5’ ends are approximately 30 Å apart. A FRET efficiency of 0.4 would correlate to a distance of 40 to 50 Å. Previous work used protein-induced fluorescence enhancement (PIFE) whereby the binding of RT to a fluorescently labeled complex induces an increase in dye fluorescence to measure RT binding kinetics36. To test whether the disagreement between vRNA-tRNA FRET data and cryo-EM structural data was a result of PIFE effects, we analyzed the fluorescence intensity of the Cy3 donor dye on the 5’ end of the tRNA in the presence and absence of 1 μM RT. Cy3 fluorescence intensity histograms showed a mean fluorescence of 2900 units in the absence of RT and 3850 units in the presence of 1 μM RT (Supplementary Figure 5). The relative increase in fluorescence in the presence of RT agrees with previous RT PIFE measurements36. Using this measurement to correct for PIFE effects on FRET efficiency results in a new FRET efficiency value of 0.6 (30–40 Å), which is in closer agreement with the 8.0Å resolution cryo-EM structural data (Figure 2A).
Discussion
The reverse transcription initiation complex in HIV is both structurally and compositionally heterogeneous. Our single-molecule experiments suggest that the vRNA-tRNA complex exhibits basal conformational heterogeneity in the absence of RT, which becomes reduced upon RT binding. By strategically labeling the RNA and protein components of the initiation complex and designing mutant vRNA and tRNA constructs, we determined possible roles for these structures in the process of reverse transcription initiation. Most importantly, we showed that the extended PBS helix observed in the RTIC structure serves to stabilize RT binding, the tRNA 5′ end modulates RT binding efficiency, and that RT binding stabilizes H1 formation.
To determine the dynamic binding properties of RT to different initiation complexes, we employed an RT/RNA FRET experiment whereby RT binding to the PBS helix was monitored through FRET between the tRNA 5’ end and C-terminus of the p66 subunit of RT (Figure 3). This system was used to understand the effect that formation of an extended 22 bp PBS helix has on RT binding and how the tRNA 5′ end modulates binding efficiency through refolding. RT dissociation rates followed a trend according to tRNA primer size in the context of 5′ truncated tRNAs. The extension of the PBS helix from 18 to 22 nucleotides decreased koff. These results suggest that the potential contacts between the additional four PBS base pairs and RNase H domain of RT in our prior cryo-EM structure serve to stabilize RT binding on the PBS helix. Binding of RT to the complex containing full-length tRNA exhibited a decreased dissociation rate, suggesting that additional regions within the tRNA or vRNA may also stabilize RT on the PBS helix. The double-exponential nature of koff in the presence of the full-length tRNA complex with PBS helices of various lengths may indicate conformational dynamics within the tRNA in which different conformations contribute to the multiexponential nature of the observed koff and result in the productive and nonproductive binding modes of RT reported previously38. The full 22 bp PBS leads to the greatest contribution of slow dissociation, consistent with additional RTRNA contacts in the wild-type complex.
The RT binding kinetics data presented here are consistent with previous bulk and singlemolecule experiments used to measure RT affinity for DNA-DNA, DNA-RNA, and RNARNA complexes29; 30; 35; 36; 37; 44. RT has a higher affinity for DNA-DNA and DNA-RNA hybrids compared to RNA-RNA hybrids, and the weak RT binding efficiency to the RNA-RNA constructs in this work support these data29; 35; 37. The presence of two exponential rates for the dissociation of RT from the full-length tRNA complexes indicates that both productive and nonproductive RT binding events occur. The RT kon rates measured for all vRNA and tRNA constructs kon values derived by PIFE for RT binding to nucleic acid substrate in a single-molecule setting36. There is a lack of RT binding kinetics data for short (<25 nucleotide) RNA-RNA complexes. The apparent kon and koff values of RT for the 18-nucleotide and 22-nucleotide tRNA in complex with vRNA are within reported ranges of RT binding kinetics for a wide variety of nucleic acid constructs25; 29.
The vRNA H1 is positioned near the active site in our cryo-EM structure, but its function is unclear as it comprises and sequesters the PAS sequence in viral RNA. The vRNA PAS sequence previously was shown to interact with the tRNA anti-PAS sequence only in HIV-1 MAL isolates. This may explain why only the vRNA-sequestered PAS interaction that forms H1 was observed using the NL4.3 HIV construct for cryo-EM studies11. We designed and applied here a FRET system that used dyes at the 5’ and 3’ ends of the vRNA to monitor the formation of H1 (Figure 5A) and to delineate how the extension of the PBS helix affects H1 stability in the context of the HIV NL4.3 isolate. This experiment was similar to Beerens et al. 2013 in that the 5’ and 3’ ends of the vRNA were labeled with donor and acceptor dyes; however, the ways in which the tRNA was annealed and the complex was anchored to the slide for smFRET experiments differed40. In our experiment, a single PAGE-purified tRNALys3 isomer or small, synthetic RNA oligonucleotide was annealed to the vRNA, which was then anchored to the slide via an extended vRNA 3’ biotin motif. The Beerens et al. 2013 method annealed a pool of total tRNA to the vRNA and anchored the complex via a biotin moiety covalently bound to a loop within the vRNA. These variations in complex preparation may explain the differences observed in FRET population distributions corresponding to vRNA H1 formation. Both experimental methods showed distinct high and low FRET states corresponding to the formation and melting of H1 respectively; however, we did not observe the previously reported intermediate FRET states observed in Beerens et al. 2013. We propose that our procedure avoids the potential for other tRNA isomers, specifically tRNALys1 and tRNALys2 with anti-PBS sequences differing by only five nucleotides, to anneal to the vRNA PBS sequences using the total tRNA method, potentially producing additional structural FRET states within the complex.
Using the 18- and 22-nucleotide truncated tRNA constructs, we tested the potential effect of the extended 22bp PBS helix on the role of H1 in reverse transcription initiation. In the presence of full-length tRNA, we observed a 70% population of H1 formation and 30% population of H1melted state. H1 formation increased slightly to 75% in the presence of the 18 nucleotide tRNA construct. This suggests that having a shorter, 18 bp PBS helix slightly reduces strain imposed on H1 through the ssRNA linkers. H1 formation drastically decreased to 22% when the 22 nucleotide tRNA was used in complex formation. This extension of the PBS helix to 22 bps may induce strain on H1, in the absence of RT, by shortening the length of the ssRNA linker. Additional interactions between the vRNA and the remainder of the full-length tRNA may contribute to the preference for H1 formation despite the ability to form the extended PBS helix. Alternatively, conformational flexibility of the 5’ end of the tRNA may have a destabilizing effect on the extended PBS helix, favoring the 18 bp form in the absence of RT.
The effect of PBS-bound RT on H1 stability varies with PBS length. Upon the addition of RT, a FRET shift favoring Helix 1 formation was observed for the full-length tRNA construct. These data are consistent with our prior structural study that suggested that H1 is formed in the crosslinked RTIC24. This H1 stabilization effect was not observed for the complex containing the 18 nucleotide tRNA construct. A shift favoring Helix 1 formation was observed for the 22 nucleotide tRNA complex for which RT has a higher affinity upon the addition of RT. These data support a model whereby the formation of a 22 bp PBS helix stabilizes RT binding, probably through coaxial stacking of the 5’ end of tRNALys3, and H1 is stabilized in the final RTIC structure.
By moving FRET labels between the 5’ and 3’ ends of H1 and the 5’ end of the tRNA, we observed multiple global conformations that interconvert on both fast (2–4s) and slow (>10s) timescales (Figure 6, Supplementary Figure 5). These data suggest a vRNA-tRNA complex that is structurally dynamic and occupies many conformational states prior to RT binding. The addition of RT to these experiments induced drastic shifts in the FRET distributions. Upon RT binding, the location of Helix 1 appears to be move farther away from the 5’ end of the tRNA primer as observed in the FRET distributions for vRNA 5’ to tRNA 5’ and vRNA 3’ to tRNA 5’ FRET which are shifted to lower FRET states. This shift to lower FRET states in these experiments is consistent, upon correction for PIFE effects, with distance measurements performed on RNA modeled into the cryo-EM electron density maps. These results further demonstrate the ability of RT to induce RNA conformational changes within the complex.
The results of this work are consistent with previous biochemical and structural studies performed on the reverse transcription initiation complex12; 14; 25; 28; 32; 33; 34; 41; 43; 45; 46. Biochemical results have provided distinct RNA secondary structural models, in which different vRNA and tRNA interactions have been proposed. Our work shows that these conformations are likely in structural equilibrium prior to RT binding (Figure 7). We show that the RNA structural heterogeneity of the initiation complex is reduced to a smaller subset of structures upon RT binding, which is facilitated by the extension of the PBS helix. Productive RT binding then further stabilizes the formation of H1. Additional regions within the tRNA may be responsible for stable RT binding, by favoring the refolded state of the tRNA, which in turn stabilizes the extended PBS interactions.
Figure 7.
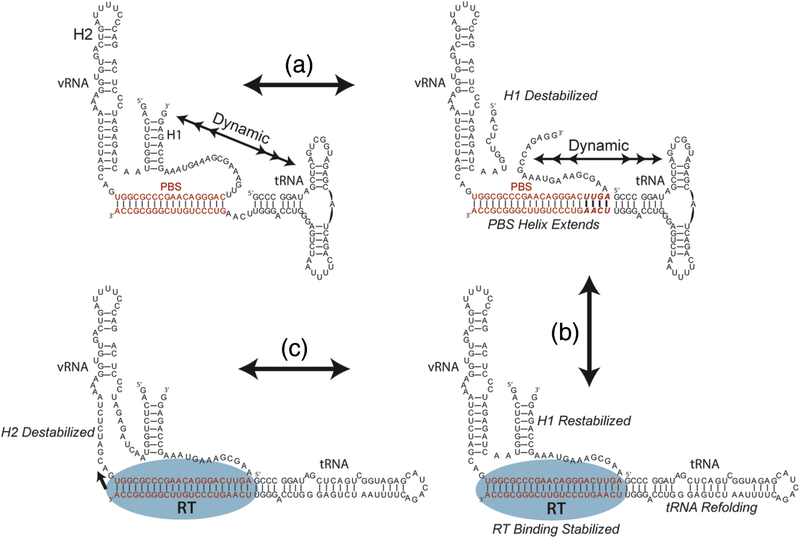
Model of the early steps of reverse transcription initiation. (a) In the absence of RT, the vRNA-tRNA is structurally dynamic as indicated by multiheaded arrows. Dynamics between formed and melted H1 and the tRNA 5’ end are observed by smFRET. The formation of an extended PBS helix destabilizes H1, and dynamics are still observed between the vRNA 5’ and 3’ ends and the tRNA 5’ end. (b) The binding of reverse transcriptase is stabilized by the extended PBS helix which can make contacts within the RNase H domain, and the dynamic properties of the RNA/RNA complex decrease. Reverse transcriptase binding facilitates refolding of the tRNA into an elongated state and stabilizes H1. (c) The stabilization of H1 may serve to induce strain on H2 which is the RT pause site during the process of initiation.
We propose a structurally dynamic model of the reverse transcription initiation complex in which multiple dynamic RNA structures form and melt in the absence of RT (Figure 7). The stable binding of RT is facilitated by the formation of four additional base pairs within the PBS helix which contacts the RNase H domain of RT and by the 5′ region of the tRNA. Stable RT binding to the initiation complex then favors the formation of vRNA H1, which is tethered between the PBS helix and H2 in vRNA. H2 is an important pause site for RT in the initiation pathway25, thus the stabilization of H1 may serve to induce strain upon the H2 checkpoint to allow reverse transcription to proceed. Using structural data to form initial hypotheses, our results represent series of dynamic snapshots that reveal the first steps in the pathway of reverse transcription initiation.
Materials and Methods
RNA reagent preparation
HIV-1 RNA and tRNA constructs were synthesized in vitro using T7 polymerase and a linearized DNA plasmid construct containing the vRNA or tRNA sequence for transcription purchased from GenScript. In vitro transcription reactions for unmodified RNA and 5’ aminoGMPlabeled RNAs was carried out as previously described24. Transcripts were purified and extracted on a sequencing PAGE gel as previously described47. Cy3-labeled tRNA, dye-labeled oligonucleotides, and biotin-labeled oligonucleotides were purchased from TriLink and verified using denaturing-PAGE and mass spectrometry. 5’ amino-GMP-labeled RNAs were dye-labeled using as previously described.
The sequence of the vRNA construct used for smFRET experiments was 5’- GGUGACUCUGGUAACUAGAGAUCCCUCAGACCCUUUUAGUCAGUGUGGAAAAUCUCU AGCAGUGGCGCCCGAACAGGGACUUGAAAGCGAAAGUAAAGCCAGAGGUCUCCUGAU CUCCCGCUCGGGAUAGGGAAUAGC-3’ where the bold nucleotides span nucleotides 121–224 of the HIV NL4.3 genome and underlined nucleotides are used to anneal dye- and biotin-labeled oligonucleotides. Mutations on the vRNA from nucleotides 200–204 were performed using a QuikChange Lightning mutagenesis kit from Agilent. Dye-labeled oligonucleotides had the sequence 5’-GCGGGAGAUCAGGCAU(C6-NH-Cy3/5)-3’, and biotin-labeled oligonucleotides 5’(Biotin)CUAUUCCCUAUCCddC-3’.
RT purification and labeling
HIV-1 reverse transcriptase was over expressed in E. coli and affinity purified as previously described24. For smFRET experiments, cysteines within the p66 and p51 subunits were mutated to serine, and a cysteine residue was inserted at the C-terminal end of p66 for dyelabeling using maleimide chemistry. Dye-labeling was carried out using 1000-fold molar excess dye in 100mM sodium phosphate buffer pH 7.0 at room temperature for 2 hours similarly to a previously described method38; 39. Excess dye was removed by two passages on a 10DG desalting column.
Dye-labeled RT was exchanged into 50mM Tris HCl pH 8.0 300mM NaCl by SEC on a Superdex 200 column. vRNA-tRNA complex formation and purification vRNAtRNA complexes were formed by mixing the vRNA and tRNA (labeled or unlabeled) in a 1:5 vRNA:tRNA molar ratio ratio (1uM vRNA, 5uM tRNA) in 10mM Bis-Tris pH 7.0 10mM NaCl. The RNAs were denatured by heating to 95C for 3 minutes then annealed by slowly cooling to room temperature over 20 minutes. 1:1 vRNA-tRNA complexes were purified from the annealing reaction by SEC on a Superdex 200 column in 10mM Bis-Tris pH 7.0 50mM NaCl. Complex purity was assessed by native PAGE. Dye- and biotin-labeled oligonucleotides were annealed in 10-fold molar excess by incubation for 5 minutes at 37°C. Excess oligonucleotides were removed by washing the slide after immobilization for smFRET experiments.
Reverse transcriptase activity assay
Reverse transcription reactions containing 50mM Tris-HCl (pH 8.0), 50mM KCl, 6mM MgCl2, 5mM β-me, 200nM vRNA-tRNA complex, and 3μM RT were pre-incubated at 37°C for 4 minutes. vRNA-tRNA complexes consisted of the vRNA construct for smFRET heat annealed to synthetic tRNALys3 with a 5’ Cyanine3 label (TriLink). Reactions were initiated by addition of a dNTP mixture to a final concentration of 100μM per nucleotide. Reactions for the ladder lanes were identical except for the addition of 25μM ddNTP for one nucleotide species in each corresponding sequencing lane. Reactions were quenched at time points with 1:1 reaction:buffer volume of loading buffer containing 50mmM Tris-HCl pH 8.0, 50mM EDTA, and 90% formamide.
Reactions were then heated for 5 min at 95 °C, loaded onto an 8.5% polyacrylamide gel which had been pre-run at 100W for 2 hours. The gel was run at 120W for 3 hours before imaging with a Typhoon Trio scanner (Amersham Biosciences).
smFRET experiments and data analysis
Biotinylated complexes were immobilized on neutravidin-derivatized quartz slides for smFRET experiments at a concentration of 50pM. Following immobilization, smFRET buffer (50mM Tris HCl pH 8.0, 50mM NaCl, 6mM MgCl2) containing oxygen-scavenging reagents (2.5mM 3,4-dihydroxybenzoic acid, 250nM protocatechuate dioxygenase, 1mM Trolox) was used to rinse the slide immediately before smFRET experiments. FRET experiments containing RT were carried out in the same buffer but containing either 10nM or 1μM labeled or unlabeled RT as indicated.
Single-molecule experiments were performed on a prism-based total internal reflection microscope as described previously48. A diode-pumped solid-state 532nm laser with 80mW power as measured at the prism was used to induce dye fluorescence. Fluorescence emission was separated into Cy3 and Cy5 channels using a Quad-View device (Photometrics) and detected using an EMCCD camera (Andor Technology). The frame rate was 100msec, and movies were either 5 minutes or 8 minutes in length as indicated.
FRET traces were analyzed using MATLAB (MathWorks) scripts written in house. Traces were first selected automatically based on spot intensity and background level. Traces were then inspected by eye for dye photophysical behavior prior to analysis. FRET lifetimes were assigned manually to generate population density histograms. RT binding events were assigned manually. RT binding kinetics were determined by fitting either single or double exponential equations to cumulative density plots of FRET lifetimes, and FRET population distributions were determined by fitting gaussians to the population density histograms. Structural figures were made in Chimera.
Supplementary Material
Figure 4.
Representative traces from single-molecule experiments using the 18 nucleotide (a), 22 nucleotide (b), and full-length tRNA constructs (c). As the size of the tRNA construct is increased, RT binding lifetimes also increase. (d) Zoom of long-lived RT binding events shows similar high/low FRET exchange as previously reported38.
Highlights.
RNA and protein together dynamically modulate reverse transcription initiation.
Formation of an extended RNA PBS helix stabilizes reverse transcriptase binding.
The initiation complex is structurally dynamic when reverse transcriptase is absent.
Reverse transcriptase binding stabilizes RNA elements within the initiation complex.
Acknowledgements
This work is supported by NIH grant GM082545 to E.V.P., NIH grant T32-GM008294 (Molecular Biophysics Training Program) to A.T.C., K.P.L, and, National Science Foundation GRFP (DGE114747) to A.T.C.
Abbreviations
- (HIV-1)
human immunodeficiency virus-1
- (vRNA)
viral RNA
- (dsDNA)
double-stranded DNA
- (5’-UTR)
5’untranslated region
- (PBS)
primer binding site
- (RT)
HIV-1 reverse transcriptase
- (PAS)
primer activation signa
- (RTIC)
reverse transcriptase initiation complex
- (cryo-EM)
cryo-electron microscopy
- (H1)
helix 1
- (H2)
helix 2
- (smFRET)
single-molecule Förster resonance energy transfer
- (SEC)
size exclusion chromatography
- (PIFE)
protein-induced fluorescence enhancement
Footnotes
Declaration of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baltimore D (1970). RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature 226, 1209–11. [DOI] [PubMed] [Google Scholar]
- 2.Temin HM & Mizutani S (1970). RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 226, 1211–3. [DOI] [PubMed] [Google Scholar]
- 3.Haseltine WA, Kleid DG, Panet A, Rothenberg E & Baltimore D (1976). Ordered Transcription of Rna Tumor-Virus Genomes. Journal of Molecular Biology 106, 109–131. [DOI] [PubMed] [Google Scholar]
- 4.Mitsuya H, Weinhold KJ, Furman PA, Stclair MH, Lehrman SN, Gallo RC, Bolognesi D, Barry DW & Broder S (1985). 3’-Azido-3’-Deoxythymidine (Bw A509u) - an Antiviral Agent That Inhibits the Infectivity and Cytopathic Effect of Human Lymphotropic-T Virus Type-Iii Lymphadenopathy-Associated Virus Invitro. Proceedings of the National Academy of Sciences of the United States of America 82, 7096–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furman PA, Fyfe JA, Stclair MH, Weinhold K, Rideout JL, Freeman GA, Lehrman SN, Bolognesi DP, Broder S, Mitsuya H & Barry DW (1986). Phosphorylation of 3’-Azido-3’- Deoxythymidine and Selective Interaction of the 5’-Triphosphate with HumanImmunodeficiencyVirus Reverse-Transcriptase. Proceedings of the National Academy of Sciences of the United States of America 83, 8333–8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba M, Tanaka H, Declercq E, Pauwels R, Balzarini J, Schols D, Nakashima H, Perno CF, Walker RT & Miyasaka T (1989). Highly Specific-Inhibition of Human Immunodeficiency Virus Type-1 by a Novel 6-Substituted Acyclouridine Derivative. Biochemical and Biophysical Research Communications 165, 1375–1381. [DOI] [PubMed] [Google Scholar]
- 7.Pauwels R, Andries K, Desmyter J, Schols D, Kukla MJ, Breslin HJ, Raeymaeckers A, Vangelder J, Woestenborghs R, Heykants J, Schellekens K, Janssen MAC, Declercq E & Janssen PAJ (1990). Potent and Selective-Inhibition of Hiv-1 Replication Invitro by a Novel Series of Tibo Derivatives. Nature 343, 470–474. [DOI] [PubMed] [Google Scholar]
- 8.Balzarini J, Holy A, Jindrich J, Naesens L, Snoeck R, Schols D & Declercq E (1993). Differential Antiherpesvirus and Antiretrovirus Effects of the (S) and (R) Enantiomers of Acyclic Nucleoside Phosphonates - Potent and Selective Invitro and Invivo Antiretrovirus Activities of (R)9-(2-Phosphonomethoxypropyl)-2,6-Diaminopurine. Antimicrobial Agents and Chemotherapy 37, 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleiman L, Jones CP & Musier-Forsyth K (2010). Formation of the tRNALys packaging complex in HIV-1. FEBS Lett 584, 359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das AT, Klaver B & Berkhout B (1997). Sequence variation of the human immunodeficiency virus primer-binding site suggests the use of an alternative tRNA(Lys) molecule in reverse transcription. J Gen Virol 78 (Pt 4), 837–40. [DOI] [PubMed] [Google Scholar]
- 11.Muthuswami R, Chen J, Burnett BP, Thimmig RL, Janjic N & McHenry CS (2002). The HIV plus-strand transfer reaction: determination of replication-competent intermediates and identification of a novel lentiviral element, the primer over-extension sequence. J Mol Biol 315, 311–23. [DOI] [PubMed] [Google Scholar]
- 12.Seif E, Niu M & Kleiman L (2015). In virio SHAPE analysis of tRNA(Lys3) annealing to HIV-1 genomic RNA in wild type and protease-deficient virus. Retrovirology 12, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beerens N & Berkhout B (2002). Switching the in vitro tRNA usage of HIV-1 by simultaneous adaptation of the PBS and PAS. RNA 8, 357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldschmidt V, Paillart JC, Rigourd ML, Ehresmann B, Aubertin AM, Ehresmann C & Marquet R (2004). Structural variability of the initiation complex of HIV-1 reverse transcription. Journal of Biological Chemistry 279, 35923–35931. [DOI] [PubMed] [Google Scholar]
- 15.Paillart JC, Dettenhofer M, Yu XF, Ehresmann C, Ehresmann B & Marquet R (2004). First snapshots of the HIV-1 RNA structure in infected cells and in virions. J Biol Chem 279, 48397–403. [DOI] [PubMed] [Google Scholar]
- 16.Ooms M, Cupac D, Abbink TE, Huthoff H & Berkhout B (2007). The availability of the primer activation signal (PAS) affects the efficiency of HIV-1 reverse transcription initiation. Nucleic Acids Res 35, 1649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson KA, Gorelick RJ, Vasa SM, Guex N, Rein A, Mathews DH, Giddings MC & Weeks KM (2008). High-throughput SHAPE analysis reveals structures in HIV-1 genomic RNA strongly conserved across distinct biological states. PLoS Biol 6, e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watts JM, Dang KK, Gorelick RJ, Leonard CW, Bess JW Jr., Swanstrom R, Burch CL & Weeks KM (2009). Architecture and secondary structure of an entire HIV-1 RNA genome. Nature 460, 711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbink TE, Beerens N & Berkhout B (2004). Forced selection of a human immunodeficiency virus type 1 variant that uses a non-self tRNA primer for reverse transcription: involvement of viral RNA sequences and the reverse transcriptase enzyme. J Virol 78, 10706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beerens N & Berkhout B (2002). The tRNA primer activation signal in the human immunodeficiency virus type 1 genome is important for initiation and processive elongation of reverse transcription. Journal of Virology 76, 2329–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beerens N, Groot F & Berkhout B (2001). Initiation of HIV-1 reverse transcription is regulated by a primer activation signal. Journal of Biological Chemistry 276, 31247–31256. [DOI] [PubMed] [Google Scholar]
- 22.Isel C, Marquet R, Keith G, Ehresmann C & Ehresmann B (1993). Modified Nucleotides of Transfer Rna(3)(Lys) Modulate Primer Template Loop-Loop Interaction in the Initiation Complex of Hiv-1 Reverse Transcription. Journal of Biological Chemistry 268, 25269–25272. [PubMed] [Google Scholar]
- 23.Liang C, Li XG, Rong LW, Inouye P, Quan YD, Kleiman L & Wainberg MA (1997). The importance of the A-rich loop in human immunodeficiency virus type 1 reverse transcription and infectivity. Journal of Virology 71, 5750–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen KP, Mathiharan YK, Kappel K, Coey AT, Chen DH, Barrero D, Madigan L, Puglisi JD, Skiniotis G & Puglisi EV (2018). Architecture of an HIV-1 reverse transcriptase initiation complex. Nature 557, 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldschmidt V, Rigourd M, Ehresmann C, Le Grice SF, Ehresmann B & Marquet R (2002). Direct and indirect contributions of RNA secondary structure elements to the initiation of HIV-1 reverse transcription. J Biol Chem 277, 43233–42. [DOI] [PubMed] [Google Scholar]
- 26.Liang C, Rong L, Gotte M, Li X, Quan Y, Kleiman L & Wainberg MA (1998). Mechanistic studies of early pausing events during initiation of HIV-1 reverse transcription. J Biol Chem 273, 21309–15. [DOI] [PubMed] [Google Scholar]
- 27.Pop MP & Biebricher CK (1996). Kinetic analysis of pausing and fidelity of human immunodeficiency virus type 1 reverse transcription. Biochemistry 35, 5054–62. [DOI] [PubMed] [Google Scholar]
- 28.Suo Z & Johnson KA (1997). RNA secondary structure switching during DNA synthesis catalyzed by HIV-1 reverse transcriptase. Biochemistry 36, 14778–85. [DOI] [PubMed] [Google Scholar]
- 29.Suo Z & Johnson KA (1997). Effect of RNA secondary structure on the kinetics of DNA synthesis catalyzed by HIV-1 reverse transcriptase. Biochemistry 36, 12459–67. [DOI] [PubMed] [Google Scholar]
- 30.Lanchy JM, Ehresmann C, Le Grice SF, Ehresmann B & Marquet R (1996). Binding and kinetic properties of HIV-1 reverse transcriptase markedly differ during initiation and elongation of reverse transcription. EMBO J 15, 7178–87. [PMC free article] [PubMed] [Google Scholar]
- 31.Jones CP, Saadatmand J, Kleiman L & Musier-Forsyth K (2013). Molecular mimicry of human tRNALys anti-codon domain by HIV-1 RNA genome facilitates tRNA primer annealing. RNA 19, 21929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarafianos SG, Marchand B, Das K, Himmel DM, Parniak MA, Hughes SH & Arnold E (2009). Structure and Function of HIV-1 Reverse Transcriptase: Molecular Mechanisms of Polymerization and Inhibition. Journal of Molecular Biology 385, 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang HF, Chopra R, Verdine GL & Harrison SC (1998). Structure of a covalently trapped catalytic complex of HIV-I reverse transcriptase: Implications for drug resistance. Science 282, 1669–1675. [DOI] [PubMed] [Google Scholar]
- 34.Sarafianos SG, Das K, Tantillo C, Clark AD, Ding J, Whitcomb JM, Boyer PL, Hughes SH & Arnold E (2001). Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA : DNA. Embo Journal 20, 1449–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kati WM, Johnson KA, Jerva LF & Anderson KS (1992). Mechanism and Fidelity of Hiv Reverse-Transcriptase. Journal of Biological Chemistry 267, 25988–25997. [PubMed] [Google Scholar]
- 36.Marko RA, Liu HW, Ablenas CJ, Ehteshami M, Gotte M & Cosa G (2013). Binding Kinetics and Affinities of Heterodimeric versus Homodimeric HIV-1 Reverse Transcriptase on DNA-DNA Substrates at the Single-Molecule Level. Journal of Physical Chemistry B 117, 45604567. [DOI] [PubMed] [Google Scholar]
- 37.Vaccaro JA, Singh HA & Anderson KS (1999). Initiation of minus-strand DNA synthesis by human immunodeficiency virus type 1 reverse transcriptase. Biochemistry 38, 15978–15985. [DOI] [PubMed] [Google Scholar]
- 38.Abbondanzieri EA, Bokinsky G, Rausch JW, Zhang JX, Le Grice SFJ & Zhuang XW (2008). Dynamic binding orientations direct activity of HIV reverse transcriptase. Nature 453, 184U2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu SX, Abbondanzieri EA, Rausch JW, Le Grice SFJ & Zhuang XW (2008). Slide into Action: Dynamic Shuttling of HIV Reverse Transcriptase on Nucleic Acid Substrates. Science 322, 1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beerens N, Jepsen MDE, Nechyporuk-Zloy V, Kruger AC, Darlix JL, Kjems J & Birkedal V (2013). Role of the primer activation signal in tRNA annealing onto the HIV-1 genome studied by single-molecule FRET microscopy. Rna-a Publication of the Rna Society 19, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coey A, Larsen K, Puglisi JD & Puglisi EV (2016). Heterogeneous structures formed by conserved RNA sequences within the HIV reverse transcription initiation site. Rna 22, 16891698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldschmidt V, Ehresmann C, Ehresmann B & Marquet R (2003). Does the HIV-1 primer activation signal interact with tRNA3(Lys) during the initiation of reverse transcription? Nucleic Acids Res 31, 850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puglisi EV & Puglisi JD (2011). Secondary structure of the HIV reverse transcription initiation complex by NMR. J Mol Biol 410, 863–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bajji AC, Sundaram M, Myszka DG & Davis DR (2002). An RNA complex of the HIV-1 Aloop and tRNA(Lys,3) is stabilized by nucleoside modifications. Journal of the American Chemical Society 124, 14302–14303. [DOI] [PubMed] [Google Scholar]
- 45.Isel C, Ehresmann C, Keith G, Ehresmann B & Marquet R (1995). Initiation of Reverse Transcription of Hiv-1 - Secondary Structure of the Hiv-1 Rna/Trna(3)(Lys) (Template/Primer) Complex. Journal of Molecular Biology 247, 236–250. [DOI] [PubMed] [Google Scholar]
- 46.Puglisi EV & Puglisi JD (1998). HIV-1 A-rich RNA loop mimics the tRNA anticodon structure. Nat Struct Biol 5, 1033–6. [DOI] [PubMed] [Google Scholar]
- 47.Petrov A, Wu TH, Puglisi EV & Puglisi JD (2013). RNA Purification by Preparative Polyacrylamide Gel Electrophoresis. Laboratory Methods in Enzymology: Rna 530, 315–330. [DOI] [PubMed] [Google Scholar]
- 48.Marshall RA, Dorywalska M & Puglisi JD (2008). Irreversible chemical steps control intersubunit dynamics during translation. Proc Natl Acad Sci U S A 105, 15364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.