Abstract
AIMS:
To determine among adolescents and young adults with youth-onset type 1 diabetes and type 2 diabetes the rates and risk factors for albuminuria regression and progression. The research hypothesis was that youth with type 2 diabetes would be more likely to show progression of albuminuria.
METHODS:
Data from SEARCH, a longitudinal observational study of youth-onset type 1 diabetes (N=1316) and type 2 diabetes (N=143) were analyzed. Urine albumin:creatinine ratio (UACR) was measured from random urine specimens at baseline and follow-up visits (mean 7 years later). Albuminuria regression was defined as halving of baseline UACR when baseline UACR was ≥30μg/mg; progression was defined as doubling of baseline UACR when follow-up UACR was ≥30μg/mg, respectively. Multivariable regression assessed risk factors associated with low-risk albuminuria category (combined persistently-low albuminuria and regression) versus moderate-risk albuminuria category (combined persistently-high albuminuria and progression).
RESULTS:
Albuminuria progression was more common in type 2 diabetes versus type 1 diabetes (15.4% versus 6.0%, p<0.001). Moderate-risk albuminuria was associated with increasing HbA1c (adjusted OR (aOR)=1.3, 95% CI 1.1–1.6) and lack of private health insurance (aOR=2.7, 95%CI 1.1–6.5) in type 1 diabetes; and African American race (OR=4.6, 95% CI 1.2–14.2), lower estimated insulin sensitivity score (aOR=2.1, 95% CI 1.4–3.3), baseline UACR (aOR=3.2, 95% CI 1.7–5.8), and follow-up estimated glomerular filtration rate (eGFR) (10-unit increase aOR=1.3, 95% CI 1.0, 1.5) in type 2 diabetes.
CONCLUSIONS:
In the first decade of diabetes duration, kidney complications in type 2 diabetes are significantly more aggressive than in type 1 diabetes and may be associated with less modifiable risk factors including race, insulin sensitivity, and eGFR. Interventions are needed even at very early stages of disease to reduce the long-term consequences of diabetic kidney disease in youth-onset diabetes.
Keywords: pediatric type 1 diabetes, pediatric type 2 diabetes, epidemiology, nephropathy
INTRODUCTION
In 2009, the prevalence of type 1 and type 2 diabetes in youth was 1.93 and 0.46 cases per 1000 under age 20 years, respectively.1 Incidence rates of both diseases among youth are increasing, particularly among minority racial and ethnic groups.2 Youth-onset (less than age 20 years) diabetes confers a greater lifetime risk for end-stage renal disease than adult-onset diabetes in part due to the longer duration of exposure to the diabetic milieu.3 Moreover, recent data show that rates of complications are significantly higher among adolescents and young adults with type 2 than with type 1 diabetes, especially diabetic kidney disease (DKD).4 Given the rising prevalence of both type 1 and type 2 diabetes, knowledge of how untreated albuminuria progresses early in the first decade of disease duration in youth-onset diabetes is critical.
In the majority of patients with diabetes, albuminuria is one of the earliest and most common markers of kidney disease.5 Over the past decade, it has become apparent that the degree of albuminuria is dynamic and regresses more often that it progresses, with rates of 40–60% regressing versus 20–30% progressing.6–9 Stable high albuminuria, as defined by albumin excretion rate exceeding 30 mg/day, increases the risk for progressive decline in estimated glomerular filtration rate (eGFR) and cardiovascular events10 and there is a known linear relationship between the magnitude of albuminuria and the risk for renal and cardivascular events and death.10,11 Regression of albuminuria may signify a lower risk for decline in eGFR, though it is not clear if this holds true for the risk of subsequent cardiovascular events.8,10,12
The shift to a natural history of albuminuria in which regression is more common than progression may be due to changes in clinical practice patterns over the last 20years.7 In studies of adults, regression of albuminuria is more frequent with improvements in hemoglobin A1c (HbA1c), blood pressure and triglycerides, as well as with the use of renin angiotensin aldosterone (RAAS) inhibitors.8,13 Other demographic predictors of regression versus progression have been debated, including differences by sex, which has been controversial.6,14,15
Data are limited regarding rates of albuminuria regression and progression in youth-onset diabetes. Adult studies carry limited generalizability to adolescents and young adults with diabetes, as younger patients are infrequently treated with RAAS blockade and they tend to experience more erratic glycemic control, especially during adolescence and young adulthood. Our primary goals for this study were: (1) to use longitudinal data to compare albuminuria regression and progression in individuals with youth-onset type 1 or type 2 diabetes and (2) to identify factors for worse albuminuria outcomes within each diabetes type. We used a novel approach to define albuminuria progression and regression that incorporates the magnitude of change in UACR in addition to crossing the threshold for moderate-risk microalbuminuria. The research hypothesis was that progression of albuminuria would be more common among youth with type 2 diabetes compared to youth with type 1 diabetes.1
1. SUBJECTS, MATERIALS, AND METHODS
2.1. Study population
Youth with diabetes diagnosed <20 years of age were identified from a population-based incidence registry at five U.S. sites by the SEARCH for Diabetes in Youth Study: South Carolina, Ohio, Colorado, Washington, and California.16 Cases 6 were newly diagnosed with type 1 diabetes or type 2 diabetes in 2002–2006 or 2008 and were identified from ongoing surveillance networks of hospitals and health care providers. Cases who could be contacted were recruited for a baseline visit (mean of 9.0±6.3 months from diagnosis for type 1 diabetes and 10.7±7.3 months from diagnosis for type 2 diabetes), and if completed, asked to return for visits at 12, 24, and 60 months post-baseline to measure risk factors for diabetes complications (Figure 1, Panel A). A subset of participants who had at least five years of diabetes duration, aged 10 years and older, were recruited from 2012–2015 for a follow-up visit.
Figure 1: Study Design and Sample Recruitment.
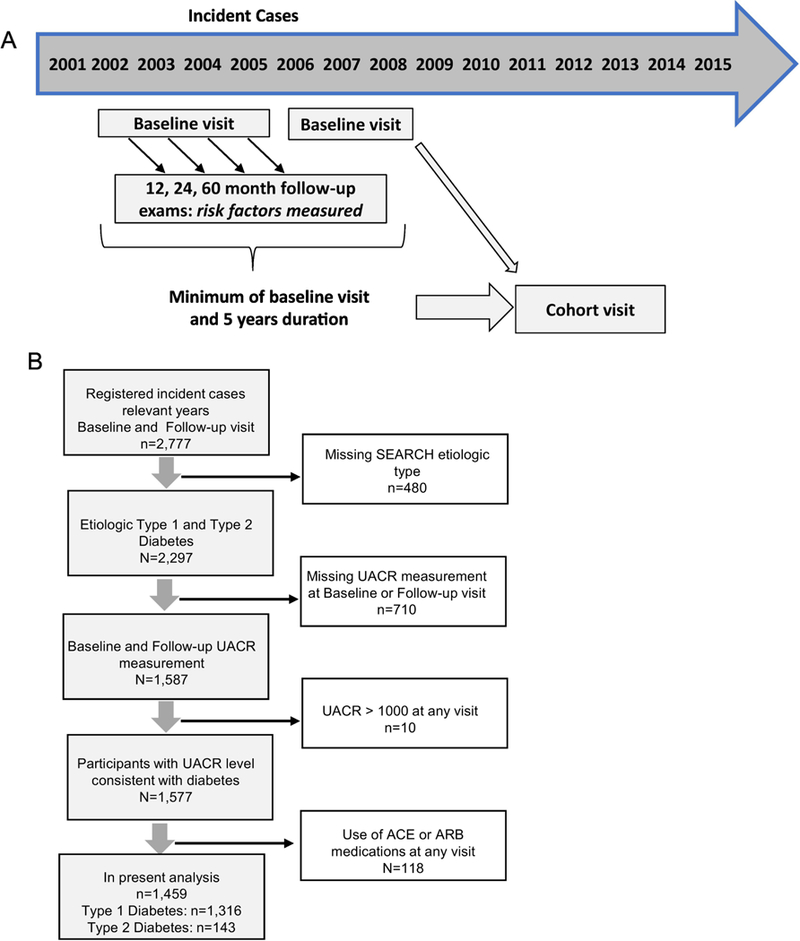
Panel A: Study design of the SEARCH Cohort Study. Panel B: Flow chart depicting participants in this report, including reasons for exclusion. The final sample included 1,316 youth with type 1 diabetes and 143 youth with type 2 daibetes.
Diabetes type was defined using an etiological classification17,18 based on one or more positive diabetes autoantibodies and estimated insulin sensitivity score (euglycemic clamp-validated equation including waist circumference, HbA1c and triglyceride levels) at the baseline visit.17 Inclusion criteria for these analyses consisted of participants with type 1 or type 2 diabetes17,18 with a urine albumin:creatinine ratio (UACR) measure available at the baseline and follow-up visits. Of the 2,297 adolescents and young adults classified as etiologic type 1 or type 2 diabetes defined by autoantibody status 1,587 had UACR available at both baseline and follow-up visits. Individuals with a UACR > 1000 mg/gm (n=10) were excluded to remove those with albuminuria due to potential for causes other than DKD. In addition, individuals taking angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) (n=118) at either timepoint were excluded to facilitate the study of the natural history of untreated albuminuria in diabetes. Baseline characteristics and albuminuria outcomes of these 118 participants treated with ACEIs or ARBs are in Supplemental Table S1. The final study sample included 1,459 individuals with diabetes: 1,316 with type 1 diabetes and 143 with type 2 diabetes (Figure 1, Panel B). The study was approved by Institutional Review Boards with jurisdiction and appropriate consent and assent were obtained for all visits.
2.2. Clinical and Laboratory measures
Trained study personnel administered questionnaires, made measurements and obtained blood samples. Race/ethnicity, sex, education and income were self-reported. Body mass index (BMI) was defined as weight (kilograms) divided by height (meters2) and converted to a Z score (BMI-Z).19 Waist circumference used the National Health and Nutrition Examination Survey protocol and was used to calculate waist to height ratio.20 The mean of 3 systolic and diastolic blood pressure levels was obtained using an aneroid manometer after at least 5 minutes of rest. A blood draw occurred after an 8 hour overnight fast, and medications, including short-acting insulin, were withheld the morning of the visit. Health insurance and smoking status were obtained from self-report at the baseline and follow-up visit.
Blood and urine samples were obtained under conditions of metabolic stability, defined as no episodes of diabetic ketoacidosis in the preceding month and the absence of fever and acute infections. Random urine specimens were collected (usually in the morning) after an overnight fast and was not collected from girls who were menstruating or in the setting of active treatment for a urinary tract infection. Urine albumin and creatinine were measured as previously described.21 Blood was processed, and fresh plasma, serum and urine samples were shipped on cold packs by overnight courier to the study central laboratory where analysis of GAD-65 antibodies, insulinoma-associated-2 antibodies, Zinc-T8 autoantibodies, cholesterol, triglycerides and HbA1c was performed as previously described.22,23 The Bouvet equation was used to calculate estimated glomerular filtration rate (eGFR) at the follow-up visit. The Bouvet equation is represented by the following: eGFR (ml/min) = 63.2 × ([plasma creatinine (μmol/L)]/96)−0.35 × ([serum cystatin C (mg/L)]/1.2)−0.56 × (weight/45)0.30 × (age/14)0.40.24 The Bouvet equation was selected to calculate eGFR given its greater accuracy for capturing hyperfiltration, an important physiologic factor in albuminuria.25
1.3. Outcome Measures:
Albuminuria was defined as a UACR measurement ≥ 30μg/mg.26,27 Using longitudinal data, four categories were defined based on baseline and follow-up visit UACR measures (Figure 2): persistently low albuminuria, persistently high albuminuria and albuminuria regression and progression. To reflect the continuous nature of UACR and to capture temporal changes in UACR even when remaining in the high albuminuria category, individuals in the ‘progression’ group showed a follow-up UACR visit that was 200% or more (i.e. double) than the baseline visit UACR, when the follow-up visit UACR was ≥30ug/mg. Individuals in the ‘regression’ group showed a follow-up visit UACR that was 50% or less (i.e. half) than the baseline visit UACR,, when the baseline visit UACR was ≥30ug/mg. Individuals who maintained a UACR ≥30ug/mg at both visits or crossed the threshold value of UACR=30ug/mg but did not meet the requirements for doubling or halving were categorized as ‘persistently high albuminuria’. Individuals with UACR <30ug/mg at both visits comprised the ‘persistently low albuminuria’ group. Our choice to classify outcomes in this manner allows us to uniquely capture youth with albuminuria at baseline who had continued worsening of albuminuria, a critical group to study given vulnerability to complications.
Figure 2. Schematic of classification of changes of albuminuria over a mean of 7 years from the baseline to follow-up visits in the SEARCH for Diabetes in Youth Cohort Study.
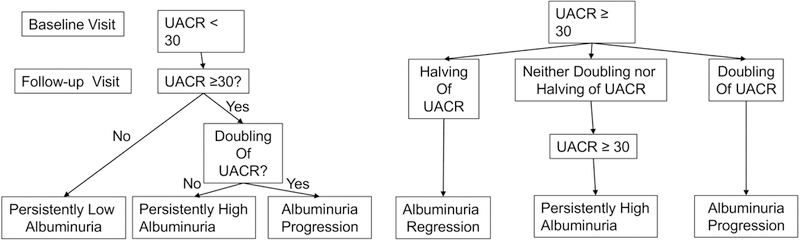
Abbreviations: UACR=urine albumin:creatinine ratio.
There were relatively few individuals with either albuminuria progression or regression. To facilitate the identification of risk factors across diabetes types, we combined longitudinal categories into two composite categories: moderate-risk albuminuria (persistently high albuminuria and progression) versus low-risk albuminuria (persistently low albuminuria and regression).
1.4. Statistical Analyses
Analyses used SAS version 9.4, (Cary, NC, USA). All analyses used a two-sided p-value of 0.05 as statistically significant and were not adjusted for multiple comparisons. Sociodemographic and clinical characteristics of the moderate-risk albuminuria group were compared with low-risk albuminuria group using chi-square and t-tests. For continuous measures presenting the median and interquartile range (IQR), the p-values are from t-tests using the log-transformed variable. For predictors with measurements available from multiple visits, a single ‘time-varying’ value was calculated using area-under-the-curve (AUC).28
Univariable and multivariable regression analyses assessed the relationship between risk factors and membership in the moderate-risk versus low-risk albuminuria group. Successively complex models were built based upon relative significance in univariate models (stratified diabetes type) and clinical relevance. In analyses of type 1 diabetes, models were sequentially adjusted for non-modifiable characteristics: age at diagnosis, diabetes duration, sex, race; modifiable clinical characteristics: HbA1c (AUC), BMI-Z (AUC), triglycerides (AUC), eGFR at follow-up; and modifiable social characteristics: follow-up smoking status, follow-up health insurance status. We opted to model eGFR using just the follow-up visit to account for the impact of hyperfiltration on albuminuria status at that time point. The final model was further adjusted for baseline UACR measure and eGFR at follow-up. Due to a smaller sample size, analyses of type 2 diabetes necessitated fewer covariates in any given model. In analyses of type 2 diabetes, the final model was adjusted for non-modifiable characteristics: age at diagnosis, diabetes duration, sex, race; modifiable clinical characteristics: insulin sensitivity score (AUC), eGFR at follow-up; and baseline UACR measure. The insulin sensitivity score was utilized only in analyses of the type 2 diabetes group due to previous work underscoring its strong association in type 2 but not type 1 diabetes.21,29
2. RESULTS
3.1. Study participants and characteristics
There were 1,316 individuals with type 1 diabetes and 143 with type 2 diabetes for this analysis. Sociodemographic and clinical characteristics of participants, stratified by diabetes type and albuminuria risk status, are displayed in Table 1. The mean time between visits was approximately 7±2 years across all groups. UACR levels stratified by diabetes type at both baseline and follow-up visits are shown in Supplementary Table S2.
Table 1.
Participant characteristics stratified by etiologic diabetes type and albuminuria risk status at follow-up visita
| Characteristics | Etiologic Type 1 Diabetes (n=1,316) | Etiologic Type 2 Diabetes (n=143) | ||||
|---|---|---|---|---|---|---|
| Moderate- risk albuminuria* (n=93) |
Low-risk albuminuria* (n=1,223) |
p- value† |
Moderate-risk albuminuria* (n=26) |
Low-risk albuminuria* (n=117) |
p- value † |
|
|
Age at diagnosis, years; mean (SD) |
9.6 (3.7) | 9.6 (4.0) | 1.0 | 14.3 (2.2) | 13.9 (2.6) | 0.5 |
| Female sex, n (%) | 37 (39.8%) | 516 (42.2%) | 0.7 | 16 (61.5%) | 71 (60.7%) | 0.9 |
| African American raceb, n (%) | 12 (12.9%) | 78 (6.4%) | 0.02 | 12 (46.2%) | 46 (39.3%) | 0.5 |
|
Lack of health insurance at follow-up visit, n (%) |
9 (9.8%) | 37 (3.0%) | <0.01 | 7 (28.0%) | 20 (17.7%) | 0.2 |
|
Diabetes duration at baseline
visit, years; mean (SD) |
0.8 (0.5) | 0.7 (0.5) | 0.6 | 1.0 (0.6) | 0.9 (0.6) | 0.5 |
|
Time between visits (years), mean (SD) |
7.2 (2.0) | 7.1 (1.9) | 0.3 | 7.1 (2.1) | 7.1 (2.0) | 0.9 |
|
Insulin Sensitivity Scorec (AUC), mean (SD) |
8.2 (2.9) | 8.8 (2.7) | 0.07 | 2.9 (1.4) | 4.4 (1.6) | <0.01 |
| UACRd, µg/mg; median (IQR) | ||||||
| Baseline | 8 (11) | 7 (7) | 0.1 | 13 (35) | 5 (4) | <0.01 |
| Follow-up visit | 53 (56) | 6 (5) | <0.01 | 113 (234) | 6 (5) | <0.01 |
|
SBP-Z (AUC), mmHg; mean (SD) |
−0.6 (0.9) | −0.5 (0.8) | 0.5 | 1.1 (1.6) | 0.7 (1.2) | 0.2 |
| HbA1c (AUC), %; mean (SD) | 9.2 (1.6) | 8.4 (1.3) | <0.01 | 9.6 (2.3) | 7.9 (2.2) | <0.01 |
| BMI-Z (AUC), mean (SD) | 0.3 (1.0) | 0.6 (0.9) | 0.02 | 2.0 (0.6) | 1.8 (0.7) | 0.4 |
|
Total Cholesterol (AUC), mg/dL; mean (SD) |
166 (27) | 165 (26) | 0.6 | 198 (36) | 169 (36) | <0.01 |
|
Triglycerides (AUC), mg/dL; median (IQR) |
71 (42) | 68 (35) | 0.06 | 150 (101) | 103 (79) | 0.01 |
| LDL (AUC), mg/dL; mean (SD) | 94 (21) | 94 (23) | 1.0 | 113 (30) | 100 (29) | 0.03 |
| HDL (AUC), mg/dL; mean (SD) | 55 (11) | 56 (12) | 0.5 | 40 (18) | 44 (10) | 0.4 |
|
Waist to Height ratio (AUC), mean (SD) |
0.5 (0.1) | 0.5 (0.1) | 0.6 | 0.7 (0.1) | 0.7 (0.1) | 0.2 |
|
Current Smoker at follow-up visit, n (%) |
19 (20.7%) | 146 (12.5%) | 0.03 | 4 (17.4%) | 45 (38.8%) | 0.05 |
|
eGFRe at follow-up visit; mean (SD) |
124.9 (26.5) | 119.9 (21.9) | 0.08 | 145.3 (36.8) | 126.4 (25.7) | 0.02 |
Categorical variables are compared using a chi-square test; continuous measures presenting means (standard deviation), are compared using t-tests; and continuous measures presenting median (IQR) from t-tests using the log-transformed variable.
Albuminuria categories were classified based on urine albumin-to-creatinine ratio (UACR) measures at baseline and UACR at follow-up visit (see Figure 2 for definitions). Moderate-risk albuminuria included albuminuria progression and persistently high albuminuria. Low-risk albuminuria included albuminuria regression and persistently normoalbuminuria.
African American race includes Hispanic and Non-Hispanic ethnicity.
Insulin Sensitivity Score (ISS) is derived from HbA1c, waist circumference and triglycerides: ISS=exp[4.64725−(0.02032×waist,cm)−(0.09779×HbA1c,%)−(0.002350×triglyceride,mg/dl)
UACR measured from random urine sample.
eGFR estimated from Bouvet equation.
Abbreviations: AUC= Area under the curve (baseline to follow-up visit). BMI=body mass index; UACR=urine albumin:creatinine ratio; LDL=low density lipoprotein; HDL=high density lipoprotein; SBP=systolic blood pressure; eGFR = estimated glomerular filtration rate.
3.2. Prevalence of albuminuria, progression, and regression
The prevalence of albuminuria at the baseline and follow-up visits was 7.8% and 7.1%, respectively for individuals with type 1 diabetes (mean baseline age = 10.5±4.0, mean follow-up age 17.5±4.3) and 9.1% and 18.2%, respectively, for individuals with type 2 diabetes (mean baseline age = 15.0±2.7, mean follow-up age 22.1±3.3, data not shown).
Figure 3 depicts the progression and regression of albuminuria at the follow-up visit for each diabetes type. Among all individuals with youth-onset diabetes, regardless of albuminuria at baseline, progression of albuminuria occurred in 7.0% of participants with type 1 and 19.6% with type 2 diabetes (p<0.001). Regression of albuminuria occurred in 7.8% of participants with type 1 and 4.5% with type 2 diabetes (p=0.13).
Figure 3: Progression and Regression of Albuminuria in Youth with Diabetes, Stratified by Etiologic Diabetes Type.
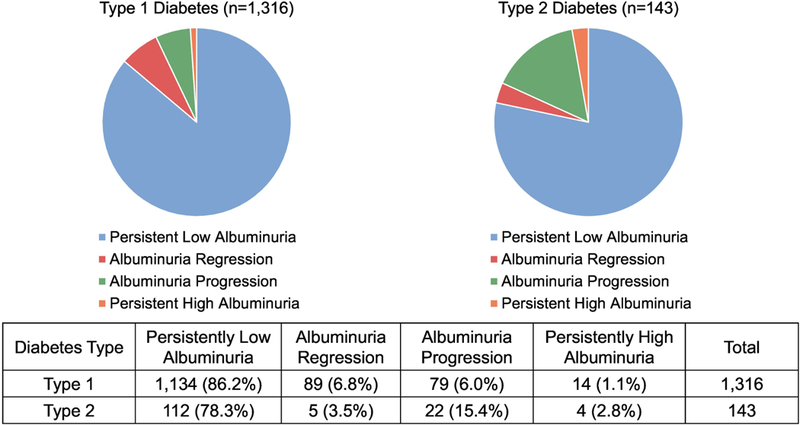
Outcomes were classified based on Urine Albumin-to-Creatinine Ratio (UACR) measures at baseline and follow-up visits. Albuminuria progression was defined as doubling of baseline UACR if the follow-up UACR was ≥30 μg/mg. Albuminuria regression was defined by halving of baseline UACR if baseline UACR was ≥30 μg/mg. Those that maintained a UACR≥30ug/mg at both visits or crossed the threshold value of UACR=30ug/mg but did not meet the requirements for doubling or ha;ving, were categorized as ‘persistently high albuminuria’. Those with UACR <30ug/mg at both visits comprised the ‘persistently low albuminuria’ group.
3.3. Factors associated with moderate-risk albuminuria
Results of the regression analyses are depicted in Tables 2a (type 1 diabetes) and 2b (type 2 diabetes). In the fully adjusted regression model for participants with type 1 diabetes, independent risk factors for membership in the moderate-risk albuminuria group were higher time-varying HbA1c (adjusted OR (aOR)=1.3, 95% CI 1.1–1.6) and lack of health insurance (aOR=2.7, 95% CI 1.1–6.5). Although African American race was significantly associated with moderate-risk albuminuria in the unadjusted model, this association was attenuated in the fully adjusted model with the addition of HbA1c, triglycerides, and health insurance status. In the fully adjusted model for type 2 diabetes, independent risk factors for membership in the moderate-risk albuminuria group included African American race compared to all others (aOR=4.6, 95% CI 1.2–14.2), time varying insulin sensitivity score (for every 1-unit decrease aOR=2.1, 95% CI 1.4–3.3), baseline UACR (aOR=3.2, 95% CI 1.7–5.8), and eGFR at the follow-up visit (for every 10-unit increase aOR=1.3, 95% CI 1.0, 1.5).
Table 2a:
Unadjusted and sequentially adjusted results of logistic regression models representing the odds ratio (OR) associated with moderate-risk versus low-risk albuminuria in youth with type 1 diabetes (n=1,136)a
| Covariates | Unadjusted | Model 1** | Model 2** | Model 3** | Model 4** | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
p-value | OR (95% CI) |
p- value |
OR (95% CI) |
p- value |
OR (95% CI) |
p- value |
OR (95% CI) |
p- value |
|
|
Age at Diagnosis (5.0-year increase) |
1.0 (0.8, 1.3) |
1.0 | 1.0 (0.8, 1.3) |
1.0 | 0.9 (0.6, 1.2) |
0.4 | 0.8 (0.5, 1.1) |
0.1 | 0.8 (0.5, 1.1) |
0.1 |
|
Sex (Female vs Male) |
0.9 (0.6, 1.4) |
0.7 | 0.9 (0.6, 1.4) |
0.6 | 1.0 (0.6, 1.5) |
0.8 | 1.0 (0.7, 1.7) |
0.8 | 1.0 (0.6, 1.7) |
0.9 |
| Raceb (AA vs Other) |
2.2 (1.2, 4.2) |
0.02 |
2.2 (1.1, 4.1) |
0.02 | 1.5 (0.7, 3.2) |
0.3 | 1.4 (0.6, 3.1) |
0.4 | 1.4 (0.6, 3.1) |
0.4 |
|
Diabetes durationc (1-year increase) |
1.0 (0.9, 1.2) |
0.5 | 1.0 (0.9, 1.2) |
0.5 | 1.0 (0.9, 1.1) |
1.0 | 1.0 (0.9, 1.1) |
0.7 | 1.0 (0.9, 1.1) |
0.7 |
|
BMI-Z (AUC) (1-unit increase) |
0.8 (0.6, 1.0) |
0.02 | -- | -- | 0.8 (0.6, 1.0) |
0.05 |
0.8 (0.6, 1.0) |
0.04 | 0.8 (0.6, 1.0) |
0.1 |
|
HbA1c (AUC) (1-percent increase) |
1.5 (1.3, 1.7) |
<0.0001 | -- | -- |
1.4 (1.2, 1.7) |
0.0001 |
1.4 (1.1, 1.6) |
0.001 |
1.3 (1.1, 1.6) |
0.002 |
|
Triglyceridesd (AUC) (1-log unit increase) |
1.7 (1.1, 2.8) |
0.03 | -- | -- | 1.6 (0.9, 2.7) |
0.1 | 1.5 (0.9, 2.6) |
0.2 | 1.5 (0.9, 2.7) |
0.2 |
|
Smoking Statuse at Follow-up |
1.8 (1.1, 3.1) |
0.03 | -- | -- | -- | -- | 1.5 (0.8, 2.9) |
0.2 | 1.6 (0.8, 3.0) |
0.2 |
|
Health Insurance at Follow-up (None vs Insured) |
3.5 (1.6, 7.4) |
0.001 | -- | -- | -- | -- |
2.7 (1.1, 6.5) |
0.02 |
2.7 (1.1, 6.5) |
0.03 |
|
Baseline UACRd (1-log unit increase) |
1.2 (1.0, 1.5) |
0.07 | -- | -- | -- | -- | -- | -- | 1.2 (1.0, 1.5) |
0.1 |
|
eGFRBouvet at Follow-up (10 unit increase)f |
1.1 (1.0, 1.2) |
0.04 | -- | -- | -- | -- | -- | -- | 1.0 (0.9, 1.1) |
0.9 |
|
LDL (AUC) (10-unit increase) |
1.0 (0.9, 1.1) |
1.0 | -- | -- | -- | -- | -- | -- | -- | -- |
|
HDL (AUC) (10-unit increase) |
0.9 (0.8, 1.1) |
0.5 | -- | -- | -- | -- | -- | -- | -- | -- |
|
SBP-Z (AUC) (1-mmHg increase) |
1.1 (0.8, 1.4) |
0.5 | -- | -- | -- | -- | -- | -- | -- | -- |
|
Waist:Height ratio (AUC) (0.1-unit increase) |
0.9 (0.6, 1.3) |
0.6 | -- | -- | -- | -- | -- | -- | -- | -- |
|
Length of Follow-up (1.0-year increase) |
1.1 (0.9, 1.2) |
0.4 | -- | -- | -- | -- | -- | -- | -- | -- |
|
Insulin Sensitivity Scoref (AUC) (1-unit decrease) |
1.1 (1.0, 1.2) |
0.07 | -- | -- | -- | -- | -- | -- | -- | -- |
Albuminuria categories were classified based on urine albumin-to-creatinine ratio (UACR) measures at baseline and UACR at follow-up visit (see Figure 2 for definitions). Moderate-risk albuminuria included albuminuria progression and persistently high albuminuria. Low-risk albuminuria included albuminuria regression and persistently normoalbuminuria.
African American race includes Hispanic and Non-Hispanic ethnicity.
Diabetes duration measured at follow-up visit.
Triglycerides and UACR were transformed using natural log scale.
Smoking status defined at follow-up status as Current vs Former/Never.
Insulin Sensitivity Score is derived from HbA1c, waist circumference and triglycerides: IS=exp[4.64725−(0.02032×waist,cm)−(0.09779×HbA1c,%)−(0.002350×triglyceride,mg/dl)
Abbreviations: AUC= Area under the curve (baseline to follow-up visit). BMI=body mass index; UACR=urine albumin:creatinine ratio; LDL=low density lipoprotein; HDL=high density lipoprotein; SBP=systolic blood pressure; eGFR = estimated glomerular filtration rate.
Adjusted models in Type 1 Diabetes:
Model 1 (non-modifiable characteristics): age at diagnosis, sex, race, diabetes duration
Model 2 (modifiable clinical characteristics): Model 1 + HbA1c, BMI-Z, TG
Model 3 (modifiable social characteristics): Model 2 + follow-up smoking status, follow-up health insurance status
Model 4 (baseline UACR): Model 3 + baseline UACR measure and follow-up eGFR
Table 2b:
Unadjusted and sequentially adjusted results of logistic regression models representing the odds ratio (OR) associated with moderate-risk versus low-risk albuminuria in youth with type 2 diabetes (n=143)a
| Covariates | Unadjusted | Model 1* | Model 2* | Model 3* | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
p-value | OR (95% CI) |
p- value |
OR (95% CI) |
p- value |
OR (95% CI) |
p- value |
|
|
Age at Diagnosis (5.0-year increase) |
1.4 (0.6, 3.3) |
0.477 | 1.3 (0.5, 3.1) |
0.5 | 0.9 (0.3, 2.4) |
0.8 | 0.4 (0.1, 1.5) |
0.2 |
| Sex (Female vs Male) | 1.0 (0.4, 2.5) |
0.936 | 1.0 (0.4, 2.4) |
1.0 | 1.3 (0.5, 3.4) |
0.6 | 0.6 (0.2, 2.0) |
0.4 |
|
Raceb (African American vs Other) |
1.3 (0.6, 3.1) |
0.521 | 1.3 (0.5, 3.2) |
0.5 | 1.2 (0.5, 3.2) |
0.7 |
4.6 (1.2, 14.2) |
0.02 |
|
Diabetes durationc (1-year
increase) |
1.1 (0.9, 1.3) |
0.548 | 1.1 (0.8, 1.3) |
0.6 | 1.1 (0.8, 1.4) |
0.6 | 1.0 (0.7, 1.3) |
0.9 |
|
Baseline UACRd (1 unit increase on log scale) |
2.5 (1.6, 3.8) |
<0.0001 | -- | -- | -- | -- |
3.2 (1.7, 5.8) |
0.0003 |
|
Insulin Sensitivity Scoree (AUC) (1.0-unit decrease) |
2.0 (1.4, 2.8) |
0.0001 | -- | -- |
2.0 (1.4, 2.8) |
0.0001 |
2.1 (1.4, 3.3) |
0.001 |
|
eGFRBouvet at Follow-up (10 unit increase) |
1.2 (1.1, 1.4 |
0.004 | -- | -- | -- | -- |
1.3 (1.0, 1.5) |
0.01 |
| BMI-Z (AUC) (1-unit increase) | 1.4 (0.7, 2.6) |
0.356 | -- | -- | -- | -- | -- | -- |
|
HbA1c (AUC) (1-percent increase) |
1.4 (1.2, 1.7) |
0.001 | -- | -- | -- | -- | -- | -- |
|
Triglyceridesd (AUC)
(1-log unit increase) |
3.4 (1.6, 7.5) |
0.002 | -- | -- | -- | -- | -- | -- |
| LDL (AUC) (10-unit increase) | 1.2 (1.0, 1.3) |
0.039 | -- | -- | -- | -- | -- | -- |
| HDL (AUC) (10-unit increase) | 0.7 (0.5, 1.2) |
0.192 | -- | -- | -- | -- | -- | -- |
|
SBP-Z (AUC) (1-mmHg increase) |
1.2 (0.9, 1.6) |
0.244 | -- | -- | -- | -- | -- | -- |
|
Waist: Height ratio (AUC) (0.1-unit increase) |
1.3 (0.9, 1.8) |
0.200 | -- | -- | -- | -- | -- | -- |
| Smoking Statusf at Follow-up |
0.3 (0.1, 1.0) |
0.058 | -- | -- | -- | -- | -- | -- |
|
Health Insurance at Follow-up (None vs Insured) |
1.8 (0.7, 4.9) |
0.245 | -- | -- | -- | -- | -- | -- |
|
Length of Follow-up (1.0-year increase) |
1.0 (0.8, 1.2) |
0.934 | -- | -- | -- | -- | -- | -- |
Albuminuria categories were classified based on urine albumin-to-creatinine ratio (UACR) measures at baseline and UACR at follow-up visit (see Figure 2 for definitions). Moderate-risk albuminuria included albuminuria progression and persistently high albuminuria. Low-risk albuminuria included albuminuria regression and persistently normoalbuminuria.
African American race includes Hispanic and Non-Hispanic ethnicity.
Diabetes duration measured at follow-up visit.
Triglycerides and UACR were transformed using natural log scale.
Insulin Sensitivity Score is derived from HbA1c, waist circumference and triglycerides: IS=exp[4.64725−(0.02032×waist,cm)−(0.09779×HbA1c,%)−(0.002350×triglyceride,mg/dl)
Smoking status defined at follow-up status as Current vs Former/Never.
Abbreviations: AUC= Area under the curve (baseline to follow-up visit). BMI=body mass index; UACR=urine albumin:creatinine ratio; LDL=low density lipoprotein; HDL=high density lipoprotein; SBP=systolic blood pressure; eGFR = estimated glomerular filtration rate.
Adjusted Models in Type 2 Diabetes:
Model 1 (Potential confounders): age at diagnosis, diabetes duration, sex, race
Model 2: (insulin sensitivity): Model 1 + Insulin Sensitivity Score (AUC)
Model 3 (baseline UACR): Model 3 + baseline UACR and follow-up eGFR
3. DISCUSSION
In this large, diverse study of adolescents and young adults with new onset diabetes, we found a low proportion of albuminuria progression or stable high albuminuria and a very high proportion of regression or stable low albuminuria over the first decade of disease. We also found higher regression and lower progression proportions among adolescents and young adults with type 1 diabetes as compared to type 2 diabetes. The prevalence of albuminuria in adolescents and young adults with type 1 diabetes has been previously cited as 4% at 5 years duration and 26% at 10 years.30,31 In the TODAY Study, a clinical trial of metformin versus lifestyle modification for type 2 diabetes, participants had a prevalence of albuminuria of 6% at baseline (<1 year diabetes duration) and 16% 4-years later.32 The SEARCH study previously reported the prevalence of albuminuria to be 9.2% in type 1 diabetes and 22.2% in type 2 diabetes at a mean of 3.7 years (IQR 0.5–5.7) and 1.9 years (IQR 0.4–3.2) following diabetes diagnosis, respectively.33
Participants with type 2 diabetes were more likely than those with type 1 diabetes to be classified as moderate-risk abuminuria over the seven-year period, suggesting that the natural history of kidney disease is more aggressive in type 2 diabetes. This finding is consistent with previous work in the SEARCH cohort1. In the present study, adolescents and young adults with type 2 diabetes were older, more often African American and without health insurance as compared to participants with type 1 diabetes. Type 2 diabetes participants also had worse clinical risk factors including higher BMI z-score, systolic blood pressure z-score and triglycerides across study visits, consistent with recent literature.4 It may be that the increased risk of albuminuria progression in type 2 diabetes is related to cardiometabolic risk factors more commonly associated with type 2 versus type 1 diabetes, such as obesity and hyperlipidemia.
Among adolescents and young adults with type 1 diabetes in the SEARCH follow-up visit, the prevalence of albuminuria after a mean of 7±2 years was 6.8%, which is fairly consistent with conservative estimates of 4–6% from other recent pediatric follow-up studies of comparable diabetes duration, including the Type 1 Diabetes Exchange Study.31 The incidence of moderate-risk albuminuria was lower than the rate of 26% reported in the Oxford Regional Prospective Study 20 years prior30 and may reflect an improved outlook for type 1 diabetes due to improvements in clinical care.
We report the proportion of regression among type 1 diabetic participants with albuminuria at baseline to be 86.2% in type 1 diabetes, which slightly exceeds the regression of 79% over 13 years reported from smaller studies of children and adolescents with type 1 diabetes with persistently elevated UACR.34 Our results are markedly higher, however, than in adult populations with type 1 diabetes in whom regression rates range 50–60% over approximately six to seven years.8,9 The higher proportion of regression in our study of adolescents and young adults versus adult studies is likely attributable to the younger age range and therefore shorter disease duration, where regression may be more common in early onset disease. Finally, the use of random urine samples could have contributed to this high proportion of regression, as transient orthostatic proteinuria is common in adolescents and young adults.35
In this study, 5.1% of adolescents and young adults with type 1 and type 2 diabetes were taking ACEIs or ARB medications, consistent with the estimated 4.4% of youth and young adults <20 years in the type 1 diabetes Exchange clinic registry.31 These data suggest that few youth with diabetes are currenty treated with antihypertensive medications despite markers of early kidney disease.
Data from observational studies of youth with type 2 diabetes are limited. The prevalence of albuminuria among adolescents and young adults with type 2 diabetes at the baseline (9%) and follow-up visit visits (18%) were consistent with previous findings from the TODAY study.32 The prevalence of albuminuria at the follow-up visit (18%) was consistent with estimates of 18.5% reported in Pima Indian youth aged 5 to 19 years36 and lower than the 27% of youth with albuminuria at diagnosis reported from the Manitoba Centre for Health Policy.3 The proportion of regression among adolescents and young adults with albuminuria at baseline exceeded estimates of 28–30% from studies of older adults with type 2 diabetes.13 Aside from the older age ranges (mean age 55–61), individuals in these studies also had longer diabetes duration at baseline, ranging from just under 6 years13 to 17–28 years. In addition, previous study participants were predominantly male, had longstanding hypertension, and had preexisting cardiovascular disease, likely indicating a somewhat different pathophysiologic mechanism for albuminuria.13
We also report factors associated with membership in the moderate-risk albuminuria groups versus low-risk albuminuria groups in type 1 and type 2 diabetes. Factors that were not significantly associated with membership in the moderate-risk albuminuria group in both diabetes types included sex, blood pressure, and diabetes duration. Whereas previous studies of type 1 diabetes have found higher progression and lower remission rates in men versus women,14 we did not find any significant effect of sex. In addition, in contrast to adult studies, systolic blood pressure z-score was not significant, though this may be due to the low prevalence of hypertension in our follow-up. We excluded 118 participants on RAAS blockers at either time point to avoid confounding by use of these medications, and this may have impacted our results. Counter to previous studies, we found no significant association with diabetes duration9 likely due to the relatively short diabetes duration and study design which limited differences in duration only by 5–6 years.8,9
Glycemic control was, as expected, a significant predictor of membership in the moderate-risk albuminuria group in analyses of both types of diabetes. This is consistent with previous cohort visit studies implicating both baseline HbA1c9,37 and rise in HbA1c over time13,38 as important predictors of albuminuria. The magnitude of the effect of HbA1c was similar in multivariable analyses of both diabetes types (aOR=1.4). Given the relatively broad distribution of HbA1c levels, one might expect a stronger impact of glycemic control on albuminuria. Previous follow-up studies, however, corroborate our findings that a change in HbA1c of 1% yields an increased risk for albuminuria of 10–40%.14,30
The albuminuria risk groups in type 1 diabetes was also uniquely associated with lack of health insurance, a proxy for low socioeconomic status and lack of access to healthcare. Adolescents and young adults with type 1 diabetes who were uninsured were also older (mean age 21.4±3.0) than those with health insurance (mean age 17.4±4.3). While other studies have shown that the socioeconomic background of people with type 1 diabetes influences the risk of developing end-stage renal disease,39 the present findings suggest that lack of access to care is associated with health outcomes even at the earliest stages of DKD, a point at which the disease is still reversible. Although health insurance status was not significant in participants with type 2 diabetes, a greater proportion of these adolescents and young adults did not have health insurance as compared to participants with type 1 diabetes, which could explain this difference.
Decreasing insulin sensitivity was strongly associated with the albuminuria risk groups in type 2 diabetes. This is consistent with our previous studies which examined insulin sensitivity and baseline UACR in SEARCH, finding a strong, inverse association between insulin sensitivity score and UACR.21 Differences between our findings and recent reports40 may be explained by differences in insulin sensitivity estimation, however, our estimating equation has been previously validated.17Although time varying insulin sensitivity was significant in sensitivity analyses of type 1 diabetes, the strength of the association between insulin sensitivity score and albuminuria progression was stronger in type 2 participants (OR=2.0 versus OR=1.3).
Diabetic kidney disease is more prevalent and aggressive in people of African American heritage.41 The effect of race differed in the two strata for diabetes type. While African American race was significant in the unadjusted analyses in type 1 diabetes, it appears this was predominantly due to confounding from glycemic control and health insurance status. In contrast, African American race was not significant in the unadjusted analyses in type 2 diabetes but became significant once we adjusted for baseline UACR (albeit with wide confidence intervals). Sensitivity analyses adjusting for HbA1c did not change this effect. It is possible that the effect of baseline UACR on the association between race and albuminuria could be due to genetic factors and warrants further investigation. Data on known kidney risk variants such as Apolipoprotein L1 gene (APOL1) may help to identify both diabetes- and non-diabetes specific pathways that may mediate the association of race and kidney outcomes that were seen at all time points.42,43
Finally, eGFR at follow-up was significantly associated with membership in the moderate-risk albuminuria group among youth with type 2 diabetes, but not youth with type 1 diabetes. This may be due, in part, to the greater proportion of youth with type 2 diabetes having an eGFR in the hyperfiltration range, as hyperfiltration can result in increased albuminuria even among those without diabetes.44 Moreover, while the mechanisms of albuminuria and change in eGFR likely have some overlap, the current thought is that they are more distinct than similar.45–47 This notion is supported by the rising prevalence of normoalbuminuric chronic kidney disease (ie. eGFR < 60 and UACR >=30), and decreasing prevalence of UACR >30.5
Finally, there was an inverse relationship between smoking and albuminuria progression in unadjusted models of adolescents and young adults with type 2 diabetes. The reasons for this reverse epidemiology of smoking is not clear and conflicts with previous reports of the association between less favorable cardiometabolic risk profile and smoking status in adolescents and young adults.48 Future research is needed to substantiate these findings.
Our study does have some limitations. GFR was estimated rather than measured, though we used both creatinine and cystatin C, increasing the accuracy of estimation.24 We also used eGFRBouvet because it is most accurate in the hyperfiltration range, an important factor to capture when assessing the impact of eGFR on albuminuria.25 A limitation of this method of estimation, however, is that the equation includes weight, and has not been validated in overweight or obesity, which are quite prevalent in youth and young adults with type 2 diabetes. UACR was measured by a single random urine sample, which may result in false positives due to either day-to-day variability in UACR or orthostatic proteinuria. Data have shown the UACR can vary by as much as 40%.49,50 which is partly why we chose to restrict the definitions of progression and regression according to magnitude of change. Despite this effort, the use of a single UACR measurement at each time point carries the risk that these day-to-day variations in UACR misrepresent longitudinal patterns of change. It should also be noted, however, that isolated, random urine samples are used for albuminuria screening in the vast majority of clinical settings, lending generalizability of our results.
Our findings represent outcomes for adolescents and young adults who are not treated with RAAS blockers and thus may not apply to those who are on medication; however, this was by intention to facilitate the study of “natural history” of the disease, rather than the progression among those treated. The small number of youth who regressed and progressed necessitated combining these groups with the larger groups of moderate-risk and low-risk albuminuria groups, respectively, for regression analyses, prohibiting study of true regression and progression. Youth with a UACR > 1000 mg/gm (n=10) were excluded to remove participants with albuminuria from causes other than DKD, which may have in inadvertently excluded a few very high-risk participants with DKD alone. The smaller size of the group with type 2 diabetes and difference in final models prohibited comparison between type 1 and type 2 diabetes. While the type 2 diabetes group was much smaller, our findings offer valuable insights into this understudied population. There was a long interval between assessments of UACR, and while there were interim visits in some individuals, there were very few urine samples collected at these visits; hence, we chose to use the covariate data from these interim visits, but not the UACR values. The long follow-up time allows us to evaluate the natural history of diabetes over a prolonged period of time.
Our study has several strengths. The SEARCH for Diabetes in Youth Study is a large, longitudinal study representing multi-ethnic individuals with youth-onset diabetes in the U.S. Longitudinal data facilitated the study of progression and regression over the first decade following diagnosis, including change in risk factors over time. Few studies have examined the natural history of early kidney disease in adolescents and young adults with both type 1 and type 2 diabetes, underscoring the importance of our findings. This report contributes to the data on differential risk of complications in youth with type 1 versus type 2 diabetes.
4. CONCLUSIONS
In summary, we compared the incidence of albuminuria progression and regression in adolescents and young adults with type 1 and type 2 diabetes over a mean of 7 years and elucidated factors associated with these changes. Our findings suggest that even in the first decade of diabetes duration, kidney complications in type 2 diabetes are significantly more aggressive than in type 1 diabetes. Risk factors for membership in the moderate-risk albuminuria group versus low-risk albuminuria group are distinct between diabetes types, where type 2 diabetes is more strongly associated with African American race, baseline albuminuria, insulin sensitivity, and eGFR at follow-up. Moreover, treatment with antihypertensive medications remains infrequent, even among adolescents and young adults with elevated UACR. Interventions at a very early stage of disease may help to reduce the long-term consequences of diabetic kidney disease in this growing population of youth-onset diabetes.
Supplementary Material
Highlights.
Little is known about the early natural history and associated risk factors of albuminuria in young adults and adolescents with youth onset type 1 and type 2 diabetes.
We found that albuminuria progression is more common and regression less common in youth onset type 2 versus type 1 diabetes.
Risk factors for moderate versus low risk albuminuria differ between youth onset type 1 versus type 2 diabetes. Specifically, the risk factors for moderate risk albuminuria are more modifiable in youth onset type 1 than type 2 diabetes.
Our findings provide further evidence for intensified glycemic control in all youth onset diabetes and focus on lifestyle changes and treatments for improved insulin sensitivity in youth onset type 2 diabetes.
ACKNOWLEDGEMENTS
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
Grant Support: SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05–069, and DP-10–001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases.
FUNDING
This work was supported by the National Institutes of Health Site Contract Numbers: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241–3, U01 DP000247, and U18DP000247–06A1), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), University of Washington School of Medicine (U58/CCU019235–4, U01 DP000244, and U18DP002710–01), Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200–2010-35171).
The authors wish to acknowledge the involvement of the South Carolina Clinical & Translational Research Institute, at the Medical University of South Carolina, NIH/National Center for Advancing Translational Sciences (NCATS) grant number UL1 TR000062; Seattle Children’s Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant Number UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (DERC NIH grant number P30 DK57516); the University of Cincinnati, NIH/NCATS grant number UL1 TR000077; and the Children with Medical Handicaps program managed by the Ohio Department of Health.
ARK was supported by funding from the University of North Carolina Renal Epidemiology Training Grant (NIH/NIDDK 5T32DK007750–16). AKM was supported by the National Institute of Diabetes and Digestive and Kidney Diseases under award number K23DK093804.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr Maahs has research support from the NIH, JDRF, NSF, and the Helmsley Charitable Trust and his institution has research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, and Roche. Dr Maahs has consulted for Abbott, the Helmsley Charitable Trust, Sanofi, and Eli Lilly and has served on an advisory board for Insulet.
ABBREVIATIONS:
- (ACEi)
Angiotensin converting enzyme inhibitor
- (ARB)
Angiotensin receptor blocker
- (DKD)
Diabetic kidney disease
- (IQR)
Interquartile range
- (UACR)
Urine albumin creatinine ratio
- (eGFR)
Estimated glomerular filtration rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interests to disclose.
References:
- 1.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311(17):1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. New England Journal of Medicine 2017;376(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care 2012;35(6):1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. Jama 2017;317(8):825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. Jama 2016;316(6):602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer IH, Rue TC, Cleary PA, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011;171(5):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaede P, Tarnow L, Vedel P, Parving HH, Pedersen O. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 2004;19(11):2784–2788. [DOI] [PubMed] [Google Scholar]
- 8.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. New England Journal of Medicine 2003;348(23):2285–2293. [DOI] [PubMed] [Google Scholar]
- 9.Giorgino F, Laviola L, Cavallo Perin P, Solnica B, Fuller J, Chaturvedi N. Factors associated with progression to macroalbuminuria in microalbuminuric Type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia 2004;47(6):1020–1028. [DOI] [PubMed] [Google Scholar]
- 10.de Boer IH, Gao X, Cleary PA, et al. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC study. Clinical Journal of the American Society of Nephrology 2016:CJN. 02870316. [DOI] [PMC free article] [PubMed]
- 11.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. Journal of the American Society of Nephrology 2007;18(4):1353–1361. [DOI] [PubMed] [Google Scholar]
- 12.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 2007;18(4):1353–1361. [DOI] [PubMed] [Google Scholar]
- 13.Gæde P, Tarnow L, Vedel P, Parving H-H, Pedersen O. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrology Dialysis Transplantation 2004;19(11):2784–2788. [DOI] [PubMed] [Google Scholar]
- 14.Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ 2004;328(7448):1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. The Journal of pediatrics 2009;155(5):668–672. e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for Diabetes in Youth Study: Rationale, Findings, and Future Directions. Diabetes Care 2014;37(12):3336–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabelea D, D’agostino R, Mason C, et al. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia 2011;54(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabelea D, Pihoker C, Talton JW, et al. Etiological approach to characterization of diabetes type. Diabetes care 2011;34(7):1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000(314):1–27. [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics Series 11, Data from the national health survey 2002(246):1–190. [PubMed] [Google Scholar]
- 21.Mottl AK, Divers J, Dabelea D, et al. The dose–response effect of insulin sensitivity on albuminuria in children according to diabetes type. Pediatric Nephrology 2016;31(6):933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonifacio E, Yu L, Williams AK, et al. Harmonization of Glutamic Acid Decarboxylase and Islet Antigen-2 Autoantibody Assays for National Institute of Diabetes and Digestive and Kidney Diseases Consortia. Journal of Clinical Endocrinology Metabolism 2010;95(7):3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampasona V, Schlosser M, Mueller PW, et al. Diabetes antibody standardization program: first proficiency evaluation of assays for autoantibodies to zinc transporter 8. Clin Chem 2011;57(12):1693–1702. [DOI] [PubMed] [Google Scholar]
- 24.Bouvet Y, Bouissou F, Coulais Y, et al. GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatric nephrology 2006;21(9):1299–1306. [DOI] [PubMed] [Google Scholar]
- 25.Sharma AP, Yasin A, Garg AX, Filler G. Diagnostic accuracy of Cystatin C–based eGFR equations at different GFR levels in children. Clinical Journal of the American Society of Nephrology 2011:CJN. 10161110. [DOI] [PubMed]
- 26.Standards of medical care in diabetes−−2011. Diabetes Care 2011;34 Suppl 1:S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007;49(2 Suppl 2):S12–154. [DOI] [PubMed] [Google Scholar]
- 28.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes care 1995;18(2):245–250. [DOI] [PubMed] [Google Scholar]
- 29.Mottl AK, Kwon K- S, Mauer M, Mayer-Davis EJ, Hogan SL, Kshirsagar AV. Normoalbuminuric diabetic kidney disease in the US population. Journal of Diabetes and its Complications 2013;27(2):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin R, Widmer B, Prevost AT, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. Bmj 2008;336(7646):697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniels M, DuBose SN, Maahs DM, et al. Factors associated with microalbuminuria in 7,549 children and adolescents with type 1 diabetes in the T1D Exchange clinic registry. Diabetes Care 2013;36(9):2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Group TS. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes. Diabetes Care 2013;36(6):1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maahs DM, Snively BM, Bell RA, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes. Diabetes Care 2007;30(10):2593–2598. [DOI] [PubMed] [Google Scholar]
- 34.Salardi S, Balsamo C, Zucchini S, et al. High rate of regression from micro-macroalbuminuria to normoalbuminuria in children and adolescents with type 1 diabetes treated or not with enalapril. Diabetes care 2011;34(2):424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandt JR, Jacobs A, Raissy HH, et al. Orthostatic proteinuria and the spectrum of diurnal variability of urinary protein excretion in healthy children. Pediatric Nephrology 2010;25(6):1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim NH, Pavkov ME, Knowler WC, et al. Predictive value of albuminuria in American Indian youth with or without type 2 diabetes. Pediatrics 2010;125(4):e844–e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M. The early natural history of nephropathy in type 1 diabetes. Diabetes 2005;54(7):2164–2171. [DOI] [PubMed] [Google Scholar]
- 38.Perkins BA, Krolewski AS. Early nephropathy in type 1 diabetes: a new perspective on who will and who will not progress. Curr Diab Rep 2005;5(6):455–463. [DOI] [PubMed] [Google Scholar]
- 39.Lievre M, Marre M, Robert J, Charpentier G, Iannascoli F, Passa P. Cross-sectional study of care, socio-economic status and complications in young French patients with type 1 diabetes mellitus. Diabetes & metabolism 2005;31(1):41–46. [DOI] [PubMed] [Google Scholar]
- 40.Bjornstad P, Nehus E, Bacha F, et al. Insulin Sensitivity and Diabetic Kidney Disease in Children and Adolescents With Type 2 Diabetes: An Observational Analysis of Data From the TODAY Clinical Trial. American Journal of Kidney Diseases 2018;71(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha SK, Shaheen M, Rajavashisth TB, Pan D, Norris KC, Nicholas SB. Association of race/ethnicity, inflammation, and albuminuria in patients with diabetes and early chronic kidney disease. Diabetes Care 2014;37(4):1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. Journal of the American Society of Nephrology 2010:ASN. 2010070730. [DOI] [PMC free article] [PubMed]
- 43.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. Jama 2014;312(20):2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melsom T, Stefansson V, Schei J, et al. Association of increasing GFR with change in albuminuria in the general population. Clinical Journal of the American Society of Nephrology 2016;11(12):2186–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacIsaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Current opinion in nephrology and hypertension 2011;20(3):246–257. [DOI] [PubMed] [Google Scholar]
- 46.Takagi M, Babazono T, Uchigata Y. Differences in risk factors for the onset of albuminuria and decrease in glomerular filtration rate in people with type 2 diabetes mellitus: implications for the pathogenesis of diabetic kidney disease. Diabetic Medicine 2015;32(10):1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porrini E, Ruggenenti P, Mogensen CE, et al. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. The Lancet Diabetes & Endocrinology 2015;3(5):382–391. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds K, Liese AD, Anderson AM, et al. Prevalence of tobacco use and association between cardiometabolic risk factors and cigarette smoking in youth with type 1 or type 2 diabetes mellitus. The Journal of pediatrics 2011;158(4):594–601. e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waikar SS, Rebholz CM, Zheng Z, et al. Biological Variability of Estimated GFR and Albuminuria in CKD. American Journal of Kidney Diseases 2018. [DOI] [PMC free article] [PubMed]
- 50.Leong A, Ekinci EI, Nguyen C, et al. Long-term intra-individual variability of albuminuria in type 2 diabetes mellitus: implications for categorization of albumin excretion rate. BMC nephrology 2017;18(1):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


