Abstract
Objective
The development of abdominal aortic aneurysm (AAA) involves extensive extracellular matrix remodeling, leading to aortic wall weakening. This process is mediated by proteases including cysteinyl cathepsins. Cystatins are their endogenous inhibitors. This study tested whether plasma cystatin B levels in AAA patients differed from those of healthy controls.
Methods
Plasma samples from AAA and age-matched controls were selected from the Viborg Vascular (VIVA) screening trial for AAA. ELISA determined plasma cystatin B. T-test, logistic regression, Pearson’s correlation, and Cox regression tested whether plasma cystatin B correlates with AAA size and growth rate, or serves as a marker for AAA.
Results
Plasma cystatin B levels were significantly higher in AAA patients than in controls (P<0.0001). Logistic regression analysis showed that cystatin B tertile at baseline associated with the presence of AAA before (odds ratio [OR]=1.656, P<0.0001) and after adjustment for PAD, COPD, and previous ischemic events (OR=1.526, P<0.0001). T-test showed significant association of cystatin B with peripheral aortic disease (PAD) at screening, hospital diagnosis of chronic obstructive pulmonary disease (COPD), previous atherosclerotic events, and use of low doses of aspirin. Pearson’s correlation test showed positive and significant associations between cystatin B and AAA size (r=0.15, P<0.001). Cox regression test showed that plasma cystatin B tertile at baseline associated with later AAA surgical repair before (hazard ratio [HR]=1.387, P<0.0001) and after adjustment for PAD, COPD, previous ischemic event, and maximal infrarenal aortic diameter (HR=1.523, P<0.0001).
Conclusion
In contrast to prior studies that showed that cystatin C associates negatively with AAA development, this study demonstrated a positive association of cystatin B with AAA sizes and associations of cystatin B tertile at baseline with AAA presence and need for later surgical repair. It is possible that these two cystatins inhibit cathepsin activity and participate in AAA with different mechanisms.
Keywords: cystatin B, cystatin C, abdominal aortic aneurysm, VIVA screening trial
INTRODUCTION
Cysteinyl cathepsins participate importantly in the pathogenesis of abdominal aortic aneurysm (AAA). Immunohistochemical analysis demonstrated elevated expression of cathepsins B, K, L, and S in macrophages, lymphocytes, luminal and microvessel endothelial cells (ECs), and smooth muscle cells (SMCs) in human AAA lesions.1–4 Yet, their dominant endogenous inhibitor cystatin C is relatively deficient.1,4 Human plasma cystatin C levels correlate negatively with AAA size and annual expansion rate,5,6 and such association persists after adjusting for renal function, smoking, diastolic blood pressure, C-reactive protein, age, and AAA size.6 In atherosclerosis-prone apolipoprotein E (ApoE)-deficient Apoe−/− mice, cystatin C-deficiency enhanced diet-induced atherosclerosis, associated thoracic and abdominal aorta dilation, and angiotensin-II perfusion-induced AAA.7,8
Similar to cystatin C, cystatin B (also called stefin B) together with cystatin A (or stefin A) is also a single-chain small molecule that forms complexes with cysteinyl cathepsins.9 While cystatin A is expressed in immune follicular dendritic cells,10 cystatin B is more widely expressed in the cytoplasma of most human cells. There is an increased expression of cystatin B in a variety of human malignant tumors, in lipopolysaccharide (LPS)-activated human blood monocytes, and in interferon-γ (IFN-γ)-induced mouse macrophages.11,12 Cystatin B-deficiency in mice or loss-of-function mutation in humans results in neurological dysfunction as a form of epilepsy.13,14 Relative to cystatin C, many fewer studies have been done to test the potential involvement of cystatin B in cardiovascular diseases (CVD). From 389 patients who developed coronary events and 409 age- and sex-matched controls from the Malmo Diet and Cancer cardiovascular cohort, plasma cathepsin L (CatL) and cystatin B levels were significantly higher in patients with coronary events, and associated with prevalent diabetes. The hazardous ratio (HR) for the incident of coronary events comparing the highest and lowest tertiles of cystatin B was 1.29 (95% CI: 1.01–1.57) after adjusting for age, sex, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride, body mass index (BMI), hypertension, and glucose, although such association becomes insignificant after additional adjustment for smoking.15 This study contradicts to most of studies from cystatin C, which plays a protective role in CVD. It remains unknown whether the expression and activity of cystatin B also differ in AAA patients or differ from cystatin C.1,4–8 Analysis of plasma cystatin B in AAA patients may help understand whether and how this inhibitor is involved in human AAA and design the next phase of studies.
In this study, we measured plasma cystatin B levels from 551 men with AAA and 198 age-matched healthy controls from the Viborg Vascular (VIVA) screening trial for AAA16–18 and tested whether plasma cystatin B associates with AAA size, growth rate, presence of AAA, or need for later surgical repair of this fatal human aortic disease.
MATERIALS AND METHODS
The VIVA trial background
The VIVA screening trial is a randomized, clinically controlled study designed to evaluate the benefits of vascular screening and modern vascular prophylaxis in a population of 50,000 men aged 65–74 years.16 A combined screening program for AAA, peripheral artery disease (PAD), and hypertension was offered at local hospitals. Participants with positive test results were offered secondary prophylaxis and/or referred to their general practitioner. The program set-up included decentralized screening by three mobile teams at 14 venues. Enrollment started October 2008 and ended January 2011. Diagnostic criteria included an aortic diameter of at least 30 mm for AAA, ankle brachial blood pressure index (ABI) below 0.9 or above 1.4 for PAD, and blood pressure above 160/100 mmHg for hypertension.17 Chronic obstructive pulmonary disease (COPD) was defined by hospital-recorded diagnosis from the national patient registry, and familial disposition to AAA was defined as a first-degree sibling with AAA. AAA sizes greater than 50 mm were referred for vascular surgical evaluation, and those below were invited annually to a control scan to check for expansion. After one year, cases with PAD were also invited for surveillance to check for ABI and optimize preventive actions. Controls were chosen as consecutive, healthy, and age-matched male attenders. Plasma and DNA samples were taken from cases with AAA, PAD, and 200 healthy controls at baseline for biobanking. The nationwide registry-based follow-ups were performed after 5 years.18 The trial is approved by the scientific ethical committee of the Mid region of Denmark (M20080018) and registered in the Clinical trials register (NCT00662480). The Brigham and Women’s Hospital Human Research Committee approved the use of de-coded patient information (2010P001930/BWH).
Designs and outcome measures
A case-control design using cases of AAA and healthy controls was used to evaluate cystatin B association with AAA. In addition, a prospective cohort design was used to investigate the potential association between cystatin B levels and annual aneurysm growth rate and need for surgical repair.
Patients
Overall, 18,749 men (uptake 74.7%) attended the screening. An AAA was diagnosed in 615 cases (3.3% [95% CI: 3.0% to 3.6%]), PAD in 2,043 (10.9% [95% CI: 10.5% to 11.0%]), and potential undiagnosed hypertension in 1,969 (10.5% [95% CI: 10.0% to 10.9%]).17 Lipid-lowering and/or antiplatelet treatment was initiated among 34.8% of all participants. Five-year follow-up data were available concerning all outcome variables.18
Plasma cystatin B measurement
Plasma samples from 551 AAA patients and 198 age-matched controls were used.5,19,20 Plasma cystatin B levels were determined blindly using the human cystatin B ELISA Kit from MyBioSource (Cat# MBS761966, San Diego, CA), according to the manufacturer’s instructions. This kit was based on a sandwich enzyme-linked, immune-sorbent assay technology. The detection range was 0.156–10 ng/ml. Plasma samples were diluted by 1:200 (i.e. 5 (μl of sample into 995 (μl of sample/standard dilution buffer). The standard, plasma sample, and biotin-conjugated detection antibody were added to each well subsequently. The absorbance was read in a microplate reader at a 450-nm wavelength, and the cystatin B concentrations were calculated based on the standard curve.
Statistical analyses
Binary responses were coded 0=no and 1= yes. Normality was tested using Shapiro-Wilks test, q-q plots, and histograms. Mean is given with standard deviation (SD), and differences between cases and controls in exposures of interest were assessed by student’s t-test. Pearsson’s correlation coefficient was used to study the association between cystatin B and maximal aortic diameter, lowest ankle brachial index, and aneurysmal growth rate. AAA growth rates were calculated based upon the ultrasound scans from the baseline and annual follow-up visits using individual linear regression between sizes and time. Logistic regression analysis was used to identify whether the tertiles of circulating cystatin B levels was independently associated with AAA or PAD compared to the controls adjusted for potential confounders identified in the univariate analyses by a p-value below 0.10. Multiple linear regression analyses was used to identify whether the exposure of interest was independently associated with maximal aortic diameter adjusted for potential confounders identified in the univariate analyses by a p-value below 0.10. Finally, we constructed a multivariate Cox proportional hazard model to study whether the tertile level of cystatin B at baseline associated with needing surgical repair or death. The model was adjusted for potential confounders identified in the univariate analyses by a p-value below 0.10. The residuals of the regression analyses were plotted in histograms and q-q plots to test for normal distribution. The statistical tests were defined in priori. SPSS 21.0 and STATA/SE 13.1 for windows were used as statistical package.
Power calculation
When the sampling occurred, the hypothesis about an association between cystatin B and AAA had not yet been formulated. However, based upon our previous study,17 we assumed if the mean of cystatin B is 0.83 and SD is 0.103,21 the smallest detectable difference between AAA and controls is ±0.037 corresponding to below 5% of the mean at 1% significance level and 99% power, suggesting a very robust study concerning the comparison between controls and AAA. We could not find any reasonable assumptions to perform a power calculation concerning aneurysmal growth rates and need for later AAA repair.
RESULTS
VIVA screening trial background
From the 18,749 men who attended the VIVA screening trial, including 615 men with AAA, the proportion of patients with familiar disposition to AAA, current smoking, hypertension, previous stroke, previous acute myocardial infarction (AMI), PAD by screening, previous atherosclerotic events, use of low-dose aspirin, and use of statin were all significantly higher in patients with AAA than those without AAA. The proportions of having hospital-diagnosed COPD and use of glucocorticoid were also higher in AAA patients than in those without AAA. As expected, the proportions of patients with diabetes mellitus were significantly lower in AAA patients than in those without AAA (Table 1).
Table 1.
Expected differences in clinical characteristics between AAA patients and controls.
| Clinical characteristics | No AAA | AAA | P | ||
|---|---|---|---|---|---|
| N* | Proportion | N* | Proportion | ||
| Familial disposition to AAA | 18074 | 0.024 | 615 | 0.037 | 0.045 |
| Current smoking | 18042 | 0.020 | 615 | 0.413 | 0.000 |
| Diabetes mellitus | 18041 | 0.109 | 615 | 0.110 | 0.905 |
| Hypertension | 18001 | 0.421 | 615 | 0.547 | 0.000 |
| Previous stroke | 18074 | 0.024 | 615 | 0.037 | 0.045 |
| Previous acute myocardial infarction | 18074 | 0.025 | 615 | 0.063 | 0.000 |
| PAD by screening | 18041 | 0.104 | 613 | 0.271 | 0.004 |
| COPD | 18074 | 0.024 | 615 | 0.042 | 0.059 |
| Previous atherosclerotic event | 18074 | 0.127 | 615 | 0.225 | 0.000 |
| Use of low dose aspirin | 17599 | 0.323 | 613 | 0.507 | 0.000 |
| Use of statins | 17599 | 0.350 | 613 | 0.541 | 0.000 |
| Use of glucocorticoid | 16961 | 0.062 | 613 | 0.080 | 0.077 |
*Some patients (No AAA and AAA) had missing information for the listed clinical characteristics.
Association of plasma cystatin B tertile at baseline with the presence of AAA
Crude logistic regression analysis showed that the presence of AAA increased by 65.6% per tertile of plasma cystatin B at baseline (unadjusted odds ratio [OR]=1.656 [95% C.I.: 1.341; 2.045], P<0.001). After adjusting for PAD at screening, outpatient or hospital-recorded COPD, and previous schemic event, the presence of AAA still increased by 52.6% per tertile of plasma cystatin B at baseline (adjusted OR=1.526 [95% C.I.: 1.224; 1.901], P<0.001) (Table 2).
Table 2.
Crude and adjusted logistic regression analyses of cystatin B tertile at baseline and associations with the presence of AAA.
| Unadjusted | Odds Ratio | Std. Err. | P Value | 95% C.I. | |
|---|---|---|---|---|---|
| Cystatin B tertiles | 1.656 | 0.178 | 0.000 | 1.341 | 2.045 |
| Constant | 1.439 | 0.181 | 0.004 | 1.125 | 1.841 |
| Adjusted | Odds Ratio | Std. Err. | P Value | 95% C.I. | |
|---|---|---|---|---|---|
| Cystatin B tertiles | 1.526 | 0.171 | 0.000 | 1.224 | 1.901 |
| PAD at screening | 7.066 | 2.686 | 0.000 | 3.354 | 14.885 |
| Outpatient or hospital-recorded COPD | 3.603 | 2.753 | 0.093 | .806 | 16.111 |
| Previous ischemic event | 2.046 | 0.549 | 0.008 | 1.208 | 3.463 |
| Constant | 1.066 | 0.146 | 0.642 | .815 | 1.393 |
Plasma cystatin B level associates with AAA size
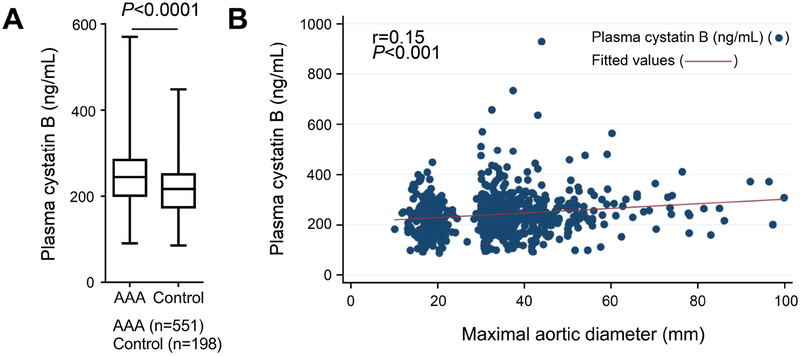
The plasma cystatin B levels were significantly higher in patients with AAA than those in age-matched controls (251.6±90.2 ng/mL vs. 220.3±64.7 ng/mL, mean±SD, P=2.49E-8) (Figure 1A). Among the dichotomous clinical characteristics, a t-test showed that plasma cystatin B levels did not associate with familiar disposition to AAA, current smoking, hypertension, previous stroke, previous AMI, use of statin, and use of glucocorticoid. Yet, plasma cystatin B levels associated significantly with PAD by screening, hospital-diagnosed COPD, previous atherosclerotic events, and use of low dose aspirin (Table 3). From the continuous clinical variables, Pearson’s correlation test showed that plasma cystatin B associated with only maximal infrarenal aortic diameter (r=0.15, P<0.001) (Figure 1B), but not with age, BMI, systolic and diastolic blood pressures, lowest ABI, and AAA growth rate (Table 3).
Figure 1.
Increased plasma cystatin B level in human AAA patients correlates with AAA sizes. A. ELISA revealed higher plasma cystatin B level in AAA patients (n=551) than that in age-matched controls (n=198). Data are mean±SD, P=2.49E-8. B. Pearson’s correlation analysis of plasma cystatin B versus maximal aortic diameters in mm.
Table 3.
Association between plasma cystatin B and dichotomous clinical characteristics (t-test) and continuous clinical variables (Pearson’s correlation).
| Dichotomous clinical characteristics | No | Yes | P | ||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean SD | ||
| Familial disposition to AAA | 617 | 241.8 (85.7) | 37 | 234.8 (61.4) | 0.645 |
| Current smoking | 469 | 238.0 (83.2) | 228 | 248.3 (86.3) | 0.236 |
| Diabetes mellitus | 579 | 240.4 (85.1) | 77 | 250.6 (79.0) | 0.227 |
| Hypertension | 329 | 236.5 (80.1) | 324 | 247.3 (88.5) | 0.219 |
| Previous stroke | 637 | 241.2 (85.2) | 20 | 253.6 (52.0) | 0.175 |
| Previous acute myocardial infarction | 625 | 242.1 (85.2) | 32 | 232.0 (65.8) | 0.837 |
| PAD at screening | 528 | 237.5 (84.3) | 123 | 256.8 (82.5) | 0.004 |
| COPD | 636 | 240.7 (84.3) | 21 | 267.9 (83.5) | 0.059 |
| Previous atherosclerotic event | 536 | 239.0 (83.6) | 121 | 253.3 (87.2) | 0.049 |
| Use of low dose aspirin | 392 | 233.0 (70.1) | 260 | 253.9 (101.6) | 0.031 |
| Use of statins | 345 | 236.5 (76.4) | 306 | 246.9 (93.0) | 0.300 |
| Use of glucocorticoid | 589 | 239.1 (80.5) | 52 | 252.5 (76.5) | 0.157 |
| Continuous clinical variables | Age | BMI | Systolic blood pressure | Diastolic blood pressure | Aorta size | Lowest ABI | AAA growth rate |
|---|---|---|---|---|---|---|---|
| R | 0.06 | 0.01 | −0.02 | −0.02 | 0.15 | −0.12 | −0.02 |
| P-value | 0.12 | 0.84 | 0.99 | 0.99 | <0.001 | 0.10 | 0.11 |
Association of plasma cystatin B tertile at baseline with the need for late surgical repair of AAA
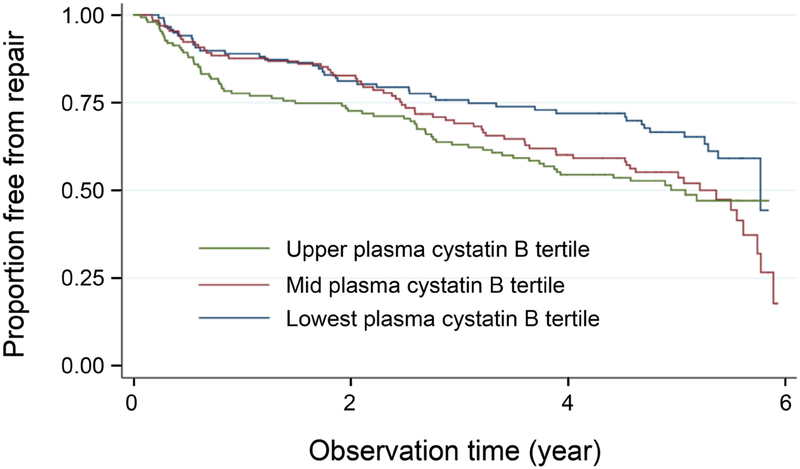
Crude Cox regression analysis showed that after a median follow-up of 4.85 years, the need for surgical repair of AAA increased by 38.7% per tertile of plasma cystatin B at baseline (unadjusted hazard ratio [HR]=1.387 [95% C.I.: 1.157, 1.662], P<0.001). After adjusting for outpatient or hospital-recorded COPD, previous schemic event, PAD at screening, and maximal infrarenal aortic diameter, the need for surgical repair of AAA increaed by 52.3% per tertile of plasma cystatin B at baseline (adjusted HR=1.523 [95% C.I.: 1.251, 1.854], P<0.001) (Figure 2 and Table 4).
Figure 2.
Cox regression analyses of cystatin B tertiles for proportion of free from late surgical repairs of AAA over the follow-up years. Crude HR=1.387 [95% C.I.: 1.157, 1.662], P<0.001. Adjusted HR=1.523 [95% C.I.: 1.251, 1.854], P<0.001.
Table 4.
Crude and adjusted Cox regression analyses of cystatin B tertile at baseline and associations with the need for later surgical repair of AAA.
| Unadjusted | Hazard Ratio | Std. Err. | P value | 95% C.I. | |
|---|---|---|---|---|---|
| Cystatin B tertiles | 1.387 | 0.128 | 0.000 | 1.157 | 1.662 |
| Adjusted | Hazard Ratio | Std. Err. | P value | 95% C.I. | |
|---|---|---|---|---|---|
| Cystatin B tertiles | 1.523 | 0.153 | 0.000 | 1.251 | 1.854 |
| Outpatient or hospital-recorded COPD | 1.455 | 0.504 | 0.279 | 0.738 | 2.868 |
| Previous ischemic event | 0.951 | 0.191 | 0.802 | 0.642 | 1.409 |
| PAD at screening | 0.723 | 0.141 | 0.097 | 0.493 | 1.061 |
| Maximal infrarenal aortic diameter | 1.082 | 0.005 | 0.000 | 1.071 | 1.092 |
DISCUSSION
The presented case-control and cohort studies demonstrated that the circulating level of cystatin B and its tertile at baseline associated with the presence and natural history of AAA (AAA size change and need for later repair during follow up). Selection bias seems unlikely, as the study group included attendees in a population-based screening trial with a relative high attendance rate (74.7%).18 Information bias seems also unlikely, as the ultrasound-based measurement of aorta was performed by a standardised and validated method showing high precision,22 and information of the need for later AAA repair was based upon nationwide registry data, where all AAA repair procedures were recorded due to law and reimbursement.23 Consequently, all had follow-up without missing data. The analyses were adjusted for known potential risk factors for AAA and the progression of AAA, yet by nature there will always be a risk of residual confounding. These conditions and the fact that the analyses were performed with a power of 90% at a 5% significance level make the novel finding of cystatin B as associated with AAA quite robust, but replication and mechanistic studies are needed for a final conclusion. Observations from this study differ from what were reported regarding cystatin C in AAA.1,4–6 Using the same samples, we reported negative correlations between plasma cystatin C with AAA size and with AAA growth rate.5,6 AAA growth has never been linear. Because the plasma samples were collected at registration, it is always possible that the correlation between plasma cystatin B and AAA size may change over time during the course of AAA growth and rupture. Plasma cystatin B levels in patients with fast-growing AAA may differ from those with relatively slow-growing or stablized AAA at follow-up years, even their measurments at registration may be comparable. Yet, our median follow-up of 4.85 years has not allowed us to see such differences.
A direct role of cystatin B in AAA or in other CVD has never been tested. Only limited information remains available regarding cystatin B in CVD. Plasma cystatin B levels have correlated with coronary events15 and body adiposity.24 In a community-based cohort of 170 8–11 year-old children, plasma cystatin B correlated positively with total body fat, abdominal fat, and pulse pressure, but negatively with maximal oxygen uptake, all of which are surrogate markers for CVD.24 However, plasma cystatin B has been broadly tested in cancer patients with mixed conclusions depending on the type of cancer. In patients with epithelial ovarian cancer,25–27 the bladder cancer transitional cell carcinoma,28 and laryngeal carcinoma,29 tissue cystatin B was overexpressed and correlated with tumor burden and disease recurrence. In patients with colorectal cancer, plasma cystatin B level correlated with survival. Patients with high levels of cystatin B had high risk of death.30 Yet, in human lung cancer31 and squamous cell carcinoma of the head and neck,32 tissue cystatin B correlated inversely with tumor burden, although it showed no influence on the probability of patient survival. One early study showed relatively higher prevalence of breast, colon, gastric, lung, lymphoma, prostate, renal, and urinary cancers in 298 patients with AAA than in 151 patients with atherosclerotic occlusive disease (P<0.014).33 A more recent study showed that patients with AAA had increased risk of developing lung and other cancers.34 There is no report showing that patients with one type of cancer a had a higher risk of AAA than those with other type of cancers. Therefore, it remains uncertain whether cystatin B links the risk between cancers and AAA.
Several major cathepsins, including cathepsins S, K, L, and C play essential roles in experimental AAA.35 Increased cystatin B in AAA subjects might implicate a protective mechanism to minimize the damages from these cathepsins. Yet we do not know the source or the consequence of increased plasma cystatin B in AAA patients. It is possible that increased plasma cystatin B represents a mechanism of aortic tissue protection. Besides extracellular matrix protein degradation, cathepsins target multiple subcellular compartments. This hypothesis is supported by the finding that upon LPS stimulation cystatin B targets the mitochondria in macrophages. Macrophages from cystatin B-deficient mice showed increased amounts of caspase-1 and −11 for IL-1β processing due to increased mitochondrial membrane destabilization and superoxide generation.36 Therefore, high levels of cystatin B in AAA patient plasma and possibly in AAA lesions may reduce IL-1β processing, thereby controlling lesion and systemic inflammation. Such speculation requires detailed studies at the molecular and cellular levels.
Together, this study reported an inverse association of plasma cystatin B with AAA size, and also demonstrated the associations between cystatin B tertile at baseline with the presence of human AAA and the need for future surgical repair. As we have recently summarized, cathepsins may play different roles in various subcellular compartments, such as lysosomes/endosomes, extracellular milieu, cell membrane, and nucleus.35 Cystatins C and cystatin B may target cathepsins at different locations, thereby exerting different activities towards the development of AAA. All these hypotheses can be important topics in general cathepsin biology and AAA pathobiology, and merit future investigation.
What this paper adds.
Prior studies demonstrated cystatin C-deficiency in human abdominal aortic aneurysm (AAA) lesions and negative correlations between plasma cystatin C and human AAA size or growth rate. Both cystatin C and cystatin B are endogenous inhibitors of cysteinyl cathepsins, but this study revealed a positive association of plasma cystatin B with AAA size. This study also revealed associations of plasma cystatin B tertile level at baseline with AAA presence and the need for later surgical repair. Different associations of cystatin C and cystatin B with AAA size suggest their possible mechanistic differences in regulating cathepsin activity during the development of human AAA.
ACKNOWLEDGEMENTS
This study is supported by awards from the American Heart Association (17P0ST33670564 to C.-L.L.), the National Natural Science Foundation of China (81570274 to J.Z.), University-College Joint Cultivation Fund of Zhengzhou University, (2016_BSTDJJ-19 to J.Z.), and the National Institute of Health [HL123568 and HL60942 to G.-P.S.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohoefer F, Reeps C, Lipp C, Rudelius M, Zimmermann A, Ockert S, et al. Histopathological analysis of cellular localization of cathepsins in abdominal aortic aneurysm wall. Int J Exp Pathol. 2012;93:252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohoefer F, Reeps C, Lipp C, Rudelius M, Haertl F, Matevossian E, et al. Quantitative expression and localization of cysteine and aspartic proteases in human abdominal aortic aneurysms. Exp Mol Med. 2014;46:e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abisi S, Burnand KG, Waltham M, Humphries J, Taylor PR, Smith A. Cysteine protease activity in the wall of abdominal aortic aneurysms. J Vasc Surg. 2007;46:1260–6. [DOI] [PubMed] [Google Scholar]
- 5.Lv BJ, Lindholt JS, Cheng X, Wang J, Shi GP. Plasma cathepsin S and cystatin C levels and risk of abdominal aortic aneurysm: a randomized population-based study. PLoS One. 2012;7:e41813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindholt JS, Erlandsen EJ, Henneberg EW. Cystatin C deficiency is associated with the progression of small abdominal aortic aneurysms. Br J Surg. 2001;88:1472–5. [DOI] [PubMed] [Google Scholar]
- 7.Schulte S, Sun J, Libby P, Macfarlane L, Sun C, Lopez-Ilasaca M, et al. Cystatin C deficiency promotes inflammation in angiotensin II-induced abdominal aortic aneurisms in atherosclerotic mice. Am J Pathol. 2010;177:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukhova GK, Wang B, Libby P, Pan JH, Zhang Y, Grubb A, et al. Cystatin C deficiency increases elastic lamina degradation and aortic dilatation in apolipoprotein E-null mice. Circ Res. 2005;96:368–75. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamson M, Barrett AJ, Salvesen G, Grubb A. Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J Biol Chem. 1986;261: 11282–9. [PubMed] [Google Scholar]
- 10.Rinne A, Dorn A, Jarvinen M, Alavaikko M, Jokinen K, Hopsu-Havu VK. Immunoelectron microscopical location of the acid cysteine proteinase inhibitor in the lymphatic tissue of the tonsils. Acta Histochem. 1986;79:137–45. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Hashimoto S, Toyoda N, Nagai S, Yamazaki N, Dong HY, et al. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood. 2000;96:2584–91. [PubMed] [Google Scholar]
- 12.Verdot L, Lalmanach G, Vercruysse V, Hartmann S, Lucius R, Hoebeke J, et al. Cystatins up-regulate nitric oxide release from interferon-gamma-activated mouse peritoneal macrophages. J Biol Chem. 1996;271:28077–81. [DOI] [PubMed] [Google Scholar]
- 13.Pennacchio LA, Lehesjoki AE, Stone NE, Willour VL, Virtaneva K, Miao J, et al. Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy (EPM1). Science. 1996;271:1731–4. [DOI] [PubMed] [Google Scholar]
- 14.Lieuallen K, Pennacchio LA, Park M, Myers RM, Lennon GG. Cystatin B-deficient mice have increased expression of apoptosis and glial activation genes. Hum Mol Genet. 2001;10:1867–71. [DOI] [PubMed] [Google Scholar]
- 15.Goncalves I, Hultman K, Duner P, Edsfeldt A, Hedblad B, Fredrikson GN, et al. High levels of cathepsin D and cystatin B are associated with increased risk of coronary events. Open Heart. 2016;3:e000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grondal N, Sogaard R, Henneberg EW, Lindholt JS. The Viborg Vascular (VIVA) screening trial of 65–74 year old men in the central region of Denmark: study protocol. Trials. 2010;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grondal N, Sogaard R, Lindholt JS. Baseline prevalence of abdominal aortic aneurysm, peripheral arterial disease and hypertension in men aged 65–74 years from a population screening study (VIVA trial). Br J Surg. 2015;102:902–6. [DOI] [PubMed] [Google Scholar]
- 18.Lindholt JS, Sogaard R. Population screening and intervention for vascular disease in Danish men (VIVA): a randomised controlled trial. Lancet. 2017;390:2256–65. [DOI] [PubMed] [Google Scholar]
- 19.Liao M, Liu CL, Lv BJ, Zhang JY, Cheng L, Cheng X, et al. Plasma cytokine levels and risks of abdominal aortic aneurysms: A population-based prospective cohort study. Ann Med. 2015;47:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv BJ, Lindholt JS, Wang J, Cheng X, Shi GP. Plasma levels of cathepsins L, K, and V and risks of abdominal aortic aneurysms: a randomized population-based study. Atherosclerosis. 2013;230:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galteau MM, Guyon M, Gueguen R, Siest G. Determination of serum cystatin C: biological variation and reference values. Clin Chem Lab Med. 2001;39:850–7. [DOI] [PubMed] [Google Scholar]
- 22.Grondal N, Bramsen MB, Thomsen MD, Rasmussen CB, Lindholt JS. The cardiac cycle is a major contributor to variability in size measurements of abdominal aortic aneurysms by ultrasound. Eur J Vasc Endovasc Surg. 2012;43:30–3. [DOI] [PubMed] [Google Scholar]
- 23.Laustsen J, Jensen LP, Hansen AK, Danish National Vascular R. Accuracy of clinical data in a population based vascular registry. Eur J Vasc Endovasc Surg. 2004;27:216–9. [DOI] [PubMed] [Google Scholar]
- 24.Dencker M, Tanha T, Karlsson MK, Wollmer P, Andersen LB, Thorsson O. Cystatin B, cathepsin L and D related to surrogate markers for cardiovascular disease in children. PLoS One. 2017;12:e0187494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takaya A, Peng WX, Ishino K, Kudo M, Yamamoto T, Wada R, et al. Cystatin B as a potential diagnostic biomarker in ovarian clear cell carcinoma. Int J Oncol. 2015;46:1573–81. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Gui L, Zhang Y, Zhang J, Shi J, Xu G. Cystatin B is a progression marker of human epithelial ovarian tumors mediated by the TGF-beta signaling pathway. Int J Oncol. 2014;44:1099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gashenko EA, Lebedeva VA, Brak IV, Tsykalenko EA, Vinokurova GV, Korolenko TA. Evaluation of serum procathepsin B, cystatin B and cystatin C as possible biomarkers of ovarian cancer. Int J Circumpolar Health. 2013;72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldman AS, Banyard J, Wu CL, McDougal WS, Zetter BR. Cystatin B as a tissue and urinary biomarker of bladder cancer recurrence and disease progression. Clin Cancer Res. 2009;15:1024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smid L, Strojan P, Budihna M, Skrk J, Vrhovec I, Zargi M, et al. Prognostic value of cathepsins B, D and steffins A and B in laryngeal carcinoma. Eur Arch Otorhinolaryngol. 1997;254 Suppl 1:S150–3. [DOI] [PubMed] [Google Scholar]
- 30.Kos J, Krasovec M, Cimerman N, Nielsen HJ, Christensen IJ, Brunner N. Cysteine proteinase inhibitors stefin A, stefin B, and cystatin C in sera from patients with colorectal cancer: relation to prognosis. Clin Cancer Res. 2000;6:505–11. [PubMed] [Google Scholar]
- 31.Ma Y, Chen Y, Petersen I. Expression and epigenetic regulation of cystatin B in lung cancer and colorectal cancer. Pathol Res Pract. 2017;213:1568–74. [DOI] [PubMed] [Google Scholar]
- 32.Strojan P, Budihna M, Smid L, Svetic B, Vrhovec I, Skrk J. Cathepsin B and L and stefin A and B levels as serum tumor markers in squamous cell carcinoma of the head and neck. Neoplasma. 2001;48:66–71. [PubMed] [Google Scholar]
- 33.Chan EL, Belem P, Ciocca RG, Madsen D, Cody RP, Mackenzie JW, et al. Incidence of cancer and abdominal aortic aneurysms. A logistic regression analysis. Ann N Y Acad Sci. 1996;800:68–73. [DOI] [PubMed] [Google Scholar]
- 34.Paraskevas KI, Mikhailidis DP, Veith FJ. Patients with peripheral arterial disease, abdominal aortic aneurysms and carotid artery stenosis are at increased risk for developing lung and other cancers. Int Angiol. 2012;31:404–5. [PubMed] [Google Scholar]
- 35.Liu CL, Guo J, Zhang X, Sukhova GK, Libby P, Shi GP. Cysteine protease cathepsins in cardiovascular disease: from basic research to clinical trials. Nat Rev Cardiol. 2018;15:351–70. [DOI] [PubMed] [Google Scholar]
- 36.Maher K, Jeric Kokelj B, Butinar M, Mikhaylov G, Mancek-Keber M, Stoka V, et al. A role for stefin B (cystatin B) in inflammation and endotoxemia. J Biol Chem. 2014;289:31736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]