Abstract
BACKGROUND:
Some studies suggest that higher body mass index is associated with increased susceptibility to bacterial vaginosis (BV), but results are conflicting.
METHODS:
Female sex workers aged 16–45 and participating in an open, prospective cohort study in Mombasa, Kenya between 2000 and 2014 were included in this analysis. Up to 2 years of follow-up were included per woman. Body mass index (BMI) was categorized as underweight (≤18.5), normal (18.5–24.9), overweight (25–29.9), and obese (≥30). Bacterial vaginosis was assessed using Nugent scores. Generalized estimating equations were used to estimate relative risks of the association between BMI and BV.
RESULTS:
At baseline, 32.1% (n=625) of 1,946 women had BV. Half of women were overweight (31.1%, n=606) or obese (20.1%, n=391). Participants contributed 14,319 follow-up visits. Adjusting for age, compared to women with normal BMI, overweight (adjusted relative risk [aRR] 0.91, 95%CI 0.81–1.02) and obese (aRR 0.82, 95%CI 0.71–0.94) women were at lower risk for BV (joint p-value=0.03).
CONCLUSIONS:
Obese women had a nearly 20% lower risk of BV compared to women with normal BMI. Potential mechanisms for this effect, including possible effects of diet, obesity-associated changes in the gut microbiome, and systemic estrogen levels, should be explored.
Keywords: Bacterial vaginosis, Body mass index, Lactobacillus
Short Summary:
A prospective cohort study of female sex workers in Mombasa, Kenya found that obese women had a nearly 20% lower risk of incident bacterial vaginosis compared to normal weight women.
BACKGROUND
Optimal vaginal microbiota is dominated by Lactobacillus species. In particular, L. crispatus and L. jensenii are most consistently associated with a healthy vaginal environment1. Bacterial vaginosis (BV), a diagnosis associated with vaginal microbiota disruption, is characterized by a reduction in Lactobacillus and replacement by a diverse bacterial community with a high concentration of anaerobic bacteria. Bacterial vaginosis is the most common vaginal infection and cause of vaginal discharge2. It is also associated with increased risk of acquiring sexually transmitted infections (STIs), HIV, pelvic inflammatory disease, and preterm birth2. Given the range of adverse reproductive consequences of BV, identification of novel risk factors may be helpful in identifying new targets for prevention.
Obesity is associated with chronic health conditions including diabetes and heart disease3. Limited research has been conducted on the relationship between body mass index (BMI) and disrupted vaginal microbiota, and results are conflicting. Three studies, one in pregnant adolescents, one in predominately African-American women in the US, and one in young South African women, found significant associations between higher BMI and vaginal microbiota disruption4–6. In contrast, in a general population study of US women and in the Vaginal Human Microbiome Project, higher BMI was not significantly associated with BV7,8.
Conflicting findings and limited prospective research examining the association between BMI and incident BV support the need for further research. This prospective analysis examined the association between BMI and incident BV using data from the Mombasa Cohort, a long-term open cohort study of Kenyan female sex workers (FSWs).
METHODS
Population
The Mombasa Cohort is a prospective, open-cohort study of FSWs initiated in 1993 to study risk factors for STI and HIV acquisition. Detailed procedures have been published9. HIV-seronegative and seropositive FSWs participating in the Mombasa Cohort between 2000, when height measurement began, and March 2014 were eligible for these analyses. The analysis population included participants ≤46 years old (proxy for menopause), and with ≥two follow-up visits with height, weight, and vaginal Gram stain data. The study was approved by the institutional review boards of Kenyatta National Hospital and the University of Washington. All participants provided written informed consent.
Procedures
Participants in the Mombasa Cohort complete a standardized face-to-face interview at enrollment and at monthly follow-up visits to ascertain demographic characteristics, sexual behaviors, and clinical symptoms. At each visit, women undergo a pelvic examination with collection of vaginal and cervical specimens for STI testing, and height and weight are measured. Syndromic management of STI symptoms was provided according to Kenyan national guidelines. Additional therapy was provided as needed, based on laboratory results available at a one-week follow-up appointment. Beginning in March 2004, HIV-seropositive women were provided with antiretroviral therapy (ART) per Kenyan national guidelines.
Vaginal swabs were collected for Gram stain, and scored to assess vaginal microbiota using Nugent score (0–3 normal, 4–6 intermediate microbiota, 7–10 BV)10. Bacterial vaginosis was also assessed using Amsel’s criteria, which require the presence of at least three of four clinical signs including: 1) clue cells on wet mount, 2) vaginal pH>4.5, 3) amine odor when vaginal fluid is exposed to 10% potassium hydroxide, and 4) abnormal vaginal discharge consistent with BV identified on clinical examination11. Vaginal specimens were inoculated onto Rogosa agar for detection of cultivable Lactobacillus; isolates were sub-cultured on tetramethylbenzidine (TMB) agar containing horseradish peroxidase to assess hydrogen peroxide (H2O2) production. Neisseria gonorrhoeae was diagnosed by culture of cervical specimens inoculated onto modified Thayer-Martin media. Beginning in 2006, the Aptima Combo-2 CT/NG Detection System (Hologic Corporation, San Diego, California) was utilized for N. gonorrhoeae and Chlamydia trachomatis detection. Trichomonas vaginalis was identified on wet mount microscopy and/or by the Aptima Trichomonas vaginalis assay (initiated in October 2010). Antibodies to herpes simplex virus type-2 (HSV-2) were detected by ELISA with an optical density index value threshold of ≥2.1 indicating positivity (HerpeSelect2 ELISA, Focus Diagnostics, Cypress, California)12. Due to the high HSV-2 prevalence in HIV-seropositive women in the cohort, HSV-2 testing was only conducted for HIV-seronegative participants. HIV-1 screening was conducted using an ELISA, with positive screens confirmed by a follow-up test13. Quantification of CD4 lymphocytes was performed every three months for HIV-seropositive participants (FACSCount, Becton Dickinson, San Jose, California).
Statistical methods
For these analyses, the baseline visit was each woman’s first visit with height, weight, and vaginal Gram stain data. The analysis included the first two years of follow-up data for each participant through March 2014. Participants who turned 46 during follow-up were administratively censored. For missing weight measurements, measurements from the prior monthly visit were carried forward. Only visits with Nugent score data were included. Visits associated with clinical trials assessing therapies to reduce recurrent vaginal infections were excluded14,15. Participant visits after trial completion were retained, because the intervention effect did not persist after cessation of the intervention16. Body mass index (kilograms/meters2) was categorized as underweight (≤18.5), normal (18.5–24.9), overweight (25–29.9), and obese (≥30).
Baseline characteristics were compared across BMI categories using X2 for categorical variables and the Kruskal-Wallis test to compare distributions of continuous variables. To examine the relationship between BMI and incident BV at follow-up visits, analyses were conducted using generalized estimating equations (GEE) specifying the log-link, Poisson family, independent correlation structure, a time offset (log of days between visits), robust standard errors, and clustering by participant to estimate relative risks (RR) and 95% confidence intervals (CIs). Body mass index, measured at each visit, was modeled as a time-varying, categorical exposure. Vaginal microbiota disruption was modeled in two ways: 1) BV (Nugent ≥7) vs No BV (Nugent 0–6), and 2) Abnormal vaginal microbiota (Nugent ≥4) vs Normal (Nugent 0–3). A sensitivity analysis was conducted using Amsel’s criteria to diagnose BV. Lastly, analyses were performed to determine whether BMI was associated with incident cultivable Lactobacillus, utilizing the model described above.
Age was included a priori in the adjusted models of the relationship between BMI and vaginal microbiota disruption, because increasing age is associated with both reductions in BV17 and increases in BMI. To determine which additional variables to include in the adjusted model, bivariate GEE regression was performed with potential confounders selected based on known associations between both BMI and BV including HIV status (seronegative, seropositive), ART status (HIV-seronegative, HIV-seropositive not taking ART, HIV-seropositive taking ART), CD4 count (HIV-seronegative, <200 cells/mm3, 200–350 cells/mm3, >350 cells/mm3), frequency of sex in last week (continuous), hormonal contraception (none/non-hormonal, implant, injectable, oral contraceptive pills), and smoking. Vaginal washing and HSV-2 serostatus have been associated with BV, but have not been independently associated with BMI, so were not assessed as potential confounders. A manual, forward, stepwise model building approach was used to evaluate potential confounders. None of these potential confounding factors changed the estimates of the associations between BMI and BV (Nugent ≥7) or abnormal vaginal microbiota (Nugent ≥4) by >10% (RR scale), so none were retained in the final adjusted models. Testing for interaction between BMI and hormonal contraceptive use (none/non-hormonal versus oral contraceptive pills/implant/injectable) was performed, setting the threshold for identifying important effect modification at p<0.10 for the interaction term.
A Kaplan-Meier plot was generated to assess whether time-to-clearance of first incident BV episode differed by BMI category. The start of an incident BV episode was defined as the midpoint between a visit with Nugent score 0–6 and a subsequent visit with Nugent score ≥7. Clearance was defined as the midpoint between the visit with the last Nugent score ≥7 and the subsequent visit with Nugent 0–6, censoring women who were BV-positive at their last study visit. Women with prevalent BV at baseline who cleared their prevalent episode and subsequently experienced incident BV were included in this analysis. A sensitivity analysis was conducted to assess clearance of first incident abnormal vaginal microbiota (Nugent ≥4 to Nugent 0–3).
RESULTS
Of the 1,946 women eligible for these analyses, 41.8% (813/1945) were HIV-seropositive at baseline. The median age of participants was 30 (interquartile range [IQR]: 26–35) (Table 1). The majority of women had vaginal microbiota disruption by Nugent score; 32.1% (n=625) had BV, 23.4% (n=456) had intermediate microbiota, and 44.5% (n=865) had a normal microbiota. Half of women were either overweight (31.1%, n=606) or obese (20.1%, n=391).
Table 1.
Baseline demographic, sexual risk behavior, clinical and contraception characteristics for 1,946 Kenyan female sex workers
| Characteristic | N | BMI Category N(%) or Median(IQR) |
||||
|---|---|---|---|---|---|---|
| Underweight (n=71) |
Normal (n=878) |
Overweight (n=606) |
Obese (n=391) |
Total (N=1946) |
||
| Demographic | ||||||
| Age | 1946 | 27 (24, 33) | 29 (25,34) | 31 (26,36) | 32 (28,36) | 30 (26,35) |
| Ever married | 1946 | 33 (46.5) | 488 (55.6) | 380 (62.7) | 261 (66.8) | 1162 (59.7) |
| Completed primary school (>8 years) | 1946 | 27 (38.0) | 354 (40.3) | 255 (42.1) | 149 (38.1) | 785 (40.3) |
| Smoker | 1942 | 17 (23.9) | 153 (17.5) | 72 (11.9) | 38 (9.7) | 280 (14.4) |
| Sexual Risk Behavior | ||||||
| Sexual abstinence in last week | 1946 | 12 (16.9) | 108 (12.3) | 72 (11.9) | 51 (13.0) | 243 (12.5) |
| Any condomless sex in last week | 1946 | 14 (19.7) | 267 (30.4) | 190 (31.4) | 123 (31.5) | 594 (30.5) |
| Frequency of sex in last weeka | 1703 | 2 (2,4) | 2 (1,3) | 2 (1,3) | 2 (1,3) | 2 (1, 3) |
| 100% condom use in last weeka | 1703 | 45 (76.3) | 503 (65.3) | 344 (64.4) | 217 (63.8) | 1109 (65.1) |
| Number of sex partners in last weeka | 1703 | 2 (1,3) | 1 (1,3) | 1 (1,2) | 1 (1,2) | 1 (1,2) |
| Vaginal washing in last week | 1944 | |||||
| None | 4 (5.6) | 94 (10.7) | 68 (11.2) | 45 (11.5) | 211 (10.9) | |
| Water only | 23 (32.4) | 220 (25.1) | 140 (23.1) | 93 (23.8) | 476 (24.5) | |
| Soap/Otherb | 44 (62.0) | 563 (64.2) | 397 (65.6) | 253 (64.7) | 1257 (64.7) | |
| Clinical | ||||||
| Vaginal microbiota | 1946 | |||||
| BV (Nugent ≥7) | 22 (31.0) | 294 (33.5) | 185 (30.5) | 124 (31.7) | 625 (32.1) | |
| Intermediate microbiota (Nugent 4–6) | 17 (23.9) | 200 (22.8) | 144 (23.8) | 95 (24.3) | 456 (23.4) | |
| Normal (Nugent 0–3) | 32 (45.1) | 384 (43.7) | 277 (45.7) | 172 (44.0) | 865 (44.5) | |
| HIV-seropositive | 1945 | 28 (39.4) | 370 (42.2) | 268 (44.2) | 147 (37.6) | 813 (41.8) |
| On antiretroviral therapyc | 813 | 12 (42.9) | 215 (58.1) | 152 (56.7) | 84 (57.1) | 463 (57.0) |
| CD4 count (cells/mm3)c,d | 782 | |||||
| <200 | 10 (35.7) | 79 (22.4) | 31 (13.2) | 15 (10.4) | 139 (17.7) | |
| 200–350 | 7 (25.0) | 115 (32.6) | 63 (24.5) | 29 (20.1) | 214 (27.4) | |
| >350 | 11 (39.3) | 150 (45.0) | 160 (62.3) | 100 (69.4) | 430 (55.0) | |
| HSV-2e | 1220 | 22 (46.8) | 341 (63.9) | 273 (73.8) | 220 (81.8) | 856 (70.2) |
| Trichomonas vaginalis | 1946 | 5 (7.0) | 48 (5.5) | 29 (4.8) | 26 (6.7) | 108 (5.6) |
| Neisseria gonorrhoeae | 1940 | 1 (1.5) | 17 (1.9) | 14 (2.3) | 7 (1.8) | 39 (2.0) |
| Chlamydia trachomatis | 223 | 1 (11.1) | 3 (2.9) | 0 (0.0) | 1 (1.9) | 5 (2.2) |
| Cervicitisf | 1924 | 0 (0.0) | 17 (2.0) | 14 (2.3) | 6 (1.6) | 37 (1.9) |
| Self-report of vaginal itching/burning in last week | 1512 | 6 (9.8) | 125 (18.3) | 72 (15.9) | 43 (13.7) | 246 (16.3) |
| Self-report of vaginal discharge in last week | 1512 | 8 (13.1) | 106 (15.5) | 55 (12.1) | 38 (12.1) | 207 (13.7) |
| Metronidazole prescribed at baseline visit | 1938 | 4 (5.7) | 42 (4.8) | 36 (6.0) | 17 (4.4) | 99 (5.1) |
| Contraception | ||||||
| Method of contraception | 1946 | |||||
| Noneg | 53 (74.7) | 548 (62.4) | 354 (58.4) | 215 (55.0) | 1170 (60.1) | |
| Oral contraceptive pills | 5 (7.0) | 50 (5.7) | 57 (9.4) | 23 (5.9) | 135 (6.9) | |
| Injectable | 10 (14.1) | 204 (23.2) | 157 (25.9) | 108 (27.6) | 479 (24.6) | |
| Intrauterine contraceptive device | 1 (1.4) | 13 (1.5) | 7 (1.2) | 13 (3.3) | 34 (1.8) | |
| Implant | 0 (0.0) | 46 (5.2) | 19 (3.1) | 17 (4.4) | 82 (4.2) | |
| Other | 2 (2.8) | 17 (1.9) | 12 (2.0) | 15 (3.8) | 46 (2.4) | |
Includes only women reporting sex in the last week. N reporting sex in last week by BMI category: Underweight = 59; Normal = 770; Overweight = 534; Obese = 340
Other includes detergent and antiseptics
Includes only HIV-seropositive women
CD4 lymphocyte count was measured after confirming the diagnosis of HIV infection. For some women, this was not confirmed until after the baseline visit for this analysis. As a result, the CD4 data presented here reflect baseline visit values for 224 HIV-seropositive participants and CD4 data from the visit after baseline for 558 HIV-seropositive participants who were diagnosed with HIV infection based on samples collected at their baseline visit. Data on CD4 lymphocyte count were not available at the baseline visit or the subsequent study visit for 31 HIV-seropositive participants.
HSV-2 testing was primarily conducted among HIV seronegative women due to high prevalence of HSV-2 among HIV seropositive women. Of the 102 HIV seropositive women with HSV-2 results at baseline, 100 were positive for HSV-2.
Defined as an average of ≥ 30 polymorphonuclear leukocytes in three high-power microscopic fields on a Gram-stained slide of cervical secretions
Includes condom use
Participants contributed 14,319 follow-up visits (2,210.6 person-years). The median number of visits per participant was 6 (IQR 3–12), and the median interval between visits was 33 days (IQR: 28–52). Neither median visit interval (p=0.25) nor median days between visits (p=0.10) differed by BMI category. Weight was missing for 5.9% (n=842) of follow-up visits, so measurements from the prior monthly visit were carried forward. Most participants’ BMI category did not change during follow-up (71.6%, 1,394/1,946), while 27.5% (535/1,946) changed one category, and 0.87% (17/1946) changed two categories.
Bacterial vaginosis (Nugent ≥7) was detected at 35.7% (5,805/16,265) of visits, and abnormal microbiota (Nugent ≥4) was detected at 56.6% (9,200/16,265) of visits. Seventy percent (1362/1,946) of participants had BV at one or more visits. Few visits with BV detection were accompanied by self-reported vaginal discharge (7.1%, 401/5,648) or metronidazole prescriptions (5.9%, 344/5,794). Bacterial vaginosis by Amsel’s criteria was detected at 20.6% (2,071/10,068) of visits with data available for all four criteria. Cultivable Lactobacillus was detected at 10.7% (1,743/16,235) of visits, and 42.9% (747/1,743) of isolates were H2O2-producing.
BMI and Vaginal Microbiota
During follow-up, BV was detected at 41.0% (226/551) of visits for underweight women, 37.5% (2,265/6,034) of visits for normal weight women, 35.2% (1,623/4,608) of visits for overweight women, and 34.1% (1,066/3,126) of visits for obese women. In unadjusted analyses, obese women were at significantly lower risk for incident BV (Nugent ≥7) compared to women with normal BMI (RR 0.84, 95%CI 0.73–0.96) (Table 2). This relationship was similar after adjustment for age (adjusted RR [aRR] 0.82, 95%CI 0.71–0.94). Compared to women with normal BMI, both overweight (aRR 0.93, 95%CI 0.85–1.01) and obese (aRR 0.84, 95%CI 0.76–0.93) women were also less likely to have abnormal vaginal microbiota (Nugent 4–10). The interaction term for BMI and hormonal contraception was not statistically significant (p=0.14).
Table 2.
Association Between Body Mass Index and Incident Bacterial Vaginosis, Cultivable Lactobacillus, and Hydrogen Peroxide Producing Lactobacillus
| Vaginal Microbiota | Unadjusted | Adjusteda | |||
|
n (%) of follow-up visits with BVb
|
RR (95%CI) | Joint P Value | RR (95% CI) | Joint P Value | |
| BV (Nugent ≥ 7 vs 0–6) | 0.07 | 0.03 | |||
| Underweight (<18.5) | 226 (41.0) | 1.02 (0.79, 1.32) | 1.01 (0.79, 1.31) | ||
| Normal (18.5–24.9) | 2,265 (37.5) | 1.0 | 1.0 | ||
| Overweight (25–29.9) | 1,623 (35.2) | 0.92 (0.82, 1.03) | 0.91 (0.81, 1.02) | ||
| Obese (≥30) | 1,066 (34.1) | 0.84 (0.73, 0.96) | 0.82 (0.71, 0.94) | ||
| Abnormal Microbiota (Nugent 4–10 vs 0–3) | 0.04 | 0.006 | |||
| Underweight (<18.5) | 341 (61.9) | 1.0 (0.82, 1.22) | 0.99 (0.82, 1.20) | ||
| Normal (18.5–24.9) | 3,494 (57.9) | 1.0 | 1.0 | ||
| Overweight (25–29.9) | 2,574 (55.9) | 0.95 (0.87, 1.03) | 0.93 (0.85, 1.01) | ||
| Obese (≥30) | 1,710 (54.7) | 0.87 (0.79, 0.96) | 0.84 (0.76, 0.93) | ||
| BV (Amsel’s Criteria)c | 0.48 | 0.23 | |||
| Underweight (<18.5) | 85 (23.6) | 1.06 (0.69, 1.63) | 1.05 (0.69, 1.60) | ||
| Normal (18.5–24.9) | 822 (21.1) | 1.0 | 1.0 | ||
| Overweight (25–29.9) | 1,623 (21.0) | 0.97 (0.81, 1.15) | 0.93 (0.78, 1.10) | ||
| Obese (≥30) | 365 (19.6) | 0.85 (0.68, 1.05) | 0.80 (0.65, 0.99) | ||
| Lactobacillus | Unadjusted | Adjusteda | |||
| n (%) of visits with Lactobacillusb | RR (95% CI) | Joint P Value | RR (95% CI) | Joint P Value | |
| Cultivable Lactobacillus | 0.58 | 0.55 | |||
| Underweight (<18.5) | 52 (9.5) | 0.90 (0.62, 1.29) | 0.90 (0.62, 1.29) | ||
| Normal (18.5–24.9) | 598 (9.9) | 1.0 | 1.0 | ||
| Overweight (25–29.9) | 512 (11.1) | 1.10 (0.93,1.31) | 1.11 (0.93, 1.32) | ||
| Obese (≥30) | 360 (11.5) | 1.07 (0.88, 1.30) | 1.08 (0.89, 1.31) | ||
| H2O2 producing Lactobacillusd | 0.91 | 0.92 | |||
| Underweight (<18.5) | 23 (4.2) | 0.87 (0.52, 1.48) | 0.87 (0.52, 1.47) | ||
| Normal (18.5–24.9) | 271 (4.5) | 1.0 | 1.0 | ||
| Overweight (25–29.9) | 211 (4.6) | 1.00 (0.79, 1.28) | 1.00 (0.78, 1.27) | ||
| Obese (≥30) | 162 (5.2) | 1.06 (0.81, 1.41) | 1.05 (0.80, 1.39) | ||
Adjusting for age at visit
Total number of follow-up visits by BMI category with Nugent score data available for analysis: Underweight = 551; Normal = 6,034; Overweight = 4,608; Obese = 3,126; Overall = 14,319
Total number of follow-up visits by BMI category with Amsel’s criteria available for analysis: Underweight = 360; Normal = 3,888; Overweight = 586; Obese = 1,957; Overall = 8,957. Assessment of amine odor when vaginal fluid was exposed to 10% potassium hydroxide was initiated in the Mombasa Cohort in 2003.
Comparing visits with detection of H2O2 producing Lactobacillus to visits with no Lactobacillus or non-H2O2 producing Lactobacillus.
In sensitivity analysis assessing whether the association between BMI and incident BV was consistent using Amsel’s criteria, obese women remained at a reduced risk for incident BV (aRR 0.80, 95%CI 0.65–0.99). Body mass index was not significantly associated with detection of cultivable Lactobacillus species by culture (Table 2).
Time-to-Clearance of BV
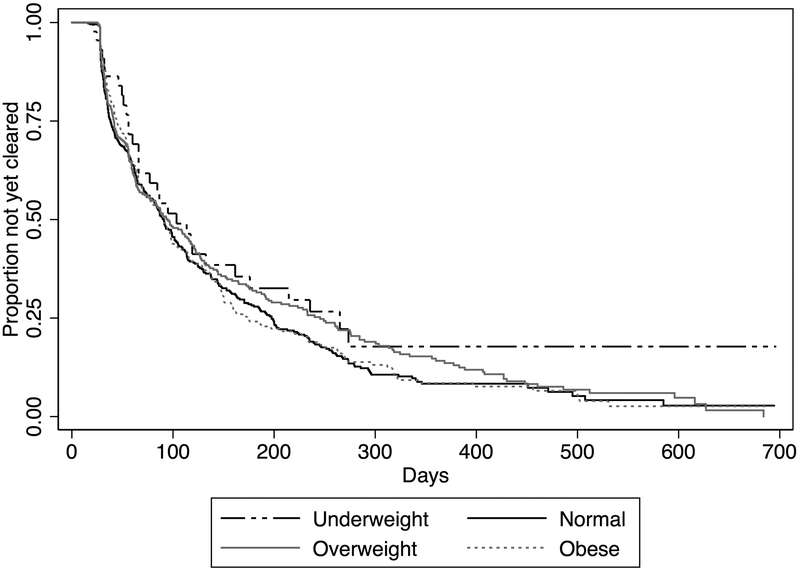
There were 1,068 first incident BV (Nugent ≥7) episodes. Only 4.3% (46/1,065) of women received a metronidazole prescription at the visit with BV detection; this did not differ by BMI category (p=0.82). The median time-to-clearance of BV was 91.5 days (95%CI: 85–99) and did not differ by BMI category (Figure 1, log rank p=0.28). Similarly, in the sensitivity analysis assessing time-to-clearance of 1,162 first incident abnormal vaginal microbiota (Nugent ≥4) episodes, median time-to-clearance did not differ by BMI category (log rank p=0.38).
Figure 1.
Days until clearance of 1,068 first incident bacterial vaginosis episodes by body mass index category – Of the 1,068 first incident BV episodes, 48 (4.5%) were in underweight women, 452 (42.3%) were in normal weight women, 345 were in overweight women (32.3%), and 114 (10.7%) in overweight women. The proportion of participants clearing their first incident BV episode within 30, 60, and 90 days was 10.7% (n=114), 33.7% (n=360), and 44.9% (n=479), respectively. Clearance rates within 30, 60, and 90 days did not differ by BMI category (30 days: p=0.31, 60 days: p=0.76, 90 days: p=0.85).
DISCUSSION
This large, prospective cohort study with frequent measurement of BMI and vaginal microbiota disruption is the first study to conclude that obese women may be at lower risk for BV. In contrast to prior studies finding a small increased likelihood of BV among women with higher BMI4,5, obese Kenyan FSWs had a nearly 20% lower risk of BV. Overweight women had a 10% reduction in BV that was of borderline significance, suggesting a dose-response effect.
Research assessing the association between BMI and BV is limited. Three studies found that higher BMI was associated with a slightly increased likelihood of BV4–6. Pregnant adolescents in the US with self-reported overweight or obese pre-pregnancy BMI were slightly more likely to be diagnosed with BV during pregnancy compared to adolescents with a normal or underweight pre-pregnancy BMI (aOR 1.16, 95%CI 1.04–1.30)4. In the US-based Longitudinal Study of Vaginal Flora cohort, including predominately African-American women, obese women had a higher likelihood of BV compared to women with a normal BMI (unadjusted OR: 1.3, 95%CI 1.2–1.8). Among 16–22 year-old, HIV-seronegative South African women, those with a higher BMI were more likely to have vaginal microbiota that was not Lactobacillus-dominant (aOR 1.2, 95%CI 1.0–1.3)5. Two of these studies were conducted in adolescents4,5, and may be less generalizable to all reproductive-aged women. In contrast, in an analysis of National Health and Nutrition Examination Survey data, higher BMI (continuous) was associated with prevalent BV in bivariate (p<0.0001, trend), but not in multivariate analysis adjusting for race/ethnicity, lifetime number of sex partners, education, and other characteristics7. Similarly, there was no association between higher BMI (continuous) and prevalent BV in a study comparing risk factors for vaginal microbiota disruption in African American women versus women of European descent in the Vaginal Human Microbiome Project (OR: 0.98, 95%CI 0.94–1.0)8. In the same study, BMI was not associated with the overall relative abundance of a group of pre-selected BV-associated bacteria including Gardnerella vaginalis, Leptotrichia/Sneathia, Prevotella, Atopobium, Megaspheara, Ureaplasma, Mycoplasma, Fusobacterium, BVAB1, BVAB2, BVAB3 (Mageeibacillus indolicus), and Mobiluncus, measured by broad-range 16S rRNA gene PCR and deep sequencing methods (adjusted multiple linear regression coefficient=-0.321, p=0.18)8. Some of these studies are limited by their cross-sectional designs7,8. In addition, the results of the study among pregnant adolescents may not be generalizable to non-pregnant women, as the vaginal microbiota becomes more dominated by Lactobacillus during pregnancy18. Variation in results could also be due to different approaches to assessing vaginal microbiota disruption. Two studies used clinical criteria to identify symptomatic BV4,8, two used Nugent score6,7, and two employed molecular methods5,8. Furthermore, because African American and African women have a higher prevalence of vaginal microbiota disruption1,19,20 and higher BMI21, compared to Caucasian women, inadequate control for important confounders like race/ethnicity could contribute to conflicting results.
In the study presented here, obese women were at lower risk for incident BV. A number of biological effects could explain this association. Menses is consistently associated with a higher rate of BV and with reductions in Lactobacilli1,2. Some overweight and obese women have ovulatory dysfunction22, which may lower their risk of incident BV. In addition, higher estrogen levels produced by adipose tissue may increase the glycogen content of vaginal epithelial cells22. Increased glycogen promotes Lactobacillus colonization and production of lactic acid, which could support a more optimal vaginal environment23. In this analysis, BMI was not associated with significantly increased detection of cultivable Lactobacillus species. However, given the low proportion of visits with cultivable Lactobacillus, power may have been insufficient to detect an association. In addition, Lactobacillus iners would not have been detected, since it does not grow on Rogosa agar24. Inability to detect L. iners may be particularly important, as increasing evidence suggests that African and African American women have more diverse vaginal bacterial communities and more frequent colonization by L. iners compared to Caucasian and Asian women1,19. Future work to disentangle possible biological mechanisms should consider speciating Lactobacillus and assessing estrogen levels.
An association between BMI and BV could also be explained by diet-driven or obesity-related changes in gut microbiota. A growing body of literature suggests that obesity is correlated with a less diverse gut microbiota which is, itself, associated with several chronic diseases3. Changes in the gut microbiota may be shared by the vaginal microbiota through translocation of bacteria, or shared host-immune characteristics25. Interestingly, while a decrease in microbial diversity generally reflects dysbiosis in the gut, a less diverse vaginal microbiota is considered to be optimal1. This study found reduced BV episodes among obese women, suggesting that the vaginal microbiota, like the gut microbiota, becomes less diverse with obesity. Potential shared drivers of reduced microbial diversity in the vagina and gut, including hormonal and immunologic effects as well as translocation of bacteria, are worthy of further consideration.
This study had a number of strengths. The longitudinal design allowed for monthly measurement of weight, combined with vaginal microbiota assessment by both Nugent score and Amsel’s criteria. In addition, Mombasa Cohort participants are a relatively homogenous group in terms of race/ethnicity, sexual activity, and socioeconomic status, introducing less potential bias from these confounders. This homogeneity is, in part, demonstrated by the lack of meaningful changes in the risk estimates when potential confounders were examined in the multivariable model building (ex: HIV status, frequency of sex). Finally, the large sample size and follow-up time provided significant power to detect an association between BMI and BV.
This analysis was also subject to a number of limitations. First, vaginal microbiota disruption diagnosed at consecutive visits may reflect persistence rather than recurrence, and we were unable to distinguish between them in this analysis. However, regardless of whether all BV episodes were incident, the analysis suggests that obese women had a lower burden of BV. Second, data were not collected on important comorbidities including diabetes and polycystic ovarian syndrome22, which may have reduced the ability to completely control for confounding factors. Third, disentangling the relationship between nutritional intake, obesity, gut microbiota, and vaginal microbiota is complex. This study did not incorporate dietary intake or gut microbiota assessment, so it was not possible to assess the effect of BMI independent of diet on BV6,26 nor consider the effect of the obesity-gut microbiota3. Fourth, given racial differences in the vaginal microbiota1,19, these results may not be generalizable to all populations. Lastly, while this observational study examined the temporal relationship between BMI and incident BV, it cannot prove that obesity is causally linked to lower BV incidence.
In summary, this is the first study to demonstrate that obese women may have a reduced incidence of BV compared to women with a normal BMI. This relationship will be important to consider when exploring other areas of women’s health where both BV and obesity can be associated with adverse outcomes, such as spontaneous pre-term birth2,27, postpartum endometritis and wound infections28,29, and infertility22,30. Determining whether BMI is causally associated with BV and elucidating the potential mechanism for reduced BV among obese women could provide insights into the pathogenesis of this common vaginal condition. Such insights could lead to novel approaches to reduce the prevalence, incidence, and recurrence of BV.
ACKNOWLEDGEMENTS
The authors acknowledge the support from the clinic, laboratory, and administrative staff and the partnerships with the Mombasa County and Coast Provincial General Hospital for their dedication. The authors thank the study participants whose commitment made this research possible. We are grateful to Dr. Julie Overbaugh (Fred Hutchinson Cancer Research Center; Seattle, WA) for her input on the manuscript and for her long-term support of the Mombasa Cohort through National Institutes of Health grant R37 AI38518.
Financial Support: This work was supported by grants from the National Institutes of Health (R37 AI38518, T32 AI07140 to EML, K24 HD88229 to RSM for mentoring). Infrastructure and logistical support for the Mombasa research site was provided by the University of Washington’s Center for AIDS Research (CFAR), a National Institutes of Health funded program (P30 AI027757) which is supported by the following research centers: National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, Eunice Kennedy Shriver National Institute of Child Health and Development, National Heart, Lung and Blood Institute, and National Center for Complementary and Integrative Health. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest/Disclosure statement: RSM receives research funding, paid to the University of Washington, from Hologic Corporation.
REFERENCES
- 1.van de Wijgert JHHM, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, et al. The vaginal microbiota: What have we learned after a decade of molecular characterization? PLoS One. 2014;9(8):e105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillier S, Marrazzo J, Holmes KK. Bacterial Vaginosis In: Holmes KK, Sparling P, Stamm W, Piot P, Wasserheit J, Corey L, et al. , editors. Sexually Transmitted Infections. 4th ed. New York, NY: McGraw Hill Companies; 2007. p. 737–68. [Google Scholar]
- 3.Duranti S, Ferrario C, van Sinderen D, Ventura M, Turroni F. Obesity and microbiota: An example of an intricate relationship. Genes Nutr. 2017;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akoh CC, Pressman EK, Cooper E, Queenan RA, Pillittere J, O’Brien KO. Prevalence and risk factors for infections in a pregnant adolescent population. J Pediatr Adolesc Gynecol. 2017;30(1):71–5. [DOI] [PubMed] [Google Scholar]
- 5.Lennard K, Dabee S, Barnabas S, Havyarimana E, Blakney A, Jaumdally SZ, et al. Microbiome composition and function predict genital tract inflammation and persistent bacterial vaginosis in adolescent South African women. Infect Immun. 2018;86(1):e00410–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu K, Goldenberg RL, et al. Dietary intake of selected nutrients affects bacterial vaginosis in women. J Nutr. 2007;137(9):2128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004: associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007. November;34(11):864–9. [DOI] [PubMed] [Google Scholar]
- 8.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology. 2014;160:2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin HL, Nyange PM, Richardson BA, Lavreys L, Mandaliya K, Jackson DJ, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of Human Immunodeficiency Virus Type 1. J Infect Dis. 1998;178(4):1053–9. [DOI] [PubMed] [Google Scholar]
- 10.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amsel R, Totten P, Spiegel C, Chen K, Eschenbach D, Holmes K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. [DOI] [PubMed] [Google Scholar]
- 12.Mujugira A, Morrow RA, Celum C, Lingappa J, Delany-Moretlwe S, Fife KH, et al. Performance of the Focus HerpeSelect-2 EIA for the detection of herpes simplex virus type 2 antibodies in seven African countries. Sex Transm Infect. 2011;87(3):238–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClelland RS, Richardson BA, Cherutich P, John-Stewart G, Miregwa B, Odem-davis K, et al. Impact of community antiretroviral therapy coverage on HIV incidence in Kenyan female sex workers: A fifteen year prospective cohort study. AIDS. 2015;29(17):2279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClelland RS, Richardson BA, Hassan WM, Chohan V, Lavreys L, Mandaliya K, et al. Improving vaginal health in women at risk for HIV-1: Results of a randomized trial. J Infect Dis. 2008;197(10):1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClelland RS, Balkus JE, Lee J, Anzala O, Kimani J, Schwebke J, et al. Randomized trial of periodic presumptive treatment with high-dose intravaginal metronidazole and miconazole to prevent vaginal infections in HIV-negative women. J Infect Dis. 2015;211(12):1875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkus JE, Jaoko W, Mandaliya K, Richardson BA, Masese L, Gitau R, et al. The posttrial effect of oral periodic presumptive treatment for vaginal infections on the incidence of bacterial vaginosis and Lactobacillus colonization. Sex Transm Dis. 2012;39(5):361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClelland R, Richardson B, Graham S, Masese L, Gitau R, Lavreys L, et al. A prospective study of risk factors for bacterial vaginosis in HIV-1-seronegative African women. Sex Transm Dis. 2008;35(6):617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci. 2011;108(Supplement 1):4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: A systematic review. Am J Obstet Gynecol. 2013;209(6):505–23. [DOI] [PubMed] [Google Scholar]
- 21.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. [DOI] [PubMed] [Google Scholar]
- 22.Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia. PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 23.Cruickshank R The conversion of the glycogen of the vagina into lactic acid. J Pathol. 1934;39(1):213–9. [Google Scholar]
- 24.Falsen E, Pascual C, Sjbden B, Ohlel M, Collins MD. Phenotypic and phylogenetic characterization of a novel Lactobacillus species from human sources: description of Lactobacillus iners sp. nov. Int J Syst Bacteriol. 1999;49:217–21. [DOI] [PubMed] [Google Scholar]
- 25.Marrazzo JM, Fiedler TL, Srinivasan S, Thomas KK, Liu C, Ko D, et al. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis. 2012;205(10):1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thoma ME, Klebanoff MA, Rovner AJ, Nansel TR, Neggers Y, Andrews WW, et al. Bacterial vaginosis is associated with variation in dietary indices. J Nutr. 2011;141(9):1698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: Systematic review and meta-analyses. BMJ. 2010;341:c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smid M, Kearney M, Stamilio D. Extreme obesity and post-cesarean wound complications in the MFMU cesarean registry. Am J Perinatol. 2015;32(14):1336–41. [DOI] [PubMed] [Google Scholar]
- 29.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obs Gynecol. 1990;75(1):52–8. [PubMed] [Google Scholar]
- 30.van Oostrum N, De Sutter P, Meys J, Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod. 2013;28(7):1809–15. [DOI] [PubMed] [Google Scholar]