Abstract
Objectives
High-grade non-muscle-invasive urothelial tumors of the bladder that fail intravesical Bacillus Calmette-Guérin immunotherapy are at the highest risk of progression. Initial evidence links heat-shock protein expression levels and outcome of bladder cancer after BCG treatment.
We aimed to determine the association between HSP60, 70 and 90 expression levels and long-term outcomes of T1HG urothelial bladder tumors treated with BCG immunotherapy.
Materials and Methods
Data of 54 consecutive patients with primary T1HG bladder tumors who underwent transurethral resection between 2002 – 2008 and received at least an induction course of BCG were reviewed. Immunohistochemical staining for HSP60, 70 and 90 were performed on resected specimens.
Study outcomes included disease recurrence and progression. The association between HSP expression levels and outcomes were evaluated with univariable and multivariable Cox proportional hazards models.
Results
During a median follow-up of 9.6 years, 25 patients had a disease recurrence and 14 patients a disease progression. Estimated 5-year recurrence and progression free survival were 59% and 81%, respectively.
On multivariable analyses, HSP60 staining >65% was associated with a higher risk for progression (HR=3.96, 95% CI 1.35–11.58, P=0.012), and HSP70 staining >5% was associated with a decreased risk for progression (HR=0.33, 95% CI 0.11–0.98, P=0.045) and recurrence (HR=0.29, 95% CI 0.13–0.65, P=0.003). HSP90 expression was not associated with disease recurrence or progression. Five patients had both a HSP60 staining >65% and a HSP70 staining ≤5% all of whom recurred at a median time of 6 months (IQR 3, 16) and 80% of whom progressed at a median time of 26 months (IQR 5, 60).
Conclusions
HSP60 and 70 cellular expression levels are associated with long-term outcome following BCG treatment of T1HG urothelial bladder tumors. These findings, if further validated, may be used to better stratify the risk of disease recurrence and progression in this group of patients.
Keywords: Urothelial carcinoma, non-muscle invasive bladder cancer, Bacillus Calmette-Guérin immunotherapy, heat shock proteins, disease progression
Introduction
Bladder cancer is the ninth most frequently-diagnosed cancer worldwide, with the highest incidence rates observed in North America and Europe.[1] At the time of diagnosis approximately 75% of bladder cancers are non-muscle invasive, 50% of which are high-grade.[2, 3] Progression rates of non-muscle invasive bladder cancer (NMIBC) range from 10% to 30% at 5-years.[3] Intravesical Bacillus Calmette-Guérin (BCG) immunotherapy is the mainstay of treatment for high-grade tumors; however, patients who fail BCG immunotherapy are at the highest risk of disease progression and may benefit from early radical cystectomy.[4] Thus, identification of predictors for BCG failure may facilitate patient counseling regarding the best treatment option for high-grade NMIBC.
Heat shock proteins are a group of molecular chaperons with low expression levels in normal physiological conditions, that are markedly upregulated after the cell is exposed to external stressors including hyperthermia, hypoxia and cytotoxic agents. They function by modifying the structures and interactions of cellular proteins, maintaining them in their biologically active conformations, thus maintaining cellular function and viability.[5] Alteration in heat shock protein (HSP) levels are observed in many cancers, and HSP overexpression usually signals a poor prognosis.[5]
Studies have shown alterations in HSP expression levels in bladder tumors compared to normal urothelium, and an association with tumor grade, stage and outcome; however, findings were inconsistent.[6–9] Furthermore, in a previous study, we demonstrated that elevated levels of urinary HSPs may be used as biomarkers for bladder cancer.[10] A single publication by Lebret et al. showed a significant correlation between lack of HSP90 expression and response to intravesical BCG in a mixed cohort of 33 patients with high grade, stage Ta-T1 disease.[11]
In the current study, we aimed to evaluate the role of HSP60, 70 and 90 in predicting the long-term response to intravesical BCG in a homogenous cohort of patients with stage T1, high-grade, urothelial tumors of the bladder.
Materials and Methods
After obtaining approval from the institutions ethics committee, we reviewed the medical records of 54 consecutive patients who underwent transurethral resection of bladder tumor (TURBT) between the years 2002–2008 with a pathological finding of primary stage T1 high-grade urothelial cancer and received at least an induction course of intravesical BCG. Patients receiving BCG or diagnosed with a high-grade tumor prior to the study period, and patients who did not receive BCG treatment or were unable to tolerate a full induction course of BCG were not included in the study cohort. Formalin-fixed paraffin-embedded tumor samples obtained at the initial TURBT were available for all patients.
Patient baseline characteristics including age, sex, smoking history (current vs. past or non-smokers) and previous events of NMIBC were collected. All patients were initially treated with a TURBT with the aim of complete resection of the bladder tumor. All surgical specimens were reviewed by a genitourinary pathologist (S.Z.), and the diagnosis of stage T1, high-grade, urothelial carcinoma was confirmed. The number of tumors, size of the largest tumor, and presence of concurrent carcinoma in situ were noted and the EORTC risk scores for recurrence and progression were calculated.[12] Restaging TURBT was performed at the discretion of the treating urologist. After initial treatment, all patients received an induction course of 6 weekly instillations of OncoTICE BCG 2–8×108 CFU. Maintenance BCG immunotherapy, per the Lamm protocol, was given based on patient characteristics and at the discretion of the treating urologist. Postoperative follow-up consisted of cystoscopy performed every three months for the first two years, every six months two to five years after surgery, and annually thereafter. Annual upper urinary tract imaging was performed during follow-up. Patients with a suspected recurrence underwent TURBT. Disease recurrence was defined as the first pathologically confirmed tumor relapse in the bladder, regardless of the tumor stage. Disease progression was defined according to the IBCG consensus definition for progression in NMIBC, in the presence of a ≥T2 stage or lymph node positive (N+) disease or distant metastasis (M1).[13] Patients who initially had a disease progression were noted to have a recurrence at the same date. All patients were followed until death or last documented follow-up.
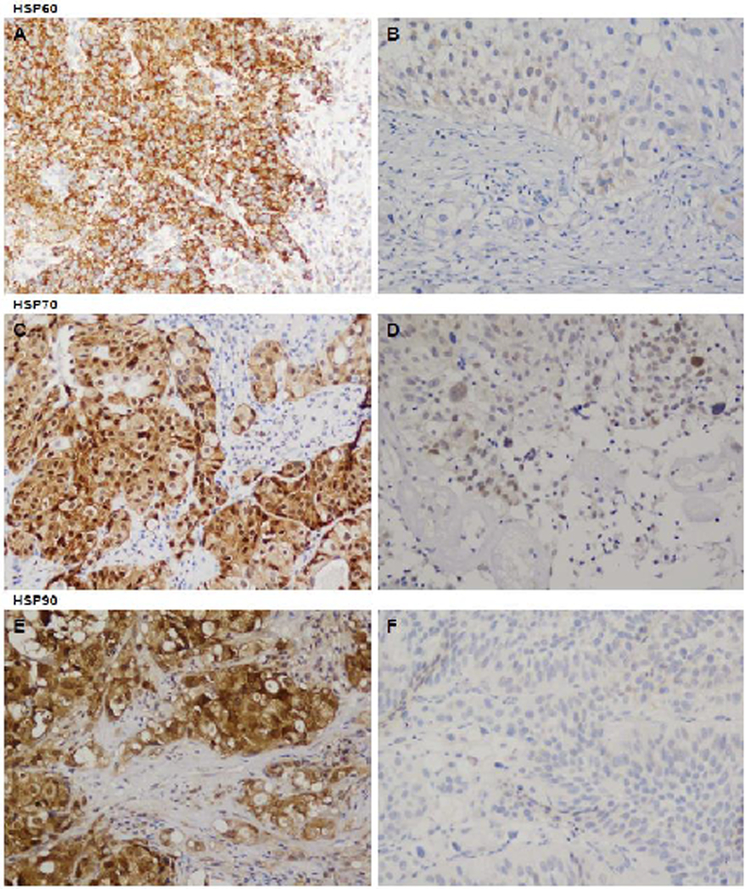
Immunohistochemical staining of tumor samples for HSP60, HSP70 and HSP90 were performed as previously described.[11] Primary antibodies used were HSP60 and HSP90 from Santa Cruz Biotechnology (Santa Cruz, CA) and HSP70 from StressGen (Victoria, British Columbia, Canada). The extent of cytoplasmatic immunostaining was evaluated by an independent uropathologist (S.Z.), who was blinded to the treatment outcome. Cells were considered positive when immunoreactivity was clearly observed in the cytoplasm (Figure 1). HSP expression was evaluated per the proportion of positively stained cells. For each tumor, percentage of immunostaining cells was estimated per 1000 cells. We used a validated web-based program to evaluate which staining percentage best predicted recurrence and progression following treatment. The optimal cutoff was defined as the point with the most significant (log-rank test) split in the survival outcome.[14]
Figure 1 -.
Immunohistochemical staining of bladder tumor specimens (x20) showing high and low intensity cytoplasmatic staining of HSP60 (A, B), HSP70 (C, D) and HSP90 (E, F), respectively
The study endpoints were disease recurrence and progression. Clinical and pathological data were reported using descriptive statistics: number and percent were calculated for categorical variables and median and interquartile range (IQR) for continuous variables. Disease recurrence and progression were treated as time-dependent variables measured from the completion of induction BCG therapy, and estimated rates were calculated using the Kaplan-Meier method, and compared using the log-rank test. Univariate and multivariate Cox regression analyses were used to evaluate the association between patient and tumor characteristics and disease recurrence and progression. To further support our findings, we evaluated the differential expression of HSP-related genes in a previously-published cohort of NMIBC.[15] All statistical analyses were two-sided. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R v3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study cohort included 47 men and 7 women at a median age of 73 years (IQR 61, 81). Five patients (9%) had a previous event of low grade NMIBC and 2 patients (4%) received previous intravesical mitomycin C. None of the patients had previous upper urinary tract tumors. All patients had a pathologically confirmed T1HG bladder tumors. Twenty-five patients (47%) had a tumor larger than 3cm, 19 (35%) had multiple tumors and 2 (4%) had concurrent carcinoma in situ. All patients received induction BCG immunotherapy; 37 patients (69%) received partial or complete maintenance BCG treatment. Baseline patient and tumor characteristics are reported in Table 1.
Table 1.
Baseline characteristics of 54 patients with stage T1, high grade, bladder tumors treated with intravesical Bacillus Calmette-Guérin immunotherapy
| Variable | Value* | ||||
|---|---|---|---|---|---|
| Sex (%) | Male | 47 (87) | |||
| Female | 7 (13) | ||||
| Median age years (IQR) | 73 (61,81) | ||||
| Smoking status (%) | Former/ Never | 42 (78) | |||
| Current | 12 (22) | ||||
| Tumor size (n=53, %) | ≤3cm | 28 (53) | |||
| >3cm | 25 (47) | ||||
| Tumor number (%) | Single | 35 (65) | |||
| Multiple | 19 (35) | ||||
| Recurrent tumor (%) | Yes | 5 (9) | |||
| No | 49 (91) | ||||
| Presence of CIS (%) | Yes | 2 (4) | |||
| No | 52 (96) | ||||
| EORTC risk score for recurrence | 0 | 0 (0) | |||
| 1–4 | 16 (30) | ||||
| 5–9 | 36 (67) | ||||
| 10–17 | 2 (4) | ||||
| EORTC risk score for progression | 0 | 0 (0) | |||
| 2–6 | 0 (0) | ||||
| 7–13 | 42 (78) | ||||
| 14–23 | 12 (22) | ||||
| RestagingTURBT(%) | Yes | 16 (30) | |||
| No | 38 (70) | ||||
| Maintenance BCG Treatment (%) | Yes | 37 (69) | |||
| No | 17 (31) | ||||
| Mean percent HSP expression (SD) | HSP60 | 31 ± 36 | |||
| HSP70 | 56 ± 44 | ||||
| HSP90 | 87 ± 28 | ||||
| Median percent HSP expression (IQR) | HSP60 | 15 (0,50) | |||
| HSP70 | 80 (0, 100) | ||||
| HSP90 | 100 (100, 100) | ||||
IQR = interquartile range; CIS = carcinoma in situ; EORTC = European Organization for Research and Treatment of Cancer; TURBT = transurethral resection of bladder tumor; BCG = Bacillus Calmette-Guérin; HSP = heat shock protein; SD = standard deviation.
Represents number of patients unless noted otherwise.
Median follow-up for patients without disease recurrence was 9.6 years (IQR 6.8, 10.6). During follow-up, 25 patients had a disease recurrence at a median time of 4.8 months (IQR 2.3, 13.9), and 14 patients had a disease progression at a median time of 24.8 months (IQR 5.5, 60.8). Estimated overall 5-year recurrence free survival was 59%, and 5-year progression free survival was 81%. Seven patients (13%), all of whom had disease progression, underwent radical cystectomy. During the study period 20 patients died of any cause and 4 patients died of bladder cancer at median times of 54.9 months (IQR 34.2, 88.5) and 39.4 months (IQR 29.4, 62.5), respectively. Estimated 5-year overall and cancer specific survival were 82% and 94%, respectively.
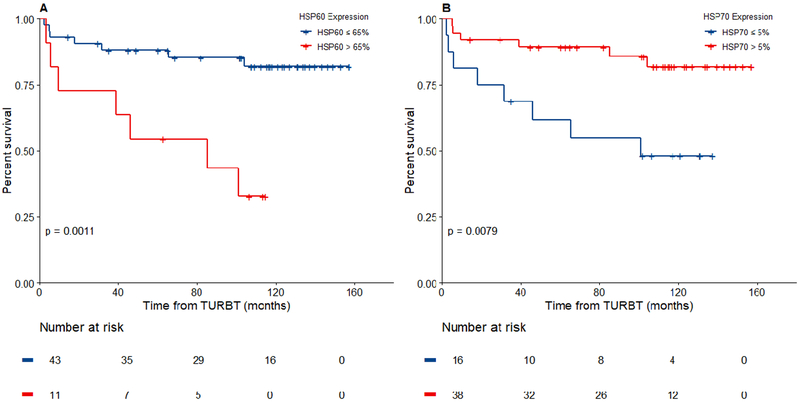
Mean HSP60, HSP70 and HSP90 expression levels in tumor cells were 31%±36%, 56%±44% and 87%±28%, respectively. Optimal HSP expression cutoff values for disease progression were 65% for HSP60 and 5% for HSP70. Similarly, optimal HSP70 cutoff value for predicting disease recurrence was 5%. No significant HSP60 cutoff value was found for predicting recurrence. HSP90 expression levels were not associated with disease recurrence and progression. HSP60 positively stained >65% of cells in 11 patients (20%); 5-year progression free survival was 54.5% for patients with HSP60 staining >65%, and 88.2% for those with HSP60 staining ≤65%, p=0.001 (Figure 2a). HSP70 staining was >5% in 38 patients (70%); 5-year progression free survival was 89.3% for patients with HSP70 staining >5% and 61.9% for those with HSP70 staining ≤5%, p=0.008 (Figure 2b). 5-year recurrence free survival was 70.9% for patients with HSP70 staining >5% and 30% for those with HSP70 staining ≤5%, p=0.002 (Figure 3b). Five patients (9%) had both a HSP60 staining >65% and a HSP70 staining ≤5%. All 5 patients recurred at a median time of 6 months (IQR 3, 16) and 4/5 patients progressed at a median time of 26 months (IQR 5, 60).
Figure 2 -.
Kaplan Meier estimates of progression free survival relative to cytoplasmatic expression levels of (A) HSP60 and (B) HSP70
Figure 3 -.
Kaplan Meier estimates of recurrence free survival relative to cytoplasmatic expression levels of (A) HSP60 and (B) HSP70
On univariable analyses, HSP60 staining >65% was associated with a higher risk for progression (hazard ratio (HR)=4.88, 95% confidence interval (CI) 1.7–14.01, P=0.003) and HSP70 staining >5% was associated with a decreased risk for progression (HR=0.26, 95% CI 0.09–0.76, P=0.014). On a multivariable model containing both HSPs, HSP60 staining >65% (HR=3.96, 95% CI 1.35–11.58, P=0.012) and HSP70 staining >5% (HR=0.33, 95% CI 0.11–0.98, P=0.045) remained significantly associated with disease progression. HSP60 and HSP70 both remained independent predictors of outcome when controlled separately for EORTC score for progression (Table 2). Univariable analyses of recurrence found that HSP70 staining >5% (HR=0.3, 95% CI 0.14–0.68, P=0.004) and BCG maintenance therapy (HR=0.27, 95% CI 0.12–0.6, P=0.001) were associated with a decreased risk for recurrence. Both variables remained independently associated with recurrence when included in a multivariable model (Table 3).
Table 2.
Univariable and multivariable predictors of disease progression in patients with stage-T1 high grade non-muscle invasive bladder cancer treated with intravesical Bacillus Calmette-Guérin immunotherapy
| Variable | Univariable | Multivariable* | Multivariabl e* | Multivariabl e* | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% Cl) | P | HR (95% Cl) | P | HR (95% Cl) | P | HR (95% Cl) | P | ||
| Sex | Male | Ref. | 0.177 | ||||||
| Female | 2.46 (0.67 – 9.03) | ||||||||
| Median age years (IQR) | 0.99 (0.95 – 1.04) | 0.841 | |||||||
| Smoking status | Former/ Never | Ref. | 0.101 | ||||||
| Current | 2.64 (0.83 – 8.42) | ||||||||
| Tumor size | ≤3cm | Ref. | 0.158 | ||||||
| >3cm | 2.24 (0.73 – 6.89) | ||||||||
| Tumor number | Single | Ref. | 0.352 | ||||||
| Multiple | 1.66 (0.57 – 4.81) | ||||||||
| Recurrent tumor | No | Ref. | 0.953 | ||||||
| Yes | 0.94 (0.12 – 7.23) | ||||||||
| Presence of CIS | No | Ref. | 0.291 | ||||||
| Yes | 2.99 (0.39 – 23) | ||||||||
| EORTC score for progression | 0 – 13 | Ref. | 0.194 | Ref. | 0.137 | Ref. | 0.349 | ||
| 14 – 23 | 2.19 (0.67 – 7.22) | 2.5 (0.75 – 8.38) | 1.77 (0.54 – 5.88) | ||||||
| Restaging TURBT | No | Ref. | 0.831 | ||||||
| Yes | 0.88 (0.28 – 2.82) | ||||||||
| Maintenan ce BCG Treatment | No | Ref. | 0.858 | ||||||
| Yes | 1.11 (0.35 – 3.55) | ||||||||
| % HSP60 expression | ≤65 | Ref. | 0.003 | Ref. | 0.012 | Ref. | 0.002 | ||
| >65 | 4.88 (1.7 – 14.01) | 3.96 (1.35 – 11.58) | 5.16 (1.79 – 14.92) | ||||||
| % HSP70 expression | ≤5 | Ref. | 0.014 | Ref. | 0.045 | Ref. | 0.02 | ||
| >5 | 0.26 (0.09 – 0.76) | 0.33 (0.11 – 0.98) | 0.28 (0.1 – 0.82) | ||||||
HR = hazards ratio; CI = confidence interval; Ref. = reference; IQR = interquartile range; CIS = carcinoma in situ; EORTC = European Organization for Research and Treatment of Cancer; TURBT = transurethral resection of bladder tumor; BCG = Bacillus Calmette-Guérin; HSP = heat shock protein
Three multivariable models were generated to avoid overfitting of a single model.
Table 3.
Univariable and multivariable predictors of disease recurrence in patients with stage-T1high grade non-muscle invasive bladder cancer treated with intravesical Bacillus Calmette-Guérin immunotherapy
| Variable | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR(95%CI) | P | HR(95%CI) | P | ||||
| Sex | Male | Ref. | 0.948 | ||||
| Female | 0.96 (0.29 – 3.2) | ||||||
| Median age years (IQR) | 0.98 (0.94 – 1.01) | 0.211 | |||||
| Smoking status | Former/ Never | Ref. | 0.258 | ||||
| Current | 1.59 (0.71 – 3.53) | ||||||
| Tumor size | ≤3cm | Ref. | 0.117 | ||||
| >3cm | 1.92 (0.85 – 4.32) | ||||||
| Tumor number | Single | Ref. | 0.794 | ||||
| Multiple | 0.89 (0.39 – 2.07) | ||||||
| Recurrent tumor | No | Ref. | 0.366 | ||||
| Yes | 1.75 (0.52 – 5.9) | ||||||
| Presence of CIS | No | Ref. | 0.7 | ||||
| Yes | 1.48 (0.2 – 10.99) | ||||||
| EORTC score for recurrence | 0 – 14 | Ref. | 0.22 | Ref. | 0.416 | ||
| 5 – 17 | 1.85 (0.69 – 4.93) | 1.51 (0.56 – 4.04) | |||||
| RestagingTURBT | No | Ref. | 0.373 | ||||
| Yes | 1.45 (0.64 – 3.29) | ||||||
| Maintenance BCG Treatment | No | Ref. | 0.001 | Ref. | <0.001 | ||
| Yes | 0.27 (0.12 – 0.6) | 0.25 (0.11 – 0.56) | |||||
| % HSP60 expression | ≤65 | Ref. | 0.074 | ||||
| >65 | 2.16(0.93 – 5.02) | ||||||
| % HSP70 expression | ≤5 | Ref. | 0.004 | Ref. | 0.003 | ||
| >5 | 0.3 (0.14 – 0.68) | 0.29 (0.13 – 0.65) | |||||
HR = hazards ratio; CI = confidence interval; Ref. = reference; IQR = interquartile range; CIS = carcinoma in situ; EORTC = European Organization for Research and Treatment of Cancer; TURBT = transurethral resection of bladder tumor; BCG = Bacillus Calmette-Guérin; HSP = heat shock protein
In a cohort of 460 NMIBC patients who underwent comprehensive transcriptional analysis, multiple genes corresponding to HSP60, HSP70 and HSP90 were highly expressed in the class 2 and 1 sub-groups versus class 3 (Supplementary Figure 1). In this study, class 1 and class 2 tumors showed luminal-like characteristics but displayed different levels of aggressiveness.[15]
Discussion
In the current study, we evaluated the association between cytoplasmatic expression levels of HSP60, 70 and 90 in T1HG bladder tumors and long-term outcomes after intravesical BCG immunotherapy. At a median follow-up of 10 years, elevated HSP60 expression was associated with an increased risk of disease progression, while elevated HSP70 expression was associated with a decreased risk of disease progression and recurrence. Moreover, a combination of the two variables was associated with a 100% risk of recurrence and 80% risk of progression during follow-up.
Non-muscle invasive bladder cancer, especially stage-T1 high-grade tumors, have the potential to recur and progress. Intravesical BCG immunotherapy with a maintenance treatment period of 1–3 years decreases the risk of an adverse outcome. In a meta-analysis of randomized controlled trials recurrence rate was 43% at a median follow-up of 4.4 years, and progression rate was 12% at a median follow-up of 4.8 years, similar to the 5-year recurrence and progression rates seen in our cohort (41% and 19%, respectively).[16] The mechanism of action of BCG is not fully understood, however current data suggest both normal and cancerous urothelial cells and immune cells play a central role. Cancer cells likely internalizes BCG and present BCG together with other cancer antigens to cells of the immune system including CD4+ and CD8+ lymphocytes, natural killer cells, granulocytes, macrophages and dendritic cells which in turn bring about an immune reaction that kills cancer cells by direct cytotoxicity.[17] Nevertheless, BCG immunotherapy is associated with multiple side effects, and patients failing BCG therapy are at the highest risk of progression, hence the importance of identifying predictors of BCG treatment outcome that will improve on current, well established, pathologic predictors.[18]
Earlier studies evaluated the association between HSP expression levels and bladder cancer with inconsistent findings. Several groups have reported a high expression of HSP70 and HSP90 in bladder cancer cells, and a positive correlation between elevated HSP70 and HSP90 levels and tumor grade and stage[6–8], Lebret et al. found a decrease in expression levels of HSP27, 60, 70 and 90 in cancerous urothelium, and loss of HSP27 and HSP60 expression were associated with higher tumor stage.[9] Moreover, the loss of expression of either HSP60 or HSP90 were associated with disease progression, and all patients with HSP60 expression level <40% eventually developed muscle invasive disease.[9] Gene expression data from Hedegaard et al. suggest that higher expression levels of HSP60, 70 and 90 might be associated with luminal-like characteristics; however, a clear association with tumor aggressiveness was not observed.[15]
The association between HSP expression levels and treatment outcome after intravesical BCG immunotherapy was evaluated in a single study by Lebret et al.[11] A cohort of 33 patients with stage Ta-T1 high-grade tumors all of whom received BCG immunotherapy were followed for a mean period of 56 months. A significant correlation was found between decreased HSP90 expression and lack of BCG response. For all 6 patients who progressed despite BCG treatment, HSP90 expression levels were <40%. No correlation was found between HSP60 expression and BCG response.[11] In the current study, we found that decreased HSP70 expression was associated with tumor progression and recurrence, however, elevated rather than decreased levels of HSP60 were associated with disease progression. Differences in results may be attributed to different cohorts studied. To isolate the effects of previous exposure to intravesical immunotherapy we evaluated a homogenous group of primary T1HG tumors who did not receive previous intravesical BCG immunotherapy.
Our findings emphasize the complex association between HSP levels and tumor cell survival. The direct association between HSP60 levels and disease progression may be explained by the cellular protective effect of HSP. Cancer cells are in a constant state of external stress, thus the upregulation and expression of HSPs is essential for their survival by preserving protein structure and function.[19] HSP60 was found to activate cytoprotective pathways centered on stabilization of survivin levels within the mitochondria and formation of HSP60-p53 complexes, which inhibit p53 function within tumor cells, both of which limit the apoptosis of cancer cells.[20] The seemingly paradoxical inverse association between HSP70 levels and disease outcome is similar to previous findings regarding HSP90.[11] The loss of HSP expression may decrease the immune response against cancer cells. HSP70 enhances tumor antigen presentation by formation of a HSP70-antigen complex which is transferred to antigen presenting cells for cross-presentation and eventually activation of cytotoxic CD8 T cells.[21] A decrease in the HSP70 expression may therefore limit the load of BCG antigens presented by antigen presenting cells, decreasing the associated therapeutic effect. Furthermore, HSP70 stimulates the cytolytic activity of naïve natural killer cells against HSP70-positive tumor target cells, thus lower levels of HSP may lead to less activity of natural killer cells against these tumor cells.[22] Both mechanisms facilitate the immune evasiveness of urothelial cancer cells limiting the response to BCG immunotherapy.
The limitations of the current study include the studies retrospective nature, and the relatively small cohort size. Furthermore, not all patients underwent restaging TURBT leading to possible under-staging, and not all patients completed maintenance BCG. However, the current cohort consists of primary T1HG tumors treated with BCG with a long follow-up, all of whom were at a high risk for disease progression. While the homogenous nature of the cohort likely affected the significance of known predictors of outcome and may limit the generalizability of the findings, these patients pose the greatest clinical dilemma regarding limited vs. radical treatment, hence the importance of discovering additional predictors of outcomes within this specific group of patients.
Conclusion
The current study supports the association between increased HSP60 and decreased HSP70 cellular expression levels and adverse long-term outcome following BCG treatment of T1HG urothelial bladder tumors. If validated, HSP immunohistochemical staining may be used in addition to other patient and tumor factors for risk stratification and patient counseling regarding the role of early cystectomy or enrollment to clinical trials aimed at enhancing the anti-tumor effect of BCG in this patient population considering the higher risk for BCG failure. Additional studies using larger cohorts are required to understand the role of each HSP sub-type in regulating the BCG-mediated immune response, assess the association between changes in HSP levels during treatment and outcome, and evaluate whether manipulating HSP expression levels may enhance the therapeutic effect of BCG immunotherapy.
Supplementary Material
Supplementary Figure 1 - HSP60, 70 and 90 related genes with significantly different expression levels in pairwise combinations between the three classes of NMIBC as defined by Hedegaard et al.
Highlights.
Heat shock proteins are molecular chaperons that maintain cellular function
Elevated HSP60 levels predict disease progression in BCG treated T1HG bladder tumors
Decreased HSP70 levels predict disease progression and recurrence in this cohort
Patients with both predictors all recurred and had an 80% progression rate at followup
Further studies may validate HSP70 and HSP60 as significant predictors for BCG failure
Acknowledgments
Funding
Dr. Renzo G. Di Natale received support through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest and Disclosure Statement
All authors have nothing to disclose.
References
- [1].Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. European urology. 2017;71:96–108. [DOI] [PubMed] [Google Scholar]
- [2].Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. European urology. 2013;63:234–41. [DOI] [PubMed] [Google Scholar]
- [3].Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, et al. Bladder cancer. Lancet. 2016;388:2796–810. [DOI] [PubMed] [Google Scholar]
- [4].Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. European urology. 2017;71:447–61. [DOI] [PubMed] [Google Scholar]
- [5].Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Syrigos KN, Harrington KJ, Karayiannakis AJ, Sekara E, Chatziyianni E, Syrigou EI, et al. Clinical significance of heat shock protein-70 expression in bladder cancer. Urology. 2003;61:677–80. [DOI] [PubMed] [Google Scholar]
- [7].Behnsawy HM, Miyake H, Kusuda Y, Fujisawa M. Small interfering RNA targeting heat shock protein 70 enhances chemosensitivity in human bladder cancer cells. Urologic oncology. 2013;31:843–8. [DOI] [PubMed] [Google Scholar]
- [8].Cardillo MR, Sale P, Di Silverio F. Heat shock protein-90, IL-6 and IL-10 in bladder cancer. Anticancer research. 2000;20:4579–83. [PubMed] [Google Scholar]
- [9].Lebret T, Watson RW, Molinie V, O’Neill A, Gabriel C, Fitzpatrick JM, et al. Heat shock proteins HSP27, HSP60, HSP70, and HSP90: expression in bladder carcinoma. Cancer. 2003;98:970–7. [DOI] [PubMed] [Google Scholar]
- [10].Margel D, Pevsner-Fischer M, Baniel J, Yossepowitch O, Cohen IR. Stress proteins and cytokines are urinary biomarkers for diagnosis and staging of bladder cancer. European urology. 2011;59:113–9. [DOI] [PubMed] [Google Scholar]
- [11].Lebret T, Watson RW, Molinie V, Poulain JE, O’Neill A, Fitzpatrick JM, et al. HSP90 expression: a new predictive factor for BCG response in stage Ta-T1 grade 3 bladder tumours. European urology. 2007;51:161–6; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- [12].Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European urology. 2006;49:466–5; discussion 75–7. [DOI] [PubMed] [Google Scholar]
- [13].Lamm D, Persad R, Brausi M, Buckley R, Witjes JA, Palou J, et al. Defining progression in nonmuscle invasive bladder cancer: it is time for a new, standard definition. J Urol. 2014;191:20–7. [DOI] [PubMed] [Google Scholar]
- [14].Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PloS one. 2012;7:e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hedegaard J, Lamy P, Nordentoft I, Algaba F, Hoyer S, Ulhoi BP, et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer cell. 2016;30:27–42. [DOI] [PubMed] [Google Scholar]
- [16].Malmstrom PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. European urology. 2009;56:247–56. [DOI] [PubMed] [Google Scholar]
- [17].Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nature reviews Urology. 2014;11:153–62. [DOI] [PubMed] [Google Scholar]
- [18].Brausi M, Oddens J, Sylvester R, Bono A, van de Beek C, van Andel G, et al. Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. European urology. 2014;65:69–76. [DOI] [PubMed] [Google Scholar]
- [19].Ischia J, So AI. The role of heat shock proteins in bladder cancer. Nature reviews Urology. 2013;10:386–95. [DOI] [PubMed] [Google Scholar]
- [20].Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. The Journal of biological chemistry. 2008;283:5188–94. [DOI] [PubMed] [Google Scholar]
- [21].Bendz H, Ruhland SC, Pandya MJ, Hainzl O, Riegelsberger S, Brauchle C, et al. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. The Journal of biological chemistry. 2007;282:31688–702. [DOI] [PubMed] [Google Scholar]
- [22].Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biological chemistry. 2003;384:267–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 - HSP60, 70 and 90 related genes with significantly different expression levels in pairwise combinations between the three classes of NMIBC as defined by Hedegaard et al.