Abstract
Most analyses of genital immunity to herpes simplex virus type 2 (HSV-2) have been performed in females, consequently immune protection of the male genital epithelium is incompletely understood. We developed a model of male genital HSV-2 infection resulting from intrarectal inoculation of guinea pigs. Vesicular lesions developed transiently on the perineum and foreskin concurrent with acute virus shedding. Virus shedding and recurrent genital lesions were also detected after establishment of a latent infection. Analysis of perineum and foreskin RNA detected transcripts for IFNγ, proinflammatory and regulatory cytokines, and for genes involved in migration and regulation of leukocytes. HSV-specific T cells were detected in lymphoid and genital tissues after resolution of the primary infection whereas virus-specific antibody secreting cells were detected only in lymphoid tissue. Taken together, the ability to quantify pathogenesis and local immunity in this guinea pig model represent an important advance towards understanding immunity to HSV-2 in males.
Keywords: male guinea pig, HSV-2, foreskin, perineum, gene expression, IFNγ, microarray
1. Introduction
HSV-2 infects at epithelial surfaces and gains access to sensory nerve endings. Following transport to the nerve cell body in the sensory ganglia, it establishes a latent infection from which it can periodically reactivate, travel back down the nerve axon and re-emerge at or near the site of original infection. Despite the presence of vigorous humoral and cellular immune responses, HSV-2 can be shed in the presence or absence of clinical lesions (Corey et al., 1983; Tronstein et al., 2011). Asymptomatic shedding occurs as frequently as 10% of days and is believed to be the major cause of the high rates of transmission worldwide (Rooney et al., 1986; Tronstein et al., 2011; Wald et al., 2000). In 2012, HSV-2 was responsible for approximately 20 million new infections worldwide (Looker et al., 2015). The seroprevalence of HSV-2 in the U.S. for the years 2007 through 2010 was estimated at 15.5% for individuals between 14 and 49 years of age (Fanfair et al., 2013) but has subsequently declined to 11.9% during 2015-2016 (McQuillan et al., 2018). HSV infection of newborns at birth as a result of exposure to virus in the birth canal of an infected mother can result in devastating morbidity with approximately 60% mortality if untreated (Brown et al., 1987; Whitley et al., 1991). The number of neonatal HSV-2 cases worldwide is currently estimated at 10,000 cases annually (Looker et al., 2017). HSV-2 infection increases the risk of acquisition of HIV (Freeman et al., 2006; Holmberg et al., 1988; Wald and Link, 2002) which is thought to be due in part to the accumulation and maintenance of HIV target cell populations at sites of HSV infection and to the disruption of the epithelial barrier following development of genital ulcers (Johnson et al., 2011; Shannon et al., 2014).
Genital HSV-2 infection is less common in men than women (Bryson et al., 1993; Mertz et al., 1992) which may reflect differences in susceptibility due to the anatomy of the epithelial surfaces or duration of exposure to virus. However, it has been reported that men experience more frequent virus reactivations than women (Benedetti et al., 1994) and both genders shed virus during periods of active recurrent lesions as well as in the absence of clinical symptoms (Wald et al., 2002). Asymptomatic shedding episodes increase the risk of virus transmission and highlight the need to develop effective vaccines that control HSV shedding in the genital tract. In experimental animal models of genital HSV-2 infection, much of what we know about the pathobiology and immunology of HSV-2 infection has come from the study of genital infection of female animals. Less is understood about the expression of immunity in the male genital epithelium. The ability to accurately detect and measure immunological events at the epithelial sites of infection and in particular local cell-mediated events is most likely critical for development of methods to confer immune protection in both genders. In the current study, the development of a male guinea pig model of HSV-2 genital infection allowed us to define the innate and adaptive immune responses that develop at the site of HSV-2 infection.
Early attempts to initiate a genital infection by direct inoculation of the penis did not result in consistent infection (Lukas et al., 1974). Later attempts using a zosteriform spread approach (Stephanopoulos et al., 1989) in which the virus inoculum was delivered to scarified skin on the medial thigh resulted in a high percentage of inoculated animals experiencing symptomatic infection including the development of vesicular lesions in the genital area and penis with shedding of virus from the urethra. We modified this model by delivering the virus intrarectally (IREC) and show that male animals consistently developed acute and recurrent HSV-2 lesions on the perineum and foreskin. We also enhanced the utility of the model by detecting and quantifying virus shedding as well as the local immune response to the genital infection. The results of this study demonstrate a robust immune response to genital HSV-2 infection including an IFNγ-dominant cell-mediated response most likely produced by Natural Killer (NK) cells and T lymphocytes. We also assessed the magnitude and location of HSV-specific memory T cells and antibody secreting cells (ASC) resident in secondary lymphoid tissue and the genital epithelium of previously infected animals. The use of this model should provide a unique testing venue for candidate vaccines in which the nature and magnitude of both systemic and genital vaccine-induced immune responses can be assessed and correlated with vaccine efficacy towards providing vaccine-mediated protection to both genders.
2. Results
2.1. HSV-2 disease at the perineum and foreskin following IREC inoculation of HSV-2
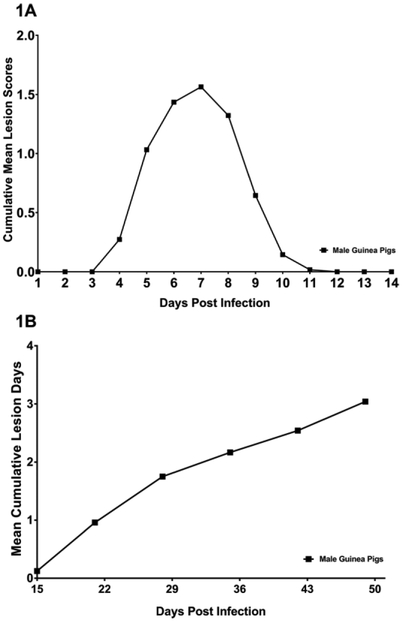
A useful model for determining the nature of local genital immunity to HSV-2 infection requires reproducible genital infection with consistent development of acute and recurrent HSV disease and virus shedding comparable to that observed in humans and female guinea pigs. To test if IREC inoculation resulted in reproducible infection and pathogenesis, thirty-one male guinea pigs (results combined from 3 separate studies) were inoculated IREC with 6.0 log10 plaque forming units (pfu) HSV-2 strain MS. Twenty-eight (90.3%) of the animals developed vesiculo-ulcerative primary genital skin disease. Lesions were observed on the perineum and penis beginning on day 4 post inoculation (p.i.), with disease severity peaking on day 7 p.i. and the skin disease resolving in all animals by day 12 p.i. (Fig. 1A). The mean severity of the primary skin disease as measured by the cumulative lesion score was 6.4 ± 3.4 (±SD) and the duration was 5.4 ± 1.2 days. Following resolution of primary disease, 24 of the animals were observed from day 15 until day 49 p.i. for the development of recurrent genital lesions. During that period, the mean number of days with recurrent lesions for animals in the group was 3.0 ± 0.5 (Fig 1B). However, there was considerable variability among individual animals. Four animals (16.6%) experienced no days with recurrences while in the remaining animals there was a range from 1-10 lesion days. These results are similar to those obtained from a group of 27 female guinea pigs infected intravaginally with the same dose of virus. During the same time period these animals experienced a mean of 2.5 ± 0.5 days with recurrent lesions (P= 0.48 compared to males, Student’s t test). Seven of the female animals (26%; P= 0.51 compared to percentage of male animals with no lesions, Fisher’s exact test) experienced no recurrent disease while the range in lesion days in the remaining animals was from 1-10 days.
Fig 1. Primary and recurrent disease in male guinea pigs following IREC HSV-2 inoculation.
Thirty-one male guinea pigs (results combined from 3 separate studies) were inoculated IREC with 6.0 log10 pfu HSV-2 strain MS and scored daily for (A) primary genital disease and the results expressed as the cumulative mean lesion score. (B) Twenty-four of these animals were observed for recurrent disease. Results are expressed as the mean cumulative lesion days.
To detect virus shedding during the primary infection, the perineum and foreskin areas of nine animals were swabbed daily on days 5-7 p.i. and assayed by qPCR (Bourne et al., 2005; Veselenak et al., 2012). At the perineum, HSV-2 DNA was detected in the swabs of all animals at each time point (Table 1). The mean number of genomes in animals shedding virus on day 5 p.i. was 1.76 ±0.14 (log 10) and increased to 3.20 ±0.32 (log 10) on day 7 p.i. HSV-2 genomes were also detected on the external foreskin of 8/9, 7/9, and 8/9 animals on days 5, 6, 7 p.i. respectively. Similar to shedding at the perineum, the mean number of genomes from animals shedding virus increased from 1.98 ± 0.42 (log 10) on day 5 p.i. to 3.31 ±0.39 (log 10) on day 7 p.i.
Table 1.
HSV-2 shedding from perineum and foreskin following IREC HSV-2 inoculation of male guinea pigs.
| Primary disease | |||||||
| Day | Incidencea | Mean HSV Genomesb | Day | Incidencea | Mean HSV Genomesb | ||
| Perineum | 5 | 9/9 | 1.76 ± 0.14 | Foreskin | 5 | 8/9 | 1.98 ± 0.42 |
| 6 | 9/9 | 2.52 ± 0.22 | 6 | 7/9 | 3.22 ± 0.53 | ||
| 7 | 9/9 | 3.20 ± 0.32 | 7 | 8/9 | 3.31 ± 0.39 | ||
| Latent disease | |||||||
| Day | Incidencea | Mean HSV Genomesc | |||||
| Foreskin | 25 | 2/5 | 1.34 ± 0.24 | ||||
| 26 | 4/5 | 1.035 ± 0.17 | |||||
| 27 | 2/5 | 0.89 ± 0.14 | |||||
| 28 | 0/5 | --- | |||||
Number of animals shedding HSV-2 per the total number of animals sampled.
Results are expressed as the mean number of genomes ± SEM (log 10).
Mean copy number ± SEM only from those animals shedding virus.
We also examined virus shedding at the foreskin during recurrent HSV disease on days 25-28 p.i. in a separate group of animals. Table 1 shows that all five animals shed virus during that period although both the daily incidence and the number of genome copies detected was lower than during active primary infection (Table 1).
2.2. Analysis of immune gene transcripts following IREC HSV-2 inoculation
The nature of early immunity to HSV-2 at the male genital epithelium is not completely understood. To define the nature of the innate and early adaptive immune response in the male genital epithelium, guinea pigs were inoculated IREC with HSV-2 strain MS and sacrificed on the day of (day 1), or days 4 or 20 after development of the first HSV-2 lesions on the perineum. Biopsies of perineum and foreskin were taken and RNA was extracted from biopsied tissue and analyzed for the transcription of 44 immune-related genes using a modified version of a previously described qPCR microarray (gpArray) (Veselenak et al., 2015; Veselenak et al., 2018; Wali et al., 2014). Three different transcription patterns based on Ct values were apparent in the perineum which roughly correlated with the time after development of first lesion (heat map, Supplemental Fig 1A). In general, comparison of gene expression at the perineum on lesion days 1, 4, or 20 relative to perineum tissue from uninfected guinea pigs (D0) revealed high expression of many genes on lesion day 1, peak expression of the majority of genes on lesion day 4 and diminished expression of most genes on lesion day 20 (Table 2). Message for CD4 was >6-fold (p < 0.05, Student’s t test) higher and >100-fold higher on lesion days 1 and 4, respectively. Transcription of CD8α followed a similar pattern, peaking at >140-fold higher expression (p < 0.05, Student’s t test) relative to naïve controls before diminishing by lesion day 20, suggesting the presence of increased numbers of T lymphocytes in the infected perineum tissue. A number of genes encoding proteins responsible for regulation of T cell proliferation, activation, or function including CD25, CD28, CD40 CD72, CD134, CTLA-4 and PD-1 were also expressed at higher levels relative to uninfected control tissue on lesion days 1 or 4. The response overall was dominated by transcription of IFNγ which was increased by a mean of >630-fold on lesion day 1 and increasing to >7800-fold on lesion day 4 compared to uninfected tissue (p < 0.05, Student’s t test). Not surprisingly, the IFNγ-regulated chemotaxins CCL2, CCL5, CCL7, CXCL10, and CXCL11 also showed a similar pattern of expression on lesion days 1 and 4 with diminishing expression on lesion day 20. Gene transcription patterns suggest the cellular response was type 1-like with increased levels of IFNγ, TNFα, IL-2, and perforin on lesion days 1 and 4. However, increased transcription of the type 2 cytokines IL-4 and IL-10 were also detected, peaking on lesion day 4 (~7-fold and ~33-fold, respectively). Genes for IL-1β, IL-17A, IL-21, and IL-27 were also up-regulated on lesion days 1 and 4 while IL-7, IL-15 and IL-33 showed low up-regulation or diminished expression on all lesion days.
Table 2.
Fold-change in gene expression in HSV-2 infected perineum tissue compared to uninfected perineum.
| Gene | Lesion day 1a | Lesion day 4 | Lesion day 20 |
|---|---|---|---|
| CCL2/MCP-1 | 15.93* | 59.98* | 1.32 |
| CCL5/RANTES | 55.67* | 1956.34* | 43.65 |
| CCL7/MCP-3 | 503.18* | 464.21* | 1.06 |
| CD4 | 6.34* | 101.67 | 3.93 |
| CD8 alpha | 2.73 | 143.47* | 3.41* |
| CD14 | 2.08 | 41.78* | 1.60 |
| CD25 | 5.06* | 83.69* | 1.52 |
| CD28 | 2.80* | 106.06 | 2.58 |
| CD39 | −2.19* | 2.79* | 1.98* |
| CD40 | 5.03* | 11.34* | 1.98* |
| CD62L | 2.53 | 2.92* | 1.86 |
| CD72 | −1.23 | 19.98* | 1.55 |
| CD94 | 8.00* | 213.59 | 5.09 |
| CD96 | 5.04* | 161.32* | 4.99 |
| CD103 | 2.14 | 9.04* | 1.55 |
| CD134 | 3.79* | 10.16* | 2.33* |
| CD152/CTLA-4 | 6.97 | 374.95 | 8.73 |
| CD223/LAG3 | 2.31 | −3.78 | −7.65 |
| CXCL10 | 2514.36* | 606.16* | 10.35* |
| CXCL11 | 12814.25* | 9896.33* | 136.50* |
| CXCL12 | −5.25* | 2.12 | 1.35 |
| IFNβ | −2.04 | −44.36 | 1.06 |
| IFN γ | 634.80* | 7818.20* | 29.45 |
| IFNAR1 | −1.05 | 1.23 | 1.51 |
| IFNAR2 | 2.41* | 6.07* | 1.34 |
| IFNGR1 | 3.21* | 3.23* | 1.69* |
| IL-1b | 80.04 | 519.62* | 10.47 |
| IL-2 | 1.74 | 240.82* | 3.53 |
| IL-4 | 1.44 | 6.96 | 1.25 |
| IL-7 | 2.48 | 1.83 | 1.37 |
| IL-10 | 5.15* | 32.77* | −4.11 |
| IL-12 p35 | −13.07* | −4.04 | −1.67 |
| IL-12 p40 | 2.01 | 26.10 | 1.79 |
| IL-15 | −1.08 | 1.75 | −164.37 |
| IL-17A | 13.61* | 55.54 | 1.68 |
| IL-21 | 7.27* | 307.65 | 2.90 |
| IL-27 | 11.93* | 28.07 | 2.14 |
| IL-33 | −21.62* | −1.57 | 2.21 |
| KLRG1 | −1.45 | 5.64 | 1.03 |
| PDCD-1 | 4.37 | 132.53 | −1.79 |
| PRF-1 | 18.24* | 276.77* | 5.22 |
| TGFβ | 11.30 | 32.29 | 25.61 |
| TNFα | 5.02* | 7.97* | 1.71 |
Values marked with an asterisk reached statistical significance compared to gene expression in uninfected perineum (p<0.05, Student’s t test).
Visible lesions were not always apparent on the foreskin on days that active lesions were present on the perineum, most likely reflecting differences in the time of zosteriform spread of the infection from the site of inoculation in the rectum, to the sensory ganglia and back to the perineum and foreskin. To ensure we evaluated immune gene expression in infected foreskin tissue on lesion days 1 and 4, immune gene transcription was analyzed only for those foreskin samples in which transcripts of the HSV-2 gD gene were detected. Because all inoculated animals experienced primary genital lesions on the perineum, all day 20 samples were considered to have come from infected tissue. Gene transcription in the foreskin appeared greatest in samples in which higher viral transcripts were detected (Supplemental Fig 1B). The three foreskin biopsies containing the highest number of HSV-2 gD transcripts (>103) expressed nearly the same array of genes as in the perineum whereas the four foreskin samples containing lower HSV-2 gD transcript levels (<5 × 102) expressed a much more restricted pattern of genes comprised predominantly of IFNγ and IFNγ –related chemokine genes. The fold-change in expression of the immune-associated genes in foreskin tissue is shown in Table 3. Transcription levels of individual genes in infected foreskins were more variable among individual animals and as a consequence, fewer of the mean increases in gene expression reached significance compared to transcription increases in the perineum. CD4 and CD8α were also upregulated in foreskins with high virus genome load suggesting the increased presence of activated T cells. Notably, IL-1β, IL-17A, and IL-27 were not expressed at increased levels relative to naïve control tissue although expression of all three genes was upregulated in the HSV-2-infected perineum (Table 1) and from previous studies (Veselenak et al., 2018) in the vaginal mucosa of HSV-2 infected guinea pigs. To provide more detailed information about the mRNA levels of these cytokines in the foreskins of individual animals during the primary infection, the mRNA samples collected from foreskins of uninfected and HSV-2 gD transcript-positive animals were analyzed individually by quantitative RT-PCR (qRT-PCR). While transcription of these genes was detected, the expression levels were somewhat variable among the 7 sampled animals and the mean number of transcripts was not significantly different than in uninfected foreskins. These results were consistent with the transcription patterns obtained by gpArray of Table 3 (G. Milligan, unpublished results).
Table 3.
Fold-change in gene expression in HSV-2 infected foreskin compared to uninfected foreskins.
| Gene | > 103 HSV gD transcriptsa |
< 5×102 HSV gD transcripts |
Day 20 |
|---|---|---|---|
| CCL2/MCP-1 | 8.80 | −8.65 | 1.55 |
| CCL5/RANTES | 65.33 | 2.31 | 6.52* |
| CCL7/MCP-3 | 5.67 | −1.44 | 1.40 |
| CD4 | 5.22 | 1.10 | 2.66 |
| CD8 alpha | 4.36 | −1.26 | 3.17 |
| CD14 | 1.66 | −2.22 | 1.73 |
| CD25 | 2.13 | −1.16 | 1.56 |
| CD28 | 3.56 | −1.47 | 1.68 |
| CD39 | −1.21 | −2.78 | 1.49 |
| CD40 | 1.63 | 1.03 | 1.99 |
| CD62L | −2.43* | −2.73 | −1.65 |
| CD72 | 8.82 | −3.15 | 8.77 |
| CD94 | 10.26 | 1.49 | 7.30 |
| CD96 | 118.12 | 31.71 | 51.97 |
| CD103 | −1.12 | 1.15 | 1.48 |
| CD134 | −1.42 | −1.55 | −1.24 |
| CD152/CTLA-4 | 49.12 | −17.78 | −1.13 |
| CXCL10 | 107.59 | 30.39 | 6.91* |
| CXCL11 | 354.47 | 21.16* | 17.89* |
| CXCL12 | 2.54 | 1.27 | 1.67 |
| IFNβ | −1.49 | −2.20 | −26.73 |
| IFN γ | 134.01 | 4.14 | 12.49 |
| IFNAR1 | −1.19 | −2.06 | 1.36 |
| IFNAR2 | 1.62 | −1.50 | 1.39 |
| IFNGR1 | 1.07 | −1.40 | 1.14 |
| IL-1b | 1.85 | 1.71 | −1.02 |
| IL-2 | 1.28 | −4.91 | 2.89 |
| IL-4 | −1.24 | −1.81 | 1.14 |
| IL-7 | 4.25 | 1.90 | 4.34* |
| IL-10 | 2.81 | −7.14 | 1.62 |
| IL-12 p35 | 1.01 | −5.13 | 1.22 |
| IL-12 p40 | −1.41 | −10.06 | 1.49 |
| IL-15 | 2.27 | −3.00 | 2.04 |
| IL-17A | 1.02 | −1.07 | 1.14 |
| IL-21 | 3.00 | −1.38 | 4.28 |
| IL-27 | 1.87 | −1.19 | 1.88 |
| IL-33 | 1.81 | 1.31 | 4.86* |
| KLRG1 | −1.29 | −3.22 | 1.57 |
| PRF-1 | 9.03 | −1.39 | 2.70 |
| TGFβ | −1.08 | −2.52 | −1.12 |
| TNFα | −1.40 | −1.74 | −1.53 |
Biopsies from lesion days 1 and 4 were segregated for analysis according to the number of HSV gD transcripts present (> 103 HSV gD (n=3) or < 5×102 HSV gD (n=4). Fold-change values marked with an asterisk reached statistical significance compared to gene expression in uninfected foreskin (p<0.05, Student’s t test).
2.3. Local and systemic HSV-specific T cell responses following IREC HSV-2 inoculation
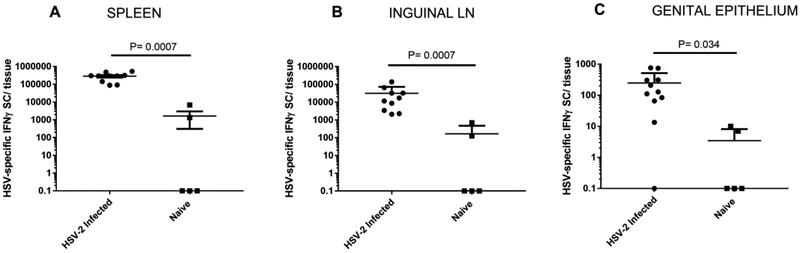
We previously showed that HSV-specific T lymphocytes are readily detectable in the genital epithelium of female guinea pigs at times greater than one year post infection (Xia et al., 2014). We used an IFNγ ELISPOT assay to test if a similar population of HSV-specific T cells became resident in the foreskin and perineum tissue of IREC HSV-2 infected male animals. As described previously in HSV-infected female guinea pigs (Xia et al., 2014), significantly increased numbers of IFNγ secreting cells (SC) were detected following HSV stimulation of lymphocytes obtained from the spleen (P = 0.0007, Mann-Whitney test), inguinal lymph nodes (ingLN) (P = 0.0007, Mann-Whitney test), and genital epithelium (P = 0.034, Mann-Whitney test) from HSV-2 infected animals compared to uninfected controls (Fig 2).
Fig 2. HSV-specific IFNγ SC are detected in secondary lymphoid tissues and genital epithelium of IREC-inoculated male guinea pigs.
Spleens (A), ingLN (B) and genital epithelium (C) from uninfected control guinea pigs and HSV-inoculated animals were harvested 1.5-4.5 months after IREC inoculation. IFNγ SC were detected by ELISPOT. Values shown are the mean numbers of IFNγ SC per tissue following HSV-stimulation ±SEM. Background IFNγ SC observed from control (medium only) stimulation have been subtracted. (* P < 0.001, ** P < 0.05; two-tailed, nonparametric Mann-Whitney test). Because the Y axis is logarithmic, zero values are depicted as 0.1. The results shown are from 10-11 animals from 2 separate experiments.
2.4. Humoral immunity following IREC HSV-2 inoculation
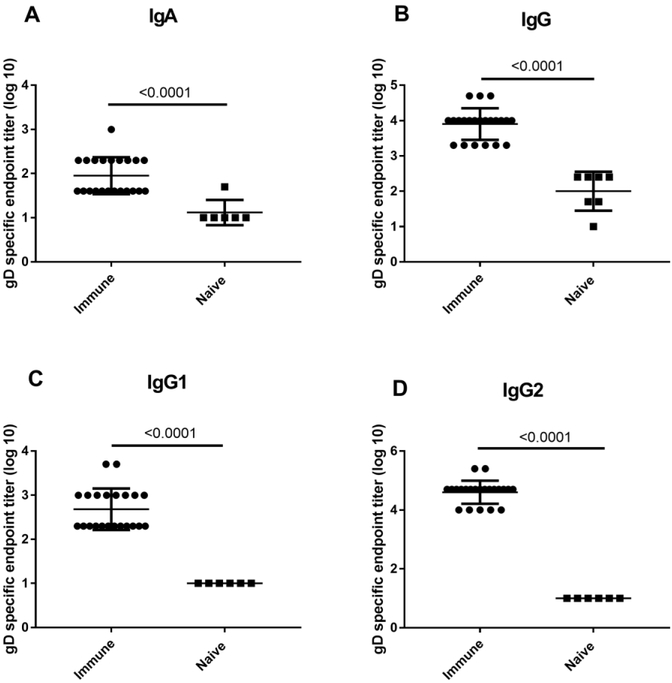
Vigorous HSV-specific antibody responses, characterized by the presence of HSV-specific antibody secreting cells (ASC) have been detected systemically in secondary lymphoid tissue, bone marrow and locally in the vaginal mucosa (Xia et al., 2014). We assessed the humoral response to IREC inoculation of male guinea pigs to determine the nature and localization of the HSV-specific antibody response. Although HSV-specific IgA antibodies were detected (endpoint titer 1.95 ± 0.089 [log10]) in the serum 1.5-4.5 months after HSV-2 infection in male animals, the majority of, the HSV gD-specific antibodies detected were primarily of the IgG isotype (endpoint titer 3.91 ± 0.095 [log10]) (Fig. 3A, B). Moreover, while both IgG1 and IgG2 subclasses were present in serum, HSV gD-specific IgG antibodies were predominantly of the IgG2 subclass (IgG1 endpoint titer 2.68 ± 0.10 [log10]; IgG2 endpoint titer 4.61 ± 0.083 [log 10]) (Fig. 3C, D). The antibody titer detected by ELISA was comparable to levels detected in our historical studies in females inoculated by the intravaginal or IREC routes (G. Milligan, unpublished results).
Fig 3. Isotypes of HSV-specific serum antibodies following IREC HSV-2 inoculation of male guinea pigs.
Serum was collected from male guinea pigs between 1.5- 4.5 months after inoculation and the endpoint titer of IgA (A), IgG (B), IgG1 (C), and IgG2 (D) gD-specific antibodies was determined by ELISA. Results shown are the mean endpoint titer ± SEM for 22 HSV-2 inoculated guinea pigs from three separate experiments. The limit of detection for these assays was a 1:10 dilution. Asterisk indicates significant differences between endpoint titers of sera from immune and naïve animals (P < 0.0001, unpaired, two-tailed Student t test).
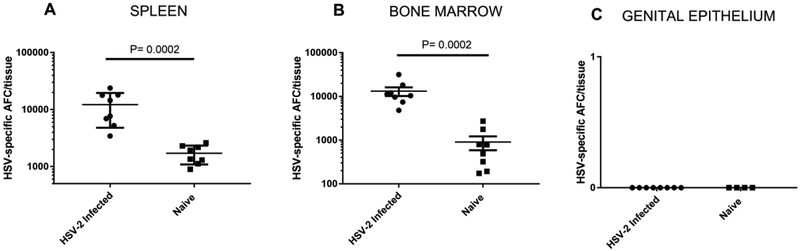
Antigen-specific antibody plays an important role in protection against reinfection of epithelial tissue, inhibits virus spread to neurons (Bourne et al., 2002), and is produced at extended periods of time after immunization or infection primarily by long lived plasma cells. We have previously detected HSV-specific ASCs in the spleens, bone marrow and genital epithelium of female guinea pigs up to 8 months after intravaginal HSV-2 infection (Milligan et al., 2005; Xia et al., 2014). To determine if HSV-specific ASC were similarly disseminated following IREC infection of male animals, we harvested spleens, bone marrow and genital epithelium from male guinea pigs infected IREC 1.5 to 4.5 months previously with HSV-2. As shown in Figure 4, significantly greater numbers of HSV-specific ASC were detected in the spleens and bone marrow of HSV-2 infected animals compared to uninfected controls (P = 0.0002, Mann-Whitney test). By contrast, HSV-specific ASC were not detected in the genital epithelium of any HSV-2 infected male guinea pig.
Fig 4. HSV-specific ASCs are detected in secondary lymphoid tissue, but not from the genital epithelium of male guinea pigs following IREC HSV-2 inoculation.
Single cell preparations of (A) spleen, (B) ingLN, and (C) genital tract digest were obtained from male guinea pigs inoculated IREC 1.5 - 4.5 months previously with HSV-2. HSV glycoprotein-specific ASC were quantified by ELISPOT. Background values obtained on a control plate coated with the non-specific antigen chicken ovalbumin were subtracted from the ASC numbers detected on HSV glycoprotein plates. The results are presented as the mean HSV-specific ASC response ± SEM from groups of eight HSV-immune, and eight non-immune male guinea pigs from two separate experiments. An asterisk indicates significant differences between the means of HSV-immune and non-immune animals (P < 0.001, nonparametric Mann-Whitney test).
3. Discussion
A previously described model of male HSV-2 genital infection utilized virus inoculated onto the scarified surface of the inner thigh and resulted in reproducible development of HSV disease characterized by self-limiting vesicular lesions on the genital epithelium (Stephanopoulos et al., 1989). In the studies described here we utilized IREC inoculation as a less invasive route that represents a clinically relevant initial site of infection in men who have sex with men (Krone et al., 1998; Krone et al., 2000). Similar to the study of Stephanopoulos et al. (Stephanopoulos et al., 1989), we observed vesicular genital epithelial lesions that developed initially on the perineum and then later on the external foreskin. This pattern of lesion development most likely reflects differences in the time required for zosteriform spread of the virus from the rectum to the sensory ganglia and back to the perineum and foreskin. The previous study detected HSV-2 shedding from the urethra in the majority of animals with a peak onset occurring on days 5-9 p.i. Similarly, we detected shedding of virus at the perineum and foreskin of 8/9 (89%) animals within a similar time frame. We extended the utility of the model by demonstrating HSV-2 shed from the foreskin after the onset of latency on days 25-28 although, as anticipated, both the incidence and amount of virus shed during latent infection were lower than during the primary infection. In addition to virus shedding, male animals also experienced spontaneous reactivations with development of recurrent lesions on the genital epithelia. Interestingly, similar to recurrent disease patterns in humans (Benedetti et al., 1994), the male animals utilized in these studies trended towards more days with recurrent lesions than did female animals inoculated by the intravaginal route however the difference did not reach significance. Taken together, the male guinea pig model exhibits acute and recurrent HSV disease as it occurs in humans and is amenable to detection and quantification of specific pathogenic events associated with genital HSV-2 disease.
We used the gpArray (Veselenak et al., 2015; Veselenak et al., 2018) to quantify the expression of 44 immune-related genes in the perineum and foreskin of male animals following IREC HSV-2 inoculation. Overall, the response in males was strikingly similar to the response previously detected in the vaginal mucosa of HSV-2 infected female animals (Veselenak et al.,2018). The cytokine response in males was dominated by the expression of the IFNγ gene and IFNγ-regulated chemokine genes including CCL2, CCL5, CCL7, CXCL10 and CXCL11 as shown previously in female guinea pigs (Veselenak et al., 2018) and mice (Cherpes et al., 2013). Similarly, CD96 which is involved in regulation of NK cell responses was highly expressed in male animals. Interestingly, lymphocyte activation gene 3 (LAG3) was among the most highly expressed genes on days 3-7 in HSV-2 infected females (Veselenak et al., 2018), but was not upregulated in the perineum or foreskin of infected male animals in the current study. The reason for the differential expression of this gene in the vaginal epithelium of females versus the perineal epithelium of males is not clear at this time but may reflect differences in the number of cells expressing LAG3, different kinetics of expression, or differential regulation of T cell responses at these two distinct genital epithelial locations. The cytokines IL-21 and IL-27 were also previously shown to be among the most highly expressed genes in the vaginal mucosa on days 3-7 p.i. In males, IL-21 was highly upregulated in the perineum (~7-fold upregulated lesion day 1 and ~307-fold upregulated on lesion day 4) and was upregulated ~3-fold in the foreskin in animals with the highest virus transcription. By contrast, IL-27 was not upregulated in the foreskin but was ~12-fold and ~28-fold upregulated in the perineum on lesion days one and four, respectively.
Vigorous adaptive immune responses have been detected in HSV-2-infected laboratory animals including memory T cell responses comprised of central-, effector-, and resident memory T cell populations. In human and mouse studies, resident memory T cells are readily detected at the epithelial sites of previous HSV infection (Milligan et al., 1998; Tang and Rosenthal, 2010; Zhu et al., 2007; Zhu et al., 2013). The importance of resident memory cells in rapid restriction of epithelial infection after emergence of virus from the sensory nerve ending appears evident given the strategic location and the rapid mobilization of effector function of these cells (Peng et al., 2012; Zhu et al., 2007; Zhu et al., 2013). We previously detected HSV-specific, IFNγ-secreting T cells in the secondary lymphoid tissue and vaginal epithelium of guinea pigs infected up to 150 days previously with HSV-2 (Xia et al., 2014). In the current study, we extended these results by demonstrating the presence of HSV-specific memory T cells in the perineum and foreskins of HSV-2-infected male guinea pigs. These memory T cells likely play a role in moderating virus shedding and in protection of the genital epithelium against virus re-infection. This model of male genital infection should prove useful in future studies to examine vaccine-induced cell-mediated protection of the male genital epithelium.
Virus-infected animals in the current study developed a vigorous HSV-specific antibody response comprised of both IgG and IgA isotypes. Despite the mucosal site of virus inoculation, the serum response was primarily IgG antibody of the IgG2 subclass which demonstrates superior effector functionality important to protection including enhanced complement fixation via the classical pathway, enhanced lymphocyte or killer cell function, opsonization for macrophage/monocytes, and virus neutralization compared to the IgG1 subclass (Ohlander et al., 1978; Sandberg et al., 1971; Togashi and Tozawa, 1982). HSV-specific ASCs have been detected in the vaginal mucosa up to 150 days after intravaginal infection (Milligan et al., 2005; Xia et al., 2014). In male animals, as expected, we also detected HSV-specific ASCs in the spleen and bone marrow. However, in contrast to the presence of HSV-specific ASCs in the vaginal epithelium of HSV-infected female guinea pigs, we did not detect any HSV-specific ASCs in digests of the perineum and foreskin tissues. It seems likely that antibodies available to protect male genital epithelial sites are predominantly serum-derived. In HSV-2 genital infection studies in female guinea pigs, passive transfer of HSV-immune serum after virus infection diminished HSV-2 titers in the dorsal root ganglia and genital tract and decreased the frequency of recurrent lesions (Bourne et al., 2002). Additionally, maternal HSV-specific antibodies reduce the transmission of virus to neonates (Yeager et al., 1980) and vaccine-elicited antibody was identified as the correlate of protection against HSV-1 infection and disease in a field trial of a subunit HSV-2 vaccine (Belshe et al., 2014). A role for and potential mechanism of antibody-mediated protection in defense of the male genital epithelium against HSV is not well understood although this model should prove useful in addressing the issue.
Little is understood about immune protection of the male genital tract but results of clinical trials with candidate genital HSV-2 vaccines point to differences in vaccine-induced protection between males and females. Two different candidate vaccines composed of recombinant HSV-2 glycoproteins and adjuvant tested in clinical trial demonstrated evidence of transient and limited protection in females, but not males, against HSV-2 disease (Corey et al., 1999; Stanberry et al., 2002). The reason for the difference in protection is uncertain but may involve the presence of HSV-specific antibodies in cervical-vaginal secretions and differences in homing of innate and HSV-specific antibody secreting cells and T cells to the genital mucosa compared to the epithelial surface of male genitalia. While ultimately neither vaccine provided sufficient efficacy for licensure, the outcome of these clinical studies is highly suggestive that differences exist in the development of or nature of the immune responses required for protection of male and female genital epithelia against HSV-2. This novel model of genital HSV-2 infection of male guinea pigs should prove useful to explore this possibility.
In summary, we have improved a model of HSV-2 genital infection in male guinea pigs that recapitulates the major pathogenesis of HSV-2 infection in humans. Self-limiting vesicular lesions on the perineum and foreskin of inoculated animals and HSV-2 shedding were reproducibly detected at the surface of these tissues. Male animals also experienced recurrent disease and shed virus in the presence or absence of recurrent lesions. These HSV disease events reflect HSV-2 pathobiology in humans and are easily quantified making the model useful for determination of efficacy of candidate vaccines. Moreover, we have developed novel methods to detect and quantify host antibody- and cell-mediated immune responses at the genital site of virus infection providing useful information for the determination of correlates and mechanisms of vaccine-mediated protection. This model should be a useful tool in the preclinical evaluation of candidate vaccines, providing important information on similarities and disparities in the protective responses of both genders to candidate HSV vaccines.
4. Materials and methods
4.1. Virus
HSV-2 strain MS stock was prepared and stored at −80°C as previously described (Bourne et al., 1999). The replication defective virus, HSV-2 dl5-29, was a kind gift from Dr. David Knipe (Harvard Medical School, Boston, MA) and viral stocks were prepared as previously described (Xia et al., 2014).
4.2. Guinea pigs
Male Hartley guinea pigs (275 to 300 g, Charles River, Burlington, MA) were maintained under specific pathogen free conditions at the Association for Assessment and Accreditation of Laboratory Animal Care-approved animal research center of the University of Texas Medical Branch. All animal research was humanely conducted and approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch with oversight of staff veterinarians. The evening of the day before virus inoculation, food was removed from the animals to minimize the amount of fecal material present in the rectum at inoculation. The following morning, the rectum was gently cleaned with a moistened swab and 0.1 ml of inoculum containing 6.0 log10 pfu HSV-2 strain MS was instilled. Following virus inoculation, food was immediately returned to the animals and they were evaluated daily until day 14 p.i. and the severity of genital vesiculo-ulcerative disease quantified using a scoring system analogous to that described previously for female guinea pigs (Valencia et al., 2013). Once the primary skin disease had resolved, the animals were monitored from days 15-50 p.i. for the development of recurrent disease.
To evaluate HSV-2 shedding during primary genital infection, the perineum and foreskin were separately swabbed on days 5, 6, and 7 p.i. and swabs were placed into 0.5 ml of assay medium as described previously (Veselenak et al., 2018). Samples were frozen at −80°C prior to processing for PCR. Thawed tubes were vortexed and 100 μl of sample was added to 100 μl Total RNA lysis buffer (Bio-Rad, Hercules, CA). Similarly, to evaluate shedding in the foreskin during recurrent infection, swab samples were collected on days 25-28 p.i. and processed as described above.
4.3. Lymphocyte isolation from spleen, lymph nodes, bone marrow, and genital epithelium
Lymphocytes from spleen, mesenteric LN (mLN), ingLN and bone marrow were dissociated by expression through a stainless steel screen. Genital epithelium tissue (perineum and foreskin) was dissected and finely minced before being enzymatically digested and the lymphocytes isolated on density gradients as previously described (Xia et al., 2014).
4.4. ELISPOT
Assays to quantify antibody secreting B cells from spleen, bone marrow, and genital epithelium from infected and uninfected animals were performed as previously described (Milligan and Bernstein, 1995; Xia et al., 2014) on HSV-glycoprotein-coated plates with ovalbumin-coated plates used as a control. Background spots detected on OVA-coated plates were routinely subtracted from the HSV glycoprotein plate total. To quantify HSV-specific IFNγ secreting cells, lymphocytes from spleen, ingLN, and genital epithelium from HSV-infected and uninfected animals were stimulated with HSV-2 dl5-29-infected or medium only-stimulated mLN antigen presenting cells for detection of IFNγ-secreting cells by ELISPOT as previously described (Xia et al., 2014).
4.5. ELISA
HSV-specific IgA, IgG, IgG1 and IgG2 titers from immune serum were obtained by ELISA as performed previously (28). ELISA plates were coated with HSV-2 recombinant glycoprotein D (gD, Meridian Life Science, Inc., Memphis, TN). Serial dilutions of serum antibody were plated and gD-bound IgG and IgA were detected by incubation with HRP-goat anti-guinea pig IgG (abcam, Cambridge, MA) and HRP-sheep anti-guinea pig IgA (MyBiosource, San Diego, CA), respectively. IgG1 and IgG2 titers were detected with biotinylated goat anti-guinea pig IgG1 and biotinylated goat anti-guinea pig IgG2 (Antibodies-Online, Inc., Atlanta, GA) followed by addition of streptavidin peroxidase (Sigma-Aldrich, St. Louis, MO). Optical density readings were obtained at 490nm (OD490) and the end point titer was defined as the serum dilution resulting in an OD490 value greater than 0.1 and greater than three standard deviations above the OD490 values from medium-only control wells.
4.6. Custom PCR microarray for detection of guinea pig immune-related transcripts
For transcriptional analysis studies, samples of perineal or foreskin tissues were taken by punch biopsy on the first day, on day four, or day 20 after lesions first became visible on the perineum (5 animals per time point). Tissue samples from the perineum and foreskin of uninfected animals (N=5 animals) were collected in parallel for comparison. All samples were placed into 0.4 ml of Dulbecco’s Modified Eagle Medium (Corning Life Sciences-Mediatech, Inc., Manassas, VA) with 2.0% newborn calf serum (Life Technologies Incorporated, Carlsbad, CA) and 1.0% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO) and an equal volume of RNA lysis buffer (Bio-Rad, Hercules, CA) was added. Samples were frozen at −80°C prior to preparation of cDNA as described previously (Veselenak et al., 2018). Transcriptional analysis was performed as described previously (Veselenak et al., 2015; Veselenak et al., 2018) for 44 immune-related genes and 4 housekeeping genes. New primers used in the current study and not described previously are listed in Table 4. Comparisons to identify significant changes in gene expression levels between tissue samples from uninfected and samples from each time point p.i. were calculated using Student’s t test with a p value cutoff of 0.05.
Table 4.
Primers for detection and quantification of guinea pig mRNA.
| Target | Forward | Reverse | Assay |
|---|---|---|---|
| CD103 | CTTGCCCAGGATGAAATC | GCCTGAACACATATCAAGA | PCR Microarray |
| CD152/CTLA-4 | CAGCTTCGAGTGTGAGTA | CTTGGATGGTGAGGTTCA | PCR Microarray |
| CD223/LAG3 | TCAGGATTCTAGCCTTCTG | CATGCTCAGCACTGTGTA | PCR Microarray |
| IL-1 beta | GCACAGTGGAATTTGAATC | CAGGAAGACAGGCTTATG | qPCR |
| IL-2 | GACTGGAGCTATTACTGA | TCCTCTAGACACTGAAGA | PCR Microarray |
| IL-4 | CTGTCCAGTGAGTGAACTTTAGCGTCTACACTTTGAATATC | PCR Microarray | |
| IL-10 | CGCTGTCATCGATTTCTTC | GCCTTTCTCTTGGAGCTTA | PCR Microarray |
| IL-17A | CCACTTCAGACCAAACTT | CATCTTGGAACAGTATTGC | PCR Microarray |
| IL-17A | GAACTTCCTCCAGAATGTC | GAGAGTCCAAGGTGATGT | qPCR |
| IL-27 | CTGCTCTACACCTATCAG | CTTGGACAGCAGTAGTAA | qPCR |
| IL-33 | GAGTCCTACAGAAGACAA | CCACGATTACTCTTACATTC | PCR Microarray |
| PDCD-1 | GCACTTTGTGCTGAATTG | AGGACACTCATCTGGAAG | PCR Microarray |
| PRF-1 | CTTCCACCATCACCAATG | CGGATACACGTGTGTTGA | PCR Microarray |
| TGFβ | TGGACACCAACTATTGCTT | CCTTGCGGAAGTCAATGT | PCR Microarray |
| TNFα | CTCAGATCAGCTTCTCAA | TGGGAGTAGATGAGGTAC | PCR Microarray |
4.7. Confirmatory qRT-PCR
PCR primers used for confirmatory qRT-PCR assays have been described previously (Veselenak et al., 2015; Wali et al., 2014) or are listed in Table 4. Cloned amplimers of the selected gene targets were generated and duplicate 10-fold dilutions (106 - 102) of cloned amplimers of the selected gene targets were included as quantitation standards. To control for cellular load and normalize cytokine copies for each sample, parallel qPCRs were conducted using the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). All reactions were performed as described previously (Veselenak et al., 2018; Wali et al., 2014). Expression levels for IL-27, IL-1β, TNFα, and IL-17A were normalized using the geometric mean of the housekeeping gene GAPDH prior to analyses.
4.8. Statistics
Statistical differences for B lymphocyte assays, T lymphocyte assays, and antibody titers were determined using a two-tailed, nonparametric Mann-Whitney test or unpaired, two-tailed Student t test as appropriate. Frequencies of lesion days were compared by Fisher’s exact test. Values for P < 0.05 were considered significant. Statistical calculations were performed using GraphPad Prism software version 5.0 (GraphPad Software, San Diego, CA).
Supplementary Material
Supplemental Fig 1. Change in expression of 44 immune-related genes in the male genital tract following IREC inoculation. Biopsies from the perineum and foreskin were taken from uninfected guinea pigs (d0, n=5) and from infected animals on the first day a lesion became apparent on the perineum (lesion d1, lesion d4 or lesion d20) and extracted mRNA was analyzed by gpArray. Perineum samples were analyzed based on the lesion day (d0, d1, d4, d20 n=5/group). To ensure foreskin samples were obtained from tissue with an active HSV-2 infection, samples were analyzed based on the presence and number of HSV-2 gD transcripts present in the samples (> 103 HSV gD (n=3) or < 5×102 HSV gD (n=4). All d20 biopsies came from animals that experienced primary skin disease (n=5). Heat maps developed from the Ct values are shown for gene expression in the perineum (A) or foreskin (B). Color on the heat map is based on expression with blue squares indicating high expression and red squares indicating lower expression. Gray squares indicate insufficient mRNA detected for analysis of the given gene.
Highlights.
Genital infection resulted from intrarectal HSV-2 inoculation of male guinea pigs
Lesions developed on the perineum and foreskin concurrent with acute virus shedding
Virus shedding detected at the foreskin after establishment of a latent infection
Transcripts detected for inflammatory cytokines and inflammatory leukocyte processes
HSV-specific T cells detected in genital tissues after resolution of acute infection
Acknowledgements
This work was supported by grants AI10596201 and AI107784, from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, Deal CD, 2014. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis 209, 828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti J, Corey L, Ashley R, 1994. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med 121, 847–854. [DOI] [PubMed] [Google Scholar]
- Bourne N, Ireland J, Stanberry LR, Bernstein DI, 1999. Effect of undecylenic acid as a topical microbicide against genital herpes infection in mice and guinea pigs. Antiviral Res 40, 139–144. [DOI] [PubMed] [Google Scholar]
- Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB, 2005. Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. J Infect Dis 192, 2117–2123. [DOI] [PubMed] [Google Scholar]
- Bourne N, Pyles RB, Bernstein DI, Stanberry LR, 2002. Modification of primary and recurrent genital herpes in guinea pigs by passive immunization. J Gen Virol 83, 2797–2801. [DOI] [PubMed] [Google Scholar]
- Brown ZA, Vontver LA, Benedetti J, Critchlow CW, Sells CJ, Berry S, Corey L, 1987. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med 317, 1246–1251. [DOI] [PubMed] [Google Scholar]
- Bryson Y, Dillon M, Bernstein DI, Radolf J, Zakowski P, Garratty E, 1993. Risk of acquisition of genital herpes simplex virus type 2 in sex partners of persons with genital herpes: a prospective couple study. J Infect Dis 167, 942–946. [DOI] [PubMed] [Google Scholar]
- Cherpes TL, Harvey SA, Phillips JM, Vicetti Miguel RD, Melan MA, Quispe Calla NE, Hendricks RL, 2013. Use of transcriptional profiling to delineate the initial response of mice to intravaginal herpes simplex virus type 2 infection. Viral Immunol 26, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L, Adams HG, Brown ZA, Holmes KK, 1983. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med 98, 958–972. [DOI] [PubMed] [Google Scholar]
- Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM Jr., Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE, 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 282, 331–340. [DOI] [PubMed] [Google Scholar]
- Fanfair RN, Zaidi A, Taylor LD, Xu F, Gottlieb S, Markowitz L, 2013. Trends in seroprevalence of herpes simplex virus type 2 among non-Hispanic blacks and non-Hispanic whites aged 14 to 49 years--United States, 1988 to 2010. Sex Transm Dis 40, 860–864. [DOI] [PubMed] [Google Scholar]
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ, 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20, 73–83. [DOI] [PubMed] [Google Scholar]
- Holmberg SD, Stewart JA, Gerber AR, Byers RH, Lee FK, O'Malley PM, Nahmias AJ, 1988. Prior herpes simplex virus type 2 infection as a risk factor for HIV infection. JAMA 259, 1048–1050. [PubMed] [Google Scholar]
- Johnson KE, Redd AD, Quinn TC, Collinson-Streng AN, Cornish T, Kong X, Sharma R, Tobian AA, Tsai B, Sherman ME, Kigozi G, Serwadda D, Wawer MJ, Gray RH, 2011. Effects of HIV-1 and herpes simplex virus type 2 infection on lymphocyte and dendritic cell density in adult foreskins from Rakai, Uganda. J Infect Dis 203, 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone MR, Tabet SR, Paradise M, Wald A, Corey L, Celum CL, 1998. Herpes simplex virus shedding among human immunodeficiency virus-negative men who have sex with men: site and frequency of shedding. J Infect Dis 178, 978–982. [DOI] [PubMed] [Google Scholar]
- Krone MR, Wald A, Tabet SR, Paradise M, Corey L, Celum CL, 2000. Herpes simplex virus type 2 shedding in human immunodeficiency virus-negative men who have sex with men: frequency, patterns, and risk factors. Clin Infect Dis 30, 261–267. [DOI] [PubMed] [Google Scholar]
- Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, Newman LM, 2015. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One 10, e0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker KJ, Magaret AS, May MT, Turner KME, Vickerman P, Newman LM, Gottlieb SL, 2017. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health 5, e300–e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas B, Wiesendanger W, Schmidt-Ruppin KH, 1974. Genital herpes in guinea-pigs. An experimental model with Herpesvirus hominis. Arch Gesamte Virusforsch 44, 153–155. [DOI] [PubMed] [Google Scholar]
- McQuillan G, Kruszon-Moran D, Flagg EW, Paulose-Ram R, 2018. Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14-49: United States, 2015-2016. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L, 1992. Risk factors for the sexual transmission of genital herpes. Ann Intern Med 116, 197–202. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI, 1995. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology 206, 234–241. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI, Bourne N, 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol 160, 6093–6100. [PubMed] [Google Scholar]
- Milligan GN, Meador MG, Chu CF, Young CG, Martin TL, Bourne N, 2005. Long-term presence of virus-specific plasma cells in sensory ganglia and spinal cord following intravaginal inoculation of herpes simplex virus type 2. J Virol 79, 11537–11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlander C, Larsson A, Perlmann P, 1978. Specificity of Fc-receptors on lymphocytes and monocytes for guinea-pig IgG1 and IgG2: phagocytosis of erythrocytes. Scand J Immunol 7, 285–296. [DOI] [PubMed] [Google Scholar]
- Peng T, Zhu J, Phasouk K, Koelle DM, Wald A, Corey L, 2012. An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of herpes simplex virus 2 infection. J Virol 86, 10587–10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney JF, Felser JM, Ostrove JM, Straus SE, 1986. Acquisition of genital herpes from an asymptomatic sexual partner. N Engl J Med 314, 1561–1564. [DOI] [PubMed] [Google Scholar]
- Sandberg AL, Oliveira B, Osler AG, 1971. Two complement interaction sites in guinea pig immunoglobulins. J Immunol 106, 282–285. [PubMed] [Google Scholar]
- Shannon B, Yi TJ, Thomas-Pavanel J, Chieza L, Janakiram P, Saunders M, Tharao W, Huibner S, Remis R, Rebbapragada A, Kaul R, 2014. Impact of asymptomatic herpes simplex virus type 2 infection on mucosal homing and immune cell subsets in the blood and female genital tract. J Immunol 192, 5074–5082. [DOI] [PubMed] [Google Scholar]
- Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G, GlaxoSmithKline Herpes Vaccine Efficacy Study, G., 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 347, 1652–1661. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos DE, Myers MG, Bernstein DI, 1989. Genital infections due to herpes simplex virus type 2 in male guinea pigs. J Infect Dis 159, 89–95. [DOI] [PubMed] [Google Scholar]
- Tang VA, Rosenthal KL, 2010. Intravaginal infection with herpes simplex virus type-2 (HSV-2) generates a functional effector memory T cell population that persists in the murine genital tract. J Reprod Immunol 87, 39–44. [DOI] [PubMed] [Google Scholar]
- Togashi H, Tozawa H, 1982. Neutralization of sendai virus by the IgG subclass antibodies of the guinea pig. Microbiol Immunol 26, 821–829. [DOI] [PubMed] [Google Scholar]
- Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, Corey L, Wald A, 2011. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 305, 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia F, Veselenak RL, Bourne N, 2013. In vivo evaluation of antiviral efficacy against genital herpes using mouse and guinea pig models. Methods Mol Biol 1030, 315–326. [DOI] [PubMed] [Google Scholar]
- Veselenak RL, Miller AL, Milligan GN, Bourne N, Pyles RB, 2015. Development and utilization of a custom PCR array workflow: analysis of gene expression in mycoplasma genitalium and guinea pig (Cavia porcellus). Mol Biotechnol 57, 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselenak RL, Milligan GN, Miller AL, Pyles RB, Bourne N, 2018. Transcriptional Analysis of the Guinea Pig Mucosal Immune Response to Intravaginal Infection with Herpes Simplex Virus Type 2. Virology 518, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselenak RL, Shlapobersky M, Pyles RB, Wei Q, Sullivan SM, Bourne N, 2012. A Vaxfectin((R))-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine 30, 7046–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Link K, 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 185, 45–52. [DOI] [PubMed] [Google Scholar]
- Wald A, Zeh J, Selke S, Warren T, Ashley R, Corey L, 2002. Genital shedding of herpes simplex virus among men. J Infect Dis 186 Suppl 1, S34–39. [DOI] [PubMed] [Google Scholar]
- Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, Krieger JN, Corey L, 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med 342, 844–850. [DOI] [PubMed] [Google Scholar]
- Wali S, Gupta R, Veselenak RL, Li Y, Yu JJ, Murthy AK, Cap AP, Guentzel MN, Chambers JP, Zhong G, Rank RG, Pyles RB, Arulanandam BP, 2014. Use of a Guinea pig-specific transcriptome array for evaluation of protective immunity against genital chlamydial infection following intranasal vaccination in Guinea pigs. PLoS One 9, e114261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R, Arvin A, Prober C, Corey L, Burchett S, Plotkin S, Starr S, Jacobs R, Powell D, Nahmias A, et al. , 1991. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med 324, 450–454. [DOI] [PubMed] [Google Scholar]
- Xia J, Veselenak RL, Gorder SR, Bourne N, Milligan GN, 2014. Virus-specific immune memory at peripheral sites of herpes simplex virus type 2 (HSV-2) infection in guinea pigs. PLoS One 9, e114652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager AS, Arvin AM, Urbani LJ, Kemp JA 3rd, 1980. Relationship of antibody to outcome in neonatal herpes simplex virus infections. Infect Immun 29, 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L, 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 204, 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, Robins H, Corey L, 2013. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature 497, 494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig 1. Change in expression of 44 immune-related genes in the male genital tract following IREC inoculation. Biopsies from the perineum and foreskin were taken from uninfected guinea pigs (d0, n=5) and from infected animals on the first day a lesion became apparent on the perineum (lesion d1, lesion d4 or lesion d20) and extracted mRNA was analyzed by gpArray. Perineum samples were analyzed based on the lesion day (d0, d1, d4, d20 n=5/group). To ensure foreskin samples were obtained from tissue with an active HSV-2 infection, samples were analyzed based on the presence and number of HSV-2 gD transcripts present in the samples (> 103 HSV gD (n=3) or < 5×102 HSV gD (n=4). All d20 biopsies came from animals that experienced primary skin disease (n=5). Heat maps developed from the Ct values are shown for gene expression in the perineum (A) or foreskin (B). Color on the heat map is based on expression with blue squares indicating high expression and red squares indicating lower expression. Gray squares indicate insufficient mRNA detected for analysis of the given gene.