Abstract
Objectives:
The current laboratory study quantified blood oxidative stress to woodsmoke exposure.
Methods:
Participants inhaled woodsmoke during 3 randomized crossover exercise trials (Clean Air (0μg/m3), Low Exposure (250μg/m3) and High Exposure (500μg/m3) woodsmoke (particulate matter <2.5μm, PM2.5)). Trolox equivalent antioxidant capacity (TEAC), uric acid (UA), 8-isoprostanes (8-ISO), lipid hydroperoxides (LOOH), protein carbonyls (PC), nitrotyrosine (3-NT), 8-isoprostane, and myeloperoxidase (MPO) were quantified in Pre, immediately Post and 1Hr post blood samples.
Results:
UA decreased following Low Exposure, while plasma TEAC levels increased Post and 1Hr. LOOH levels decreased 1Hr Post (High Exposure), while 8-Iso increased following both smoke trials. PC and MPO were unchanged following all trials, while 3-NT increased over Clean Air.
Conclusion:
Blood oxidative stress occurred largely independent of PM2.5 concentrations. Future studies should employ longer duration smoke and exercise combined with physiologic parameters.
Keywords: air pollution, antioxidants, free radicals, reactive oxygen species
Introduction
The dangers of air pollution remain an important public health topic (1). Burning biomass in the US is the primary source of woodsmoke particulate matter (PM) inhalation and is associated with numerous adverse health effects (2–4), the etiology of which includes oxidative stress. In particular, woodsmoke combustion yields a complex mixture including carbon monoxide, aldehydes, polycyclic aromatic hydrocarbons, volatile organic compounds and PM (5). Inhalation of these and other woodsmoke components promote systemic oxidative stress through direct and indirect means and may compromise physiologic function and long-term health. In this regard, comprehensive understanding of woodsmoke inhalation during firefighting and oxidative stress remains limited.
The detrimental effects of oxidative stress accrue in a dose-dependent fashion. In the context of wildland firefighting occupational exposures to woodsmoke vary with the length of exposure, PM content, and concentration. Wildland firefighters are an important investigative population in this regard because over the course of an average year they typically experience greater woodsmoke exposures than the general public (6). Several logistical aspects of the work environment compound the exposure problem including limited access to protective gear. Moreover, if protective equipment is available it is often perceived as cumbersome during the demanding physical task of firefighting and frequently goes unused. In addition to PM exposure, acute physical activity is well demonstrated to promote systemic oxidative stress in transient fashion (7–9). A series of recent studies by our group indicate that the post-exercise environment impacts oxidative stress as quantified by a panel of blood biomarkers (10–12). Indeed, the relationship between exercise and environment on oxidative stress during wildland firefighting is influenced by high heat, higher elevations, high intensity or long duration working conditions (see review (13)) and perhaps woodsmoke inhalation.
Given this understanding, a rationale exists to suspect that wildland firefighters experience significant oxidative stress when they inhale woodsmoke. However, few well controlled investigations of firefighting-type woodsmoke investigations exist. An important early investigation revealed that circulating leukocytes exhibited oxidative damage to DNA following prescribed exercise where participants received 4 hours of laboratory controlled smoke inhalation (14). A number of scientific methodologies should be employed in future investigations to better understand the impact of smoke exposure during physical activity. Specific approaches should include application of a low-to-moderate intensity exercise stimulus that parallels day long firefighting conditions. Well controlled smoke exposures, at particulate concentrations representative of wildland fire scenarios should also be employed. Finally, use of a comprehensive blood biomarker panel to quantify systemic oxidative stress are needed to better understand the acute biochemical response to woodsmoke inhalation during firefighting.
Based upon this scientific need, the current investigation was undertaken to quantify blood oxidative stress responses to episodic woodsmoke inhalation. Exposure trials were performed within the context of a laboratory-controlled firefighting simulation. A randomized repeated measures study design was employed to expose individuals to “Clean Air” (0 μg/m3), “Low Exposure” (250 μg/m3) and “High Exposure” (500 μg/m3) woodsmoke PM2.5. A panel of oxidative stress biomarkers was used to assess outcomes in blood plasma collected before, immediately post and 1Hr following the 3 exposure trials. Based on prior findings it was broadly hypothesized that exposure to episodic woodsmoke events during controlled physical activity would result in transient elevation of blood oxidative damage markers and a corresponding decline in blood plasma antioxidant content. Additionally, it was hypothesized that indices of oxidative stress would be altered in proportion to the smoke exposure dose.
Materials and Methods
Participants
Prior to participant recruitment and testing study approval was obtained from the University of Montana Institutional Review Board. Study participants were recruited from the Missoula, Montana community and written informed consent was obtained prior to data collection. Study participants had no history of chronic lung disease or respiratory problems and no exposure to woodsmoke at home or work. Each participant completed a personal information questionnaire and the Physical Activity Readiness Questionnaire (PARQ) and was required to have a VO2 max greater than 40 ml.kg−1.min-1. Prior to arrival at the lab, participants confirmed they had not developed any respiratory infections and/or other health related changes between recruitment and testing completion.
Study Design
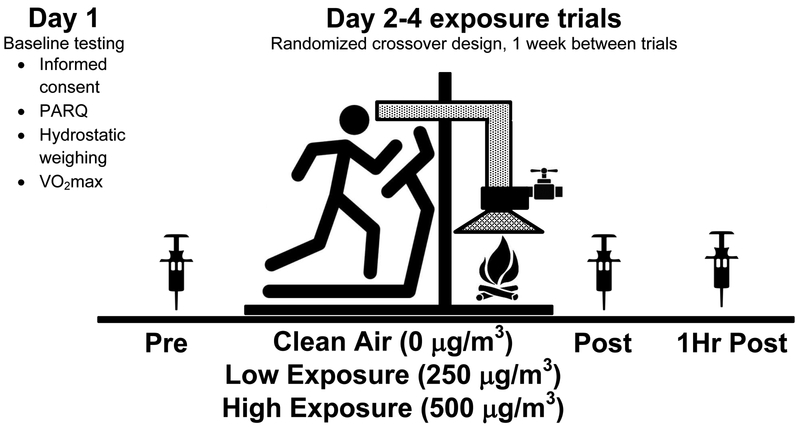
The study design included an initial lab visit to determine eligibility and perform baseline testing. Experimentation Days 2–4 were the exercise/exposure trials. Participants were asked to fast for three hours prior to coming in on Day 1. On days 2–4, the subjects participated in three exposure trials occurring one week apart in a randomized crossover fashion. The three exposure trials included a Clean Air (0 μg/m3), Low Exposure (250 μg/m3) and High Exposure (500 μg/m3) woodsmoke PM2.5. The study design is presented in Figure 1.
Figure 1. Study design.
Four testing days included baseline examination on Day 1 (informed consent, PARQ, hydrostatic weighing, and VO2max) followed by 3 days of identical exercise during the three smoke exposure conditions (“Clean Air” 0 μg/m3; Low Exposure 250 μg/m3; High Exposure 500 μg/m3`) Episodic woodsmoke exposures were introduced in a randomized crossover design with trials separated by 1 week. In all three trials participants performed an identical treadmill session for 1.5 hours. Blood samples were taken Pre-, Post-, and 1Hr Post exercise for subsequent examination of blood oxidative stress biomarkers.
Baseline testing (Day 1)
Participants characteristic data were collected on Day 1. Percent body fat was determined using hydrodensitometry. The underwater weight of each person was obtained using a digital scale (Exertech, Dresbach, MN). Participants repeated trials until 3 hydrostatic weight values within 100g were obtained. Underwater weights were corrected for estimates of residual lung volume (residual lung volume = (0.0115*Age) + (0.019*Height)-2.24). Body volume estimates were converted to percent fat using the Siri Equation (%BF= (4.95/ρ−4.50)*100) (15). Participants completed a peak maximal aerobic power test on an electronically powered treadmill (Model Q5, Quinton Instrument Company, Bothell, WA) to estimate peak aerobic fitness. Percent grade and speed were increased incrementally every 3 minutes until volitional fatigue.
Exposure trials (Days 2–4)
Woodsmoke preparation
The Pulmonary Physiology Core located within the Center for Environmental Health Sciences at the University of Montana was used to conduct the woodsmoke exposure trials. A woodstove (Englander, England Stove Works, Inc., Monroe, VA) was used to generate wood smoke for exposure trials. Western larch (Larix occidentalis Nutt) cured to 15% moisture content was burned throughout the study. The fires were prepared 25 minutes prior to the start of each exposure trial by using 1kg of wood and kindling with 1–2 pages of newspaper. Three hundred grams of wood was then added every 15–20 minutes over the two-hour period. The fire was maintained by removing ash if necessary to ensure that a constant layer of ash (approximately 0.5–1 inch deep) was present.
Exposure trials and smoke dose titration
Participants were exposed once to each of the three woodsmoke particle doses in a randomized fashion while exercising for 1.5 hours. The Low Exposure PM concentration (250 μg/m3) equated to inhalation doses experienced during cooking or heating with biomass (16). The High Exposure woodsmoke exposure (500 μg/m3) concentrations also provide clinical relevance in that these particulate concentrations elicit acute inflammatory responses (17). Throughout each trial PM2.5 and carbon monoxide levels were monitored continuously. Woodsmoke PM concentrations were maintained throughout the testing duration and confirmed with two systemized PM2.5 monitors (DustTrak, TSI, Model 8530, Shoreview, MN). Clean air was adjusted manually as a function of observed PM levels just upstream from subject exposure. Woodsmoke was delivered directly from the dilution and mixing chamber to the subject via a modified mask respirator with continuous PM2.5 measurements measured by the DustTrak II (DustTrak, TSI, Model 8530, Shoreview, MN). Temperature, humidity, carbon monoxide, and carbon dioxide in the mask and exercise room were monitored with a Q-Trak (TSI Inc., Shoreview, MN).
Exposure trials and exercise prescription
During the exposure trials subjects performed treadmill exercise at a continuous workload (3.5 mph and 5.7% grade, <57% of estimated VO 2 max) for 1.5 hours (with a short break e.g. 20–30 seconds) every 15 minutes to simulate fire line workloads.
Blood sampling
Blood samples were collected pre- (Pre), post – (Post), and 1- (1Hr) hours post exposure from the antecubital vein with sodium heparinized vacutainers (Becton Dickinson, Franklin Lakes, NJ). Samples were centrifuged at 6000xg for 10 minutes at 4°C, aliquoted and stored immediately at −80°C until subsequent analysis of oxidative damage and antioxidant biomarkers.
Biochemical oxidative stress panel
To quantify blood oxidative, stress a panel of markers was examined for both oxidative damage and for antioxidant content. Collectively, oxidative stress comes in 2 main forms, a depletion in native antioxidants and then creation of damage markers (which can be both lipid and protein). For this investigation we utilized two assays for damage to lipids: included lipid hydroperoxides (LOOH) and 8-Isoprostane (8-ISO). To assess protein damage due to oxidative modification, protein carbonyls (PC) and 3-nitrotyrosine (3-NT) assays were performed. Finally, the antioxidant markers were quantified relative to equivalents of a water soluble vitamin E analog, Trolox equivalent antioxidant capacity (TEAC), and the ubiquitous water soluble antioxidant, uric acid (UA). To better understand whether potential oxidative stress was a direct effect of the smoke or due to subsequent inflammation, additional markers of redox related inflammatory markers included myeloperoxidase (MPO) activity and the respective protein content were examined. Individual aliquots were assayed within a few months of sample collection and were subjected to a single freeze-thaw cycle. In an effort to further preserve sample viability on the day of assay, plasma aliquots were kept on ice and in the dark to prevent environmental redox alterations. Specific methods for these various assays is provided below.
Biochemical assays for antioxidant capacity
TEAC – Trolox equivalent antioxidant capacity was performed to measure antioxidants scavenging of 2, 2’ azinobis 3-ethyl-benzothiazoline-6-sulfonic acid radical anions using a quantifiable colorimetric reaction. The assay work solution was made by combining 2.5 mM Trolox solution with 50 mM Glycine buffer. Glycine peroxidase to 50 mM glycine buffer and ABTS solution was added in addition to 22 mM H2O2. Calculated TEAC values from unknown plasma samples was performed based on a standard reaction compared to the water-soluble vitamin E analogue Trolox (18).UA – Uric acid concentrations were performed using a colormetric assay. Measurements of H2O2 quenching was determined by peroxidase catalyzed oxidation of chromogenic substrates and measured spectrophotometrically using a reaction mixture containing 3-methyl-benzothiazoline-2-one hydrazone and 3-dimethylaminobenzoic acid. Final plasma UA values were determined by comparison with internal standard responses (19).
Biochemical assays for oxidative damage
LOOH – Plasma LOOH was quantified by implementing the ferrous oxidation-xylenol orange assay. Briefly, plasma samples were incubated with/without a reducing agent and then incubated with a colormetric work solution containing ferrous ammonium sulfate, butylated hydroxytolene, and xylenol orange. During the reaction oxidized ferrous ions react with the ferrous sensitive dye contained in xylenol orange to form a complex that is quantified through absorbance spectroscopy at a wavelength of 595 nm and compared to cumene hydroperoxide standard reactions (20).8-ISO – Blood plasma 8-isoprostane concentrations were measured using a specific enzyme immunoassay kit according to manufacturer instructions (Cayman Chemical, Ann Arbor, MI). Quantification is based on the competition between 8-isoprostane and an 8-isoprostane-acetylchoinesterase conjugate (8-Isoprostane Tracer). The enzymatic reaction is read by spectroscopy at 412 nm (21). For protein markers of oxidative damage plasma sample protein concentrations were analyzed via absorbance spectroscopy according to the methods of Bradford (22).PC – Plasma samples were quantified by a commercially available enzyme linked immunosorbent assay kit according to manufacturer instructions (Biocell Corporation Ltd, Papatoetoe, NZ) (23). The samples were diluted based on the standard procedures for samples containing 4–35 mg protein per ml. Following the enzyme linked immunosorbent assay procedure the absorbance was read at 450 nm. 3-NT - Nitrotyrosine content in plasma was quantified using commercial enzyme linked immunosorbent assay kits and according to kit instructions (Cell Biolabs INC, San Diego, CA) which are based on prior work (24). Kit assays were developed spectrophotometrically at 450 nm.
Biochemical assays of redox sensitive inflammatory markers
MPO – Quantitative measures of MPO protein content were obtained by an enzyme immunoassay technique (R&D Systems, Minneapolis, MN) and based on established work (25). Samples were analyzed by kit manufacturer instructions and the colormetric assay was developed and quantified at 450 nm. MPO activity was measured by quantifying products of MPO using a colormetric activity enzyme immunoassay kit (Sigma Aldrich, St. Louis, MO).
Statistical Analysis
Given the full repeated measures study design employed currently of this preliminary study, planned comparisons were used to determine differences among the three trials for Pre, Post, and 1HR time points. Additional time-dependent relationships were examined within each of the three trials. Because of the pilot nature of this investigation, an a priori decision was made to perform planned comparisons paired-sample t-tests for all oxidative stress variables (26). All values are presented as means ± standard error (SEM). Significance was set at p ≤ 0.05 a priori.
Results
Participant characteristics and performance data
Study participant physical characteristics and performance data are presented in Table 1. Nine of the ten subjects completed all trials, with one subject completing only the Low Exposure and High Exposure trials. The mean body fat for the recruited participants was 14.1% and aerobic power was of 53.6 ml.−1kg.−1min. There were no self-reported occurrences of respiratory complications during or following the exposure trials.
Table 1.
Participant characteristics and exercise performance data.
| Characteristics | |
|---|---|
| Participants (n) | 10 |
| Age (years) | 26.4 ± 3.5 |
| Height (cm) | 178.1 ± 3.0 |
| Body mass (kg) | 79.0 ± 11.8 |
| Percent fat (%) | 14.1 ± 3.4 |
| Exercise performance | |
| Estimated VO2 peak (ml. −1kg.−1min) | 53.6 ± 6.9 |
Plasma antioxidant capacity
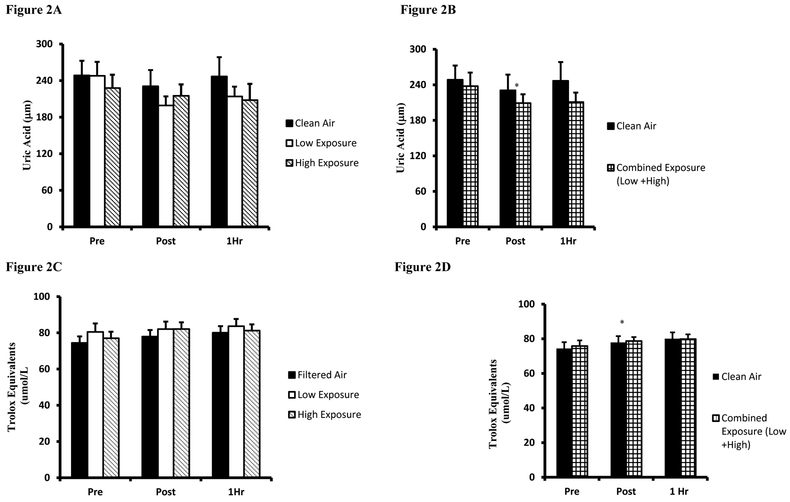
Mean responses for plasma antioxidant capacity assessed by UA is presented in Figure 2A. UA values were largely unaltered by exercise or smoke inhalation, with the lone difference being a Pre-Post decline during the Low Exposure trial (p=0.042). Given that a dose-response effect was not observed between Low/High Exposure trials, the values from both trials were combined and compared to Clean Air using identical analyses described above, Figure 2B. Combined Low/High Exposure values were similarly decreased Pre-Post Exposure (p=0.032) and indicate that when inhaling woodsmoke at a concentration higher than 250 μg/m3, an immediate post-exercise drop in the antioxidant UA occurred. TEAC outcomes for the three trials are presented in Figure 2C. Plasma TEAC levels were elevated Post (Clean Air and High Exposure, p=0.015 and p=0.001 respectively) and at 1Hr (Clean Air and High Exposure, p=0.001 and p=0.031 respectively). Similar to UA, combined Low/High Exposure analyses were compared to Clean Air using as averaged Low and High Exposures means, Figure 2D. Combined Low/High plasma TEAC values were higher than Pre at both Post and 1Hr sampling points.
Figure 2. Plasma antioxidant biomarkers.
A. Uric acid values are expressed as Uric Acid equivalents (µM) for Clean Air (black bars), Low Exposure (white bars), and High Exposure (striped bars). B. Uric acid values are expressed as Uric Acid equivalents (µM) between Clean Air (black bars) and Combined (Low/High Exposure Average, checkered bars). C. Trolox equivalent antioxidant capacity values are expressed as Trolox equivalent antioxidant capacity equivalents (µmol/L). D. Trolox equivalent antioxidant capacity. values are expressed as Uric acid equivalents (µM). Means are expressed± SEM. *significantly different from Pre; # significantly different from Post.
Biomarkers for plasma oxidative damage
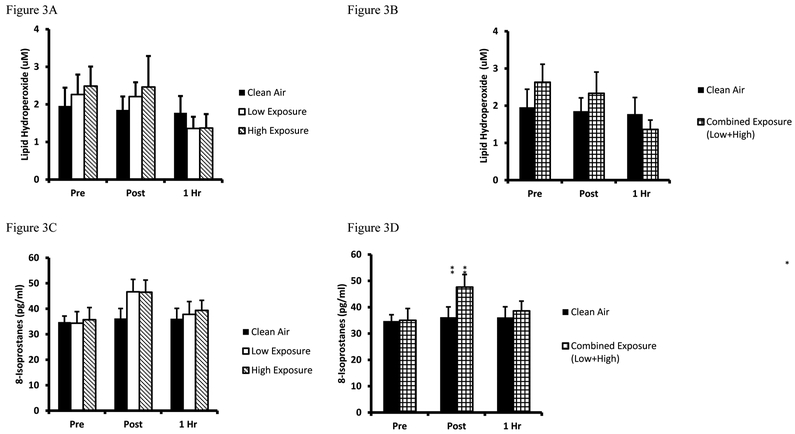
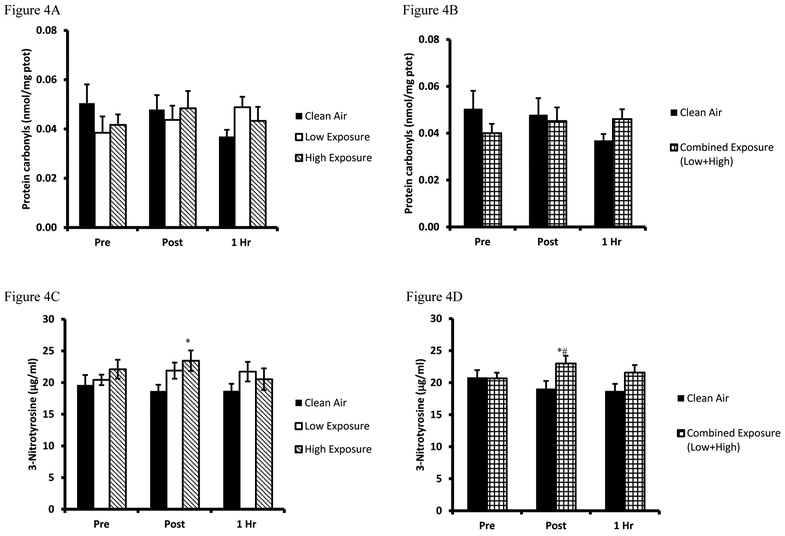
Biomarkers for oxidative damage were quantified by the LOOH and 8-ISO, and protein modification biomarkers PC and 3-NT. LOOH mean values are presented in Figure 3A while the combined Low/High analyses are presented in Figure 3B. Analysis of LOOH findings revealed a decreased 1Hr post (High exposure, p=0.036) compared to baseline values. Combined exposure analyses were performed and confirmed a decrease in combined exposure Pre-1Hr post (p=0.011). Results from 8-ISO are presented in Figure 3C while the combined Low/High values are presented in Figure 3D. Analysis of the 8-ISO data revealed a mean Pre-Post increase for both exposure trials (Low Exposure p=0.004, High exposure p=0.009). Combined exposure analysis confirmed the increase from Pre- to immediately Post (p=0.002). Findings for plasma PC are presented in Figure 4A and combined Low/High data in Figure 4B, respectively. Plasma PC levels were unaltered in response to any of the 3 exercise/exposure trials, although in combined Low/High Exposure analyses revealed a numerical difference that approached significance as compared to Clean Air (p=0.053). Results from 3NT analyses are presented for the 3 trials in Figure 4C and combined Low/High analyses are presented in Figure 4D. Analysis of the 3-NT assay results indicated Post trial differences existed between Clean Air and High Exposure (p=0.014). The Low Exposure trial yielded a numeric difference between Pre-Post that approached significance (p=0.076), and Clean Air-Low Exposure neared significance for Post (p=0.069) and 1Hr (p=0.088) recovery sample times. Combined Low/High Exposure analyses indicated a Pre-Post elevation in plasma 3-NT (p=0.049), and Clean Air-Low/High Exposure differences existed Post trial (p=0.012).
Figure 3. Plasma lipid oxidative damage biomarkers.
A. Lipid hydroperoxides are expressed as lipid hydroperioxide equivalents (µM) for Clean Air (black bars), Low Exposure (white bars), and High Exposure (striped bars). B. Lipid hydroperoxide between Clean Air (white bars) and Combined (Low/High Exposure Average, checkered bars). C. 8-isoprostanes values are expressed in standard comparison to 8-isoprostanes protein content (pg/ml) values are expressed as lipid hydroperioxide equivalents (µM). D. 8-Isoprostanes between Clean Air (white bars) and Combined (Low/High Exposure Average, checkered bars), are expressed in standard comparison to 8-isoprostanes protein content (pg/ml). Means are expressed ± SEM. *significantly different from Pre; # significantly different from Post.
Figure 4. Plasma protein oxidative damage biomarkers.
A. Protein carbonyl values are expressed in standard comparison to Protein Carbonyl equivalents (µM) for Clean Air (black bars), Low Exposure (white bars), and High Exposure (striped bars). B. Protein carbonyl values between Clean Air (white bars) and Combined (Low/High Exposure Average, checkered bars), are expressed in standard comparison to Protein Carbonyl equivalents (µM). C. Nitrotyrosine values are expressed in standard comparison to Nitrotrysine protein content (µg/ml). D. Nitrotyrosine values between Clean Air (white bars) and Combined (Low/High Exposure Average, checkered bars) are expressed in standard comparison to Nitrotrysine protein content (µg/ml). Means are expressed ± SEM.
Biochemical Assays for Redox Sensitive Inflammatory Markers
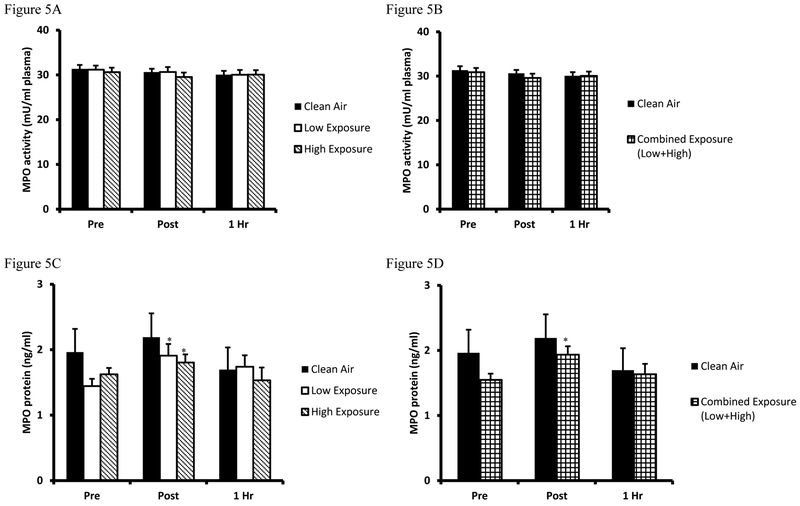
Biomarkers for inflammatory response were measured by MPO activity (Figure 5A and combined analyses in Figure 5B, respectively) and protein concentration (Figure 5C and Figure 5D combined analyses, respectively). Results from the MPO activity assay indicate that mean values were unchanged by exercise or smoke inhalation. Independent of MPO activity, analyses of plasma MPO protein concentrations indicate slight increases were present in Post samples from Low Exposure and High Exposure (p=0.035 and p=0.019, respectively) trials. Combined exposure analysis confirmed an increase immediately Post (p=0.005) as compared to Pre baseline values.
Figure 5. Plasma MPO concentration and MPO activity.
A. Myeloperoxidase activity values are expressed in standard comparison amount of myeloperoxidase activity (mU/ml plasma) for Clean Air (black bars), Low Exposure (white bars), and High Exposure (striped bars). B. Myeloperoxidase activity between Clean Air (white bars) and Combined (Low/High Exposure Average, checkered bars) are expressed in standard comparison amount of myeloperoxidase activity (mU/ml plasma). C. Myeloperoxidase activity values are expressed in standard comparison amount of myeloperoxidase protein content (ng/ml plasma) for Clean Air (black bars), Low Exposure (white bars), and High Exposure (striped bars). D. Myeloperoxidase activity between Clean Air (white bars) and Combined (Low/High Exposure Average, checkered bars) are expressed in standard comparison amount of myeloperoxidase protein content (ng/ml plasma). Means are expressed ± SEM.
Discussion
Wildland firefighters routinely inhale potentially detrimental woodsmoke doses as an unavoidable part of the job (6). While the extent to which woodsmoke inhalation contributes to deleterious health outcomes is not well quantified, the contribution of oxidative stress is suspected to be central to both the acute challenge (17, 27) and pathological sequelae (21, 24, 28). To date, however, scientific links between physiologic outcomes and oxidative stress due to woodsmoke exposures are poorly established. Based on this need, the current investigation was undertaken with the intent of identifying the extent to which a comprehensive series of blood plasma oxidative stress biomarkers exhibited transient alterations following controlled woodsmoke exposures. To achieve this end, a well-controlled laboratory study was designed to simulate physical activity in wildland firefighters. Participants were exposed to Clean Air and Low/High Exposure woodsmoke concentrations in a randomized crossover fashion. Key findings reveal that elevations in oxidative damage markers were observed. Concomitant to this elevation in circulating oxidative damage markers, a modest but significant drop in the water-soluble antioxidant uric was observed. Our interpretation of these findings is that the simulated firefighting walking task successfully elicited a modest spike in blood oxidative stress. This conclusion serves as a foundation for future laboratory and field based investigations of oxidative stress and wildland firefighting.
Oxidative damage and controlled woodsmoke exposure
Of the four oxidative damage markers used in the current oxidative stress panel, 8-ISO and 3-NT were elevated following the two woodsmoke exposure trials, and these effects appeared to be independent of smoke dose. We cannot currently confirm why 8-ISO and 3-NT were increased while PC exhibited no change and LOOH appeared to drop slightly. Nonetheless, there is a biochemical rationale that supports our findings with respect to the two lipid peroxidation assays, 8-ISO and LOOH, used currently. 8-ISO is a relatively stable prostaglandin compound produced in vivo by free radical-catalyzed peroxidation (21). Thus, parent molecule prostaglandins are likely produced enzymatically, meaning they aren’t protected within a lipid bilayer, and quickly degrade to the relatively stable 8-ISO metabolite examined. In contrast, LOOH are more often derived from lipid bilayers within membranes, and as such, less labile as compared to 8-ISO production (21, 29). Given the short duration of exercise, smoke exposure, and 1 hour recovery time, negative findings for LOOH were not altogether surprising. Past investigations by our group have demonstrated that higher intensity exercise and longer duration sampling time points are associated with significantly elevated LOOH (7, 8). Thus, there is reason to believe that longer duration smoke exposure and physical activity, may have elicited an increase in LOOH as well. As a function of methodological recommendations for future research, longer duration smoke and exercise exposure would also better simulate a full day of firefighting.
In the current investigation two indices of protein oxidative damage were investigated, 3-NT and PC. The key finding in this regard was that plasma 3-NT was elevated following woodsmoke exposure, while PC were unaltered. As with lipid peroxidation, this disparate finding cannot be explained currently, although there is a biochemical rationale to support the collective outcome. Tyrosine residues are quickly modified in response to oxidant production (30). In the context of air pollution, published findings indicate that exposure to inhaled diesel exhaust particles contributed toß rapid post-translational modification of protein tyrosine nitration (31). In contrast to 3-NT, plasma PC values were unaltered in the current investigation and are supported by our prior findings that PC can peak hours to days following the initial oxidant stimulus (8). These findings are an important reminder that future applications should be mindful of oxidant loads experienced during exercise and woodsmoke exposure, in addition to the post-exposure sampling time frame for biosample collection.
Plasma antioxidant content and controlled woodsmoke exposure.
We hypothesized that plasma antioxidants would be decreased in response to woodsmoke exposure during our exercise trials. Contrary to our hypothesis, a rise in plasma TEAC occurred independent of smoke exposure. A rise in post-exercise TEAC is curious in that elevated plasma TEAC values are typically associated with fatiguing exercise of long duration (32, 33) or high intensity (8, 34). The rise in TEAC is generally associated with elevated UA, a consequence of accelerated purine metabolism in fatigued skeletal muscle (35). However, in the current study the exercise was performed at a dose well below a fatigue threshold. Moreover, and most importantly, post-exercise plasma UA dropped statistically following the Low Exposure trial (and in the Low/High combined analysis). In support of the current findings, a prior investigation also observed drop in plasma antioxidant capacity following woodsmoke exposure, although methodological differences in that investigation somewhat limit comparisons to the current investigation (28). Based on this understanding, conclusions about plasma antioxidant capacity following the current smoke exposure trials suggest that antioxidant content dropped slightly. As with the oxidative damage biomarker conclusions, however, increasing the exercise and smoke doses and extending the biosample time frame in recovery may provide more telling results.
Redox dependent inflammation markers
Based on prior understanding of pulmonary-related inflammation following woodsmoke inhalation (17), MPO protein and activity were quantified currently in order to gain preliminary insight on redox related inflammation processes. MPO activity was unchanged by the 3 exercise/exposure trials while MPO protein content was only modestly elevated following woodsmoke exposure. While it may be tempting to speculate that elevated MPO protein content is indicative of oxidative stress related to inflammation, the absolute values are below values considered as “clinically significant” (36, 37). Although we did not find significant alterations in MPO, previous work implicate MPO as a sensitive acute marker of oxidative stress following exercise (7) and mild pulmonary inflammatory responses (5). Is support, findings from a companion study to this investigation revealed that MPO concentrations were elevated in proportion to smoke concentration (38). Collectively, these findings may indicate that blood oxidative stress and inflammation my increase subsequent to the respiratory system, and reinforces recommendations for the inclusion of additional physical activity and longer duration smoke exposure in subsequent laboratory investigations.
Study limitations and recommendations for future research
Despite tight scientific controls for many aspects of the investigation, study limitations also existed which impact both study conclusions and recommendations for future research. The two smoke concentrations used currently was lower on the PM2.5 air spectrum often experienced by wildland firefighting. In fact, a classic study of air quality experienced by wildland firefighters revealed peak PM2.5 concentrations of 2930 μg/m3, with average levels of 720 μg/m3 while on the fire line (39). In contrast the Low Exposure PM (250 μg/m3) load used currently is comparable to domestic biomass smoke exposure (16). Smoke dose in the current study was also limited by the fact that exposure time was approximately 1/10th of a typical wildland firefighter work shifts (3). In support, subjects from a prior investigation were exposed to woodsmoke for a 4-hour period and exhibited a notable oxidative stress and inflammatory markers (32). Extending upon this notion of woodsmoke dose, it is notable that wildland firefighters work extended shifts on consecutive days. Thus, future laboratory and field investigations should employ multiple day exposures. Finally, the exercise trial used currently was a continuous bout of exercise at relatively low intensity. However, ventilation rates and oxidative stress typically accrue in an intensity-dependent fashion (7, 40), and wildland practices often include brief bouts of vigorous work interspersed by continuous low intensity activity. Thus, future lab-based studies may be benefited by the scheduled inclusion of intermittent 20–30 second bouts of vigorous activity. Alternately, additional proof of concept experimentation should be conducted to better resolve whether or when a comprehensive (antioxidant depletion in addition to lipid and protein oxidative damage) oxidative stress response occurs to a given dose of woodsmoke inhalation.
Conclusions
The average wildland firefighting career averages 8 seasons (41) and deleterious health outcomes are common. Our overarching research goal is to quantify oxidative stress following woodsmoke exposure as the phenomenon is linked to physiologic declines (e.g., respiratory, vascular dysfunction) and clinical outcomes. Upon acquisition of this collective knowledge, protective equipment and firefighting strategies can be optimized to preserve wildland firefighter health. Given the need for further investigations, findings from this study indicate that the current biomarker panel should be extended to future examination of oxidative stress following smoke exposure. In subsequent laboratory or field investigations, longer smoke exposure times and longer duration exercise/physical activity challenges should also be included. Finally, given the current findings of oxidative stress, next generation investigations should also explore associations to physiologic outcomes. Our research group recently published a parallel investigation from this subject cohort and found no changes in respiratory function (42), though there is reason to suspect that aforementioned smoke and exercise doses might alter pulmonary responses (43). Thus, linking oxidative stress responses to potential changes in vascular, cardiac and respiratory parameters may provide new insights into clinical outcomes. Once a testing scenario is optimized, the detrimental impact of woodsmoke exposure can evolve to discovery of potent counter measures to oxidative stress in wildland firefighters.
Acknowledgments
Funding Sources: NCRR (COBRE P20RR 017670)
Abbreviations
- 3-NT
3-nitrotyrosine
- 8-ISO
8-isoprostanes
- LOOH
Lipid hydroperoxides
- MPO
Myeloperoxidase
- PARQ
Physical Activity Readiness Questionnaire
- PC
Protein carbonyls
- PM
Particulate matter
- PM2.5
Particulate matter <2.5μm
- SEM
standard error
- TEAC
Trolox equivalent antioxidant capacity
- UA
Uric Acid
Footnotes
Conflicts of Interest: None declared
References
- 1.Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol. 2008;52:719–726. [DOI] [PubMed] [Google Scholar]
- 2.Adetona O, Simpson CD, Onstad G, Naeher LP. Exposure of wildland firefighters to carbon monoxide, fine particles, and levoglucosan. The Annals of occupational hygiene. 2013;57:979–991. [DOI] [PubMed] [Google Scholar]
- 3.Adetona O, Zhang JJ, Hall DB, Wang JS, Vena JE, Naeher LP. Occupational exposure to woodsmoke and oxidative stress in wildland firefighters. Sci Total Environ. 2013;449:269–275. [DOI] [PubMed] [Google Scholar]
- 4.Rehfuess E, Mehta S, Pruss-Ustun A. Assessing household solid fuel use: multiple implications for the Millennium Development Goals. Environmental health perspectives. 2006;114:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naeher LP, Brauer M, Lipsett M, et al. Woodsmoke health effects: a review. Inhalation toxicology. 2007;19:67–106. [DOI] [PubMed] [Google Scholar]
- 6.Swiston JR, Davidson W, Attridge S, Li GT, Brauer M, van Eeden SF. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur Respir J. 2008;32:129–138. [DOI] [PubMed] [Google Scholar]
- 7.Quindry JC, Stone WL, King J, Broeder CE. The effects of acute exercise on neutrophils and plasma oxidative stress. Med Sci Sports Exerc. 2003;35:1139–1145. [DOI] [PubMed] [Google Scholar]
- 8.Hudson MB, Hosick PA, McCaulley GO, et al. The effect of resistance exercise on humoral markers of oxidative stress. Med Sci Sports Exerc. 2008;40:542–548. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131. [DOI] [PubMed] [Google Scholar]
- 10.Ballmann C, McGinnis G, Peters B, et al. Exercise-induced oxidative stress and hypoxic exercise recovery. Eur J Appl Physiol. 2014. [DOI] [PubMed] [Google Scholar]
- 11.McGinnis G, Kliszczewiscz B, Barberio MD, et al. Acute Hypoxia and Exercise-Induced Blood Oxidative Stress. In Review. 2014. [DOI] [PubMed] [Google Scholar]
- 12.Peters B, Ballmann C, McGinnis G, Epstein E, Hyatt H, Slivka D, Cuddy J, Hailes W, Dumke C, Ruby B, & Quindry J Graded Hypoxia and Blood Oxidative Stress During Exercise Recovery. Journal of sports sciences. 2015. [DOI] [PubMed] [Google Scholar]
- 13.Quindry J, Dumke C, Slivka D, Ruby B. Impact of extreme exercise at high altitude on oxidative stress in humans. J Physiol. 2016;594:5093–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danielsen PH, Brauner EV, Barregard L, et al. Oxidatively damaged DNA and its repair after experimental exposure to wood smoke in healthy humans. Mutation research. 2008;642:37–42. [DOI] [PubMed] [Google Scholar]
- 15.Kravitz L, Vivian H. Body Composition Assessment. [Google Scholar]
- 16.Dills RL, Paulsen M, Ahmad J, Kalman DA, Elias FN, Simpson CD. Evaluation of urinary methoxyphenols as biomarkers of woodsmoke exposure. Environmental science & technology. 2006;40:2163–2170. [DOI] [PubMed] [Google Scholar]
- 17.Ghio AJ, Soukup JM, Case M, et al. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occupational and environmental medicine. 2012;69:170–175. [DOI] [PubMed] [Google Scholar]
- 18.Erel O A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. [DOI] [PubMed] [Google Scholar]
- 19.Kovar KA, el Bolkiny MN, Rink R, Hamid MA. An enzymatic assay for the colorimetric and fluorimetric determination of uric acid in sera. Archiv der Pharmazie. 1990;323:235–237. [DOI] [PubMed] [Google Scholar]
- 20.Nourooz-Zadeh J Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods in enzymology. 1999;300:58–62. [DOI] [PubMed] [Google Scholar]
- 21.Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med. 1999;160:216–220. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 23.Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med. 1997;23:361–366. [DOI] [PubMed] [Google Scholar]
- 24.Ceriello A, Mercuri F, Quagliaro L, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia. 2001;44:834–838. [DOI] [PubMed] [Google Scholar]
- 25.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. The Journal of investigative dermatology. 1982;78:206–209. [DOI] [PubMed] [Google Scholar]
- 26.Field A Discovering Statistics Using IBM SPSS Statistics SAGE Publications Ltd; 2013. [Google Scholar]
- 27.Danielsen PH, Moller P, Jensen KA, et al. Oxidative stress, DNA damage, and inflammation induced by ambient air and wood smoke particulate matter in human A549 and THP-1 cell lines. Chemical research in toxicology. 2011;24:168–184. [DOI] [PubMed] [Google Scholar]
- 28.Kurmi OP, Dunster C, Ayres JG, Kelly FJ. Oxidative potential of smoke from burning wood and mixed biomass fuels. Free Radic Res. 2013;47:829–835. [DOI] [PubMed] [Google Scholar]
- 29.Montuschi P, Barnes PJ, Roberts LJ, 2nd. Isoprostanes: markers and mediators of oxidative stress. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:1791–1800. [DOI] [PubMed] [Google Scholar]
- 30.Cai Z, Yan LJ. Protein Oxidative Modifications: Beneficial Roles in Disease and Health. Journal of biochemical and pharmacological research. 2013;1:15–26. [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao GG, Nel AE, Loo JA. Nitrotyrosine-modified proteins and oxidative stress induced by diesel exhaust particles. Electrophoresis. 2005;26:280–292. [DOI] [PubMed] [Google Scholar]
- 32.Barregard L, Sallsten G, Gustafson P, et al. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhalation toxicology. 2006;18:845–853. [DOI] [PubMed] [Google Scholar]
- 33.Lee MS, Eum KD, Fang SC, Rodrigues EG, Modest GA, Christiani DC. Oxidative stress and systemic inflammation as modifiers of cardiac autonomic responses to particulate air pollution. International journal of cardiology. 2014;176:166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller LE, McGinnis GR, Kliszczewicz B, et al. Blood Oxidative Stress Markers During a High Altitude Trek. Int J Sport Nutr Exerc Metab. 2012. [DOI] [PubMed] [Google Scholar]
- 35.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free radical biology & medicine. 1993;14:303–311. [DOI] [PubMed] [Google Scholar]
- 36.Bury TB, Pirnay F. effect of prolonged exercise on neutrophil myeloperoxidase secretion. International Journal of Sports Medicine. 1995;16:410–412. [DOI] [PubMed] [Google Scholar]
- 37.Pyne DB, Baker MS, Telford RD, Weidemann MJ. exercse and the neutrophil oxidative burst: biological and experimental variability. European Journal of Applied Physiology and Occupational Physiology. 1996;74:564–571. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson MD, Semmens EO, Dumke C, Quindry JC, Ward TJ. Measured Pulmonary and Systemic Markers of Inflammation and Oxidative Stress Following Wildland Firefighter Simulations. J Occup Environ Med. 2016;58:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinhardt TE, Ottmar RD. Baseline measurements of smoke exposure among wildland firefighters. Journal of occupational and environmental hygiene. 2004;1:593–606. [DOI] [PubMed] [Google Scholar]
- 40.Allessio H, Hagerman A, Fulkerson B, Ambrose J, Rice R, Wiley L. Generation of reactive oxygen species after exhaustive and isometric exercise. Medicine Science in Sports and Exercise. 2000;32:1576–1581. [DOI] [PubMed] [Google Scholar]
- 41.Booze TF, Reinhardt TE, Quiring SJ, Ottmar RD. A screening-level assessment of the health risks of chronic smoke exposure for wildland firefighters. Journal of occupational and environmental hygiene. 2004;1:296–305. [DOI] [PubMed] [Google Scholar]
- 42.Ferguson MD, Semmens EO, Weiler E, et al. Lung function measures following simulated wildland firefighter exposures. Journal of occupational and environmental hygiene. 2017:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quindry JC, Brown DD, McCaw ST, Thomas DQ. Effect of exercise-induced changes in residual lung volume on the determination of body composition. J Strength Cond Res. 2002;16:591–598. [PubMed] [Google Scholar]