Abstract
Peptide and protein-based cancer vaccines usually fail to elicit efficient immune responses against tumors. However, delivery of these peptides and proteins as components within caged protein nanoparticles has shown promising improvements in vaccine efficacy. Advantages of protein nanoparticles over other vaccine platforms include their highly organized structures and symmetry, biodegradability, ability to specifically functionalize at three different interfaces (inside, outside, and between subunits in macromolecular assembly), and ideal size for vaccine delivery. In this review, we discuss different classes of virus-like particles and caged protein nanoparticles that have been used as vehicles to deliver and increase the interaction of cancer vaccine components with the immune system. We review the effectiveness of these protein nanoparticles towards inducing and elevating specific immune responses, which are needed to overcome the low immunogenicity of the tumor microenvironment.
Text for Graphical Abstract:
In this review, we discuss several different protein-based nanoparticles as delivery vehicles to increase the interaction of cancer vaccine components (e.g., adjuvants, tumor-associated antigens) with the immune system. These important components can be efficiently internalized and processed by dendritic cells, which then present the antigen to the T cells for specific T cell responses that lead to specific tumor lysis and elimination. The elevated immune responses that are elicited by these nanoparticle vaccines are advantageous to overcome the low immunogenicity of the tumor microenvironment.
Keywords: cancer vaccines, virus-like particles, caged protein nanoparticles, tumor antigens
Introduction
Boosting a patient’s native immune system by immunotherapy has been a promising approach in cancer treatment.1 The goal of cancer vaccines is to promote the immune system to recognize distinct antigenic markers expressed primarily by cancer cells and to target such cells for lysis.2 These markers, known as tumor associated antigens (TAAs), vary widely among different cancer types, and the identities of many TAAs have been elucidated.3 In many clinically-examined cancer vaccines, TAAs are co-administered with adjuvant, which are immune activator molecules. Although these cancer vaccines have been shown to elicit an immune response, the clinical outcome is usually weak and insufficient to overcome the low immunogenicity of the tumor microenvironment.1
In recent years, different strategies have been developed to increase vaccine efficacy, such as vaccination with the whole tumor lysate,4 combination of antigens with adjuvants,5 and formulation in carriers such as nanoparticles (e.g., PLG, PLGA, gold nanoparticles),6–8 liposomes,9 and microparticles.10 Virus-like particles (VLP) and caged protein (CP) nanoparticles have also attracted significant interest as cancer vaccine platforms for inducing antigen-specific immune responses against cancerous cells. We define VLPs as protein structures isolated from viruses which are lacking the infectious viral genome to a mammalian host, and CPs as self-assembled protein structures with physical properties and geometries similar to viruses but not from a viral source. VLP and CP nanoparticles as vaccine platforms have the potential to improve vaccine efficacy by promoting antigen localization to dendritic cell-enriched draining lymph nodes,11 enhancing endocytosis of antigens by antigen presenting cells (APCs),12 and increasing antigen presentation to the adaptive immune cells.13 In this review, we discuss the different types of VLP and CP nanoparticles and their physical properties, biodistribution, and cellular uptake towards enhancing vaccine efficacy. Although VLP and CP nanoparticles have also shown success as platforms to induce higher immune responses to infectious diseases, vaccines for communicable diseases have been reviewed by others,14,15 and will not be covered in this discussion.
Antigen-based Cancer Vaccines
Tumor-associated antigens (TAAs) are antigenic proteins produced by tumor cells which can trigger an immune response in the host.16 Immunotherapy using vaccines is based on the premise that TAAs can induce specific cytotoxic T cell responses to cancer cells, resulting in tumor destruction without harming normal cells.17 However, clinically-examined cancer vaccines, consisting of whole tumor antigen (proteins) or epitopes (smaller peptides), are often insufficient to overcome the low immunogenicity of the tumor microenvironment.16
To address this limitation, different approaches have been examined to increase the antitumor responses for improved vaccine efficacy. One strategy is the combination of these antigen-based cancer vaccines with immune activator molecules known as adjuvants.17,18 Common adjuvants used in clinical trials include aluminum salts, oil-in- water emulsions (MF59), and monophosphoryl lipid A (MPL) with aluminum salt.19 Recently, ligands of Toll-like receptors in APCs such as CpG20–22, poly-IC23,24, and imidazoquinoline25,26 have also attracted considerable interest as cancer vaccine adjuvants in preclinical and clinical trials.
Alternative approaches for increasing vaccine efficacy such as using multiple antigen peptide epitopes27–29 and personalized peptide formulations have been developed and supported by clinical studies.30,31 Vaccination with multiple-peptide epitopes from different sources can decrease the possibility of tumor escape and increase the anti-tumor responses relative to single epitope immunization.32,33 Also, since tumor cells and TAAs are heterogeneous among patients, a personalized selection of peptides against individually-expressed antigens can increase efficacy.34
Despite these improvements, clinical outcomes of cancer vaccines have still been limited by factors such as identification of optimal antigens, adjuvants, and importantly, delivery system. Our focus in this review is to discuss and survey VLP and CP nanoparticles that have been used as delivery systems to increase effectiveness of cancer vaccines.
Delivery Systems
Generation of potent specific immune responses to cancer is dependent on antigen uptake by APCs, particularly dendritic cells (DCs). Efficient uptake by DCs is subject to the important antigen properties of size, shape, and surface charge.35,36 Additional key steps in generation of response include proper activation of DCs, trafficking of DCs to lymph nodes (LNs), sufficient communication of DCs with adaptive immune cells such as CD8 T cells, and activation of cytotoxic T cells for targeted tumor lysis (Figure 1).37
Figure 1. Common mechanism of tumor cell elimination.
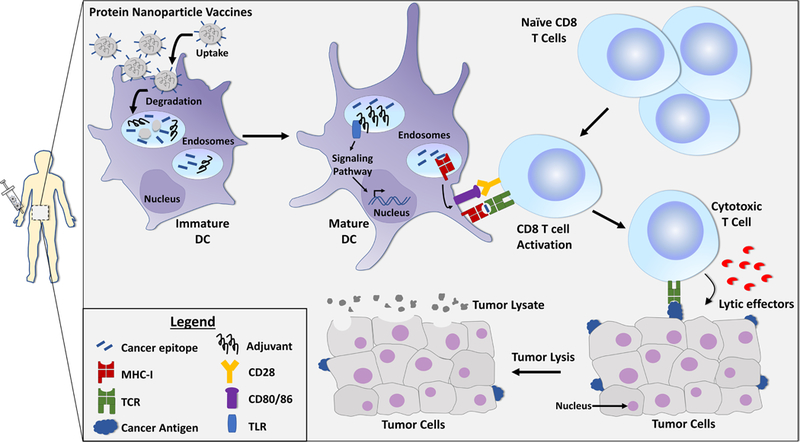
Protein nanoparticle (NP) cancer vaccines that are injected in vivo can accumulate in the LNs and spleen. Immature DCs residing in these tissues internalize and degrade the NPs and process the antigens and adjuvants for potential danger signals. If DCs are activated through an adjuvant-TLR interaction, they present the antigens to the T cells in the context of MHC- I molecules for specific and longer-term T cell responses (i.e., cross-presentation). Upon T cell activation and recognition of tumor-associated antigens on cancer cells, T cells secrete lytic effectors (such as perforin), leading to tumor lysis and elimination. Abbreviations in the figure include: MHC-I (major histocompatibility complex, class I), TCR (T-cell receptor), CD28 (cluster of differentiation 28, costimulatory molecule), CD80/86 (cluster of differentiation 80/86, costimulatory molecules), TLR (Toll-like receptor).
The use of nanoparticles in vaccines is supported by the premise that a higher cellular uptake and an elevated interaction of antigens with the immune cells can be achieved by using an optimally-designed delivery system.38 Vaccine delivery materials that have been examined for cancer immunotherapy include liposomes, polymers, nanoparticles, and hydrogels.8,39 Nanoparticles based on proteins, in particular VLPs and CPs, have symmetries and physical properties that are similar to viruses and can potentially increase the interaction of the vaccine components with APCs. We briefly discuss how delivery system properties, such as size, shape, and surface charge, can affect the cellular uptake and induce potentially more effective anti-tumor immune responses.
Size
Nanoparticle studies have shown that there is an optimal size range for passive transport to the lymphatic system and APCs.36 Particles between 20–45 nm are drained significantly by LNs, with a relatively high retention time measured up to 120 hrs post-injection.40 Particles of this size range are internalized by almost 50% of LN-resident DCs compared to 10% internalization by APCs of 100-nm particles.40–42 However, particles below 10 nm are not internalized by DCs efficiently.36 Furthermore, relatively high (~76%) lymphatic uptake was observed for 40-nm liposomes compared to larger liposomes (>150 nm), the latter of which remained almost completely at the site of injection.43
As discussed, conventional formulations of peptide- and protein-based cancer vaccines usually yield relatively weak immune responses, which can be attributed to insufficient uptake and interaction of antigens with APCs.36 The size-uptake studies suggest that delivering the soluble protein or peptide antigens (which are typically much smaller than 5 nm) within nanoparticles of ~20–50 nm can lead to more efficient lymphatic drainage and a higher antigen uptake by DCs, resulting in stronger adaptive immunity to the antigens.
Shape
Particle shape can also affect the uptake of nanoparticles by immune cells.36,44,45 Although investigations have shown that non-spherical particles such as rod-shaped particles have higher circulation times, they also demonstrated decreased cellular uptake compared to spherical NPs.46 In fact, spherical NPs have the highest cell internalization rate compared to cubic, rod and disk-like shaped NPs.47 Spherical polystyrene particles (diameters ~200 nm) conjugated to ovalbumin antigen (OVA) generated stronger in vivo Th1 and Th2 immune responses relative to rod-shaped particles.48 In addition, enhanced LN transport and uptake by APCs was observed for the spherical virus-like particle, cowpea mosaic virus (CPMV), compared to the rodshaped virus-like particles, potato virus X (PVX).49 Others have demonstrated that not only shape but the initial orientation of particles can affect phagocytosis by macrophages.45
Surface charge
Surface charge is another parameter that can affect the cellular uptake of NPs.36,50 Neutral NPs demonstrated minimal cellular interaction and cellular uptake compared to the charged NPs.50 Positively charged nanoparticles have the greatest efficiency in cellular internalization, possibly due to their interactions with the negatively charged groups on the cell membrane.50 However, evidence also demonstrated uptake of negatively charged nanoparticles despite their unfavorable electrostatic interaction with cell membranes. For example, cellular uptake of both negatively and positively charged micellar NPs has been observed through different endocytic pathways (e.g., clathrin-mediated endocytosis, caveolae-mediated endocytosis, and macropinocytosis).51
With regard to tumor tissue, in vivo biodistribution of chitosan and micellar-based NPs suggested that NPs with relatively weak negative surface charges tend to accumulate in tumors more than NPs with positive or highly-negative zeta potential values;51,52 this is somewhat consistent with a recent extensive survey demonstrating that NPs with neutral surface charges tend to result in higher delivery efficiencies to tumors, relative to NPs with more positive or negative charges.53 An important, but sometimes overlooked, consideration is the adsorption of blood components on nanoparticle surfaces (called the protein corona), which can modify NP surface properties. Therefore, physicochemical properties of a NP immediately after synthesis can be different than the one that cells encounter in vivo.54–56
Virus-Like Nanoparticles
Viruses activate immune responses due to their repetitive surface structure and presence of pathogen associated molecular patterns (PAMPs).57 PAMPs are viral and microbial components that are recognized as foreign by the pattern recognition receptors, and their detection leads to a cascade of cytokine production and activation of innate immunity. VLPs share these similar components as viruses, providing an efficient platform to enhance immunogenicity and stability of low immunogenic antigens. Furthermore, they exhibit an additional advantage of lacking mammalian-replicable genetic material, rendering VLPs to be non-infectious to the host. VLPs have been explored as delivery systems for cancer vaccines (see Table 1), and this section will specifically focus on icosahedral plant viruses, rod-shaped plant viruses, and bacteriophage Qβ (Figure 2A). Specific examples are also highlighted in Figures 3A and 3B.
Table 1.
Summary of virus-like & caged protein nanoparticles that have been explored as delivery platforms for cancer vaccines.
| Protein NP | Antigen | Adjuvant | Target/ Cancer |
In vivo/ In vitro |
Clinical Study |
Response Investigated |
Ref |
|---|---|---|---|---|---|---|---|
| CPMV | Her2 | - | Breast | In vivo | - | Antibody | 49 |
| CPMV | Tn | Freund’s | Breast, colon, prostate | In vivo | - | Antibody | 60 |
| PVX | Her2 | - | Breast | In vivo | - | Antibody | 115 |
| PVX | Recombinant Id I | Alum | B-cell lymphoma | In vivo | - | T cell and antibody | 70 |
| TMV | Tn | Freund’s | - | In vivo | - | Antibody | 67 |
| TMV | SIINFEKL | CpG | - | In vitro, In vivo | - | T cell | 68 |
| TMV | p15e | CpG | Melanoma | In vitro, In vivo | - | T cell | 69 |
| Bacteriophage Qβ |
Melan-A26–35 | CpG and IFA | Melanoma | In vivo | Yes | T cell |
74, 75 |
| Recombinant HSP 110 | Her2/Neu | - | - | Ex vivo | - | T cell and antibody | 95 |
| Recombinant HSP 70 | MAGE-A1 | - | Melanoma | Ex vivo, In vivo | - | T cell | 94 |
| Recombinant HSP 110 | gp100 | - | Melanoma | Ex vivo, In vivo | - | T cell | 93 |
| Tumor derived HSP96 | - | - | Colorectal | yes | T cell | 85 | |
| Tumor derived HSP96 | - | - | Melanoma | yes | T cells | 86 | |
| Tumor derived HSP96 | - | - | Glioblastoma | Yes | T cell | 88 | |
| Tumor derived HSP70 | - | - | Lung | Yes | NK cell | 89 | |
| E2 | SIINFEKL | CpG | - | In vitro | - | T cell | 13 |
| E2 | gp100 | CpG | Melanoma | In vivo | - | T cell | 99 |
| E2 | NY-ESO-1 | CpG | Melanoma | Ex vivo | - | T cell | 102 |
| E2 | MAGE-A3 | CpG | Melanoma | Ex vivo | - | T cell | 102 |
| Ferritin | SIINFEKL | - | - | In vitro, Ex vivo | - | T cell and antibody | 103 |
| Ferritin | RFP | RFP-Melanoma | Ex vivo, In vivo | - | T cell | 106 | |
| Vault protein | SIINFEKL | - | - | In vitro, In vivo | - | T cell and antibody | 112 |
| Vault protein | CCL21 ligand | - | Lung | In vivo | - | Immune cells | 114 |
Figure 2. Protein structures of different virus-like particles (panel A), and caged protein nanoparticles (panel B).
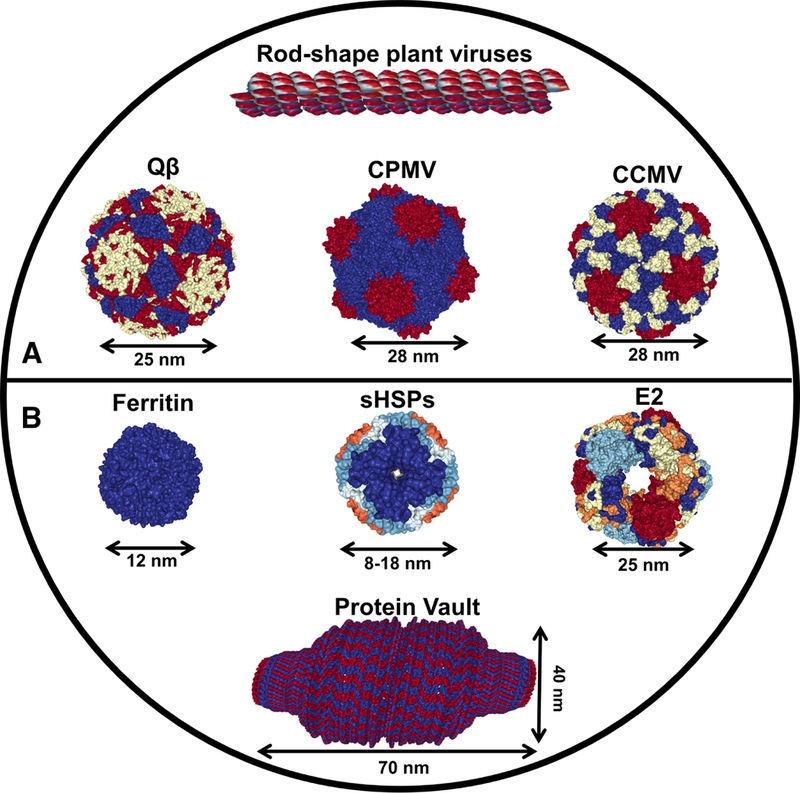
Structural images are from Protein Data Bank (PDB; http://www.rcsb.org/pdb/home/home.do). Structure of TMV is reconstructed from helical structure (PDB ID code: 3J06), Qβ (1QBE), CPMV (1NY7), CCMV (1ZA7), ferritin (1MFR), small HSPs (3VQK), E2 (1B5S), and protein vault (2QZV).
Figure 3. Examples of protein-based cancer vaccines.
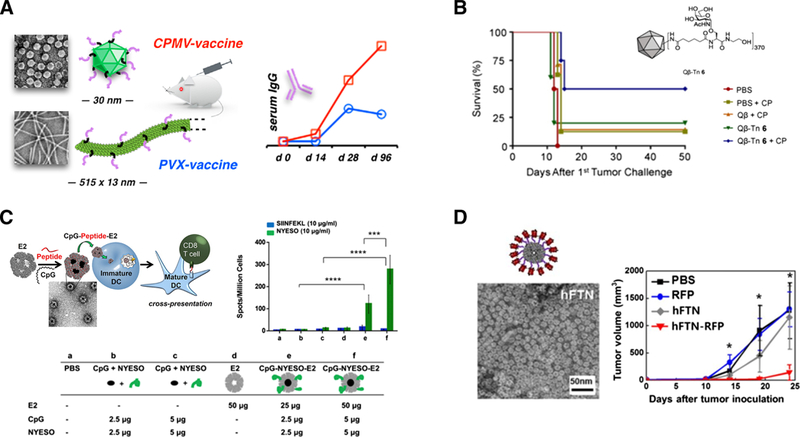
(A) TEM images and antibody responses of plant virus-based cancer vaccines. HER2 antigen conjugated to CPMV and PVX nanoparticles resulted in higher antibody responses. Reprinted from Shukla et al., Biomaterials 121, 15–27, copyright (2017); with permission from Elsevier. (B) Tumor-associated carbohydrate antigens conjugated to Qp resulted in increased survival of mice challenged with mammary tumor cells. Reprinted from Yin et al., ACS ChemBiol. 10, 2364–2372, copyright (2015); with permission from American Chemical Society. (C) E2 nanoparticles in cancer vaccine studies. Upper left: Schematic of E2 interaction with immune cells. High DC activation and antigen crosspresentation result when antigen and DC-activating molecules are both attached to E2 nanoparticles. Reprinted from Molino et al. ACS Nano 7, 9743–9752, copyright (2013); with permission from American Chemical Society. Upper right and bottom: Conjugation of human cancer-testis antigen and adjuvant to E2 nanoparticle increased specific IFN-γ secretion. Reprinted from Neek et al., Biomaterials 156, 194–203, copyright (2018); with permission from Elsevier. (D) TEM image of ferritin nanoparticles (left). Immunization with red fluorescence protein (RFP) significantly decreased the RFP-expressing melanoma tumor growth in mice. Reprinted from Lee et al., Scientific Reports 6: 35182 (2016). doi:10.1038/srep35182. License for use at http://creativecommons.org/licenses/by/4.0/.
Icosahedral plant viruses
One of the most studied icosahedral plant viruses is the cowpea mosaic virus (CPMV) which is a 28-nm capsid composed of 120 protein subunits (60 large and 60 small). CPMV has been shown to bind and be internalized by APCs in vitro and in vivo.58 Intraperitoneal injection of CPMV resulted in localization of these virus nanoparticles in the lymph nodes and APCs, leading to APC activation.49 Furthermore, in different metastatic cancer models, vaccination with empty CPMV without any antigens led to a longer survival time in mice. This unusual observation was correlated with an increase in the recruitment of tumor-infiltrating neutrophils and the production of cytokines that activate adaptive immune responses.59 In these mice immunized with CPMV, no noticeable signs of injury or inflammation were observed in the histology of reported organs.59
Attachment of cancer antigens onto CPMV has been effective in inducing antigen-specific responses. Subcutaneous immunization with CPMV conjugated with HER2 breast cancer epitopes (CH401 and P4) significantly increased HER2-specific antibody responses and tumor protection in murine models (Figure 3A).49 Delivery of low immunogenic Tn antigen, a tumor-associated carbohydrate antigen found in various cancers, with Freud’s adjuvant enhanced the production of Tn-specific IgG antibodies; these antibodies were capable of recognizing breast cancer cells.60
Other icosahedral plant viruses, such as alfafa mosaic virus and cowpea chlorotic mottle virus (CCMV), are also being engineered for antigen delivery. Antigen- conjugated incorporation of the two plant viruses have shown to elicit specific antibody and CD8+ T cell responses to infectious diseases.61,62 Although applied to communicable diseases, the generation of high antigen specific CD8+ T cell responses opens a promising potential of using those icosahedral plant viruses in cancer vaccines.63
Rod-shaped plant viruses
Some of the rod-shaped plant viruses investigated as vaccine constructs include tobacco mosaic virus (TMV) and potato virus X (PVX). These viruses have dimensions of 300 × 18 nm and 515 × 13 nm, respectively, and the filamentous structure and high aspect ratio of TMV has been reported to enhance tumor homing and penetration for drug delivery and tumor imaging.64 Furthermore, the large surface areas (relative to icosahedral viruses) allow for presenting a greater number of antigens and conjugating larger antigens. TMV and PVX showed efficient DC uptake which resulted in DC activation,65,66 and immunization with epitope-conjugated TMV in murine models increased the specific CD8 T cell responses in different infectious diseases.15
Vaccines using tumor-associated epitopes have also been developed with TMV and PVX. Immunization of Tn-conjugated TMV at tyrosine 139 resulted in higher Tn- specific IgG and IgM antibody responses when co-administered with Freund’s adjuvant.67 However, attachment of target antigens to the N-terminus of TMV monomers did not yield in any immune response,67 suggesting the importance of conjugation site in inducing immune responses. It was also reported that bivalent conjugation of two melanoma antigens (p15e and Trp2) to TMV increased tumor protection, compared to a mixture of monovalently conjugated p15e-TMV and Trp2-TMV. Antigen delivery (SIINFEKL or p15e) with TMV through reducible chemical conjugation and genetic modification was compared, and it was observed that the genetic insertion of antigen to TMV was not as effective in eliciting an immune response as chemical conjugation.69
PVX has also been used as an antigen delivery platform for different models of cancers. The entire idiotypic (Id) tumor antigen, derived from BCL1 lymphoma, has been presented on PVX through the streptavidin-biotin interaction. Id-tumor antigens are immunogenically weak, but immunization with Id-PVX resulted in higher anti-Id IgG antibody responses and higher survival rate after lymphoma challenge.70 HER2 breast cancer epitopes (CH401 and P4) have also been conjugated with PVX to generate increased antibody responses in murine model compared to soluble free epitopes.49
Bacteriophage Qβ
Bacteriophage Qβ is an E. coli RNA phage with a 25-nm self-assembled icosahedral capsid that consists of 178 capsid proteins. Vaccination with epitope- conjugated Qβ has led to activation of DCs, increase in pro-inflammatory cytokines, and antibody responses when formulated with CpG adjuvant.71 The toxicology of Qβ and the use of therapeutic Qβ for chronic disease immunotherapy have been reviewed elsewhere.72
Qβ has shown promise in antigen-specific cancer immunotherapy for several cancer models. Tumor-associated carbohydrate antigens have been conjugated to Qβ and resulted in increased survival of mice challenged with mammary tumor cells (Figure 3B).73 Melanoma-specific Melan-A/Mart-1 peptide was conjugated to Qβ with TLR9 adjuvant CpG (particle formulation CMP-001), and its efficacy has been examined in HLA-A2 transgenic murine models as well as in melanoma patients in a phase I/II clinical investigation.74 A phase II clinical study was performed using the same melanoma antigens with IFA and imiquimod, and 16/21 patients generated specific T cell responses ex vivo,75 with only mild or moderate local injection site responses reported.75 The same vaccine formulation is currently in two clinical studies examining combination therapy with blockade antibody anti-PD1 (Pembrolizumab) in patients with advanced stage melanoma (NCT0308464076 and NCT0268018477).
Caged Protein Nanoparticles
As previously described, highly-organized structures and symmetries of caged protein (CP) nanoparticles, their biodegradability, and their optimal size for delivery make them attractive vaccine platforms. CP NPs are protein assemblies that have virus-like structures and geometries, but are not from viral sources. Examples of CP NPs that have been used in the field of cancer immunotherapy are heat-shock proteins, E2, ferritin, and protein vault nanoparticles (Figure 2B), and we discuss these below. A summary is also presented in Table 1, with examples in Figures 3C and 3D.
Heat-shock proteins
Heat-shock proteins (HSPs), the most abundant class of chaperone proteins, are produced by cells in response to stress conditions.78 HSPs range in molecular sizes from 8 to 150 kDa, and are classified based on their molecular weights (e.g., hsp40, hsp70, hsp110).79 These proteins have been used as platforms for drug delivery.80 Furthermore, HSPs are overexpressed in a wide range of human cancers, and they are often involved in tumor cell proliferation, invasion, and progression.81
This overexpression of HSPs on tumors has made them an attractive source for anticancer vaccines. It has been observed that HSPs isolated from tumor cell lysates are bound to cancer antigens from the parental tumor.82 Therefore, one approach in using HSPs for cancer treatment is to extract these HSP-tumor antigen complexes from tumors and immunize with them.83 In this strategy, the tumor antigens bound to HSPs will be taken up more efficiently by DCs84 and activate the typical process described in Figure 1.84
Vaccination with HSP-tumor antigen complexes has been investigated for different cancer types in preclinical and clinical studies, and has primarily resulted in an increase in the CD8 T cell responses.85–89 There are also still ongoing clinical trials using autologous HSP-tumor antigen complexes, including gastric (NCT02317471),90 glioblastoma (NCT02122822),91 and liver (NCT02133079)92 cancers. Although studies have demonstrated efficacy and safety, one major limitation is the overall yield; the amount of vaccine obtained is dependent on the volume of tumor tissue isolated from the patients.83
The use of recombinant protein in designing HSP cancer vaccines partially addresses this yield limitation of tumor-derived HSPs. Recombinant HSPs serve as a carrier for the antigens of interest, which can be loaded through the chaperone-binding properties.93 Immunization of mice with recombinant HSPs containing gp100,93 MAGE, 94 or HER295 antigens resulted in a higher level of antigen-specific IFN-γ production, supporting T-cell activity. This translated to an increase in survival time for tumor- bearing animals immunized with HSPs-antigen complexes.93,94
E2 Protein Nanoparticle
The self-assembled protein nanoparticle E2 is derived from the E2 subunit of the pyruvate dehydrogenase complex from Bacillus stearothermophilus. It has been used in drug delivery and vaccines.96–99 The assembled NP is composed of 60 identical monomers that form a highly thermostable dodecahedral caged structure100 with a diameter of 25 nm, which is within the favored size range for lymphatic transport and DC uptake.40,41 This caged structure has an internal 12-nm cavity and twelve 5-nm openings leading to this hollow cavity. The scaffold has three interfaces (internal hollow cavity, subunit-subunit interface, and the exterior surface) which can be molecularly modified for site-directed functionalization.99–101
Biodistribution studies have shown that E2 is taken up effectively by DCs, with almost 50% of DCs within the draining LNs being associated with E2 NPs at 6 hrs post-injection.11 Furthermore, an even higher in vitro and in vivo uptake by APCs was observed when E2 was designed with DNA attached to the surface, compared to E2 alone.11 This efficient uptake by DCs suggests that the E2 NP could be an effective platform for the development of cancer vaccines.
Supporting this premise, studies have demonstrated a significantly higher DC activation and antigen cross-presentation when an OVA antigen (SIINFEKL) and DC- activating DNA (CpG) are both attached to E2 (Figure 3C, upper left).13 Simultaneous temporal and spatial delivery of OVA and CpG to DCs increased CD8 T cell activation in vitro,13 with concurrent delivery of antigen and CpG being essential to achieve the highest T cell activation.13 In vivo studies in C57BL/6 mice showed higher antigen- specific CD8 T cell proliferation and IFN-γ secretion when an epitope of the TAA gp100 was co-delivered with CpG using E2, compared to free antigen and CpG.99 This enhanced activity translated to an increased animal survival time in the aggressive B16- F10 melanoma tumor model.99
Applicability for solely-human TAAs was also reported. In a transgenic mouse model humanized with the HLA-A2 gene, significantly higher IFN-γ secretion and cell lysis activity were observed when the immunodominant epitopes of HLA-A2 restricted human cancer-testis antigens and CpG were coupled to E2 (Figure 3C, upper right and bottom).102 Furthermore, combined delivery of cancer epitopes from different antigen sources within E2 yielded an additive effect that increased lytic activity towards human cancer cells bearing the antigens.102 These investigations demonstrate that formulation of TAAs within E2 NPs can significantly enhance cell-mediated immune responses.
Ferritin
Ferritin protein cage nanoparticles self-assemble from identical subunits; for example, ferritin isolated from Pyrococcus furiosus comprises 24 subunits, forming a 12-nm diameter protein complex with a hollow cavity of 8 nm.103 Ferritins have been used for drug delivery, imaging, and targeting applications.104,105 A recent study demonstrated that ferritin protein cages carrying ovalbumin peptides were efficiently phagocytosed by DCs and resulted in a high specific CD8 T cell induction which selectively killed antigen-specific target cells.103 Human ferritin can also deliver red fluorescence protein (RFP) efficiently to LNs, with a high retention time up to six days after injection.106 This passive targeting to LNs resulted in higher RFP-specific cytotoxic CD8 T cell responses, which decelerated growth of RFP-expressing melanoma cells in vivo and increased animal survival time (Figure 3D).106
Protein vault nanoparticles
Vault nanoparticles are mammalian self-assembling ellipsoidal structures with an internal hollow cavity. These particles are highly uniform and are approximately 40 nm in width and 70 nm in length, with a mass of ~13 MDa.107–109 Vault nanoparticles have been used in applications such as cell targeting110 and drug delivery.111 Recent investigations suggested that vault nanoparticles have adjuvant properties that favor cell-mediated over humoral-mediated immune responses, and therefore can be advantageous for use in cancer vaccines to induce cell-mediated responses.112 A greater number of OVA CD8+ memory T cells and a higher level of specific IFN-γ production was observed when the same dose of OVA antigen was delivered through vault nanoparticles compared to liposomes.112 Elevated induction of CD8 T cells by vault nanoparticles could be a result of highly efficient internalization of these nanoparticles by DCs.113
Vault nanoparticles were also used to efficiently deliver CCL21 to the tumor microenvironment. This ligand plays an important role in the homing and localization of immune cells. Intratumoral injection of CCL21-modified DCs (with no vault nanoparticles) enhanced immune cell recruitment and inhibited lung tumor growth in a preclinical study.114 Although this CCL21-modified DC treatment was somewhat effective, the extensive work to isolate and culture autologous DC, often with a low DC yield, is a limitation. As an alternative strategy, vault nanoparticles were demonstrated to deliver the CCL21 ligand efficiently; this approach promoted the recruitment of T lymphocytes and DCs into the tumor microenvironment and resulted in antitumor activity towards an in vivo 3LL lung cancer model.114
Possible Immune Responses to the Delivery Platforms
Although immunogenicity is important in vaccine delivery, immune recognition to the nanoparticle platform itself could be a potential problem that leads to an antibody response resulting in neutralization and rapid clearance of the vaccine.116 Administration of protein nanoparticles such as CCMV, CPMV, and HSPs have yielded higher B cell counts and specific IgG antibody titers, which resulted in rapid clearance.117,118
Approaches to slow down clearance of different protein-based delivery systems have been investigated. PEGylation of particles such as PVX and bacteriophage Qβ led to increased plasma circulation and reduced non-specific immune recognition.66,119 With recent studies suggesting anti-PEG antibody production after repeated administration of PEGylated nanoparticles,120 alternatives to PEG are being developed. For example, the “self-marker” membrane protein CD47 ectodomain and its self-peptide have been displayed on nanobeads to avoid phagocyte-mediated clearance and resulted in 10-fold enhanced plasma circulation time.121 Furthermore, CD47 peptide decorated on the surface of VLP was shown to decrease phagocytosis in vitro.122 Other methods that mimic PEG using amino acids such as XTENylation123 and PASylation124 have also led to immune evasion and increased circulation time when conjugated to proteins, but further investigations are needed to validate these strategies on protein nanoparticles for antigen delivery.
Future Opportunities
Protein-based nanoparticle platforms present exciting opportunities to significantly improve cancer vaccines effectiveness. Co-delivery of high payloads of adjuvants and antigens within NPs promotes antigen-specific immune responses against cancers. While progress has been made in this field, there is still potential for improvement and for mechanistic understanding. One strategy to increase the efficacy of protein-based NP vaccines is combination of these vaccines with other FDA- approved treatments, such as blockade checkpoint inhibitors or immune-regulating drugs. An example of a drug with which to investigate co-delivery would be α-CTLA4 (ipilimumab); it was approved by the FDA in 2011 for treatment of patients with late-stage melanoma125 and belongs to the class of checkpoint blockade antibodies (e.g., α- CTLA4, α-PD1) shown to enhance anti-tumor responses.126,127
Synergistic anti-tumor activity has been observed when checkpoint blockade treatments are combined with cancer vaccines that are formulated with the immune regulatory cytokine GM-CSF in PLG.128 The synergistic effect results from the simultaneous boost in the T cell response (due to the vaccine), the recruitment of immune cells (e.g., APCs; due to GM-CSF), and a decrease in the T cell inhibition (due to the checkpoint inhibitor). Similar combination strategies of protein-based nanoparticle vaccines with other treatments also have strong potential to improve the efficacy of NP- based vaccines.129
Summary
The delivery of cancer antigens within protein-based NPs can potentially increase the antigen uptake and interaction by APCs, resulting in an increase in cell- mediated immune responses specific to the particular cancer antigen. These advantages are likely enabled by physical properties, co-delivery of bioactive chemical elements, and geometries of the NPs that are similar to viruses. Recent studies have shown that the effectiveness of antigen-based cancer vaccines is improved when NPs are used as delivery platforms, and we are now observing the emergence of this approach in immunotherapy-based vaccines. Future studies will reveal the feasibility of using protein-based NPs and their involvement in combination therapy in the clinical field of cancer treatment.
Acknowledgment
We gratefully acknowledge support from the National Institute of Health (R21EB017995), the University of California Cancer Research Coordinating Committee (CTR-18–524776), and the University of California, Irvine (Graduate Division and the Henry Samueli School of Engineering).
Funding: National Institute of Health (R21EB017995), the University of California Cancer Research Coordinating Committee (CTR-18–524776), and the University of California, Irvine (Graduate Division and the Henry Samueli School of Engineering).
Abbreviations:
- APC
antigen presenting cell
- CCMV
cowpea chlorotic mottle virus
- CP
caged protein
- CpG
unmethylated cytosine-phosphodiester-guanine rich single-stranded oligonucleotides
- CPMV
cowpea mosaic virus
- DC
dendritic cell
- E2
caged E2-subunit assembly of pyruvate dehydrogenase
- DC
dendritic cell
- HSP
heat shock protein
- Id
idiotypic antigen
- LN
lymph node
- MHC
major histocompatibility complex
- NP
nanoparticle
- OVA
ovalbumin
- PVX
potato virus X
- TAA
tumor associated antigen
- RFP
red fluorescence protein
- TMV
tobacco mosaic virus
- VLP
virus-like particle
- Qβ
bacteriophage Qβ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004; 10: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Even-Desrumeaux K, Baty D, Chames P. State of the Art in T umor Antigen and Biomarker Discovery. Cancer 2011;3: 2554–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vesely MD, Schreiber RD. Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy. Ann. N. Y. Acad. Sci 2013; 1284: 1–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyes D, Salazar L, Espinoza E, Pereda C, Castellón E, Valdevenito R,et al. Tumour cell lysate-loaded dendritic cell vaccine induces biochemical and memory immune response in castration-resistant prostate cancer patients. Br. J. Cancer 2013; 109: 1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat. Med. 2013; 19: 1597–1608. [DOI] [PubMed] [Google Scholar]
- 6.Zhao L, Seth A, Wibowo N, Zhao C, Mitter N, Yu C, Middelberg APJ. Nanoparticle vaccines. Vaccines 2014; 32: 327–337. [DOI] [PubMed] [Google Scholar]
- 7.Park YM, Lee SJ, Kim YS, Lee MH, Cha GS, Jung ID, et al. Nanoparticle-based vaccine delivery for cancer immunotherapy. Immune Netw 2013; 13: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnamachari Y, Geary SM, Lemke CD, Salem AK. Nanoparticle delivery systems in cancer vaccines. Pharm. Res. 2011; 28: 215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther. Adv. Vaccines 2014; 2: 159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co- glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv. Drug Deliv. Rev. 2005; 57:391–410. [DOI] [PubMed] [Google Scholar]
- 11.Molino NM, Neek M, T ucker JA, Nelson EL, Wang SW. Display of DNA on Nanoparticles for Targeting Antigen Presenting Cells. ACS Biomater. Sci. Eng. 2017; 3: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008; 38:1404–1413. [DOI] [PubMed] [Google Scholar]
- 13.Molino NM, Anderson AKL, Nelson EL, Wang SW. Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano 2013; 7: 9743–9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohovie MJ, Nagasawa M, Swartz JR. Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioeng. Transl. 2016; Med. 2: 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lebel MÈ, Chartrand K, Leclerc D, Lamarre A. Plant Viruses as Nanoparticle-Based Vaccines and Adjuvants. Vaccines 2015; 3:620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.le EV, Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol. Immunother. 2005; 54: 187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A. Cancer Immunotherapy With Peptide-Based Vaccines: What Have We Achieved ? Where Are We Going ? J. Natl. Cancer Inst. 1991; 94: 805–818. [DOI] [PubMed] [Google Scholar]
- 18.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 2014; 11: 509–524. [DOI] [PubMed] [Google Scholar]
- 19.Temizoz B, Kuroda E, Ishii KJ. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016; 28: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins D, Marshall JD, Traquina P, Nest GV, Livingston BD. Immunostimulatory DNA as a vaccine adjuvant. Expert Rev Vaccines 2007; 6: 747–759. [DOI] [PubMed] [Google Scholar]
- 21.Shirota H, Tross D, Klinman DM. CpG Oligonucleotides as Cancer Vaccine Adjuvants. Vaccines 2015; 3: 390–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheiermann J, Klinman DM. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014; 32: 6377–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ammi R, Waele J, Willemen Y, Brussel IV, Schrijvers M, Lion E, Smits EL J. Pharmacology & Therapeutics Poly (I :C) as cancer vaccine adjuvant : Knocking on the door of medical breakthroughs. Pharmacol. Ther. 2015; 146: 120–131. [DOI] [PubMed] [Google Scholar]
- 24.Wischke C, Zimmermann J, Wessinger B, Schendler A, Borchert HH., Peters JH, et al. . Poly(I:C) coated PLGA microparticles induce dendritic cell maturation. Int. J. Pharm. 2009; 365: 61–68. [DOI] [PubMed] [Google Scholar]
- 25.Johnston D, Zaidi B. TLR7 imidazoquinoline ligand 3M-019 is a potent adjuvant for pure protein prototype vaccines. Cancer Immunol. Immunother. 2007; 56: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith AJ, Li Y, Bazin HG, St-Jean JR, Larocque D, Evans JT, Baldridge JR. Evaluation of novel synthetic TLR7 / 8 agonists as vaccine adjuvants. Vaccine 2016; 34: 4304–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshitake Y, Fukuma D, Yuno A, Hirayama M, Nakayama H, Tanaka T, et al. Phase II Clinical Trial of Multiple Peptide Vaccination for Advanced Head and Neck Cancer Patients Revealed Induction of Immune Responses and Improved OS. Clin. Cancer Res. 2014; 21: 312–21. [DOI] [PubMed] [Google Scholar]
- 28.Yoshitake Y, Fukuma D, Yuno A, Hirayama M, Nakayama H, Tanaka T, et al. N A phase I study of combination vaccine treatment of five therapeutic epitope-peptides for metastatic colorectal cancer; safety , immunological response , and clinical outcome. J. Transl. Med. 2014; 12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aruga A, Takeshita N, Kotera Y, Okuyama R, Matsushita N, Ohta T. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J. Transl. Med. 2014; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasada T, Yamada A, Noguchi M, Itoh K. Personalized Peptide Vaccine for Treatment of Advanced Cancer. Curr Med Chem 2014; 21: 2332–2345. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto S, Yamada T, Terazaki Y, Yoshiyama K, Sugawara S, Takamori S, et al. Feasibility Study of Personalized Peptide Vaccination for Advanced Small Cell Lung Cancer. Clin. Lung Cancer 2017; 18: 1–10. [DOI] [PubMed] [Google Scholar]
- 32.Banchereau J, Palucka K, Dhodapkar M, Burkeholder ., Taquet N, Rolland A, et al. Immune and Clinical Responses in Patients with Metastatic Melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001; 61: 6451–6458. [PubMed] [Google Scholar]
- 33.Fay JW, Palucka AK, Johnston DA, Burkeholder S. Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34 + progenitor- derived dendritic cells. Cancer Immunol. Immunother. 2006; 55: 1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura T, Egawa S, Uemura H. Personalized peptide vaccines and their relation to other therapies in urological cancer. Nat. Rev. Urol. 2017; 14: 501–510. [DOI] [PubMed] [Google Scholar]
- 35.Fehres CM, Unger WWJ, Garcia-Vallejo JJ, van Kooyk Y. Understanding the biology of antigen cross-presentation for the design of vaccines against cancer. Front. Immunol. 2014; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010; 10: 787–96. [DOI] [PubMed] [Google Scholar]
- 37.Bousso P, Robey E. Dynamics of CD8 + T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 2003; 4: 579–585. [DOI] [PubMed] [Google Scholar]
- 38.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014; 66: 2–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat. Rev. Cancer 2014; 14: 559–567. [DOI] [PubMed] [Google Scholar]
- 40.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 2006; 112: 26–34. [DOI] [PubMed] [Google Scholar]
- 41.Reddy ST, Vlies AJ, Simeoni E, O’Neil CP, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007; 25: 1159–1164. [DOI] [PubMed] [Google Scholar]
- 42.Swartz MA. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 2001; 50: 3–20. [DOI] [PubMed] [Google Scholar]
- 43.Oussoren C, Zuidema J, Crommelin DJA, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. Influence of liposomal size, lipid composition and lipid dose. Biochim. Biophys. Acta - Biomembr. 1997; 1328: 261–272. [DOI] [PubMed] [Google Scholar]
- 44.Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H. Gold Nanoparticles as a Vaccine Platform : Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano 2013; 7: 3926–3938. [DOI] [PubMed] [Google Scholar]
- 45.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. 2006; 103: 4930–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conniot J, Silva JM, Fernandes JG, Silva LC, Gaspar R, Brocchini S, et al. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front. Chem. 2014; 2: 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Kröger M, Liu WK. Shape effect in cellular uptake of PEGylated nanoparticles: comparison between sphere, rod, cube and disk. Nanoscale 2015; 7: 16631–16646. [DOI] [PubMed] [Google Scholar]
- 48.Kumar S, Anselmo AC, Banerjee A, Zakrewsky M, Mitragotri S. Shape and size- dependent immune response to antigen-carrying nanoparticles. J. Control. Release 2015; 220: 141–148. [DOI] [PubMed] [Google Scholar]
- 49.Shukla S, Myers JT, Woods SE, Gong X, Czapar AE, Commandeur U, et al. Plant viral nanoparticles-based HER2 vaccine : Immune response in fl uenced by differential transport , localization and cellular interactions of particulate carriers. Biomaterials 2017; 121: 15–27. [DOI] [PubMed] [Google Scholar]
- 50.Verma A, Stellacci F. Effect of surface properties on nanoparticle-cell interactions. Small 2010; 6: 12–21. [DOI] [PubMed] [Google Scholar]
- 51.Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011; 32: 3435–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010; 31: 3657–3666. [DOI] [PubMed] [Google Scholar]
- 53.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016; 1: 1–12. [Google Scholar]
- 54.Walkey CD, Olsen JB, Song F, Liu R, Guo H, Olsen DWH, et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 2014; 8: 2439–2455. [DOI] [PubMed] [Google Scholar]
- 55.Ritz S, Schöttler S, Kotman N, Baier G, Musyanovych A, Kuharev J, et al. Protein Corona of Nanoparticles: Distinct Proteins Regulate the Cellular Uptake. Biomacromolecules 2015; 16: 1311–1321. [DOI] [PubMed] [Google Scholar]
- 56.Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012; 134: 2139–2147. [DOI] [PubMed] [Google Scholar]
- 57.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signals 0s taht spur autophagy and inmunity. Immunol Rev 2013; 249: 158–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez MJ, Plummer EM, Rae CS, Manchester M. Interaction of Cowpea mosaic virus (CPMV) nanoparticles with antigen presenting cells in vitro and in vivo. PLoS One 2009; 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, Fiering S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2015; 11: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miermont A, Barnhill H, Strable E, Lu X, Wall KA, Wang Q, et al. Cowpea mosaic virus capsid: A promising carrier for the development of carbohydrate based antitumor vaccines. Chem. - A Eur. J. 2008; 14: 4939–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ortega-Berlanga B, Musiychuk K, Shoji Y, Chichester JA, Yusibov V, Patino-Rodrlguez O, et al. Engineering and expression of a RhoA peptide against respiratory syncytial virus infection in plants. Planta 2016; 243: 451–458. [DOI] [PubMed] [Google Scholar]
- 62.Hassani-Mehraban A, Creutzburg S, Heereveld L, Kormelink R. Feasibility of Cowpea chlorotic mottle virus-like particles as scaffold for epitope presentations. BMC Biotechnol. 2015; 15: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li S, Symonds ALJ, Miao T, Sanderson I, Wang P. Modulation of antigen-specific T-cells as immune therapy for chronic infectious diseases and cancer. Front. Immunol. 2014; 5: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shukla S, Ablack AL, Wen AM, Lee KL, Lewis JD, Steinmetz NF. Increased tumor homing and tissue penetration of the filamentous plant viral nanoparticle potato virus X. Mol. Pharm. 2013; 10: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kemnade JO, Seethammagari M, Collinson-Pautz M, Kaur H, Spencer DM, McCormick AA. Tobacco mosaic virus efficiently targets DC uptake, activation and antigen-specific T cell responses in vivo. Vaccine 2014; 32: 4228–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee KL, Shukla S, Wu M, Ayat NR, El Sanadi CE, Wen AM, et al. Stealth filaments: Polymer chain length and conformation affect the in vivo fate of PEGylated potato virus X. Acta Biomater. 2015; 19: 166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin ZJ, Nguyen HG, Chowdhury S, Bentley P, Bruckman MA, Miermont A, et al. Tobacco Mosaic Virus as a New Carrier for T umor Associated Carbohydrate Antigens. Bioconjug. Chem. 2012; 23: 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCormick AA, Corbo TA, Wykoff-Clary S, Palmer KE, Pogue GP. Chemical conjugate TMV - Peptide bivalent fusion vaccines improve cellular immunity and tumor protection. Bioconjug. Chem. 2006; 17: 1330–1338. [DOI] [PubMed] [Google Scholar]
- 69.McCormick AA, Corbo TA, Wykoff-Clary S, Nguyen LV, Smith ML. Palmer KE, Pogue GP. TMV-peptide fusion vaccines induce cell-mediated immune responses and tumor protection in two murine models. Vaccine 2006; 24, 6414–6423. [DOI] [PubMed] [Google Scholar]
- 70.Jobsri J, Allen A, Rajagopal D, Shipton M, Kanyuka K, Lomonossoff GP, et al. Plant virus particles carrying tumour antigen activate TLR7 and induce high levels of protective antibody. PLoS One 2015; 10: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwarz K, Meijerink E, Speiser DE, Tissot AC, Cielens I, Renhof R, et al. Efficient homologous prime-boost strategies for T cell vaccination based on virus-like particles. Eur. J. Immunol. 2005; 35: 816–821. [DOI] [PubMed] [Google Scholar]
- 72.Jennings GT, Bachmann MF. Immunodrugs: Therapeutic VLP-Based Vaccines for Chronic Diseases. Annu. Rev. Pharmacol. Toxicol. 2009; 49: 243–263. [DOI] [PubMed] [Google Scholar]
- 73.Yin Z, Chowdhury S, Craig M, Baniel C. Significant Impact of Immunogen Desgin on the Diversity of Antibodies Generated by Carbohydrate-Based Anticancer Vaccine. ACS Chem Biol. 2015; 10: 2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Speiser DE, Schwarz K, Baumgaertner P, Manolova V, Devevre E, Sterry W. et al. Memory and effector CD8 T-cell responses after nanoparticle vaccination of melanoma patients. J. Immunother. 2010; 33: 848–858. [DOI] [PubMed] [Google Scholar]
- 75.Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, Hammann- Haenni A, et al. Nano-particle vaccination combined with TLR-7 and −9 ligands triggers memory and effector CD8+ T-cell responses in melanoma patients. Eur. J. Immunol. 2012; 42: 3049–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phase 1b Study Evaluating Alternative Routes of Administration of CMP-001 in Combination With Pembrolizumab in Subjects With Advanced Melanoma. Available at: https://clinicaltrials.gov/ct2/show/NCT03084640. (Accessed: 2nd March 2018)
- 77.Clinical Study of CMP-001 in Combination With Pembrolizumab or as a Monotherapy. Available at: https://clinicaltrials.gov/ct2/show/NCT02680184. (Accessed: 2nd March 2018)
- 78.Feder ME, Hofmann GE. Heat-Shock Proteins, Molecular Chaperones, and the Stress Response: Evolutionary and Ecological Physiology, Annu. Rev. Physiol. 1999; 61: 243–282. [DOI] [PubMed] [Google Scholar]
- 79.Whitley D, Goldberg SP, Jordan WD. Heat shock proteins: A review of the molecular chaperones. J. Vasc. Surg. 1999; 29: 748–751. [DOI] [PubMed] [Google Scholar]
- 80.Molino NM, Wang SW. Caged protein nanoparticles for drug delivery. Curr. Opin. Biotechnol. 2014; 28: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia-Soto NSA. Secreted heat shock protein gp96-Ig : next-generation vaccines for cancer and infectious diseases. Immunol. Res. 2013; 57: 311–325. [DOI] [PubMed] [Google Scholar]
- 82.Srivastava P Interaction of Heat-Shock Proteins with Peptides and Antigen Presenting Cells: Chaperoning of the Innate and Adaptive Immune Responses. Annu. Rev. Immunol. 2002; 20: 395–425. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Zheng L. Tumor immunotherapy based on tumor - derived heat shock proteins ( Review). Oncol. Lett 2013; 6: 1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, Salle H D, Hanau D. Cutting Edge: Receptor-Mediated Endocytosis of Heat Shock Proteins by Professional Antigen-Presenting Cells. J. Immunol. 1999; 162: 3757–60. [PubMed] [Google Scholar]
- 85.Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, et al. Vaccination with Autologous Tumor-derived Heat-Shock Protein Gp96 after Liver Resection for Metastatic Colorectal Cancer. Clin Cancer Res. 2003; . 9: 3235–3245. [PubMed] [Google Scholar]
- 86.Belli BF, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, et al. Vaccination of Metastatic Melanoma Patients With Autologous Tumor-Derived Heat Shock Protein gp96- Peptide Complexes: Clinical and Immunologic Findings. J. Clin. Oncol. 2002; 20: 4169–4180. [DOI] [PubMed] [Google Scholar]
- 87.Pilla L, Patuzzo R, Rivoltini L, Maio M, Pennacchioli E, Larnja E, et al. A phase II trial of vaccination with autologous , tumor-derived heat-shock protein peptide complexes Gp96 , in combination with GM-CSF and interferon- a in metastatic melanoma patients. Cancer Immunol. Immunother. 2006; 55: 958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crane CA, Han SJ, Ahn B, Oehlke J, Kivett V, Fedoroff A, et al. Individual patient- specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clin. Cancer Res. 2013; 19: 205–214. [DOI] [PubMed] [Google Scholar]
- 89.Specht HM, Ahrens N, Blankenstein C, Duell T, Fietkau R, Gaipl US, et al. Heat shock protein 70 (Hsp70) peptide activated Natural Killer (NK) cells for the treatment of patients with non-small cell lung cancer (NSCLC) after radiochemotherapy (RCTx) - from preclinical studies to a clinical phase II trial. Front. Immunol. 2015; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Immunotherapy of Gastric Cancer With Autologous T umor Derived Heat Shock Protein gp96. Available at: https://clinicaltrials.gov/ct2/show/NCT02317471?cond=NCT02317471&rank=1. (Accessed: 2nd March 2018)
- 91.Research for Immunotherapy of Glioblastoma With Autologous Heat Shock Protein gp96. Available at: https://clinicaltrials.gov/ct2/show/NCT02122822?cond=NCT02122822&rank=1. (Accessed: 2nd March 2018)
- 92.Immunotherapy of Tumor With Autologous Tumor Derived Heat Shock Protein gp96. Available at: https://clinicaltrials.gov/ct2/show/NCT02133079?cond=NCT02133079&rank=1. (Accessed: 2nd March 2018)
- 93.Wang X, Chen X, Manjili MH, Repasky E, Henderson R, Subjeck JR. Targeted Immunotherapy Using Reconstituted Chaperone Complexes of Heat Shock Protein 110 and Melanoma-associated Antigen gp100 1. Cancer Res. 2003; 63: 2553–2560. [PubMed] [Google Scholar]
- 94.Ge W, Li Y, Zhang ZLS, Hu YSP, Yang XW, Si HS, Sui XZY. The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70 / SEA complex protein vaccine following peroral administration route. Cancer Immunol. Immunother. 2009; 58: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manjili MH, Henderson R, Wang X, Chen X, Li Y, Repasky E, Kazim L, Subjeck JR. Development of a recombinant HSP110-HER-2 / neu vaccine using the chaperoning properties of HSP110. Cancer Res. 2002; 62: 1737–1742. [PubMed] [Google Scholar]
- 96.Ren D, Kratz F, Wang SW. Engineered drug-protein nanoparticle complexes for folate receptor targeting. Biochem. Eng. J. 2014; 89: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ren D, Kratz F, Wang SW. Protein nanocapsules containing doxorubicin as a pH- responsive delivery system. Small 2011; 7: 1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jaworski JP, Krebs SJ, Trovato M, Kovarik DN, Brower Z, Sutton WF, et al. Coimmunization with multimeric scaffolds and DNA rapidly induces potent autologous HIV-1 neutralizing antibodies and CD8 + T cells. PLoS One 2012; 7: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Molino NM, Neek M, Tucker JA, Nelson EL, Wang SW Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials 2016; 86: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dalmau M, Lim S, Chen HC, Ruiz C, Wang SW. Thermostability and molecular encapsulation within an engineered caged protein scaffold. Biotechnol. Bioeng. 2008; 101: 654–664. [DOI] [PubMed] [Google Scholar]
- 101.Molino NM, Bilotkach K, Fraser D, Ren D, Wang SW. Complement activation and cell uptake responses toward polymer-functionalized protein nanocapsules. Biomacromolecules 2010; 13: 974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neek M, Tucker JA, Kim TI., Molino NM, Nelson EL, Wang SW. Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials 2018; 156: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han J, Kang YJ, Shin C, Ra J, Shin H, Hong SY, et al. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell ( DC ) -based vaccine development. Nanomedicine Nanotechnology, Biol. Med 2014; 10: 561–569 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Zhen Z, Tang W, Todd , Xie J. Ferritins as nanoplatforms for imaging and drug delivery Ferritins as nanoplatforms for imaging and drug delivery. Expert Opin. Drug Deliv. 2014; 11:1913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Truffi M, Fiandra L, Sorrentino L, Monieri M., Corsi F, Mazzucchelli S. Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol. Res. 2016; 107: 57–65. [DOI] [PubMed] [Google Scholar]
- 106.Lee B, Ko HK, Ryu JH, Ahn KY, Lee Y, Oh SJ, et al. Engineered human ferritin nanoparticles for direct delivery of tumor antigens to lymph node and cancer immunotherapy. Nat. Publ. Gr. 2016; 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buehler DC, Marsden MD, Shen S, Toso DB, Wu X, Loo JA, et al. Bioengineered vaults: self-assembling protein shell-lipophilic core nanoparticles for drug delivery. ACS Nano . 2014; 8: 7723–7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matsumoto NM, Prabhakaran P, Rome LH, Maynard HD. Smart vaults: thermally- responsive protein nanocapsules. ACS Nano 2013; 7: 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benner NL, Zang X, Buehler DC, Kickhoefer VA, Rome ME, Rome LH, Wender PA. Vault Nanoparticles: Chemical modifications for imaging and enhanced delivery. ACS Nano 2017; 11: 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kickhoefer VA, Han M, Raval-Fernandes S, Poderycki MJ, Moniz RJ, Vaccari D, et al. Targeting vault nanoparticles to specific cell surface receptors. ACS Nano 2009; 3: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buehler DC, Toso DB, Kickhoefer VA, Zhou ZH, Rome LH. Vaults Engineered for Hydrophobic Drug Delivery. Small 2011; 7: 1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kar UK, Jiang J, Champion CI, Salehi S, Srivastava ., Sharma S, et al. Vault nanocapsules as adjuvants favor cell-mediated over antibody-mediated immune responses following immunization of mice. 2012; 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Champion CI, Kickhoefer VA, Liu G, Moniz RJ, Freed AS, Bergmann LL, et al. A vault nanoparticle vaccine induces protective mucosal immunity. PLoS One 2009, 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baratelli F, Huang M, Valerie A, Kar UK, Srivastava MK, Rome LH, et al. Novel CCL21- Vault Nanocapsule Intratumoral Delivery Inhibits Lung Cancer Growth. PLoS One 2011;6: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shukla S, Wen AM, Commandeur U, Steinmetz NF. Presentation of HER2 epitopes using a filamentous plant virus-based vaccination platform. J. Mater. Chem. B 2014; 2: 6249 – 6258 [DOI] [PubMed] [Google Scholar]
- 116.Da Silva DM, Pastrana DV, Schiller JT, Kast WM. Effect of preexisting neutralizing antibodies on the anti-tumor immune response induced by chimeric human papillomavirus virus-like particle vaccines. Virology 2001; 290: 350–360. [DOI] [PubMed] [Google Scholar]
- 117.Singh P, Prasuhn D, Yeh RM, Destito G, Rae CS, Osborn K, et al. Bio-distribution, toxicity and pathology of cowpea mosaic virus nanoparticles in vivo. J. Control. Release 2007; 120: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaiser CR, Flenniken ML, Gillitzer E, Harmsen AL, Harmsen AG, Jutila MA, et al. Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo. Int. J. Nanomedicine 2007; 2: 715–733. [PMC free article] [PubMed] [Google Scholar]
- 119.Prasuhn DE, Singh P, Strable E, Brown S, Manchester M, Finn MG. Plasma clearance of bacteriophage Qb particles as a function of surface charge. J. Am. Chem. Soc. 2008; 130: 1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang Q, Lai SK. Anti-PEG immunity: emergence , characteristics , and unaddressed. Nanomed Nanobiotechnology 2015; 7: 655–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal ‘self’ peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013; 339: 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwarz B, Madden P, Avera J, Gordon B, Larson K, Miettinen HM, et al. Symmetry Controlled, Genetic Presentation of Bioactive Proteins on the P22 Virus-like Particle Using an External Decoration Protein. ACS Nano 2015; 9: 9134–9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schellenberger V, Wang C, Geething NC, Spink BJ, Campbell A, To W, et al. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat. Biotechnol. 2009; 27: 1186–1190. [DOI] [PubMed] [Google Scholar]
- 124.Theobald I, Wachinger K, Kisling S, Haller D, Skerra A. PASylation : a biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins. Protein Engineering, Design & Selection. 2013; 26: 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, et al. Development of ipilimumab: A novel immunotherapeutic approach for the treatment of advanced melanoma. Ann. N. Y. Acad. Sci. 2013; 1291: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dyck L, Wilk MM, Raverdeau M, Misiak A, Boon L, Mills KHG. Anti-PD-1 inhibits Foxp3+Treg cell conversion and unleashes intratumoural effector T cells thereby enhancing the efficacy of a cancer vaccine in a mouse model. Cancer Immunol. Immunother 2016; 65: 1491–1498 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. 2010; 107: 4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ali OA, Lewin SA, Dranoff G, Mooney DJ. Vaccines Combined with Immune Checkpoint Antibodies Promote Cytotoxic T-cell Activity and Tumor Eradication. Cancer Immunol. Res. 2016; 4: 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014; 74: 4042–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]


