Abstract
Dermal wound healing is the process of repairing and remodeling skin following injury. Delayed or aberrant cutaneous healing pose a challenge for the health-care system. The lack of detailed understanding of cellular and molecular mechanisms involved in this process hamper the development of effective targeted treatments. In a recent study, Parfejevs and colleagues, by using state-of-the-art technologies, including in vivo sophisticated Cre/loxP techniques in combination with a mouse model of excisional cutaneous wounding, reveal that Schwann cells induce adult dermal wound healing. Strikingly, genetic ablation of Schwann cells delays wound contraction and closure, decreases myofibroblast formation, and impairs skin re-epithelization after injury. From a drug development perspective, Schwann cells are a new cellular candidate to be activated to accelerate skin healing. Here, we summarize and evaluate recent advances in the understanding of Schwann cells roles in the skin microenvironment.
Keywords: Schwann cells, skin, wound, healing, microenvironment
INTRODUCTION
Skin, cutis, is the largest organ of the human organism. It serves as the main boundary between the surroundings and the internal tissues (1). The skin maintains favorable visceral physiologic conditions by providing an equilibrium between liquid intake and loss, regulating the concentration of electrolytes, as well as controlling heat loss (2). The cutaneous tissue also shields internal organs from external harm provoked by diversified physical, chemical, and infectious agents (3). Wounds in the skin induced by burns, traumas, and chronic disorders are a major public health problem affecting millions, and resulting in the impairment of life quality, and in prolonged hospitalization period, which culminate in considerable health costs (4, 5).
Skin wound healing is a complicated and dynamic biological process with the aim of restoring the cutaneous’ barrier role. It comprises distinct yet overlapping well-coordinated and highly regulated sequence of events, which include clot formation, inflammation, tissue remodeling, new tissue formation, and revascularization (6, 7). Cutaneous repair occurs with an intricate cascade of interactions between cells present in the skin microenvironment, extracellular matrix proteins, and growth factors (8). A wide range of strategies have been developed for accelerating wound closure in skin lesions based on what we know so far about cutaneous healing (9). Deciphering the details of cellular and molecular mechanisms involved in dermal restoration is central in skin research. Discovering key cells and the underlying mechanisms participating in wound healing will create novel therapeutic strategies for skin repair. In particular, how stromal cells associated with the peripheral nervous system, Schwann cells, contribute to the skin recovery after lesion is not entirely clear. These cells are defined by their intimate relationship with peripheral axons throughout life (10, 11).
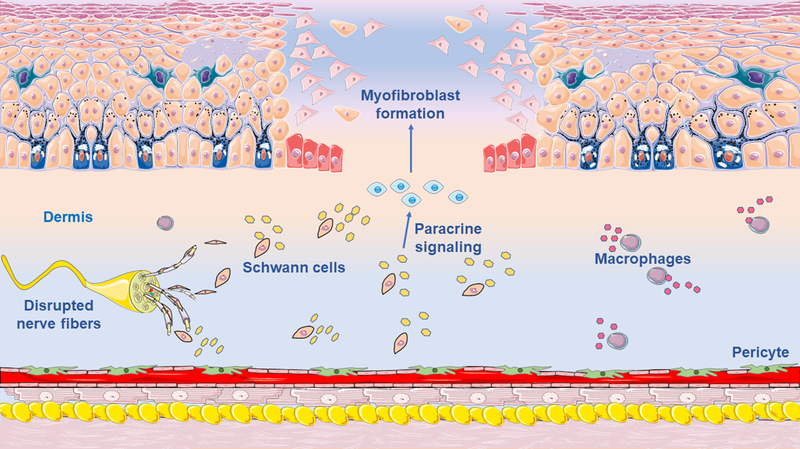
Now, in a recent article in Nature Communications, Parfejevs and colleagues reveal that Schwann cells contribute to adult dermal wound healing (12). The authors examined the role of Schwann cells in the injured skin by using elegant state-of-the-art techniques, including in vivo sophisticated Cre/loxP techniques in combination with a mouse model of excisional cutaneous wounding. In vivo lineage-tracing technologies to track specifically Schwann cells (Plp-CreER/tdTomato and Dhh-Cre/tdTomato mice) demonstrated that, after dermal lesion, endogenous Schwann cells disseminated from the disrupted peripheral nerves into the granulation tissue of the wounded skin, de-differentiated, and proliferated (12) (Figure 1). Strikingly, specific genetic ablation of Schwann cells, by using Plp-CreER/Sox10 floxed mice, delayed wound contraction and closure, decreased myofibroblasts formation, and impaired re-epithelization in the affected skin. Interestingly, conditional activation and expansion of Schwann cells, by Pten inactivation, triggered TGFβ signaling, and enhanced myofibroblasts generation. Additionally, by co-cultures of Schwann cells with fibroblasts, Parfejevs and colleagues suggested that Schwann cells promote differentiation into myofibroblasts via paracrine activation of transforming growth factor β (TGFβ) signaling. Although this work identifies a novel role for cutaneous Schwann cells, which could be used as a therapeutic target to improve dermal wound healing, some major questions still remain unanswered. For example, since TGFβ signaling is one of the main molecules orchestrating the wound healing process, it remains to be evaluated whether Schwann cells and/or their secreted molecules activate TGF-β signaling in vivo. Moreover, which myofibroblasts precursors are activated in the skin healing microenvironment, since Schwann cells do not differentiate into myofibroblasts? Or in which cell types important for the post-cutaneous injury scenario are Schwann cells differentiating?
Figure 1. Skin lesion activates Schwann cells to induce wound healing.
Cutaneous healing involves the cell-to-cell cross-talk between several cell types. Deciphering the cellular and molecular mechanisms that drive the wound closure is a central question in regenerative medicine. Parfejevs and colleagues now show that Schwann cells from the disrupted dermal peripheral nerves contribute to myofibroblasts formation, wound contraction, and re-epithelization of the injured skin (12). Future studies may reveal the complexity of the dermal microenvironment important for wound healing in much greater detail.
Here, we discuss the findings from this study, and evaluate recent advances and unresolved questions in our understanding of the role of Schwann cells in the cutaneous microenvironment.
PERSPECTIVES / FUTURE DIRECTIONS ORIGIN OF MYOFIBROBLASTS IN THE SKIN HEALING
Dermal wound closure is accompanied by skin contraction and the creation of scar tissue, that is defined by vigorous fibrous tissue deposition, which in excess may lead to cutaneous debilitating pathologies (13). The lack of detailed knowledge about the biological mechanisms involved in dermal fibrosis limits the success of clinical applications. The key cell producing fibrous tissue through extracellular matrix deposition is the myofibroblast (14). Understanding which cells generate cutaneous myofibroblasts is essential, as gaining control of those cells will allow the arrestment, or even the reversion of fibrosis production (15). This has been the focus of extensive basic research with the goal to improve the design of targeted anti-fibrotic therapies. Although the biological processes underlying fibrosis formation in the skin are not fully understood, multiple cell types have been suggested as the generators of myofibroblasts, including resident fibroblasts (16), epithelial cells (17), circulating progenitor cells (18), pericytes (19–23), and endothelial cells (24, 25). Parfejevs and colleagues demonstrated that Schwann cells do not differentiate into myofibroblasts in the adult skin during healing (12). However, since cell-to-cell communications may play positive roles during fibrous tissue deposition, it will be interesting to investigate which cells capable of originating myofibroblasts are activated during skin healing by Schwann cells to form matrix-producing cells, and how.
SCHWANN CELLS DERIVED SIGNALS IMPORTANT AFTER CUTANEOUS INJURY
Schwann cells release multiple bioactive molecules able to regulate behavior, proliferation and migration of other cellular populations (26–28). Therefore, Schwann cells may induce a reparative and regenerative milieu in the skin after lesion.
TGFβ signaling is one of the main pathways orchestrating wound healing, resulting in the secretion of extracellular matrix components (29). Parfejevs and colleagues show that in vivo genetically induced expansion of injury-activated Schwann cells induce myofibroblast appearance in the dermal wound via TGFβ signaling (12). The authors demonstrated that in vitro activated Schwann cells co-cultured with dermal fibroblasts also induced myofibroblast differentiation via TGFβ signaling (12). Nevertheless, it remains to be evaluated whether Schwann cells activates TGFβ signaling in dermal fibroblasts also in vivo. Also, it remains unsolved whether TGFβ itself is directly provided by injury-activated Schwann cells, and which molecules secreted by Schwann cells are important for this pathway activation. Schwann cells may produce activated TGFβ in other tissues (28), however whether they produce this molecule in the lesioned skin in vivo is still unknown. Additionally, whether Schwann cell-derived TGFβ is important for wound closure remains to be studied. TGFβ has not been yet conditionally deleted from cutaneous Schwann cells or from other possible sources in the skin, so there is no direct evidence that Schwann cells are the main/only functionally important source of TGFβ for myofibroblast differentiation. Transgenic mouse models have been widely applied to explore the roles of different cell populations within tissues-microenvironments. The ability, not only to ablate cells, but also to delete single genes in specific cell types in adult mice has allowed us to answer specific questions regarding the roles of distinct cell populations in the regulation of several physiologic and pathologic processes. The exact molecular mechanisms involved in cutaneous myofibroblast differentiation in vivo are yet unclear, and will need to be revealed in future works. The crossing of TGFβ floxed mice (30) with Schwann cell-specific inducible CreER drivers, such as PlpCreER, will allow us to specifically delete TGFβ in Schwann cells in vivo. In addition to studies in transgenic mice, transcriptomic and single Schwann cell analysis represent fundamental tools that will help us understand the role of Schwann cells in the skin microenvironment.
Platelet-derived growth factor β (PDGFβ) / Platelet-derived growth factor receptor β (PDGFRβ) signaling regulates events critical to fibrous tissue deposition. Activation of this pathway stimulates matrix-producing cells activation, migration, and proliferation (31). Parfejevs and colleagues exhibit that Schwann cells upregulate the expression of PDGFβ after dermal lesion (12). Interestingly, several stromal cells present in the skin microenvironment, important in wound healing progress, express the cell-surface tyrosine kinase receptor, PDGFRβ, such as pericytes (32–45), vascular smooth muscle cells (46, 47), and tissue-resident fibroblasts (48). Therefore, PDGFβ/PDGFRβ signaling may play crucial role in the communication between these important cell types during dermal healing. Future studies should explore the role of this pathway after injury in the skin. Importantly, inhibitors of this pathway, including sunitinib and imatinib have been proposed as anti-cancer drugs (49–51), and are Food and Drug Administration (FDA) approved (52).
SCHWANN CELLS PLASTICITY IN THE SKIN
Schwann cells were until recently considered cells committed exclusively to the glial fate. Surprisingly, recent elegant studies indicated that, in specific conditions, Schwann cells may also behave as stem cells forming other cell populations as well. Schwann cells have the ability to differentiate into melanocytes (53), chromaffin cells (11, 54), odontoblasts (55), endoneural fibroblasts (56), parasympathetic (57, 58) and enteric neurons (59). Due to this multipotency, Schwann cells could be potential targets for tissue repair and regenerative medicine. Although Parfejevs and colleagues determined that Schwann cells do not form myofibroblasts during skin healing (12), it remains to be studied whether dermal Schwann cells have the ability to differentiate into other cell types important post-cutaneous injury. Genetic fate-tracing mouse models should be explored further for assessing Schwann cell plasticity in vivo in the skin.
The decisions of stem cells to continue quiescent, self-renew or differentiate are dependent on the interaction of these cells with other cells in their surrounding niches (60–68). Recently, multiple cell types have been identified as potential niche-supporting cells for stem cells in the skin (69). Nevertheless, how the composition of the cutaneous stem cell niche is affected during skin healing remains poorly understood. Schwann cells have been shown to be essential components of the stem cell niche in the bone marrow (28, 70–76). Whether Schwann cells maintain dermal stem cells as well remains unknown. Interestingly, a recent study has revealed that osteoactivin is essential to activate mesenchymal stem cells in the skin, accelerating healing (77, 78). Future studies should explore whether dermal Schwann cells upregulate osteoactivin after lesion, and whether they contribute to endogenous activation of mesenchymal stem cells.
THE SKIN MICROENVIRONMENT
The skin involves an intricate microenvironment which contains, in addition to Schwann cells, several other types of stromal cells, immune cells (79, 80), innervations (11, 81), and extracellular matrix proteins. This complex mixture of cells cooperate to perform the necessary roles for the skin functioning, and the interplay between the distinct cellular components will define the dermal outcomes in distinct pathophysiological circumstances. Deciphering the individual and combinatorial signals that influence skin healing will help develop effective therapeutic interventions. Thus, what is the cross-talk between distinct Schwann cells involved in skin healing and other skin microenvironment cells remains to be examined. For instance, future studies are required to evaluate the importance of Schwann cell’ interactions with immune cells in skin healing. Further insights into the cellular and molecular processes involved in wound healing will have important implications for our understanding of skin homeostasis and disease.
SCHWANN CELLS’ HETEROGENEITY
Schwann cells are heterogeneous. They have been shown to be subdivided into subpopulations (10). Parfejevs and colleagues, in their study, consider Schwann cells as a homogeneous cell population (12). Schwann cells line nerve projections, protecting them and facilitating their survival. The skin is a richly innervated organ, with a variety of nerve fibers, including sensory and autonomic axons, influencing a multitude of physiological and pathophysiological cutaneous functions (82). It remains to be studied whether Schwann cells that support distinct types of nerve fibers also present differences in their behavior during dermal healing. The heterogeneity of Schwann cells in the skin should be defined in future studies. Therefore, it still needs to be evaluated whether cutaneous Schwann cells correspond to a homogeneous cell population or not. Are Schwann cells associate with sensory nerves the same cells as the ones attached to sympathetic fibers? Future research shall examine whether particular Schwann cell phenotypes relate to a precise behaviors after cutaneous lesion. The presence of dermal Schwann cells not expressing Plp, as well as their role in skin microenvironment, should be explored in future studies. Additionally, Schwann cells are subdivided in myelinating, associated with motor neurons, and non-myelinating Schwann cells. Future studies will need to reveal whether both myelinating, from sciatic nerves, and non-myelinating Schwann cells migrate to the wound in vivo after dermal injury. Also, it will be interesting to explore whether a subset of Schwann cells that participate in wound healing migrate from outside the skin.
CLINICAL RELEVANCE
Millions suffer with non-healing wounds around the world (83). Various disorders are characterized by poor skin healing, including skin atrophy, deformity, neuropathy, anaemia, microvascular disease, local factors, or the toxic effects of drugs used in therapy. Parfejevs and colleagues demonstrated that Schwann cells’ activation improved dermal wound healing (12). It will be interesting to explore whether Schwann cells could be also used as a cell therapy for skin healing. These results suggest that Schwann cell transplantation may improve wound closure. Moreover, wound restoration is important in multiple organs, and the healing process varies among distinct tissues. As Schwann cells are present in multiple tissues, are Schwann cells important for healing in other organs as well?
Although skin healing mouse model aims to recreate features of human wound after lesion, wound healing in mice may differ from the one in humans (84). Considering the peculiarity of each specie is essential to interpret the data correctly. Albeit the use of human patients have several limitations, such as ethical issues, logistical problems, and patient variability with regards to the extent and duration of the lesion, the combined use of different tools will reduce the limitations of each technique. Therefore, future analyses of human skin Schwann cells should reveal the translatability of this important Schwann cells novel role.
CONCLUSION
The study by Parfejevs and colleagues reveals a new important role of Schwann cells in the skin. Nevertheless, our understanding of dermal Schwann cell biology still remains limited, and future studies should shed light on the complexity and interactions of different cellular components of the cutaneous microenvironment during wound healing. A great challenge for the future will be to translate the research from mouse models into human patients. How human Schwann cells contribute to different stages of dermal wound healing remains to be determined. Improving the availability of human tissue samples will be essential to reach this goal.
ACKNOWLEDGMENTS
Alexander Birbrair is supported by a grant from Instituto Serrapilheira/Serra-1708–15285, a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); a grant from FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED-00570–16)], and a grant from FAPEMIG [Rede De Pesquisa Em Doenças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED-00313–16)]; Akiva Mintz is supported by the National Institute of Health (1R01CA179072–01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13-121-01-CDD).
DISCLOSURES
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Spencer JM, Amonette R. Tanning beds and skin cancer: artificial sun + old sol = real risk. Clinics in dermatology 1998;16(4):487–501. [DOI] [PubMed] [Google Scholar]
- 2.Macintyre L, Baird M. Pressure garments for use in the treatment of hypertrophic scars--a review of the problems associated with their use. Burns : journal of the International Society for Burn Injuries 2006;32(1):10–5. [DOI] [PubMed] [Google Scholar]
- 3.Burgdorf WH, Hoenig LJ. Changing skin colors. JAMA dermatology 2015;151(2):199. [DOI] [PubMed] [Google Scholar]
- 4.Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. Journal of the American Academy of Dermatology 2001;44(3):401–21; quiz 22–4. [DOI] [PubMed] [Google Scholar]
- 5.Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation 2014;130(4):333–46. [DOI] [PubMed] [Google Scholar]
- 6.Valls MD, Cronstein BN, Montesinos MC. Adenosine receptor agonists for promotion of dermal wound healing. Biochemical pharmacology 2009;77(7):1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, et al. Type-2 pericytes participate in normal and tumoral angiogenesis. American journal of physiology Cell physiology 2014;307(1):C25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453(7193):314–21. [DOI] [PubMed] [Google Scholar]
- 9.Pereira Lde P, Mota MR, Brizeno LA, Nogueira FC, Ferreira EG, Pereira MG, et al. Modulator effect of a polysaccharide-rich extract from Caesalpinia ferrea stem barks in rat cutaneous wound healing: Role of TNF-alpha, IL-1beta, NO, TGF-beta. Journal of ethnopharmacology 2016;187:213–23. [DOI] [PubMed] [Google Scholar]
- 10.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nature reviews Neuroscience 2005;6(9):671–82. [DOI] [PubMed] [Google Scholar]
- 11.Lousado L, Prazeres P, Andreotti JP, Paiva AE, Azevedo PO, Santos GSP, et al. Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell death & disease 2017;8(10):e3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parfejevs V, Debbache J, Shakhova O, Schaefer SM, Glausch M, Wegner M, et al. Injury-activated glial cells promote wound healing of the adult skin in mice. Nature communications 2018;9(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding J, Tredget EE. The Role of Chemokines in Fibrotic Wound Healing. Advances in wound care 2015;4(11):673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 biology reports 2010;2:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Science translational medicine 2013;5(167):167sr1. [DOI] [PubMed] [Google Scholar]
- 16.Barnes JL, Glass WF 2nd., Renal interstitial fibrosis: a critical evaluation of the origin of myofibroblasts. Contrib Nephrol 2011;169:73–93. [DOI] [PubMed] [Google Scholar]
- 17.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proceedings of the National Academy of Sciences of the United States of America 2006;103(35):13180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scholten D, Reichart D, Paik YH, Lindert J, Bhattacharya J, Glass CK, et al. Migration of fibrocytes in fibrogenic liver injury. The American journal of pathology 2011;179(1):189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clinical science 2015;128(2):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nature medicine 2012;18(8):1262–70. [DOI] [PubMed] [Google Scholar]
- 21.Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, et al. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem cell research & therapy 2014;5(6):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. American journal of physiology Cell physiology 2013;305(11):C1098–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Frontiers in aging neuroscience 2014;6:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature medicine 2007;13(8):952–61. [DOI] [PubMed] [Google Scholar]
- 25.Paiva AE, Lousado L, Almeida VM, Andreotti JP, Santos GSP, Azevedo PO, et al. Endothelial Cells as Precursors for Osteoblasts in the Metastatic Prostate Cancer Bone. Neoplasia 2017;19(11):928–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. The Journal of neuroscience : the official journal of the Society for Neuroscience 2002;22(15):6696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, et al. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. The Journal of cell biology 2012;198(1):127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 2011;147(5):1146–58. [DOI] [PubMed] [Google Scholar]
- 29.Finnson KW, McLean S, Di Guglielmo GM, Philip A. Dynamics of Transforming Growth Factor Beta Signaling in Wound Healing and Scarring. Advances in wound care 2013;2(5):195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azhar M, Yin M, Bommireddy R, Duffy JJ, Yang J, Pawlowski SA, et al. Generation of mice with a conditional allele for transforming growth factor beta 1 gene. Genesis 2009;47(6):423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Moon A, Kim HR. Both platelet-derived growth factor receptor (PDGFR)-alpha and PDGFR-beta promote murine fibroblast cell migration. Biochemical and biophysical research communications 2001;282(3):697–700. [DOI] [PubMed] [Google Scholar]
- 32.Santos GSP, Prazeres P, Mintz A, Birbrair A. Role of pericytes in the retina. Eye 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azevedo PO, Sena IFG, Andreotti JP, Carvalho-Tavares J, Alves-Filho JC, Cunha TM, et al. Pericytes modulate myelination in the central nervous system. Journal of cellular physiology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prazeres PHDM, Turquetti AOM, Azevedo PO, Barreto RSN, Miglino MA, Mintz A, et al. Perivascular cell αv integrins as a target to treat skeletal muscle fibrosis. Int J Biochem Cell Biol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prazeres P, Almeida VM, Lousado L, Andreotti JP, Paiva AE, Santos GSP, et al. Macrophages Generate Pericytes in the Developing Brain. Cellular and molecular neurobiology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, et al. Pericytes are heterogeneous in their origin within the same tissue. Developmental biology 2017;427(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa MA, Paiva AE, Andreotti JP, Cardoso MV, Cardoso CD, Mintz A, et al. Pericytes constrict blood vessels after myocardial ischemia. Journal of molecular and cellular cardiology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coatti GC, Frangini M, Valadares MC, Gomes JP, Lima NO, Cavacana N, et al. Pericytes Extend Survival of ALS SOD1 Mice and Induce the Expression of Antioxidant Enzymes in the Murine Model and in IPSCs Derived Neuronal Cells from an ALS Patient. Stem cell reviews 2017. [DOI] [PubMed] [Google Scholar]
- 39.Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, et al. Novel Peripherally Derived Neural-Like Stem Cells as Therapeutic Carriers for Treating Glioblastomas. Stem cells translational medicine 2017;6(2):471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birbrair A, Delbono O. Pericytes are Essential for Skeletal Muscle Formation. Stem cell reviews 2015;11(4):547–8. [DOI] [PubMed] [Google Scholar]
- 41.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, et al. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem cells and development 2013;22(16):2298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, et al. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 2013;10(1):67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PloS one 2011;6(2):e16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, et al. Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res 2013;319(1):45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos GSP, Magno LAV, Romano-Silva MA, Mintz A, Birbrair A. Pericytes plasticity in the brain. Neuroscience Bulletin 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997;277(5323):242–5. [DOI] [PubMed] [Google Scholar]
- 47.Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener 2010;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. The Journal of experimental medicine 2017;214(3):579–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Silva WN, Mintz A, et al. Targeting glioblastoma-derived pericytes improves chemotherapeutic outcome. Angiogenesis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paiva AE, Lousado L, Guerra DAP, Azevedo PO, Sena IFG, Andreotti JP, et al. Pericytes in the Premetastatic Niche. Cancer research 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azevedo PO, Paiva AE, Santos GSP, Lousado L, Andreotti JP, Sena IFG, et al. Cross-talk between lung cancer and bones results in neutrophils that promote tumor progression. . Cancer and Metastasis Reviews 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. The Journal of clinical investigation 2003;111(9):1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Muller T, et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 2009;139(2):366–79. [DOI] [PubMed] [Google Scholar]
- 54.Furlan A, Dyachuk V, Kastriti ME, Calvo-Enrique L, Abdo H, Hadjab S, et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 2017;357(6346). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014;513(7519):551–4. [DOI] [PubMed] [Google Scholar]
- 56.Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, et al. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 2004;131(22):5599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dyachuk V, Furlan A, Shahidi MK, Giovenco M, Kaukua N, Konstantinidou C, et al. Neurodevelopment. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science 2014;345(6192):82–7. [DOI] [PubMed] [Google Scholar]
- 58.Espinosa-Medina I, Outin E, Picard CA, Chettouh Z, Dymecki S, Consalez GG, et al. Neurodevelopment. Parasympathetic ganglia derive from Schwann cell precursors. Science 2014;345(6192):87–90. [DOI] [PubMed] [Google Scholar]
- 59.Uesaka T, Nagashimada M, Enomoto H. Neuronal Differentiation in Schwann Cell Lineage Underlies Postnatal Neurogenesis in the Enteric Nervous System. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015;35(27):9879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014;505(7483):327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nature medicine 2014;20(8):833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schofield R The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 63.Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, et al. How plastic are pericytes? Stem cells and development 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Annals of the New York Academy of Sciences 2016;1370(1):82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borges IDT, Sena IFG, de Azevedo PO, Andreotti JP, de Almeida VM, de Paiva AE, et al. Lung as a Niche for Hematopoietic Progenitors. Stem Cell Reviews and Reports 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andreotti JP, Prazeres PHDM, Magno LAV, Romano-Silva MA, Mintz A, Birbrair A. Neurogenesis in the postnatal cerebellum after injury. Int J Dev Neurosci 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Azevedo PO, Lousado L, Paiva AE, Andreotti JP, Santos GSP, Sena IFG, et al. Endothelial cells maintain neural stem cells quiescent in their niche. Neuroscience 2017;363:62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pereira LX, Viana CTR, Orellano LAA, Almeida SA, Vasconcelos AC, Goes AM, et al. Synthetic matrix of polyether-polyurethane as a biological platform for pancreatic regeneration. Life sciences 2017;176:67–74. [DOI] [PubMed] [Google Scholar]
- 69.Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nature medicine 2014;20(8):847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 2017;19(3):214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 2016;351(6269):176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Birbrair A Stem Cell Microenvironments and Beyond. Advances in experimental medicine and biology 2017;1041:1–3. [DOI] [PubMed] [Google Scholar]
- 73.Sena IFG, Borges IT, Lousado L, Azevedo PO, Andreotti JP, Almeida VM, et al. LepR+ cells dispute hegemony with Gli1+ cells in bone marrow fibrosis. Cell cycle 2017:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sena IFG, Prazeres P, Santos GSP, Borges IT, Azevedo PO, Andreotti JP, et al. Identity of Gli1+ cells in the bone marrow. Experimental hematology 2017;54:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Batista ML, Jr., Mintz A, et al. Adipocytes role in the bone marrow niche. Cytometry Part A : the journal of the International Society for Analytical Cytology 2018;93(2):167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alvarenga EC, Silva WN, Vasconcellos R, Paredes-Gamero EJ, Mintz A, Birbrair A. Promyelocytic leukemia protein in mesenchymal stem cells is essential for leukemia progression. Annals of Hematology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu B, Alboslemy T, Safadi F, Kim MH. Glycoprotein Nonmelanoma Clone B Regulates the Crosstalk between Macrophages and Mesenchymal Stem Cells toward Wound Repair. The Journal of investigative dermatology 2018;138(1):219–27. [DOI] [PubMed] [Google Scholar]
- 78.Silva WN, Prazeres P, Paiva AE, Lousado L, Turquetti AOM, Barreto RSN, et al. Macrophage-derived GPNMB accelerates skin healing. Experimental dermatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andreotti JP, Paiva AE, Prazeres PHDM, Guerra DAP, Silva WN, Vaz RS, et al. Natural Killer cells role in the uterine microenvironment during pregnancy Cell Mol Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andreotti JP, Paiva AE, Prazeres P, Guerra DAP, Silva WN, Vaz RS, et al. The role of natural killer cells in the uterine microenvironment during pregnancy. Cellular & molecular immunology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andreotti JP, Lousado L, Magno LAV, Birbrair A. Hypothalamic Neurons Take Center Stage in the Neural Stem Cell Niche. Cell stem cell 2017;21(3):293–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laverdet B, Danigo A, Girard D, Magy L, Demiot C, Desmouliere A. Skin innervation: important roles during normal and pathological cutaneous repair. Histology and histopathology 2015;30(8):875–92. [DOI] [PubMed] [Google Scholar]
- 83.Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clinics in dermatology 2007;25(1):19–25. [DOI] [PubMed] [Google Scholar]
- 84.Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cellular and molecular life sciences : CMLS 2014;71(17):3241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]