Abstract
Using samples collected for VRE surveillance, we evaluated unit-admission prevalence of CRE perirectal colonization and whether CRE carriers (unknown to staff) were on contact precautions for other indications. CRE colonization at unit-admission was infrequent (3.9%). Most CRE carriers were not on contact precautions, representing a reservoir for healthcare-associated CRE transmission.
INTRODUCTION
Carbapenem-resistant Enterobacteriaceae (CRE) represent an urgent antibiotic resistance threat.1 The Centers for Disease Control and Prevention (CDC) recommends contact isolation precautions for CRE colonized or infected patients to limit healthcare-associated transmission.2 Most U.S. inpatient facilities, however, do not perform routine screening to detect CRE. Our objective was to measure the prevalence of CRE perirectal colonization upon hospital unit admission (results unknown to clinical staff) and to evaluate whether CRE carriers were already on contact precautions for other indications at the time of unit entry.
METHODS
Study Setting and Population.
This study included adults admitted to the Johns Hopkins Hospital (JHH) medical intensive care unit (MICU) or solid organ transplant unit (Transplant Unit) from May 1, 2016 – July 1, 2017. Both units have a longstanding vancomycin-resistant Enterococcus (VRE) surveillance program and collect admission perirectal Eswabs (Copan) from patients.
Microbiology Methods.
Residual Amies media was stored at 4˚C and, within 4 days of swab collection, directly plated onto MacConkey agar with ertapenem and meropenem disks.3 Colonies growing within 27 mm of ertapenem and 32 mm of meropenem were identified by matrix-assisted laser-desorption ionization time-of-flight mass spectrometry (Bruker Daltonics), and carbapenem antimicrobial susceptibility testing (ertapenem, meropenem and imipenem) was performed by disk diffusion applying Clinical and Laboratory Standards Institute guidelines.4 Enterobacteriaceae resistant to ertapenem, meropenem, and/or imipenem were categorized as CRE. CRE-positive isolates were tested for carbapenemase production (CP-CRE) by the modified carbapenem inactivation method (mCIM).5 CRE status was de-identified until study completion and blinded to clinical and infection control staff.
Infection Control Data Collection.
Infection control databases were queried to identify patients who were placed on contact precautions at unit admission because of a flagged history of: (1) methicillin-resistant Staphylococcus aureus (MRSA); (2) Vancomycin-resistant Enterococcus (VRE); (3) Clostridioides difficile; (4) Multidrug-resistant Gram-negative (MDRGN) bacteria; (5) CRE (which are classified separately from other MDRGNs at JHH); (6) Respiratory viruses; and (7) Other indications, including “CRE rule-out” for patients recently hospitalized internationally (≤ 6 mos.),2 enteric pathogens, and contact precautions without associated infection control flag(s).
Statistical Methods.
Descriptive statistics for contact precaution status and indications were calculated. The relationship between these variables and CRE or CP-CRE colonization was evaluated using univariable logistic regression with general estimating equations and robust standard errors to account for patient-clustering due to repeat unit admissions. Results were summarized by odds ratios (ORs) and corresponding 95% confidence intervals (CIs). Analyses were performed in Stata, version 13.0 (StataCorp, College Station, TX). This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board, with a waiver of consent.
RESULTS
There were 3,784 unit admissions during the study period: 2,034 (54%) in the MICU and 1,750 (46%) in the Transplant Unit. Of these encounters, 3,249 (86%), representing 2,424 unique patients, had stored perirectal admission screening swabs.
Overall, 126 of 3249 admission swabs (3.9%), from 117 unique patients, tested positive for one or more CRE (95% CI: 3.2 – 4.6%). The CRE prevalence was higher among MICU admissions compared to Transplant Unit admissions (4.7% vs. 2.8%, p=0.01). Of the 126 CRE-positive swabs, 26 (21%) were positive for carbapenemase production (from 24 unique patients), yielding a CP-CRE admission prevalence of 0.8% (95% CI: 0.5 – 1.2%). The prevalence of CP-CRE was similar in both units (0.8% in the MICU vs. 0.9% in the Transplant Unit, p=0.74). The majority of CP-CRE were Klebsiella pneumoniae (46%), followed by Enterobacter cloacae (35%), Citrobacter amalonaticus (11%), and Escherichia coli (8%).
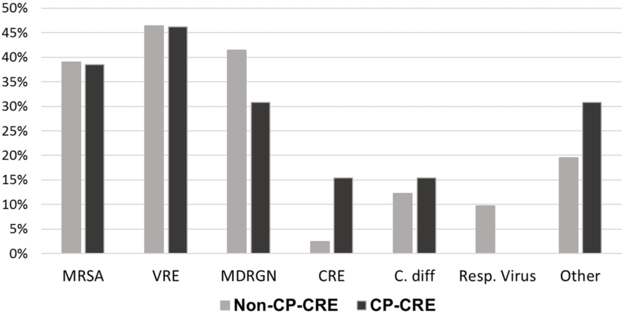
During the study period, 817 (25%) patients were on contact precautions at unit admission. The majority of patients with perirectal CRE and CP-CRE colonization (72 [57%] and 13 [50%], respectively) were not on contact precautions at unit entry. Relative to non-carriers, however, CRE and CP-CRE carriers were still more likely to be on contact precautions (ORs, respectively: 2.18 [95% CI: 1.50 – 3.15] and 2.93 [95% CI: 1.28 – 6.72]). The most common infection control flag indication(s) among CRE carriers were a history of VRE (46%), MRSA (39%), or MDRGN organisms (39%) (Figure). Patients with an MDRGN history were nearly 3.5 times more likely to test CRE-positive (OR 3.42, 95% CI: 1.83 – 6.36) (Table). Three CRE carriers (all CP-CRE-negative MICU patients) had documented recent international hospitalization: one patient was not on contact precautions at unit admission, and two patients were already isolated for history of MDRGNs.
Figure.
Indications for Contact Precautions at Unit Admission in Patients Who Test Positive for Non-Carbapenemasing-Producing (non-CP-CRE) and Carbapenemase-Producing (CP-CRE) Colonizationa
Indications for contact precautions among non-CP-CRE (n=159) and CP-CRE (n=13) colonized patients who were on contact precautions at unit admission. There were 126 CRE carriers (overall) during the study period (100 non-CP-CRE and 26 CP-CRE), 57% of whom (72, 59 non-CP-CRE and 13 CP-CRE) were on contact precautions at unit admission.
Abbreviations: MRSA – methicillin-resistant Staphylococcus aureus; VRE – Vancomycin-resistant Enteroccocus; MDRGN – multidrug-resistant Gram-negative bacteria (defined as Gram-negative rods other than non-fermenters resistant to 3 of 5 antibiotic classes, non-fermenters resistant to 4 of 5 antibiotic classes, trimethoprim and sulfamethoxazole-resistant Stenotrophomonas spp., extended-spectrum β-lactamase (ESBL)-producing bacteria, and/or specified Enterobacteriaceae resistant to ceftriaxone); CRE – Carbapenem-resistant Enterobacteriaceae (defined as resistance to any carbapenem); C. diff – Clostridioides difficile; Resp. Virus – Respiratory viruses; and Other – Other indications, including enteric pathogens, “CRE Rule-Out” for recent internationally hospitalized patients, and unspecified reasons.
a Percentages exceed 100%, due to >1 possible indication per patient.
Table.
Association between Colonization and Indication for Contact Precautions at Unit Admission, Comparing CRE or CP-CRE Carriers to Non-Carriers
| Covariate | CRE Odds Ratio (95% CI) |
P Value | CP-CRE Odds Ratio (95% CI) |
P Value |
|---|---|---|---|---|
| On Contact Precautions | 2.18 (1.50 – 3.15) | <0.001 | 2.93 (1.28 – 6.72) | 0.01 |
| Indication(s)a: | 1.00 | N/A | 1.00 | N/A |
| MRSA | 1.68 (0.90 – 3.13) | 0.01 | 1.60 (0.38 – 6.77) | 0.52 |
| VRE | 1.38 (0.75 – 2.54) | 0.30 | 1.31 (0.35 – 4.97) | 0.69 |
| MDRGN | 3.42 (1.83 – 6.36) | <0.001 | 2.20 (0.54 – 9.02) | 0.27 |
| CRE | 3.31 (0.58 – 18.87) | 0.18 | 8.95 (0.96 – 83.60) | 0.05 |
| C. diff | 1.05 (0.43 – 2.55) | 0.92 | 0.82 (0.06 – 12.0) | 0.88 |
| Resp. Virus | 0.60 (0.20 – 1.74) | 0.34 | (No observations) | N/A |
| Other | 0.68 (0.34 – 1.33) | 0.26 | 1.24 (0.34 – 4.50) | 0.74 |
Abbreviations: CI, Confidence Interval; MRSA, Methicillin-resistant Staphylococcus aureus; VRE, Vancomycin-resistant Enterococcus; MDRGN, Multidrug-resistant Gram-negative; C. diff, Clostridioides difficile; Resp. Virus, Respiratory virus.
Indications analyses restricted to patients who were on contact precautions at admission.
Two of 26 patients who had CP-CRE isolated on admission perirectal surveillance were already on contact precautions with a CRE ‘flag,’ because of a prior CRE-positive culture (unrelated to study screening). In sixteen additional encounters, patients were isolated based upon an institutional CRE flag, but they tested CP-CRE-negative. The sensitivity and specificity of a CRE flag for predicting CP-CRE colonization at unit admission was 7.7% and 99.5%, respectively.
DISCUSSION
Identifying CRE-colonized patients at hospital unit admission can facilitate timely infection control interventions, such as placing colonized patients on contact precautions, in order to limit healthcare-associated transmission. The CDC recommends CRE colonization screening in limited instances (e.g., patients with recent international hospitalization),2 but most U.S. hospitals do not perform routine CRE colonization screening. Evaluating patients admitted to a medical intensive care unit and a solid organ transplant unit, we found that CRE colonization unit-admission prevalence was infrequent (3.9%), and only 21% of CREs were carbapenemase-producers. These findings are similar to the proportions of CRE (3.1%) and CP-CRE (32% of CRE) among clinical isolates reported to the National Healthcare Safety Network in 2015 and 2017, respectively.6
The majority of CRE and CP-CRE colonized patients were not on contact precautions at unit admission. Of particular concern, only one CP-CRE carrier (two encounters) had a known history of CRE, which may reflect a true lack of prior positive cultures, or incomplete data from institutions outside the Johns Hopkins Health System. Moreover, no CP-CRE colonized patients were recently hospitalized internationally. Our findings suggest that many CP-CRE carriers — and the potential they pose for onward transmission — are missed for infection control interventions under existing institutional protocols.
Although most CRE-colonized patients were not on contact precautions at unit admission, CRE and CP-CRE-colonized patients were still two-to-three times more likely than non-carriers to be on contact precautions. The most common indications were histories of VRE, MRSA, and/or MDRGNs. These findings are consistent with the overlap in risk factors (e.g., antibiotic use, exposure to high-risk healthcare facilities) between CRE and other drug-resistant organisms.7–9 Moreover, an MDRGN history was associated with colonization with CRE, but not CP-CRE, which may reflect differing acquisition pathways between CRE types.10 Identifying additional risk factors for CRE colonization, particularly among patients who lack MDRGN histories, could enhance targeted screening efforts.
This was a single-center study with some missing swabs, and our results should be validated in other cohorts. In addition, contact precautions policies vary between hospitals, which could impact generalizability of these findings. We only ascertained contact precaution status at unit admission, and patient status may have changed during unit stay. Screening method may also affect organism recovery, although CDC guidance endorses peri-rectal swabs for CRE surveillance2.
In summary, the majority of CRE-colonized patients in this study were not on contact precautions at unit admission. Given low colonization prevalence, further research on CRE colonization risk factors among U.S. inpatients is necessary in order to develop algorithms for identifying and screening patients at greatest risk of harboring CRE.
ACKNOWLEDGMENTS
We would like to thank Verna Scheeler, Michael Anderson, Dina Khamash, and Sean Thompson for their assistance with study coordination, and data collection and validation, as well as Belita Opene, Shawna Lewis, and Krizia Chambers for their work processing surveillance cultures. We would also like to thank members of the JHU Clinical Microbiology Laboratory staff for helping with collection of surveillance swabs for the study.
Financial Support: This work was supported by the CDC Prevention Epicenters Program CDC 1U54CK000447, the CDC MIND-Healthcare Program 1U01CK000536, AHRQ R36HS025089, and The Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases.
Footnotes
Conflicts of Interest: Dr. Milstone reports personal fees from BD Diagnostics, Inc., Dr. Rock reports grant support from The Clorox Company, and Dr. Tamma reports grants from Merck, all outside the scope of the submitted work. Dr. Simner reports grants and personal fees from Accelerate Diagnostics, grants from BD Diagnostics, Inc., grants from bioMerieux, Inc., grants from Check-Points Diagnostics, BV, grants from Hardy Diagnostics, personal fees from Roche Diagnostics, personal fees from Opgen Inc, and personal fees from Oxford Nanopore, all outside the scope of the submitted work. All other authors report no potential conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. In:2013. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Facility Guidance for Control of Carbapenem-resistant Enterobacteriaceae (CRE): November 2015. Update. In:2015. [Google Scholar]
- 3.Simner PJ, Martin I, Opene B, Tamma PD, Carroll KC, Milstone AM. Evaluation of Multiple Methods for the Detection of Gastrointestinal Colonization of Carbapenem-Resistant Organisms from Rectal Swabs. J Clin Microbiol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 28th ed. Supplement M100S In. Wayne, PA: CLSI; 2018. [Google Scholar]
- 5.Pierce VM, Simner PJ, Lonsway DR, et al. Modified Carbapenem Inactivation Method for Phenotypic Detection of Carbapenemase Production among Enterobacteriaceae. J Clin Microbiol. 2017;55(8):2321–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodworth KR, Walters MS, Weiner LM, et al. Vital Signs: Containment of Novel Multidrug-Resistant Organisms and Resistance Mechanisms - United States, 2006-2017. MMWR Morb Mortal Wkly Rep. 2018;67(13):396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logan LK, Weinstein RA. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J Infect Dis. 2017;215(suppl_1):S28–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinnell JA, Miller LG, Eells SJ, Cui E, Huang SS. A systematic literature review and meta-analysis of factors associated with methicillin-resistant Staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol. 2013;34(10):1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simner PJ, Goodman KE, Carroll KC, Harris AD, Han JH, Tamma PD. Using Patient Risk Factors to Identify Whether Carbapenem-Resistant Enterobacteriaceae Infections Are Caused by Carbapenemase-Producing Organisms. Open Forum Infectious Diseases. 2018;5(5):ofy094–ofy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman KE, Simner PJ, Tamma PD, Milstone AM. Infection control implications of heterogeneous resistance mechanisms in carbapenem-resistant Enterobacteriaceae (CRE). Expert review of anti-infective therapy. 2016;14(1):95–108. [DOI] [PubMed] [Google Scholar]